Abstract
Objective:
The inappropriate use of antibiotics remains the primary factor in antimicrobial drug resistance. In this study, we evaluate the use of meropenem in surgical/medical wards of Imam Khomeini Tertiary Referral Hospital, Sari, Iran.
Methods:
This retrospective observational study was used to assess rational use of meropenem. The study was conducted by reviewing medical records of 100 admitted patients who received meropenem during March 2013 to January 2014.
Findings:
Meropenem was prescribed most frequently in Intensive Care Unit (22%), and pneumonia was the most common diagnosis (35%). The third-generation cephalosporins were the most frequently prescribed antimicrobials after meropenem (53%). In 21% of the patients, imipenem was changed to meropenem. Most of the inappropriate uses were seen in terms of frequency of meropenem use (34%), followed by duration of meropenem therapy (28%).
Conclusion:
Comparing our study results has shown higher inappropriate use. It is necessary to take action to improve prescribing habit in order to reduce the unnecessary usage of antibiotic thus enhance rational antibiotic use.
Keywords: Antimicrobial resistance, appropriate drug use, drug use evaluation, Meropenem
INTRODUCTION
Antibiotics are the most frequently prescribed drugs among all hospitalized patients. About one-third of hospitalized patients receive antimicrobial therapy.[1] However, excessive and inappropriate use of the antibiotics remains the primary factor in the emergence and spread of antibiotic-resistant bacteria.[2] Optimizing medication utilization has the potential to reduce the development of antimicrobial resistance and to lower overall health care costs by providing cost-effective treatments.[3]
As one of the broad-spectrum antibiotics, meropenem is widely used to treat a wide variety of infections.[4] It is considered as a potent drug for treatment of multidrug resistant Gram-negative infections due to the stability of these agents against the majority of beta-lactamases and their high rate of permeation through bacterial outer membranes. However, there have been reports of the emergence of resistant to meropenem.[5,6] A recent study reported an emergence of imipenem-susceptible, meropenem-resistant Klebsiella pneumonia.[7] The high incident of empirical prescription for this drug in hospitals will potentially increase the prevalence of resistance, making it an important candidate for execution of drug use evaluation (DUE) studies. The aim of this study was to evaluate the use of meropenem in Imam Khomeini Hospital located in Mazandaran Province, Sari, Northern Iran, to provide an overview of its use in hospital in order to promote the rational prescribing, dispensing, and administration of meropenem.
METHODS
This retrospective observational study of meropenem usage was approved by the Ethics Committee of Mazandaran University of Medical Sciences and was conducted in the General Surgery, Surgical Intensive Care Unit (ICU), Medical ICU, Internal, Oncology, Orthopedic, Urology, Neurology and Obstetrics and Gynecology Wards of Imam Khomeini Hospital, Sari, Iran. Imam Khomeini Hospital is a Teaching Hospital Affiliated to Mazandaran University of Medical Sciences and is a 300-bed referral and Tertiary Care Hospital in North of Iran. The medical records of admitted patients who received meropenem during March 2013 to January 2014 were reviewed.
Demographic data, antibiotic medication history (agents, doses, dose intervals, routes of administration, number of doses, initiation times, and durations of administration), site(s) of infection, indication of meropenem use, initiation time, dosing regimen, rate and duration of administration, other co-prescribed antimicrobials, meropenem prescriber's specialty, renal function status, and microbiology laboratory results were collected and recorded in data gathering form. Analysis of the appropriateness of the use of meropenem was evaluated based on recommendations provided by American Hospital Formulary Services[8] and the Sanford Guide to Antimicrobial Therapy. Meropenem has approved indication for complicated skin/skin structure infections and intra-abdominal infections. It also has off-label indication for community-acquired pneumonia and febrile neutropenia. The appropriate use of meropenem requires considering appropriate dose, frequency, and duration of treatment. The usual dose for most of the indications is 500–1000 mg, every 8 h, and the maximum recommended dose is 2 g intravenous, every 8 h. The dose should be modified in renal impairment.[9]
Data were gathered and analyzed using the statistical software, SPSS (Version 19, IBM Corporation, Armonk, NY, USA), the qualitative variables are presented by their frequency of distribution. The quantitative variables are summarized as mean with standard deviation.
RESULTS
Meropenem was given to 153 adult hospitalized patients from August March 2013 to January 2014. One hundred of 153 patients were included in the study based on their medical record number randomly selected by “random number table.” Sixty-three of patients were male. Mean age of patients was 47.6 ± 20.7 years (range 14–83 years) [Table 1].
Table 1.
