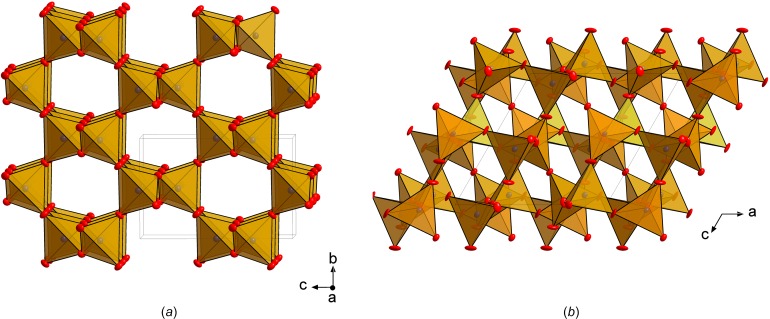
The crystal structures of two phosphorus oxonitride polymorphs (cristobalite- and coesite-type) were redetermined by means of single-crystal X-ray diffraction data.
Keywords: crystal structure, phosphorus oxonitride, silica analogues, redetermination
Abstract
Hitherto, phosphorus oxonitride (PON) could not be obtained in the form of single crystals and only powder diffraction experiments were feasible for structure studies. In the present work we have synthesized two polymorphs of phosphorus oxonitride, cristobalite-type (cri-PON) and coesite-type (coe-PON), in the form of single crystals and reinvestigated their crystal structures by means of in house and synchrotron single-crystal X-ray diffraction. The crystal structures of cri-PON and coe-PON are built from PO2N2 tetrahedral units, each with a statistical distribution of oxygen and nitrogen atoms. The crystal structure of the coe-PON phase has the space group C2/c with seven atomic sites in the asymmetric unit [two P and three (N,O) sites on general positions, one (N,O) site on an inversion centre and one (N,O) site on a twofold rotation axis], while the cri-PON phase possesses tetragonal I-42d symmetry with two independent atoms in the asymmetric unit [the P atom on a fourfold inversion axis and the (N,O) site on a twofold rotation axis]. In comparison with previous structure determinations from powder data, all atoms were refined with anisotropic displacement parameters, leading to higher precision in terms of bond lengths and angles.
Chemical context
The pseudo-binary system P3N5/P2O5 has been investigated intensively because the properties of related ceramic materials are promising for industrial applications. A mid-member of this system is phosphorus oxonitride (PON), whose chemical stability is essential for its use as an insulator or for fireproofing. This compound has attracted significant attention as a ternary base compound of electrolytes for rechargeable thin-film Li/Li-ion batteries. Phosphorus oxonitride is an isoelectronic analogue of silica (SiO2) with the charge-balanced substitution P5+ + N3− = Si4+ + O2−. The crystal structures of the polymorphic forms of SiO2 and PON are built of tetrahedral SiO4 and PO2N2 units, respectively. At present, five modifications of PON have been identified. Four of them are isostructural to known silica polymorphs, viz. α-quartz- (Léger et al., 1999 ▸), β-cristobalite- (Léger et al., 2001 ▸), moganite- (Chateau et al., 1999 ▸) and coesite-type (Baumann et al., 2015 ▸). The fifth one, δ-PON, has a structure type different from any of the silica modifications (Baumann et al., 2012 ▸). A rich variety of polymorphs is a result of the many ways in which the tetrahedra can be linked to form corner-sharing networks. Most of the phases in the P3N5/P2O5 system are usually obtained either in an amorphous state or in the form of powders consisting of very small crystallites. We succeeded in synthesizing single crystals of pure cristobalite- (cri) and coesite-type (coe) PON of a size suitable for single-crystal X-ray diffraction and report here the results of the structure refinements.
Structural commentary
The structure of cri-PON (Fig. 1 ▸
a) can be derived from that of β-cristobalite by tilting each PO2N2 tetrahedron about the 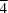 axes alternately clockwise and anticlockwise. This leads to the lowering of symmetry from Fd
axes alternately clockwise and anticlockwise. This leads to the lowering of symmetry from Fd
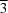 m to I
m to I
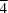 2d, however, the topology remains the same. The length of the P—(O,N) bond in cri-PON is 1.5796 (10) Å, which is in a good agreement with the average of expected P—N (1.626 Å) and P—O (1.537 Å) distances (Huminicki & Hawthorne, 2002 ▸). All P—(O,N) distances within the PO2N2 units are equal, but there is a noticeable (O,N)—P—(O,N) angle variation between 107.86 (2) and 112.73 (5)° due to the compression of the tetrahedra along the c-axis direction.
