Crystal structures of three co-crystals of bis(4-alkoxybenzoic acid) and 4,4′-bipyridyl have been determined at 93 K. The asymmetric unit of each compound comprises two crystallographically independent acid molecules and one base molecule, which are held together by O—H⋯N hydrogen bonds, forming a linear hydrogen-bonded 2:1 unit.
Keywords: crystal structure; 4,4′-bipyridyl; 4-alkoxybenzoic acid; hydrogen-bonded liquid crystal
Abstract
The crystal structures of three hydrogen-bonded co-crystals of 4-alkoxybenzoic acid–4,4′-bipyridyl (2/1), namely, 2C9H10O3·C10H8N2, (I), 2C10H12O3·C10H8N2, (II) and 2C11H14O3·C10H8N2, (III), have been determined at 93 K. Although the structure of (I) has been determined in the space group P21 with Z = 4 [Lai et al. (2008 ▸). J. Struct. Chem. 49, 1137–1140], the present study shows that the space group is P21/n with Z = 4. In each crystal, the components are linked by O—H⋯N hydrogen bonds, forming a linear hydrogen-bonded 2:1 unit of the acid and the base. The 2:1 unit of (I) adopts nearly pseudo-C 2 symmetry, viz. twofold rotation around an axis passing through the mid-point of the central C—C bond of 4,4′-bipyridyl, while the units of (II) and (III), except for the terminal alkyl chains, have pseudo-inversion symmetry. The 2:1 units of (I), (II) and (III) are linked via C—H⋯O hydrogen bonds, forming sheet, double-tape and tape structures, respectively.
Chemical context
The 4-alkoxybenzoic acid–4,4′-bipyridyl (2/1) system, in which the two acids and the base are held together by intermolecular O—H⋯N hydrogen bonds, shows thermotropic liquid crystallinity (Kato et al., 1990 ▸, 1993 ▸; Grunert et al., 1997 ▸). The compounds of 4-methoxy-, 4-ethoxy- and 4-n-propoxybenzoic acid show nematic phases, while the compound of 4-n-butoxybenzoic acid exhibits a smectic A phase and then a nematic phase with increasing temperature (Kato et al., 1990 ▸, 1993 ▸). The crystal structure of 4-methoxybenzoic acid–4,4′-bipyridyl (2/1) was reported recently (Mukherjee & Desiraju, 2014 ▸; Ramon et al., 2014 ▸). Although the structure of 4-ethoxybenzoic acid–4,4′-bipyridyl (2/1) in space group P21 was also reported (Lai et al., 2008 ▸), the molecular structure is distorted probably due to the wrong choice of space group. In the present study, we have analysed the structure of 4-ethoxybenzoic acid–4,4′-bipyridyl (2/1), (I), as well as the structures of 4-n-propoxybenzoic acid–4,4′-bipyridyl (2/1), (II), and 4-n-butoxybenzoic acid–4,4′-bipyridyl(2/1), (III).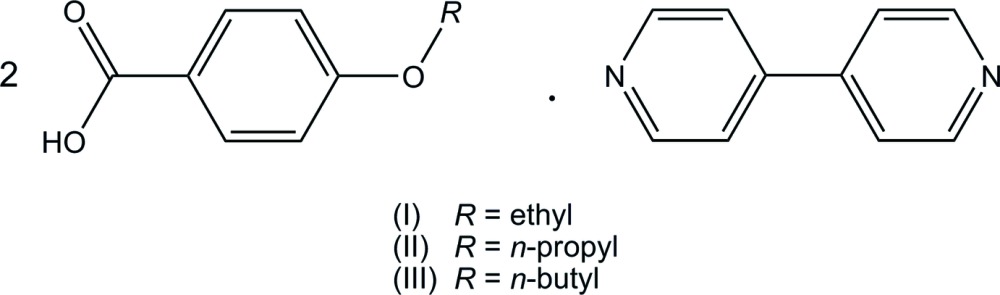
Structural commentary
The molecular structure of (I) is shown in Fig. 1 ▸. Compound (I) crystallizes in the space group P21/n with Z = 4. For the structure (space group P21) previously determined by Lai et al. (2008 ▸), ADDSYM in PLATON (Spek, 2009 ▸) detected missed symmetry elements, viz. a centre of inversion and a glide plane. The molecular structures of (II) and (III) are shown in Figs. 2 ▸ and 3 ▸, respectively. The asymmetric units each comprise two crystallographically independent 4-alkoxybenzoic acid molecules and one 4,4′-bipyridyl molecule, and the two acids and the base are held together by O—H⋯N hydrogen bonds (Tables 1 ▸, 2 ▸ and 3 ▸), forming a linear hydrogen-bonded 2:1 aggregate. Similar to the reported structure of the 2:1 unit of 4-methoxybenzoic acid–4,4′-bipyridyl (2/1) (Mukherjee & Desiraju, 2014 ▸; Ramon et al., 2014 ▸), the 2:1 unit of (I) also adopts nearly pseudo-C 2 symmetry, viz. twofold rotation around an axis passing through the mid-point of the central C21—C26 bond of the 4,4′-bipyridyl molecule. On the other hand, the 2:1 units of (II) and (III), except for the terminal alkyl chains, have pseudo-inversion symmetry.
Figure 1.
The molecular structure of compound (I), showing the atom-numbering scheme. Displacement ellipsoids of non-H atoms are drawn at the 50% probability level and H atoms are drawn as circles of arbitrary size. The O—H⋯N hydrogen bonds are indicated by dashed lines.
Figure 2.
The molecular structure of compound (II), showing the atom-numbering scheme. Displacement ellipsoids of non-H atoms are drawn at the 50% probability level and H atoms are drawn as circles of arbitrary size. The O—H⋯N hydrogen bonds are indicated by dashed lines.
Figure 3.
The molecular structure of compound (III), showing the atom-numbering scheme. Displacement ellipsoids of non-H atoms are drawn at the 50% probability level and H atoms are drawn as circles of arbitrary size. The O—H⋯N hydrogen bonds are indicated by dashed lines.
Table 1. Hydrogen-bond geometry (Å, °) for (I) .
Cg1 and Cg2 are the centroids of the C1–C6 and C10–C15 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N1 | 0.942 (19) | 1.72 (2) | 2.6587 (11) | 177.2 (19) |
| O4—H4⋯N2 | 0.948 (19) | 1.690 (19) | 2.6312 (11) | 171.4 (19) |
| C12—H12⋯O2i | 0.95 | 2.43 | 3.3712 (11) | 172 |
| C14—H14⋯O1ii | 0.95 | 2.56 | 3.2288 (11) | 128 |
| C24—H24⋯O3ii | 0.95 | 2.57 | 3.4407 (12) | 153 |
| C9—H9A⋯Cg2iii | 0.98 | 2.68 | 3.6450 (11) | 169 |
| C18—H18C⋯Cg1iv | 0.98 | 2.67 | 3.6253 (11) | 164 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Table 2. Hydrogen-bond geometry (Å, °) for (II) .
Cg1 and Cg2 are the centroids of the C1–C6 and C11–C16 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N1 | 1.03 (2) | 1.61 (2) | 2.6407 (10) | 174.3 (19) |
| O4—H4⋯N2 | 1.01 (2) | 1.67 (2) | 2.6728 (11) | 173.9 (18) |
| C3—H3⋯O5i | 0.95 | 2.57 | 3.3981 (11) | 146 |
| C25—H25⋯O3ii | 0.95 | 2.57 | 3.4581 (11) | 156 |
| C9—H9B⋯Cg2iii | 0.99 | 2.84 | 3.6750 (1) | 142 |
| C19—H19A⋯Cg1iv | 0.99 | 2.72 | 3.5781 (1) | 146 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  .
.
Table 3. Hydrogen-bond geometry (Å, °) for (III) .
Cg1 and Cg2 are the centroids of the C1–C6 and C12–C17 rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N1 | 1.04 (3) | 1.56 (3) | 2.600 (3) | 173 (3) |
| O4—H4⋯N2 | 1.00 (4) | 1.64 (4) | 2.636 (3) | 172 (4) |
| C24—H24⋯O5i | 0.95 | 2.47 | 3.408 (3) | 171 |
| C29—H29⋯O2ii | 0.95 | 2.53 | 3.456 (3) | 164 |
| C2—H2⋯Cg2iii | 0.95 | 2.98 | 3.754 (3) | 139 |
| C8—H8B⋯Cg2iv | 0.99 | 2.68 | 3.518 (3) | 143 |
| C19—H19B⋯Cg1v | 0.99 | 2.77 | 3.586 (3) | 140 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
The dihedral angles between the pyridine rings of 4,4′-bipyridyl are 27.95 (5), 28.84 (4) and 38.76 (12)° for (I), (II) and (III), respectively. The pyridine ring and the carboxyl group hydrogen-bonded to it are twisted slightly to each other. The dihedral angles between the N1/C19–C23 and O1/O2/C7 planes, and the N2/C24–C28 and O4/O5/C16 planes are 6.54 (11) and 10.31 (11)°, respectively, in (I), those between the N1/C21–C25 and O1/O2/C7 planes, and the N2/C26–C30 and O4/O5/C17 planes are 12.13 (10) and 13.96 (10)°, respectively, in (II), and those between the N1/C23–C27 and O1/O2/C7 planes, and the N2/C28–C32 and O4/O5/C18 planes are 13.7 (3) and 8.5 (3)°, respectively, in (III).
The molecular structures of the ethoxy- and propoxybenzoic acids in (I) and (II) are approximately planar. The dihedral angles made by the benzene ring with the carboxyl group and the alkoxy group in each ethoxybenzoic acid in (I) are 9.60 (10), 1.13 (11), 4.48 (9) and 7.57 (9)°, respectively, between the C1–C6 and O1/O2/C7 planes, the C10–C15 and O4/O5/C16 planes, the C1–C6 and O3/C8/C9 planes, and the C10–C15 and O6/C17/C18 planes. The corresponding dihedral angles in (II) are 2.42 (10), 2.48 (10), 2.96 (7) and 5.82 (7)°, respectively, between the C1–C6 and O1/O2/C7 planes, the C11–C16 and O4/O5/C17 planes, the C1–C6 and O3/C8/C9/C10 planes, and the C11–C16 and O6/C18/C19/C20 planes. The butoxybenzoic acid molecules in (III) are also planar, except for the terminal ethyl groups which deviate from the molecular plane with dihedral angles of 66.6 (3) and 60.7 (3)°, respectively, between the C4/O3/C8 and C9/C10/C11planes, and the C15/O6/C19 and C20/C21/C22 planes. The dihedral angles made by the benzene ring with the carboxyl group and the alkoxy group are 5.6 (3), 5.4 (3), 5.2 (2) and 4.3 (2)°, respectively, between the C1–C6 and O1/O2/C7 planes, the C12–C17 and O4/O5/C18 planes, the C1–C6 and O3/C8/C9 planes, and the C11–C16 and O6/C19/C20 planes.
Supramolecular features
In the crystal of (I), the 2:1 units are linked by C—H⋯O hydrogen bonds (Table 1 ▸), forming a sheet structure parallel to (103) (Fig. 4 ▸). In addition, the units are stacked in a column through π–π interactions between the acid and base rings along the a axis (Fig. 5 ▸). The centroid–centroid distances between the C1–C6 and N1/C19–C23(x − 1, y, z) rings, and between the C10–C15 and N2/C24–C28 (x + 1, y, z) rings are 3.7052 (5) and 3.7752 (6) Å, respectively. C—H⋯π interactions (Table 1 ▸) are also observed between the columns and between the sheets.
Figure 4.
A partial packing diagram of compound (I), showing the sheet structure formed by O—H⋯N and C—H⋯O hydrogen bonds (dashed lines). H atoms not involved in the hydrogen bonds have been omitted. [Symmetry codes: (i) −x + 1, −y + 1, −z + 1; (ii) x +  , −y +
, −y +  , z −
, z −  .]
.]
Figure 5.
A partial packing diagram of compound (I), showing the column structure formed by π–π stacking interactions (dashed lines). H atoms not involved in the O—H⋯N hydrogen bonds have been omitted. [Symmetry codes: (iii) x + 1, y, z; (iv) x − 1, y, z.]
In the crystal of (II) and (III), the 2:1 units are linked by C—H⋯O interactions (Tables 2 ▸ and 3 ▸), forming a double-tape structure along the a axis (Fig. 6 ▸) and a tape structure along the b axis (Fig. 7 ▸), respectively. Between the tapes in (II) and (III) C—H⋯π interactions are observed (Tables 2 ▸ and 3 ▸). A packing diagram of (III) viewed along the a axis, which is approximately perpendicular to the mean plane of the 2:1 unit, is shown in Fig. 8 ▸. The units are arranged into a layer parallel to the bc plane, which leads to a smectic structure. On the other hand, no such a layer structure is observed in compounds (I) and (II), which form nematic liquid phases.
