The title compounds are two closely related oxalate-bridged dinuclear copper complexes. The histamine ligand is in a gauche conformation and coordinates to copper ions in a bidentate chelating fashion. The dinuclear complexes are linked to form three-dimensional networks via different types of hydrogen-bond and weak interactions.
Keywords: crystal structure, dinuclear copper(II) complex, oxalate ligand, histamine ligand, hydrogen bonding
Abstract
The title compounds, μ-oxalato-κ4 O 1,O 2:O 1′,O 2′-bis[[4-(2-aminoethyl)-1H-imidazole-κ2 N 3,N 4](azido-κN 1)copper(II)], [Cu2(C2O4)(N3)2(C5H9N3)2], (I), and μ-oxalato-κ4 O 1,O 2:O 1′,O 2′-bis[[4-(2-aminoethyl)-1H-imidazole-κ2 N 3,N 4](dicyanamido-κN 1)copper(II)], [Cu2(C2O4)(C2N3)2(C5H9N3)2], (II), are two oxalate-bridged dinuclear copper complexes. Each CuII ion adopts a five-coordinate square-pyramidal coordination sphere where the basal N2O2 plane is formed by two O atoms of the oxalate ligand and two N atoms of a bidentate chelating histamine molecule. The apical coordination site in compound (I) is occupied by a monodentate azide anion through one of its terminal N atoms. The apical coordination site in compound (II) is occupied by a monodentate dicyanamide anion through one of its terminal N atoms. The molecules in both structures are centrosymmetric. In the crystals of compounds (I) and (II), the dinuclear complexes are linked through N—H⋯X and C—H⋯X (X = N, O) hydrogen bonds where the donors are provided by the histamine ligand and the acceptor atoms are provided by the azide, dicyanamide, and oxalate ligands. In compound (I), the coordinatively unsaturated copper ions interact with the histamine ligand via a C—H⋯Cu interaction. The coordinatively unsaturated copper ions in compound (II) interact via a weak N⋯Cu interaction with the dicyanamide ligand of a neighboring molecule. The side chain of the histamine ligand is disordered over three sets of sites in (II).
Chemical context
The oxalate ligand often plays an important role as a versatile bridging ligand in the formation of coordination polymers of various dimensionalities, including dinuclear complexes, chains, two-dimensional layered structures etc. (Coronado et al., 2003 ▸; Pardo et al., 2010 ▸). The oxalate dianion can coordinate to two metal ions in a bis-bidentate fashion to form a dinuclear unit, although other coordination modes of oxalate have also been reported (Hernández-Molina et al., 2001 ▸). In our effort to design and synthesize coordination polymers in a more rational and controlled fashion, we decided to use oxalate-based dinuclear complexes as molecular building blocks in preparing ladder-like coordination polymers. One strategy would be to introduce a linear bridging ligand to link the dinuclear units into ladder-like structures. Some potential choices of linear bridging ligands include azide and dicyanamide anions which have been widely used as bridging ligands in the design and synthesis of coordination polymers. The azide anion mainly coordinates in an end-on or end-to-end fashion (Escuer & Aromí, 2006 ▸; Stamatatos & Christou, 2009 ▸), while dicyanamide exhibits several different coordination modes (Batten & Murray, 2003 ▸). During our attempts to react azide and dicyanamide with oxalate-bridged biscopper(II) complexes, we obtained the title compounds as dinuclear units interacting via hydrogen-bonding and weak interactions.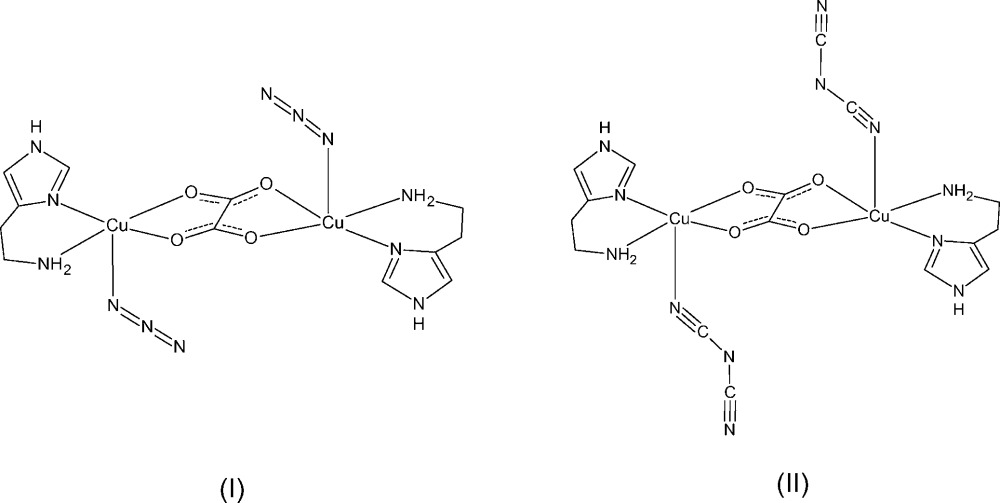
Structural commentary
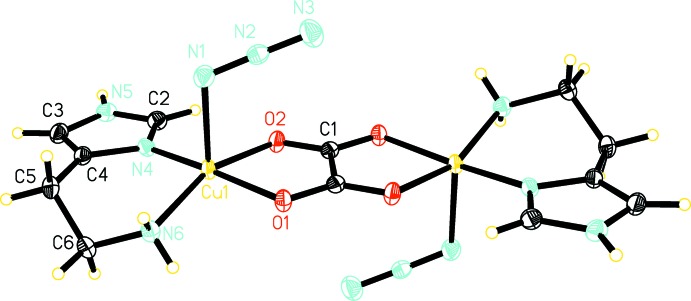
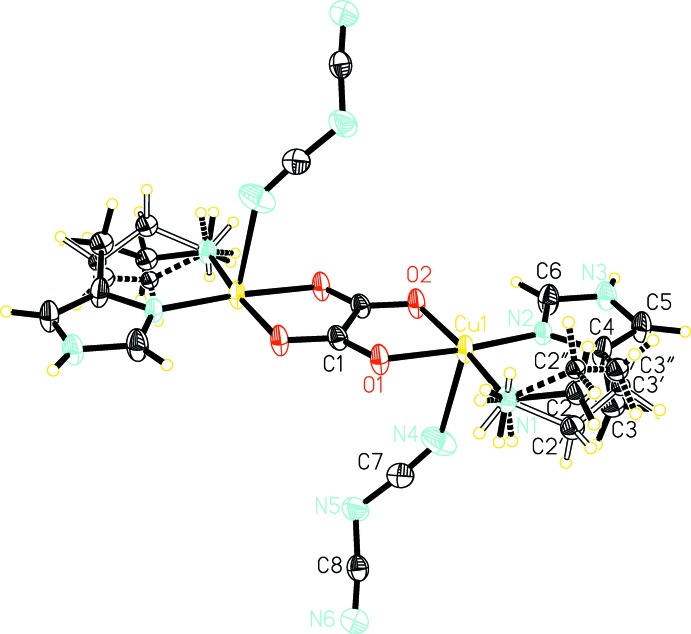
Compound (I) crystallizes in the orthorhombic space group Pbca (Fig. 1 ▸) and compound (II) crystallizes in the monoclinic space group P21/c (Fig. 2 ▸). Both complexes are binuclear with a bridging oxalate anion coordinating in a bis-bidentate fashion to two copper cations, and both binuclear complexes are centrosymmetric with a center of inversion located at the center of the bridging oxalate anion. The copper ions in both compounds have a five-coordinate square-pyramidal coordination geometry. In compound (I), the basal N2O2 plane defined by N4, N6, O1, and O2 has an r.m.s. deviation of 0.116 Å and the Cu1 atom is displaced from this basal plane toward the apical site by 0.240707 (11) Å. In compound (II), the basal N2O2 plane defined by N1, N2, O1, and O2 has an r.m.s. deviation of 0.023 Å and the Cu1 atom is displaced from this basal plane by 0.0274291 (17) Å. The four Cu—O and Cu—N bonds in the basal plane have similar lengths in both of the title compounds, with the Cu—O bonds being slightly longer than the Cu—N bonds. These bond length values are in good agreement with those reported for other oxalate-bridged dinuclear copper complexes (Felthouse et al., 1976 ▸; Gleizes et al., 1992 ▸; Mukherjee et al., 2004 ▸; Zhang et al., 2012 ▸). The apical coordination site of the copper ions is occupied by a monodentate nonbridging azide anion in compound (I) and by a monodentate nearly bridging (see below) dicyanamide anion in compound (II). The apical Cu—N bond in both title compounds is significantly longer than the Cu—O and Cu—N bonds in the basal plane.