2d, however, the topology remains the same. The length of the P—(O,N) bond in cri-PON is 1.5796 (10) Å, which is in a good agreement with the average of expected P—N (1.626 Å) and P—O (1.537 Å) distances (Huminicki & Hawthorne, 2002 ▸). All P—(O,N) distances within the PO2N2 units are equal, but there is a noticeable (O,N)—P—(O,N) angle variation between 107.86 (2) and 112.73 (5)° due to the compression of the tetrahedra along the c-axis direction.
Figure 1.
Crystal structures of cri-PON (a) and coe-PON (b) shown in polyhedral representation. Displacement parameters are drawn at the 50% probability level. Mixed (N,O) sites are shown in red; P atoms are shown in brown.
The structure of coe-PON (Fig. 1 ▸ b) is isotypic with coesite (SiO2) (Angel et al., 2003 ▸). The framework of coe-PON is constructed of four-member rings comprised of corner-sharing PO2N2 tetrahedra. These rings are linked in such a manner that crankshaft-like chains are formed. The average P—(O,N) distance in coe-PON (1.572 Å) is slightly shorter than that of 1.581 Å reported by Baumann et al. (2015 ▸) likely due to the difference in temperatures at which the experiments were conducted. The tetrahedra are irregularly distorted, with P—(O,N) distances varying between 1.5530 (9) and 1.588 (3) Å, and (O,N)—P—(O,N) angles between 106.79 (19) and 112.0 (2)°.
In comparison with the refinements from powder diffraction data (Léger et al., 2001 ▸; Baumann et al., 2015 ▸), single-crystal diffraction data revealed a detailed electron density map, which allowed us in addition to a substitutional O-N disorder, to detect a possible positional disorder (for details see Refinement section), which may affect physical properties of coe-PON.
Synthesis and crystallisation
Cristobalite-type PON was synthesized from phosphoric triamide by a two-step condensation process. POCl3 (99%, Sigma Aldrich) was reacted with liquid NH3 (5.0, Air Liquide) to yield a mixture of PO(NH2)3 and NH4Cl, which was subsequently heated to 893 K for 5 h in a stream of dry ammonia. The amorphous reaction product was crystallized at 1023 K for 7 d in an evacuated fused silica ampoule, yielding pure cristobalite-type PON. Coesite-type PON was obtained by high-pressure/high-temperature reaction of cri-PON in a modified Walker-type multi-anvil apparatus. The starting material was tightly packed in a h-BN capsule, which was centered in a MgO:Cr octahedron (Ceramic Substrates & Components, Isle of Wight, UK) with an edge length of 10 mm. The latter was subsequently compressed between eight truncated tungsten carbide cubes (5 mm truncation edge length, Hawedia, Marklkofen, Germany) using a 1000 t hydraulic press (Voggenreiter, Mainleus, Germany). The sample was compressed to 15.5 GPa, the temperature raised to 1573 K within 15 min and held constant for 60 min. The sample was cooled by turning off the heating, decompressed and mechanically isolated.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. Structure refinements of both coe-PON and cri-PON were performed using occupancies of oxygen and nitrogen atoms fixed to 0.5 for each site. As a result of the very similar scattering powers of N and O atoms, an attempt to refine the occupancies resulted in unreliable values with large standard uncertainties. The cri-PON crystal was twinned by inversion with an equal amount of the two twin domains. The refinement of the coe-PON structure revealed a residual electron density peak of 1.41 e−·Å−3 at a distance 1.22 Å from atom P2 and 1.50, 1.65 and 1.65 Å from atoms O1, O2 and O5, respectively. This density may be explained by a static disorder of the P2 atom between two positions. The disorder is, however, too weak to give additional reliable residual density peaks for the assignments of oxygen and nitrogen atoms.