Figure 6.
A partial packing diagram of compound (II), showing the double-tape structure formed by C—H⋯O interactions. H atoms not involved in the C—H⋯O and O—H⋯N hydrogen bonds (dashed lines) have been omitted. [Symmetry codes: (i) −x + 1, −y + 2, −z; (ii) −x, −y + 2, −z.]
Figure 7.
A partial packing diagram of compound (III), showing the tape structure formed by C—H⋯O interactions. H atoms not involved in the C—H⋯O and O—H⋯N hydrogen bonds (dashed lines) have been omitted. [Symmetry codes: (i) x, y − 1, z; (ii) x, y + 1, z.]
Figure 8.
A packing diagram of compound (III) viewed along the a axis, showing a layer aggregate. H atoms not involved in the O—H⋯N hydrogen bonds (dashed lines) have been omitted.
Database survey
A search of the Cambridge Structural Database (Version 5.36, last update February 2015; Groom & Allen, 2014 ▸) for co-crystals of 4,4′-bipyridyl with 4-alkoxybenzoic acid gave five structures (refcodes: NOPXIZ, ORASAC, RIRGUV, YAKVAI and YANCUM), except for 4-methoxybenzoic acid–4,4′-bipyridyl (2/1) and 4-ethoxybenzoic acid–4,4′-bipyridyl (2/1). Of these compounds, NOPXIZ, 4-[(S)-(−)-2-methylbutoxy]benzoic acid–4,4′-bipyridyl (2/10, shows smectic A and nematic phases (Grunert et al., 1997 ▸).
Synthesis and crystallization
Single crystals of compound (I) were obtained by slow evaporation from an acetone solution (150 ml) of 4,4′-bipyridyl (70 mg) with 4-ethoxybenzoic acid (150 mg) at room temperature. Crystals of compounds (II) and (III) were obtained from ethanol solutions of 4,4′-bipyridyl with 4-n-propoxybenzoic acid and 4-n-butoxybenzoic acid, respectively, at room temperature [ethanol solution (150 ml) of 4,4′-bipyridyl (65 mg) and 4-n-propoxybenzoic acid (150 mg) for (II), and ethanol solution (150 ml) of 4,4′-bipyridyl (60 mg) and 4-n-butoxybenzoic acid (150 mg) for (III)].
Liquid crystalline phases of these compounds were confirmed by measurements of DSC (differential scanning calorimetry) and polarizing microscope. DSC measurements were performed by using Perkin Elmer Pyris 1 in the temperature range from 103 K to the melting temperature at a heating rate of 10 K min−1. Phase transition temperatures (K) and enthalpies (kJ mol−1) determined by DSC are as follows:
(I) 373 (2) [5.4 (4)] K1 → K2, 424 (1) [50 (3)] K2 → N, 442 (1) [7.2 (6)] N → I;
(II) 365 (1) [2.9 (6)] K1 → K2, 369 (1) [3.9 (2)] K2 → K3, 417 (1) [39 (1)] K3 → N, 430 (1) [5.7 (2)] N → I;
(III) 358 (1) [2.5 (2)] K1 → K2, 386 (1) [0.30 (3)] K2 → K3, 403 (1) [11.1 (5)] K3 → K4, 407 (1) [24.5 (6)] K4 → SA, 425 (1) [2.2 (6)] SA → N, 432 (1) [6.4 (1)] N → I.
Ki, SA, N and I denote crystal, smectic A, nematic and isotropic phases, respectively. The observed transition temperatures and enthalpies from the solid phase to the liquid crystalline phase are in good agreement with those reported Kato et al. (1990 ▸, 1993 ▸). Some unreported thermal anomalies, 373 (2) K for (I), 365 (1) and 369 (1) K for (II), and 358 (1) and 386 (1) K for (III), were also observed.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 4 ▸. For all compounds, C-bound H atoms were positioned geometrically with C—H = 0.95–0.99 Å and were refined as riding with U iso(H) = 1.2U eq(C) or 1.5U eq(methyl C). The O-bound H atoms were located in a difference Fourier map and refined freely [refined O—H = 0.942 (19)–1.04 (3) Å].
Table 4. Experimental details.
| (I) | (II) | (III) | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | 2C9H10O3·C10H8N2 | 2C10H12O3·C10H8N2 | 2C11H14O3·C10H8N2 |
| M r | 488.52 | 516.57 | 544.63 |
| Crystal system, space group | Monoclinic, P21/n | Triclinic, P

|
Triclinic, P

|
| Temperature (K) | 93 | 93 | 93 |
| a, b, c (Å) | 9.1090 (2), 20.9348 (5), 12.8738 (4) | 10.7592 (4), 10.8838 (3), 11.6462 (4) | 7.6645 (10), 8.5087 (13), 22.606 (3) |
| α, β, γ (°) | 90, 102.9429 (10), 90 | 86.6411 (11), 89.2313 (13), 73.8867 (12) | 80.498 (3), 86.486 (3), 80.082 (3) |
| V (Å3) | 2392.60 (11) | 1307.95 (8) | 1431.5 (4) |
| Z | 4 | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 0.10 | 0.09 | 0.09 |
| Crystal size (mm) | 0.28 × 0.25 × 0.10 | 0.50 × 0.40 × 0.10 | 0.53 × 0.41 × 0.11 |
| Data collection | |||
| Diffractometer | Rigaku R-AXIS RAPIDII | Rigaku R-AXIS RAPIDII | Rigaku R-AXIS RAPIDIIr |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 28629, 6941, 6004 | 15909, 7507, 5980 | 12433, 5612, 3432 |
| R int | 0.035 | 0.069 | 0.075 |
| (sin θ/λ)max (Å−1) | 0.703 | 0.703 | 0.617 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.045, 0.127, 1.04 | 0.047, 0.135, 1.04 | 0.069, 0.193, 1.01 |
| No. of reflections | 6941 | 7507 | 5610 |
| No. of parameters | 335 | 354 | 371 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.39, −0.37 | 0.35, −0.32 | 0.24, −0.41 |
Supplementary Material
Crystal structure: contains datablock(s) I, II, III, General. DOI: 10.1107/S2056989015018435/lh5794sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015018435/lh5794Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989015018435/lh5794IIsup3.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989015018435/lh5794IIIsup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Crystal data
| 2C9H10O3·C10H8N2 | F(000) = 1032.00 |
| Mr = 488.52 | Dx = 1.356 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71075 Å |
| a = 9.1090 (2) Å | Cell parameters from 23666 reflections |
| b = 20.9348 (5) Å | θ = 3.0–30.0° |
| c = 12.8738 (4) Å | µ = 0.10 mm−1 |
| β = 102.9429 (10)° | T = 93 K |
| V = 2392.60 (11) Å3 | Needle, colorless |
| Z = 4 | 0.28 × 0.25 × 0.10 mm |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Data collection
| Rigaku R-AXIS RAPIDII diffractometer | Rint = 0.035 |
| Detector resolution: 10.000 pixels mm-1 | θmax = 30.0° |
| ω scans | h = −12→12 |
| 28629 measured reflections | k = −28→29 |
| 6941 independent reflections | l = −18→18 |
| 6004 reflections with I > 2σ(I) |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: mixed |
| wR(F2) = 0.127 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0817P)2 + 0.3767P] where P = (Fo2 + 2Fc2)/3 |
| 6941 reflections | (Δ/σ)max = 0.001 |
| 335 parameters | Δρmax = 0.39 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Reflections were merged by SHELXL according to the crystal class for the calculation of statistics and refinement. |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.33398 (8) | 0.30866 (3) | 0.77777 (6) | 0.02333 (16) | |
| O2 | −0.37344 (8) | 0.40959 (3) | 0.72097 (6) | 0.02177 (15) | |
| O3 | −0.92113 (7) | 0.36336 (3) | 0.94659 (6) | 0.01856 (14) | |
| O4 | 0.74748 (8) | 0.35124 (3) | 0.34692 (6) | 0.02249 (16) | |
| O5 | 0.77838 (8) | 0.45585 (3) | 0.38227 (6) | 0.02363 (16) | |
| O6 | 1.33565 (7) | 0.38813 (3) | 0.17479 (6) | 0.01967 (15) | |
| N1 | −0.10882 (9) | 0.31350 (4) | 0.67751 (7) | 0.02016 (17) | |
| N2 | 0.51947 (9) | 0.35033 (4) | 0.44136 (7) | 0.02191 (17) | |
| C1 | −0.54350 (10) | 0.36269 (4) | 0.81521 (7) | 0.01557 (17) | |
| C2 | −0.57223 (10) | 0.31282 (4) | 0.88043 (7) | 0.01700 (17) | |
| H2 | −0.5048 | 0.2777 | 0.8950 | 0.020* | |
| C3 | −0.69860 (10) | 0.31438 (4) | 0.92393 (8) | 0.01764 (17) | |
| H3 | −0.7169 | 0.2806 | 0.9686 | 0.021* | |
| C4 | −0.79903 (10) | 0.36588 (4) | 0.90180 (7) | 0.01553 (17) | |
| C5 | −0.77115 (10) | 0.41585 (4) | 0.83675 (7) | 0.01658 (17) | |
| H5 | −0.8388 | 0.4509 | 0.8216 | 0.020* | |
| C6 | −0.64371 (10) | 0.41374 (4) | 0.79445 (7) | 0.01672 (17) | |
| H6 | −0.6246 | 0.4478 | 0.7505 | 0.020* | |
| C7 | −0.40997 (10) | 0.36318 (4) | 0.76677 (7) | 0.01667 (17) | |
| C8 | −1.02432 (10) | 0.41622 (4) | 0.92520 (8) | 0.01880 (18) | |