Demographic and clinical characteristics of the patients (n=100)
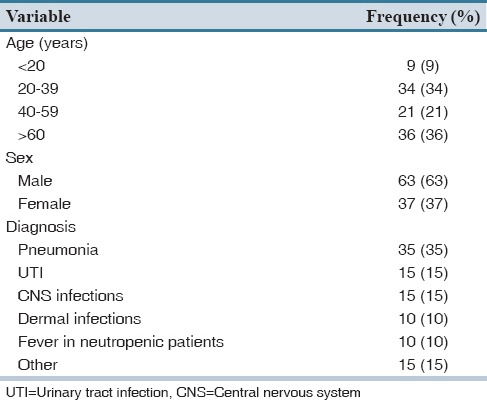
Meropenem was prescribed most frequently in ICU (22%), followed by oncology (19%), surgical (19%) and internal medicine (17%) wards. In 35% of the cases, meropenem was prescribed for pneumonia.
Mean duration of hospitalization was 15.6 ± 11 days (range 2–55 days). In 63% of the hospitalized patients, antimicrobial therapy was started from the 1st day of admission. The mean duration of antimicrobial therapy was 13.5 ± 10.6 days. In addition to meropenem, patients received 2.75 ± 1.47 antibiotics during their hospital stay [Table 2]. Third generation cephalosporins (53%) and vancomycin (52%) were among the most frequently prescribed antimicrobials. In 21% of the patients, imipenem was changed to meropenem and in 1 case it was due to imipenem induced seizure.
Table 2.
Antibiotic prescribing data (n=100)
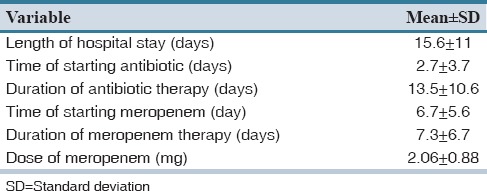
An infectious disease consultation was recorded in 52% of the cases. The renal function tests were evaluated in 96% of patients. Nine patients had abnormal results, and only three of them received dosage adjustment based on their renal function. The prescribed daily dose of meropenem for each patient was 2.06 ± 0.88 g [Table 2]. Patients received 1.32 ± 0.84 antimicrobial agent concomitant with meropenem.
All the patients received meropenem as an empiric therapy. Microbiological cultures were utilized in the course of therapy for 38% of patients receiving meropenem. In 34.4% of the cases, samples for culture obtained before antimicrobial therapy, for 15.6%, it was performed within 24 h after starting antimicrobial therapy. The most common isolated micro-organisms were Acinetobacter spp., Escherichia coli, Enterococcus spp., and Pseudomonas aeruginosa. Antibiogram was performed only for three of them.
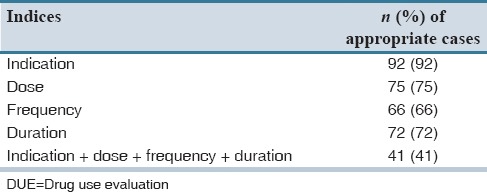
It was found that 95% of meropenem indications were appropriate, 92.5% and 52.5% of indications contained correct dose and frequency of the drug respectively, in 51.6% of the patients, the antibiotic therapy duration was not appropriate [Table 3].
Table 3.
Distribution of appropriate use of meropenem based on DUE criteria (n=100)
DISCUSSION
This study provides the data on the use of meropenem in patients admitted to surgical/medical wards of Imam Khomeini Hospital. Previously we evaluate the rational use of imipenem in this center.[10] Meropenem and imipenem are two carbapenems with similar spectrum.[11] However, important differences exist between the two compounds in favor of meropenem.[12] These include the greater in vitro activity of meropenem against the predominant Gram-negative pathogens[11] and its stability to renal dehydropeptidase-I (DHP-I), which permits its administration without a DHP-I inhibitor such as cilastatin (which can accumulate in renal failure).[13] Also, meropenem is well-tolerated by the central nervous system with regard to seizures.[12]
In our study, appropriate meropenem use obtained in only 41% of the patients. Previous studies evaluating meropenem use in hospitals have reported that 21–46.5% of prescriptions can be inappropriate.[14,15,16,17] Comparing our study results has shown higher inappropriate use. In our study, most of the inappropriate prescribing occurred in surgery ward. Surgeons might be a suitable primary target for the interventional studies. The panel discussions including different specialties, seminars, and pamphlets about antimicrobial decision making and antimicrobial rational use will be helpful.[3]
Our results show that meropenem therapy was started for all patients based on empiric therapy, and microbiological cultures were utilized only for 38% of the patients. It seems to be reasonable to promote practice guidelines about utilizing culture and sensitivity testing when considering the use of broad spectrum antibiotic like meropenem. Also in our study, antibiotic recommendations by infectious disease specialist consultation were done for 52 patients despite its rate is much higher than the rate of imipenem consultation.[10] Our hospital should consider guidelines regarding broad-spectrum antimicrobials, which include a requirement of an infectious disease consultation prior to initiation of these drugs.