Figure 1.
The molecular structure of compound (I). Displacement ellipsoids are drawn at the 50% probability level. Unlabeled atoms are related by inversion symmetry (−x + 1, −y + 1, −z + 1).
Figure 2.
The molecular structure of compound (II). Displacement ellipsoids are drawn at the 50% probability level. Unlabeled atoms are related by inversion symmetry (−x + 1, −y + 1, −z). All disordered components are shown.
The distance between the two CuII ions bridged by oxalate is 5.24755 (18) Å in compound (I) and 5.2151 (3) Å in compound (II). These distances are within the typical range of values for oxalate-bridged dinuclear copper complexes (Felthouse et al., 1976 ▸; Gleizes et al., 1992 ▸; Mukherjee et al., 2004 ▸; Xu, 2011 ▸).
In both of the title compounds, the histamine molecule adopts the Nτ—H tautomer form where imidazole atom N5 in compound (I) and N3 in compound (II) are protonated. The histamine ligand coordinates to the copper ion in a bidentate chelating fashion via the nonprotonated N atom on the imidazole ring as well as the N atom on the ethylamino side chain, resulting in a gauche conformation for the histamine ligand.
Supramolecular features
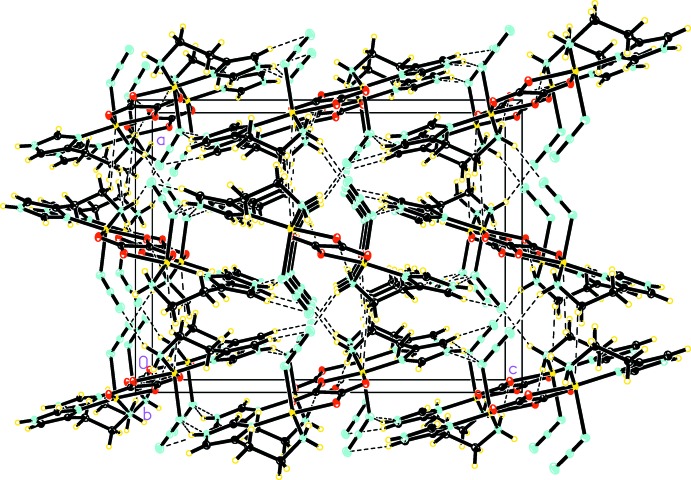
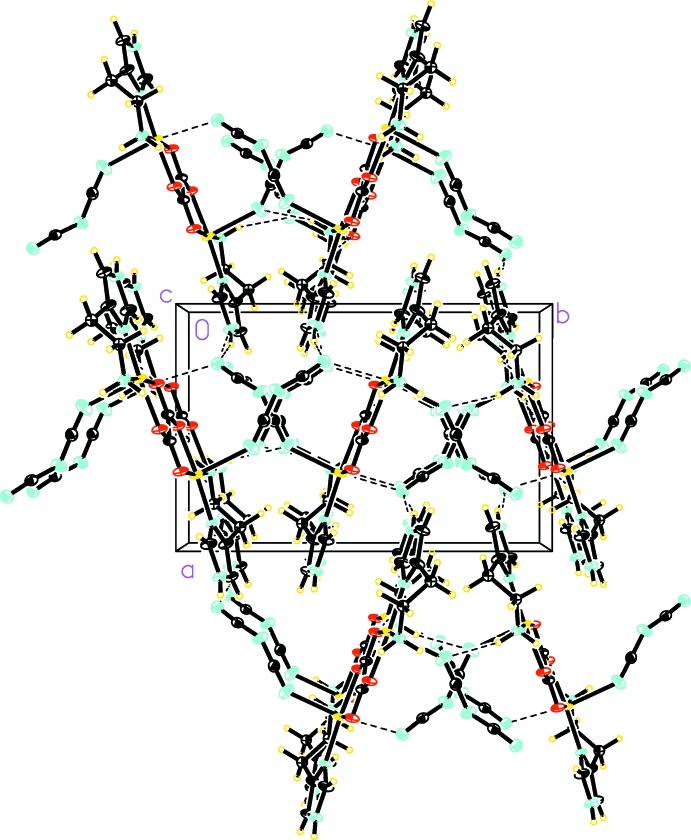
In the crystals of the title compounds, hydrogen-bonding and weak interactions exist between the dinuclear complexs. As a result, the dinuclear complexes are linked to form rows which then assemble into sheets, and finally sheets stack to form three-dimensional networks.
The two title compounds exhibit a common set of hydrogen bonds between dinuclear complexes where the histamine molecule is the sole source of hydrogen-bond donors. The NH2 group of the histamine ethylamino side chain functions as a donor to form an N—H⋯O hydrogen bond where the acceptor is the O1 atom of the oxalate ligand. This hydrogen bond results in the formation of rows of parallel dinuclear complexes along the crystallographic b axis in compound (I) and along the crystallographic c axis in compound (II). Within each row, dinuclear complexes are placed side-to-side with each other rather than stacking directly above and below each other. As a result, there is essentially no overlap between the dinuclear planes. The same NH2 group of histamine is also the donor for a N—H⋯N hydrogen bond where the acceptor is the N3 atom of the azide in compound (I) and N4 of the dicyanamide in compound (II). This hydrogen bond links rows of dinuclear complexes to form sheets parallel to the ab plane in compound (I) (Fig. 3 ▸) and to the bc plane in compound (II) (Fig. 4 ▸). The protonated N—H group on the imidazole ring is another hydrogen-bond donor for a second N—H⋯N hydrogen bond, where the acceptor is N1 of azide in compound (I) and N6 of dicyanamide in compound (II). This hydrogen bond operates between dinuclear complexes from neighboring sheets and assembles the sheets into a three-dimensional network. For numerical values and symmetry operators for (I) and (II), see Tables 2 ▸ and 3 ▸.
