Abstract
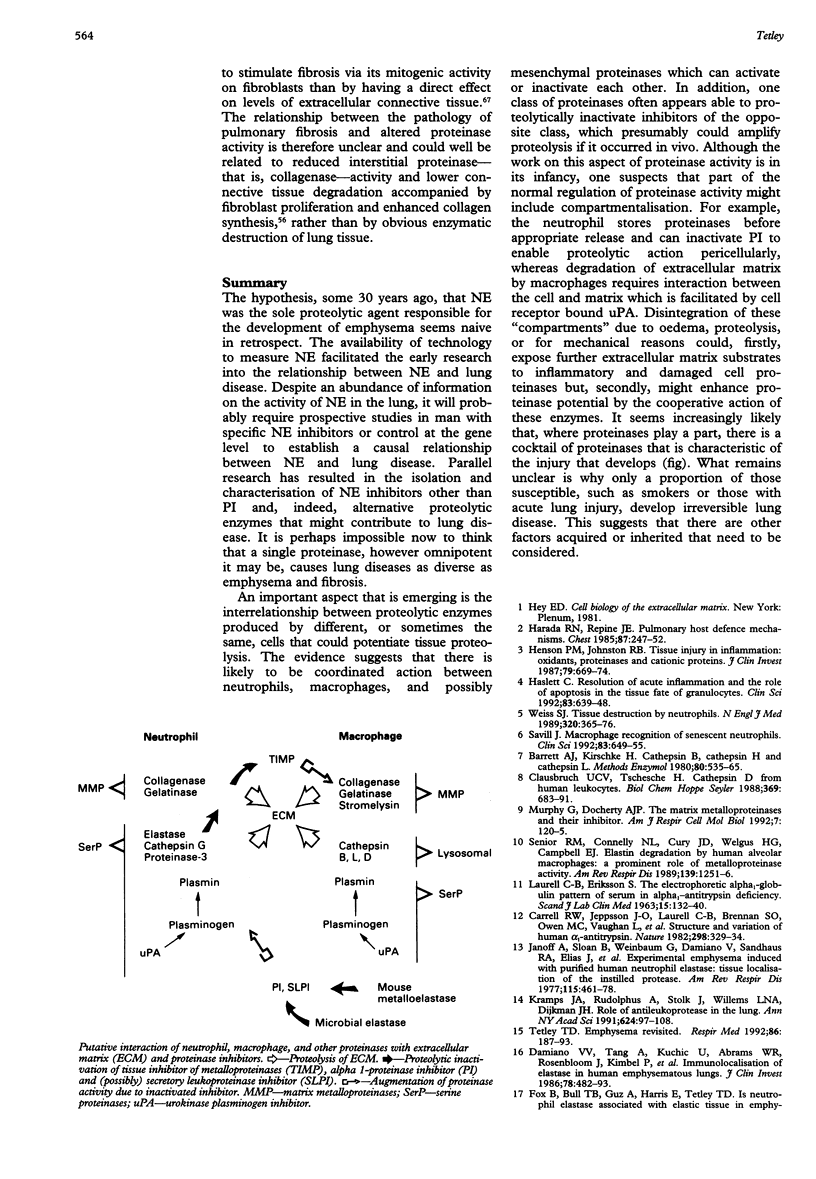
The hypothesis, some 30 years ago, that NE was the sole proteolytic agent responsible for the development of emphysema seems naive in retrospect. The availability of technology to measure NE facilitated the early research into the relationship between NE and lung disease. Despite an abundance of information on the activity of NE in the lung, it will probably require prospective studies in man with specific NE inhibitors or control at the gene level to establish a causal relationship between NE and lung disease. Parallel research has resulted in the isolation and characterisation of NE inhibitors other than PI and, indeed, alternative proteolytic enzymes that might contribute to lung disease. It is perhaps impossible now to think that a single proteinase, however omnipotent it may be, causes lung diseases as diverse as emphysema and fibrosis. An important aspect that is emerging is the interrelationship between proteolytic enzymes produced by different, or sometimes the same, cells that could potentiate tissue proteolysis. The evidence suggests that there is likely to be coordinated action between neutrophils, macrophages, and possibly mesenchymal proteinases which can activate or inactivate each other. In addition, one class of proteinases often appears able to proteolytically inactivate inhibitors of the opposite class, which presumably could amplify proteolysis if it occurred in vivo. Although the work on this aspect of proteinase activity is in its infancy, one suspects that part of the normal regulation of proteinase activity might include compartmentalisation. For example, the neutrophil stores proteinases before appropriate release and can inactivate PI to enable proteolytic action pericellularly, whereas degradation of extracellular matrix by macrophages requires interaction between the cell and matrix which is facilitated by cell receptor bound uPA. Disintegration of these "compartments" due to oedema, proteolysis, or for mechanical reasons could, firstly, expose further extracellular matrix substrates to inflammatory and damaged cell proteinases but, secondly, might enhance proteinase potential by the cooperative action of these enzymes. It seems increasingly likely that, where proteinases play a part, there is a cocktail of proteinases that is characteristic of the injury that develops (fig). What remains unclear is why only a proportion of those susceptible, such as smokers or those with acute lung injury, develop irreversible lung disease. This suggests that there are other factors acquired or inherited that need to be considered.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amitani R., Wilson R., Rutman A., Read R., Ward C., Burnett D., Stockley R. A., Cole P. J. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Jan;4(1):26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- Baird B. R., Cheronis J. C., Sandhaus R. A., Berger E. M., White C. W., Repine J. E. O2 metabolites and neutrophil elastase synergistically cause edematous injury in isolated rat lungs. J Appl Physiol (1985) 1986 Dec;61(6):2224–2229. doi: 10.1152/jappl.1986.61.6.2224. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Breuer R., Lucey E. C., Stone P. J., Christensen T. G., Snider G. L. Proteolytic activity of human neutrophil elastase and porcine pancreatic trypsin causes bronchial secretory cell metaplasia in hamsters. Exp Lung Res. 1985;9(1-2):167–175. doi: 10.3109/01902148509061535. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr Role of enzyme receptors and inhibitors in regulating proteolytic activities of macrophages. Ann N Y Acad Sci. 1991;624:87–96. doi: 10.1111/j.1749-6632.1991.tb17009.x. [DOI] [PubMed] [Google Scholar]