Table 1. Experimental details.
| cri-PON | coe-PON | |
|---|---|---|
| Crystal data | ||
| Chemical formula | PON | PON |
| M r | 60.98 | 60.98 |
| Crystal system, space group | Tetragonal, I
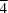 2d 2d
|
Monoclinic, C2/c |
| Temperature (K) | 293 | 100 |
| a, b, c (Å) | 4.6135 (2), 4.6135 (2), 6.9991 (5) | 6.9464 (6), 12.0340 (4), 6.9463 (5) |
| α, β, γ (°) | 90, 90, 90 | 90, 119.914 (10), 90 |
| V (Å3) | 148.97 (2) | 503.30 (7) |
| Z | 4 | 16 |
| Radiation type | Mo Kα | Synchrotron, λ = 0.69428 Å |
| μ (mm−1) | 1.24 | 1.35 |
| Crystal size (mm) | 0.02 × 0.02 × 0.02 | 0.02 × 0.02 × 0.02 |
| Data collection | ||
| Diffractometer | Bruker SMART APEX CCD | PILATUS@SNBL |
| Absorption correction | Multi-scan (CrysAlis PRO; Agilent, 2014 ▸) | Multi-scan (CrysAlis PRO; Agilent, 2014 ▸) |
| T min, T max | 0.791, 1.000 | 0.949, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 445, 92, 92 | 2415, 535, 469 |
| R int | 0.016 | 0.038 |
| (sin θ/λ)max (Å−1) | 0.666 | 0.640 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.016, 0.043, 1.45 | 0.037, 0.102, 1.05 |
| No. of reflections | 92 | 535 |
| No. of parameters | 8 | 57 |
| Δρmax, Δρmin (e Å−3) | 0.21, −0.28 | 1.41, −0.54 |
| Absolute structure | Refined as a perfect inversion twin. | – |
| Absolute structure parameter | 0.5 | – |
Supplementary Material
Crystal structure: contains datablock(s) coe-PON, New_Global_Publ_Block, cri-PON. DOI: 10.1107/S205698901501899X/wm5203sup1.cif
Structure factors: contains datablock(s) cri-PON. DOI: 10.1107/S205698901501899X/wm5203cri-PONsup2.hkl
Structure factors: contains datablock(s) coe-PON. DOI: 10.1107/S205698901501899X/wm5203coe-PONsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We gratefully acknowledge financial support by the Fonds der Chemischen Industrie (FCI) and the Deutsche Forschungsgemeinschaft (DFG) (priority program SPP1236, project SCHN 377–13). ND thanks the German Research Foundation for financial support through the DFG Heisenberg Program. ND and LD gratefully acknowledge the Federal Ministry of Education and Research (BMBF, Germany) for funding.
supplementary crystallographic information
(cri-PON) Phosphorus oxonitride. Crystal data
| NOP | Dx = 2.719 Mg m−3 |
| Mr = 60.98 | Mo Kα radiation, λ = 0.71069 Å |
| Tetragonal, I42d | Cell parameters from 431 reflections |
| a = 4.6135 (2) Å | θ = 5.3–28.2° |
| c = 6.9991 (5) Å | µ = 1.24 mm−1 |
| V = 148.97 (2) Å3 | T = 293 K |
| Z = 4 | Prism, colourless |
| F(000) = 120 | 0.02 × 0.02 × 0.02 mm |
(cri-PON) Phosphorus oxonitride. Data collection
| Three-circle diffractometer | 92 independent reflections |
| Radiation source: rotating-anode X-ray tube, Rigaku Rotor Flex FR-D | 92 reflections with I > 2σ(I) |