Figure 3.
The crystal packing of compound (I), showing the hydrogen bonds as dashed lines.
Figure 4.
The crystal packing of compound (II), showing the hydrogen bonds as dashed lines. Atoms of disordered components have been omitted for clarity.
Table 2. Hydrogen-bond geometry (Å, °) for (II) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1E⋯O1i | 0.91 | 2.29 | 3.131 (3) | 154 |
| N1—H1F⋯N4ii | 0.91 | 2.50 | 3.377 (4) | 161 |
| N3—H3⋯N6iii | 0.91 (5) | 2.10 (5) | 2.954 (3) | 157 (4) |
| C2—H2A⋯O2iv | 0.99 | 2.52 | 3.277 (1) | 133 |
Symmetry codes: (i) 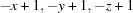 ; (ii)
; (ii) 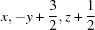 ; (iii)
; (iii) 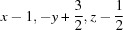 ; (iv)
; (iv) 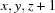 .
.
Table 3. Experimental details.
| (I) | (II) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | [Cu2(C2O4)(N3)2(C5H9N3)2] | [Cu2(C2O4)(C2N3)2(C5H9N3)2] |
| M r | 521.46 | 569.50 |
| Crystal system, space group | Orthorhombic, P b c a | Monoclinic, P21/c |
| Temperature (K) | 173 | 173 |
| a, b, c (Å) | 13.4419 (7), 7.4576 (4), 17.7662 (9) | 9.6816 (7), 14.7236 (11), 7.4604 (6) |
| α, β, γ (°) | 90, 90, 90 | 90, 90.112 (1), 90 |
| V (Å3) | 1780.96 (16) | 1063.46 (14) |
| Z | 4 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 2.44 | 2.05 |
| Crystal size (mm) | 0.17 × 0.11 × 0.11 | 0.32 × 0.27 × 0.18 |
| Data collection | ||
| Diffractometer | Bruker SMART CCD area detector | Bruker SMART CCD area detector |
| Absorption correction | Integration (SADABS; Bruker, 1998 ▸) | Analytical (SADABS; Bruker, 1998 ▸) |
| T min, T max | 0.682, 0.835 | 0.340, 0.503 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 10138, 2027, 1768 | 6331, 2374, 2172 |
| R int | 0.061 | 0.061 |
| (sin θ/λ)max (Å−1) | 0.650 | 0.650 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.026, 0.070, 1.08 | 0.034, 0.096, 1.15 |
| No. of reflections | 2027 | 2374 |
| No. of parameters | 145 | 198 |
| No. of restraints | 0 | 55 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.78, −0.26 | 0.34, −0.42 |
In addition to the traditional N—H⋯X (X = N, O) hydrogen bonds, both title compounds also exhibit a weak C—H⋯X (X = N, O) hydrogen bond between neighboring dinuclear complexes. In compound (I), this weak hydrogen bond is C3—H3⋯N3v where the donor is the C—H group on the imidazole ring and the acceptor is N3 of the azide. The C3—H3⋯N3v hydrogen bonds (Table 2 ▸) operate between dinuclear complexes from different sheets and link sheets to form a three-dimensional network. In compound (II), this weak hydrogen bond is C2—H2A⋯O2iv (Table 3 ▸), where the donor is an aliphatic C—H group on the ethylamino side chain of histamine and the acceptor is the O2iv of oxalate. The C2—H2A⋯O2iv bonds operate between dinuclear complexes in the same row. Weak hydrogen bonds of the C—H⋯X (X = N, O) types are prevalent in crystal structures and are formed with many different types of acceptor. The geometrical features of these weak hydrogen bonds exhibit a wide range of variation depending on the strength of the donors and acceptors. The values of the bond lengths and angles for the two title compounds in this study are within the typical range for C—H⋯X (X = N, O) hydrogen bonds (Mascal, 1998 ▸; Sigel et al., 1998 ▸; Janiak & Scharmann, 2003 ▸; Youm et al., 2006 ▸).
In compound (I), the coordinatively unsaturated copper ions interact with the histamine ligand via C6—H6C/H6D⋯Cu1vi [symmetry code: (vi) 1/2-x, − + y, z] interactions (Braga et al., 1998 ▸). These interactions exist along the a axis between neighboring rows of dinuclear complexes. The H6C/H6D⋯Cu1vi distances are 3.14625 (16) and 3.19821 (12) Å for H6C and H6D, respectively. The C6⋯Cu1vi separation is 3.64696 (16) Å. These distances are significantly longer than those found in the traditional and weak hydrogen bonds described above. The C6—H6C/H6D⋯Cu1vi angles are 112.8720 (3) and 109.287 (4)° for H6C and H6D, respectively. These distances and bond angle values are in good agreement with other similar interactions found in the literature (Brookhart & Green, 1983 ▸; Braga et al., 1998 ▸; Yang et al., 2004 ▸; Yamauchi et al., 2008 ▸).
+ y, z] interactions (Braga et al., 1998 ▸). These interactions exist along the a axis between neighboring rows of dinuclear complexes. The H6C/H6D⋯Cu1vi distances are 3.14625 (16) and 3.19821 (12) Å for H6C and H6D, respectively. The C6⋯Cu1vi separation is 3.64696 (16) Å. These distances are significantly longer than those found in the traditional and weak hydrogen bonds described above. The C6—H6C/H6D⋯Cu1vi angles are 112.8720 (3) and 109.287 (4)° for H6C and H6D, respectively. These distances and bond angle values are in good agreement with other similar interactions found in the literature (Brookhart & Green, 1983 ▸; Braga et al., 1998 ▸; Yang et al., 2004 ▸; Yamauchi et al., 2008 ▸).
In compound (II), the dicyanamide ligands are largely perpendicular to the dinuclear plane, making it possible for the coordinatively unsaturated copper ions to interact directly with the terminal noncoordinating N6 atom of the dicyanamide ligand of a neighboring dinuclear complex. The N6⋯Cu1v [symmetry code: (v) 1-x,  + y, 1/2-z] distance is 2.60123 (18) Å, indicating a much stronger interaction than the C—H⋯Cu interaction in compound (I). Similar to the C—H⋯Cu interactions in compound (I), the N6⋯Cu1v interactions in compound (II) also operate between neighboring rows of dinuclear complexes along the b axis.
+ y, 1/2-z] distance is 2.60123 (18) Å, indicating a much stronger interaction than the C—H⋯Cu interaction in compound (I). Similar to the C—H⋯Cu interactions in compound (I), the N6⋯Cu1v interactions in compound (II) also operate between neighboring rows of dinuclear complexes along the b axis.
Synthesis and crystallization
Compound (I) was synthesized by mixing copper(II) perchlorate hexahydrate (1.0 mmol), histamine dihydrochloride (1.0 mmol), sodium oxalate (0.5 mmol), and sodium azide (1.0 mmol) in deionized water (25 ml) to form an aqueous solution. The solution was allowed to stand in air. After a few days, dark-green prismatic crystals were collected, washed with deionized water, and dried in air (yield 63%). Selected IR (KBr, cm−1): 3271, 3228 (primary amine N—H), 2041 (N=N), 1637 (C—O), 1585 (C=C), 1078 (imidazole C—N). Elemental analysis calculated for C12H18Cu2N12O4: C 27.64, H 3.48, N 32.24%. Found: C 27.53, H 3.17, N 32.42%.