| H8A | −1.0723 | 0.4179 | 0.8482 | 0.023* | |
| H8B | −0.9696 | 0.4568 | 0.9453 | 0.023* | |
| C9 | −1.14267 (11) | 0.40745 (5) | 0.98921 (8) | 0.02217 (19) | |
| H9A | −1.0945 | 0.4070 | 1.0653 | 0.033* | |
| H9B | −1.1954 | 0.3669 | 0.9695 | 0.033* | |
| H9C | −1.2150 | 0.4427 | 0.9744 | 0.033* | |
| C10 | 0.95579 (10) | 0.40171 (4) | 0.30216 (7) | 0.01621 (17) | |
| C11 | 1.04428 (10) | 0.45571 (4) | 0.30003 (8) | 0.01774 (17) | |
| H11 | 1.0160 | 0.4949 | 0.3273 | 0.021* | |
| C12 | 1.17334 (10) | 0.45373 (4) | 0.25886 (8) | 0.01782 (18) | |
| H12 | 1.2328 | 0.4910 | 0.2585 | 0.021* | |
| C13 | 1.21379 (10) | 0.39607 (4) | 0.21808 (7) | 0.01639 (17) | |
| C14 | 1.12647 (10) | 0.34139 (4) | 0.22033 (8) | 0.01837 (18) | |
| H14 | 1.1544 | 0.3022 | 0.1928 | 0.022* | |
| C15 | 0.99956 (10) | 0.34406 (4) | 0.26245 (8) | 0.01753 (17) | |
| H15 | 0.9416 | 0.3065 | 0.2645 | 0.021* | |
| C16 | 0.81942 (10) | 0.40630 (5) | 0.34748 (7) | 0.01747 (17) | |
| C17 | 1.43238 (10) | 0.44212 (5) | 0.17318 (8) | 0.02026 (19) | |
| H17A | 1.4830 | 0.4543 | 0.2468 | 0.024* | |
| H17B | 1.3729 | 0.4791 | 0.1388 | 0.024* | |
| C18 | 1.54764 (11) | 0.42313 (5) | 0.11099 (8) | 0.0229 (2) | |
| H18A | 1.5998 | 0.3843 | 0.1421 | 0.034* | |
| H18B | 1.6209 | 0.4578 | 0.1139 | 0.034* | |
| H18C | 1.4970 | 0.4150 | 0.0366 | 0.034* | |
| C19 | −0.09136 (10) | 0.36667 (5) | 0.62319 (8) | 0.01935 (18) | |
| H19 | −0.1662 | 0.3990 | 0.6159 | 0.023* | |
| C20 | 0.03056 (10) | 0.37669 (5) | 0.57728 (7) | 0.01765 (17) | |
| H20 | 0.0388 | 0.4153 | 0.5402 | 0.021* | |
| C21 | 0.14130 (10) | 0.32950 (4) | 0.58603 (7) | 0.01586 (17) | |
| C22 | 0.12277 (10) | 0.27418 (5) | 0.64248 (8) | 0.01962 (18) | |
| H22 | 0.1954 | 0.2409 | 0.6508 | 0.024* | |
| C23 | −0.00263 (11) | 0.26821 (5) | 0.68641 (8) | 0.02095 (19) | |
| H23 | −0.0137 | 0.2303 | 0.7245 | 0.025* | |
| C24 | 0.46423 (11) | 0.29264 (5) | 0.45714 (9) | 0.0242 (2) | |
| H24 | 0.5105 | 0.2559 | 0.4352 | 0.029* | |
| C25 | 0.34301 (11) | 0.28424 (5) | 0.50393 (9) | 0.0228 (2) | |
| H25 | 0.3080 | 0.2424 | 0.5140 | 0.027* | |
| C26 | 0.27224 (10) | 0.33746 (4) | 0.53636 (7) | 0.01618 (17) | |
| C27 | 0.32923 (10) | 0.39752 (4) | 0.51958 (8) | 0.01849 (18) | |
| H27 | 0.2847 | 0.4352 | 0.5400 | 0.022* | |
| C28 | 0.45223 (11) | 0.40137 (5) | 0.47250 (8) | 0.02086 (19) | |
| H28 | 0.4906 | 0.4425 | 0.4619 | 0.025* | |
| H1 | −0.254 (2) | 0.3118 (9) | 0.7426 (17) | 0.059 (5)* | |
| H4 | 0.663 (2) | 0.3549 (10) | 0.3782 (17) | 0.066 (6)* |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0216 (3) | 0.0188 (3) | 0.0345 (4) | 0.0023 (3) | 0.0167 (3) | 0.0000 (3) |
| O2 | 0.0200 (3) | 0.0207 (3) | 0.0274 (4) | −0.0013 (3) | 0.0114 (3) | 0.0013 (3) |
| O3 | 0.0148 (3) | 0.0197 (3) | 0.0238 (3) | 0.0017 (2) | 0.0099 (2) | 0.0022 (2) |
| O4 | 0.0194 (3) | 0.0226 (3) | 0.0293 (4) | −0.0033 (3) | 0.0136 (3) | −0.0007 (3) |
| O5 | 0.0217 (3) | 0.0227 (3) | 0.0293 (4) | 0.0038 (3) | 0.0117 (3) | −0.0003 (3) |
| O6 | 0.0165 (3) | 0.0182 (3) | 0.0276 (4) | −0.0019 (2) | 0.0120 (3) | −0.0021 (3) |
| N1 | 0.0170 (3) | 0.0235 (4) | 0.0220 (4) | −0.0013 (3) | 0.0086 (3) | −0.0038 (3) |
| N2 | 0.0168 (3) | 0.0281 (4) | 0.0228 (4) | 0.0005 (3) | 0.0088 (3) | 0.0003 (3) |
| C1 | 0.0141 (4) | 0.0167 (4) | 0.0167 (4) | −0.0013 (3) | 0.0053 (3) | −0.0030 (3) |
| C2 | 0.0152 (4) | 0.0166 (4) | 0.0198 (4) | 0.0006 (3) | 0.0052 (3) | −0.0012 (3) |
| C3 | 0.0163 (4) | 0.0169 (4) | 0.0207 (4) | −0.0003 (3) | 0.0062 (3) | 0.0019 (3) |
| C4 | 0.0134 (4) | 0.0176 (4) | 0.0165 (4) | −0.0009 (3) | 0.0054 (3) | −0.0019 (3) |
| C5 | 0.0161 (4) | 0.0157 (4) | 0.0189 (4) | 0.0009 (3) | 0.0059 (3) | −0.0003 (3) |
| C6 | 0.0173 (4) | 0.0165 (4) | 0.0175 (4) | −0.0010 (3) | 0.0061 (3) | −0.0007 (3) |
| C7 | 0.0150 (4) | 0.0179 (4) | 0.0181 (4) | −0.0015 (3) | 0.0058 (3) | −0.0043 (3) |
| C8 | 0.0169 (4) | 0.0185 (4) | 0.0228 (4) | 0.0022 (3) | 0.0082 (3) | −0.0003 (3) |
| C9 | 0.0175 (4) | 0.0280 (5) | 0.0230 (5) | 0.0027 (4) | 0.0087 (3) | 0.0021 (4) |
| C10 | 0.0148 (4) | 0.0179 (4) | 0.0168 (4) | 0.0010 (3) | 0.0051 (3) | 0.0023 (3) |
| C11 | 0.0177 (4) | 0.0159 (4) | 0.0209 (4) | 0.0019 (3) | 0.0070 (3) | 0.0005 (3) |
| C12 | 0.0164 (4) | 0.0156 (4) | 0.0227 (4) | −0.0010 (3) | 0.0070 (3) | 0.0012 (3) |
| C13 | 0.0142 (4) | 0.0176 (4) | 0.0183 (4) | 0.0008 (3) | 0.0056 (3) | 0.0018 (3) |
| C14 | 0.0179 (4) | 0.0150 (4) | 0.0240 (4) | 0.0009 (3) | 0.0084 (3) | −0.0001 (3) |
| C15 | 0.0171 (4) | 0.0159 (4) | 0.0209 (4) | −0.0009 (3) | 0.0069 (3) | 0.0016 (3) |
| C16 | 0.0153 (4) | 0.0207 (4) | 0.0172 (4) | 0.0015 (3) | 0.0053 (3) | 0.0026 (3) |
| C17 | 0.0181 (4) | 0.0192 (4) | 0.0260 (5) | −0.0034 (3) | 0.0104 (4) | −0.0015 (3) |
| C18 | 0.0187 (4) | 0.0272 (5) | 0.0255 (5) | −0.0047 (4) | 0.0108 (4) | −0.0055 (4) |
| C19 | 0.0159 (4) | 0.0230 (4) | 0.0201 (4) | 0.0026 (3) | 0.0061 (3) | −0.0022 (3) |
| C20 | 0.0159 (4) | 0.0205 (4) | 0.0174 (4) | 0.0009 (3) | 0.0057 (3) | −0.0007 (3) |
| C21 | 0.0134 (4) | 0.0196 (4) | 0.0153 (4) | −0.0008 (3) | 0.0047 (3) | −0.0034 (3) |
| C22 | 0.0173 (4) | 0.0200 (4) | 0.0233 (4) | 0.0022 (3) | 0.0084 (3) | 0.0000 (3) |
| C23 | 0.0204 (4) | 0.0212 (4) | 0.0237 (4) | −0.0006 (3) | 0.0102 (4) | 0.0007 (3) |
| C24 | 0.0212 (4) | 0.0241 (4) | 0.0313 (5) | 0.0035 (4) | 0.0141 (4) | −0.0014 (4) |
| C25 | 0.0218 (4) | 0.0194 (4) | 0.0313 (5) | 0.0000 (4) | 0.0147 (4) | −0.0019 (4) |
| C26 | 0.0139 (4) | 0.0197 (4) | 0.0160 (4) | 0.0009 (3) | 0.0055 (3) | −0.0010 (3) |
| C27 | 0.0186 (4) | 0.0194 (4) | 0.0191 (4) | 0.0006 (3) | 0.0077 (3) | −0.0007 (3) |
| C28 | 0.0201 (4) | 0.0227 (4) | 0.0218 (4) | −0.0028 (3) | 0.0090 (3) | 0.0000 (3) |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Geometric parameters (Å, º)
| O1—C7 | 1.3259 (11) | C10—C16 | 1.4891 (12) |
| O1—H1 | 0.942 (19) | C11—C12 | 1.3941 (12) |
| O2—C7 | 1.2208 (12) | C11—H11 | 0.9500 |
| O3—C4 | 1.3637 (10) | C12—C13 | 1.3984 (13) |
| O3—C8 | 1.4380 (11) | C12—H12 | 0.9500 |
| O4—C16 | 1.3252 (12) | C13—C14 | 1.3979 (12) |
| O4—H4 | 0.948 (19) | C14—C15 | 1.3836 (12) |
| O5—C16 | 1.2213 (12) | C14—H14 | 0.9500 |
| O6—C13 | 1.3592 (10) | C15—H15 | 0.9500 |
| O6—C17 | 1.4361 (11) | C17—C18 | 1.5092 (13) |
| N1—C23 | 1.3409 (13) | C17—H17A | 0.9900 |
| N1—C19 | 1.3429 (13) | C17—H17B | 0.9900 |
| N2—C28 | 1.3370 (13) | C18—H18A | 0.9800 |
| N2—C24 | 1.3412 (14) | C18—H18B | 0.9800 |
| C1—C6 | 1.3920 (12) | C18—H18C | 0.9800 |
| C1—C2 | 1.4006 (13) | C19—C20 | 1.3859 (12) |
| C1—C7 | 1.4860 (12) | C19—H19 | 0.9500 |
| C2—C3 | 1.3887 (12) | C20—C21 | 1.3983 (12) |
| C2—H2 | 0.9500 | C20—H20 | 0.9500 |
| C3—C4 | 1.4012 (12) | C21—C22 | 1.3974 (13) |
| C3—H3 | 0.9500 | C21—C26 | 1.4835 (12) |
| C4—C5 | 1.3984 (12) | C22—C23 | 1.3892 (12) |
| C5—C6 | 1.3890 (12) | C22—H22 | 0.9500 |
| C5—H5 | 0.9500 | C23—H23 | 0.9500 |
| C6—H6 | 0.9500 | C24—C25 | 1.3825 (13) |
| C8—C9 | 1.5077 (13) | C24—H24 | 0.9500 |
| C8—H8A | 0.9900 | C25—C26 | 1.3971 (13) |
| C8—H8B | 0.9900 | C25—H25 | 0.9500 |
| C9—H9A | 0.9800 | C26—C27 | 1.3956 (13) |
| C9—H9B | 0.9800 | C27—C28 | 1.3905 (12) |
| C9—H9C | 0.9800 | C27—H27 | 0.9500 |
| C10—C11 | 1.3923 (12) | C28—H28 | 0.9500 |
| C10—C15 | 1.4030 (12) | ||
| C7—O1—H1 | 109.2 (12) | C15—C14—C13 | 120.28 (8) |
| C4—O3—C8 | 116.70 (7) | C15—C14—H14 | 119.9 |
| C16—O4—H4 | 112.0 (13) | C13—C14—H14 | 119.9 |
| C13—O6—C17 | 118.02 (7) | C14—C15—C10 | 120.54 (8) |
| C23—N1—C19 | 117.60 (8) | C14—C15—H15 | 119.7 |
| C28—N2—C24 | 117.50 (8) | C10—C15—H15 | 119.7 |
| C6—C1—C2 | 119.00 (8) | O5—C16—O4 | 123.31 (8) |
