Abstract
Objective:
Methicillin-resistant Staphylococcus aureus (MRSA) ranks top among the nosocomial pathogens. Nasal formulation of mupirocin is found to eradicate MRSA from colonized individuals, but the emergence of resistant strains is a matter of concern.
Methods:
Nasal swabs were collected from 200 health care workers (HCWs) who were screened for MRSA. Kirby–Bauer disc diffusion method was used to perform antibiotic susceptibility test. MRSA detection was done using a cefoxitin 30 µg disc and interpreted according to the Clinical and Laboratory Standards Institute guidelines. Determination of mupirocin resistance was performed using Epsilometer test (E-test).
Findings:
About 14% of HCWs showed nasal carriage of MRSA. Nursing orderlies were the predominant carriers. E-test showed four mupirocin resistant isolates. The antibiogram of the MRSA isolates revealed the higher resistance to antibiotics as compared to methicillin-sensitive Staphylococcus aureus. All the MRSA isolates were sensitive to linezolid.
Conclusion:
HCWs in our hospital showed high nasal carriage rate of MRSA, particularly the nursing orderlies which is statistically significant. It is advisable to detect mupirocin resistance among the isolates obtained from the HCWs so that in case of resistance, alternative treatment should be sought.
Keywords: Health care workers, methicillin-resistant Staphylococcus aureus, mupirocin-resistant S. aureus, nasal carriage
INTRODUCTION
Staphylococcus aureus and its resistant form methicillin-resistant S. aureus (MRSA), is one of the most common nosocomial pathogens which not only causes increased morbidity and mortality but also increases the length of hospital stay and cost.[1,2] The health care workers (HCWs) serve as a link between hospitals, long-term care facilities, and nursing homes on one hand and the community on the other. They may serve as reservoirs, vectors, or victims of MRSA cross-transmission.[3]
S. aureus is a member of commensal microflora and many body sites such as hands, rectum, perineum, axillae, vagina, gastrointestinal tract, and intact or inflamed skin are frequently colonized for varying time periods, and the main reservoir of MRSA is the anterior nares.[4] The main sources of MRSA in the hospital environment are the asymptomatically colonized patients and HCWs.[5]
The drug of choice for the treatment of serious MRSA infection is vancomycin till date.[6] With the emergence of vancomycin-resistant MRSA, treatment, options have become more limited. Mupirocin, a topical glycopeptide antibiotic is now commonly used for nasal decolonization of the HCWs as part of a routine surveillance to prevent the emergence and transmission of MRSA in health care facilities.[7] The increased pressure of antibiotic use has led to the emergence of mupirocin resistant S. aureus (MupRSA), and the clinicians are left with few alternatives to prevent the spread of MRSA.[8]
The aim of the present study is to estimate the nasal carriage of MRSA as well as MupRSA among the HCWs of our hospital. Thus, a real-time prevalence of MRSA carriage and its resistance to mupirocin will help the institution to develop a better MRSA control and infection control policy by instituting use of alternative options to prevent the colonization and spread of infection in case of resistance.
METHODS
Nasal swabs from HCWs working in different departments of Mayo Institute of Medical Sciences, Barabanki, Uttar Pradesh, were collected after obtaining their informed consent. Those hospitalized within the previous 1-year or on antibiotics within last 1-week of collection of the swabs were excluded from the study. Ethical clearance was obtained prior to commencement of the study. Data, including demographic profile, work profile, and medical history, were recorded in a preformed questionnaire.
Sample collection
Nasal swabs were collected using a sterile cotton swab with transport tube. The swab was rotated in the anterior nares for 3 s. In case of sneezing, resampling was done. After collecting swabs were re-inserted in the transport tube, labeled properly, and transported to the laboratory for further processing.
Sample processing
All the swabs were inoculated on 5% sheep blood agar and mannitol salt agar and incubated at 37°C for 24 h. After incubation, growth was identified as S. aureus on the basis of colony morphology, Gram stain, catalase, dimethyl sulfoxide oxidase, DNase, and coagulase test (slide and tube).
