Abstract
Objective:
This study was aimed to determine the prevalence of drug-related problems (DRPs), identify the most common drugs, and drug classes involved in DRPs as well as associated factors with the occurrence of DRPs.
Methods:
A prospective cross-sectional study was conducted on 225 patients admitted to medical wards of Tikur Anbessa Specialized Hospital, Addis Ababa from March to June 2014. Data regarding patient characteristics, medications, diagnosis, length of hospitalization, investigation, and laboratory results were collected using data abstraction forms through review of patients’ medical card and medication charts. Identified DRPs were recorded and classified using DRP registration forms. The possible intervention measures for the identified DRPs were proposed and communicated to either the physician or the patient. Data were entered into Epi Info 7 and analyzed using SPSS version 21 (IBM Corp. Released 2012, Armonk, NY: IBM Corp).
Findings:
DRPs were found in 52% of study subjects. A drug-drug interaction (48% of all DRPs) was the most common DRP followed by adverse drug reaction (23%). Anti-infectives and gastrointestinal medicines were commonly involved in DRPs. Drugs with the highest drug risk ratio were gentamycin, warfarin, nifedipine, and cimetidine. The number of drugs taken by the patient per day is an important risk factor for DRPs.
Conclusion:
DRPs are common among medical ward patients. Polypharmacy has a significant association with the occurrence of DRP. Drugs such as gentamycin, warfarin, nifedipine, and cimetidine have the highest probability of causing DRP. So, patients who are taking either of these drugs or polypharmacy should be closely assessed for identification and timely correction of DRPs.
Keywords: Drug-related problem, medical wards, polypharmacy, Tikur Anbessa, specialized hospital
INTRODUCTION
In the case of most diseases, drug therapy will enhance health-related quality of life. However, inappropriate use of drugs may be harmful and lead to drug therapy problems.[1] In order to achieve a quality health care service inappropriate use of drugs that potentially lead to problems should be identified and corrected. Drug-related problems (DRPs) are defined as events or circumstances involving drug therapy that actually or potentially interfere with desired health outcomes.[2]
An infinite number of DRPs exist because of the rapidly expanding array of drug products available, the growing number of diseases being recognized and diagnosed, and the growing number of patients entering the health care system. All patient problems involving medications can be grouped into one of the seven types of DRPs. These include unnecessary drug therapy, the need for additional drug therapy, ineffective drug, dosage too low, adverse drug reaction (ADR), dosage too high, and noncompliance.[3]
DRPs may lead to reduced quality of life, increased hospital stay, increased overall health care cost and even increases the risk of morbidity and mortality.[4] More people die of inappropriate drug treatment than from breast cancer, acquired immune deficiency syndrome, and traffic accidents altogether.[5] An Institute of Medicine report estimated that between 44,000 and 98,000 people in the U.S hospitals die each year because of medical errors.[5] It is estimated that the annual cost of drug-related morbidity and mortality is nearly 177 billion dollars in the United States. Twice as much money is used to solve DRPs and adverse drug events than on the drug themselves.[6]
Identification and intervention on actual and potential DRPs, along with awareness of drugs carrying a high-risk for DRPs, are important elements of drug therapy and may contribute to diminish drug-related morbidity and mortality. The objective of this study was to assess DRPs in medical wards of Tikur Anbessa Specialized Hospital (TASH). The findings of this study might also help in influencing the development of appropriate policies, plans, and intervention programs for the prevention and management of DRPs. This in turn, might improve the quality of care for patients admitted to hospitals.
METHODS
This prospective cross-sectional study was conducted in the medical wards of TASH, Addis Ababa, Ethiopia from March 15, 2014, to June 15, 2014. The hospital has more than 600 beds and gives diagnostic and treatment service for about 370,000–400,000 patients per year.[7] Two hundred twenty-five patients were included in the study by using simple random sampling method. Patients admitted to intensive care unit, patients with <48 h length of stay and those who refused to participate were excluded from the study.