- Christner P., Fein A., Goldberg S., Lippmann M., Abrams W., Weinbaum G. Collagenase in the lower respiratory tract of patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985 May;131(5):690–695. doi: 10.1164/arrd.1985.131.5.690. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Kuhn C., 3rd Bleomycin-induced pulmonary fibrosis in hamsters: effect of neutrophil depletion on lung collagen synthesis. Am Rev Respir Dis. 1982 Oct;126(4):737–739. doi: 10.1164/arrd.1982.126.4.737. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B., MacArthur C., Idell S., Maunder R., Martin T., Dinarello C. A., Griffith D., McLarty J. A peptide from alveolar macrophages that releases neutrophil enzymes into the lungs in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1988 May;137(5):1151–1158. doi: 10.1164/ajrccm/137.5.1151. [DOI] [PubMed] [Google Scholar]
- Damiano V. V., Tsang A., Kucich U., Abrams W. R., Rosenbloom J., Kimbel P., Fallahnejad M., Weinbaum G. Immunolocalization of elastase in human emphysematous lungs. J Clin Invest. 1986 Aug;78(2):482–493. doi: 10.1172/JCI112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estreicher A., Mühlhauser J., Carpentier J. L., Orci L., Vassalli J. D. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990 Aug;111(2):783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera T., Abboud R. T., Richter A., Johal S. S. Acute effect of smoking on elastaselike esterase activity and immunologic neutrophil elastase levels in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1986 Apr;133(4):568–573. doi: 10.1164/arrd.1986.133.4.568. [DOI] [PubMed] [Google Scholar]
- Fox B., Bull T. B., Guz A., Harris E., Tetley T. D. Is neutrophil elastase associated with elastic tissue in emphysema? J Clin Pathol. 1988 Apr;41(4):435–440. doi: 10.1136/jcp.41.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita J., Nelson N. L., Daughton D. M., Dobry C. A., Spurzem J. R., Irino S., Rennard S. I. Evaluation of elastase and antielastase balance in patients with chronic bronchitis and pulmonary emphysema. Am Rev Respir Dis. 1990 Jul;142(1):57–62. doi: 10.1164/ajrccm/142.1.57. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., James H. L., Zinkgraf S., Perlman M. B., Keogh B. A. Lower respiratory tract abnormalities in rheumatoid interstitial lung disease. Potential role of neutrophils in lung injury. Am Rev Respir Dis. 1987 Oct;136(4):811–817. doi: 10.1164/ajrccm/136.4.811. [DOI] [PubMed] [Google Scholar]
- Ghafouri M. A., Patil K. D., Kass I. Sputum changes associated with the use of ipratropium bromide. Chest. 1984 Sep;86(3):387–393. doi: 10.1378/chest.86.3.387. [DOI] [PubMed] [Google Scholar]
- Glassroth J. L., Bernardo J., Lucey E. C., Center D. M., Jung-Legg Y. J., Snider G. L. Interstitial pulmonary fibrosis induced in hamsters by intratracheally administered chrysotile asbestos. Histology, lung mechanics, and inflammatory events. Am Rev Respir Dis. 1984 Aug;130(2):242–248. doi: 10.1164/arrd.1984.130.2.242. [DOI] [PubMed] [Google Scholar]
- Harada R. N., Repine J. E. Pulmonary host defense mechanisms. Chest. 1985 Feb;87(2):247–252. doi: 10.1378/chest.87.2.247. [DOI] [PubMed] [Google Scholar]
- Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci (Lond) 1992 Dec;83(6):639–648. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnston R. B., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987 Mar;79(3):669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S., Kucich U., Fein A., Kueppers F., James H. L., Walsh P. N., Weinbaum G., Colman R. W., Cohen A. B. Neutrophil elastase-releasing factors in bronchoalveolar lavage from patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985 Nov;132(5):1098–1105. doi: 10.1164/arrd.1985.132.5.1098. [DOI] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Jordana M. Mast cells and fibrosis--who's on first? Am J Respir Cell Mol Biol. 1993 Jan;8(1):7–8. doi: 10.1165/ajrcmb/8.1.7. [DOI] [PubMed] [Google Scholar]
