The title compound is an example of a 12-metallacrown-4 self-assembled supramolecular coordination complex with ring MnIII ions. A YIII ion and Na+ ion are captured on opposite sides of the metallacrown cavity, and the YIII ion is tethered to the metallacrown with four trimethylacetate anions.
Keywords: heterotrimetallic, metallacrown, self-assembled coordination complex, crystal structure
Abstract
The synthesis and crystal structure for the title compound, [YNaMn4(C7H4NO3)4(C5H9O2)4(H2O)3.76(C3H7NO)0.24]·8.04C3H7NO·0.62H2O or [YIIINa(OTMA)4[12-MCMn(III)N(shi)-4](H2O)3.76(DMF)0.24·8.04DMF·0.62H2O, where OTMA is trimethylacetate, MC is metallacrown, shi3− is salicylhydroximate, and DMF is N,N-dimethylformamide, is reported. The macrocyclic metallacrown consists of an –[MnIII–N–O]4– ring repeat unit, and the metallacrown captures one YIII ion and one NaI ion in the central cavity on opposite faces of the metallacrown. Overall the metallacrown is domed towards the side of the NaI ion. Both the YIII and NaI ions are eight-coordinate, and the trimethylacetate anions bridge the central YIII to each ring MnIII ion. The ring MnIII ions are six-coordinate with a tetragonally distorted octahedral geometry.
Chemical context
Since 1989 metallacrowns (MCs) have served as an excellent example of the controllable self-assembly of supramolecular coordination complexes (Mezei et al., 2007 ▸). Considered the structural and functional inorganic analogues to crown ethers, metallacrowns self-assemble in solution to form coordination complexes with multiple metal centers. Not only can homometallic complexes be synthesized, but heterobimetallic and heterotrimetallic metallacrowns can also be prepared through one-step reactions (Mezei et al., 2007 ▸; Azar et al., 2014 ▸). The deliberate formation of supramolecular coordination complexes, especially those with multiple metal types, remains a synthetic challenge (Cook & Stang, 2015 ▸; Saalfrank et al., 2008 ▸); however, metallacrowns provide a class of molecules that allows the investigation of the formation of multi-metal supramolecular coordination complexes.
Recently we reported the first synthetic strategy for heterotrimetallic metallacrowns: Ln III M(OAc)4[12-MCMn(III)N(shi)-4], where Ln III is PrIII to YbIII (except PmIII) and YIII, M is NaI or KI, −OAc is acetate, and shi3− is salicylhydroximate (Azar et al., 2014 ▸). In the previous report, we demonstrated the ability to systematically replace the central metal ions; however, the metallacrown framework has other points of alteration, in particular the bridging carboxylate anion. In these alkali metal–lanthanide–manganese ion complexes, four acetate anions serve as bridges between the central lanthanide ion and the ring MnIII ions. Potentially the acetate anions could be replaced with other carboxylate monoanions.
Herein we report the synthesis and crystal structure of YIIINa(OTMA)4[12-MCMn(III)N(shi)-4](H2O)3.76(DMF)0.24·8.04DMF·0.62H2O, (1), where OTMA is trimethylacetate and DMF is N,N-dimethylformamide. This metallacrown demonstrates the ability to vary the bridging carboxylate monoanion of this heterotrimetallic class of metallacrowns.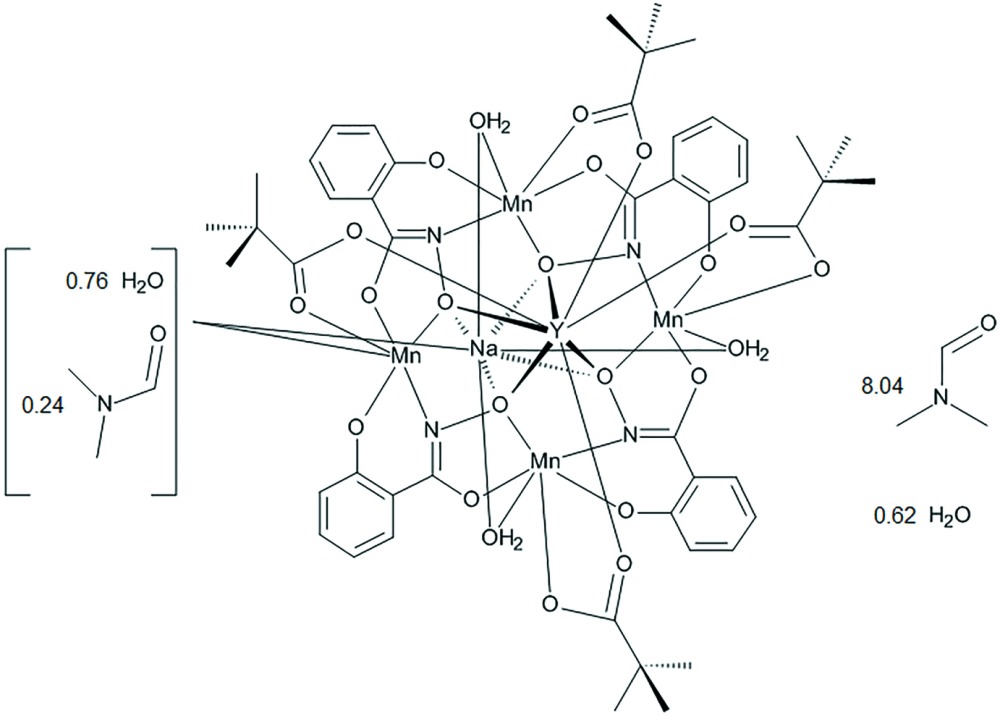
Structural commentary
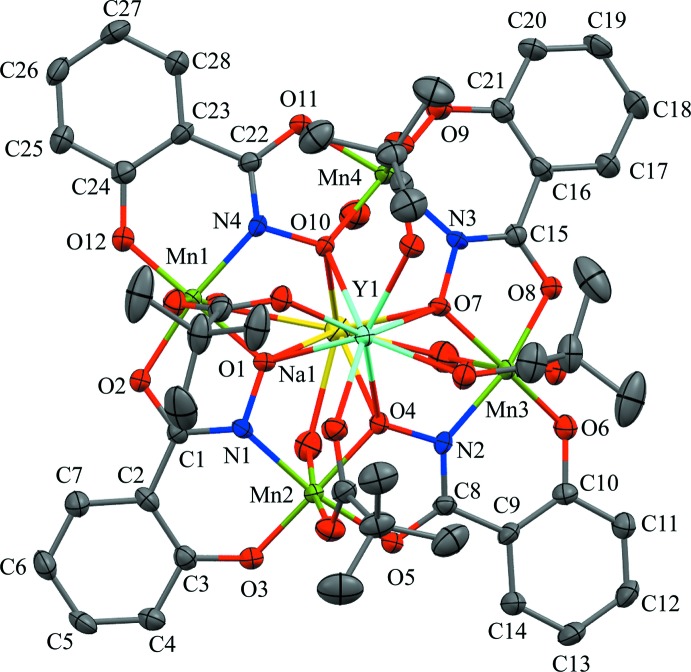
The structure of the title compound YIIINa(OTMA)4[12-MCMn(III)N(shi)-4](H2O)3.76(DMF)0.24·8.04DMF·0.62H2O, (1), is based on the typical [12-MCMn(III)N(shi)-4] core. Four shi3− framework ligands and four MnIII ions self-assemble to form an overall square geometry with a –[Mn-N-O]4– repeat unit. The MC ring forms a central cavity with a pseudo-fourfold rotation axis that is capable of binding central metal ions, in this structure an YIII ion and a NaI ion. The two ions are bound on opposite faces of the MC, and the metallacrown is slightly domed with the YIII ion residing on the convex side of the central cavity and the NaI ion residing on the underside of the dome. The YIII ion is also connected to the MC core by four trimethylacetate monoanions that serve to bridge the YIII ion to each ring MnIII ion. The molecular structure is shown in Figs. 1 ▸ and 2 ▸.
Figure 1.
The molecular structure of (1) in top view with displacement ellipsoids at the 50% probability level. For clarity, H atom and lattice solvent molecules have been omitted, and only atom labels for all non-H atoms of the 12-MC-4 framework have been provided. Color scheme: aqua – YIII, green – MnIII, yellow – Na+, red – oxygen, blue – nitrogen, and gray – carbon.
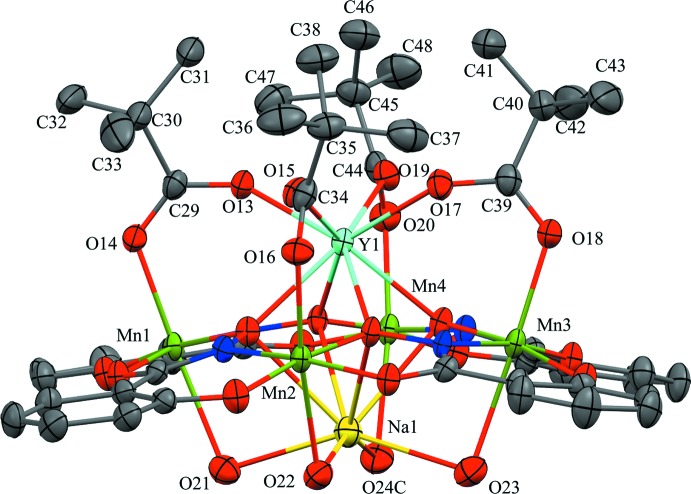
Figure 2.
The molecular structure of (1) in side view. For clarity, only atom labels for all non-H atoms of the trimethylacetate anions and the coordinating water molecules and of the metal ions have been provided. For the solvent coordination site to Mn4, a water molecule and DMF molecule are disordered with an occupancy ratio of 0.758 (8):0.242 (8). Only the water molecule is displayed. See Fig. 1 ▸ for display details.
The ring MnIII ions and the central YIII ion are assigned a 3+ oxidation state based on average bond lengths, calculated bond-valence-sum (BVS) values (Liu & Thorp, 1993 ▸), and overall molecular charge considerations. For Mn1, Mn2, Mn3, and Mn4, the average bond lengths are 2.05, 2.04, 2.06, and 2.05 Å, respectively, and the calculated BVS values for Mn1–Mn4 are 3.04, 3.06, 3.07, and 3.05 v. u., respectively. In addition, each MnIII possesses elongated axial bond lengths, which would be expected for a high-spin d 4 ion. The Y1 ion has an average bond length and BVS value of 2.35 Å and 3.32 v. u., respectively. Molecular charge neutrality considerations also support the assigned oxidation states as the four shi3− ligands and four trimethylacetate monoanions (total 16- charge) are balanced by the presence of four MnIII ions, one YIII ion, and one NaI ion (total 16+ charge).
The YIII ion is eight-coordinate with a distorted square antiprismatic geometry. The first coordination sphere is provided by two planes of four oxygen atoms each. One plane consists of four carboxylate oxygen atoms from the bridging trimethylacetate anions, and the second plane is formed by four oxime oxygen atoms of the MC ring. The YIII ion lies closer to the mean plane of the carboxylate oxygen atoms (OcarMP), 1.07 Å, than the mean plane of the oxime oxygen atoms (OoxMP), 1.57 Å. Also, the two planes are twisted relative to each other with an average skew angle of 50.02o about the YIII ion (AlDamen et al., 2008 ▸, 2009 ▸). The skew angles were calculated with the program Mercury (Macrae et al., 2006 ▸) and determined as previously described (Azar et al., 2014 ▸). For an ideal square-prismatic geometry, the skew angle is 0o, while for an ideal square-antiprismatic geometry, the skew angle is 45o. Given the measured skew angle and the placement of the YIII ion relative to the two planes of oxygen atoms, the best description of the geometry is distorted square antiprismatic.
The NaI ion is eight-coordinated with a severely distorted square-antiprismatic geometry. As in the YIII ion, the first coordination sphere is supplied by two planes of four oxygen atoms each. One plane is composed of the four oxime oxygen atoms of the MC ring, and the second plane consists of oxygen atoms from solvent molecules. Three of the four coordination sites are occupied by water molecules, while a water molecule and DMF molecule are disordered over the fourth site with an occupancy ratio of 0.758 (8):0.242 (8) (complete refinement details are given below). The NaI ion lies closer to the mean plane of the solvent oxygen atoms (OsolventMP), 0.67 Å, than the mean plane of the oxime oxygen atoms, 1.97 Å. Also, the two planes are twisted relative to each other with an average skew angle of 29.18o about the NaI ion. Lastly, the solvent oxygen atoms bridge the central NaI ion to the ring MnIII ions. The water and DMF molecules disordered over the coordination site to the NaI ion bridge the NaI ion to Mn4.
Each ring MnIII is six-coordinate with a tetragonally distorted octahedral geometry. The equatorial plane is comprised of a six-membered chelate ring and a trans five-membered chelate ring. The six-membered chelate ring is formed from the oxime nitrogen atom and the phenolate oxygen atom of one shi3− ligand, and the five-membered chelate ring is formed from the oxime oxygen atom and the carbonyl oxygen atom of a second shi3− ligand. Each MnIII ion possesses an elongated axial axis, which is composed of a carboxylate oxygen atom from a bridging trimethylacetate anion and a bridging solvent oxygen atom from either a water or a DMF molecule. The MnIII—Osolvent bond lengths are rather long (2.4–2.5 Å), which is likely due to the simultaneous coordination to the central NaI ion.
The metallacrown is slightly domed toward the central NaI ion. As previously reported, the doming effect is not likely due to the presence of either central metal ion, but likely due to the displacement of each ring MnIII ion from the equatorial mean plane of its first coordination sphere ligand atoms (Azar et al., 2014 ▸). For (1), the average distance of the ring MnIII ions above the equatorial ligand atom mean plane is 0.15 Å. Another indication of the doming effect in the MC is the angle between the axial carboxylate oxygen atom, the ring MnIII ion, and the calculated centroid of the oxime oxygen atoms (Mercury; Macrae et al., 2006 ▸). In a planar MC, this angle would be 90o. For the title compound, the average angle about the MnIII ions is 101.74o, which indicates that the MC is slightly domed.
In addition to the MC, several solvent molecules are located in the lattice some of which are only partially occupied (complete refinement details are given below). Three different DMF molecules are flipped disordered over two sites, one DMF molecule is disordered over two sites with different orientations, and two DMF molecules are partially occupied. In addition, the disordered water/DMF binding site of the NaI ion is correlated to two DMF molecules, one of which is disordered over two sites with different orientations, and to two partially occupied water molecules. Overall there is a total of 8.04 DMF and 0.62 water molecules located in the lattice.
Supramolecular features
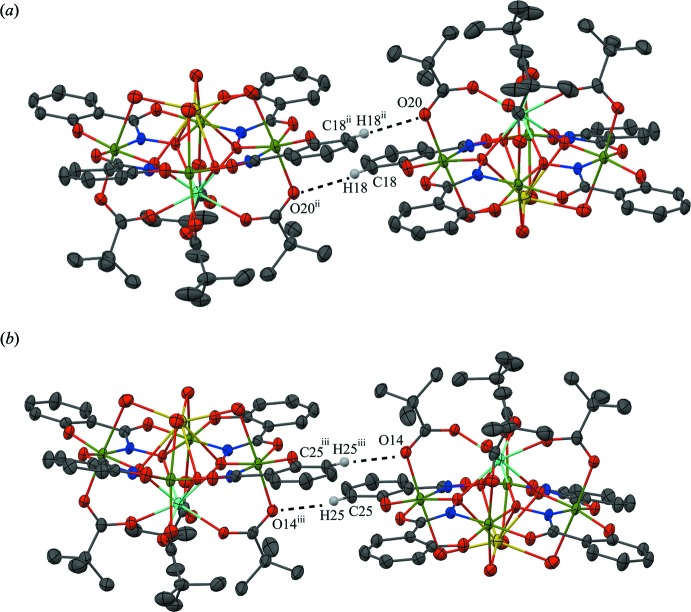
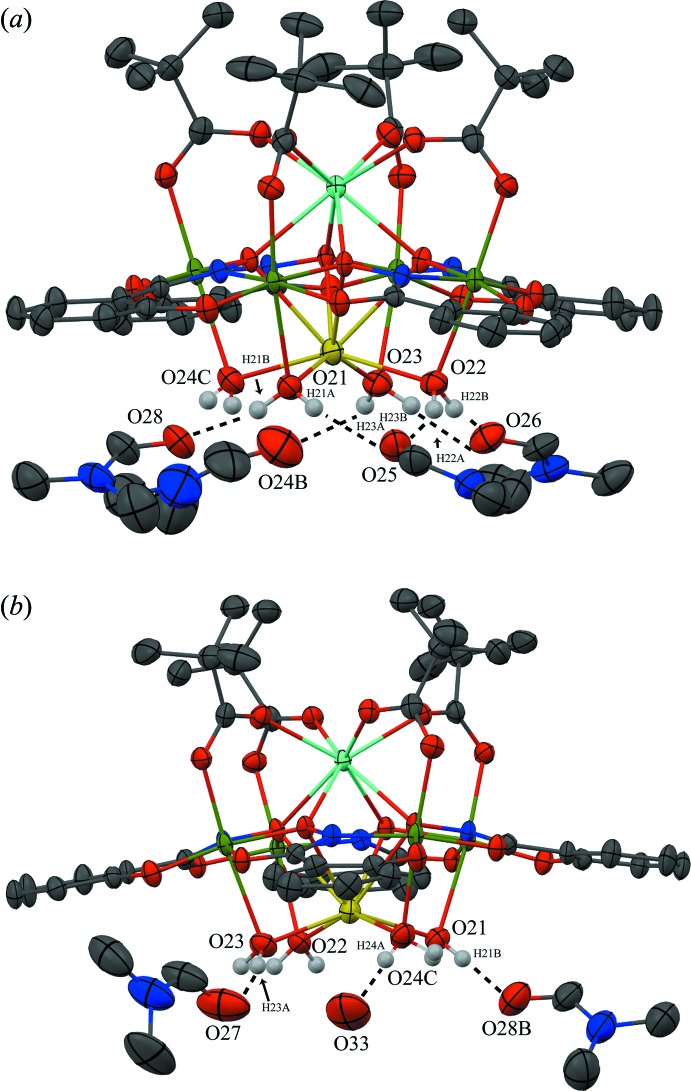
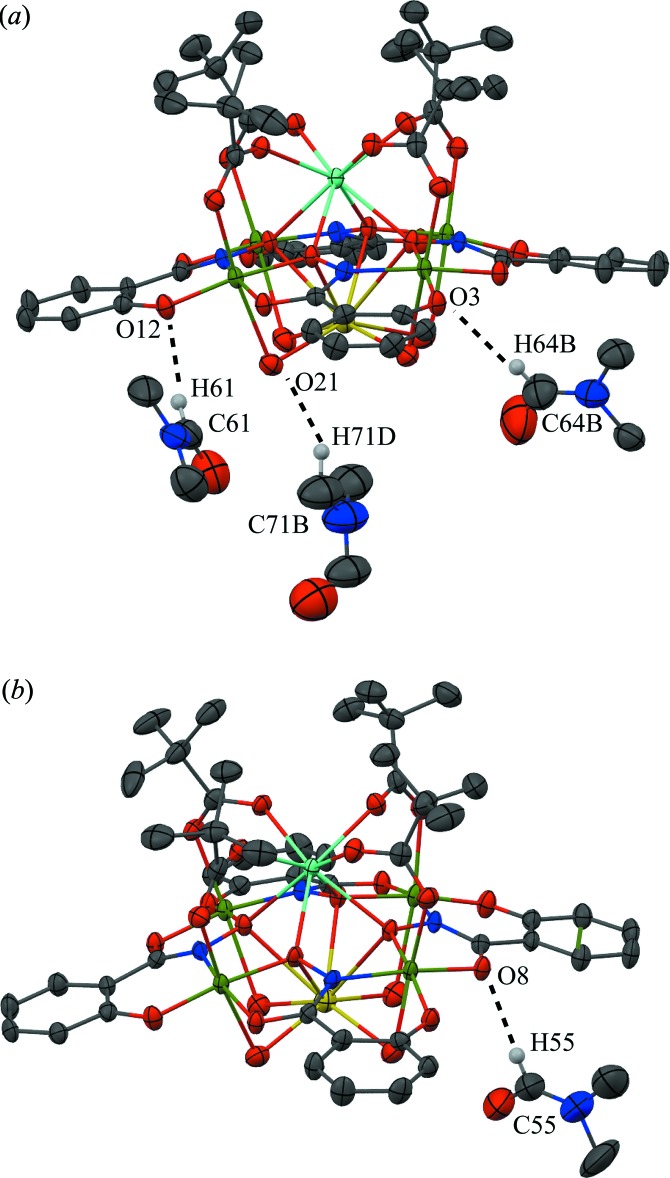
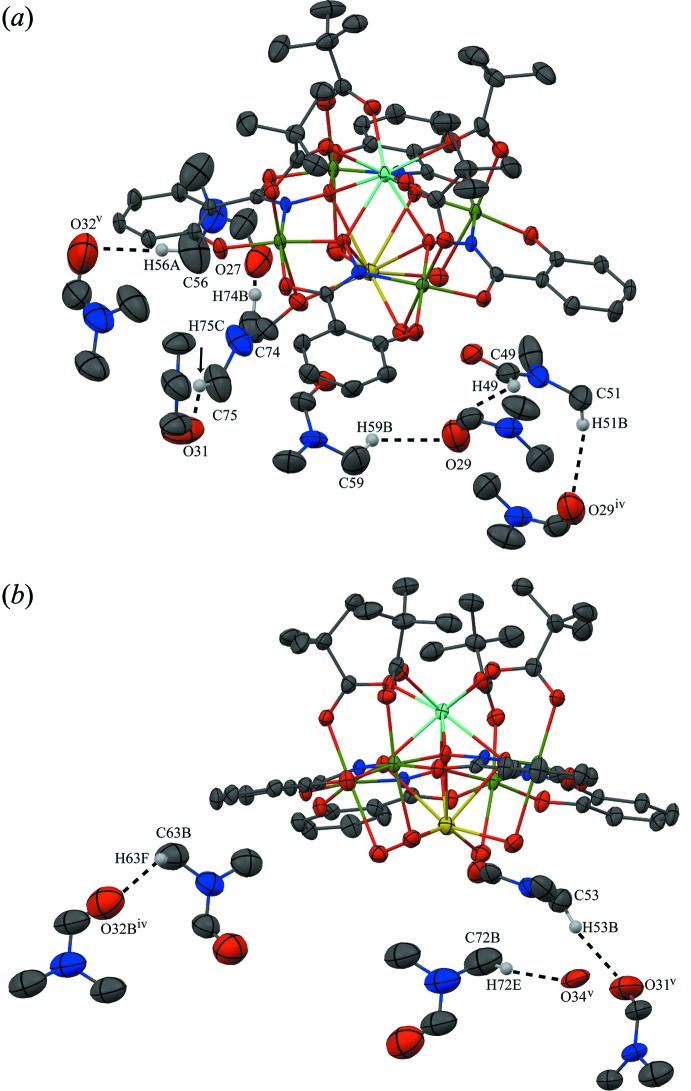
No strong directional intermolecular interactions are observed between the YIIINa(OTMA)4[12-MCMn(III)N(shi)-4](H2O)3.76(DMF)0.24 molecules, but intermolecular C—H⋯O interactions exist between adjacent metallacrowns (Table 1 ▸). The interactions exist between the carboxylate oxygen atoms (O14 and O20) of the trimethylacetate anions and the benzene carbon atoms (C18 and C25) of the shi3− ligands on adjacent metallacrowns (Fig. 3 ▸). In addition, the water molecules (O21, O22, O23, and O24C) coordinating to the NaI ion are hydrogen bonded to several lattice water and DMF molecules (Fig. 4 ▸), and the lattice DMF molecules interact with the MC molecule through C—H⋯O interactions (Fig. 5 ▸). The C—H⋯O interactions occur between either a phenolate oxygen atoms (O3 and O12) of shi3− ligands, a carboxylate oxygen atom (O8) of a shi3− ligand, or a coordinating water oxygen atom (O21) and carbonyl carbon atoms (C55, C61, and C64B) or a methyl carbon atom (C71B) of lattice DMF molecules (Fig. 5 ▸). Lastly, several C—H⋯O interactions exist between adjacent solvent molecules (Fig. 6 ▸). The carbonyl (C49) or methyl (C51, C53, C56, C59, C63B, C72B, C74, and C75) carbon atoms of DMF molecules interact with either an oxygen atom (O34) of a lattice water molecule or carbonyl oxygen atoms (O27, O29, O31, O32, and O32B) of lattice DMF molecules. The hydrogen bonding and weak C—H⋯O interactions, in addition to pure van der Waals forces, contribute to the overall packing of the molecules.