Relevant information about each patient such as patient characteristics, physical examination, laboratory results, current medications, comorbidities, length of hospitalization, and relevant previous medical, and medication histories were recorded using data abstraction format through reviewing patients’ medical cards and medication charts. Supplementary information and clarifications on some patient's medical information were obtained through discussion with the patient and the physician. Taking 5–9 drugs/day were considered as minor polypharmacy and 10 or more drugs were considered as major polypharmacy.[8,9]
Data were collected, using a structured format, by two pharmacists who had basic knowledge on pharmaceutical care services who were instructed on how to obtain data from patient cards and medication charts and on how to approach the patients and health care professionals. Then the collected data were given to the authors of this research daily and we cheeked the appropriateness of drug therapy using updated references such as Medscape (WebMD, LLC), UpToDate® (version 21.2, Wolters Kluwer, Netherlands), Epocrates® (Epocrates, Inc., San Francisco) and Micromedex® (Micromedex 2.0., Truven Health Analytics Inc.) in order to determine the presence of DRP. Medscape drug interaction checker was used to identify drug-drug interactions. If DRP was identified it was recorded and classified using DRP registration format (taken from Cipolle et al., 2004 with modification).[3] Then the possible intervention measures were proposed and communicated to either the internist/resident/senior physician or the patient in order to resolve or prevent DRPs.
The data collection process was supervised, all filled DRP registration formats, and data abstraction formats were reviewed and cheeked for their completeness every day. After data was checked for completeness, it was edited, cleaned, and analyzed. The collected data were entered into Epi Info 7 software version 7.1.4 (Centers for Disease Control and Prevention, Atlanta, GA) and analyzed using IBM SPSS statistics for Windows version 21 (IBM Corp. Released 2012, Armonk, NY: IBM Corp). Cross-tabulation was used in the bivariate analysis. A test of association was done using binary and multiple logistic regressions. P < 0.05 was considered significant. Drug risk ratio (frequency of involvement in DRP divided by the frequency of prescription) was used to identify drugs that were prone to create DRP. Descriptive statistics was used to characterize DRPs. Results of the study were organized in the form of frequencies and percentages.
Letter of ethical clearance was obtained from the Ethical Review Committee of Addis Ababa University. Verbal consent from a patient was requested to extract data from patients’ medical card and medication charts. Privacy and confidentiality were ensured throughout the study. Thus, name and address of the patient was not recorded in the DRP registration and data abstraction forms.
RESULTS
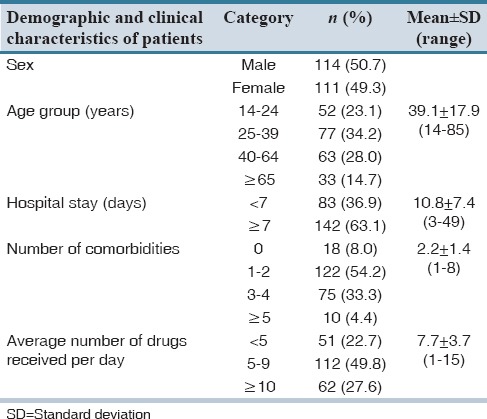
A total of 225 patients were included in the study, of which 114 (50.7%) were males. The mean age was 39.1 years with the maximum number of patients being in the age group of 25–39 years. The majority of the patients (122 cases, 54.2%) were found to have one to two comorbidities. Only 18 (8%) of the study subjects were without any comorbid condition. A total of 1729 medications were prescribed. An average number of drugs per day for a patient were 7.7. The majority of the study subjects (112 cases, 49.8%) received 5–9 drugs per day. Patients’ demographic characteristics along with other factors that may influence DRPs such as number of comorbidities, length of hospital stay, and an average number of drugs received per day are shown in Table 1.
Table 1.
Demographic details and clinical characteristics of the study subjects
The disease distribution of the study subjects showed a higher incidence of infections (37.9%) followed by cardiovascular diseases (21.1%), malignancies (8.1%), electrolyte abnormalities (7.5%), and others (25.4%). Commonly prescribed drug classes in the study subjects were anti-infectives (629), central nervous system drugs (294), gastrointestinal medicine (244), and cardiovascular drugs (171).
DRPs were found in 52% of the study subjects. A total of 152 DRPs were identified from 117 patients during the study period, in which, one DRP was identified in 82 (70.1%) patients, 2 DRPS in 31 (26.5%) patients, and more than 2 DRPs in 4 (3.4%) patients. Average number of DRP per patient was 0.68.