Compound (II) was synthesized in a similar manner, except that the sodium azide was replaced by sodium dicyanamide (1.0 mmol). After a few days, deep-blue plates of crystals were collected, washed with deionized water, and dried in air (yield 55%). Selected IR (KBr, cm−1): 3296, 3253 (primary amine N—H), 2254, 2204, 2146 (C≡N), 1646 (C—O), 1571 (C=C), 1079 (imidazole C—N). Elemental analysis calculated for C16H18Cu2N12O4: C 33.74, H 3.18, N 29.51%. Found: C 33.59, H 2.90, N 29.79%.
Refinement details
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. All H atoms, except the amine protons, were placed in geometrically idealized positions and allowed to ride on their parent atoms, with C—H = 0.93/1.00 Å and U iso(H) = 1.2/1.5U eq(C). Methyl H atoms were allowed to rotate around the corresponding C—C bond. In compound (II), the C2H4 unit of the histamine side chain is disordered and was refined anisotropically over three positions with their site-occupation factors constrained to unity. Equivalent bond lengths were restrained to be similar [SAME command in SHELXL2014 (Sheldrick, 2015 ▸), s.u. = 0.005 Å], and disordered atoms were subjected to a rigid bond restraint (RIGU command in SHELXL2014, s.u. = 0.004 Å2). As a consequence of the disorder, the two protons on the adjacent N atom are disordered and also were included in three idealized positions and were treated riding on their parent atoms. The other amino protons were obtained from difference Fourier maps and refined freely. In compound (I), the largest residual difference desnity peak (0.78 e Å−3) found at (0.3411, 0.4069, 0.9096) could be due to a minor alternative position for an additional CuII ion. Due to its small size, copper disorder was not included in the final refinement model.
Table 1. Hydrogen-bond geometry (Å, °) for (I) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N5—H5⋯N1i | 0.88 | 2.18 | 2.904 (2) | 140 |
| N5—H5⋯N3ii | 0.88 | 2.69 | 3.258 (2) | 124 |
| N6—H6A⋯N3iii | 0.87 (3) | 2.29 (3) | 3.099 (3) | 154 (2) |
| N6—H6B⋯O1iv | 0.89 (3) | 2.17 (3) | 3.061 (2) | 175 (2) |
| C3—H3⋯N3v | 0.95 | 2.54 | 3.476 (8) | 169 |
Symmetry codes: (i) 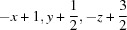 ; (ii)
; (ii) 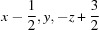 ; (iii)
; (iii) 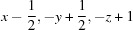 ; (iv)
; (iv) 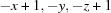 ; (v)
; (v) 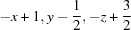 .
.
Supplementary Material
Crystal structure: contains datablock(s) I, II, global. DOI: 10.1107/S2056989015019908/zl2645sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015019908/zl2645Isup5.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989015019908/zl2645IIsup6.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
CL wishes to acknowledge the financial support of the Vice President Research Fund, Grenfell Campus, Memorial University of Newfoundland. KAA wishes to acknowledge the National Science Foundation and the University of Florida for funding the purchase of the X-ray equipment.
supplementary crystallographic information
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Crystal data
| [Cu2(C2O4)(N3)2(C5H9N3)2] | Dx = 1.945 Mg m−3 |
| Mr = 521.46 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, Pbca | Cell parameters from 88 reflections |
| a = 13.4419 (7) Å | θ = 2.0–28.0° |
| b = 7.4576 (4) Å | µ = 2.44 mm−1 |
| c = 17.7662 (9) Å | T = 173 K |
| V = 1780.96 (16) Å3 | Prism, green |
| Z = 4 | 0.17 × 0.11 × 0.11 mm |
| F(000) = 1056 |
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Data collection
| Bruker SMART CCD area-detector diffractometer | 2027 independent reflections |
| Radiation source: fine-focus sealed tube | 1768 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.061 |
| ω scans | θmax = 27.5°, θmin = 2.3° |
| Absorption correction: integration (SADABS; Bruker, 1998) | h = −13→17 |
| Tmin = 0.682, Tmax = 0.835 | k = −9→9 |
| 10138 measured reflections | l = −20→23 |
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Refinement
| Refinement on F2 | Secondary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.026 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.070 | w = 1/[σ2(Fo2) + (0.0279P)2 + 1.2918P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max = 0.002 |
| 2027 reflections | Δρmax = 0.78 e Å−3 |
| 145 parameters | Δρmin = −0.26 e Å−3 |
| 0 restraints | Extinction correction: SHELXL2014 (Sheldrick, 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0022 (3) |
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger.All H atoms were positioned geometrically (C—H = 0.93/1.00 Å) and allowed to ride with Uiso(H)= 1.2/1.5Ueq(C). Methyl ones were allowed to rotate around the corresponding C—C.The amino protons were obtained from a Difference Fourier map and refined freely.A small peak (0.78) was found at 0.3411 0.4069 0.9096, which had the following geometry around it: We believe it is a small trace of a Cu center but was not included in the final refinement model for its small size.ENVIRONMENT OF Q1Ligand Symcode Dist. Angles symm operationO1 6566 2.581 x. 5 - y. 5 + z N1 5656 2.039 107.5 1 - x. 5 + y 1.5 - z N2 5656 2.580 119.2 26.6 1 - x. 5 + y 1.5 - z N3 4557 1.902 108.9 127.6 101.4 -.5 + x y. 5 - z N5 1555 1.928 97.9 94.0 113.7 116.5 |