| Detector resolution: 16.6 pixels mm-1 | Rint = 0.016 |
| ω scans | θmax = 28.3°, θmin = 5.3° |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2014) | h = −5→5 |
| Tmin = 0.791, Tmax = 1.000 | k = −5→6 |
| 445 measured reflections | l = −5→9 |
(cri-PON) Phosphorus oxonitride. Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0229P)2 + 0.0508P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.016 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.043 | Δρmax = 0.21 e Å−3 |
| S = 1.45 | Δρmin = −0.28 e Å−3 |
| 92 reflections | Absolute structure: Refined as a perfect inversion twin. |
| 8 parameters | Absolute structure parameter: 0.5 |
(cri-PON) Phosphorus oxonitride. Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refined as a 2-component perfect inversion twin. |
(cri-PON) Phosphorus oxonitride. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| P | 0.5000 | 0.5000 | 0.0000 | 0.0106 (3) | |
| N | 0.3630 (5) | 0.2500 | 0.1250 | 0.0155 (5) | 0.5 |
| O | 0.3630 (5) | 0.2500 | 0.1250 | 0.0155 (5) | 0.5 |
(cri-PON) Phosphorus oxonitride. Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P | 0.0112 (4) | 0.0112 (4) | 0.0094 (4) | 0.000 | 0.000 | 0.000 |
| N | 0.0151 (11) | 0.0149 (12) | 0.0165 (9) | 0.000 | 0.000 | 0.0065 (10) |
| O | 0.0151 (11) | 0.0149 (12) | 0.0165 (9) | 0.000 | 0.000 | 0.0065 (10) |
(cri-PON) Phosphorus oxonitride. Geometric parameters (Å, º)
| P—Oi | 1.5796 (10) | P—Oiii | 1.5796 (10) |
| P—Ni | 1.5796 (10) | P—Niii | 1.5796 (10) |
| P—Oii | 1.5796 (10) | P—N | 1.5796 (10) |
| P—Nii | 1.5796 (10) | N—Piv | 1.5796 (10) |
| Oi—P—Ni | 0.0 | Ni—P—Niii | 107.86 (2) |
| Oi—P—Oii | 107.86 (2) | Oii—P—Niii | 112.7 |
| Ni—P—Oii | 107.86 (2) | Nii—P—Niii | 112.73 (5) |
| Oi—P—Nii | 107.9 | Oiii—P—Niii | 0.0 |
| Ni—P—Nii | 107.86 (2) | Oi—P—N | 112.7 |
| Oii—P—Nii | 0.0 | Ni—P—N | 112.73 (5) |
| Oi—P—Oiii | 107.86 (2) | Oii—P—N | 107.9 |
| Ni—P—Oiii | 107.86 (2) | Nii—P—N | 107.86 (2) |
| Oii—P—Oiii | 112.73 (5) | Oiii—P—N | 107.9 |
| Nii—P—Oiii | 112.73 (5) | Niii—P—N | 107.86 (2) |
| Oi—P—Niii | 107.9 | P—N—Piv | 132.83 (16) |
Symmetry codes: (i) −x+1, −y+1, z; (ii) y, −x+1, −z; (iii) −y+1, x, −z; (iv) x, −y+1/2, −z+1/4.
(coe-PON) Phosphorus oxonitride. Crystal data
| NOP | F(000) = 480 |
| Mr = 60.98 | Dx = 3.219 Mg m−3 |
| Monoclinic, C2/c | Synchrotron radiation, λ = 0.69428 Å |
| a = 6.9464 (6) Å | Cell parameters from 1202 reflections |
| b = 12.0340 (4) Å | θ = 3.3–26.3° |
| c = 6.9463 (5) Å | µ = 1.35 mm−1 |
| β = 119.914 (10)° | T = 100 K |
| V = 503.30 (7) Å3 | Prism, colourless |
| Z = 16 | 0.02 × 0.02 × 0.02 mm |
(coe-PON) Phosphorus oxonitride. Data collection
| PILATUS@SNBL diffractometer | 535 independent reflections |