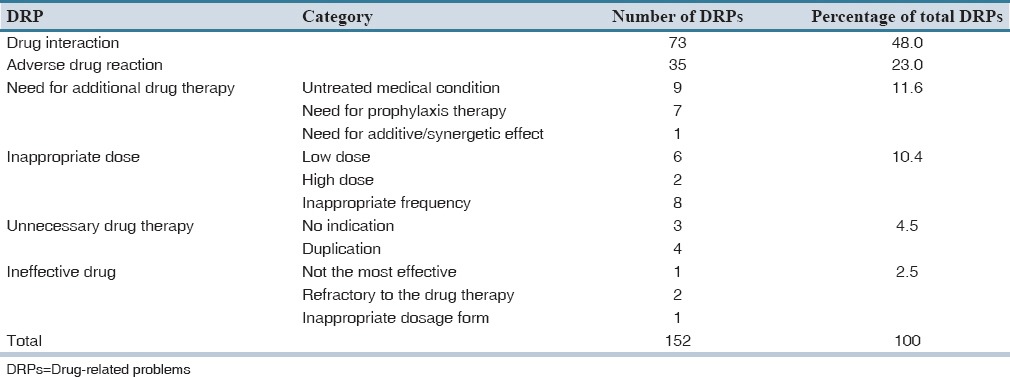
Drug interaction was the most common DRP (73 [48%] of all DRPs) followed by ADR (23%). Of the 17 DRPs classified as need for additional drug therapy, 9 (52.9%) were because of untreated medical condition, and 7 (41.2%) were due to need for prophylaxis therapy to reduce the risk of developing a new condition. The type and number of DRPs identified were characterized as shown in Table 2.
Table 2.
Types of DRPs identified
A total of 64 drugs were involved in different types of DRPs. Among these, the most frequently involved drugs in DRPs were cimetidine (36), tramadol (20), heparin (18), warfarin (16), ceftriaxone (12), prednisolone (11), and cotrimoxazol (10). Gentamycin, warfarin, nifedipine, cimetidine, simvastatin, prednisolone, digoxin, and pethidine were drugs with the highest drug risk ratio. These drugs had a risk ratio of above 0.33 which indicates that DRP may occur in more than one-third of the times these drugs are used. Figure 1 shows frequency and drug risk ratio of commonly used drugs which were involved in DRP.
Figure 1.
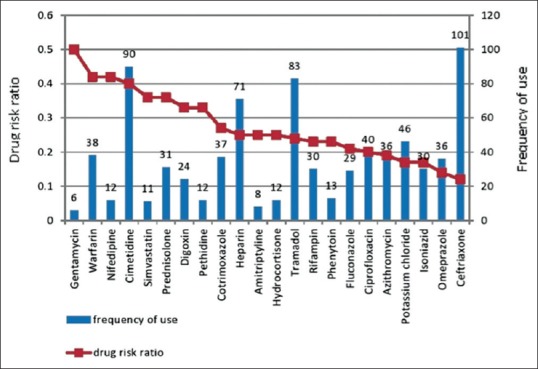
Frequency and drug risk ratio of commonly used drugs involved in drug-related problems
Appropriate intervention, measures were taken to correct the identified DRPs. The most commonly applied intervention was informing the physicians to monitor for the adverse effect of a drug (69) which may occur due to a potential drug-drug interaction or potential ADR because of risk factors in a patient such as renal failure, history of allergy, and comorbidity. The rest of the interventions were as following: To discontinue the drug (33), to change the frequency of administration (24), to do laboratory monitoring (16), to change the dose (13), to change the drug (9), and to monitor for the drug's effectiveness (4).