Determination of methicillin-resistant Staphylococcus aureus
All confirmed S. aureus isolates were further tested for detection of methicillin resistance by Kirby–Bauer disc diffusion method using cefoxitin 30 µg discs (HiMedia Laboratories, Mumbai, Maharashtra, India) as per Clinical and Laboratory Standards Institute (CLSI) 2013 guidelines.[9] Zone of inhibition of size of ≤21 mm was taken as resistant and ≥22 mm as sensitive.
Determination of antibiotic susceptibility pattern of Staphylococcus
It was done by Kirby–Bauer disc diffusion method; antibiotic discs used were penicillin (10 units), ciprofloxacin (5 µg), clindamycin (2 µg), erythromycin (15 µg), levofloxacin (5 µg), linezolid (30 µg), rifampin (5 µg), tetracycline (30 µg), and cotrimoxazole (1.25/23.75 µg). Zone diameter interpretation for determining sensitive, intermediate or resistant was done as per CLSI guidelines.[9]
Determination of mupirocin resistant Staphylococcus aureus
Mupirocin resistance was determined by Epsilometer test (E-test) using HiComb mupirocin strip and interpreted as per CLSI guidelines.[9] Isolates with minimum inhibitory concentrations (MICs) ≥512 μg/ml were considered as high-level resistant (MuH), those with MICs 8–256 μg/ml were considered as low-level resistant (MuL), and with ≤4 μg/ml were considered as mupirocin sensitive.
Statistical analysis
The results were recorded and analyzed statistically in Microsoft office Excel Sheet 2010. Chi-square test was used for statistical analysis. P ≤ 0.05 was considered as statistically significant.
RESULTS
In the present study, 200 HCWs working in our hospital were included in the study. Out of these HCWs, 92 (46%) were females and 108 (54%) were males. The age ranged between 18 and 65 years. Of the 200 nonduplicate nasal swabs processed in the laboratory, Staphylococcus spp. was isolated in 162 (81%) samples which comprised of 96 (48%) S. aureus isolates.
Table 1 shows the distribution of various samples on the basis of the source. HCWs of various categories such as doctor, nurse, technician, nursing orderlies, nursing student, and others (included pharmacists, receptionists, and security guards) working in our hospital were taken in nearly equal representation.
Table 1.
Distribution of samples on the basis of source
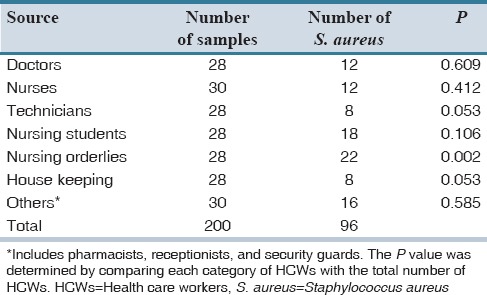
The distribution percentage of MRSA and methicillin-sensitive S. aureus (MSSA) strains in the total specimens received is documented in Table 2. In both Tables 1 and 2, the P value was determined by comparing each category of HCWs with the total number of HCWs. Of 96 S. aureus isolates, 28 (14%) were detected as MRSA strains. Nursing orderlies showed a higher carrier rate of MRSA as compared to other HCWs which was statistically significant (P < 0.05).
Table 2.