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.44296 (2) | 0.24198 (3) | 0.59064 (2) | 0.01472 (11) | |
| O1 | 0.47496 (11) | 0.27517 (17) | 0.48108 (7) | 0.0173 (3) | |
| O2 | 0.46843 (11) | 0.50801 (17) | 0.59516 (7) | 0.0181 (3) | |
| N1 | 0.60261 (13) | 0.1548 (2) | 0.60977 (10) | 0.0221 (4) | |
| N2 | 0.65931 (13) | 0.2515 (2) | 0.57879 (9) | 0.0182 (3) | |
| N3 | 0.71372 (14) | 0.3492 (3) | 0.54767 (10) | 0.0301 (4) | |
| N4 | 0.39863 (12) | 0.23846 (19) | 0.69487 (9) | 0.0159 (3) | |
| N5 | 0.36694 (12) | 0.3189 (2) | 0.81078 (9) | 0.0199 (3) | |
| H5 | 0.3614 | 0.3858 | 0.8514 | 0.024* | |
| N6 | 0.37467 (14) | 0.0143 (2) | 0.56327 (9) | 0.0182 (3) | |
| H6A | 0.3427 (19) | 0.035 (3) | 0.5215 (14) | 0.025 (6)* | |
| H6B | 0.420 (2) | −0.065 (4) | 0.5489 (14) | 0.027 (6)* | |
| C1 | 0.49805 (13) | 0.5671 (2) | 0.53301 (10) | 0.0156 (4) | |
| C2 | 0.39970 (14) | 0.3740 (3) | 0.74353 (10) | 0.0187 (4) | |
| H2A | 0.4207 | 0.4926 | 0.7322 | 0.022* | |
| C3 | 0.34345 (15) | 0.1401 (3) | 0.80562 (11) | 0.0203 (4) | |
| H3A | 0.3182 | 0.0661 | 0.8447 | 0.024* | |
| C4 | 0.36342 (14) | 0.0895 (2) | 0.73347 (10) | 0.0164 (4) | |
| C5 | 0.35239 (15) | −0.0881 (2) | 0.69514 (11) | 0.0187 (4) | |
| H5A | 0.3107 | −0.1673 | 0.7268 | 0.022* | |
| H5B | 0.4188 | −0.1445 | 0.6903 | 0.022* | |
| C6 | 0.30567 (14) | −0.0729 (3) | 0.61751 (11) | 0.0188 (4) | |
| H6C | 0.2882 | −0.1941 | 0.5990 | 0.023* | |
| H6D | 0.2436 | −0.0019 | 0.6210 | 0.023* |
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.01840 (16) | 0.01299 (14) | 0.01277 (15) | −0.00233 (8) | 0.00262 (8) | 0.00036 (8) |
| O1 | 0.0229 (7) | 0.0142 (6) | 0.0148 (6) | −0.0025 (5) | 0.0022 (5) | 0.0010 (5) |
| O2 | 0.0246 (7) | 0.0158 (6) | 0.0140 (6) | −0.0019 (5) | 0.0021 (5) | 0.0016 (5) |
| N1 | 0.0205 (9) | 0.0218 (8) | 0.0240 (8) | 0.0025 (7) | 0.0006 (7) | 0.0050 (7) |
| N2 | 0.0192 (8) | 0.0197 (8) | 0.0156 (7) | 0.0012 (6) | −0.0015 (7) | −0.0017 (6) |
| N3 | 0.032 (1) | 0.0341 (10) | 0.0243 (9) | −0.0096 (8) | 0.0050 (8) | −0.0009 (8) |
| N4 | 0.0156 (8) | 0.0166 (8) | 0.0155 (8) | −0.0024 (6) | 0.0020 (6) | 0.0001 (6) |
| N5 | 0.0212 (8) | 0.0242 (8) | 0.0143 (8) | 0.0004 (7) | 0.0006 (7) | −0.0039 (6) |
| N6 | 0.0220 (8) | 0.0173 (8) | 0.0152 (8) | −0.0018 (7) | 0.0017 (7) | −0.0013 (6) |
| C1 | 0.0149 (8) | 0.0159 (9) | 0.0158 (9) | 0.0004 (7) | −0.0012 (7) | 0.0006 (7) |
| C2 | 0.0198 (9) | 0.0190 (9) | 0.0172 (9) | −0.0007 (7) | −0.0003 (7) | −0.0016 (7) |
| C3 | 0.0199 (9) | 0.0235 (10) | 0.0174 (9) | 0.0007 (7) | 0.0014 (7) | 0.0041 (7) |
| C4 | 0.0155 (9) | 0.0173 (8) | 0.0163 (9) | 0.0002 (7) | 0.0007 (7) | 0.0030 (7) |
| C5 | 0.0213 (10) | 0.0144 (8) | 0.0204 (9) | 0.0000 (7) | 0.0028 (7) | 0.0031 (7) |
| C6 | 0.0194 (9) | 0.0159 (9) | 0.0210 (9) | −0.0037 (7) | 0.0022 (8) | −0.0002 (7) |
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Geometric parameters (Å, º)
| Cu1—N4 | 1.9454 (16) | N6—C6 | 1.487 (2) |
| Cu1—N6 | 1.9904 (17) | N6—H6A | 0.87 (3) |
| Cu1—O1 | 2.0088 (13) | N6—H6B | 0.89 (3) |
| Cu1—O2 | 2.0148 (13) | C1—O1i | 1.257 (2) |
| Cu1—N1 | 2.2679 (17) | C1—C1i | 1.542 (4) |
| O1—C1i | 1.257 (2) | C2—H2A | 0.9500 |
| O2—C1 | 1.254 (2) | C3—C4 | 1.363 (3) |
| N1—N2 | 1.185 (2) | C3—H3 | 0.9500 |
| N2—N3 | 1.171 (2) | C4—C5 | 1.497 (3) |
| N4—C2 | 1.330 (2) | C5—C6 | 1.520 (3) |
| N4—C4 | 1.388 (2) | C5—H5A | 0.9900 |
| N5—C2 | 1.338 (2) | C5—H5B | 0.9900 |
| N5—C3 | 1.374 (3) | C6—H6C | 0.9900 |
| N5—H5 | 0.8800 | C6—H6D | 0.9900 |
| N4—Cu1—N6 | 94.58 (7) | H6A—N6—H6B | 102 (2) |
| N4—Cu1—O1 | 171.72 (6) | O2—C1—O1i | 126.59 (17) |
| N6—Cu1—O1 | 88.11 (6) | O2—C1—C1i | 116.9 (2) |
| N4—Cu1—O2 | 91.58 (6) | O1i—C1—C1i | 116.48 (19) |
| N6—Cu1—O2 | 158.12 (7) | N4—C2—N5 | 110.11 (17) |
| O1—Cu1—O2 | 83.16 (5) | N4—C2—H2A | 124.9 |
| N4—Cu1—N1 | 98.24 (6) | N5—C2—H2A | 124.9 |
| N6—Cu1—N1 | 103.21 (7) | C4—C3—N5 | 106.62 (16) |
| O1—Cu1—N1 | 88.73 (6) | C4—C3—H3A | 126.7 |
| O2—Cu1—N1 | 96.63 (6) | N5—C3—H3A | 126.7 |
| C1i—O1—Cu1 | 111.73 (11) | C3—C4—N4 | 108.08 (16) |
| C1—O2—Cu1 | 111.40 (11) | C3—C4—C5 | 130.79 (17) |
| N2—N1—Cu1 | 111.39 (13) | N4—C4—C5 | 121.13 (16) |
| N3—N2—N1 | 178.6 (2) | C4—C5—C6 | 112.82 (16) |
| C2—N4—C4 | 106.89 (16) | C4—C5—H5A | 109.0 |
| C2—N4—Cu1 | 127.24 (13) | C6—C5—H5A | 109.0 |
| C4—N4—Cu1 | 125.82 (12) | C4—C5—H5B | 109.0 |
| C2—N5—C3 | 108.30 (16) | C6—C5—H5B | 109.0 |
| C2—N5—H5 | 125.8 | H5A—C5—H5B | 107.8 |
| C3—N5—H5 | 125.8 | N6—C6—C5 | 111.28 (16) |
| C6—N6—Cu1 | 120.15 (12) | N6—C6—H6C | 109.4 |
| C6—N6—H6A | 108.7 (16) | C5—C6—H6C | 109.4 |
| Cu1—N6—H6A | 106.6 (16) | N6—C6—H6D | 109.4 |
| C6—N6—H6B | 108.9 (16) | C5—C6—H6D | 109.4 |
| Cu1—N6—H6B | 108.7 (17) | H6C—C6—H6D | 108.0 |
Symmetry code: (i) −x+1, −y+1, −z+1.