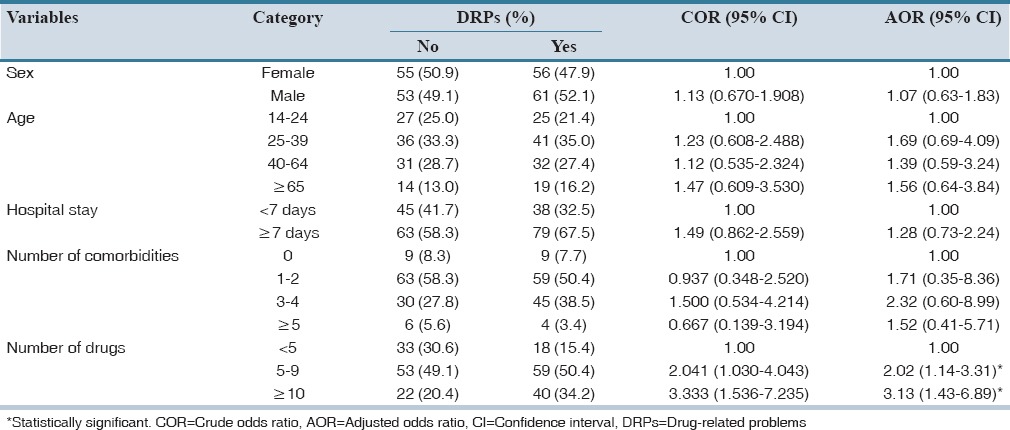
The identification of risk factors for DRPs may be helpful in finding patients at risk. These patients can then be given special attention, with the hope of avoiding overt DRPs. Sex, age, the average number of drugs per day, length of hospital stay, and number of comorbidities were analyzed to determine whether they could predict the occurrence of DRPs or not. The average number of drugs taken by the patient per day was shown to be a risk factor for the occurrence of DRPs while age, sex, length of hospital stay, and number of comorbidities were not. As shown in Table 3, patients who took an average of 5–9 drugs per day (minor polypharmacy) are 2 times more likely to develop DRPs as compared to patients who took <5 drugs per day while those taking more than 10 drugs per day (major polypharmacy) are 3.1 times more prone in developing DRP.
Table 3.
Associated factors for the occurrence of DRPs
DISCUSSION
The goal of drug therapy is to achieve defined therapeutic outcomes and improve the patient's quality of life while minimizing patient risk. However, inappropriate use of drugs during disease management may lead to drug therapy problems. This study was carried out to assess DRPs in medical inpatients of one of a tertiary care teaching hospital in Ethiopia.
This study showed that 52% of patients admitted to the medical wards of TASH had DRPs. This result is lower than what was found in Jimma, Ethiopia (73.5%).[10] The lower rate of DRP in this study might be because the data collection of the study in Jimma were done at 2011 when clinical pharmacists were not involved in the ward-based activities, while in the period and setting of the current study, pharmacists were involved in the ward activities. The study in Malaysia also found a prevalence of 90.5%.[11]
The most frequently encountered DRPs in the present study were drug-drug interaction. Similar studies from different countries showed drug-drug interaction was among the top ranking types of DRPs.[8,12,13,14] Even though the majority of drug-drug interactions in our study were only potential, there are some interactions that had resulted in significant adverse events like bleeding, raised international normalized ratio (INR), hypoglycemia, and constipation. The most common intervention measure given for this class of DRP was informing the physicians to monitor the patient for serious adverse events. In addition, monitoring for the effectiveness of some drugs and limiting the daily dose of a drug (e.g., paracetamol dose not more than 2 g/day when given with imatinib or rifampin) were taken as intervention measures. As drug interactions can affect patient's clinical outcome, quality of life, as well as contribute to unnecessary healthcare cost, the high prevalence rate (48% of all the DRPs) in this study would make this an important area requiring future focus of pharmacists on reviewing patients’ medication charts and checking for potential drug-drug interactions.
The second most common DRP identified in this study was ADR (23%). This was in close proximity with the result of Harvard Medical Practice Study showing up to 20% of hospitalized patients experiencing at least one ADR during their hospital stay.[15] Some of the adverse drug events identified in this study were bleeding/increased INR due to warfarin; hepatotoxicity due to anti-tuberculosis (TB) medications, propylthiouracil, and cancer chemotherapy; allergic reaction due to cotrimoxazole, vancomycin, and furosemide; gastritis due to acetylsalicylic acid (ASA), cotrimoxazole and anti-TB drugs; nephrotoxicity due to vancomycin and tenofovir; atrioventricular block due to digoxin and megaloblastic anemia due to phenobarbital.