| C6—C1—C7 | 118.51 (8) | O5—C16—C10 | 123.32 (8) |
| C2—C1—C7 | 122.49 (8) | O4—C16—C10 | 113.37 (8) |
| C3—C2—C1 | 120.48 (8) | O6—C17—C18 | 107.60 (8) |
| C3—C2—H2 | 119.8 | O6—C17—H17A | 110.2 |
| C1—C2—H2 | 119.8 | C18—C17—H17A | 110.2 |
| C2—C3—C4 | 119.90 (8) | O6—C17—H17B | 110.2 |
| C2—C3—H3 | 120.0 | C18—C17—H17B | 110.2 |
| C4—C3—H3 | 120.0 | H17A—C17—H17B | 108.5 |
| O3—C4—C5 | 123.84 (8) | C17—C18—H18A | 109.5 |
| O3—C4—C3 | 116.20 (8) | C17—C18—H18B | 109.5 |
| C5—C4—C3 | 119.96 (8) | H18A—C18—H18B | 109.5 |
| C6—C5—C4 | 119.40 (8) | C17—C18—H18C | 109.5 |
| C6—C5—H5 | 120.3 | H18A—C18—H18C | 109.5 |
| C4—C5—H5 | 120.3 | H18B—C18—H18C | 109.5 |
| C5—C6—C1 | 121.25 (8) | N1—C19—C20 | 123.18 (8) |
| C5—C6—H6 | 119.4 | N1—C19—H19 | 118.4 |
| C1—C6—H6 | 119.4 | C20—C19—H19 | 118.4 |
| O2—C7—O1 | 123.00 (8) | C19—C20—C21 | 119.42 (9) |
| O2—C7—C1 | 123.05 (8) | C19—C20—H20 | 120.3 |
| O1—C7—C1 | 113.95 (8) | C21—C20—H20 | 120.3 |
| O3—C8—C9 | 108.56 (8) | C22—C21—C20 | 117.29 (8) |
| O3—C8—H8A | 110.0 | C22—C21—C26 | 121.30 (8) |
| C9—C8—H8A | 110.0 | C20—C21—C26 | 121.41 (8) |
| O3—C8—H8B | 110.0 | C23—C22—C21 | 119.50 (8) |
| C9—C8—H8B | 110.0 | C23—C22—H22 | 120.2 |
| H8A—C8—H8B | 108.4 | C21—C22—H22 | 120.2 |
| C8—C9—H9A | 109.5 | N1—C23—C22 | 123.01 (9) |
| C8—C9—H9B | 109.5 | N1—C23—H23 | 118.5 |
| H9A—C9—H9B | 109.5 | C22—C23—H23 | 118.5 |
| C8—C9—H9C | 109.5 | N2—C24—C25 | 122.95 (9) |
| H9A—C9—H9C | 109.5 | N2—C24—H24 | 118.5 |
| H9B—C9—H9C | 109.5 | C25—C24—H24 | 118.5 |
| C11—C10—C15 | 118.58 (8) | C24—C25—C26 | 119.73 (9) |
| C11—C10—C16 | 119.52 (8) | C24—C25—H25 | 120.1 |
| C15—C10—C16 | 121.89 (8) | C26—C25—H25 | 120.1 |
| C10—C11—C12 | 121.64 (8) | C27—C26—C25 | 117.37 (8) |
| C10—C11—H11 | 119.2 | C27—C26—C21 | 122.05 (8) |
| C12—C11—H11 | 119.2 | C25—C26—C21 | 120.57 (8) |
| C11—C12—C13 | 118.94 (8) | C28—C27—C26 | 118.92 (8) |
| C11—C12—H12 | 120.5 | C28—C27—H27 | 120.5 |
| C13—C12—H12 | 120.5 | C26—C27—H27 | 120.5 |
| O6—C13—C14 | 115.41 (8) | N2—C28—C27 | 123.53 (9) |
| O6—C13—C12 | 124.58 (8) | N2—C28—H28 | 118.2 |
| C14—C13—C12 | 120.01 (8) | C27—C28—H28 | 118.2 |
| C6—C1—C2—C3 | −0.16 (13) | C11—C10—C15—C14 | −1.18 (14) |
| C7—C1—C2—C3 | −179.95 (8) | C16—C10—C15—C14 | 179.68 (8) |
| C1—C2—C3—C4 | 0.57 (14) | C11—C10—C16—O5 | 1.11 (14) |
| C8—O3—C4—C5 | −0.72 (13) | C15—C10—C16—O5 | −179.75 (9) |
| C8—O3—C4—C3 | 179.48 (8) | C11—C10—C16—O4 | −178.52 (8) |
| C2—C3—C4—O3 | 179.25 (8) | C15—C10—C16—O4 | 0.62 (13) |
| C2—C3—C4—C5 | −0.56 (14) | C13—O6—C17—C18 | −173.89 (8) |
| O3—C4—C5—C6 | −179.65 (8) | C23—N1—C19—C20 | −0.45 (14) |
| C3—C4—C5—C6 | 0.14 (14) | N1—C19—C20—C21 | 0.69 (14) |
| C4—C5—C6—C1 | 0.28 (14) | C19—C20—C21—C22 | −0.57 (13) |
| C2—C1—C6—C5 | −0.27 (14) | C19—C20—C21—C26 | 178.73 (8) |
| C7—C1—C6—C5 | 179.53 (8) | C20—C21—C22—C23 | 0.28 (14) |
| C6—C1—C7—O2 | 9.58 (13) | C26—C21—C22—C23 | −179.03 (9) |
| C2—C1—C7—O2 | −170.62 (9) | C19—N1—C23—C22 | 0.13 (15) |
| C6—C1—C7—O1 | −170.43 (8) | C21—C22—C23—N1 | −0.05 (15) |
| C2—C1—C7—O1 | 9.37 (13) | C28—N2—C24—C25 | −0.27 (16) |
| C4—O3—C8—C9 | −174.93 (8) | N2—C24—C25—C26 | 0.41 (17) |
| C15—C10—C11—C12 | 0.53 (14) | C24—C25—C26—C27 | −0.13 (15) |
| C16—C10—C11—C12 | 179.70 (8) | C24—C25—C26—C21 | 179.56 (9) |
| C10—C11—C12—C13 | 0.48 (14) | C22—C21—C26—C27 | −152.77 (9) |
| C17—O6—C13—C14 | −178.14 (8) | C20—C21—C26—C27 | 27.95 (13) |
| C17—O6—C13—C12 | 1.77 (13) | C22—C21—C26—C25 | 27.55 (13) |
| C11—C12—C13—O6 | 179.23 (9) | C20—C21—C26—C25 | −151.72 (10) |
| C11—C12—C13—C14 | −0.87 (14) | C25—C26—C27—C28 | −0.26 (14) |
| O6—C13—C14—C15 | −179.85 (8) | C21—C26—C27—C28 | −179.94 (9) |
| C12—C13—C14—C15 | 0.24 (14) | C24—N2—C28—C27 | −0.16 (15) |
| C13—C14—C15—C10 | 0.80 (14) | C26—C27—C28—N2 | 0.43 (15) |
(I) 4-Ethoxybenzoic acid–4,4'-bipyridyl (2/1). Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C1–C6 and C10–C15 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N1 | 0.942 (19) | 1.72 (2) | 2.6587 (11) | 177.2 (19) |
| O4—H4···N2 | 0.948 (19) | 1.690 (19) | 2.6312 (11) | 171.4 (19) |
| C12—H12···O2i | 0.95 | 2.43 | 3.3712 (11) | 172 |
| C14—H14···O1ii | 0.95 | 2.56 | 3.2288 (11) | 128 |
| C24—H24···O3ii | 0.95 | 2.57 | 3.4407 (12) | 153 |
| C9—H9A···Cg2iii | 0.98 | 2.68 | 3.6450 (11) | 169 |
| C18—H18C···Cg1iv | 0.98 | 2.67 | 3.6253 (11) | 164 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x+3/2, −y+1/2, z−1/2; (iii) x−2, y, z+1; (iv) x+2, y, z−1.
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Crystal data
| 2C10H12O3·C10H8N2 | Z = 2 |
| Mr = 516.57 | F(000) = 548.00 |
| Triclinic, P1 | Dx = 1.312 Mg m−3 |
| a = 10.7592 (4) Å | Mo Kα radiation, λ = 0.71075 Å |
| b = 10.8838 (3) Å | Cell parameters from 13551 reflections |
| c = 11.6462 (4) Å | θ = 3.0–30.0° |
| α = 86.6411 (11)° | µ = 0.09 mm−1 |
| β = 89.2313 (13)° | T = 93 K |
| γ = 73.8867 (12)° | Block, colorless |
| V = 1307.95 (8) Å3 | 0.50 × 0.40 × 0.10 mm |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Data collection
| Rigaku R-AXIS RAPIDII diffractometer | Rint = 0.069 |
| Detector resolution: 10.000 pixels mm-1 | θmax = 30.0°, θmin = 3.0° |
| ω scans | h = −15→15 |
| 15909 measured reflections | k = −13→15 |
| 7507 independent reflections | l = −16→16 |
| 5980 reflections with I > 2σ(I) |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.047 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.135 | w = 1/[σ2(Fo2) + (0.090P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.001 |
| 7507 reflections | Δρmax = 0.35 e Å−3 |
| 354 parameters | Δρmin = −0.32 e Å−3 |
| 0 restraints | Extinction correction: SHELXL-2014/7, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.019 (4) |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Reflections were merged by SHELXL according to the crystal class for the calculation of statistics and refinement. |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.21741 (6) | 0.84171 (6) | −0.00993 (6) | 0.02370 (16) | |
| O2 | 0.26015 (7) | 0.62711 (6) | 0.01082 (6) | 0.02497 (16) | |
| O3 | −0.31475 (6) | 0.79522 (6) | −0.19102 (6) | 0.02084 (15) | |
| O4 | 1.32849 (7) | 0.66282 (6) | 0.36794 (6) | 0.02574 (16) | |
| O5 | 1.28863 (7) | 0.87599 (6) | 0.33722 (6) | 0.02456 (15) | |
| O6 | 1.86048 (6) | 0.70388 (6) | 0.55057 (5) | 0.01982 (14) | |
| N1 | 0.44816 (7) | 0.80971 (7) | 0.08178 (6) | 0.01957 (16) | |
| N2 | 1.08835 (7) | 0.69161 (7) | 0.29159 (6) | 0.01975 (16) | |
| C1 | 0.05619 (8) | 0.74854 (8) | −0.06643 (7) | 0.01587 (16) | |
| C2 | −0.02538 (8) | 0.86942 (8) | −0.09987 (7) | 0.01713 (16) | |
| H2 | 0.0038 | 0.9435 | −0.0934 | 0.021* | |
| C3 | −0.14819 (8) | 0.88217 (8) | −0.14234 (7) | 0.01796 (17) | |
| H3 | −0.2024 | 0.9644 | −0.1660 | 0.022* | |
| C4 | −0.19207 (8) | 0.77306 (8) | −0.15022 (7) | 0.01676 (17) | |
| C5 | −0.11202 (8) | 0.65191 (8) | −0.11692 (7) | 0.01732 (16) | |
| H5 | −0.1416 | 0.5779 | −0.1222 | 0.021* | |
| C6 | 0.01143 (8) | 0.64099 (8) | −0.07598 (7) | 0.01671 (16) | |
| H6 | 0.0665 | 0.5586 | −0.0541 | 0.020* | |
| C7 | 0.18746 (8) | 0.73163 (8) | −0.01851 (7) | 0.01780 (17) | |
| C8 | −0.36390 (8) | 0.68649 (8) | −0.20224 (7) | 0.01802 (17) | |
| H8A | −0.3609 | 0.6385 | −0.1270 | 0.022* | |
| H8B | −0.3108 | 0.6283 | −0.2576 | 0.022* | |
| C9 | −0.50170 (9) | 0.73524 (9) | −0.24460 (8) | 0.02173 (18) | |
| H9B | −0.5034 | 0.7767 | −0.3228 | 0.026* | |
| H9A | −0.5522 | 0.7999 | −0.1931 | 0.026* | |
| C10 | −0.56236 (9) | 0.62384 (9) | −0.24724 (10) | 0.0281 (2) | |
| H10A | −0.6465 | 0.6532 | −0.2862 | 0.042* | |
| H10B | −0.5742 | 0.5925 | −0.1684 | 0.042* | |