(I) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](azido-κN1)copper(II)] . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N5—H5···N1ii | 0.88 | 2.18 | 2.904 (2) | 140 |
| N5—H5···N3iii | 0.88 | 2.69 | 3.258 (2) | 124 |
| N6—H6A···N3iv | 0.87 (3) | 2.29 (3) | 3.099 (3) | 154 (2) |
| N6—H6B···O1v | 0.89 (3) | 2.17 (3) | 3.061 (2) | 175 (2) |
| C3—H3···N3vi | 0.95 | 2.54 | 3.476 (8) | 169 |
Symmetry codes: (ii) −x+1, y+1/2, −z+3/2; (iii) x−1/2, y, −z+3/2; (iv) x−1/2, −y+1/2, −z+1; (v) −x+1, −y, −z+1; (vi) −x+1, y−1/2, −z+3/2.
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Crystal data
| [Cu2(C2O4)(C2N3)2(C5H9N3)2] | F(000) = 576 |
| Mr = 569.50 | Dx = 1.778 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.6816 (7) Å | Cell parameters from 87 reflections |
| b = 14.7236 (11) Å | θ = 2.0–28.0° |
| c = 7.4604 (6) Å | µ = 2.05 mm−1 |
| β = 90.112 (1)° | T = 173 K |
| V = 1063.46 (14) Å3 | Plate, blue |
| Z = 2 | 0.32 × 0.27 × 0.18 mm |
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Data collection
| Bruker SMART CCD area-detector diffractometer | 2374 independent reflections |
| Radiation source: fine-focus sealed tube | 2172 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.061 |
| ω scans | θmax = 27.5°, θmin = 2.1° |
| Absorption correction: analytical (SADABS; Bruker, 1998) | h = −12→10 |
| Tmin = 0.340, Tmax = 0.503 | k = −18→18 |
| 6331 measured reflections | l = −8→9 |
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.034 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.096 | w = 1/[σ2(Fo2) + (0.0382P)2 + 1.0333P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.15 | (Δ/σ)max = 0.001 |
| 2374 reflections | Δρmax = 0.34 e Å−3 |
| 198 parameters | Δρmin = −0.42 e Å−3 |
| 55 restraints | Extinction correction: SHELXL2014 (Sheldrick, 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.022 (2) |
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. All H atoms were positioned geometrically (C—H = 0.93/1.00 Å) and allowed to ride with Uiso(H)= 1.2/1.5Ueq(C). Methyl ones were allowed to rotate around the corresponding C—C.A C2H4 is disordered and was refined in three parts with their site occupation factors adding up to one. As a consequence of this disorder, the two protons on the adjacent N atom are disordered and also were calculated is three idealized positioned and were treated riding on their parent atom. SADI and RIGU were applied to the disorder geometry to maintain equivalent bond lengths of corresponding bonds.Proton H3 were obtained from a Difference Fourier map and refined freely. |
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Cu1 | 0.31334 (3) | 0.56430 (2) | 0.21722 (4) | 0.02660 (15) | |
| O1 | 0.50672 (18) | 0.51395 (14) | 0.2322 (2) | 0.0288 (4) | |
| O2 | 0.32980 (18) | 0.53232 (14) | −0.0421 (2) | 0.0294 (4) | |
| N1 | 0.3199 (2) | 0.58973 (16) | 0.4781 (3) | 0.0274 (4) | |
| H1A | 0.3702 | 0.5447 | 0.5303 | 0.033* | 0.516 (3) |
| H1B | 0.3682 | 0.6422 | 0.4933 | 0.033* | 0.516 (3) |
| H1C | 0.3102 | 0.5359 | 0.5368 | 0.033* | 0.243 (3) |
| H1D | 0.4057 | 0.6112 | 0.5044 | 0.033* | 0.243 (3) |
| H1E | 0.3862 | 0.5547 | 0.5302 | 0.033* | 0.240 (3) |
| H1F | 0.3426 | 0.6490 | 0.4961 | 0.033* | 0.240 (3) |
| N2 | 0.1213 (2) | 0.60545 (14) | 0.1895 (3) | 0.0235 (4) | |
| N3 | −0.0839 (2) | 0.62944 (17) | 0.0764 (3) | 0.0322 (5) | |
| H3 | −0.145 (5) | 0.634 (3) | −0.015 (7) | 0.073 (14)* | |
| N4 | 0.4224 (3) | 0.7083 (2) | 0.1465 (4) | 0.0516 (7) | |
| N5 | 0.6458 (3) | 0.7761 (2) | 0.0675 (4) | 0.0409 (6) | |
| N6 | 0.7630 (3) | 0.89650 (17) | 0.2373 (3) | 0.0360 (5) | |
| C1 | 0.5513 (2) | 0.49455 (17) | 0.0797 (3) | 0.0236 (5) | |
| C2 | 0.1885 (7) | 0.5987 (6) | 0.5821 (12) | 0.0267 (19) | 0.516 (3) |
| H2A | 0.2099 | 0.6155 | 0.7077 | 0.032* | 0.516 (3) |
| H2B | 0.1392 | 0.5399 | 0.5830 | 0.032* | 0.516 (3) |
| C3 | 0.0970 (10) | 0.6712 (6) | 0.4982 (8) | 0.0285 (19) | 0.516 (3) |
| H3A | 0.0213 | 0.6867 | 0.5818 | 0.034* | 0.516 (3) |
| H3B | 0.1520 | 0.7268 | 0.4765 | 0.034* | 0.516 (3) |
| C2' | 0.2169 (9) | 0.6548 (8) | 0.5547 (15) | 0.031 (2) | 0.243 (3) |
| H2'A | 0.2272 | 0.7153 | 0.4985 | 0.037* | 0.243 (3) |
| H2'B | 0.2311 | 0.6613 | 0.6856 | 0.037* | 0.243 (3) |
| C3' | 0.0738 (14) | 0.6165 (13) | 0.5163 (10) | 0.033 (4) | 0.243 (3) |
| H3'A | 0.0053 | 0.6442 | 0.5981 | 0.039* | 0.243 (3) |
| H3'B | 0.0736 | 0.5500 | 0.5358 | 0.039* | 0.243 (3) |