Distribution of MRSA and MSSA isolates on the basis of source
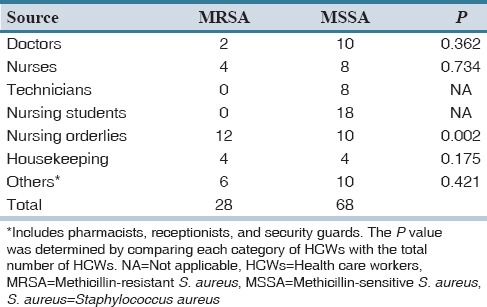
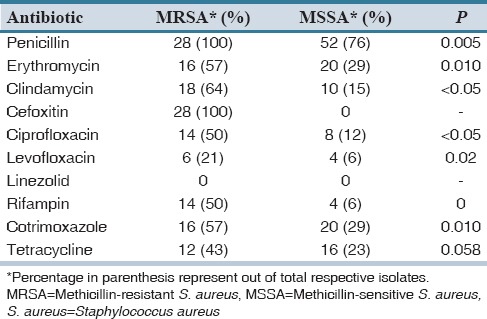
Table 3 displays the antibiotic susceptibility pattern of MRSA and MSSA strains for different group of antibiotics effective primarily against the Gram-positive organism. None of MRSA isolates was sensitive to penicillin, nearly 60% were resistant to clindamycin and erythromycin and around 50% were resistant to tetracycline and cotrimoxazole. All MRSA isolates were susceptible to linezolid. MSSA isolates sensitive to penicillin were 23%, nearly 60% were sensitive to clindamycin, ciprofloxacin and levofloxacin and around 50% were sensitive to tetracycline and cotrimoxazole. All MSSA isolates were susceptible to linezolid. Resistance to most of the antibiotics was significantly associated with MRSA strains (P < 0.05).
Table 3.
Antibiotic resistance pattern of MRSA and MSSA isolates
Mupirocin resistance was seen in 4 (7%) of 28 MRSA isolates by E-test; 3 (75%) isolates were MuH, and 1 (25%) isolate was MuL. No mupirocin resistance was detected in MSSA. The MuH strains were isolated from the nasal swabs of nursing orderlies whereas the MuL strain was isolated from a nurse.
DISCUSSION
Nasal carriage of S. aureus acts as an important reservoir of infection among the colonized HCWs and may transmit the infection to co-workers and in the community. The prevalence of S. aureus nasal carriage among HCWs is 48% in the present study which is higher than the study conducted by Truong et al. (35.8%) and Yazgi et al. (34.9%) and comparable to other studies conducted by Singh et al. (47.5%) and Al-Abdli and Baiu.[10,11,12,13] Norazah et al., in a study conducted in Malaysia in 2002, reported a higher prevalence of S. aureus nasal carriage which varied from 45% to 76%.[14]
MRSA strains are well known for their high tendency to spread among the HCWs and from the HCWs to the patients which may in-turn lead to the increase in the treatment cost burden by prolonging the duration of hospital stay and or administration of expensive medications. In our study, nasal carriage of MRSA was found in 14% of the HCWs. This is consistent with the study conducted in different hospital setting worldwide which has been reported in the range of 5.8% to 17.8%.[15,16,17,18] Lower percentage of MRSA carriage has been reported from Nepal.[19] This difference in prevalence of S. aureus and MRSA in various hospitals may be attributed to the inter-laboratory variation in the methods of detection as well as the effectiveness of hospital infection control policy.
In our study, the MRSA carriage was particularly high among the nursing orderlies (6%), which was statistically significant (P < 0.05) [Table 2]. The higher prevalence of MRSA among this group of HCWs could be due to their lack of knowledge with regard to hand hygiene, contact precautions, and infection control policies.
In this study, all the isolates were susceptible to linezolid. As expected, most of the MRSA isolates were resistant to penicillin. But, the clinically significant observation of the study is the significant association of resistance shown by MRSA to other antibiotics used for the treatment of staphylococcal infections. Other studies report a higher resistance rates for fluoroquinolones and aminoglycosides such as the Pulimood et al., that has reported a high ciprofloxacin resistance of 90% and Qureshi et al., had reported a resistance of 98.9%.[20,21] In contrast, this study has demonstrated 50% of the strains resistant to ciprofloxacin and rifampin and a further lower resistant rate to tetracycline (42%) and levofloxacin (21.4%).