We performed a DUE study for meropenem and attempted to gather basic data to examine the appropriate use of antibiotics. Our findings highlight the meropenem prescription defects in Imam Khomeini Hospital, including high rate of empiric prescription, lack of attention to dosage adjustment in patients with renal failure, initiation of antimicrobial therapy from the first day of hospitalization in high percentage of patients, and inadequate culture and sensitivity tests.
AUTHORS’ CONTRIBUTION
Salehifar designed the study and contributed in data analysis and revising the manuscript; A. Shiva supervised data collection and contributed in data analysis and writing the initial draft of the manuscript; M. Moshayedi contributed in data collection and contributed in writing the initial draft of the manusvript; T. Samiei Kashi and A. Chabra contributed in data collection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Erbay A, Bodur H, Akinci E, Colpan A. Evaluation of antibiotic use in intensive care units of a tertiary care hospital in Turkey. J Hosp Infect. 2005;59:53–61. doi: 10.1016/j.jhin.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh W, Dean W. Factors associated with the inappropriate use of antimicrobials. Zoonoses Public Health. 2015;62(Suppl 1):22–8. doi: 10.1111/zph.12169. [DOI] [PubMed] [Google Scholar]
- 3.May L, Cosgrove S, L’Archeveque M, Talan DA, Payne P, Jordan J, et al. A call to action for antimicrobial stewardship in the emergency department: Approaches and strategies. Ann Emerg Med. 2013;62:69–77.e2. doi: 10.1016/j.annemergmed.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, et al. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int J Antimicrob Agents. 2011;37:244–7. doi: 10.1016/j.ijantimicag.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Suh B, Bae IK, Kim J, Jeong SH, Yong D, Lee K. Outbreak of meropenem-resistant Serratia marcescens comediated by chromosomal AmpC beta-lactamase overproduction and outer membrane protein loss. Antimicrob Agents Chemother. 2010;54:5057–61. doi: 10.1128/AAC.00768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejikeugwu PC, Ugwu CM, Araka CO, Gugu TH, Iroha IR, Adikwu MU. Imipenem and meropenem resistance amongst ESBL producing Escherichia coli and Klebsiella pneumoniae clinical isolates. Int Res J Microbiol. 2012;3:339–44. [Google Scholar]
- 7.Shigemoto N, Kuwahara R, Kayama S, Shimizu W, Onodera M, Yokozaki M, et al. Emergence in Japan of an imipenem-susceptible, meropenem-resistant Klebsiella pneumoniae carrying blaIMP-6. Diagn Microbiol Infect Dis. 2012;72:109–12. doi: 10.1016/j.diagmicrobio.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy GK, editor . Bethesda (MD): American Society of Health-System Pharmacists, Inc; 2015. AHFS Drug Information. [Google Scholar]
- 9.Gilbert DN, Moellering RC, Eliopoulos GM, Chambers HF, Saag MS. Sperryville: Antimicrobial Therapy; 2014. The Sanford Guide to Antimicrobial Therapy. [Google Scholar]
- 10.Shiva A, Salehifar E, Amini M, Ala S, Rafati MR, Ganji R. Drug utilization evaluation of imipenem in an educational hospital in Mazandaran Province. Pharm Sci. 2014;20:12–7. [Google Scholar]
- 11.Hawkey PM, Livermore DM. Carbapenem antibiotics for serious infections. BMJ. 2012;344:e3236. doi: 10.1136/bmj.e3236. [DOI] [PubMed] [Google Scholar]
- 12.Hornik CP, Herring AH, Benjamin DK Jr, Capparelli EV, Kearns GL, van den Anker J, et al. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatr Infect Dis J. 2013;32:748–53. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito T, Sawazaki R, Ujiie K, Oda M, Saitoh H. Possible factors involved in oral inactivity of meropenem, a carbapenem antibiotic. Pharmacol Pharm. 2012;3:201–6. [Google Scholar]
- 14.Tarcea Bizo P, Dumitras D, Popa A. Evaluation of restricted antibiotic use in a hospital in Romania. Int J Clin Pharm. 2015;37:452–6. doi: 10.1007/s11096-015-0096-1. [DOI] [PubMed] [Google Scholar]
- 15.Khan MU, Yousuf RI, Shoaib MH. Drug utilization evaluation of meropenem and correlation of side effects with renal status of patients in a teaching based hospital. Pak J Pharm Sci. 2014;27:1503–8. [PubMed] [Google Scholar]
- 16.Mahini S, Hayatshahi A, Torkamandi H, Gholami KH, Javadi MR. Carbapenem utilization in critically Ill patients. J Pharm Care. 2014;1:141–4. [Google Scholar]
- 17.Raveh D, Muallem-Zilcha E, Greenberg A, Wiener-Well Y, Schlesinger Y, Yinnon AM. Prospective drug utilization evaluation of three broad-spectrum antimicrobials: Cefepime, piperacillin-tazobactam and meropenem. QJM. 2006;99:397–406. doi: 10.1093/qjmed/hcl050. [DOI] [PubMed] [Google Scholar]