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| C18H18O20i | 0.95 | 2.60 | 3.359(5) | 137 |
| C25H25O14ii | 0.95 | 2.59 | 3.374(5) | 141 |
| C49H49O29 | 0.95 | 2.58 | 3.180(8) | 121 |
| C51H51BO29iii | 0.98 | 2.56 | 3.376(9) | 141 |
| C53H53BO31iv | 0.98 | 2.48 | 3.377(9) | 152 |
| C55H55O8 | 0.95 | 2.36 | 3.098(8) | 135 |
| C56H56AO32iv | 0.98 | 2.56 | 3.499(17) | 162 |
| C59H59BO29 | 0.98 | 2.56 | 3.262(11) | 129 |
| C61H61O12 | 0.95 | 2.52 | 3.457(8) | 169 |
| C63BH63FO32B iii | 0.98 | 2.53 | 3.34(6) | 140 |
| C64BH64BO3 | 0.95 | 2.50 | 3.40(3) | 157 |
| C71BH71DO21 | 0.98 | 2.60 | 3.41(5) | 141 |
| C72BH72EO34iv | 0.98 | 2.36 | 3.31(7) | 163 |
| C74H74BO27 | 0.98 | 2.27 | 2.87(3) | 119 |
| C75H75CO31 | 0.98 | 2.15 | 2.99(3) | 143 |
| O21H21AO25 | 0.82(2) | 2.00(3) | 2.767(4) | 155(5) |
| O21H21BO28 | 0.83(2) | 2.05(3) | 2.792(5) | 148(5) |
| O21H21BO28B | 0.83(2) | 1.87(3) | 2.70(2) | 172(5) |
| O22H22AO25 | 0.84(2) | 1.96(3) | 2.727(4) | 151(5) |
| O22H22BO26 | 0.83(2) | 1.93(3) | 2.688(4) | 151(5) |
| O23H23AO27 | 0.84(2) | 2.06(3) | 2.871(7) | 164(5) |
| O23H23AO24B | 0.84(2) | 2.06(5) | 2.696(19) | 132(5) |
| O23H23BO26 | 0.86(2) | 1.98(3) | 2.789(5) | 155(5) |
| O24CH24AO33 | 0.86(2) | 1.91(4) | 2.78(3) | 179(5) |
Symmetry codes: (i)  ; (ii)
; (ii) 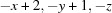 ; (iii)
; (iii) 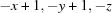 ; (iv)
; (iv) 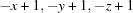 .
.
Figure 3.
Intermolecular C—H⋯O interactions between adjacent metallacrowns. For clarity the interactions have been divided into two sections (a) and (b), only the H atoms (white) involved in the interactions have been included, and only the atoms involved in the interactions have been labelled. See Fig. 1 ▸ for display details. [Symmetry codes: (ii) −x + 2, −y + 1; (iii) −x + 2, −y + 1, −z.]
Figure 4.
Intermolecular hydrogen bonding between the water molecules coordinating to the Na+ ion and the water and DMF molecules of the lattice. For clarity the hydrogen bonding has been divided into two sections (a) and (b), only the H atoms (white) involved in the hydrogen bonding have been included, and only the atoms involved in the hydrogen bonding have been labelled. See Fig. 1 ▸ for display details.
Figure 5.
Intermolecular C—H⋯O interactions between the metallacrown and the DMF molecules of the lattice. For clarity the interactions have been divided into two sections (a) and (b), only the H atoms (white) involved in the interactions have been included, and only the atoms involved in the interactions have been labelled. See Fig. 1 ▸ for display details.
Figure 6.
Intermolecular C—H⋯O interactions between adjacent water and DMF molecules. For clarity the interactions have been divided into two sections (a) and (b), only the H atoms (white) involved in the interactions have been included, and only the atoms involved in the interactions have been labelled. See Fig. 1 ▸ for display details. [Symmetry codes: (iv) −x + 1, −y + 1, −z; (v) −x + 1, −y + 1, −z + 1.]
Database survey
The crystal structure of one other yttrium-based heterotrimetallic 12-MC-4 has been reported: YIIINa(OAc)4[12-MCMn(III)N(shi)-4](H2O)4·6DMF, 2 (Azar et al., 2014 ▸). In the title compound (1), trimethylacetate anions bridge the central YIII ion to the ring MnIII ions, while in the previously reported compound (2) acetate anions bridge the YIII ion and the MnIII ions. Also for the previously reported compound (2), there are two independent MCs in each unit cell; thus, the labels (2A) and (2B) will be used to distinguish the two MCs. The replacement of acetate for trimethylacetate does not severely distort the [12-MCMn(III)N(shi)-4] framework. Comparing the two carboxylate monoanion structures, several key features of both MCs are very similar (Table 2 ▸). These features were calculated and measured using the program Mercury (Macrae et al., 2006 ▸) and in the same manner as previously described (Azar et al., 2014 ▸). Comparable measured values for the MC cavity radii, average adjacent MnIII—MnIII distances, cross cavity MnIII—MnIII distances, and cross cavity oxime oxygen (Oox—Oox) distances demonstrate that the [12-MCMn(III)N(shi)-4] framework is not significantly affected by the identity of the bridging carboxylate anion. In addition, the determined metrics of the central YIII ions and Na+ ions are very similar in both (1) and (2) (Table 2 ▸). The greatest deviations between the structures is the distance of the NaI ion from the mean plane of the solvent oxygen atoms. This is likely due to the difference in the first coordination sphere of the NaI ions. In (2A) and (2B) only water molecules bind to the NaI ions, while in (1) a mixture of water and DMF molecules bind to the NaI ion.
Table 2. Structural feature comparison () of YIIINa(OTMA)4[12-MCMn(III)N(shi)-4](H2O)3.76(DMF)0.248.04DMF0.62H2O (1) and YIIINa(OAc)4[12-MCMn(III)N(shi)-4](H2O)46DMF (2).
| Compound | YIII crystal radius | MC cavity radius | Avg. adjacent MnIIIMnIII distance | Avg. cross-cavity MnIIIMnIII distance | Avg. cross-cavity OoxOox distance | YIIIOcarMP distance | YIIIOoxMP distance | YIIIMnMP distance | NaIOsolventMP distance | NaIOoxMP distance |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | 1.05 | 0.55 | 4.62 | 6.53 | 3.71 | 1.07 | 1.57 | 1.91 | 0.67 | 1.97 |
| (2A) | 1.05 | 0.55 | 4.61 | 6.52 | 3.70 | 1.04 | 1.57 | 1.92 | 0.79 | 1.92 |
| (2B) | 1.05 | 0.55 | 4.61 | 6.52 | 3.70 | 1.03 | 1.58 | 1.93 | 0.79 | 1.91 |
The identity of the bridging ligand does not significantly alter the domed feature of the metallacrown. As stated in the Structural commentary for (1), the average distance of the ring MnIII ions above the equatorial ligand atom mean plane is 0.15 Å, and the average angle about the MnIII ions with respect to the axial carboxylate oxygen atom and the calculated centroid of the oxime oxygen atoms is 101.74o. For (2A) and (2B), the MnIII ions in both structures are on average 0.17 Å above the equatorial ligand atom mean plane, and the average angles about the MnIII ions with respect to the axial carboxylate oxygen atom and the calculated centroid of the oxime oxygen atoms are 102.31 and 102.04o, respectively.
Synthesis and crystallization
The title compound (1) was synthesized by first mixing yttrium(III) nitrate hexahydrate (0.125 mmol), sodium trimethylacetate hydrate (4 mmol based on an assumption of three waters of hydration), and salicylhydroxamic acid (2 mmol) in 10 mL of DMF resulting in a cloudy, white mixture. In a separate beaker, manganese(II) acetate tetrahydrate (2 mmol) was dissolved in 10 mL of DMF resulting in an orange–red solution. The two solutions were mixed resulting in a dark-brown solution and then allowed to stir overnight. The solution was then filtered to remove a dark-brown precipitate, which was discarded. Slow evaporation of the dark-brown filtrate yielded X-ray quality black/dark-brown crystals after 9 days. The yield was 20% based on yttrium(III) nitrate hexahydrate.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸. The following low angle reflections were affected by the beam stop and were omitted from the refinement: 1 0 0, 0 1 0, 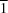
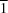 1, and
1, and 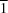 1 0. For all of the disordered solvate water and DMF molecules, neighboring atoms were restrained to have similar U
ij components of their ADPs if closer than 1.7 Å (SIMU restraints in SHELXL).
1 0. For all of the disordered solvate water and DMF molecules, neighboring atoms were restrained to have similar U
ij components of their ADPs if closer than 1.7 Å (SIMU restraints in SHELXL).
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [YNaMn4(C7H4NO3)4(C5H9O2)4(C3H7NO)0.24(H2O)3.76]8.04C3H7NO0.62H2O |
| M r | 2021.04 |
| Crystal system, space group | Triclinic, P
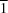
|
| Temperature (K) | 100 |
| a, b, c () | 14.8659(9), 17.3261(10), 19.2709(11) |
| , , () | 83.488(3), 82.499(3), 72.805(3) |
| V (3) | 4686.5(5) |
| Z | 2 |
| Radiation type | Cu K |
| (mm1) | 5.83 |
| Crystal size (mm) | 0.15 0.14 0.10 |
| Data collection | |
| Diffractometer | Bruker X8 Prospector CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2014 ▸) |
| T min, T max | 0.572, 0.753 |
| No. of measured, independent and observed [I > 2(I)] reflections | 59383, 16375, 14639 |
| R int | 0.045 |
| (sin /)max (1) | 0.596 |
| Refinement | |
| R[F 2 > 2(F 2)], wR(F 2), S | 0.053, 0.142, 1.02 |
| No. of reflections | 16375 |
| No. of parameters | 1537 |
| No. of restraints | 1505 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| max, min (e 3) | 1.73, 0.58 |
The geometries of the DMF molecules associated with N7, N8B, N9, N9B, N10, N10B, N11, N12, N12B, N13, and N13B were restrained to be similar to the DMF molecule associated with N5 (esd = 0.02 Å). For the DMF molecules associated with N7B and N11B, the geometries were restrained to be similar to the DMF molecule associated with N5 (esd = 0.001 Å). For the DMF molecules associated with N8B, N11B, and N13B, the carbon, oxygen, and nitrogen atoms were restrained to lie in the same plane (e.s.d. = 0.01 Å3).
A water molecule (O24C) and DMF molecule associated with N13 are disordered over a binding site to Na1. The atoms O24 and O24C were given identical coordinates, and to avoid correlation of the thermal parameters, the ADPs of O24 and O24C were constrained to be identical. Subject to these and the above conditions, the occupancy ratio of the disordered water and DMF molecules refined to 0.758 (8) to 0.242 (8). Correlated to the occupation of the binding site to Na1 is a DMF molecule associated with N13B and a DMF molecule associated with N7 that is disordered over two sites with different orientations. Subject to the above restraints, the occupancy ratio of the DMF molecule associated with N13B refined to 0.252 (5), and the occupancy ratio of the disordered DMF molecule associated with N7 refined to 0.748 (5):0.252 (5). In addition, two partially occupied water molecules associated with O33 and O34 are correlated to these water and DMF molecules. The occupancy of the water molecule of O33 and the water molecule of O34 are 0.257 (14) and 0.361 (13), respectively.
Several DMF molecules are disordered, and the above restraints were used to model the data. The DMF molecule associated with N8 is flipped disordered over two sites, and the occupancy ratio refined to 0.813 (7):0.187 (7). The DMF molecule associated with N9 is flipped disordered over two sites, and the occupancy ratio refined to 0.813 (7):0.187 (7). The DMF molecule associated with N10 is disordered over two sites with different orientations, and the occupancy ratio refined to 0.795 (6):0.205 (6). The DMF molecule associated with N11 is flipped disordered over two sites, and the occupancy ratio refined to 0.790 (9):0.210 (9). Two DMF molecules associated with N12 and N12B are partially occupied. The occupancy of the DMF molecule N12 and the DMF molecule 12B are 0.662 (8) and 0.129 (7), respectively.
For the water molecules, the oxygen–hydrogen bond lengths were restrained to 0.84 (2) Å. The hydrogen–hydrogen distances for the water molecules associated with O24, O33, and O34 were restrained to 1.36 (2) Å. For the water molecule O24C, the hydrogen atoms were restrained to a distance of at least 2.90 (2) Å from Na1. For the water molecules associated with O33 and O34, the hydrogen atoms were refined as riding on the oxygen atoms.
For the methyl group carbon atoms C56B, C62B, C63B, C69, C69B, C71B, C72B, C74, C74B, C75, and C75B, hydrogen atoms were placed in tetrahedral positions with an ideal staggered geometry (AFIX 33). All other methyl group hydrogen atoms were allowed to rotate. All other hydrogen atoms were placed in calculated positions and refined as riding on their carrier atoms with C—H distances of 0.95 Å for sp 2 carbon atoms and 0.98 Å for methyl carbon atoms. The U iso values for hydrogen atoms were set to a multiple of the value of the carrying carbon atom (1.2 times for sp 2-hybridized carbon atoms or 1.5 times for methyl carbon atoms and water oxygen atoms).
Several larger than desired residual electron density peaks remain after refinement of the data, which is typical for this class of compounds. The origin of these peaks is usually caused either by minor twinning, excessive twinning with multiple components that is beyond what can be completely handled with current integration and absorption correction software, pseudosymmetry (and correlation), or additional disorder not defined well enough to be modeled. In the case of the presented structure, the residual electron density is mostly due to additional disorder. The 3rd, 4th, 5th and 7th largest residual electron density peaks are due to alternative positions of manganese atoms of a minor moiety of the metallacrown unit (whole molecule disorder). The height of these peaks, 1.3 to 1.2 electrons per Å3, indicate the presence of less than 5% of the second moiety, and most other atoms (carbon, nitrogen, and oxygen) are not resolved. The 2nd largest residual density peak (1.71 electrons per Å3) is located close to the yttrium atom and is within the typical range of residual electron density peaks close to heavy atoms. The two remaining residual electron density peaks, the largest (1.73 electrons per Å3) and 6th largest (1.23 electrons per Å3) are due to minor twinning by a 180.0 degree rotation about the 1 1 0 reciprocal lattice direction (twin law 0.215 0.785 −0.203, 1.215 −0.215 −0.203, 0 0 −1). Refinement as a non-merohedric twin does reduce these peaks to 1.14 and 0.71 electrons per Å3, respectively; however, the R1 value slightly increases to 0.0553 from 0.0525. Also, the other larger residual electron density peaks (see above) are not improved by inclusion of twinning, nor is the structural model in any way changed. Considering the very minor effect, non-merohedric twinning was not used.
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015018216/bg2568sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015018216/bg2568Isup2.hkl
CCDC reference: 1428526
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
CMZ and JRT thank the Undergraduate Research Grant Program and the CFEST Faculty Training and Continued Education program at Shippensburg University for financial support. MZ thanks the NSF (grant DMR 1337296) for funding for the X-ray diffractometer.