Nasal formulation of mupirocin, a topical antibiotic agent that interferes with bacterial protein synthesis, is recommended by the Food and Drug Administration of United States for use in the eradication of nasal carriage of S. aureus in adult patients and HCWs. Though the use of mupirocin is limited for infection control and other prophylactic measures, emergence of MupRSA is being reported from across the globe with the prevalence of 0.5% in Nigeria to 14.6% in India.[22,23] In our study, 4 (2%) isolates were found to be mupirocin resistant of which three isolates were high levels resistant. In the presence of mupirocin resistance strains, treatment with mupirocin may be ineffective, especially with high-level resistance strains. Although low-level mupirocin resistant strains can be controlled by normal dosage schedule of mupirocin but few studies suggest that treatment failure may occur after few weeks. This emphasizes the importance of identification of both high and low-level resistant strains.[24,25,26]
MRSA nasal colonization of HCWs in our hospital is high, particularly among the nursing orderlies, who are in prolonged contact with the patient leading to the high possibility of nurse-to-patient transmission of these bacteria and dissemination of them in hospital setting. As a routine, screening and treatment of HCWs should be done for MRSA status in every hospital. It is also advisable to detect mupirocin resistance among the isolates obtained from the HCWs so that in case of resistance, alternative treatment options should be initiated.
AUTHORS’ CONTRIBUTION
Conceived and designed the experiments: LA, AKS. Performed the experiment in the laboratory: LA, CS. Analyzed the data: LA, AKS, AA. Wrote the first draft of the manuscript: LA. Contributed to the writing of the manuscript: LA, AKS, AA. Agree with manuscript results and conclusions: LA, AKS, CS, AA. Made critical revisions and approved final version: LA, AKS. All authors reviewed and approved of the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was conducted as part of Indian Council of Medical Research Short Term Studentship 2014.
REFERENCES
- 1.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: Attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–30. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 2.Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, et al. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in Europe. Euro Surveill. 2010;15:19688. doi: 10.2807/ese.15.41.19688-en. [DOI] [PubMed] [Google Scholar]
- 3.Albrich WC, Harbarth S. Health-care workers: Source, vector, or victim of MRSA? Lancet Infect Dis. 2008;8:289–301. doi: 10.1016/S1473-3099(08)70097-5. [DOI] [PubMed] [Google Scholar]
- 4.Solberg CO. Spread of Staphylococcus aureus in hospitals: Causes and prevention. Scand J Infect Dis. 2000;32:587–95. doi: 10.1080/003655400459478. [DOI] [PubMed] [Google Scholar]
- 5.Mathanraj S, Sujatha S, Sivasangeetha K, Parija SC. Screening for methicillin-resistant Staphylococcus aureus carriers among patients and health care workers of a tertiary care hospital in South India. Indian J Med Microbiol. 2009;27:62–4. [PubMed] [Google Scholar]
- 6.Paul M, Bishara J, Yahav D, Goldberg E, Neuberger A, Ghanem-Zoubi N, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by methicillin resistant Staphylococcus aureus: Randomised controlled trial. BMJ. 2015;350:h2219. doi: 10.1136/bmj.h2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lally RT, Lanz E, Schrock CG. Rapid control of an outbreak of Staphylococcus aureus on a neonatal intensive care department using standard infection control practices and nasal mupirocin. Am J Infect Control. 2004;32:44–7. doi: 10.1016/s0196-6553(03)00088-9. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi P, Singh AK, Singh AK, Shukla S, Agarwal L. Prevalence of mupirocin resistant Staphylococcus aureus isolates among patients admitted to a tertiary care hospital. N Am J Med Sci. 2014;6:403–7. doi: 10.4103/1947-2714.139293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayne, PA: Clinical and Laboratory Standard Institute; 2013. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Eleventh Edition and Performance Standards for Antimicrobial Susceptibility Testing: Twenty- Third Informational Supplement. CLSI Document M02-A11 and M100-S23. [Google Scholar]