| Radiation source: Beamline BM1A, SNBL ESRF, Grenoble, France | 469 reflections with I > 2σ(I) |
| Detector resolution: 5.8 pixels mm-1 | Rint = 0.038 |
| φ scans | θmax = 26.4°, θmin = 3.3° |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2014) | h = −8→8 |
| Tmin = 0.949, Tmax = 1.000 | k = −15→15 |
| 2415 measured reflections | l = −8→8 |
(coe-PON) Phosphorus oxonitride. Refinement
| Refinement on F2 | 57 parameters |
| Least-squares matrix: full | 0 restraints |
| R[F2 > 2σ(F2)] = 0.037 | w = 1/[σ2(Fo2) + (0.054P)2 + 4.3556P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.102 | (Δ/σ)max < 0.001 |
| S = 1.05 | Δρmax = 1.41 e Å−3 |
| 535 reflections | Δρmin = −0.54 e Å−3 |
(coe-PON) Phosphorus oxonitride. Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
(coe-PON) Phosphorus oxonitride. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| P1 | 0.28266 (16) | 0.09026 (8) | 0.04006 (16) | 0.0067 (4) | |
| P2 | 0.31812 (17) | 0.35749 (7) | 0.42525 (17) | 0.0084 (4) | |
| N1 | 0.2117 (5) | 0.4603 (2) | 0.4818 (6) | 0.0148 (8) | 0.5 |
| O1 | 0.2117 (5) | 0.4603 (2) | 0.4818 (6) | 0.0148 (8) | 0.5 |
| N2 | 0.2500 | 0.2500 | 0.5000 | 0.0116 (10) | 0.5 |
| O2 | 0.2500 | 0.2500 | 0.5000 | 0.0116 (10) | 0.5 |
| N3 | 0.2322 (6) | 0.3532 (3) | 0.1704 (5) | 0.0188 (8) | 0.5 |
| O3 | 0.2322 (6) | 0.3532 (3) | 0.1704 (5) | 0.0188 (8) | 0.5 |
| N4 | 0.5000 | 0.1336 (3) | 0.2500 | 0.0110 (10) | 0.5 |
| O4 | 0.5000 | 0.1336 (3) | 0.2500 | 0.0110 (10) | 0.5 |
| N5 | 0.0792 (5) | 0.1273 (3) | 0.0656 (6) | 0.0186 (8) | 0.5 |
| O5 | 0.0792 (5) | 0.1273 (3) | 0.0656 (6) | 0.0186 (8) | 0.5 |
(coe-PON) Phosphorus oxonitride. Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| P1 | 0.0054 (7) | 0.0057 (6) | 0.0070 (7) | 0.0003 (3) | 0.0016 (5) | 0.0013 (3) |
| P2 | 0.0075 (7) | 0.0071 (6) | 0.0095 (7) | 0.0000 (4) | 0.0035 (5) | 0.0011 (4) |
| N1 | 0.0191 (18) | 0.0047 (15) | 0.0250 (19) | 0.0010 (12) | 0.0142 (16) | −0.0018 (12) |
| O1 | 0.0191 (18) | 0.0047 (15) | 0.0250 (19) | 0.0010 (12) | 0.0142 (16) | −0.0018 (12) |
| N2 | 0.013 (2) | 0.006 (2) | 0.016 (2) | −0.0007 (16) | 0.008 (2) | 0.0032 (17) |
| O2 | 0.013 (2) | 0.006 (2) | 0.016 (2) | −0.0007 (16) | 0.008 (2) | 0.0032 (17) |
| N3 | 0.028 (2) | 0.0177 (16) | 0.0062 (18) | −0.0054 (14) | 0.0048 (16) | 0.0015 (13) |
| O3 | 0.028 (2) | 0.0177 (16) | 0.0062 (18) | −0.0054 (14) | 0.0048 (16) | 0.0015 (13) |
| N4 | 0.008 (2) | 0.009 (2) | 0.013 (2) | 0.000 | 0.003 (2) | 0.000 |
| O4 | 0.008 (2) | 0.009 (2) | 0.013 (2) | 0.000 | 0.003 (2) | 0.000 |
| N5 | 0.0034 (17) | 0.0255 (18) | 0.0213 (19) | 0.0014 (13) | 0.0020 (15) | −0.0079 (15) |
| O5 | 0.0034 (17) | 0.0255 (18) | 0.0213 (19) | 0.0014 (13) | 0.0020 (15) | −0.0079 (15) |