supplementary crystallographic information
Crystal data
| [YNaMn4(C7H4NO3)4(C5H9O2)4(C3H7NO)\ 0.24(H2O)3.76]·8.04C3H7NO·0.62H2O | Z = 2 |
| Mr = 2021.04 | F(000) = 2106.3 |
| Triclinic, P1 | Dx = 1.432 Mg m−3 |
| a = 14.8659 (9) Å | Cu Kα radiation, λ = 1.54178 Å |
| b = 17.3261 (10) Å | Cell parameters from 9921 reflections |
| c = 19.2709 (11) Å | θ = 2.7–66.8° |
| α = 83.488 (3)° | µ = 5.83 mm−1 |
| β = 82.499 (3)° | T = 100 K |
| γ = 72.805 (3)° | Plate, black |
| V = 4686.5 (5) Å3 | 0.15 × 0.14 × 0.10 mm |
Data collection
| Bruker X8 Prospector CCD diffractometer | 16375 independent reflections |
| Radiation source: I-mu-S microsource X-ray tube | 14639 reflections with I > 2σ(I) |
| Laterally graded multilayer (Goebel) mirror monochromator | Rint = 0.045 |
| ω and phi scans | θmax = 66.9°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Bruker, 2014) | h = −17→17 |
| Tmin = 0.572, Tmax = 0.753 | k = −20→20 |
| 59383 measured reflections | l = −22→22 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.053 | Hydrogen site location: mixed |
| wR(F2) = 0.142 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0737P)2 + 11.125P] where P = (Fo2 + 2Fc2)/3 |
| 16375 reflections | (Δ/σ)max = 0.005 |
| 1537 parameters | Δρmax = 1.73 e Å−3 |
| 1505 restraints | Δρmin = −0.58 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. For all of the disordered solvate water and DMF molecules, neighboring atoms were restrained to have similar Uij components of their ADPs if closter than 1.7 Angstoms (SIMU restraints in SHELXL).The geometries of the DMF molecules associated with N7, N8B, N9, N9B, N10, N10B, N11, N12, N12B, N13, and N13B were restrained to be similar to the DMF molecule associated with N5 (e.s.d. = 0.02 Angstrom). For the DMF molecules associated with N7B and N11B, the geometries were restrained to be similar to the DMF molecule associated with N5 (e.s.d. = 0.001 Angstrom). For the DMF molecules associated with N8B, N11B, and N13B, the carbon, oxygen, and nitrogen atoms were restrained to lie in the same plane (0.01 Angstroms cubed).A water molecule (O24C) and DMF molecule associated with N13 are disordered over a binding site to Na1. The atoms O24 and O24C were given identical coordinates, and to avoid correlation of the thermal parameters, the ADP of O24 and O24C were constrained to be identical. Subject to these and the above conditions, the occupancy ratio of the disordered water and DMF molecules refined to 0.758 (8) to 0.242 (8). Correlated to the occupation of the binding site is a DMF molecule associated with N13B and a DMF molecule associated with N7 that is disordered over two sites with different orientations. Subject to the above restraints, the occupancy ratio of the DMF molecule associated with N13B refined to 0.252 (5), and the occupancy ratio of the disordered DMF molecule associated with N7 refined to 0.748 (5) to 0.252 (5). In addition, two partially occupied water molecules associated with O33 and O34 are correlated to these water and DMF molecules. The occupancy of the water molecule of O33 and the water molecule of O34 are 0.257 (14) and 0.361 (13), respectively.Several DMF molecules are disordered, and the above restraints were used to model the data. The DMF molecule associated with N8 is flipped disordered over two sites, and the occupancy ratio refined to 0.813 (7) to 0.187 (7). The DMF molecule associated with N9 is flipped disordered over two sites, and the occupancy ratio refined to 0.813 (7) to 0.187 (7). The DMF molecule associated with N10 is disordered over two sites with different orientations, and the occupancy ratio refined to 0.795 (6) to 0.205 (6). The DMF molecule associated with N11 is flipped disordered over two sites, and the occupancy ratio refined to 0.790 (9) to 0.210 (9). Two DMF molecules associated with N12 and N12B are partially occupied. The occupancy of the DMF molecule N12 and the DMF molecule 12B are 0.662 (8) and 0.129 (7), respectively.For the water molecules, the oxygen-hydrogen bond distances were restrained to 0.84 (2) Angstrom. The hydrogen-hydrogen distances for the water molecules associated with O24, O33, and O34 were restrained to 1.36 (2) Angstroms. For the water molecule O24C, the hydrogen atoms were restrained to a distance of at least 2.90 (2) Angstroms from Na1. For the water molecules associated with O33 and O34, the hydrogen atoms were refined as riding on the oxygen atoms.For the methyl group carbon atoms 56B, 62B, 63B, 69, 69B, 71B, 72B, 74, 74B, 75, and 75B, hydrogen atoms were placed in tetrahedral positions with an ideal staggered geometry (AFIX 33). All other methyl group hydrogen atoms were allowed to rotate. All other hydrogen atoms were placed in calculated positions and refined as riding on their carrier atoms with C—H distances of 0.95 Angstrom for sp2 carbon atoms and 0.98 Angstrom for methyl carbon atoms. The Uiso values for hydrogen atoms were set to a multiple of the value of the carrying carbon atom (1.2 times for sp2 hybridized carbon atoms or 1.5 times for methyl carbon atoms and water oxygen atoms).The following low angle reflections were affected by the beam stop and were omitted from the refinement: 1 0 0, 0 1 0, −1 − 1 1, and −1 1 0. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.7096 (2) | 0.7443 (2) | 0.05094 (17) | 0.0206 (7) | |
| C2 | 0.6469 (3) | 0.7852 (2) | −0.00412 (18) | 0.0240 (7) | |
| C3 | 0.5705 (3) | 0.8557 (2) | 0.00655 (18) | 0.0241 (7) | |
| C4 | 0.5182 (3) | 0.8919 (2) | −0.05003 (19) | 0.0277 (8) | |
| H4 | 0.4674 | 0.9399 | −0.0441 | 0.033* | |
| C5 | 0.5393 (3) | 0.8590 (3) | −0.1141 (2) | 0.0319 (9) | |
| H5 | 0.5032 | 0.8849 | −0.1518 | 0.038* | |
| C6 | 0.6124 (3) | 0.7886 (3) | −0.1243 (2) | 0.0359 (9) | |
| H6 | 0.6255 | 0.7656 | −0.1683 | 0.043* | |
| C7 | 0.6660 (3) | 0.7522 (2) | −0.0695 (2) | 0.0302 (8) | |
| H7 | 0.7164 | 0.7041 | −0.0763 | 0.036* | |
| C8 | 0.5234 (2) | 0.9402 (2) | 0.26837 (18) | 0.0212 (7) | |
| C9 | 0.4547 (3) | 0.9900 (2) | 0.31967 (19) | 0.0245 (7) | |
| C10 | 0.4589 (3) | 0.9758 (2) | 0.39318 (19) | 0.0246 (7) | |
| C11 | 0.3913 (3) | 1.0274 (2) | 0.4371 (2) | 0.0289 (8) | |
| H11 | 0.3937 | 1.0185 | 0.4865 | 0.035* | |
| C12 | 0.3214 (3) | 1.0907 (2) | 0.4107 (2) | 0.0340 (9) | |
| H12 | 0.2768 | 1.1254 | 0.4418 | 0.041* | |
| C13 | 0.3150 (3) | 1.1048 (3) | 0.3384 (2) | 0.0388 (10) | |
| H13 | 0.2660 | 1.1481 | 0.3202 | 0.047* | |
| C14 | 0.3812 (3) | 1.0545 (2) | 0.2938 (2) | 0.0330 (9) | |
| H14 | 0.3771 | 1.0636 | 0.2446 | 0.040* | |
| C15 | 0.7409 (3) | 0.7195 (2) | 0.45908 (18) | 0.0226 (7) | |
| C16 | 0.7741 (3) | 0.6612 (2) | 0.51876 (18) | 0.0248 (8) | |
| C17 | 0.8440 (3) | 0.5863 (2) | 0.50983 (19) | 0.0258 (8) | |
| C18 | 0.8731 (3) | 0.5362 (2) | 0.5704 (2) | 0.0312 (8) | |
| H18 | 0.9202 | 0.4858 | 0.5656 | 0.037* | |
| C19 | 0.8348 (3) | 0.5590 (2) | 0.6365 (2) | 0.0349 (9) | |
| H19 | 0.8564 | 0.5244 | 0.6765 | 0.042* | |
| C20 | 0.7645 (3) | 0.6322 (3) | 0.6456 (2) | 0.0374 (10) | |
| H20 | 0.7379 | 0.6473 | 0.6913 | 0.045* | |
| C21 | 0.7344 (3) | 0.6823 (2) | 0.5869 (2) | 0.0315 (9) | |
| H21 | 0.6860 | 0.7318 | 0.5927 | 0.038* | |
| C22 | 0.9346 (2) | 0.5280 (2) | 0.24097 (18) | 0.0223 (7) | |
| C23 | 0.9801 (3) | 0.4625 (2) | 0.1944 (2) | 0.0255 (8) | |
| C24 | 0.9629 (3) | 0.4678 (2) | 0.12331 (19) | 0.0254 (8) | |
| C25 | 1.0121 (3) | 0.4030 (2) | 0.0831 (2) | 0.0307 (8) | |
| H25 | 1.0020 | 0.4059 | 0.0351 | 0.037* | |
| C26 | 1.0744 (3) | 0.3355 (2) | 0.1116 (2) | 0.0357 (9) | |
| H26 | 1.1068 | 0.2924 | 0.0831 | 0.043* | |
| C27 | 1.0910 (3) | 0.3289 (2) | 0.1818 (2) | 0.0398 (10) | |
| H27 | 1.1337 | 0.2816 | 0.2014 | 0.048* | |
| C28 | 1.0442 (3) | 0.3923 (2) | 0.2223 (2) | 0.0319 (9) | |
| H28 | 1.0555 | 0.3885 | 0.2702 | 0.038* | |
| C29 | 0.9785 (3) | 0.7356 (2) | 0.0956 (2) | 0.0275 (8) | |
| C30 | 1.0344 (3) | 0.7837 (3) | 0.0444 (2) | 0.0411 (11) | |
| C31 | 1.0753 (4) | 0.8352 (3) | 0.0832 (3) | 0.0509 (13) | |
| H31A | 1.1078 | 0.8671 | 0.0492 | 0.076* | |
| H31B | 1.1204 | 0.8000 | 0.1142 | 0.076* | |
| H31C | 1.0240 | 0.8719 | 0.1112 | 0.076* | |
| C32 | 1.1134 (4) | 0.7254 (4) | 0.0017 (3) | 0.0678 (18) | |
| H32A | 1.0862 | 0.6939 | −0.0244 | 0.102* | |
| H32B | 1.1565 | 0.6886 | 0.0333 | 0.102* | |
| H32C | 1.1484 | 0.7561 | −0.0314 | 0.102* | |
| C33 | 0.9635 (5) | 0.8392 (3) | −0.0041 (3) | 0.0616 (16) | |
| H33A | 0.9130 | 0.8765 | 0.0239 | 0.092* | |
| H33B | 0.9361 | 0.8061 | −0.0283 | 0.092* | |
| H33C | 0.9962 | 0.8702 | −0.0388 | 0.092* | |
| C34 | 0.7526 (3) | 0.9651 (2) | 0.12915 (19) | 0.0290 (8) | |
| C35 | 0.7756 (3) | 1.0463 (2) | 0.1244 (2) | 0.0371 (10) | |
| C36 | 0.7445 (4) | 1.0949 (3) | 0.0567 (3) | 0.0551 (13) | |
| H36A | 0.7590 | 1.1468 | 0.0535 | 0.083* | |
| H36B | 0.6762 | 1.1046 | 0.0562 | 0.083* | |
| H36C | 0.7782 | 1.0645 | 0.0166 | 0.083* | |
| C37 | 0.7184 (3) | 1.0906 (3) | 0.1889 (3) | 0.0492 (12) | |
| H37A | 0.6511 | 1.0959 | 0.1883 | 0.074* | |
| H37B | 0.7276 | 1.1446 | 0.1870 | 0.074* | |
| H37C | 0.7406 | 1.0593 | 0.2322 | 0.074* | |
| C38 | 0.8809 (3) | 1.0344 (3) | 0.1280 (3) | 0.0458 (11) | |
| H38A | 0.9175 | 1.0059 | 0.0878 | 0.069* | |
| H38B | 0.9002 | 1.0023 | 0.1717 | 0.069* | |
| H38C | 0.8925 | 1.0873 | 0.1270 | 0.069* | |
| C39 | 0.7880 (3) | 0.9357 (2) | 0.3575 (2) | 0.0286 (8) | |
| C40 | 0.8520 (3) | 0.9791 (3) | 0.3829 (2) | 0.0401 (10) | |
| C41 | 0.9221 (3) | 1.0004 (3) | 0.3245 (3) | 0.0461 (11) | |
| H41A | 0.9637 | 0.9507 | 0.3053 | 0.069* | |
| H41B | 0.9602 | 1.0291 | 0.3434 | 0.069* | |
| H41C | 0.8876 | 1.0353 | 0.2872 | 0.069* | |
| C42 | 0.9077 (4) | 0.9190 (4) | 0.4386 (3) | 0.0657 (17) | |
| H42A | 0.9432 | 0.8685 | 0.4175 | 0.099* | |
| H42B | 0.8635 | 0.9075 | 0.4779 | 0.099* | |
| H42C | 0.9519 | 0.9430 | 0.4557 | 0.099* | |
| C43 | 0.7913 (5) | 1.0551 (4) | 0.4162 (4) | 0.0710 (19) | |
| H43A | 0.7548 | 1.0922 | 0.3810 | 0.107* | |
| H43B | 0.8321 | 1.0816 | 0.4345 | 0.107* | |
| H43C | 0.7479 | 1.0406 | 0.4548 | 0.107* | |
| C44 | 1.0183 (3) | 0.7055 (2) | 0.32290 (19) | 0.0292 (8) | |
| C45 | 1.1210 (3) | 0.7093 (3) | 0.3051 (2) | 0.0350 (9) | |
| C46 | 1.1251 (3) | 0.7950 (3) | 0.2804 (3) | 0.0467 (11) | |
| H46A | 1.0880 | 0.8152 | 0.2403 | 0.070* | |
| H46B | 1.1911 | 0.7944 | 0.2663 | 0.070* | |
| H46C | 1.0989 | 0.8304 | 0.3187 | 0.070* | |
| C47 | 1.1641 (3) | 0.6538 (3) | 0.2449 (3) | 0.0471 (11) | |
| H47A | 1.1253 | 0.6716 | 0.2055 | 0.071* | |
| H47B | 1.1658 | 0.5978 | 0.2615 | 0.071* | |
| H47C | 1.2286 | 0.6567 | 0.2295 | 0.071* | |
| C48 | 1.1755 (4) | 0.6780 (3) | 0.3692 (3) | 0.0542 (13) | |
| H48A | 1.1759 | 0.6217 | 0.3828 | 0.081* | |
| H48B | 1.1449 | 0.7114 | 0.4082 | 0.081* | |
| H48C | 1.2408 | 0.6808 | 0.3579 | 0.081* | |
| O26 | 0.3919 (2) | 0.8164 (2) | 0.29808 (18) | 0.0521 (8) | |
| C52 | 0.3186 (3) | 0.8741 (3) | 0.3082 (3) | 0.0495 (12) | |
| H52 | 0.2980 | 0.9116 | 0.2696 | 0.059* | |
| N6 | 0.2689 (3) | 0.8853 (3) | 0.3696 (2) | 0.0510 (10) | |
| C53 | 0.2997 (5) | 0.8315 (4) | 0.4300 (3) | 0.0709 (17) | |
| H53A | 0.3443 | 0.7810 | 0.4145 | 0.106* | |
| H53B | 0.2448 | 0.8196 | 0.4581 | 0.106* | |
| H53C | 0.3309 | 0.8573 | 0.4585 | 0.106* | |
| C54 | 0.1848 (4) | 0.9538 (4) | 0.3792 (4) | 0.0719 (19) | |
| H54A | 0.1966 | 0.9910 | 0.4092 | 0.108* | |
| H54B | 0.1316 | 0.9342 | 0.4013 | 0.108* | |
| H54C | 0.1695 | 0.9822 | 0.3334 | 0.108* | |
| O27 | 0.4821 (5) | 0.7058 (5) | 0.5099 (3) | 0.099 (2) | 0.748 (5) |
| C55 | 0.4849 (6) | 0.7581 (7) | 0.5502 (4) | 0.082 (2) | 0.748 (5) |
| H55 | 0.5191 | 0.7953 | 0.5308 | 0.099* | 0.748 (5) |
| N7 | 0.4468 (6) | 0.7670 (6) | 0.6144 (4) | 0.089 (2) | 0.748 (5) |
| C56 | 0.3832 (8) | 0.7197 (8) | 0.6489 (6) | 0.123 (4) | 0.748 (5) |
| H56A | 0.4048 | 0.6947 | 0.6943 | 0.184* | 0.748 (5) |
| H56B | 0.3839 | 0.6773 | 0.6190 | 0.184* | 0.748 (5) |
| H56C | 0.3186 | 0.7556 | 0.6563 | 0.184* | 0.748 (5) |
| C57 | 0.4594 (8) | 0.8258 (8) | 0.6569 (6) | 0.106 (3) | 0.748 (5) |
| H57A | 0.4864 | 0.8647 | 0.6270 | 0.127* | 0.748 (5) |
| H57B | 0.5023 | 0.7980 | 0.6923 | 0.127* | 0.748 (5) |
| H57C | 0.3980 | 0.8545 | 0.6804 | 0.127* | 0.748 (5) |
| O27B | 0.486 (2) | 0.7133 (14) | 0.7576 (9) | 0.175 (10) | 0.252 (5) |
| C55B | 0.4904 (18) | 0.7687 (13) | 0.7122 (8) | 0.116 (6) | 0.252 (5) |
| H55B | 0.5196 | 0.8075 | 0.7223 | 0.140* | 0.252 (5) |
| N7B | 0.4572 (12) | 0.7783 (8) | 0.6510 (7) | 0.098 (4) | 0.252 (5) |
| C56B | 0.4258 (19) | 0.7137 (11) | 0.6292 (11) | 0.125 (7) | 0.252 (5) |
| H56D | 0.4030 | 0.7296 | 0.5827 | 0.188* | 0.252 (5) |
| H56E | 0.4787 | 0.6641 | 0.6271 | 0.188* | 0.252 (5) |
| H56F | 0.3743 | 0.7040 | 0.6631 | 0.188* | 0.252 (5) |
| C57B | 0.4632 (18) | 0.8464 (12) | 0.6004 (10) | 0.096 (5) | 0.252 (5) |