| H10C | −0.5053 | 0.5544 | −0.2889 | 0.042* | |
| C11 | 1.49360 (8) | 0.75522 (8) | 0.41394 (7) | 0.01674 (16) | |
| C12 | 1.57341 (9) | 0.63400 (8) | 0.44879 (7) | 0.01858 (17) | |
| H12 | 1.5434 | 0.5605 | 0.4418 | 0.022* | |
| C13 | 1.69517 (9) | 0.62010 (8) | 0.49316 (7) | 0.01892 (17) | |
| H13 | 1.7485 | 0.5373 | 0.5164 | 0.023* | |
| C14 | 1.74023 (8) | 0.72791 (8) | 0.50403 (7) | 0.01712 (17) | |
| C15 | 1.66235 (9) | 0.84947 (8) | 0.46796 (7) | 0.01804 (17) | |
| H15 | 1.6927 | 0.9229 | 0.4740 | 0.022* | |
| C16 | 1.54008 (8) | 0.86179 (8) | 0.42315 (7) | 0.01771 (17) | |
| H16 | 1.4873 | 0.9443 | 0.3984 | 0.021* | |
| C17 | 1.36100 (9) | 0.77237 (8) | 0.36890 (7) | 0.01841 (17) | |
| C18 | 1.91667 (8) | 0.80929 (8) | 0.55338 (7) | 0.01815 (17) | |
| H18A | 1.8635 | 0.8761 | 0.6016 | 0.022* | |
| H18B | 1.9206 | 0.8477 | 0.4747 | 0.022* | |
| C19 | 2.05137 (9) | 0.75818 (9) | 0.60313 (8) | 0.02037 (18) | |
| H19B | 2.1028 | 0.6888 | 0.5564 | 0.024* | |
| H19A | 2.0465 | 0.7220 | 0.6825 | 0.024* | |
| C20 | 2.11746 (9) | 0.86578 (9) | 0.60422 (9) | 0.0277 (2) | |
| H20A | 2.0643 | 0.9360 | 0.6475 | 0.042* | |
| H20B | 2.1277 | 0.8972 | 0.5251 | 0.042* | |
| H20C | 2.2027 | 0.8330 | 0.6408 | 0.042* | |
| C21 | 0.52853 (9) | 0.69053 (9) | 0.08870 (8) | 0.02069 (18) | |
| H21 | 0.4970 | 0.6220 | 0.0666 | 0.025* | |
| C22 | 0.65531 (8) | 0.66285 (8) | 0.12658 (8) | 0.01907 (17) | |
| H22 | 0.7088 | 0.5771 | 0.1302 | 0.023* | |
| C23 | 0.70378 (8) | 0.76194 (8) | 0.15928 (7) | 0.01608 (16) | |
| C24 | 0.61977 (8) | 0.88583 (8) | 0.15197 (7) | 0.01863 (17) | |
| H24 | 0.6488 | 0.9563 | 0.1730 | 0.022* | |
| C25 | 0.49411 (8) | 0.90534 (8) | 0.11387 (7) | 0.01989 (17) | |
| H25 | 0.4380 | 0.9900 | 0.1103 | 0.024* | |
| C26 | 1.02841 (8) | 0.80376 (8) | 0.23616 (7) | 0.02020 (18) | |
| H26 | 1.0730 | 0.8678 | 0.2273 | 0.024* | |
| C27 | 0.90438 (8) | 0.83033 (8) | 0.19124 (7) | 0.01839 (17) | |
| H27 | 0.8655 | 0.9110 | 0.1527 | 0.022* | |
| C28 | 0.83731 (8) | 0.73745 (8) | 0.20317 (7) | 0.01565 (16) | |
| C29 | 0.90043 (8) | 0.62059 (8) | 0.26043 (7) | 0.01836 (17) | |
| H29 | 0.8586 | 0.5544 | 0.2701 | 0.022* | |
| C30 | 1.02416 (9) | 0.60203 (8) | 0.30285 (7) | 0.02023 (18) | |
| H30 | 1.0656 | 0.5222 | 0.3417 | 0.024* | |
| H1 | 0.305 (2) | 0.8292 (19) | 0.0308 (18) | 0.088 (6)* | |
| H4 | 1.240 (2) | 0.6740 (19) | 0.3337 (16) | 0.082 (6)* |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0188 (3) | 0.0171 (3) | 0.0356 (4) | −0.0057 (3) | −0.0076 (3) | 0.0000 (3) |
| O2 | 0.0195 (3) | 0.0176 (3) | 0.0355 (4) | −0.0016 (2) | −0.0077 (3) | 0.0014 (3) |
| O3 | 0.0148 (3) | 0.0155 (3) | 0.0318 (3) | −0.0040 (2) | −0.0071 (2) | 0.0017 (2) |
| O4 | 0.0198 (3) | 0.0180 (3) | 0.0396 (4) | −0.0060 (3) | −0.0084 (3) | 0.0027 (3) |
| O5 | 0.0202 (3) | 0.0193 (3) | 0.0314 (3) | −0.0018 (3) | −0.0051 (3) | 0.0043 (3) |
| O6 | 0.0175 (3) | 0.0163 (3) | 0.0255 (3) | −0.0048 (2) | −0.0064 (2) | 0.0022 (2) |
| N1 | 0.0156 (3) | 0.0211 (4) | 0.0216 (3) | −0.0048 (3) | −0.0021 (3) | 0.0009 (3) |
| N2 | 0.0166 (3) | 0.0199 (3) | 0.0219 (3) | −0.0035 (3) | −0.0022 (3) | −0.0019 (3) |
| C1 | 0.0154 (4) | 0.0154 (4) | 0.0163 (3) | −0.0036 (3) | −0.0007 (3) | 0.0006 (3) |
| C2 | 0.0171 (4) | 0.0139 (3) | 0.0207 (4) | −0.0049 (3) | −0.0016 (3) | 0.0000 (3) |
| C3 | 0.0165 (4) | 0.0132 (3) | 0.0229 (4) | −0.0025 (3) | −0.0028 (3) | 0.0018 (3) |
| C4 | 0.0143 (4) | 0.0161 (4) | 0.0188 (4) | −0.0028 (3) | −0.0019 (3) | 0.0007 (3) |
| C5 | 0.0178 (4) | 0.0139 (4) | 0.0205 (4) | −0.0049 (3) | −0.0018 (3) | 0.0003 (3) |
| C6 | 0.0168 (4) | 0.0134 (3) | 0.0186 (3) | −0.0024 (3) | −0.0017 (3) | 0.0012 (3) |
| C7 | 0.0167 (4) | 0.0173 (4) | 0.0190 (4) | −0.0041 (3) | −0.0011 (3) | −0.0008 (3) |
| C8 | 0.0172 (4) | 0.0164 (4) | 0.0206 (4) | −0.0051 (3) | −0.0024 (3) | 0.0003 (3) |
| C9 | 0.0169 (4) | 0.0194 (4) | 0.0287 (4) | −0.0052 (3) | −0.0052 (3) | 0.0013 (3) |
| C10 | 0.0205 (4) | 0.0241 (4) | 0.0409 (5) | −0.0080 (4) | −0.0064 (4) | −0.0010 (4) |
| C11 | 0.0161 (4) | 0.0166 (4) | 0.0168 (3) | −0.0036 (3) | −0.0004 (3) | 0.0003 (3) |
| C12 | 0.0196 (4) | 0.0153 (4) | 0.0209 (4) | −0.0052 (3) | −0.0017 (3) | 0.0005 (3) |
| C13 | 0.0196 (4) | 0.0136 (4) | 0.0220 (4) | −0.0024 (3) | −0.0031 (3) | 0.0017 (3) |
| C14 | 0.0163 (4) | 0.0168 (4) | 0.0170 (3) | −0.0027 (3) | −0.0011 (3) | 0.0005 (3) |
| C15 | 0.0189 (4) | 0.0146 (4) | 0.0203 (4) | −0.0042 (3) | −0.0012 (3) | 0.0000 (3) |
| C16 | 0.0177 (4) | 0.0148 (4) | 0.0187 (4) | −0.0016 (3) | −0.0006 (3) | 0.0010 (3) |
| C17 | 0.0176 (4) | 0.0185 (4) | 0.0179 (4) | −0.0034 (3) | −0.0003 (3) | 0.0006 (3) |
| C18 | 0.0178 (4) | 0.0161 (4) | 0.0206 (4) | −0.0051 (3) | −0.0021 (3) | 0.0010 (3) |
| C19 | 0.0177 (4) | 0.0193 (4) | 0.0239 (4) | −0.0052 (3) | −0.0035 (3) | 0.0017 (3) |
| C20 | 0.0213 (4) | 0.0252 (4) | 0.0376 (5) | −0.0091 (4) | −0.0056 (4) | 0.0043 (4) |
| C21 | 0.0184 (4) | 0.0204 (4) | 0.0240 (4) | −0.0064 (3) | −0.0024 (3) | −0.0019 (3) |
| C22 | 0.0163 (4) | 0.0164 (4) | 0.0238 (4) | −0.0031 (3) | −0.0019 (3) | −0.0010 (3) |
| C23 | 0.0142 (4) | 0.0172 (4) | 0.0160 (3) | −0.0035 (3) | 0.0002 (3) | 0.0010 (3) |
| C24 | 0.0174 (4) | 0.0158 (4) | 0.0223 (4) | −0.0042 (3) | −0.0012 (3) | −0.0003 (3) |
| C25 | 0.0164 (4) | 0.0180 (4) | 0.0235 (4) | −0.0025 (3) | −0.0016 (3) | 0.0025 (3) |
| C26 | 0.0171 (4) | 0.0214 (4) | 0.0227 (4) | −0.0066 (3) | −0.0014 (3) | 0.0006 (3) |
| C27 | 0.0167 (4) | 0.0167 (4) | 0.0213 (4) | −0.0043 (3) | −0.0016 (3) | 0.0018 (3) |
| C28 | 0.0138 (4) | 0.0164 (4) | 0.0161 (3) | −0.0030 (3) | 0.0000 (3) | −0.0017 (3) |
| C29 | 0.0169 (4) | 0.0157 (4) | 0.0219 (4) | −0.0036 (3) | −0.0018 (3) | −0.0002 (3) |
| C30 | 0.0180 (4) | 0.0175 (4) | 0.0235 (4) | −0.0021 (3) | −0.0037 (3) | −0.0002 (3) |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Geometric parameters (Å, º)
| O1—C7 | 1.3335 (10) | C11—C17 | 1.4853 (12) |
| O1—H1 | 1.03 (2) | C12—C13 | 1.3802 (12) |
| O2—C7 | 1.2208 (11) | C12—H12 | 0.9500 |
| O3—C4 | 1.3612 (10) | C13—C14 | 1.4010 (11) |
| O3—C8 | 1.4368 (10) | C13—H13 | 0.9500 |
| O4—C17 | 1.3331 (11) | C14—C15 | 1.3993 (12) |
| O4—H4 | 1.01 (2) | C15—C16 | 1.3904 (12) |
| O5—C17 | 1.2190 (11) | C15—H15 | 0.9500 |
| O6—C14 | 1.3603 (10) | C16—H16 | 0.9500 |
| O6—C18 | 1.4397 (10) | C18—C19 | 1.5109 (11) |
| N1—C25 | 1.3430 (11) | C18—H18A | 0.9900 |
| N1—C21 | 1.3430 (12) | C18—H18B | 0.9900 |
| N2—C30 | 1.3420 (11) | C19—C20 | 1.5301 (12) |
| N2—C26 | 1.3433 (12) | C19—H19B | 0.9900 |
| C1—C6 | 1.3944 (11) | C19—H19A | 0.9900 |
| C1—C2 | 1.4004 (12) | C20—H20A | 0.9800 |
| C1—C7 | 1.4848 (11) | C20—H20B | 0.9800 |
| C2—C3 | 1.3848 (11) | C20—H20C | 0.9800 |
| C2—H2 | 0.9500 | C21—C22 | 1.3851 (12) |
| C3—C4 | 1.4023 (11) | C21—H21 | 0.9500 |
| C3—H3 | 0.9500 | C22—C23 | 1.3950 (11) |
| C4—C5 | 1.3963 (12) | C22—H22 | 0.9500 |
| C5—C6 | 1.3886 (11) | C23—C24 | 1.3981 (11) |
| C5—H5 | 0.9500 | C23—C28 | 1.4790 (11) |
| C6—H6 | 0.9500 | C24—C25 | 1.3840 (12) |
| C8—C9 | 1.5084 (11) | C24—H24 | 0.9500 |
| C8—H8A | 0.9900 | C25—H25 | 0.9500 |
| C8—H8B | 0.9900 | C26—C27 | 1.3875 (11) |
| C9—C10 | 1.5301 (12) | C26—H26 | 0.9500 |
| C9—H9B | 0.9900 | C27—C28 | 1.3955 (11) |
| C9—H9A | 0.9900 | C27—H27 | 0.9500 |
| C10—H10A | 0.9800 | C28—C29 | 1.3990 (12) |
| C10—H10B | 0.9800 | C29—C30 | 1.3834 (11) |
| C10—H10C | 0.9800 | C29—H29 | 0.9500 |
| C11—C16 | 1.3946 (11) | C30—H30 | 0.9500 |
| C11—C12 | 1.4002 (12) | ||
| C7—O1—H1 | 112.5 (11) | C16—C15—C14 | 119.37 (8) |
| C4—O3—C8 | 117.72 (6) | C16—C15—H15 | 120.3 |