Among patients who needed additional drug therapy, 29.4% were those who needed potassium chloride for treating hypokalemia and those who needed stress ulcer prophylaxis. There were also patients who needed pyridoxine, cotrimoxazole, and anti-convulsants as prophylaxis for neuropathy, Pneumocystis carinii pneumonia, and seizure, respectively. Ineffective drug use (2.6%) was less frequently occurring DRP in this study. This result is in close agreement with a study in Jimma, which showed ineffective drug therapy (2.7%) as the less frequently occurring DRP among all the DRPs identified.[16]
It is necessary to be aware of those drugs with the highest drug risk ratio (high rate of causing DRP) since these are drugs most frequently expose the patient to DRP when taking them. In this study, gentamycin, warfarin, nifedipine, cimetidine, simvastatin, prednisolone, digoxin, and pethidine were drugs with the highest drug risk ratio. Similarly, the study in Norway mentions warfarin, prednisolone, and digoxin among drugs with the high-risk ratio.[17]
Cimetidine was one of the frequently prescribed drugs in medical wards. It has a higher drug risk ratio because of its frequent involvement in interaction with various drugs such as tramadol, prednisolone, dexamethasone, warfarin, fluconazole, cotrimoxazole, pethidine, hydrocortisone, diazepam, codeine, nimodipine, simvastatin, and digoxin. The mechanism of interaction of cimetidine with these drugs was either by inhibiting cytochrome P450 enzyme or by increasing gastric pH. Involving in drug interaction was also a very common reason for being a high-risk drug for digoxin, pethidine, and simvastatin. Gentamycin was found to cause nephrotoxicity because of its interaction with furosemide. In 42% of patients who took warfarin there was either an actual ADR (bleeding) or raised INR, or serious drug interaction with other drugs like cimetidine, ceftriaxone, cotrimoxazole, heparin, ciprofloxacin, ASA, fluconazole, and azithromycin. Nifedipine was involved in dose-related problem and drug interaction with phenytoin and simvastatin.
In the attempt to identify risk factors, the result of this study supported published findings that the number of drugs taken by a patient is an important risk factor for DRPs, but sex and age did not have significant correlation with the occurrence of DRP. The number of medications used was found to be a risk factor for increasing DRPs by a number of studies.[8,18,19,20,21] However, sex and age were not found to affect DRPs.[8,19,20,22] In our study, length of hospital stay and a number of comorbidities were not found to significantly affect the occurrence of DRP. Similarly, the study in Jimma by Tigabu et al. did not show a significant association between the likelihood of DRP occurrence and length of hospital stay or a number of comorbidity.[10]
In this study, drug therapy problems related to adherence to the prescribed medications were not addressed because of time and budget constraint. The result of the study may not be generalized to all hospitals because it was a single-centered study conducted in a hospital serving referred patients who have severe illnesses and more comorbidity. So we suggest further multi-centered studies to be done.
AUTHORS’ CONTRIBUTION
Mohammed Biset had contributed in proposal writing, data analysis and interpretation; write up of the final research and manuscript preparation and finalization.
Teshome Nedi had contributed in proposal writing, data analysis and interpretation and write up of the final research.
Yewondwossen Tadesse had contributed in proposal writing, questionnaire design and write up of the final research.
Financial support and sponsorship
This study was done with the financial support of Addis Ababa University.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fita R, Syed A, Dewa P, Wasilah R. Involvement of Ward Pharmacist During Theraputic Process in Hospitalized Geriatric Patients in Dr. Sarjito Hospital, Yogyakarta, Indonesia. 2008. [Last cited on 2014 Nov 09]. Available from: http://www.academia.edu/3579827 .
- 2.Classification for Drug Related Problems V 6.20. Pharmaceutical Care Network Europe Foundation; c2003-2010. [About 1 screen] [Last updated on 2010 Jan 14; Last cited on 2014 Feb 10]. Available from: http://wwwpcneorg/upload/files/11_PCNE_classification_V6-2.pdf .