| C2" | 0.1832 (11) | 0.5704 (13) | 0.564 (3) | 0.029 (4) | 0.240 (3) |
| H2"A | 0.1949 | 0.5669 | 0.6956 | 0.035* | 0.240 (3) |
| H2"B | 0.1480 | 0.5110 | 0.5214 | 0.035* | 0.240 (3) |
| C3" | 0.079 (3) | 0.6445 (17) | 0.5181 (13) | 0.033 (6) | 0.240 (3) |
| H3"A | 0.1213 | 0.7048 | 0.5408 | 0.040* | 0.240 (3) |
| H3"B | −0.0028 | 0.6384 | 0.5956 | 0.040* | 0.240 (3) |
| C4 | 0.0362 (3) | 0.6377 (2) | 0.3221 (4) | 0.0328 (6) | |
| C5 | −0.0920 (3) | 0.6523 (2) | 0.2523 (4) | 0.0332 (6) | |
| H5A | −0.1711 | 0.6739 | 0.3143 | 0.040* | |
| C6 | 0.0449 (3) | 0.6013 (2) | 0.0430 (4) | 0.0345 (6) | |
| H6A | 0.0770 | 0.5810 | −0.0705 | 0.041* | |
| C7 | 0.5280 (3) | 0.74143 (19) | 0.1173 (4) | 0.0320 (6) | |
| C8 | 0.7025 (3) | 0.84044 (18) | 0.1632 (3) | 0.0283 (5) |
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.01728 (19) | 0.0447 (2) | 0.01783 (19) | 0.00787 (12) | 0.00023 (11) | −0.00297 (12) |
| O1 | 0.0178 (8) | 0.0499 (11) | 0.0187 (8) | 0.0068 (7) | 0.0004 (6) | −0.0011 (8) |
| O2 | 0.0172 (8) | 0.0505 (11) | 0.0204 (8) | 0.0075 (7) | 0.0008 (6) | −0.0015 (8) |
| N1 | 0.0223 (10) | 0.0381 (12) | 0.0218 (10) | 0.0056 (9) | −0.0013 (8) | −0.0024 (9) |
| N2 | 0.0189 (9) | 0.0293 (11) | 0.0222 (10) | 0.0040 (8) | 0.0009 (7) | −0.0011 (8) |
| N3 | 0.0219 (10) | 0.0457 (13) | 0.0289 (11) | 0.0071 (9) | −0.0016 (9) | 0.0006 (10) |
| N4 | 0.0501 (16) | 0.0543 (17) | 0.0504 (16) | −0.0225 (14) | 0.0002 (13) | 0.0072 (14) |
| N5 | 0.0345 (12) | 0.0526 (15) | 0.0356 (13) | −0.0118 (11) | 0.0026 (10) | −0.0124 (11) |
| N6 | 0.0357 (12) | 0.0359 (13) | 0.0365 (13) | −0.0036 (10) | −0.0028 (10) | 0.0009 (11) |
| C1 | 0.0183 (10) | 0.0333 (12) | 0.0193 (11) | 0.0009 (9) | −0.0004 (9) | 0.0010 (9) |
| C2 | 0.029 (3) | 0.030 (5) | 0.021 (3) | 0.005 (2) | 0.007 (2) | −0.002 (3) |
| C3 | 0.031 (3) | 0.028 (4) | 0.027 (3) | 0.010 (3) | 0.001 (2) | −0.004 (2) |
| C2' | 0.028 (4) | 0.039 (6) | 0.025 (5) | 0.006 (4) | −0.001 (4) | −0.008 (4) |
| C3' | 0.025 (5) | 0.052 (10) | 0.022 (5) | 0.005 (5) | 0.005 (4) | −0.013 (5) |
| C2" | 0.031 (5) | 0.044 (10) | 0.012 (5) | 0.011 (5) | 0.000 (4) | −0.007 (6) |
| C3" | 0.033 (8) | 0.041 (14) | 0.027 (6) | 0.008 (7) | −0.004 (5) | −0.018 (5) |
| C4 | 0.0243 (12) | 0.0475 (16) | 0.0265 (12) | 0.0116 (11) | 0.0012 (10) | −0.0070 (11) |
| C5 | 0.0218 (12) | 0.0462 (16) | 0.0315 (13) | 0.0110 (11) | 0.0019 (10) | −0.0003 (12) |
| C6 | 0.0244 (12) | 0.0556 (17) | 0.0236 (12) | 0.0098 (12) | −0.0021 (10) | −0.0032 (12) |
| C7 | 0.0346 (14) | 0.0300 (13) | 0.0315 (13) | 0.0002 (11) | −0.0052 (11) | 0.0028 (11) |
| C8 | 0.0248 (12) | 0.0339 (13) | 0.0261 (12) | 0.0035 (10) | 0.0008 (10) | 0.0038 (10) |
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Geometric parameters (Å, º)
| Cu1—N2 | 1.966 (2) | N6—C8 | 1.153 (4) |
| Cu1—N1 | 1.983 (2) | C1—O2i | 1.249 (3) |
| Cu1—O2 | 1.9978 (18) | C1—C1i | 1.557 (4) |
| Cu1—O1 | 2.0165 (17) | C2—C3 | 1.521 (7) |
| Cu1—N4 | 2.426 (3) | C2—H2A | 0.9900 |
| O1—C1 | 1.251 (3) | C2—H2B | 0.9900 |
| O2—C1i | 1.249 (3) | C3—C4 | 1.521 (5) |
| N1—C2 | 1.497 (5) | C3—H3A | 0.9900 |
| N1—C2' | 1.497 (6) | C3—H3B | 0.9900 |
| N1—C2" | 1.498 (6) | C2'—C3' | 1.522 (8) |
| N1—H1A | 0.9100 | C2'—H2'A | 0.9900 |
| N1—H1B | 0.9100 | C2'—H2'B | 0.9900 |
| N1—H1C | 0.9100 | C3'—C4 | 1.526 (6) |
| N1—H1D | 0.9100 | C3'—H3'A | 0.9900 |
| N1—H1E | 0.9100 | C3'—H3'B | 0.9900 |
| N1—H1F | 0.9100 | C2"—C3" | 1.521 (8) |
| N2—C6 | 1.320 (3) | C2"—H2"A | 0.9900 |
| N2—C4 | 1.373 (3) | C2"—H2"B | 0.9900 |
| N3—C6 | 1.338 (3) | C3"—C4 | 1.524 (6) |
| N3—C5 | 1.357 (4) | C3"—H3"A | 0.9900 |
| N3—H3 | 0.91 (5) | C3"—H3"B | 0.9900 |
| N4—C7 | 1.154 (4) | C4—C5 | 1.362 (4) |
| N5—C7 | 1.304 (4) | C5—H5A | 0.9500 |
| N5—C8 | 1.306 (4) | C6—H6A | 0.9500 |
| N2—Cu1—N1 | 94.21 (9) | H2A—C2—H2B | 108.2 |
| N2—Cu1—O2 | 92.75 (8) | C4—C3—C2 | 110.7 (7) |
| N1—Cu1—O2 | 173.01 (8) | C4—C3—H3A | 109.5 |
| N2—Cu1—O1 | 175.44 (8) | C2—C3—H3A | 109.5 |
| N1—Cu1—O1 | 89.27 (8) | C4—C3—H3B | 109.5 |
| O2—Cu1—O1 | 83.74 (7) | C2—C3—H3B | 109.5 |
| N2—Cu1—N4 | 96.87 (10) | H3A—C3—H3B | 108.1 |
| N1—Cu1—N4 | 92.03 (10) | N1—C2'—C3' | 107.3 (9) |
| O2—Cu1—N4 | 87.70 (10) | N1—C2'—H2'A | 110.3 |
| O1—Cu1—N4 | 85.92 (10) | C3'—C2'—H2'A | 110.3 |
| C1—O1—Cu1 | 110.86 (15) | N1—C2'—H2'B | 110.3 |
| C1i—O2—Cu1 | 111.54 (15) | C3'—C2'—H2'B | 110.3 |
| C2—N1—Cu1 | 120.0 (4) | H2'A—C2'—H2'B | 108.5 |