| H57D | 0.5255 | 0.8554 | 0.5993 | 0.115* | 0.252 (5) |
| H57E | 0.4137 | 0.8952 | 0.6142 | 0.115* | 0.252 (5) |
| H57F | 0.4546 | 0.8342 | 0.5537 | 0.115* | 0.252 (5) |
| O28 | 0.7538 (3) | 0.4474 (3) | 0.2625 (3) | 0.0522 (12) | 0.813 (7) |
| C58 | 0.8097 (5) | 0.3802 (4) | 0.2689 (4) | 0.0566 (16) | 0.813 (7) |
| H58 | 0.8491 | 0.3708 | 0.3058 | 0.068* | 0.813 (7) |
| N8 | 0.8218 (5) | 0.3195 (4) | 0.2308 (4) | 0.0559 (14) | 0.813 (7) |
| C59 | 0.7602 (8) | 0.3316 (6) | 0.1739 (5) | 0.090 (3) | 0.813 (7) |
| H59A | 0.7993 | 0.3163 | 0.1299 | 0.134* | 0.813 (7) |
| H59B | 0.7235 | 0.3888 | 0.1687 | 0.134* | 0.813 (7) |
| H59C | 0.7170 | 0.2979 | 0.1856 | 0.134* | 0.813 (7) |
| C60 | 0.8925 (7) | 0.2423 (5) | 0.2366 (5) | 0.078 (2) | 0.813 (7) |
| H60A | 0.9309 | 0.2324 | 0.1914 | 0.117* | 0.813 (7) |
| H60B | 0.8613 | 0.1993 | 0.2497 | 0.117* | 0.813 (7) |
| H60C | 0.9331 | 0.2424 | 0.2727 | 0.117* | 0.813 (7) |
| O28B | 0.7439 (16) | 0.4344 (12) | 0.2246 (14) | 0.068 (5) | 0.187 (7) |
| C58B | 0.8040 (16) | 0.3744 (12) | 0.2020 (13) | 0.060 (3) | 0.187 (7) |
| H58B | 0.8433 | 0.3831 | 0.1607 | 0.072* | 0.187 (7) |
| N8B | 0.8185 (17) | 0.3000 (13) | 0.2296 (14) | 0.065 (4) | 0.187 (7) |
| C59B | 0.765 (3) | 0.276 (2) | 0.2927 (17) | 0.093 (8) | 0.187 (7) |
| H59D | 0.7304 | 0.3243 | 0.3178 | 0.139* | 0.187 (7) |
| H59E | 0.8078 | 0.2377 | 0.3232 | 0.139* | 0.187 (7) |
| H59F | 0.7191 | 0.2507 | 0.2797 | 0.139* | 0.187 (7) |
| C60B | 0.892 (2) | 0.2353 (17) | 0.198 (2) | 0.082 (7) | 0.187 (7) |
| H60D | 0.9536 | 0.2436 | 0.1996 | 0.123* | 0.187 (7) |
| H60E | 0.8811 | 0.2349 | 0.1484 | 0.123* | 0.187 (7) |
| H60F | 0.8898 | 0.1833 | 0.2228 | 0.123* | 0.187 (7) |
| O24B | 0.5766 (16) | 0.6104 (12) | 0.4575 (11) | 0.094 (5) | 0.252 (5) |
| C73B | 0.6222 (16) | 0.5394 (13) | 0.4589 (17) | 0.093 (5) | 0.252 (5) |
| H73B | 0.6528 | 0.5246 | 0.4139 | 0.112* | 0.252 (5) |
| N13B | 0.6396 (13) | 0.4791 (12) | 0.5058 (12) | 0.101 (4) | 0.252 (5) |
| C74B | 0.605 (3) | 0.480 (2) | 0.5799 (14) | 0.142 (11) | 0.252 (5) |
| H74D | 0.6289 | 0.4257 | 0.6033 | 0.213* | 0.252 (5) |
| H74E | 0.6259 | 0.5190 | 0.6015 | 0.213* | 0.252 (5) |
| H74F | 0.5352 | 0.4953 | 0.5848 | 0.213* | 0.252 (5) |
| C75B | 0.700 (2) | 0.4037 (16) | 0.4804 (17) | 0.101 (6) | 0.252 (5) |
| H75D | 0.7097 | 0.3615 | 0.5194 | 0.152* | 0.252 (5) |
| H75E | 0.6709 | 0.3875 | 0.4441 | 0.152* | 0.252 (5) |
| H75F | 0.7617 | 0.4111 | 0.4606 | 0.152* | 0.252 (5) |
| O29 | 0.6538 (5) | 0.4705 (4) | 0.0592 (3) | 0.094 (2) | 0.813 (7) |
| C61 | 0.7226 (5) | 0.4828 (4) | 0.0228 (4) | 0.0627 (18) | 0.813 (7) |
| H61 | 0.7729 | 0.4889 | 0.0455 | 0.075* | 0.813 (7) |
| N9 | 0.7307 (5) | 0.4879 (4) | −0.0460 (3) | 0.0588 (15) | 0.813 (7) |
| C62 | 0.6558 (7) | 0.4829 (6) | −0.0858 (5) | 0.085 (3) | 0.813 (7) |
| H62A | 0.6515 | 0.5217 | −0.1272 | 0.127* | 0.813 (7) |
| H62B | 0.5953 | 0.4956 | −0.0562 | 0.127* | 0.813 (7) |
| H62C | 0.6703 | 0.4279 | −0.1007 | 0.127* | 0.813 (7) |
| C63 | 0.8174 (6) | 0.4974 (5) | −0.0839 (6) | 0.091 (3) | 0.813 (7) |
| H63A | 0.8511 | 0.4480 | −0.1072 | 0.137* | 0.813 (7) |
| H63B | 0.8573 | 0.5071 | −0.0510 | 0.137* | 0.813 (7) |
| H63C | 0.8029 | 0.5436 | −0.1191 | 0.137* | 0.813 (7) |
| O29B | 0.593 (2) | 0.474 (2) | −0.0371 (18) | 0.112 (7) | 0.187 (7) |
| C61B | 0.670 (2) | 0.460 (2) | −0.075 (2) | 0.072 (4) | 0.187 (7) |
| H61B | 0.6843 | 0.4166 | −0.1042 | 0.087* | 0.187 (7) |
| N9B | 0.732 (2) | 0.501 (2) | −0.0781 (14) | 0.073 (4) | 0.187 (7) |
| C62B | 0.783 (3) | 0.505 (2) | −0.0198 (18) | 0.085 (6) | 0.187 (7) |
| H62D | 0.8250 | 0.5392 | −0.0348 | 0.128* | 0.187 (7) |
| H62E | 0.7376 | 0.5281 | 0.0194 | 0.128* | 0.187 (7) |
| H62F | 0.8203 | 0.4503 | −0.0048 | 0.128* | 0.187 (7) |
| C63B | 0.770 (3) | 0.529 (2) | −0.1455 (16) | 0.096 (8) | 0.187 (7) |
| H63D | 0.8149 | 0.5587 | −0.1388 | 0.144* | 0.187 (7) |
| H63E | 0.8024 | 0.4829 | −0.1729 | 0.144* | 0.187 (7) |
| H63F | 0.7183 | 0.5657 | −0.1707 | 0.144* | 0.187 (7) |
| O30 | 0.1094 (4) | 1.1136 (3) | 0.2412 (3) | 0.0700 (15) | 0.795 (6) |
| C64 | 0.1762 (5) | 1.1008 (3) | 0.1954 (3) | 0.0485 (15) | 0.795 (6) |
| H64 | 0.2132 | 1.1378 | 0.1889 | 0.058* | 0.795 (6) |
| N10 | 0.2021 (4) | 1.0394 (3) | 0.1536 (3) | 0.0428 (12) | 0.795 (6) |
| C65 | 0.1457 (6) | 0.9836 (6) | 0.1555 (5) | 0.053 (2) | 0.795 (6) |
| H65A | 0.0982 | 0.9918 | 0.1964 | 0.064* | 0.795 (6) |
| H65B | 0.1869 | 0.9278 | 0.1587 | 0.064* | 0.795 (6) |
| H65C | 0.1138 | 0.9935 | 0.1125 | 0.064* | 0.795 (6) |
| C66 | 0.2827 (6) | 1.0294 (5) | 0.1004 (5) | 0.060 (2) | 0.795 (6) |
| H66A | 0.2614 | 1.0314 | 0.0540 | 0.091* | 0.795 (6) |
| H66B | 0.3290 | 0.9769 | 0.1101 | 0.091* | 0.795 (6) |
| H66C | 0.3120 | 1.0730 | 0.1013 | 0.091* | 0.795 (6) |
| O30B | 0.315 (2) | 0.8658 (15) | 0.1790 (17) | 0.128 (9) | 0.205 (6) |
| C64B | 0.3147 (19) | 0.9280 (15) | 0.1396 (17) | 0.080 (5) | 0.205 (6) |
| H64B | 0.3701 | 0.9276 | 0.1087 | 0.096* | 0.205 (6) |
| N10B | 0.2436 (15) | 0.9936 (12) | 0.1381 (13) | 0.063 (4) | 0.205 (6) |
| C65B | 0.149 (2) | 0.996 (3) | 0.173 (2) | 0.061 (7) | 0.205 (6) |
| H65D | 0.1260 | 1.0420 | 0.2013 | 0.073* | 0.205 (6) |
| H65E | 0.1528 | 0.9454 | 0.2025 | 0.073* | 0.205 (6) |
| H65F | 0.1062 | 1.0008 | 0.1368 | 0.073* | 0.205 (6) |
| C66B | 0.253 (2) | 1.0673 (16) | 0.099 (2) | 0.068 (6) | 0.205 (6) |
| H66D | 0.2186 | 1.0773 | 0.0574 | 0.102* | 0.205 (6) |
| H66E | 0.3202 | 1.0620 | 0.0844 | 0.102* | 0.205 (6) |
| H66F | 0.2271 | 1.1128 | 0.1287 | 0.102* | 0.205 (6) |
| O32 | 0.4946 (10) | 0.3953 (7) | 0.2133 (6) | 0.149 (4) | 0.662 (8) |
| C70 | 0.5017 (10) | 0.4644 (8) | 0.2209 (7) | 0.104 (3) | 0.662 (8) |
| H70 | 0.5351 | 0.4896 | 0.1843 | 0.125* | 0.662 (8) |
| N12 | 0.4647 (8) | 0.5032 (6) | 0.2776 (6) | 0.097 (3) | 0.662 (8) |
| C71 | 0.4145 (10) | 0.4772 (9) | 0.3425 (7) | 0.119 (4) | 0.662 (8) |
| H71A | 0.3821 | 0.5243 | 0.3692 | 0.178* | 0.662 (8) |
| H71B | 0.4600 | 0.4378 | 0.3708 | 0.178* | 0.662 (8) |
| H71C | 0.3679 | 0.4521 | 0.3308 | 0.178* | 0.662 (8) |
| C72 | 0.4817 (9) | 0.5813 (7) | 0.2843 (8) | 0.104 (4) | 0.662 (8) |
| H72A | 0.5500 | 0.5745 | 0.2792 | 0.156* | 0.662 (8) |
| H72B | 0.4542 | 0.6002 | 0.3305 | 0.156* | 0.662 (8) |
| H72C | 0.4522 | 0.6212 | 0.2476 | 0.156* | 0.662 (8) |
| O32B | 0.393 (4) | 0.438 (3) | 0.258 (3) | 0.125 (6) | 0.129 (7) |
| C70B | 0.383 (3) | 0.489 (4) | 0.302 (3) | 0.102 (5) | 0.129 (7) |
| H70B | 0.3305 | 0.4985 | 0.3364 | 0.123* | 0.129 (7) |
| N12B | 0.447 (4) | 0.529 (4) | 0.299 (2) | 0.102 (4) | 0.129 (7) |
| C71B | 0.501 (4) | 0.539 (4) | 0.231 (2) | 0.104 (7) | 0.129 (7) |
| H71D | 0.5457 | 0.5696 | 0.2364 | 0.156* | 0.129 (7) |
| H71E | 0.5366 | 0.4855 | 0.2158 | 0.156* | 0.129 (7) |
| H71F | 0.4580 | 0.5684 | 0.1968 | 0.156* | 0.129 (7) |
| C72B | 0.485 (5) | 0.540 (4) | 0.362 (3) | 0.113 (7) | 0.129 (7) |
| H72D | 0.5305 | 0.5710 | 0.3489 | 0.170* | 0.129 (7) |
| H72E | 0.4334 | 0.5684 | 0.3947 | 0.170* | 0.129 (7) |
| H72F | 0.5167 | 0.4863 | 0.3836 | 0.170* | 0.129 (7) |
| N1 | 0.7053 (2) | 0.78168 (17) | 0.10739 (14) | 0.0199 (6) | |
| N2 | 0.6028 (2) | 0.89077 (17) | 0.28938 (14) | 0.0204 (6) | |
| N3 | 0.7847 (2) | 0.70711 (17) | 0.39560 (14) | 0.0206 (6) | |
| N4 | 0.8901 (2) | 0.60026 (17) | 0.21382 (15) | 0.0203 (6) | |
| O1 | 0.76938 (17) | 0.73567 (14) | 0.15509 (12) | 0.0214 (5) | |
| O2 | 0.76892 (17) | 0.67437 (14) | 0.04144 (12) | 0.0239 (5) | |
| O3 | 0.54324 (18) | 0.88879 (15) | 0.06758 (13) | 0.0281 (6) | |
| O4 | 0.66176 (17) | 0.84794 (15) | 0.23461 (12) | 0.0221 (5) | |
| O5 | 0.50616 (17) | 0.94708 (15) | 0.20380 (12) | 0.0244 (5) | |
| O6 | 0.52302 (18) | 0.91461 (16) | 0.42318 (13) | 0.0292 (6) | |
| O7 | 0.74262 (17) | 0.76747 (14) | 0.34465 (12) | 0.0212 (5) | |
| O8 | 0.67041 (18) | 0.78276 (15) | 0.47023 (12) | 0.0252 (5) | |
| O9 | 0.88376 (19) | 0.55938 (16) | 0.44801 (13) | 0.0314 (6) | |
| O10 | 0.85166 (17) | 0.65657 (14) | 0.26463 (12) | 0.0206 (5) | |
| O11 | 0.94163 (18) | 0.51425 (15) | 0.30769 (12) | 0.0259 (5) | |
| O12 | 0.90092 (19) | 0.53000 (15) | 0.09247 (13) | 0.0284 (6) | |
| O13 | 0.95902 (18) | 0.75362 (15) | 0.15842 (13) | 0.0259 (5) | |
| O14 | 0.95353 (18) | 0.68252 (15) | 0.07062 (13) | 0.0277 (6) | |
| O15 | 0.81358 (18) | 0.90155 (15) | 0.14887 (13) | 0.0277 (6) | |
| O16 | 0.67229 (19) | 0.96743 (16) | 0.11445 (14) | 0.0303 (6) | |
| O17 | 0.80583 (19) | 0.91298 (15) | 0.29535 (13) | 0.0296 (6) | |
| O18 | 0.71982 (19) | 0.92547 (16) | 0.39915 (13) | 0.0295 (6) | |
| O19 | 0.95190 (18) | 0.76711 (15) | 0.30493 (13) | 0.0274 (6) | |
| O20 | 1.00531 (19) | 0.64178 (16) | 0.35380 (14) | 0.0300 (6) | |
| O21 | 0.7043 (2) | 0.59229 (17) | 0.17790 (15) | 0.0327 (6) | |
| H21A | 0.664 (3) | 0.599 (3) | 0.150 (2) | 0.049* | |
| H21B | 0.715 (4) | 0.5427 (13) | 0.189 (3) | 0.049* | |
| O22 | 0.5360 (2) | 0.76725 (18) | 0.19837 (15) | 0.0337 (6) | |
| H22A | 0.518 (4) | 0.742 (3) | 0.171 (2) | 0.050* | |
| H22B | 0.482 (2) | 0.778 (3) | 0.219 (3) | 0.050* | |
| O23 | 0.5523 (2) | 0.74493 (19) | 0.36839 (16) | 0.0385 (7) | |
| H23A | 0.534 (4) | 0.725 (3) | 0.4070 (17) | 0.058* | |
| H23B | 0.4944 (19) | 0.766 (3) | 0.359 (3) | 0.058* | |
| O24C | 0.7301 (2) | 0.57216 (17) | 0.34800 (16) | 0.0374 (7) | 0.758 (8) |
| H24A | 0.693 (3) | 0.566 (2) | 0.3854 (16) | 0.056* | 0.758 (8) |
| H24B | 0.742 (4) | 0.5272 (10) | 0.3291 (16) | 0.056* | 0.758 (8) |
| O24 | 0.7301 (2) | 0.57216 (17) | 0.34800 (16) | 0.0374 (7) | 0.242 (8) |
| C73 | 0.7336 (14) | 0.5073 (10) | 0.3849 (10) | 0.053 (3) | 0.242 (8) |
| H73 | 0.7839 | 0.4622 | 0.3699 | 0.064* | 0.242 (8) |
| N13 | 0.6797 (15) | 0.4908 (12) | 0.4407 (11) | 0.083 (4) | 0.242 (8) |
| C74 | 0.589 (2) | 0.554 (2) | 0.455 (2) | 0.086 (6) | 0.242 (8) |
| H74A | 0.5533 | 0.5370 | 0.4973 | 0.129* | 0.242 (8) |
| H74B | 0.6016 | 0.6050 | 0.4618 | 0.129* | 0.242 (8) |
| H74C | 0.5515 | 0.5626 | 0.4149 | 0.129* | 0.242 (8) |
| C75 | 0.672 (3) | 0.4096 (14) | 0.458 (2) | 0.105 (7) | 0.242 (8) |
| H75A | 0.6286 | 0.4087 | 0.5014 | 0.157* | 0.242 (8) |
| H75B | 0.6465 | 0.3926 | 0.4199 | 0.157* | 0.242 (8) |
| H75C | 0.7341 | 0.3723 | 0.4658 | 0.157* | 0.242 (8) |
| O33 | 0.614 (2) | 0.5511 (17) | 0.4696 (16) | 0.097 (4) | 0.257 (14) |
| H33E | 0.6474 | 0.5763 | 0.4848 | 0.145* | 0.257 (14) |
| H33F | 0.5717 | 0.5468 | 0.5014 | 0.145* | 0.257 (14) |
| O34 | 0.6567 (9) | 0.3773 (8) | 0.5029 (7) | 0.073 (4) | 0.361 (13) |
| H34A | 0.6407 | 0.4059 | 0.5379 | 0.109* | 0.361 (13) |
| H34B | 0.6056 | 0.3767 | 0.4882 | 0.109* | 0.361 (13) |
| O25 | 0.5452 (2) | 0.6557 (2) | 0.10663 (18) | 0.0504 (8) | |
| C49 | 0.5246 (4) | 0.6532 (3) | 0.0472 (3) | 0.0533 (12) | |
| H49 | 0.5702 | 0.6184 | 0.0167 | 0.064* | |
| N5 | 0.4446 (3) | 0.6948 (3) | 0.0228 (2) | 0.0572 (11) | |
| C50 | 0.3745 (4) | 0.7508 (5) | 0.0669 (3) | 0.087 (2) | |
| H50A | 0.3800 | 0.8058 | 0.0546 | 0.130* | |
| H50B | 0.3852 | 0.7350 | 0.1163 | 0.130* | |
| H50C | 0.3110 | 0.7494 | 0.0596 | 0.130* | |
| C51 | 0.4265 (5) | 0.6905 (4) | −0.0491 (3) | 0.0772 (19) | |
| H51A | 0.4089 | 0.7452 | −0.0727 | 0.116* | |
| H51B | 0.3748 | 0.6660 | −0.0485 | 0.116* | |
| H51C | 0.4838 | 0.6573 | −0.0745 | 0.116* | |
| O31 | 0.8265 (6) | 0.2547 (5) | 0.4425 (5) | 0.124 (3) | 0.790 (9) |
| C67 | 0.9164 (7) | 0.2283 (6) | 0.4274 (5) | 0.086 (2) | 0.790 (9) |
| H67 | 0.9419 | 0.1729 | 0.4181 | 0.104* | 0.790 (9) |
| N11 | 0.9754 (5) | 0.2716 (5) | 0.4240 (4) | 0.0768 (19) | 0.790 (9) |
| C68 | 0.9530 (8) | 0.3518 (6) | 0.4479 (6) | 0.089 (3) | 0.790 (9) |
| H68A | 0.9751 | 0.3877 | 0.4109 | 0.133* | 0.790 (9) |
| H68B | 0.9844 | 0.3492 | 0.4901 | 0.133* | 0.790 (9) |
| H68C | 0.8844 | 0.3729 | 0.4588 | 0.133* | 0.790 (9) |
| C69 | 1.0730 (6) | 0.2372 (6) | 0.3946 (6) | 0.093 (3) | 0.790 (9) |
| H69A | 1.1089 | 0.2762 | 0.3953 | 0.139* | 0.790 (9) |
| H69B | 1.1020 | 0.1872 | 0.4226 | 0.139* | 0.790 (9) |
| H69C | 1.0737 | 0.2249 | 0.3460 | 0.139* | 0.790 (9) |
| O31B | 0.9091 (16) | 0.1627 (9) | 0.4682 (14) | 0.123 (6) | 0.210 (9) |
| C67B | 0.8859 (10) | 0.2372 (8) | 0.4658 (12) | 0.092 (4) | 0.210 (9) |
| H67B | 0.8207 | 0.2645 | 0.4760 | 0.110* | 0.210 (9) |
| N11B | 0.9444 (10) | 0.2819 (8) | 0.4504 (12) | 0.091 (4) | 0.210 (9) |
| C68B | 1.0447 (11) | 0.2424 (14) | 0.435 (2) | 0.100 (7) | 0.210 (9) |
| H68D | 1.0534 | 0.1878 | 0.4207 | 0.150* | 0.210 (9) |
| H68E | 1.0773 | 0.2388 | 0.4765 | 0.150* | 0.210 (9) |
| H68F | 1.0710 | 0.2740 | 0.3962 | 0.150* | 0.210 (9) |
| C69B | 0.9116 (19) | 0.3701 (8) | 0.449 (2) | 0.098 (7) | 0.210 (9) |
| H69D | 0.9656 | 0.3922 | 0.4361 | 0.147* | 0.210 (9) |
| H69E | 0.8661 | 0.3911 | 0.4137 | 0.147* | 0.210 (9) |
| H69F | 0.8808 | 0.3864 | 0.4950 | 0.147* | 0.210 (9) |