- 3.Cipolle R, Strand L, Morley P. 2nd ed. New York: McGraw Hill; 2004. Pharmaceutical Care Practice: The Clinicians Guide. [Google Scholar]
- 4.Satish K, Prasanna D, Rajesh V, Prashant C. Assessment of clinical pharmacist intervention in tertiary care teaching hospital of southern India. Asian J Pharm Clin Res. 2013;6:258–61. [Google Scholar]
- 5.Kohl L, Corrigan J, Donaldson M, editors. Washington: National Academy Press; 2003. [Last cited on 2014 Feb 12]. To Err is Human: Building a Safer Health System. Available from: http://www.books.nap.edu/html/to_err_is_human/exec_summ.html . [PubMed] [Google Scholar]
- 6.Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: Updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001;41:192–9. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 7.Seattle Alliance Outreach. [Last updated on 2014 Jan 15; Last cited on 2014 Feb 16]. Available from: http://www.ethio.medic.com/indexphp/healthcare-facilities .
- 8.Koh Y, Kutty FB, Li SC. Drug-related problems in hospitalized patients on polypharmacy: The influence of age and gender. Ther Clin Risk Manag. 2005;1:39–48. doi: 10.2147/tcrm.1.1.39.53597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DC, Chen JH, Kuo HK, We CJ, Lu IS, Chiu LS, et al. Drug-related problems (DRPs) identified from geriatric medication safety review clinics. Arch Gerontol Geriatr. 2012;54:168–74. doi: 10.1016/j.archger.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Tigabu BM, Daba D, Habte B. Drug-related problems among medical ward patients in Jimma university specialized hospital, Southwest Ethiopia. J Res Pharm Pract. 2014;3:1–5. doi: 10.4103/2279-042X.132702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaman Huri H, Fun Wee H. Drug related problems in type 2 diabetes patients with hypertension: A cross-sectional retrospective study. BMC Endocr Disord. 2013;13:2. doi: 10.1186/1472-6823-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers A, Robays H, De Paepe P, Van Maele G, Perehudoff K, Petrovic M. Evaluation of clinical pharmacist recommendations in the geriatric ward of a Belgian university hospital. Clin Interv Aging. 2013;8:703–9. doi: 10.2147/CIA.S42162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Hajje AH, Atoui F, Awada S, Rachidi S, Zein S, Salameh P. Drug-related problems identified by clinical pharmacist's students and pharmacist's interventions. Ann Pharm Fr. 2012;70:169–76. doi: 10.1016/j.pharma.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Abbasinazari M, Hajhossein Talasaz A, Eshraghi A, Sahraei Z. Detection and management of medication errors in internal wards of a teaching hospital by clinical pharmacists. Acta Med Iran. 2013;51:482–6. [PubMed] [Google Scholar]
- 15.Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324:370–6. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 16.Mekonnen AB, Yesuf EA, Odegard PS, Wega SS. Implementing ward based clinical pharmacy services in an Ethiopian University Hospital. Pharm Pract (Granada) 2013;11:51–7. doi: 10.4321/s1886-36552013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol. 2007;63:187–95. doi: 10.1111/j.1365-2125.2006.02744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: Their structure and function. DICP. 1990;24:1093–7. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 19.Blix HS, Viktil KK, Reikvam A, Moger TA, Hjemaas BJ, Pretsch P, et al. The majority of hospitalised patients have drug-related problems: Results from a prospective study in general hospitals. Eur J Clin Pharmacol. 2004;60:651–8. doi: 10.1007/s00228-004-0830-4. [DOI] [PubMed] [Google Scholar]
- 20.Aburuz SM, Bulatova NR, Yousef AM, Al-Ghazawi MA, Alawwa IA, Al-Saleh A. Comprehensive assessment of treatment related problems in hospitalized medicine patients in Jordan. Int J Clin Pharm. 2011;33:501–11. doi: 10.1007/s11096-011-9497-y. [DOI] [PubMed] [Google Scholar]
- 21.Nickel CH, Ruedinger JM, Messmer AS, Maile S, Peng A, Bodmer M, et al. Drug-related emergency department visits by elderly patients presenting with non-specific complaints. Scand J Trauma Resusc Emerg Med. 2013;21:15. doi: 10.1186/1757-7241-21-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento Y, Carvalho W, Acurcio F. Drug related problems observed in a pharmaceutical care service, Belo Horizonte, Brazil. Braz J Pharm Sci. 2009;45:321–30. [Google Scholar]