| C2'—N1—Cu1 | 118.4 (4) | C2'—C3'—C4 | 108.6 (8) |
| C2"—N1—Cu1 | 110.9 (8) | C2'—C3'—H3'A | 110.0 |
| C2—N1—H1A | 107.3 | C4—C3'—H3'A | 110.0 |
| Cu1—N1—H1A | 107.3 | C2'—C3'—H3'B | 110.0 |
| C2—N1—H1B | 107.3 | C4—C3'—H3'B | 110.0 |
| Cu1—N1—H1B | 107.3 | H3'A—C3'—H3'B | 108.4 |
| H1A—N1—H1B | 106.9 | N1—C2"—C3" | 110.5 (17) |
| C2'—N1—H1C | 107.7 | N1—C2"—H2"A | 109.5 |
| Cu1—N1—H1C | 107.7 | C3"—C2"—H2"A | 109.5 |
| C2'—N1—H1D | 107.7 | N1—C2"—H2"B | 109.5 |
| Cu1—N1—H1D | 107.7 | C3"—C2"—H2"B | 109.5 |
| H1C—N1—H1D | 107.1 | H2"A—C2"—H2"B | 108.1 |
| C2"—N1—H1E | 109.5 | C2"—C3"—C4 | 110.4 (15) |
| Cu1—N1—H1E | 109.5 | C2"—C3"—H3"A | 109.6 |
| C2"—N1—H1F | 109.5 | C4—C3"—H3"A | 109.6 |
| Cu1—N1—H1F | 109.5 | C2"—C3"—H3"B | 109.6 |
| H1E—N1—H1F | 108.0 | C4—C3"—H3"B | 109.6 |
| C6—N2—C4 | 106.1 (2) | H3"A—C3"—H3"B | 108.1 |
| C6—N2—Cu1 | 126.94 (18) | C5—C4—N2 | 109.1 (2) |
| C4—N2—Cu1 | 126.83 (17) | C5—C4—C3 | 129.0 (4) |
| C6—N3—C5 | 108.2 (2) | N2—C4—C3 | 120.2 (4) |
| C6—N3—H3 | 120 (3) | C5—C4—C3" | 127.2 (10) |
| C5—N3—H3 | 132 (3) | N2—C4—C3" | 123.3 (10) |
| C7—N4—Cu1 | 142.8 (3) | C5—C4—C3' | 127.6 (6) |
| C7—N5—C8 | 119.7 (3) | N2—C4—C3' | 118.1 (7) |
| O2i—C1—O1 | 126.6 (2) | N3—C5—C4 | 106.0 (2) |
| O2i—C1—C1i | 116.7 (3) | N3—C5—H5A | 127.0 |
| O1—C1—C1i | 116.7 (3) | C4—C5—H5A | 127.0 |
| N1—C2—C3 | 110.1 (7) | N2—C6—N3 | 110.6 (2) |
| N1—C2—H2A | 109.6 | N2—C6—H6A | 124.7 |
| C3—C2—H2A | 109.6 | N3—C6—H6A | 124.7 |
| N1—C2—H2B | 109.6 | N4—C7—N5 | 174.2 (3) |
| C3—C2—H2B | 109.6 | N6—C8—N5 | 173.6 (3) |
Symmetry code: (i) −x+1, −y+1, −z.
(II) µ-Oxalato-κ4O1,O2:O1',O2'-bis[[4-(2-aminoethyl)-1H-imidazole-κ2N3,N4](dicyanamido-κN1)copper(II)] . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1E···O1ii | 0.91 | 2.29 | 3.131 (3) | 154 |
| N1—H1F···N4iii | 0.91 | 2.50 | 3.377 (4) | 161 |
| N3—H3···N6iv | 0.91 (5) | 2.10 (5) | 2.954 (3) | 157 (4) |
| C2—H2A···O2v | 0.99 | 2.52 | 3.277 (1) | 133 |
Symmetry codes: (ii) −x+1, −y+1, −z+1; (iii) x, −y+3/2, z+1/2; (iv) x−1, −y+3/2, z−1/2; (v) x, y, z+1.
References
- Batten, S. R. & Murray, K. S. (2003). Coord. Chem. Rev 246, 103–130.
- Braga, D., Grepioni, F. & Desiraju, G. R. (1998). Chem. Rev. 98, 1375–1405. [DOI] [PubMed]
- Brookhart, M. & Green, M. L. H. (1983). J. Organomet. Chem. 250, 395–408.
- Bruker (1998). SMART, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Coronado, E., Giménez, M. C., Gómez-García, C. J. & Romero, F. M. (2003). Polyhedron, 22, 3115–3122.
- Escuer, A. & Aromí, G. (2006). Eur. J. Inorg. Chem. 23, 4721–4736.
- Felthouse, T. R., Laskowski, E. J., Bieksza, D. S. & Hendrickson, D. N. (1976). J. Chem. Soc. Chem. Commun. pp. 777–778.
- Gleizes, A., Julve, M., Verdaguer, M., Real, J. A., Faus, J. & Solons, X. (1992). J. Chem. Soc. Dalton Trans. pp. 3209–3216.
- Hernández-Molina, A., Lorenzo-Luis, P. A. & Ruiz-Pérez, C. (2001). CrystEngComm, 16, 1–4.
- Janiak, C. & Scharmann, T. G. (2003). Polyhedron, 22, 1123–1133.
- Mascal, M. (1998). Chem. Commun. pp. 303–304.
- Mukherjee, P. S., Konar, S., Dalai, S., Zangrando, E. & Chaudhuri, N. R. (2004). Indian J. Chem. Sect. A, 43, 760–762.
- Pardo, E., Train, C., Lescouëzec, R., Boubekeur, K., Ruiz, E., Lloret, F. & Verdaguer, M. (2010). Dalton Trans. 39, 4951–4958. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Sigel, R. K., Freisinger, E., Metzger, S. & Lippert, B. (1998). J. Am. Chem. Soc. 120, 12000–12007.
- Stamatatos, T. & Christou, G. (2009). Inorg. Chem. 48, 3308–3322. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Xu, Q. (2011). Acta Cryst. E67, m1585. [DOI] [PMC free article] [PubMed]
- Yamauchi, O., Yajima, T., Fujii, R., Shimazaki, Y., Yabusaki, M., Takani, M., Tashiro, M., Motoyama, T., Kakuto, M. & Nakabayashi, Y. (2008). J. Inorg. Biochem. 102, 1218–1226. [DOI] [PubMed]
- Yang, L., Chen, Q., Yan, L., Xiong, S., Li, G. & Ma, J. S. (2004). Eur. J. Inorg. Chem. pp. 1478–1487.
- Youm, K. T., Ko, J. & Jun, M. J. (2006). Polyhedron, 25, 2717–2720.
- Zhang, Z., Shao, D. L., Geng, Z. R. & Wang, Z. L. (2012). Z. Anorg. Allg. Chem 638, 821–825.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, II, global. DOI: 10.1107/S2056989015019908/zl2645sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015019908/zl2645Isup5.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989015019908/zl2645IIsup6.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report