| Na1 | 0.66419 (10) | 0.68887 (9) | 0.26918 (8) | 0.0300 (3) | |
| Mn1 | 0.84339 (4) | 0.63331 (3) | 0.12120 (3) | 0.01926 (13) | |
| Mn2 | 0.60623 (4) | 0.87295 (3) | 0.14702 (3) | 0.01915 (13) | |
| Mn3 | 0.63617 (4) | 0.84893 (3) | 0.38408 (3) | 0.02020 (13) | |
| Mn4 | 0.87530 (4) | 0.60976 (3) | 0.35842 (3) | 0.02184 (14) | |
| Y1 | 0.83176 (2) | 0.80033 (2) | 0.23623 (2) | 0.01941 (9) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0219 (17) | 0.0207 (17) | 0.0181 (16) | −0.0060 (14) | −0.0003 (13) | 0.0003 (13) |
| C2 | 0.0255 (18) | 0.0303 (19) | 0.0174 (17) | −0.0098 (15) | −0.0057 (14) | 0.0018 (14) |
| C3 | 0.0284 (19) | 0.0244 (18) | 0.0206 (17) | −0.0095 (15) | −0.0038 (14) | 0.0006 (14) |
| C4 | 0.0278 (19) | 0.0282 (19) | 0.0253 (19) | −0.0062 (16) | −0.0061 (15) | 0.0039 (15) |
| C5 | 0.032 (2) | 0.041 (2) | 0.0209 (18) | −0.0080 (18) | −0.0116 (16) | 0.0074 (16) |
| C6 | 0.042 (2) | 0.046 (2) | 0.0195 (18) | −0.010 (2) | −0.0057 (17) | −0.0055 (17) |
| C7 | 0.030 (2) | 0.033 (2) | 0.0254 (19) | −0.0042 (16) | −0.0035 (16) | −0.0047 (16) |
| C8 | 0.0224 (17) | 0.0175 (16) | 0.0205 (17) | −0.0027 (14) | −0.0013 (14) | 0.0027 (13) |
| C9 | 0.0219 (18) | 0.0208 (17) | 0.0249 (18) | 0.0022 (14) | 0.0018 (14) | −0.0048 (14) |
| C10 | 0.0212 (18) | 0.0238 (18) | 0.0268 (18) | −0.0026 (15) | −0.0039 (14) | −0.0020 (15) |
| C11 | 0.028 (2) | 0.032 (2) | 0.0239 (18) | −0.0050 (16) | −0.0003 (15) | −0.0042 (15) |
| C12 | 0.033 (2) | 0.029 (2) | 0.032 (2) | 0.0035 (17) | 0.0058 (17) | −0.0097 (17) |
| C13 | 0.036 (2) | 0.031 (2) | 0.036 (2) | 0.0115 (18) | −0.0021 (18) | −0.0039 (18) |
| C14 | 0.036 (2) | 0.030 (2) | 0.0242 (19) | 0.0041 (17) | −0.0035 (16) | −0.0010 (16) |
| C15 | 0.0255 (18) | 0.0220 (17) | 0.0204 (17) | −0.0069 (15) | −0.0025 (14) | −0.0018 (14) |
| C16 | 0.0308 (19) | 0.0275 (19) | 0.0162 (17) | −0.0098 (16) | −0.0041 (14) | 0.0034 (14) |
| C17 | 0.0292 (19) | 0.0273 (19) | 0.0206 (18) | −0.0087 (15) | −0.0029 (15) | 0.0012 (14) |
| C18 | 0.037 (2) | 0.0269 (19) | 0.0268 (19) | −0.0052 (17) | −0.0076 (16) | 0.0051 (16) |
| C19 | 0.048 (3) | 0.034 (2) | 0.0216 (19) | −0.0111 (19) | −0.0101 (17) | 0.0105 (16) |
| C20 | 0.051 (3) | 0.039 (2) | 0.0174 (18) | −0.008 (2) | −0.0013 (17) | 0.0017 (16) |
| C21 | 0.039 (2) | 0.028 (2) | 0.0230 (19) | −0.0044 (17) | −0.0024 (16) | 0.0002 (15) |
| C22 | 0.0214 (17) | 0.0188 (17) | 0.0236 (18) | −0.0016 (14) | −0.0023 (14) | 0.0001 (14) |
| C23 | 0.0251 (18) | 0.0180 (17) | 0.0293 (19) | 0.0007 (14) | −0.0006 (15) | −0.0056 (14) |
| C24 | 0.0239 (18) | 0.0234 (18) | 0.0272 (18) | −0.0045 (15) | −0.0021 (15) | −0.0016 (15) |
| C25 | 0.034 (2) | 0.0271 (19) | 0.030 (2) | −0.0048 (16) | −0.0002 (16) | −0.0096 (16) |
| C26 | 0.036 (2) | 0.027 (2) | 0.041 (2) | 0.0009 (17) | −0.0035 (18) | −0.0173 (18) |
| C27 | 0.040 (2) | 0.023 (2) | 0.048 (3) | 0.0074 (18) | −0.009 (2) | −0.0071 (18) |
| C28 | 0.039 (2) | 0.0261 (19) | 0.0263 (19) | −0.0001 (17) | −0.0062 (17) | −0.0055 (16) |
| C29 | 0.0265 (19) | 0.0259 (19) | 0.0265 (19) | −0.0033 (15) | 0.0034 (15) | −0.0052 (15) |
| C30 | 0.050 (3) | 0.046 (3) | 0.033 (2) | −0.027 (2) | 0.016 (2) | −0.0150 (19) |
| C31 | 0.062 (3) | 0.054 (3) | 0.046 (3) | −0.036 (3) | 0.025 (2) | −0.024 (2) |
| C32 | 0.070 (4) | 0.076 (4) | 0.071 (4) | −0.051 (3) | 0.045 (3) | −0.045 (3) |
| C33 | 0.106 (5) | 0.055 (3) | 0.033 (3) | −0.045 (3) | 0.002 (3) | 0.006 (2) |
| C34 | 0.032 (2) | 0.0258 (19) | 0.0226 (18) | −0.0020 (16) | 0.0029 (15) | 0.0021 (15) |
| C35 | 0.032 (2) | 0.027 (2) | 0.051 (3) | −0.0097 (17) | −0.0041 (19) | 0.0052 (18) |
| C36 | 0.050 (3) | 0.043 (3) | 0.073 (4) | −0.022 (2) | −0.010 (3) | 0.019 (3) |
| C37 | 0.041 (3) | 0.033 (2) | 0.075 (4) | −0.009 (2) | −0.005 (2) | −0.012 (2) |
| C38 | 0.037 (2) | 0.039 (2) | 0.063 (3) | −0.016 (2) | −0.003 (2) | 0.004 (2) |
| C39 | 0.032 (2) | 0.0227 (18) | 0.028 (2) | −0.0026 (16) | −0.0017 (16) | −0.0037 (15) |
| C40 | 0.045 (3) | 0.048 (3) | 0.034 (2) | −0.023 (2) | 0.0065 (19) | −0.0156 (19) |
| C41 | 0.046 (3) | 0.051 (3) | 0.050 (3) | −0.026 (2) | 0.006 (2) | −0.015 (2) |
| C42 | 0.061 (3) | 0.112 (5) | 0.039 (3) | −0.049 (4) | −0.010 (2) | 0.001 (3) |
| C43 | 0.072 (4) | 0.071 (4) | 0.084 (4) | −0.044 (3) | 0.029 (3) | −0.047 (3) |
| C44 | 0.031 (2) | 0.032 (2) | 0.0231 (18) | −0.0049 (17) | −0.0065 (15) | −0.0053 (16) |
| C45 | 0.028 (2) | 0.035 (2) | 0.042 (2) | −0.0083 (17) | −0.0079 (18) | −0.0016 (18) |
| C46 | 0.038 (2) | 0.043 (3) | 0.062 (3) | −0.017 (2) | −0.007 (2) | −0.004 (2) |
| C47 | 0.038 (3) | 0.040 (2) | 0.058 (3) | −0.010 (2) | 0.009 (2) | −0.005 (2) |
| C48 | 0.040 (3) | 0.064 (3) | 0.060 (3) | −0.016 (2) | −0.020 (2) | 0.008 (3) |
| O26 | 0.0374 (18) | 0.068 (2) | 0.051 (2) | −0.0147 (17) | 0.0045 (15) | −0.0159 (17) |
| C52 | 0.032 (2) | 0.068 (3) | 0.054 (3) | −0.019 (2) | 0.001 (2) | −0.021 (3) |
| N6 | 0.038 (2) | 0.065 (3) | 0.056 (3) | −0.022 (2) | 0.0059 (19) | −0.025 (2) |
| C53 | 0.082 (4) | 0.074 (4) | 0.063 (4) | −0.038 (4) | 0.021 (3) | −0.021 (3) |
| C54 | 0.040 (3) | 0.086 (4) | 0.094 (5) | −0.013 (3) | 0.005 (3) | −0.049 (4) |
| O27 | 0.104 (5) | 0.158 (7) | 0.057 (4) | −0.082 (5) | 0.000 (3) | 0.004 (4) |
| C55 | 0.067 (4) | 0.142 (6) | 0.053 (4) | −0.057 (4) | 0.002 (3) | −0.010 (4) |
| N7 | 0.072 (4) | 0.138 (6) | 0.063 (4) | −0.049 (4) | 0.008 (3) | 0.002 (4) |
| C56 | 0.091 (7) | 0.172 (9) | 0.088 (7) | −0.045 (7) | 0.037 (6) | 0.031 (7) |
| C57 | 0.069 (5) | 0.168 (9) | 0.083 (6) | −0.035 (6) | 0.009 (5) | −0.033 (6) |
| O27B | 0.155 (18) | 0.20 (2) | 0.144 (18) | −0.016 (18) | −0.013 (16) | −0.023 (17) |
| C55B | 0.094 (9) | 0.164 (11) | 0.085 (9) | −0.031 (10) | 0.005 (9) | −0.014 (9) |
| N7B | 0.079 (6) | 0.148 (7) | 0.073 (6) | −0.042 (6) | 0.000 (6) | −0.010 (6) |
| C56B | 0.097 (11) | 0.166 (11) | 0.096 (10) | −0.030 (11) | 0.016 (10) | 0.011 (10) |
| C57B | 0.062 (9) | 0.147 (11) | 0.088 (10) | −0.051 (9) | 0.004 (9) | −0.003 (10) |
| O28 | 0.067 (3) | 0.032 (2) | 0.057 (3) | −0.014 (2) | −0.005 (2) | −0.002 (2) |
| C58 | 0.071 (4) | 0.038 (3) | 0.063 (3) | −0.019 (3) | −0.007 (3) | −0.006 (3) |
| N8 | 0.070 (3) | 0.039 (3) | 0.065 (3) | −0.025 (2) | 0.002 (3) | −0.018 (2) |
| C59 | 0.121 (7) | 0.078 (5) | 0.066 (5) | −0.016 (5) | −0.011 (5) | −0.023 (4) |
| C60 | 0.084 (5) | 0.048 (4) | 0.102 (6) | −0.016 (4) | −0.002 (5) | −0.022 (4) |
| O28B | 0.088 (9) | 0.047 (8) | 0.072 (9) | −0.024 (7) | −0.005 (9) | −0.008 (8) |
| C58B | 0.075 (6) | 0.044 (5) | 0.066 (6) | −0.025 (5) | −0.004 (5) | −0.010 (5) |
| N8B | 0.078 (6) | 0.047 (6) | 0.071 (5) | −0.020 (5) | −0.001 (5) | −0.011 (5) |
| C59B | 0.104 (14) | 0.073 (13) | 0.088 (13) | −0.018 (12) | 0.013 (13) | −0.002 (12) |
| C60B | 0.089 (11) | 0.060 (11) | 0.097 (12) | −0.025 (10) | 0.004 (12) | −0.015 (11) |
| O24B | 0.121 (11) | 0.086 (10) | 0.077 (9) | −0.044 (9) | −0.003 (9) | 0.014 (9) |
| C73B | 0.097 (8) | 0.096 (7) | 0.081 (8) | −0.041 (7) | 0.019 (7) | 0.017 (7) |
| N13B | 0.099 (7) | 0.107 (7) | 0.090 (7) | −0.033 (6) | −0.001 (6) | 0.018 (6) |
| C74B | 0.113 (18) | 0.152 (19) | 0.138 (19) | −0.003 (17) | −0.020 (17) | −0.001 (18) |
| C75B | 0.093 (11) | 0.119 (11) | 0.088 (11) | −0.036 (10) | −0.020 (10) | 0.033 (10) |
| O29 | 0.122 (5) | 0.080 (4) | 0.072 (4) | −0.020 (4) | −0.003 (4) | 0.002 (3) |
| C61 | 0.066 (4) | 0.052 (3) | 0.073 (4) | −0.014 (3) | −0.016 (3) | −0.016 (3) |
| N9 | 0.065 (3) | 0.050 (3) | 0.068 (4) | −0.027 (2) | 0.000 (3) | −0.013 (3) |
| C62 | 0.101 (6) | 0.078 (6) | 0.078 (6) | −0.024 (5) | −0.024 (5) | −0.010 (5) |
| C63 | 0.081 (5) | 0.067 (5) | 0.126 (7) | −0.037 (4) | 0.026 (5) | −0.013 (5) |
| O29B | 0.116 (13) | 0.098 (13) | 0.123 (14) | −0.035 (12) | −0.002 (12) | −0.012 (12) |
| C61B | 0.079 (7) | 0.062 (7) | 0.083 (7) | −0.028 (7) | −0.008 (7) | −0.011 (7) |
| N9B | 0.077 (6) | 0.060 (6) | 0.086 (6) | −0.024 (5) | −0.009 (6) | −0.015 (6) |
| C62B | 0.084 (10) | 0.067 (10) | 0.102 (10) | −0.018 (9) | −0.007 (10) | −0.011 (10) |
| C63B | 0.092 (13) | 0.076 (13) | 0.120 (14) | −0.032 (12) | 0.013 (13) | −0.019 (13) |
| O30 | 0.066 (3) | 0.068 (3) | 0.068 (3) | −0.003 (3) | −0.003 (3) | −0.021 (3) |
| C64 | 0.057 (4) | 0.038 (3) | 0.055 (4) | −0.016 (3) | −0.020 (3) | 0.000 (3) |
| N10 | 0.049 (3) | 0.040 (3) | 0.048 (3) | −0.025 (2) | −0.011 (2) | −0.001 (2) |
| C65 | 0.061 (4) | 0.056 (5) | 0.057 (5) | −0.036 (3) | −0.009 (3) | −0.010 (4) |
| C66 | 0.068 (5) | 0.063 (5) | 0.060 (4) | −0.035 (4) | −0.001 (4) | −0.007 (4) |
| O30B | 0.116 (16) | 0.104 (16) | 0.139 (18) | −0.010 (14) | 0.035 (15) | −0.011 (15) |
| C64B | 0.079 (9) | 0.078 (9) | 0.078 (9) | −0.023 (8) | 0.008 (9) | −0.001 (9) |
| N10B | 0.068 (7) | 0.064 (7) | 0.064 (7) | −0.031 (6) | −0.002 (6) | −0.001 (6) |
| C65B | 0.068 (11) | 0.056 (11) | 0.065 (12) | −0.026 (10) | −0.003 (10) | −0.015 (10) |
| C66B | 0.072 (11) | 0.068 (12) | 0.065 (10) | −0.030 (10) | −0.006 (10) | 0.013 (11) |
| O32 | 0.180 (10) | 0.154 (9) | 0.113 (7) | −0.032 (8) | −0.060 (7) | 0.006 (7) |
| C70 | 0.114 (7) | 0.106 (7) | 0.109 (7) | −0.050 (6) | −0.043 (6) | 0.014 (6) |
| N12 | 0.087 (5) | 0.100 (6) | 0.115 (6) | −0.053 (5) | −0.015 (5) | 0.026 (5) |
| C71 | 0.107 (8) | 0.116 (8) | 0.130 (9) | −0.049 (7) | −0.002 (7) | 0.042 (7) |
| C72 | 0.094 (7) | 0.098 (7) | 0.129 (9) | −0.055 (6) | −0.002 (7) | 0.018 (7) |
| O32B | 0.123 (10) | 0.127 (10) | 0.126 (10) | −0.045 (10) | −0.020 (10) | 0.025 (10) |
| C70B | 0.094 (8) | 0.104 (9) | 0.119 (9) | −0.055 (8) | −0.019 (8) | 0.027 (8) |
| N12B | 0.097 (7) | 0.103 (8) | 0.118 (8) | −0.054 (7) | −0.024 (7) | 0.026 (7) |
| C71B | 0.101 (11) | 0.103 (11) | 0.117 (12) | −0.053 (11) | −0.020 (11) | 0.025 (11) |
| C72B | 0.103 (11) | 0.110 (11) | 0.128 (12) | −0.048 (11) | −0.011 (11) | 0.029 (11) |
| N1 | 0.0207 (14) | 0.0223 (14) | 0.0152 (13) | −0.0033 (12) | −0.0052 (11) | 0.0015 (11) |
| N2 | 0.0187 (14) | 0.0221 (14) | 0.0166 (14) | −0.0011 (12) | 0.0032 (11) | −0.0049 (11) |
| N3 | 0.0258 (15) | 0.0182 (14) | 0.0143 (13) | −0.0022 (12) | −0.0036 (11) | 0.0034 (11) |
| N4 | 0.0233 (15) | 0.0165 (14) | 0.0184 (14) | −0.0014 (11) | 0.0005 (11) | −0.0054 (11) |
| O1 | 0.0227 (12) | 0.0190 (11) | 0.0187 (11) | 0.0004 (10) | −0.0047 (9) | 0.0000 (9) |
| O2 | 0.0271 (13) | 0.0220 (12) | 0.0192 (12) | −0.0005 (10) | −0.0028 (10) | −0.0048 (10) |
| O3 | 0.0286 (14) | 0.0257 (13) | 0.0247 (13) | 0.0023 (11) | −0.0066 (11) | −0.0033 (10) |
| O4 | 0.0200 (12) | 0.0258 (12) | 0.0155 (11) | 0.0008 (10) | 0.0007 (9) | −0.0041 (9) |
| O5 | 0.0258 (13) | 0.0237 (12) | 0.0183 (12) | 0.0015 (10) | −0.0030 (10) | −0.0011 (10) |
| O6 | 0.0281 (14) | 0.0337 (14) | 0.0199 (12) | −0.0012 (11) | 0.0007 (10) | −0.0023 (11) |
| O7 | 0.0229 (12) | 0.0192 (11) | 0.0160 (11) | 0.0004 (10) | −0.0012 (9) | 0.0031 (9) |
| O8 | 0.0286 (13) | 0.0238 (13) | 0.0175 (12) | 0.0004 (11) | 0.0002 (10) | −0.0022 (10) |
| O9 | 0.0361 (15) | 0.0253 (13) | 0.0231 (13) | 0.0032 (11) | −0.0007 (11) | 0.0028 (10) |
| O10 | 0.0256 (12) | 0.0157 (11) | 0.0169 (11) | −0.0005 (9) | 0.0000 (9) | −0.0033 (9) |
| O11 | 0.0317 (14) | 0.0206 (12) | 0.0196 (12) | 0.0016 (10) | −0.0043 (10) | 0.0002 (10) |
| O12 | 0.0357 (15) | 0.0243 (13) | 0.0225 (13) | −0.0022 (11) | −0.0049 (11) | −0.0056 (10) |
| O13 | 0.0248 (13) | 0.0289 (13) | 0.0217 (13) | −0.0053 (11) | 0.0026 (10) | −0.0052 (10) |
| O14 | 0.0317 (14) | 0.0274 (13) | 0.0239 (13) | −0.0101 (11) | 0.0043 (11) | −0.0052 (11) |
| O15 | 0.0290 (14) | 0.0254 (13) | 0.0247 (13) | −0.0045 (11) | 0.0003 (11) | 0.0023 (10) |
| O16 | 0.0322 (15) | 0.0269 (13) | 0.0327 (14) | −0.0108 (11) | −0.0058 (11) | 0.0036 (11) |
| O17 | 0.0367 (15) | 0.0253 (13) | 0.0258 (13) | −0.0081 (11) | 0.0005 (11) | −0.0047 (11) |
| O18 | 0.0293 (14) | 0.0326 (14) | 0.0270 (13) | −0.0101 (11) | 0.0041 (11) | −0.0093 (11) |
| O19 | 0.0278 (14) | 0.0272 (13) | 0.0255 (13) | −0.0034 (11) | −0.0066 (11) | −0.0030 (10) |
| O20 | 0.0293 (14) | 0.0296 (14) | 0.0297 (14) | −0.0068 (11) | −0.0073 (11) | 0.0044 (11) |
| O21 | 0.0360 (16) | 0.0293 (14) | 0.0341 (15) | −0.0104 (13) | −0.0069 (12) | −0.0006 (12) |
| O22 | 0.0302 (15) | 0.0360 (16) | 0.0348 (16) | −0.0112 (13) | 0.0029 (12) | −0.0051 (12) |
| O23 | 0.0369 (16) | 0.0433 (17) | 0.0347 (16) | −0.0144 (14) | 0.0015 (13) | 0.0018 (13) |
| O24C | 0.0448 (17) | 0.0280 (14) | 0.0382 (16) | −0.0118 (13) | −0.0002 (13) | 0.0009 (12) |
| O24 | 0.0448 (17) | 0.0280 (14) | 0.0382 (16) | −0.0118 (13) | −0.0002 (13) | 0.0009 (12) |
| C73 | 0.057 (6) | 0.050 (6) | 0.053 (6) | −0.026 (5) | 0.004 (5) | 0.009 (5) |
| N13 | 0.084 (7) | 0.083 (6) | 0.079 (6) | −0.036 (6) | 0.007 (6) | 0.023 (6) |
| C74 | 0.089 (11) | 0.098 (10) | 0.072 (10) | −0.040 (10) | 0.000 (9) | 0.018 (9) |