| C17—O4—H4 | 113.0 (11) | C14—C15—H15 | 120.3 |
| C14—O6—C18 | 117.65 (6) | C15—C16—C11 | 121.16 (8) |
| C25—N1—C21 | 117.73 (7) | C15—C16—H16 | 119.4 |
| C30—N2—C26 | 117.69 (7) | C11—C16—H16 | 119.4 |
| C6—C1—C2 | 118.87 (7) | O5—C17—O4 | 123.34 (8) |
| C6—C1—C7 | 119.01 (7) | O5—C17—C11 | 123.60 (8) |
| C2—C1—C7 | 122.11 (7) | O4—C17—C11 | 113.06 (7) |
| C3—C2—C1 | 120.70 (7) | O6—C18—C19 | 107.89 (7) |
| C3—C2—H2 | 119.6 | O6—C18—H18A | 110.1 |
| C1—C2—H2 | 119.6 | C19—C18—H18A | 110.1 |
| C2—C3—C4 | 119.63 (7) | O6—C18—H18B | 110.1 |
| C2—C3—H3 | 120.2 | C19—C18—H18B | 110.1 |
| C4—C3—H3 | 120.2 | H18A—C18—H18B | 108.4 |
| O3—C4—C5 | 124.21 (8) | C18—C19—C20 | 110.05 (7) |
| O3—C4—C3 | 115.45 (7) | C18—C19—H19B | 109.6 |
| C5—C4—C3 | 120.34 (8) | C20—C19—H19B | 109.6 |
| C6—C5—C4 | 119.14 (8) | C18—C19—H19A | 109.6 |
| C6—C5—H5 | 120.4 | C20—C19—H19A | 109.6 |
| C4—C5—H5 | 120.4 | H19B—C19—H19A | 108.2 |
| C5—C6—C1 | 121.31 (8) | C19—C20—H20A | 109.5 |
| C5—C6—H6 | 119.3 | C19—C20—H20B | 109.5 |
| C1—C6—H6 | 119.3 | H20A—C20—H20B | 109.5 |
| O2—C7—O1 | 123.50 (8) | C19—C20—H20C | 109.5 |
| O2—C7—C1 | 123.11 (8) | H20A—C20—H20C | 109.5 |
| O1—C7—C1 | 113.39 (7) | H20B—C20—H20C | 109.5 |
| O3—C8—C9 | 107.90 (7) | N1—C21—C22 | 123.03 (8) |
| O3—C8—H8A | 110.1 | N1—C21—H21 | 118.5 |
| C9—C8—H8A | 110.1 | C22—C21—H21 | 118.5 |
| O3—C8—H8B | 110.1 | C21—C22—C23 | 119.47 (8) |
| C9—C8—H8B | 110.1 | C21—C22—H22 | 120.3 |
| H8A—C8—H8B | 108.4 | C23—C22—H22 | 120.3 |
| C8—C9—C10 | 109.79 (7) | C22—C23—C24 | 117.30 (8) |
| C8—C9—H9B | 109.7 | C22—C23—C28 | 121.74 (8) |
| C10—C9—H9B | 109.7 | C24—C23—C28 | 120.94 (8) |
| C8—C9—H9A | 109.7 | C25—C24—C23 | 119.67 (8) |
| C10—C9—H9A | 109.7 | C25—C24—H24 | 120.2 |
| H9B—C9—H9A | 108.2 | C23—C24—H24 | 120.2 |
| C9—C10—H10A | 109.5 | N1—C25—C24 | 122.80 (8) |
| C9—C10—H10B | 109.5 | N1—C25—H25 | 118.6 |
| H10A—C10—H10B | 109.5 | C24—C25—H25 | 118.6 |
| C9—C10—H10C | 109.5 | N2—C26—C27 | 123.00 (8) |
| H10A—C10—H10C | 109.5 | N2—C26—H26 | 118.5 |
| H10B—C10—H10C | 109.5 | C27—C26—H26 | 118.5 |
| C16—C11—C12 | 118.80 (8) | C26—C27—C28 | 119.40 (8) |
| C16—C11—C17 | 119.71 (7) | C26—C27—H27 | 120.3 |
| C12—C11—C17 | 121.49 (8) | C28—C27—H27 | 120.3 |
| C13—C12—C11 | 120.75 (8) | C27—C28—C29 | 117.37 (7) |
| C13—C12—H12 | 119.6 | C27—C28—C23 | 121.57 (8) |
| C11—C12—H12 | 119.6 | C29—C28—C23 | 121.05 (8) |
| C12—C13—C14 | 120.08 (8) | C30—C29—C28 | 119.54 (8) |
| C12—C13—H13 | 120.0 | C30—C29—H29 | 120.2 |
| C14—C13—H13 | 120.0 | C28—C29—H29 | 120.2 |
| O6—C14—C15 | 124.82 (7) | N2—C30—C29 | 123.00 (8) |
| O6—C14—C13 | 115.36 (7) | N2—C30—H30 | 118.5 |
| C15—C14—C13 | 119.82 (8) | C29—C30—H30 | 118.5 |
| C6—C1—C2—C3 | −0.36 (13) | C12—C11—C16—C15 | −1.18 (13) |
| C7—C1—C2—C3 | −178.83 (7) | C17—C11—C16—C15 | 178.18 (7) |
| C1—C2—C3—C4 | 1.02 (13) | C16—C11—C17—O5 | 0.98 (13) |
| C8—O3—C4—C5 | −1.09 (12) | C12—C11—C17—O5 | −179.69 (8) |
| C8—O3—C4—C3 | 179.21 (7) | C16—C11—C17—O4 | −178.26 (7) |
| C2—C3—C4—O3 | 178.88 (7) | C12—C11—C17—O4 | 1.08 (12) |
| C2—C3—C4—C5 | −0.84 (13) | C14—O6—C18—C19 | −177.34 (7) |
| O3—C4—C5—C6 | −179.70 (7) | O6—C18—C19—C20 | 178.05 (7) |
| C3—C4—C5—C6 | −0.01 (13) | C25—N1—C21—C22 | −0.30 (13) |
| C4—C5—C6—C1 | 0.69 (13) | N1—C21—C22—C23 | −0.11 (14) |
| C2—C1—C6—C5 | −0.51 (13) | C21—C22—C23—C24 | 0.12 (12) |
| C7—C1—C6—C5 | 178.01 (7) | C21—C22—C23—C28 | 178.42 (8) |
| C6—C1—C7—O2 | 1.75 (13) | C22—C23—C24—C25 | 0.27 (12) |
| C2—C1—C7—O2 | −179.78 (8) | C28—C23—C24—C25 | −178.04 (8) |
| C6—C1—C7—O1 | −177.75 (7) | C21—N1—C25—C24 | 0.72 (13) |
| C2—C1—C7—O1 | 0.72 (12) | C23—C24—C25—N1 | −0.72 (13) |
| C4—O3—C8—C9 | 178.04 (7) | C30—N2—C26—C27 | 0.30 (13) |
| O3—C8—C9—C10 | −174.77 (7) | N2—C26—C27—C28 | −0.11 (13) |
| C16—C11—C12—C13 | 0.99 (13) | C26—C27—C28—C29 | −0.23 (12) |
| C17—C11—C12—C13 | −178.35 (8) | C26—C27—C28—C23 | 178.56 (8) |
| C11—C12—C13—C14 | 0.13 (13) | C22—C23—C28—C27 | 152.95 (9) |
| C18—O6—C14—C15 | −5.96 (12) | C24—C23—C28—C27 | −28.82 (12) |
| C18—O6—C14—C13 | 174.06 (7) | C22—C23—C28—C29 | −28.31 (12) |
| C12—C13—C14—O6 | 178.89 (7) | C24—C23—C28—C29 | 149.93 (8) |
| C12—C13—C14—C15 | −1.09 (13) | C27—C28—C29—C30 | 0.37 (12) |
| O6—C14—C15—C16 | −179.08 (7) | C23—C28—C29—C30 | −178.42 (8) |
| C13—C14—C15—C16 | 0.91 (13) | C26—N2—C30—C29 | −0.15 (13) |
| C14—C15—C16—C11 | 0.23 (13) | C28—C29—C30—N2 | −0.19 (13) |
(II) 4-n-Propoxybenzoic acid–4,4'-bipyridyl (2/1) . Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C1–C6 and C11–C16 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N1 | 1.03 (2) | 1.61 (2) | 2.6407 (10) | 174.3 (19) |
| O4—H4···N2 | 1.01 (2) | 1.67 (2) | 2.6728 (11) | 173.9 (18) |
| C3—H3···O5i | 0.95 | 2.57 | 3.3981 (11) | 146 |
| C25—H25···O3ii | 0.95 | 2.57 | 3.4581 (11) | 156 |
| C9—H9B···Cg2iii | 0.99 | 2.84 | 3.6750 (1) | 142 |
| C19—H19A···Cg1iv | 0.99 | 2.72 | 3.5781 (1) | 146 |
Symmetry codes: (i) −x+1, −y+2, −z; (ii) −x, −y+2, −z; (iii) x−2, y, z−1; (iv) x+2, y, z+1.
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Crystal data
| 2C11H14O3·C10H8N2 | Z = 2 |
| Mr = 544.63 | F(000) = 580.00 |
| Triclinic, P1 | Dx = 1.264 Mg m−3 |
| a = 7.6645 (10) Å | Mo Kα radiation, λ = 0.71075 Å |
| b = 8.5087 (13) Å | Cell parameters from 9788 reflections |
| c = 22.606 (3) Å | θ = 3.2–30.1° |
| α = 80.498 (3)° | µ = 0.09 mm−1 |
| β = 86.486 (3)° | T = 93 K |
| γ = 80.082 (3)° | Platelet, colorless |
| V = 1431.5 (4) Å3 | 0.53 × 0.41 × 0.11 mm |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Data collection
| Rigaku R-AXIS RAPIDII diffractometer | Rint = 0.075 |
| Detector resolution: 10.000 pixels mm-1 | θmax = 26.0° |
| ω scans | h = −8→9 |
| 12433 measured reflections | k = −10→10 |
| 5612 independent reflections | l = −27→27 |
| 3432 reflections with I > 2σ(I) |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.069 | Hydrogen site location: mixed |
| wR(F2) = 0.193 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0965P)2] where P = (Fo2 + 2Fc2)/3 |
| 5610 reflections | (Δ/σ)max < 0.001 |
| 371 parameters | Δρmax = 0.24 e Å−3 |
| 0 restraints | Δρmin = −0.41 e Å−3 |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Reflections were merged by SHELXL according to the crystal class for the calculation of statistics and refinement. |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.1390 (2) | −0.1143 (2) | −0.18237 (8) | 0.0358 (4) | |
| O2 | 0.3054 (2) | −0.3129 (2) | −0.12317 (8) | 0.0399 (5) | |
| O3 | 0.1632 (2) | −0.6711 (2) | −0.33646 (8) | 0.0347 (4) | |
| O4 | 0.2363 (2) | 0.7005 (2) | 0.21058 (8) | 0.0380 (5) | |
| O5 | 0.3659 (3) | 0.8609 (3) | 0.13954 (8) | 0.0433 (5) | |
| O6 | 0.3442 (2) | 1.2321 (2) | 0.36266 (7) | 0.0336 (4) | |
| N1 | 0.1708 (3) | 0.0497 (3) | −0.09736 (10) | 0.0375 (5) | |
| N2 | 0.2511 (3) | 0.5220 (3) | 0.12499 (9) | 0.0343 (5) | |
| C1 | 0.2120 (3) | −0.3677 (3) | −0.21459 (10) | 0.0288 (5) | |
| C2 | 0.1061 (3) | −0.3154 (3) | −0.26457 (11) | 0.0318 (6) | |
| H2 | 0.0429 | −0.2080 | −0.2710 | 0.038* | |
| C3 | 0.0926 (3) | −0.4186 (3) | −0.30463 (11) | 0.0325 (6) | |
| H3 | 0.0206 | −0.3821 | −0.3385 | 0.039* | |
| C4 | 0.1843 (3) | −0.5760 (3) | −0.29530 (11) | 0.0306 (5) | |
| C5 | 0.2917 (3) | −0.6282 (3) | −0.24626 (11) | 0.0329 (6) | |
| H5 | 0.3555 | −0.7354 | −0.2399 | 0.040* | |
| C6 | 0.3055 (3) | −0.5231 (3) | −0.20661 (11) | 0.0323 (6) | |
| H6 | 0.3804 | −0.5586 | −0.1734 | 0.039* | |
| C7 | 0.2243 (3) | −0.2636 (3) | −0.16939 (11) | 0.0323 (6) | |
| C8 | 0.2479 (3) | −0.8367 (3) | −0.32529 (12) | 0.0338 (6) | |
| H8A | 0.2005 | −0.8918 | −0.2872 | 0.041* | |