(coe-PON) Phosphorus oxonitride. Geometric parameters (Å, º)
| P1—O3i | 1.568 (3) | P2—O5iii | 1.584 (3) |
| P1—N3i | 1.568 (3) | P2—N5iii | 1.584 (3) |
| P1—O1ii | 1.573 (3) | P2—N1 | 1.588 (3) |
| P1—N1ii | 1.573 (3) | N1—P1iv | 1.574 (3) |
| P1—N5 | 1.574 (3) | N2—P2v | 1.5530 (9) |
| P1—N4 | 1.5755 (17) | N3—P1i | 1.568 (3) |
| P2—N2 | 1.5530 (9) | N4—P1vi | 1.5755 (17) |
| P2—N3 | 1.562 (3) | N5—P2vii | 1.584 (3) |
| O3i—P1—N3i | 0.0 | N2—P2—N3 | 110.10 (13) |
| O3i—P1—O1ii | 109.55 (17) | N2—P2—O5iii | 109.69 (13) |
| N3i—P1—O1ii | 109.55 (17) | N3—P2—O5iii | 112.0 (2) |
| O3i—P1—N1ii | 109.55 (17) | N2—P2—N5iii | 109.69 (13) |
| N3i—P1—N1ii | 109.55 (17) | N3—P2—N5iii | 112.0 (2) |
| O1ii—P1—N1ii | 0.0 | O5iii—P2—N5iii | 0.0 |
| O3i—P1—N5 | 109.7 (2) | N2—P2—N1 | 108.03 (12) |
| N3i—P1—N5 | 109.7 (2) | N3—P2—N1 | 110.13 (18) |
| O1ii—P1—N5 | 111.04 (17) | O5iii—P2—N1 | 106.79 (19) |
| N1ii—P1—N5 | 111.04 (17) | N5iii—P2—N1 | 106.79 (19) |
| O3i—P1—N4 | 107.83 (16) | P1iv—N1—P2 | 135.5 (2) |
| N3i—P1—N4 | 107.83 (16) | P2—N2—P2v | 180.0 |
| O1ii—P1—N4 | 111.09 (19) | P2—N3—P1i | 148.5 (2) |
| N1ii—P1—N4 | 111.09 (19) | P1vi—N4—P1 | 141.3 (3) |
| N5—P1—N4 | 107.57 (15) | P1—N5—P2vii | 141.3 (2) |
Symmetry codes: (i) −x+1/2, −y+1/2, −z; (ii) −x+1/2, y−1/2, −z+1/2; (iii) x+1/2, −y+1/2, z+1/2; (iv) −x+1/2, y+1/2, −z+1/2; (v) −x+1/2, −y+1/2, −z+1; (vi) −x+1, y, −z+1/2; (vii) x−1/2, −y+1/2, z−1/2.
References
- Agilent (2014). CrysAlis PRO. Agilent Technologies, Yarnton, England.
- Angel, R. J., Shaw, C. S. J. & Gibbs, G. V. (2003). Phys. Chem. Miner. 30, 167–176.
- Baumann, D., Niklaus, R. & Schnick, W. (2015). Angew. Chem. Int. Ed. 54, 4388–4391. [DOI] [PubMed]
- Baumann, D., Sedlmaier, S. J. & Schnick, W. (2012). Angew. Chem. Int. Ed. 51, 4707–4709. [DOI] [PubMed]
- Brandenburg, K. (2006). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Chateau, C., Haines, J., Léger, J. M., Lesauze, A. & Marchand, R. (1999). Am. Mineral. 84, 207–210.
- Huminicki, D. M. C. & Hawthorne, F. C. (2002). Rev. Mineral. Geochem. 48, 123–253.
- Léger, J.-M., Haines, J., de Oliveira, L. S., Chateau, C., Le Sauze, A., Marchand, R. & Hull, S. (1999). J. Phys. Chem. Solids, 60, 145–152.
- Léger, J. M., Haines, J., Chateau, C., Bocquillon, G., Schmidt, M. W., Hull, S., Gorelli, F., Lesauze, A. & Marchand, R. (2001). Phys. Chem. Miner. 28, 388–398.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) coe-PON, New_Global_Publ_Block, cri-PON. DOI: 10.1107/S205698901501899X/wm5203sup1.cif
Structure factors: contains datablock(s) cri-PON. DOI: 10.1107/S205698901501899X/wm5203cri-PONsup2.hkl
Structure factors: contains datablock(s) coe-PON. DOI: 10.1107/S205698901501899X/wm5203coe-PONsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report