| C75 | 0.097 (11) | 0.116 (11) | 0.092 (11) | −0.030 (10) | −0.008 (10) | 0.032 (10) |
| O33 | 0.100 (8) | 0.099 (7) | 0.085 (7) | −0.042 (7) | 0.017 (6) | 0.018 (6) |
| O34 | 0.073 (7) | 0.091 (7) | 0.070 (7) | −0.056 (5) | −0.011 (5) | 0.026 (5) |
| O25 | 0.053 (2) | 0.0454 (18) | 0.056 (2) | −0.0126 (16) | −0.0182 (17) | −0.0072 (16) |
| C49 | 0.058 (3) | 0.045 (3) | 0.064 (3) | −0.019 (2) | −0.019 (3) | −0.004 (2) |
| N5 | 0.053 (2) | 0.057 (3) | 0.067 (3) | −0.024 (2) | −0.023 (2) | 0.014 (2) |
| C50 | 0.047 (3) | 0.114 (6) | 0.078 (4) | −0.006 (4) | −0.005 (3) | 0.031 (4) |
| C51 | 0.092 (5) | 0.077 (4) | 0.077 (4) | −0.041 (4) | −0.040 (4) | 0.017 (3) |
| O31 | 0.128 (6) | 0.150 (6) | 0.128 (6) | −0.099 (5) | 0.031 (5) | −0.039 (5) |
| C67 | 0.108 (5) | 0.105 (5) | 0.071 (5) | −0.079 (4) | 0.019 (4) | −0.014 (4) |
| N11 | 0.096 (5) | 0.097 (4) | 0.060 (4) | −0.073 (4) | 0.006 (3) | 0.004 (3) |
| C68 | 0.119 (7) | 0.103 (6) | 0.072 (5) | −0.082 (6) | 0.018 (6) | −0.020 (5) |
| C69 | 0.084 (6) | 0.085 (6) | 0.109 (8) | −0.034 (5) | −0.010 (6) | 0.021 (6) |
| O31B | 0.132 (11) | 0.143 (11) | 0.108 (11) | −0.070 (10) | 0.009 (10) | −0.009 (10) |
| C67B | 0.108 (7) | 0.114 (6) | 0.076 (7) | −0.069 (6) | 0.003 (6) | −0.015 (6) |
| N11B | 0.111 (7) | 0.108 (6) | 0.075 (6) | −0.074 (6) | 0.011 (6) | −0.010 (6) |
| C68B | 0.105 (12) | 0.109 (11) | 0.084 (12) | −0.040 (11) | 0.009 (12) | 0.008 (11) |
| C69B | 0.123 (13) | 0.110 (11) | 0.071 (11) | −0.061 (11) | 0.011 (12) | 0.000 (11) |
| Na1 | 0.0306 (8) | 0.0275 (7) | 0.0303 (8) | −0.0058 (6) | −0.0019 (6) | −0.0044 (6) |
| Mn1 | 0.0231 (3) | 0.0161 (3) | 0.0155 (3) | −0.0002 (2) | −0.0028 (2) | −0.0023 (2) |
| Mn2 | 0.0202 (3) | 0.0180 (3) | 0.0155 (3) | 0.0006 (2) | −0.0024 (2) | −0.0015 (2) |
| Mn3 | 0.0203 (3) | 0.0211 (3) | 0.0140 (3) | 0.0012 (2) | −0.0003 (2) | −0.0006 (2) |
| Mn4 | 0.0272 (3) | 0.0174 (3) | 0.0152 (3) | 0.0012 (2) | −0.0008 (2) | 0.0001 (2) |
| Y1 | 0.02044 (16) | 0.01900 (16) | 0.01589 (15) | −0.00179 (11) | −0.00074 (11) | −0.00104 (11) |
Geometric parameters (Å, º)
| C1—O2 | 1.287 (4) | C74B—H74D | 0.9800 |
| C1—N1 | 1.313 (4) | C74B—H74E | 0.9800 |
| C1—C2 | 1.481 (5) | C74B—H74F | 0.9800 |
| C2—C7 | 1.404 (5) | C75B—H75D | 0.9800 |
| C2—C3 | 1.414 (5) | C75B—H75E | 0.9800 |
| C3—O3 | 1.329 (4) | C75B—H75F | 0.9800 |
| C3—C4 | 1.402 (5) | O29—C61 | 1.222 (9) |
| C4—C5 | 1.379 (5) | C61—N9 | 1.311 (8) |
| C4—H4 | 0.9500 | C61—H61 | 0.9500 |
| C5—C6 | 1.386 (6) | N9—C63 | 1.441 (9) |
| C5—H5 | 0.9500 | N9—C62 | 1.460 (9) |
| C6—C7 | 1.385 (6) | C62—H62A | 0.9800 |
| C6—H6 | 0.9500 | C62—H62B | 0.9800 |
| C7—H7 | 0.9500 | C62—H62C | 0.9800 |
| C8—O5 | 1.288 (4) | C63—H63A | 0.9800 |
| C8—N2 | 1.320 (5) | C63—H63B | 0.9800 |
| C8—C9 | 1.475 (5) | C63—H63C | 0.9800 |
| C9—C14 | 1.408 (5) | O29B—C61B | 1.245 (16) |
| C9—C10 | 1.417 (5) | C61B—N9B | 1.310 (15) |
| C10—O6 | 1.332 (4) | C61B—H61B | 0.9500 |
| C10—C11 | 1.395 (5) | N9B—C63B | 1.441 (15) |
| C11—C12 | 1.371 (6) | N9B—C62B | 1.455 (15) |
| C11—H11 | 0.9500 | C62B—H62D | 0.9800 |
| C12—C13 | 1.397 (6) | C62B—H62E | 0.9800 |
| C12—H12 | 0.9500 | C62B—H62F | 0.9800 |
| C13—C14 | 1.381 (6) | C63B—H63D | 0.9800 |
| C13—H13 | 0.9500 | C63B—H63E | 0.9800 |
| C14—H14 | 0.9500 | C63B—H63F | 0.9800 |
| C15—O8 | 1.289 (4) | O30—C64 | 1.225 (7) |
| C15—N3 | 1.317 (5) | C64—N10 | 1.342 (7) |
| C15—C16 | 1.475 (5) | C64—H64 | 0.9500 |
| C16—C21 | 1.409 (5) | N10—C65 | 1.450 (8) |
| C16—C17 | 1.414 (5) | N10—C66 | 1.453 (8) |
| C17—O9 | 1.329 (4) | C65—H65A | 0.9800 |
| C17—C18 | 1.408 (5) | C65—H65B | 0.9800 |
| C18—C19 | 1.376 (6) | C65—H65C | 0.9800 |
| C18—H18 | 0.9500 | C66—H66A | 0.9800 |
| C19—C20 | 1.396 (6) | C66—H66B | 0.9800 |
| C19—H19 | 0.9500 | C66—H66C | 0.9800 |
| C20—C21 | 1.383 (5) | O30B—C64B | 1.247 (15) |
| C20—H20 | 0.9500 | C64B—N10B | 1.303 (14) |
| C21—H21 | 0.9500 | C64B—H64B | 0.9500 |
| C22—O11 | 1.293 (4) | N10B—C66B | 1.443 (14) |
| C22—N4 | 1.315 (4) | N10B—C65B | 1.462 (15) |
| C22—C23 | 1.474 (5) | C65B—H65D | 0.9800 |
| C23—C28 | 1.406 (5) | C65B—H65E | 0.9800 |
| C23—C24 | 1.414 (5) | C65B—H65F | 0.9800 |
| C24—O12 | 1.332 (4) | C66B—H66D | 0.9800 |
| C24—C25 | 1.401 (5) | C66B—H66E | 0.9800 |
| C25—C26 | 1.369 (6) | C66B—H66F | 0.9800 |
| C25—H25 | 0.9500 | O32—C70 | 1.258 (11) |
| C26—C27 | 1.393 (6) | C70—N12 | 1.319 (12) |
| C26—H26 | 0.9500 | C70—H70 | 0.9500 |
| C27—C28 | 1.378 (6) | N12—C71 | 1.467 (11) |
| C27—H27 | 0.9500 | N12—C72 | 1.469 (11) |
| C28—H28 | 0.9500 | C71—H71A | 0.9800 |
| C29—O14 | 1.251 (5) | C71—H71B | 0.9800 |
| C29—O13 | 1.261 (4) | C71—H71C | 0.9800 |
| C29—C30 | 1.537 (6) | C72—H72A | 0.9800 |
| C30—C32 | 1.521 (6) | C72—H72B | 0.9800 |
| C30—C31 | 1.522 (6) | C72—H72C | 0.9800 |
| C30—C33 | 1.538 (8) | O32B—C70B | 1.250 (16) |
| C31—H31A | 0.9800 | C70B—N12B | 1.323 (15) |
| C31—H31B | 0.9800 | C70B—H70B | 0.9500 |
| C31—H31C | 0.9800 | N12B—C72B | 1.440 (16) |
| C32—H32A | 0.9800 | N12B—C71B | 1.468 (16) |
| C32—H32B | 0.9800 | C71B—H71D | 0.9800 |
| C32—H32C | 0.9800 | C71B—H71E | 0.9800 |
| C33—H33A | 0.9800 | C71B—H71F | 0.9800 |
| C33—H33B | 0.9800 | C72B—H72D | 0.9800 |
| C33—H33C | 0.9800 | C72B—H72E | 0.9800 |
| C34—O16 | 1.251 (5) | C72B—H72F | 0.9800 |
| C34—O15 | 1.259 (5) | N1—O1 | 1.415 (4) |
| C34—C35 | 1.534 (6) | N1—Mn2 | 1.964 (3) |
| C35—C36 | 1.518 (7) | N2—O4 | 1.408 (4) |
| C35—C38 | 1.527 (6) | N2—Mn3 | 1.956 (3) |
| C35—C37 | 1.553 (7) | N3—O7 | 1.411 (4) |
| C36—H36A | 0.9800 | N3—Mn4 | 1.962 (3) |
| C36—H36B | 0.9800 | N4—O10 | 1.406 (4) |
| C36—H36C | 0.9800 | N4—Mn1 | 1.963 (3) |
| C37—H37A | 0.9800 | O1—Mn1 | 1.925 (2) |
| C37—H37B | 0.9800 | O1—Y1 | 2.439 (2) |
| C37—H37C | 0.9800 | O1—Na1 | 2.717 (3) |
| C38—H38A | 0.9800 | O2—Mn1 | 1.956 (2) |
| C38—H38B | 0.9800 | O3—Mn2 | 1.850 (3) |
| C38—H38C | 0.9800 | O4—Mn2 | 1.925 (2) |
| C39—O18 | 1.250 (5) | O4—Y1 | 2.419 (2) |
| C39—O17 | 1.274 (5) | O4—Na1 | 2.752 (3) |
| C39—C40 | 1.529 (6) | O5—Mn2 | 1.956 (2) |
| C40—C43 | 1.516 (7) | O6—Mn3 | 1.850 (3) |
| C40—C41 | 1.523 (6) | O7—Mn3 | 1.919 (2) |
| C40—C42 | 1.547 (8) | O7—Y1 | 2.429 (2) |
| C41—H41A | 0.9800 | O7—Na1 | 2.676 (3) |
| C41—H41B | 0.9800 | O8—Mn3 | 1.943 (2) |
| C41—H41C | 0.9800 | O9—Mn4 | 1.846 (3) |
| C42—H42A | 0.9800 | O10—Mn4 | 1.927 (2) |
| C42—H42B | 0.9800 | O10—Y1 | 2.427 (2) |
| C42—H42C | 0.9800 | O10—Na1 | 2.667 (3) |
| C43—H43A | 0.9800 | O11—Mn4 | 1.954 (2) |
| C43—H43B | 0.9800 | O12—Mn1 | 1.854 (3) |
| C43—H43C | 0.9800 | O13—Y1 | 2.261 (2) |
| C44—O20 | 1.248 (5) | O14—Mn1 | 2.140 (3) |
| C44—O19 | 1.271 (5) | O15—Y1 | 2.270 (2) |
| C44—C45 | 1.540 (6) | O16—Mn2 | 2.143 (3) |
| C45—C46 | 1.522 (6) | O17—Y1 | 2.281 (3) |
| C45—C48 | 1.526 (6) | O18—Mn3 | 2.132 (3) |
| C45—C47 | 1.542 (6) | O19—Y1 | 2.261 (2) |
| C46—H46A | 0.9800 | O20—Mn4 | 2.152 (3) |
| C46—H46B | 0.9800 | O21—Na1 | 2.460 (3) |
| C46—H46C | 0.9800 | O21—Mn1 | 2.466 (3) |
| C47—H47A | 0.9800 | O21—H21A | 0.82 (2) |
| C47—H47B | 0.9800 | O21—H21B | 0.83 (2) |
| C47—H47C | 0.9800 | O22—Mn2 | 2.423 (3) |
| C48—H48A | 0.9800 | O22—Na1 | 2.463 (3) |
| C48—H48B | 0.9800 | O22—H22A | 0.84 (2) |
| C48—H48C | 0.9800 | O22—H22B | 0.83 (2) |
| O26—C52 | 1.252 (6) | O23—Na1 | 2.449 (3) |
| C52—N6 | 1.312 (6) | O23—H23A | 0.84 (2) |
| C52—H52 | 0.9500 | O23—H23B | 0.86 (2) |
| N6—C53 | 1.440 (8) | O24C—Na1 | 2.424 (3) |
| N6—C54 | 1.455 (7) | O24C—Mn4 | 2.469 (3) |
| C53—H53A | 0.9800 | O24C—H24A | 0.863 (19) |
| C53—H53B | 0.9800 | O24C—H24B | 0.859 (19) |
| C53—H53C | 0.9800 | O24—C73 | 1.251 (12) |
| C54—H54A | 0.9800 | O24—Na1 | 2.424 (3) |
| C54—H54B | 0.9800 | O24—Mn4 | 2.469 (3) |
| C54—H54C | 0.9800 | C73—N13 | 1.313 (14) |
| O27—C55 | 1.271 (9) | C73—H73 | 0.9500 |
| C55—N7 | 1.298 (9) | N13—C75 | 1.447 (15) |
| C55—H55 | 0.9500 | N13—C74 | 1.483 (15) |
| N7—C57 | 1.444 (10) | C74—H74A | 0.9800 |
| N7—C56 | 1.477 (10) | C74—H74B | 0.9800 |
| C56—H56A | 0.9800 | C74—H74C | 0.9800 |
| C56—H56B | 0.9800 | C75—H75A | 0.9800 |
| C56—H56C | 0.9800 | C75—H75B | 0.9800 |
| C57—H57A | 0.9800 | C75—H75C | 0.9800 |
| C57—H57B | 0.9800 | O33—H33E | 0.8447 |
| C57—H57C | 0.9800 | O33—H33F | 0.8356 |
| O27B—C55B | 1.232 (6) | O34—H34A | 0.8494 |
| C55B—N7B | 1.311 (6) | O34—H34B | 0.8475 |
| C55B—H55B | 0.9500 | O25—C49 | 1.232 (5) |
| N7B—C56B | 1.453 (7) | C49—N5 | 1.311 (6) |
| N7B—C57B | 1.460 (6) | C49—H49 | 0.9500 |
| C56B—H56D | 0.9800 | N5—C50 | 1.452 (7) |
| C56B—H56E | 0.9800 | N5—C51 | 1.459 (6) |
| C56B—H56F | 0.9800 | C50—H50A | 0.9800 |
| C57B—H57D | 0.9800 | C50—H50B | 0.9800 |
| C57B—H57E | 0.9800 | C50—H50C | 0.9800 |
| C57B—H57F | 0.9800 | C51—H51A | 0.9800 |
| O28—C58 | 1.218 (8) | C51—H51B | 0.9800 |
| C58—N8 | 1.306 (9) | C51—H51C | 0.9800 |
| C58—H58 | 0.9500 | O31—C67 | 1.284 (10) |
| N8—C60 | 1.439 (10) | C67—N11 | 1.304 (8) |
| N8—C59 | 1.476 (11) | C67—H67 | 0.9500 |
| C59—H59A | 0.9800 | N11—C68 | 1.445 (9) |
| C59—H59B | 0.9800 | N11—C69 | 1.461 (10) |
| C59—H59C | 0.9800 | C68—H68A | 0.9800 |
| C60—H60A | 0.9800 | C68—H68B | 0.9800 |
| C60—H60B | 0.9800 | C68—H68C | 0.9800 |
| C60—H60C | 0.9800 | C69—H69A | 0.9800 |
| O28B—C58B | 1.233 (15) | C69—H69B | 0.9800 |
| C58B—N8B | 1.304 (14) | C69—H69C | 0.9800 |
| C58B—H58B | 0.9500 | O31B—C67B | 1.232 (6) |
| N8B—C60B | 1.444 (15) | C67B—N11B | 1.311 (6) |
| N8B—C59B | 1.455 (15) | C67B—H67B | 0.9500 |
| C59B—H59D | 0.9800 | N11B—C68B | 1.452 (7) |
| C59B—H59E | 0.9800 | N11B—C69B | 1.459 (6) |
| C59B—H59F | 0.9800 | C68B—H68D | 0.9800 |
| C60B—H60D | 0.9800 | C68B—H68E | 0.9800 |
| C60B—H60E | 0.9800 | C68B—H68F | 0.9800 |
| C60B—H60F | 0.9800 | C69B—H69D | 0.9800 |
| O24B—C73B | 1.215 (15) | C69B—H69E | 0.9800 |
| C73B—N13B | 1.290 (14) | C69B—H69F | 0.9800 |
| C73B—H73B | 0.9500 | Na1—Y1 | 3.5343 (15) |
| N13B—C75B | 1.448 (15) | Na1—Mn4 | 3.6079 (16) |
| N13B—C74B | 1.454 (15) | Na1—Mn3 | 3.6382 (15) |
| O2—C1—N1 | 121.6 (3) | O32—C70—N12 | 122.8 (13) |
| O2—C1—C2 | 119.4 (3) | O32—C70—H70 | 118.6 |
| N1—C1—C2 | 119.0 (3) | N12—C70—H70 | 118.6 |
| C7—C2—C3 | 119.6 (3) | C70—N12—C71 | 129.9 (11) |
| C7—C2—C1 | 117.7 (3) | C70—N12—C72 | 119.3 (10) |
| C3—C2—C1 | 122.8 (3) | C71—N12—C72 | 110.5 (11) |
| O3—C3—C4 | 117.6 (3) | N12—C71—H71A | 109.5 |
| O3—C3—C2 | 124.1 (3) | N12—C71—H71B | 109.5 |
| C4—C3—C2 | 118.2 (3) | H71A—C71—H71B | 109.5 |
| C5—C4—C3 | 121.1 (4) | N12—C71—H71C | 109.5 |
| C5—C4—H4 | 119.5 | H71A—C71—H71C | 109.5 |
| C3—C4—H4 | 119.5 | H71B—C71—H71C | 109.5 |
| C4—C5—C6 | 120.9 (3) | N12—C72—H72A | 109.5 |
| C4—C5—H5 | 119.5 | N12—C72—H72B | 109.5 |
| C6—C5—H5 | 119.5 | H72A—C72—H72B | 109.5 |
| C7—C6—C5 | 119.2 (4) | N12—C72—H72C | 109.5 |
| C7—C6—H6 | 120.4 | H72A—C72—H72C | 109.5 |
| C5—C6—H6 | 120.4 | H72B—C72—H72C | 109.5 |
| C6—C7—C2 | 121.0 (4) | O32B—C70B—N12B | 120 (3) |
| C6—C7—H7 | 119.5 | O32B—C70B—H70B | 120.2 |
| C2—C7—H7 | 119.5 | N12B—C70B—H70B | 120.2 |
| O5—C8—N2 | 121.2 (3) | C70B—N12B—C72B | 122 (3) |
| O5—C8—C9 | 119.8 (3) | C70B—N12B—C71B | 117 (2) |
| N2—C8—C9 | 119.0 (3) | C72B—N12B—C71B | 118 (2) |
| C14—C9—C10 | 118.9 (3) | N12B—C71B—H71D | 109.5 |
| C14—C9—C8 | 117.9 (3) | N12B—C71B—H71E | 109.5 |
| C10—C9—C8 | 123.2 (3) | H71D—C71B—H71E | 109.5 |
| O6—C10—C11 | 117.7 (3) | N12B—C71B—H71F | 109.5 |
| O6—C10—C9 | 123.7 (3) | H71D—C71B—H71F | 109.5 |
| C11—C10—C9 | 118.6 (3) | H71E—C71B—H71F | 109.5 |
| C12—C11—C10 | 121.5 (4) | N12B—C72B—H72D | 109.5 |
| C12—C11—H11 | 119.3 | N12B—C72B—H72E | 109.5 |
| C10—C11—H11 | 119.3 | H72D—C72B—H72E | 109.5 |
| C11—C12—C13 | 120.7 (4) | N12B—C72B—H72F | 109.5 |
| C11—C12—H12 | 119.7 | H72D—C72B—H72F | 109.5 |
| C13—C12—H12 | 119.7 | H72E—C72B—H72F | 109.5 |
| C14—C13—C12 | 118.9 (4) | C1—N1—O1 | 112.7 (3) |
| C14—C13—H13 | 120.6 | C1—N1—Mn2 | 130.4 (2) |
| C12—C13—H13 | 120.6 | O1—N1—Mn2 | 114.56 (19) |
| C13—C14—C9 | 121.4 (4) | C8—N2—O4 | 112.6 (3) |
| C13—C14—H14 | 119.3 | C8—N2—Mn3 | 130.1 (2) |
| C9—C14—H14 | 119.3 | O4—N2—Mn3 | 115.3 (2) |
| O8—C15—N3 | 121.0 (3) | C15—N3—O7 | 112.3 (3) |
| O8—C15—C16 | 119.3 (3) | C15—N3—Mn4 | 130.6 (2) |
| N3—C15—C16 | 119.7 (3) | O7—N3—Mn4 | 115.36 (19) |
| C21—C16—C17 | 119.6 (3) | C22—N4—O10 | 112.9 (3) |
| C21—C16—C15 | 117.9 (3) | C22—N4—Mn1 | 129.9 (2) |
| C17—C16—C15 | 122.5 (3) | O10—N4—Mn1 | 115.28 (19) |
| O9—C17—C18 | 117.5 (3) | N1—O1—Mn1 | 112.17 (18) |
| O9—C17—C16 | 124.5 (3) | N1—O1—Y1 | 121.55 (18) |
| C18—C17—C16 | 118.0 (3) | Mn1—O1—Y1 | 120.20 (11) |
| C19—C18—C17 | 121.3 (4) | N1—O1—Na1 | 106.95 (17) |
| C19—C18—H18 | 119.4 | Mn1—O1—Na1 | 102.09 (10) |
| C17—C18—H18 | 119.4 | Y1—O1—Na1 | 86.36 (8) |
| C18—C19—C20 | 121.0 (4) | C1—O2—Mn1 | 111.7 (2) |
| C18—C19—H19 | 119.5 | C3—O3—Mn2 | 130.0 (2) |
| C20—C19—H19 | 119.5 | N2—O4—Mn2 | 112.58 (18) |
| C21—C20—C19 | 118.9 (4) | N2—O4—Y1 | 121.43 (18) |