- 10.Truong H, Shah SS, Ludmir J, Twananana EO, Bafana M, Wood SM, et al. Staphylococcus aureus skin and soft tissue infections at a tertiary hospital in Botswana. S Afr Med J. 2011;101:413–6. [PubMed] [Google Scholar]
- 11.Yazgi H, Ertek M, Ozbek A, Kadanali A. Nasal carriage of Staphylococcus aureus in hospital personnel and the normal population and antibiotic resistance of the isolates. Mikrobiyol Bul. 2003;37:137–42. [PubMed] [Google Scholar]
- 12.Singh AK, Gupta M, Agarwal A, Gupta P, Singh M. Prevalence of methicillin-resistant Staphylococcus aureus colonisation and its antibiotic susceptibility profile among healthcare personnel in a tertiary care setup of Northern India. Int J Curr Microbiol Appl Sci. 2013;2:293–9. [Google Scholar]
- 13.Al-Abdli NE, Baiu S. Nasal carriage of Staphylococcus in health care workers in Benghazi hospitals. Am J Microbiol Res. 2014;2:110–2. [Google Scholar]
- 14.Norazah A, Koh YT, Kamel AG, Alias R, Lim VK. Mupirocin resistance among Malaysian isolates of methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 2001;17:411–4. doi: 10.1016/s0924-8579(01)00314-4. [DOI] [PubMed] [Google Scholar]
- 15.Mulqueen J, Cafferty F, Cormican M, Keane JD, Rossney A. Nasal carriage of meticillin-resistant Staphylococcus aureus in GPs in the West of Ireland. Br J Gen Pract. 2007;57:811–3. [PMC free article] [PubMed] [Google Scholar]
- 16.Eveillard M, Martin Y, Hidri N, Boussougant Y, Joly-Guillou ML. Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: Prevalence, duration, and transmission to households. Infect Control Hosp Epidemiol. 2004;25:114–20. doi: 10.1086/502360. [DOI] [PubMed] [Google Scholar]
- 17.Akoua Koffi C, Dje K, Toure R, Guessennd N, Acho B, Faye Kette H, et al. Nasal carriage of meticillin-resistant Staphylococcus aureus among health care personnel in Abidjan (Côte d’lvoire) Dakar Med. 2004;49:70–4. [PubMed] [Google Scholar]
- 18.Cesur S, Cokça F. Nasal carriage of methicillin-resistant Staphylococcus aureus among hospital staff and outpatients. Infect Control Hosp Epidemiol. 2004;25:169–71. doi: 10.1086/502371. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha B, Pokhrel BM, Mohapatra TM. Staphylococcus aureus nasal carriage among health care workers in a Nepal Hospital. Braz J Infect Dis. 2009;13:322. doi: 10.1590/S1413-86702009000500001. [DOI] [PubMed] [Google Scholar]
- 20.Pulimood TB, Lalitha MK, Jesudason MV, Pandian R, Selwyn J, John TJ. The spectrum of antimicrobial resistance among methicillin resistant Staphylococcus aureus (MRSA) in a tertiary care centre in India. Indian J Med Res. 1996;103:212–5. [PubMed] [Google Scholar]
- 21.Qureshi AH, Rafi S, Qureshi SM, Ali AM. The current susceptibility patterns of methicillin resistant Staphylococcus aureus to conventional anti Staphylococcus antimicrobials at Rawalpindi. Pak J Med Sci. 2004;20:361–4. [Google Scholar]
- 22.Shittu AO, Lin J, Kolawole D. Antimicrobial susceptibility patterns of Staphylococcus aureus and characterization of MRSA in Southwestern Nigeria. Wounds. 2006;18:77–84. [Google Scholar]
- 23.Gadepalli R, Dhawan B, Mohanty S, Kapil A, Das BK, Chaudhry R, et al. Mupirocin resistance in Staphylococcus aureus in an Indian hospital. Diagn Microbiol Infect Dis. 2007;58:125–7. doi: 10.1016/j.diagmicrobio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Hudson IR. The efficacy of intranasal mupirocin in the prevention of staphylococcal infections: A review of recent experience. J Hosp Infect. 1994;27:81–98. doi: 10.1016/0195-6701(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 25.Singh AK, Venkatesh V, Singh M. Mupirocin resistance in clinical isolates of Staphylococcus aureus in a tertiary care hospital set up in North India. Int J Med Res Health Sci. 2013;2:840–7. [Google Scholar]
- 26.Walker ES, Vasquez JE, Dula R, Bullock H, Sarubbi FA. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: Does mupirocin remain effective? Infect Control Hosp Epidemiol. 2003;24:342–6. doi: 10.1086/502218. [DOI] [PubMed] [Google Scholar]