| H8B | 0.3771 | −0.8427 | −0.3219 | 0.041* | |
| C9 | 0.2121 (3) | −0.9175 (4) | −0.37668 (11) | 0.0352 (6) | |
| H9A | 0.2542 | −1.0350 | −0.3663 | 0.042* | |
| H9B | 0.0824 | −0.9016 | −0.3814 | 0.042* | |
| C10 | 0.2976 (3) | −0.8574 (4) | −0.43586 (12) | 0.0381 (6) | |
| H10A | 0.4271 | −0.8701 | −0.4313 | 0.046* | |
| H10B | 0.2521 | −0.7409 | −0.4475 | 0.046* | |
| C11 | 0.2622 (4) | −0.9474 (4) | −0.48561 (13) | 0.0474 (7) | |
| H11A | 0.3074 | −1.0629 | −0.4745 | 0.071* | |
| H11B | 0.3221 | −0.9055 | −0.5230 | 0.071* | |
| H11C | 0.1343 | −0.9319 | −0.4914 | 0.071* | |
| C12 | 0.3175 (3) | 0.9341 (3) | 0.23705 (11) | 0.0300 (5) | |
| C13 | 0.2420 (3) | 0.9052 (3) | 0.29435 (11) | 0.0300 (5) | |
| H13 | 0.1816 | 0.8155 | 0.3053 | 0.036* | |
| C14 | 0.2538 (3) | 1.0058 (3) | 0.33557 (11) | 0.0301 (5) | |
| H14 | 0.2019 | 0.9852 | 0.3747 | 0.036* | |
| C15 | 0.3423 (3) | 1.1382 (3) | 0.31945 (11) | 0.0305 (5) | |
| C16 | 0.4185 (3) | 1.1671 (3) | 0.26282 (11) | 0.0325 (6) | |
| H16 | 0.4781 | 1.2572 | 0.2518 | 0.039* | |
| C17 | 0.4079 (3) | 1.0642 (3) | 0.22178 (11) | 0.0320 (6) | |
| H17 | 0.4629 | 1.0829 | 0.1830 | 0.038* | |
| C18 | 0.3088 (3) | 0.8303 (3) | 0.19068 (11) | 0.0323 (6) | |
| C19 | 0.4228 (3) | 1.3756 (3) | 0.34655 (12) | 0.0375 (6) | |
| H19A | 0.3595 | 1.4478 | 0.3129 | 0.045* | |
| H19B | 0.5485 | 1.3469 | 0.3337 | 0.045* | |
| C20 | 0.4099 (3) | 1.4595 (4) | 0.40073 (12) | 0.0376 (6) | |
| H20A | 0.4719 | 1.5539 | 0.3912 | 0.045* | |
| H20B | 0.4720 | 1.3845 | 0.4340 | 0.045* | |
| C21 | 0.2211 (3) | 1.5162 (4) | 0.42172 (12) | 0.0372 (6) | |
| H21A | 0.1552 | 1.5829 | 0.3875 | 0.045* | |
| H21B | 0.1624 | 1.4210 | 0.4354 | 0.045* | |
| C22 | 0.2123 (4) | 1.6142 (4) | 0.47241 (13) | 0.0452 (7) | |
| H22A | 0.2700 | 1.5464 | 0.5075 | 0.068* | |
| H22B | 0.0881 | 1.6524 | 0.4830 | 0.068* | |
| H22C | 0.2731 | 1.7070 | 0.4596 | 0.068* | |
| C23 | 0.2365 (4) | −0.0184 (4) | −0.04412 (13) | 0.0472 (7) | |
| H23 | 0.2711 | −0.1324 | −0.0368 | 0.057* | |
| C24 | 0.2571 (4) | 0.0681 (4) | 0.00093 (12) | 0.0421 (7) | |
| H24 | 0.3000 | 0.0144 | 0.0388 | 0.051* | |
| C25 | 0.2135 (3) | 0.2354 (3) | −0.01037 (11) | 0.0307 (5) | |
| C26 | 0.1489 (3) | 0.3070 (3) | −0.06606 (11) | 0.0332 (6) | |
| H26 | 0.1191 | 0.4211 | −0.0756 | 0.040* | |
| C27 | 0.1286 (3) | 0.2094 (3) | −0.10754 (12) | 0.0347 (6) | |
| H27 | 0.0819 | 0.2593 | −0.1453 | 0.042* | |
| C28 | 0.2950 (3) | 0.5728 (4) | 0.06786 (11) | 0.0365 (6) | |
| H28 | 0.3359 | 0.6735 | 0.0581 | 0.044* | |
| C29 | 0.2834 (3) | 0.4853 (3) | 0.02218 (11) | 0.0329 (6) | |
| H29 | 0.3116 | 0.5271 | −0.0182 | 0.040* | |
| C30 | 0.2296 (3) | 0.3348 (3) | 0.03651 (11) | 0.0298 (5) | |
| C31 | 0.1889 (3) | 0.2808 (3) | 0.09596 (11) | 0.0334 (6) | |
| H31 | 0.1551 | 0.1775 | 0.1075 | 0.040* | |
| C32 | 0.1978 (3) | 0.3784 (3) | 0.13857 (11) | 0.0332 (6) | |
| H32 | 0.1648 | 0.3420 | 0.1790 | 0.040* | |
| H1 | 0.160 (4) | −0.054 (4) | −0.1475 (15) | 0.060 (9)* | |
| H4 | 0.242 (5) | 0.642 (5) | 0.1754 (16) | 0.065 (10)* |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0412 (10) | 0.0304 (11) | 0.0354 (10) | −0.0006 (8) | −0.0033 (8) | −0.0091 (8) |
| O2 | 0.0485 (11) | 0.0369 (12) | 0.0331 (10) | 0.0009 (9) | −0.0080 (8) | −0.0085 (8) |
| O3 | 0.0326 (9) | 0.0333 (11) | 0.0377 (10) | 0.0069 (8) | −0.0063 (7) | −0.0156 (8) |
| O4 | 0.0436 (10) | 0.0351 (12) | 0.0371 (10) | −0.0067 (8) | −0.0014 (8) | −0.0110 (9) |
| O5 | 0.0581 (12) | 0.0399 (13) | 0.0319 (10) | −0.0076 (10) | 0.0004 (9) | −0.0064 (9) |
| O6 | 0.0371 (9) | 0.0322 (11) | 0.0333 (9) | −0.0080 (8) | 0.0011 (7) | −0.0089 (8) |
| N1 | 0.0455 (12) | 0.0327 (14) | 0.0337 (12) | −0.0007 (10) | −0.0019 (9) | −0.0092 (10) |
| N2 | 0.0328 (10) | 0.0357 (14) | 0.0340 (11) | 0.0000 (9) | −0.0018 (9) | −0.0097 (10) |
| C1 | 0.0261 (11) | 0.0307 (15) | 0.0292 (12) | −0.0020 (10) | 0.0019 (9) | −0.0074 (10) |
| C2 | 0.0290 (11) | 0.0315 (15) | 0.0338 (13) | 0.0009 (10) | −0.0018 (10) | −0.0079 (11) |
| C3 | 0.0280 (11) | 0.0358 (16) | 0.0321 (13) | 0.0023 (11) | −0.0042 (10) | −0.0071 (11) |
| C4 | 0.0250 (11) | 0.0340 (15) | 0.0327 (13) | 0.0001 (10) | −0.0016 (9) | −0.0100 (11) |
| C5 | 0.0329 (12) | 0.0283 (15) | 0.0357 (13) | 0.0050 (10) | −0.0041 (10) | −0.0088 (11) |
| C6 | 0.0293 (11) | 0.0369 (16) | 0.0304 (12) | −0.0021 (11) | −0.0018 (10) | −0.0078 (11) |
| C7 | 0.0324 (12) | 0.0317 (15) | 0.0328 (13) | −0.0040 (11) | 0.0008 (10) | −0.0074 (11) |
| C8 | 0.0357 (12) | 0.0288 (15) | 0.0360 (14) | 0.0005 (11) | 0.0002 (10) | −0.0088 (11) |
| C9 | 0.0351 (12) | 0.0359 (16) | 0.0360 (13) | −0.0052 (11) | 0.0022 (10) | −0.0115 (12) |
| C10 | 0.0383 (13) | 0.0402 (17) | 0.0365 (14) | −0.0050 (12) | 0.0019 (11) | −0.0111 (12) |
| C11 | 0.0518 (16) | 0.054 (2) | 0.0385 (15) | −0.0066 (15) | 0.0001 (13) | −0.0161 (14) |
| C12 | 0.0268 (11) | 0.0271 (14) | 0.0351 (13) | 0.0014 (10) | −0.0069 (10) | −0.0060 (11) |
| C13 | 0.0261 (11) | 0.0291 (14) | 0.0331 (13) | 0.0000 (10) | −0.0030 (10) | −0.0043 (11) |
| C14 | 0.0280 (11) | 0.0326 (15) | 0.0296 (12) | −0.0038 (10) | −0.0021 (10) | −0.0053 (11) |
| C15 | 0.0265 (11) | 0.0289 (15) | 0.0353 (13) | −0.0004 (10) | −0.0035 (10) | −0.0058 (11) |
| C16 | 0.0312 (12) | 0.0315 (15) | 0.0338 (13) | −0.0040 (11) | −0.0019 (10) | −0.0035 (11) |
| C17 | 0.0290 (11) | 0.0341 (15) | 0.0309 (13) | −0.0009 (10) | −0.0023 (10) | −0.0037 (11) |
| C18 | 0.0333 (12) | 0.0297 (15) | 0.0317 (13) | 0.0019 (11) | −0.0065 (10) | −0.0035 (11) |
| C19 | 0.0354 (13) | 0.0357 (17) | 0.0442 (15) | −0.0095 (12) | 0.0020 (11) | −0.0120 (12) |
| C20 | 0.0351 (13) | 0.0387 (17) | 0.0419 (15) | −0.0062 (12) | −0.0037 (11) | −0.0135 (12) |
| C21 | 0.0377 (13) | 0.0335 (16) | 0.0397 (14) | 0.0009 (11) | −0.0032 (11) | −0.0101 (12) |
| C22 | 0.0491 (16) | 0.0396 (18) | 0.0474 (16) | −0.0021 (13) | 0.0005 (13) | −0.0149 (14) |
| C23 | 0.071 (2) | 0.0278 (16) | 0.0404 (15) | 0.0043 (14) | −0.0095 (14) | −0.0083 (13) |
| C24 | 0.0577 (17) | 0.0306 (16) | 0.0344 (14) | 0.0049 (13) | −0.0063 (12) | −0.0055 (12) |
| C25 | 0.0292 (11) | 0.0310 (15) | 0.0314 (13) | −0.0004 (10) | 0.0003 (10) | −0.0086 (11) |
| C26 | 0.0368 (13) | 0.0280 (15) | 0.0337 (13) | 0.0002 (11) | −0.0044 (10) | −0.0061 (11) |
| C27 | 0.0351 (13) | 0.0339 (16) | 0.0340 (13) | 0.0014 (11) | −0.0027 (10) | −0.0087 (11) |
| C28 | 0.0382 (13) | 0.0349 (16) | 0.0377 (14) | −0.0058 (12) | −0.0014 (11) | −0.0093 (12) |
| C29 | 0.0327 (12) | 0.0326 (15) | 0.0327 (13) | −0.0018 (11) | 0.0012 (10) | −0.0071 (11) |
| C30 | 0.0273 (11) | 0.0295 (14) | 0.0314 (12) | 0.0026 (10) | −0.0020 (9) | −0.0086 (11) |
| C31 | 0.0346 (12) | 0.0316 (15) | 0.0326 (13) | −0.0004 (11) | −0.0004 (10) | −0.0064 (11) |
| C32 | 0.0323 (12) | 0.0324 (15) | 0.0312 (13) | 0.0036 (11) | 0.0003 (10) | −0.0042 (11) |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Geometric parameters (Å, º)
| O1—C7 | 1.319 (3) | C12—C18 | 1.489 (3) |
| O1—H1 | 1.04 (3) | C13—C14 | 1.382 (3) |
| O2—C7 | 1.225 (3) | C13—H13 | 0.9500 |
| O3—C4 | 1.363 (3) | C14—C15 | 1.399 (3) |
| O3—C8 | 1.435 (3) | C14—H14 | 0.9500 |
| O4—C18 | 1.322 (3) | C15—C16 | 1.376 (3) |
| O4—H4 | 1.00 (4) | C16—C17 | 1.392 (3) |
| O5—C18 | 1.214 (3) | C16—H16 | 0.9500 |
| O6—C15 | 1.362 (3) | C17—H17 | 0.9500 |
| O6—C19 | 1.438 (3) | C19—C20 | 1.506 (3) |
| N1—C27 | 1.325 (4) | C19—H19A | 0.9900 |
| N1—C23 | 1.335 (4) | C19—H19B | 0.9900 |
| N2—C28 | 1.334 (3) | C20—C21 | 1.518 (4) |
| N2—C32 | 1.337 (3) | C20—H20A | 0.9900 |
| C1—C6 | 1.381 (4) | C20—H20B | 0.9900 |
| C1—C2 | 1.398 (3) | C21—C22 | 1.516 (4) |