| C21—C20—H20 | 120.6 | Mn2—O4—Y1 | 120.63 (11) |
| C19—C20—H20 | 120.6 | N2—O4—Na1 | 104.91 (17) |
| C20—C21—C16 | 121.3 (4) | Mn2—O4—Na1 | 102.99 (10) |
| C20—C21—H21 | 119.4 | Y1—O4—Na1 | 85.99 (8) |
| C16—C21—H21 | 119.4 | C8—O5—Mn2 | 111.9 (2) |
| O11—C22—N4 | 121.0 (3) | C10—O6—Mn3 | 129.7 (2) |
| O11—C22—C23 | 119.4 (3) | N3—O7—Mn3 | 112.68 (18) |
| N4—C22—C23 | 119.6 (3) | N3—O7—Y1 | 121.91 (18) |
| C28—C23—C24 | 119.2 (3) | Mn3—O7—Y1 | 119.81 (11) |
| C28—C23—C22 | 117.9 (3) | N3—O7—Na1 | 103.38 (17) |
| C24—C23—C22 | 122.9 (3) | Mn3—O7—Na1 | 103.48 (10) |
| O12—C24—C25 | 117.7 (3) | Y1—O7—Na1 | 87.50 (8) |
| O12—C24—C23 | 124.1 (3) | C15—O8—Mn3 | 112.2 (2) |
| C25—C24—C23 | 118.2 (3) | C17—O9—Mn4 | 130.9 (2) |
| C26—C25—C24 | 121.3 (4) | N4—O10—Mn4 | 112.48 (18) |
| C26—C25—H25 | 119.3 | N4—O10—Y1 | 121.36 (18) |
| C24—C25—H25 | 119.3 | Mn4—O10—Y1 | 119.71 (11) |
| C25—C26—C27 | 121.0 (4) | N4—O10—Na1 | 106.01 (17) |
| C25—C26—H26 | 119.5 | Mn4—O10—Na1 | 102.31 (10) |
| C27—C26—H26 | 119.5 | Y1—O10—Na1 | 87.74 (8) |
| C28—C27—C26 | 118.8 (4) | C22—O11—Mn4 | 111.9 (2) |
| C28—C27—H27 | 120.6 | C24—O12—Mn1 | 129.6 (2) |
| C26—C27—H27 | 120.6 | C29—O13—Y1 | 139.9 (2) |
| C27—C28—C23 | 121.4 (4) | C29—O14—Mn1 | 123.2 (2) |
| C27—C28—H28 | 119.3 | C34—O15—Y1 | 139.9 (2) |
| C23—C28—H28 | 119.3 | C34—O16—Mn2 | 123.8 (2) |
| O14—C29—O13 | 124.8 (4) | C39—O17—Y1 | 140.9 (2) |
| O14—C29—C30 | 116.8 (3) | C39—O18—Mn3 | 124.7 (2) |
| O13—C29—C30 | 118.4 (3) | C44—O19—Y1 | 139.1 (2) |
| C32—C30—C31 | 110.1 (4) | C44—O20—Mn4 | 123.9 (2) |
| C32—C30—C29 | 109.5 (4) | Na1—O21—Mn1 | 95.42 (10) |
| C31—C30—C29 | 111.4 (4) | Na1—O21—H21A | 116 (4) |
| C32—C30—C33 | 110.1 (4) | Mn1—O21—H21A | 111 (4) |
| C31—C30—C33 | 109.4 (4) | Na1—O21—H21B | 121 (4) |
| C29—C30—C33 | 106.2 (4) | Mn1—O21—H21B | 114 (4) |
| C30—C31—H31A | 109.5 | H21A—O21—H21B | 100 (5) |
| C30—C31—H31B | 109.5 | Mn2—O22—Na1 | 98.28 (11) |
| H31A—C31—H31B | 109.5 | Mn2—O22—H22A | 118 (4) |
| C30—C31—H31C | 109.5 | Na1—O22—H22A | 117 (4) |
| H31A—C31—H31C | 109.5 | Mn2—O22—H22B | 122 (4) |
| H31B—C31—H31C | 109.5 | Na1—O22—H22B | 116 (4) |
| C30—C32—H32A | 109.5 | H22A—O22—H22B | 88 (5) |
| C30—C32—H32B | 109.5 | Na1—O23—H23A | 133 (4) |
| H32A—C32—H32B | 109.5 | Na1—O23—H23B | 115 (4) |
| C30—C32—H32C | 109.5 | H23A—O23—H23B | 90 (5) |
| H32A—C32—H32C | 109.5 | Na1—O24C—Mn4 | 95.00 (10) |
| H32B—C32—H32C | 109.5 | Na1—O24C—H24A | 115 (2) |
| C30—C33—H33A | 109.5 | Mn4—O24C—H24A | 119 (4) |
| C30—C33—H33B | 109.5 | Na1—O24C—H24B | 112.6 (19) |
| H33A—C33—H33B | 109.5 | Mn4—O24C—H24B | 113 (4) |
| C30—C33—H33C | 109.5 | H24A—O24C—H24B | 103 (3) |
| H33A—C33—H33C | 109.5 | C73—O24—Na1 | 157.0 (10) |
| H33B—C33—H33C | 109.5 | C73—O24—Mn4 | 108.0 (10) |
| O16—C34—O15 | 124.4 (4) | Na1—O24—Mn4 | 95.00 (10) |
| O16—C34—C35 | 116.8 (3) | O24—C73—N13 | 131.1 (17) |
| O15—C34—C35 | 118.8 (4) | O24—C73—H73 | 114.5 |
| C36—C35—C38 | 110.3 (4) | N13—C73—H73 | 114.5 |
| C36—C35—C34 | 109.2 (4) | C73—N13—C75 | 120.8 (19) |
| C38—C35—C34 | 111.7 (3) | C73—N13—C74 | 115.0 (18) |
| C36—C35—C37 | 110.5 (4) | C75—N13—C74 | 112.9 (19) |
| C38—C35—C37 | 109.0 (4) | N13—C74—H74A | 109.5 |
| C34—C35—C37 | 106.0 (3) | N13—C74—H74B | 109.5 |
| C35—C36—H36A | 109.5 | H74A—C74—H74B | 109.5 |
| C35—C36—H36B | 109.5 | N13—C74—H74C | 109.5 |
| H36A—C36—H36B | 109.5 | H74A—C74—H74C | 109.5 |
| C35—C36—H36C | 109.5 | H74B—C74—H74C | 109.5 |
| H36A—C36—H36C | 109.5 | N13—C75—H75A | 109.5 |
| H36B—C36—H36C | 109.5 | N13—C75—H75B | 109.5 |
| C35—C37—H37A | 109.5 | H75A—C75—H75B | 109.5 |
| C35—C37—H37B | 109.5 | N13—C75—H75C | 109.5 |
| H37A—C37—H37B | 109.5 | H75A—C75—H75C | 109.5 |
| C35—C37—H37C | 109.5 | H75B—C75—H75C | 109.5 |
| H37A—C37—H37C | 109.5 | H33E—O33—H33F | 107.6 |
| H37B—C37—H37C | 109.5 | H34A—O34—H34B | 106.1 |
| C35—C38—H38A | 109.5 | O25—C49—N5 | 125.1 (5) |
| C35—C38—H38B | 109.5 | O25—C49—H49 | 117.5 |
| H38A—C38—H38B | 109.5 | N5—C49—H49 | 117.5 |
| C35—C38—H38C | 109.5 | C49—N5—C50 | 119.1 (5) |
| H38A—C38—H38C | 109.5 | C49—N5—C51 | 121.8 (5) |
| H38B—C38—H38C | 109.5 | C50—N5—C51 | 119.0 (5) |
| O18—C39—O17 | 123.6 (4) | N5—C50—H50A | 109.5 |
| O18—C39—C40 | 117.6 (3) | N5—C50—H50B | 109.5 |
| O17—C39—C40 | 118.7 (3) | H50A—C50—H50B | 109.5 |
| C43—C40—C41 | 110.0 (4) | N5—C50—H50C | 109.5 |
| C43—C40—C39 | 109.3 (4) | H50A—C50—H50C | 109.5 |
| C41—C40—C39 | 112.9 (4) | H50B—C50—H50C | 109.5 |
| C43—C40—C42 | 109.9 (5) | N5—C51—H51A | 109.5 |
| C41—C40—C42 | 108.7 (4) | N5—C51—H51B | 109.5 |
| C39—C40—C42 | 105.8 (4) | H51A—C51—H51B | 109.5 |
| C40—C41—H41A | 109.5 | N5—C51—H51C | 109.5 |
| C40—C41—H41B | 109.5 | H51A—C51—H51C | 109.5 |
| H41A—C41—H41B | 109.5 | H51B—C51—H51C | 109.5 |
| C40—C41—H41C | 109.5 | O31—C67—N11 | 125.0 (9) |
| H41A—C41—H41C | 109.5 | O31—C67—H67 | 117.5 |
| H41B—C41—H41C | 109.5 | N11—C67—H67 | 117.5 |
| C40—C42—H42A | 109.5 | C67—N11—C68 | 125.4 (8) |
| C40—C42—H42B | 109.5 | C67—N11—C69 | 118.5 (8) |
| H42A—C42—H42B | 109.5 | C68—N11—C69 | 116.1 (7) |
| C40—C42—H42C | 109.5 | N11—C68—H68A | 109.5 |
| H42A—C42—H42C | 109.5 | N11—C68—H68B | 109.5 |
| H42B—C42—H42C | 109.5 | H68A—C68—H68B | 109.5 |
| C40—C43—H43A | 109.5 | N11—C68—H68C | 109.5 |
| C40—C43—H43B | 109.5 | H68A—C68—H68C | 109.5 |
| H43A—C43—H43B | 109.5 | H68B—C68—H68C | 109.5 |
| C40—C43—H43C | 109.5 | N11—C69—H69A | 109.5 |
| H43A—C43—H43C | 109.5 | N11—C69—H69B | 109.5 |
| H43B—C43—H43C | 109.5 | H69A—C69—H69B | 109.5 |
| O20—C44—O19 | 124.0 (4) | N11—C69—H69C | 109.5 |
| O20—C44—C45 | 117.9 (3) | H69A—C69—H69C | 109.5 |
| O19—C44—C45 | 118.2 (4) | H69B—C69—H69C | 109.5 |
| C46—C45—C48 | 110.2 (4) | O31B—C67B—N11B | 125.1 (6) |
| C46—C45—C44 | 111.7 (3) | O31B—C67B—H67B | 117.5 |
| C48—C45—C44 | 109.6 (4) | N11B—C67B—H67B | 117.5 |
| C46—C45—C47 | 109.0 (4) | C67B—N11B—C68B | 119.1 (5) |
| C48—C45—C47 | 110.1 (4) | C67B—N11B—C69B | 121.9 (5) |
| C44—C45—C47 | 106.1 (3) | C68B—N11B—C69B | 119.0 (5) |
| C45—C46—H46A | 109.5 | N11B—C68B—H68D | 109.5 |
| C45—C46—H46B | 109.5 | N11B—C68B—H68E | 109.5 |
| H46A—C46—H46B | 109.5 | H68D—C68B—H68E | 109.5 |
| C45—C46—H46C | 109.5 | N11B—C68B—H68F | 109.5 |
| H46A—C46—H46C | 109.5 | H68D—C68B—H68F | 109.5 |
| H46B—C46—H46C | 109.5 | H68E—C68B—H68F | 109.5 |
| C45—C47—H47A | 109.5 | N11B—C69B—H69D | 109.5 |
| C45—C47—H47B | 109.5 | N11B—C69B—H69E | 109.5 |
| H47A—C47—H47B | 109.5 | H69D—C69B—H69E | 109.5 |
| C45—C47—H47C | 109.5 | N11B—C69B—H69F | 109.5 |
| H47A—C47—H47C | 109.5 | H69D—C69B—H69F | 109.5 |
| H47B—C47—H47C | 109.5 | H69E—C69B—H69F | 109.5 |
| C45—C48—H48A | 109.5 | O24—Na1—O23 | 87.79 (11) |
| C45—C48—H48B | 109.5 | O24C—Na1—O23 | 87.79 (11) |
| H48A—C48—H48B | 109.5 | O24—Na1—O21 | 85.54 (11) |
| C45—C48—H48C | 109.5 | O24C—Na1—O21 | 85.54 (11) |
| H48A—C48—H48C | 109.5 | O23—Na1—O21 | 146.26 (12) |
| H48B—C48—H48C | 109.5 | O24—Na1—O22 | 149.78 (12) |
| O26—C52—N6 | 123.2 (5) | O24C—Na1—O22 | 149.78 (12) |
| O26—C52—H52 | 118.4 | O23—Na1—O22 | 84.02 (11) |
| N6—C52—H52 | 118.4 | O21—Na1—O22 | 85.33 (11) |
| C52—N6—C53 | 120.0 (5) | O24—Na1—O10 | 68.52 (9) |
| C52—N6—C54 | 121.3 (5) | O24C—Na1—O10 | 68.52 (9) |
| C53—N6—C54 | 118.6 (5) | O23—Na1—O10 | 124.35 (11) |
| N6—C53—H53A | 109.5 | O21—Na1—O10 | 83.38 (9) |
| N6—C53—H53B | 109.5 | O22—Na1—O10 | 138.49 (10) |
| H53A—C53—H53B | 109.5 | O24—Na1—O7 | 84.56 (10) |
| N6—C53—H53C | 109.5 | O24C—Na1—O7 | 84.56 (10) |
| H53A—C53—H53C | 109.5 | O23—Na1—O7 | 69.82 (10) |
| H53B—C53—H53C | 109.5 | O21—Na1—O7 | 141.90 (11) |
| N6—C54—H54A | 109.5 | O22—Na1—O7 | 119.17 (10) |
| N6—C54—H54B | 109.5 | O10—Na1—O7 | 58.78 (8) |
| H54A—C54—H54B | 109.5 | O24—Na1—O1 | 121.82 (10) |
| N6—C54—H54C | 109.5 | O24C—Na1—O1 | 121.82 (10) |
| H54A—C54—H54C | 109.5 | O23—Na1—O1 | 140.89 (11) |
| H54B—C54—H54C | 109.5 | O21—Na1—O1 | 67.70 (9) |
| O27—C55—N7 | 128.1 (9) | O22—Na1—O1 | 80.57 (9) |
| O27—C55—H55 | 116.0 | O10—Na1—O1 | 58.19 (8) |
| N7—C55—H55 | 116.0 | O7—Na1—O1 | 86.88 (8) |
| C55—N7—C57 | 123.4 (8) | O24—Na1—O4 | 142.11 (11) |
| C55—N7—C56 | 122.2 (9) | O24C—Na1—O4 | 142.11 (11) |
| C57—N7—C56 | 114.4 (8) | O23—Na1—O4 | 83.54 (10) |
| N7—C56—H56A | 109.5 | O21—Na1—O4 | 120.40 (10) |
| N7—C56—H56B | 109.5 | O22—Na1—O4 | 65.63 (9) |
| H56A—C56—H56B | 109.5 | O10—Na1—O4 | 86.37 (8) |
| N7—C56—H56C | 109.5 | O7—Na1—O4 | 57.82 (7) |
| H56A—C56—H56C | 109.5 | O1—Na1—O4 | 57.35 (7) |
| H56B—C56—H56C | 109.5 | O24—Na1—Y1 | 106.73 (9) |
| N7—C57—H57A | 109.5 | O24C—Na1—Y1 | 106.73 (9) |
| N7—C57—H57B | 109.5 | O23—Na1—Y1 | 107.52 (9) |
| H57A—C57—H57B | 109.5 | O21—Na1—Y1 | 106.08 (8) |
| N7—C57—H57C | 109.5 | O22—Na1—Y1 | 103.48 (8) |
| H57A—C57—H57C | 109.5 | O10—Na1—Y1 | 43.32 (5) |
| H57B—C57—H57C | 109.5 | O7—Na1—Y1 | 43.36 (5) |
| O27B—C55B—N7B | 125.0 (6) | O1—Na1—Y1 | 43.53 (5) |
| O27B—C55B—H55B | 117.5 | O4—Na1—Y1 | 43.05 (5) |
| N7B—C55B—H55B | 117.5 | O24—Na1—Mn4 | 42.98 (8) |
| C55B—N7B—C56B | 118.9 (5) | O24C—Na1—Mn4 | 42.98 (8) |
| C55B—N7B—C57B | 121.7 (5) | O23—Na1—Mn4 | 99.01 (9) |
| C56B—N7B—C57B | 118.8 (5) | O21—Na1—Mn4 | 98.38 (8) |
| N7B—C56B—H56D | 109.5 | O22—Na1—Mn4 | 167.24 (9) |
| N7B—C56B—H56E | 109.5 | O10—Na1—Mn4 | 31.45 (5) |
| H56D—C56B—H56E | 109.5 | O7—Na1—Mn4 | 51.71 (6) |
| N7B—C56B—H56F | 109.5 | O1—Na1—Mn4 | 89.51 (6) |
| H56D—C56B—H56F | 109.5 | O4—Na1—Mn4 | 102.24 (7) |
| H56E—C56B—H56F | 109.5 | Y1—Na1—Mn4 | 63.77 (3) |
| N7B—C57B—H57D | 109.5 | O24—Na1—Mn3 | 99.33 (8) |
| N7B—C57B—H57E | 109.5 | O24C—Na1—Mn3 | 99.33 (8) |
| H57D—C57B—H57E | 109.5 | O23—Na1—Mn3 | 44.12 (8) |
| N7B—C57B—H57F | 109.5 | O21—Na1—Mn3 | 169.26 (9) |
| H57D—C57B—H57F | 109.5 | O22—Na1—Mn3 | 94.84 (8) |
| H57E—C57B—H57F | 109.5 | O10—Na1—Mn3 | 89.48 (6) |
| O28—C58—N8 | 127.6 (7) | O7—Na1—Mn3 | 30.86 (5) |
| O28—C58—H58 | 116.2 | O1—Na1—Mn3 | 101.70 (7) |
| N8—C58—H58 | 116.2 | O4—Na1—Mn3 | 50.83 (5) |
| C58—N8—C60 | 126.4 (8) | Y1—Na1—Mn3 | 63.41 (3) |
| C58—N8—C59 | 117.5 (7) | Mn4—Na1—Mn3 | 79.24 (3) |
| C60—N8—C59 | 116.1 (7) | O12—Mn1—O1 | 172.51 (12) |
| N8—C59—H59A | 109.5 | O12—Mn1—O2 | 96.78 (11) |
| N8—C59—H59B | 109.5 | O1—Mn1—O2 | 81.85 (10) |
| H59A—C59—H59B | 109.5 | O12—Mn1—N4 | 90.93 (11) |
| N8—C59—H59C | 109.5 | O1—Mn1—N4 | 89.15 (11) |
| H59A—C59—H59C | 109.5 | O2—Mn1—N4 | 166.95 (11) |
| H59B—C59—H59C | 109.5 | O12—Mn1—O14 | 94.63 (11) |
| N8—C60—H60A | 109.5 | O1—Mn1—O14 | 92.76 (10) |
| N8—C60—H60B | 109.5 | O2—Mn1—O14 | 90.79 (11) |
| H60A—C60—H60B | 109.5 | N4—Mn1—O14 | 99.10 (11) |
| N8—C60—H60C | 109.5 | O12—Mn1—O21 | 91.16 (11) |
| H60A—C60—H60C | 109.5 | O1—Mn1—O21 | 81.40 (10) |
| H60B—C60—H60C | 109.5 | O2—Mn1—O21 | 85.59 (10) |
| O28B—C58B—N8B | 126 (2) | N4—Mn1—O21 | 83.73 (11) |
| O28B—C58B—H58B | 117.0 | O14—Mn1—O21 | 173.51 (10) |
| N8B—C58B—H58B | 117.0 | O12—Mn1—Na1 | 126.89 (9) |
| C58B—N8B—C60B | 120.2 (19) | O1—Mn1—Na1 | 46.81 (8) |
| C58B—N8B—C59B | 124.0 (19) | O2—Mn1—Na1 | 101.65 (8) |
| C60B—N8B—C59B | 115.9 (18) | N4—Mn1—Na1 | 65.33 (9) |
| N8B—C59B—H59D | 109.5 | O14—Mn1—Na1 | 133.92 (7) |
| N8B—C59B—H59E | 109.5 | O21—Mn1—Na1 | 42.23 (7) |
| H59D—C59B—H59E | 109.5 | O3—Mn2—O4 | 172.75 (12) |
| N8B—C59B—H59F | 109.5 | O3—Mn2—O5 | 96.84 (11) |
| H59D—C59B—H59F | 109.5 | O4—Mn2—O5 | 81.62 (10) |
| H59E—C59B—H59F | 109.5 | O3—Mn2—N1 | 90.58 (11) |
| N8B—C60B—H60D | 109.5 | O4—Mn2—N1 | 89.78 (11) |
| N8B—C60B—H60E | 109.5 | O5—Mn2—N1 | 167.69 (11) |
| H60D—C60B—H60E | 109.5 | O3—Mn2—O16 | 94.43 (11) |
| N8B—C60B—H60F | 109.5 | O4—Mn2—O16 | 92.66 (11) |
| H60D—C60B—H60F | 109.5 | O5—Mn2—O16 | 90.25 (11) |
| H60E—C60B—H60F | 109.5 | N1—Mn2—O16 | 98.98 (11) |
| O24B—C73B—N13B | 136 (3) | O3—Mn2—O22 | 92.31 (11) |
| O24B—C73B—H73B | 111.9 | O4—Mn2—O22 | 80.55 (10) |
| N13B—C73B—H73B | 111.9 | O5—Mn2—O22 | 86.75 (10) |
| C73B—N13B—C75B | 115.0 (18) | N1—Mn2—O22 | 83.14 (11) |
| C73B—N13B—C74B | 126.8 (19) | O16—Mn2—O22 | 172.91 (10) |
| C75B—N13B—C74B | 118.1 (18) | O3—Mn2—Na1 | 127.54 (9) |
| N13B—C74B—H74D | 109.5 | O4—Mn2—Na1 | 46.51 (8) |
| N13B—C74B—H74E | 109.5 | O5—Mn2—Na1 | 101.65 (8) |
| H74D—C74B—H74E | 109.5 | N1—Mn2—Na1 | 66.07 (8) |
| N13B—C74B—H74F | 109.5 | O16—Mn2—Na1 | 133.63 (8) |
| H74D—C74B—H74F | 109.5 | O22—Mn2—Na1 | 41.26 (7) |
| H74E—C74B—H74F | 109.5 | O6—Mn3—O7 | 170.54 (12) |
| N13B—C75B—H75D | 109.5 | O6—Mn3—O8 | 95.86 (11) |
| N13B—C75B—H75E | 109.5 | O7—Mn3—O8 | 81.69 (10) |
| H75D—C75B—H75E | 109.5 | O6—Mn3—N2 | 91.04 (11) |
| N13B—C75B—H75F | 109.5 | O7—Mn3—N2 | 89.58 (11) |
| H75D—C75B—H75F | 109.5 | O8—Mn3—N2 | 166.28 (12) |
| H75E—C75B—H75F | 109.5 | O6—Mn3—O18 | 95.75 (11) |
| O29—C61—N9 | 123.3 (7) | O7—Mn3—O18 | 93.46 (10) |
| O29—C61—H61 | 118.4 | O8—Mn3—O18 | 91.75 (11) |
| N9—C61—H61 | 118.4 | N2—Mn3—O18 | 99.36 (11) |
| C61—N9—C63 | 118.8 (7) | O6—Mn3—Na1 | 126.47 (9) |
| C61—N9—C62 | 122.6 (7) | O7—Mn3—Na1 | 45.66 (8) |
| C63—N9—C62 | 118.6 (8) | O8—Mn3—Na1 | 99.23 (8) |
| N9—C62—H62A | 109.5 | N2—Mn3—Na1 | 67.21 (9) |
| N9—C62—H62B | 109.5 | O18—Mn3—Na1 | 134.40 (7) |
| H62A—C62—H62B | 109.5 | O9—Mn4—O10 | 172.02 (12) |
| N9—C62—H62C | 109.5 | O9—Mn4—O11 | 97.27 (11) |
| H62A—C62—H62C | 109.5 | O10—Mn4—O11 | 81.63 (10) |
| H62B—C62—H62C | 109.5 | O9—Mn4—N3 | 90.42 (11) |
| N9—C63—H63A | 109.5 | O10—Mn4—N3 | 89.20 (11) |
| N9—C63—H63B | 109.5 | O11—Mn4—N3 | 166.41 (12) |
| H63A—C63—H63B | 109.5 | O9—Mn4—O20 | 94.64 (12) |
| N9—C63—H63C | 109.5 | O10—Mn4—O20 | 93.27 (10) |
| H63A—C63—H63C | 109.5 | O11—Mn4—O20 | 90.16 (11) |
| H63B—C63—H63C | 109.5 | N3—Mn4—O20 | 100.40 (12) |
| O29B—C61B—N9B | 125 (3) | O9—Mn4—O24 | 91.44 (12) |
| O29B—C61B—H61B | 117.4 | O10—Mn4—O24 | 80.60 (10) |
| N9B—C61B—H61B | 117.4 | O11—Mn4—O24 | 85.58 (11) |
| C61B—N9B—C63B | 120 (2) | N3—Mn4—O24 | 83.03 (11) |
| C61B—N9B—C62B | 124 (2) | O20—Mn4—O24 | 172.98 (10) |
| C63B—N9B—C62B | 114.4 (19) | O9—Mn4—O24C | 91.44 (12) |
| N9B—C62B—H62D | 109.5 | O10—Mn4—O24C | 80.60 (10) |
| N9B—C62B—H62E | 109.5 | O11—Mn4—O24C | 85.58 (11) |
| H62D—C62B—H62E | 109.5 | N3—Mn4—O24C | 83.03 (11) |
| N9B—C62B—H62F | 109.5 | O20—Mn4—O24C | 172.98 (10) |
| H62D—C62B—H62F | 109.5 | O9—Mn4—Na1 | 126.87 (9) |
| H62E—C62B—H62F | 109.5 | O10—Mn4—Na1 | 46.24 (8) |
| N9B—C63B—H63D | 109.5 | O11—Mn4—Na1 | 101.43 (8) |
| N9B—C63B—H63E | 109.5 | N3—Mn4—Na1 | 65.06 (9) |
| H63D—C63B—H63E | 109.5 | O20—Mn4—Na1 | 134.11 (7) |
| N9B—C63B—H63F | 109.5 | O24—Mn4—Na1 | 42.02 (7) |
| H63D—C63B—H63F | 109.5 | O24C—Mn4—Na1 | 42.02 (7) |
| H63E—C63B—H63F | 109.5 | O19—Y1—O13 | 77.98 (9) |
| O30—C64—N10 | 126.2 (6) | O19—Y1—O15 | 123.27 (10) |
| O30—C64—H64 | 116.9 | O13—Y1—O15 | 77.25 (9) |
| N10—C64—H64 | 116.9 | O19—Y1—O17 | 76.42 (9) |
| C64—N10—C65 | 121.1 (6) | O13—Y1—O17 | 124.40 (10) |
| C64—N10—C66 | 122.1 (5) | O15—Y1—O17 | 77.15 (9) |
| C65—N10—C66 | 116.6 (6) | O19—Y1—O4 | 145.05 (9) |
| N10—C65—H65A | 109.5 | O13—Y1—O4 | 136.80 (8) |
| N10—C65—H65B | 109.5 | O15—Y1—O4 | 77.39 (9) |
| H65A—C65—H65B | 109.5 | O17—Y1—O4 | 82.48 (9) |
| N10—C65—H65C | 109.5 | O19—Y1—O10 | 78.54 (9) |
| H65A—C65—H65C | 109.5 | O13—Y1—O10 | 82.23 (9) |
| H65B—C65—H65C | 109.5 | O15—Y1—O10 | 145.10 (9) |
| N10—C66—H66A | 109.5 | O17—Y1—O10 | 137.52 (9) |
| N10—C66—H66B | 109.5 | O4—Y1—O10 | 99.89 (8) |
| H66A—C66—H66B | 109.5 | O19—Y1—O7 | 82.80 (9) |
| N10—C66—H66C | 109.5 | O13—Y1—O7 | 145.03 (8) |
| H66A—C66—H66C | 109.5 | O15—Y1—O7 | 137.39 (8) |
| H66B—C66—H66C | 109.5 | O17—Y1—O7 | 77.84 (9) |
| O30B—C64B—N10B | 124 (2) | O4—Y1—O7 | 65.56 (8) |
| O30B—C64B—H64B | 118.0 | O10—Y1—O7 | 65.37 (8) |
| N10B—C64B—H64B | 118.0 | O19—Y1—O1 | 138.13 (8) |
| C64B—N10B—C66B | 121.2 (18) | O13—Y1—O1 | 77.21 (9) |
| C64B—N10B—C65B | 122.4 (19) | O15—Y1—O1 | 82.85 (9) |
| C66B—N10B—C65B | 116.4 (19) | O17—Y1—O1 | 145.20 (9) |
| N10B—C65B—H65D | 109.5 | O4—Y1—O1 | 65.40 (8) |
| N10B—C65B—H65E | 109.5 | O10—Y1—O1 | 65.11 (8) |
| H65D—C65B—H65E | 109.5 | O7—Y1—O1 | 99.25 (8) |
| N10B—C65B—H65F | 109.5 | O19—Y1—Na1 | 117.64 (7) |
| H65D—C65B—H65F | 109.5 | O13—Y1—Na1 | 117.35 (7) |
| H65E—C65B—H65F | 109.5 | O15—Y1—Na1 | 119.08 (7) |
| N10B—C66B—H66D | 109.5 | O17—Y1—Na1 | 118.24 (7) |
| N10B—C66B—H66E | 109.5 | O4—Y1—Na1 | 50.95 (6) |
| H66D—C66B—H66E | 109.5 | O10—Y1—Na1 | 48.94 (6) |
| N10B—C66B—H66F | 109.5 | O7—Y1—Na1 | 49.15 (6) |
| H66D—C66B—H66F | 109.5 | O1—Y1—Na1 | 50.11 (6) |
| H66E—C66B—H66F | 109.5 | ||
| O2—C1—C2—C7 | −10.7 (5) | O32—C70—N12—C72 | 176.0 (13) |
| N1—C1—C2—C7 | 167.2 (3) | O32B—C70B—N12B—C72B | −134 (7) |
| O2—C1—C2—C3 | 170.0 (3) | O32B—C70B—N12B—C71B | 25 (9) |
| N1—C1—C2—C3 | −12.1 (5) | O2—C1—N1—O1 | −1.9 (5) |
| C7—C2—C3—O3 | 175.8 (3) | C2—C1—N1—O1 | −179.7 (3) |
| C1—C2—C3—O3 | −4.9 (6) | O2—C1—N1—Mn2 | −163.5 (2) |
| C7—C2—C3—C4 | −2.3 (5) | C2—C1—N1—Mn2 | 18.6 (5) |
| C1—C2—C3—C4 | 177.0 (3) | O5—C8—N2—O4 | −1.1 (5) |
| O3—C3—C4—C5 | −177.0 (4) | C9—C8—N2—O4 | −179.3 (3) |
| C2—C3—C4—C5 | 1.3 (6) | O5—C8—N2—Mn3 | −164.1 (2) |
| C3—C4—C5—C6 | 0.6 (6) | C9—C8—N2—Mn3 | 17.7 (5) |
| C4—C5—C6—C7 | −1.5 (6) | O8—C15—N3—O7 | −2.7 (5) |
| C5—C6—C7—C2 | 0.4 (6) | C16—C15—N3—O7 | 178.6 (3) |
| C3—C2—C7—C6 | 1.5 (6) | O8—C15—N3—Mn4 | −166.5 (3) |
| C1—C2—C7—C6 | −177.8 (4) | C16—C15—N3—Mn4 | 14.8 (5) |
| O5—C8—C9—C14 | −12.0 (5) | O11—C22—N4—O10 | −1.3 (5) |
| N2—C8—C9—C14 | 166.3 (4) | C23—C22—N4—O10 | −179.1 (3) |
| O5—C8—C9—C10 | 167.7 (3) | O11—C22—N4—Mn1 | −164.5 (3) |
| N2—C8—C9—C10 | −14.1 (5) | C23—C22—N4—Mn1 | 17.8 (5) |
| C14—C9—C10—O6 | 177.2 (4) | C1—N1—O1—Mn1 | 0.6 (3) |
| C8—C9—C10—O6 | −2.4 (6) | Mn2—N1—O1—Mn1 | 165.36 (13) |
| C14—C9—C10—C11 | −1.5 (6) | C1—N1—O1—Y1 | 153.2 (2) |
| C8—C9—C10—C11 | 178.9 (3) | Mn2—N1—O1—Y1 | −42.1 (3) |
| O6—C10—C11—C12 | −178.4 (4) | C1—N1—O1—Na1 | −110.5 (3) |
| C9—C10—C11—C12 | 0.3 (6) | Mn2—N1—O1—Na1 | 54.2 (2) |
| C10—C11—C12—C13 | 0.9 (7) | N1—C1—O2—Mn1 | 2.1 (4) |
| C11—C12—C13—C14 | −1.0 (7) | C2—C1—O2—Mn1 | 179.9 (2) |
| C12—C13—C14—C9 | −0.2 (7) | C4—C3—O3—Mn2 | −165.7 (3) |
| C10—C9—C14—C13 | 1.4 (6) | C2—C3—O3—Mn2 | 16.2 (5) |
| C8—C9—C14—C13 | −178.9 (4) | C8—N2—O4—Mn2 | −1.5 (3) |
| O8—C15—C16—C21 | −7.5 (5) | Mn3—N2—O4—Mn2 | 164.14 (13) |
| N3—C15—C16—C21 | 171.1 (3) | C8—N2—O4—Y1 | 152.7 (2) |
| O8—C15—C16—C17 | 172.9 (3) | Mn3—N2—O4—Y1 | −41.6 (3) |
| N3—C15—C16—C17 | −8.4 (5) | C8—N2—O4—Na1 | −112.8 (3) |
| C21—C16—C17—O9 | 177.8 (4) | Mn3—N2—O4—Na1 | 52.9 (2) |
| C15—C16—C17—O9 | −2.7 (6) | N2—C8—O5—Mn2 | 3.2 (4) |
| C21—C16—C17—C18 | −1.7 (6) | C9—C8—O5—Mn2 | −178.6 (3) |
| C15—C16—C17—C18 | 177.8 (3) | C11—C10—O6—Mn3 | −165.1 (3) |
| O9—C17—C18—C19 | −179.2 (4) | C9—C10—O6—Mn3 | 16.3 (5) |
| C16—C17—C18—C19 | 0.3 (6) | C15—N3—O7—Mn3 | 0.7 (3) |
| C17—C18—C19—C20 | 0.9 (7) | Mn4—N3—O7—Mn3 | 167.18 (13) |
| C18—C19—C20—C21 | −0.6 (7) | C15—N3—O7—Y1 | 154.2 (2) |
| C19—C20—C21—C16 | −0.8 (7) | Mn4—N3—O7—Y1 | −39.3 (3) |
| C17—C16—C21—C20 | 2.0 (6) | C15—N3—O7—Na1 | −110.3 (3) |
| C15—C16—C21—C20 | −177.5 (4) | Mn4—N3—O7—Na1 | 56.1 (2) |
| O11—C22—C23—C28 | −11.8 (5) | N3—C15—O8—Mn3 | 3.3 (4) |
| N4—C22—C23—C28 | 166.0 (4) | C16—C15—O8—Mn3 | −178.0 (3) |
| O11—C22—C23—C24 | 169.0 (3) | C18—C17—O9—Mn4 | −172.5 (3) |
| N4—C22—C23—C24 | −13.2 (6) | C16—C17—O9—Mn4 | 7.9 (6) |
| C28—C23—C24—O12 | 177.2 (4) | C22—N4—O10—Mn4 | −0.6 (3) |
| C22—C23—C24—O12 | −3.6 (6) | Mn1—N4—O10—Mn4 | 165.20 (13) |
| C28—C23—C24—C25 | −1.1 (6) | C22—N4—O10—Y1 | 151.1 (2) |
| C22—C23—C24—C25 | 178.1 (4) | Mn1—N4—O10—Y1 | −43.1 (3) |
| O12—C24—C25—C26 | −177.5 (4) | C22—N4—O10—Na1 | −111.6 (3) |
| C23—C24—C25—C26 | 0.8 (6) | Mn1—N4—O10—Na1 | 54.2 (2) |
| C24—C25—C26—C27 | 0.1 (7) | N4—C22—O11—Mn4 | 2.5 (4) |
| C25—C26—C27—C28 | −0.8 (7) | C23—C22—O11—Mn4 | −179.8 (3) |
| C26—C27—C28—C23 | 0.5 (7) | C25—C24—O12—Mn1 | −165.3 (3) |
| C24—C23—C28—C27 | 0.4 (6) | C23—C24—O12—Mn1 | 16.5 (5) |
| C22—C23—C28—C27 | −178.8 (4) | O14—C29—O13—Y1 | −60.1 (6) |
| O14—C29—C30—C32 | −47.8 (6) | C30—C29—O13—Y1 | 117.9 (4) |
| O13—C29—C30—C32 | 134.0 (4) | O13—C29—O14—Mn1 | 16.7 (5) |
| O14—C29—C30—C31 | −169.9 (4) | C30—C29—O14—Mn1 | −161.3 (3) |
| O13—C29—C30—C31 | 11.9 (6) | O16—C34—O15—Y1 | −59.2 (6) |
| O14—C29—C30—C33 | 71.0 (5) | C35—C34—O15—Y1 | 118.9 (4) |
| O13—C29—C30—C33 | −107.1 (4) | O15—C34—O16—Mn2 | 18.4 (5) |
| O16—C34—C35—C36 | −45.5 (5) | C35—C34—O16—Mn2 | −159.8 (3) |
| O15—C34—C35—C36 | 136.2 (4) | O18—C39—O17—Y1 | −54.7 (6) |
| O16—C34—C35—C38 | −167.8 (4) | C40—C39—O17—Y1 | 126.3 (4) |
| O15—C34—C35—C38 | 13.9 (5) | O17—C39—O18—Mn3 | 12.7 (5) |
| O16—C34—C35—C37 | 73.6 (5) | C40—C39—O18—Mn3 | −168.3 (3) |
| O15—C34—C35—C37 | −104.7 (4) | O20—C44—O19—Y1 | −60.2 (6) |
| O18—C39—C40—C43 | −47.7 (6) | C45—C44—O19—Y1 | 119.3 (4) |
| O17—C39—C40—C43 | 131.4 (4) | O19—C44—O20—Mn4 | 17.2 (5) |
| O18—C39—C40—C41 | −170.5 (4) | C45—C44—O20—Mn4 | −162.3 (3) |
| O17—C39—C40—C41 | 8.5 (6) | Na1—O24—C73—N13 | −63 (4) |
| O18—C39—C40—C42 | 70.7 (5) | Mn4—O24—C73—N13 | 116 (3) |
| O17—C39—C40—C42 | −110.3 (4) | O24—C73—N13—C75 | 155 (3) |
| O20—C44—C45—C46 | −169.2 (4) | O24—C73—N13—C74 | 14 (4) |
| O19—C44—C45—C46 | 11.2 (5) | O25—C49—N5—C50 | 2.1 (8) |
| O20—C44—C45—C48 | −46.8 (5) | O25—C49—N5—C51 | 178.0 (5) |
| O19—C44—C45—C48 | 133.6 (4) | O31—C67—N11—C68 | 11.3 (16) |
| O20—C44—C45—C47 | 72.0 (4) | O31—C67—N11—C69 | −169.6 (10) |
| O19—C44—C45—C47 | −107.5 (4) | O31B—C67B—N11B—C68B | −0.1 (4) |
| O26—C52—N6—C53 | 2.1 (7) | O31B—C67B—N11B—C69B | −180.0 (4) |
| O26—C52—N6—C54 | 179.3 (5) | C24—O12—Mn1—O2 | 179.9 (3) |
| O27—C55—N7—C57 | 176.1 (11) | C24—O12—Mn1—N4 | −10.7 (3) |
| O27—C55—N7—C56 | −6.7 (18) | C24—O12—Mn1—O14 | 88.5 (3) |
| O27B—C55B—N7B—C56B | −10 (4) | C24—O12—Mn1—O21 | −94.4 (3) |
| O27B—C55B—N7B—C57B | 179 (3) | C24—O12—Mn1—Na1 | −70.0 (3) |
| O28—C58—N8—C60 | 175.8 (8) | C3—O3—Mn2—O5 | −179.4 (3) |
| O28—C58—N8—C59 | −1.4 (12) | C3—O3—Mn2—N1 | −9.2 (3) |
| O28B—C58B—N8B—C60B | 180.0 (4) | C3—O3—Mn2—O16 | 89.8 (3) |
| O28B—C58B—N8B—C59B | 0.0 (4) | C3—O3—Mn2—O22 | −92.4 (3) |
| O24B—C73B—N13B—C75B | 179.9 (4) | C3—O3—Mn2—Na1 | −69.0 (3) |
| O24B—C73B—N13B—C74B | 0.1 (5) | C10—O6—Mn3—O8 | −179.4 (3) |
| O29—C61—N9—C63 | −176.1 (8) | C10—O6—Mn3—N2 | −11.3 (3) |
| O29—C61—N9—C62 | 2.7 (13) | C10—O6—Mn3—O18 | 88.2 (3) |
| O29B—C61B—N9B—C63B | −131 (5) | C10—O6—Mn3—Na1 | −73.4 (3) |
| O29B—C61B—N9B—C62B | 64 (7) | C17—O9—Mn4—O11 | −171.4 (3) |
| O30—C64—N10—C65 | 5.1 (11) | C17—O9—Mn4—N3 | −2.7 (3) |
| O30—C64—N10—C66 | 179.9 (7) | C17—O9—Mn4—O20 | 97.8 (3) |
| O30B—C64B—N10B—C66B | 171 (4) | C17—O9—Mn4—O24 | −85.7 (3) |
| O30B—C64B—N10B—C65B | −13 (6) | C17—O9—Mn4—O24C | −85.7 (3) |
| O32—C70—N12—C71 | 3 (2) | C17—O9—Mn4—Na1 | −61.2 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C18—H18···O20i | 0.95 | 2.60 | 3.359 (5) | 137 |
| C25—H25···O14ii | 0.95 | 2.59 | 3.374 (5) | 141 |
| C49—H49···O29 | 0.95 | 2.58 | 3.180 (8) | 121 |
| C51—H51B···O29iii | 0.98 | 2.56 | 3.376 (9) | 141 |
| C53—H53B···O31iv | 0.98 | 2.48 | 3.377 (9) | 152 |
| C55—H55···O8 | 0.95 | 2.36 | 3.098 (8) | 135 |
| C56—H56A···O32iv | 0.98 | 2.56 | 3.499 (17) | 162 |
| C59—H59B···O29 | 0.98 | 2.56 | 3.262 (11) | 129 |
| C61—H61···O12 | 0.95 | 2.52 | 3.457 (8) | 169 |
| C63B—H63F···O32Biii | 0.98 | 2.53 | 3.34 (6) | 140 |
| C64B—H64B···O3 | 0.95 | 2.50 | 3.40 (3) | 157 |
| C71B—H71D···O21 | 0.98 | 2.60 | 3.41 (5) | 141 |
| C72B—H72E···O34iv | 0.98 | 2.36 | 3.31 (7) | 163 |
| C74—H74B···O27 | 0.98 | 2.27 | 2.87 (3) | 119 |
| C75—H75C···O31 | 0.98 | 2.15 | 2.99 (3) | 143 |
| O21—H21A···O25 | 0.82 (2) | 2.00 (3) | 2.767 (4) | 155 (5) |
| O21—H21B···O28 | 0.83 (2) | 2.05 (3) | 2.792 (5) | 148 (5) |
| O21—H21B···O28B | 0.83 (2) | 1.87 (3) | 2.70 (2) | 172 (5) |
| O22—H22A···O25 | 0.84 (2) | 1.96 (3) | 2.727 (4) | 151 (5) |
| O22—H22B···O26 | 0.83 (2) | 1.93 (3) | 2.688 (4) | 151 (5) |
| O23—H23A···O27 | 0.84 (2) | 2.06 (3) | 2.871 (7) | 164 (5) |
| O23—H23A···O24B | 0.84 (2) | 2.06 (5) | 2.696 (19) | 132 (5) |
| O23—H23B···O26 | 0.86 (2) | 1.98 (3) | 2.789 (5) | 155 (5) |
| O24C—H24A···O33 | 0.86 (2) | 1.91 (4) | 2.78 (3) | 179 (5) |
Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) −x+2, −y+1, −z; (iii) −x+1, −y+1, −z; (iv) −x+1, −y+1, −z+1.
References
- AlDamen, M. A., Cardona-Serra, S., Clemente-Juan, J. M., Coronado, E., Gaita-Ariño, A., Martí-Gastaldo, C., Luis, F. & Montero, O. (2009). Inorg. Chem. 48, 3467–3479. [DOI] [PubMed]
- AlDamen, M. A., Clemente-Juan, J. M., Coronado, E., Martí-Gastaldo, C. & Gaita-Ariño, A. (2008). J. Am. Chem. Soc. 130, 8874–8875. [DOI] [PubMed]
- Azar, M. R., Boron, T. T., Lutter, J. C., Daly, C. I., Zegalia, K. A., Nimthong, R., Ferrence, G. M., Zeller, M., Kampf, J. W., Pecoraro, V. L. & Zaleski, C. M. (2014). Inorg. Chem. 53, 1729–1742. [DOI] [PubMed]
- Bruker (2014). APEX2, SADABS, and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Cook, T. R. & Stang, P. J. (2015). Chem. Rev. 115, 7001–7045. [DOI] [PubMed]
- Hübschle, C. B., Sheldrick, G. M. & Dittrich, B. (2011). J. Appl. Cryst. 44, 1281–1284. [DOI] [PMC free article] [PubMed]
- Liu, W. & Thorp, H. H. (1993). Inorg. Chem. 32, 4102–4105.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Mezei, G., Zaleski, C. M. & Pecoraro, V. L. (2007). Chem. Rev. 107, 4933–5003. [DOI] [PubMed]
- Saalfrank, R. W., Maid, H. & Scheurer, A. (2008). Angew. Chem. Int. Ed. 47, 8794–8824. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015018216/bg2568sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015018216/bg2568Isup2.hkl
CCDC reference: 1428526
Additional supporting information: crystallographic information; 3D view; checkCIF report