| C1—C7 | 1.475 (3) | C21—H21A | 0.9900 |
| C2—C3 | 1.381 (3) | C21—H21B | 0.9900 |
| C2—H2 | 0.9500 | C22—H22A | 0.9800 |
| C3—C4 | 1.390 (4) | C22—H22B | 0.9800 |
| C3—H3 | 0.9500 | C22—H22C | 0.9800 |
| C4—C5 | 1.389 (3) | C23—C24 | 1.382 (4) |
| C5—C6 | 1.387 (3) | C23—H23 | 0.9500 |
| C5—H5 | 0.9500 | C24—C25 | 1.389 (4) |
| C6—H6 | 0.9500 | C24—H24 | 0.9500 |
| C8—C9 | 1.505 (3) | C25—C26 | 1.386 (3) |
| C8—H8A | 0.9900 | C25—C30 | 1.483 (3) |
| C8—H8B | 0.9900 | C26—C27 | 1.383 (3) |
| C9—C10 | 1.504 (4) | C26—H26 | 0.9500 |
| C9—H9A | 0.9900 | C27—H27 | 0.9500 |
| C9—H9B | 0.9900 | C28—C29 | 1.384 (3) |
| C10—C11 | 1.523 (3) | C28—H28 | 0.9500 |
| C10—H10A | 0.9900 | C29—C30 | 1.394 (4) |
| C10—H10B | 0.9900 | C29—H29 | 0.9500 |
| C11—H11A | 0.9800 | C30—C31 | 1.382 (3) |
| C11—H11B | 0.9800 | C31—C32 | 1.383 (3) |
| C11—H11C | 0.9800 | C31—H31 | 0.9500 |
| C12—C13 | 1.387 (3) | C32—H32 | 0.9500 |
| C12—C17 | 1.390 (3) | ||
| C7—O1—H1 | 107 (2) | C15—C16—C17 | 119.9 (2) |
| C4—O3—C8 | 117.16 (19) | C15—C16—H16 | 120.1 |
| C18—O4—H4 | 105 (2) | C17—C16—H16 | 120.1 |
| C15—O6—C19 | 117.34 (19) | C12—C17—C16 | 120.5 (2) |
| C27—N1—C23 | 117.5 (2) | C12—C17—H17 | 119.7 |
| C28—N2—C32 | 118.2 (2) | C16—C17—H17 | 119.7 |
| C6—C1—C2 | 118.9 (2) | O5—C18—O4 | 123.1 (2) |
| C6—C1—C7 | 118.8 (2) | O5—C18—C12 | 123.0 (2) |
| C2—C1—C7 | 122.3 (2) | O4—C18—C12 | 113.9 (2) |
| C3—C2—C1 | 120.5 (2) | O6—C19—C20 | 108.0 (2) |
| C3—C2—H2 | 119.7 | O6—C19—H19A | 110.1 |
| C1—C2—H2 | 119.7 | C20—C19—H19A | 110.1 |
| C2—C3—C4 | 120.0 (2) | O6—C19—H19B | 110.1 |
| C2—C3—H3 | 120.0 | C20—C19—H19B | 110.1 |
| C4—C3—H3 | 120.0 | H19A—C19—H19B | 108.4 |
| O3—C4—C5 | 123.5 (2) | C19—C20—C21 | 113.9 (2) |
| O3—C4—C3 | 116.6 (2) | C19—C20—H20A | 108.8 |
| C5—C4—C3 | 119.9 (2) | C21—C20—H20A | 108.8 |
| C6—C5—C4 | 119.6 (2) | C19—C20—H20B | 108.8 |
| C6—C5—H5 | 120.2 | C21—C20—H20B | 108.8 |
| C4—C5—H5 | 120.2 | H20A—C20—H20B | 107.7 |
| C1—C6—C5 | 121.1 (2) | C22—C21—C20 | 112.6 (2) |
| C1—C6—H6 | 119.5 | C22—C21—H21A | 109.1 |
| C5—C6—H6 | 119.5 | C20—C21—H21A | 109.1 |
| O2—C7—O1 | 122.9 (2) | C22—C21—H21B | 109.1 |
| O2—C7—C1 | 122.4 (2) | C20—C21—H21B | 109.1 |
| O1—C7—C1 | 114.7 (2) | H21A—C21—H21B | 107.8 |
| O3—C8—C9 | 108.3 (2) | C21—C22—H22A | 109.5 |
| O3—C8—H8A | 110.0 | C21—C22—H22B | 109.5 |
| C9—C8—H8A | 110.0 | H22A—C22—H22B | 109.5 |
| O3—C8—H8B | 110.0 | C21—C22—H22C | 109.5 |
| C9—C8—H8B | 110.0 | H22A—C22—H22C | 109.5 |
| H8A—C8—H8B | 108.4 | H22B—C22—H22C | 109.5 |
| C10—C9—C8 | 114.8 (2) | N1—C23—C24 | 123.5 (3) |
| C10—C9—H9A | 108.6 | N1—C23—H23 | 118.3 |
| C8—C9—H9A | 108.6 | C24—C23—H23 | 118.3 |
| C10—C9—H9B | 108.6 | C23—C24—C25 | 118.5 (3) |
| C8—C9—H9B | 108.6 | C23—C24—H24 | 120.7 |
| H9A—C9—H9B | 107.5 | C25—C24—H24 | 120.7 |
| C9—C10—C11 | 112.5 (2) | C26—C25—C24 | 118.2 (2) |
| C9—C10—H10A | 109.1 | C26—C25—C30 | 120.8 (2) |
| C11—C10—H10A | 109.1 | C24—C25—C30 | 121.0 (2) |
| C9—C10—H10B | 109.1 | C27—C26—C25 | 118.9 (3) |
| C11—C10—H10B | 109.1 | C27—C26—H26 | 120.6 |
| H10A—C10—H10B | 107.8 | C25—C26—H26 | 120.6 |
| C10—C11—H11A | 109.5 | N1—C27—C26 | 123.4 (2) |
| C10—C11—H11B | 109.5 | N1—C27—H27 | 118.3 |
| H11A—C11—H11B | 109.5 | C26—C27—H27 | 118.3 |
| C10—C11—H11C | 109.5 | N2—C28—C29 | 122.9 (2) |
| H11A—C11—H11C | 109.5 | N2—C28—H28 | 118.5 |
| H11B—C11—H11C | 109.5 | C29—C28—H28 | 118.5 |
| C13—C12—C17 | 119.2 (2) | C28—C29—C30 | 118.8 (2) |
| C13—C12—C18 | 123.0 (2) | C28—C29—H29 | 120.6 |
| C17—C12—C18 | 117.7 (2) | C30—C29—H29 | 120.6 |
| C14—C13—C12 | 120.5 (2) | C31—C30—C29 | 118.0 (2) |
| C14—C13—H13 | 119.7 | C31—C30—C25 | 120.5 (2) |
| C12—C13—H13 | 119.7 | C29—C30—C25 | 121.5 (2) |
| C13—C14—C15 | 119.8 (2) | C30—C31—C32 | 119.5 (2) |
| C13—C14—H14 | 120.1 | C30—C31—H31 | 120.3 |
| C15—C14—H14 | 120.1 | C32—C31—H31 | 120.3 |
| O6—C15—C16 | 124.4 (2) | N2—C32—C31 | 122.5 (2) |
| O6—C15—C14 | 115.6 (2) | N2—C32—H32 | 118.7 |
| C16—C15—C14 | 120.0 (2) | C31—C32—H32 | 118.7 |
| C6—C1—C2—C3 | 1.2 (3) | C18—C12—C17—C16 | 179.0 (2) |
| C7—C1—C2—C3 | −177.4 (2) | C15—C16—C17—C12 | 1.4 (4) |
| C1—C2—C3—C4 | 0.2 (4) | C13—C12—C18—O5 | 175.6 (2) |
| C8—O3—C4—C5 | 4.5 (3) | C17—C12—C18—O5 | −5.2 (4) |
| C8—O3—C4—C3 | −176.1 (2) | C13—C12—C18—O4 | −5.6 (3) |
| C2—C3—C4—O3 | 179.5 (2) | C17—C12—C18—O4 | 173.5 (2) |
| C2—C3—C4—C5 | −1.1 (4) | C15—O6—C19—C20 | 179.9 (2) |
| O3—C4—C5—C6 | 180.0 (2) | O6—C19—C20—C21 | −62.8 (3) |
| C3—C4—C5—C6 | 0.5 (4) | C19—C20—C21—C22 | −174.4 (2) |
| C2—C1—C6—C5 | −1.8 (3) | C27—N1—C23—C24 | 2.1 (4) |
| C7—C1—C6—C5 | 176.9 (2) | N1—C23—C24—C25 | −2.6 (5) |
| C4—C5—C6—C1 | 0.9 (4) | C23—C24—C25—C26 | 1.0 (4) |
| C6—C1—C7—O2 | −4.7 (4) | C23—C24—C25—C30 | 178.9 (3) |
| C2—C1—C7—O2 | 174.0 (2) | C24—C25—C26—C27 | 0.8 (4) |
| C6—C1—C7—O1 | 176.3 (2) | C30—C25—C26—C27 | −177.1 (2) |
| C2—C1—C7—O1 | −5.1 (3) | C23—N1—C27—C26 | −0.2 (4) |
| C4—O3—C8—C9 | −178.40 (19) | C25—C26—C27—N1 | −1.3 (4) |
| O3—C8—C9—C10 | 66.9 (3) | C32—N2—C28—C29 | 1.6 (4) |
| C8—C9—C10—C11 | 178.0 (2) | N2—C28—C29—C30 | −2.3 (4) |
| C17—C12—C13—C14 | 1.0 (4) | C28—C29—C30—C31 | 0.5 (4) |
| C18—C12—C13—C14 | −179.8 (2) | C28—C29—C30—C25 | 179.6 (2) |
| C12—C13—C14—C15 | 0.1 (4) | C26—C25—C30—C31 | 140.9 (3) |
| C19—O6—C15—C16 | 3.6 (4) | C24—C25—C30—C31 | −37.0 (3) |
| C19—O6—C15—C14 | −175.7 (2) | C26—C25—C30—C29 | −38.2 (3) |
| C13—C14—C15—O6 | 178.8 (2) | C24—C25—C30—C29 | 144.0 (3) |
| C13—C14—C15—C16 | −0.5 (4) | C29—C30—C31—C32 | 1.8 (4) |
| O6—C15—C16—C17 | −179.5 (2) | C25—C30—C31—C32 | −177.3 (2) |
| C14—C15—C16—C17 | −0.3 (4) | C28—N2—C32—C31 | 0.9 (4) |
| C13—C12—C17—C16 | −1.8 (4) | C30—C31—C32—N2 | −2.6 (4) |
(III) 4-n-Butoxybenzoic acid–4,4'-bipyridyl (2/1) . Hydrogen-bond geometry (Å, º)
Cg1 and Cg2 are the centroids of the C1–C6 and C12–C17 rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N1 | 1.04 (3) | 1.56 (3) | 2.600 (3) | 173 (3) |
| O4—H4···N2 | 1.00 (4) | 1.64 (4) | 2.636 (3) | 172 (4) |
| C24—H24···O5i | 0.95 | 2.47 | 3.408 (3) | 171 |
| C29—H29···O2ii | 0.95 | 2.53 | 3.456 (3) | 164 |
| C2—H2···Cg2iii | 0.95 | 2.98 | 3.754 (3) | 139 |
| C8—H8B···Cg2iv | 0.99 | 2.68 | 3.518 (3) | 143 |
| C19—H19B···Cg1v | 0.99 | 2.77 | 3.586 (3) | 140 |
Symmetry codes: (i) x, y−1, z; (ii) x, y+1, z; (iii) −x, −y+1, −z; (iv) −x+1, −y, −z; (v) −x+1, −y+1, −z.
References
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Groom, C. R. & Allen, F. H. (2014). Angew. Chem. Int. Ed. 53, 662–671. [DOI] [PubMed]
- Grunert, M., Howie, A., Kaeding, A. & Imrie, C. T. (1997). J. Mater. Chem. 7, 211–214.
- Kato, T., Fréchet, J. M. J., Wilson, P. G., Saito, T., Uryu, T., Fujishima, A., Jin, C. & Kaneuchi, F. (1993). Chem. Mater. 5, 1094–1100.
- Kato, T., Wilson, P. G., Fujishima, A. & Fréchet, J. M. J. (1990). Chem. Lett. pp. 2003–2006.
- Lai, L.-L., Lee, L.-J., Luo, D.-W., Liu, Y.-H. & Wang, Y. (2008). J. Struct. Chem. 49, 1137–1140.
- Mukherjee, A. & Desiraju, G. R. (2014). Cryst. Growth Des. 14, 1375–1385.
- Ramon, G., Davies, K. & Nassimbeni, L. R. (2014). CrystEngComm, 16, 5802–5810.
- Rigaku (2006). RAPID-AUTO. Rigaku Corporation, Tokyo, Japan.
- Rigaku (2010). CrystalStructure. Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, II, III, General. DOI: 10.1107/S2056989015018435/lh5794sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015018435/lh5794Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989015018435/lh5794IIsup3.hkl
Structure factors: contains datablock(s) III. DOI: 10.1107/S2056989015018435/lh5794IIIsup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report