- Kercsmar C. M., Davis P. B. Resistance of human tracheal epithelial cells to killing by neutrophils, neutrophil elastase, and Pseudomonas elastase. Am J Respir Cell Mol Biol. 1993 Jan;8(1):56–62. doi: 10.1165/ajrcmb/8.1.56. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Rudolphus A., Stolk J., Willems L. N., Dijkman J. H. Role of antileukoprotease in the human lung. Ann N Y Acad Sci. 1991;624:97–108. doi: 10.1111/j.1749-6632.1991.tb17010.x. [DOI] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Lewis R. W., Harwood J. L., Tetley T. D., Harris E., Richards R. J. Degradation of human and rat surfactant apoprotein by neutrophil elastase and cathepsin G. Biochem Soc Trans. 1993 May;21(2):206S–206S. doi: 10.1042/bst021206s. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- McGuire W. W., Spragg R. G., Cohen A. B., Cochrane C. G. Studies on the pathogenesis of the adult respiratory distress syndrome. J Clin Invest. 1982 Mar;69(3):543–553. doi: 10.1172/JCI110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Docherty A. J. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992 Aug;7(2):120–125. doi: 10.1165/ajrcmb/7.2.120. [DOI] [PubMed] [Google Scholar]
- O'Connor C., Odlum C., Van Breda A., Power C., Fitzgerald M. X. Collagenase and fibronectin in bronchoalveolar lavage fluid in patients with sarcoidosis. Thorax. 1988 May;43(5):393–400. doi: 10.1136/thx.43.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene F. P., Martin S. E., Parker M. M., Schlesinger T., Roach P., Burch C., Shelhamer J. H., Parrillo J. E. Adult respiratory distress syndrome in patients with severe neutropenia. N Engl J Med. 1986 Aug 28;315(9):547–551. doi: 10.1056/NEJM198608283150904. [DOI] [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I., Kishi J., Hayakawa T., Watorek W., Travis J., Nagase H. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988 Feb 29;229(1):157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Rao N. V., Wehner N. G., Marshall B. C., Sturrock A. B., Huecksteadt T. P., Rao G. V., Gray B. H., Hoidal J. R. Proteinase-3 (PR-3): a polymorphonuclear leukocyte serine proteinase. Ann N Y Acad Sci. 1991;624:60–68. doi: 10.1111/j.1749-6632.1991.tb17006.x. [DOI] [PubMed] [Google Scholar]
- Rickard K. A., Taylor J., Rennard S. I. Observations of development of resistance to detachment of cultured bovine bronchial epithelial cells in response to protease treatment. Am J Respir Cell Mol Biol. 1992 Apr;6(4):414–420. doi: 10.1165/ajrcmb/6.4.414. [DOI] [PubMed] [Google Scholar]
- Savill J. Macrophage recognition of senescent neutrophils. Clin Sci (Lond) 1992 Dec;83(6):649–655. doi: 10.1042/cs0830649. [DOI] [PubMed] [Google Scholar]
- Selman M., Pardo A. Potential role of proteases in pulmonary fibrosis. Ann N Y Acad Sci. 1991;624:297–306. doi: 10.1111/j.1749-6632.1991.tb17028.x. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Connolly N. L., Cury J. D., Welgus H. G., Campbell E. J. Elastin degradation by human alveolar macrophages. A prominent role of metalloproteinase activity. Am Rev Respir Dis. 1989 May;139(5):1251–1256. doi: 10.1164/ajrccm/139.5.1251. [DOI] [PubMed] [Google Scholar]
- Simon R. H., DeHart P. D., Todd R. F., 3rd Neutrophil-induced injury of rat pulmonary alveolar epithelial cells. J Clin Invest. 1986 Nov;78(5):1375–1386. doi: 10.1172/JCI112724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. F., Guz A., Cooke N. T., Burton G. H., Tetley T. D. Extracellular elastolytic activity in human lung lavage: a comparative study between smokers and non-smokers. Clin Sci (Lond) 1985 Jul;69(1):17–27. doi: 10.1042/cs0690017. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Ciccolella D. E., Morris S. M., Stone P. J., Lucey E. C. Putative role of neutrophil elastase in the pathogenesis of emphysema. Ann N Y Acad Sci. 1991;624:45–59. doi: 10.1111/j.1749-6632.1991.tb17005.x. [DOI] [PubMed] [Google Scholar]
- Sommerhoff C. P., Nadel J. A., Basbaum C. B., Caughey G. H. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Invest. 1990 Mar;85(3):682–689. doi: 10.1172/JCI114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponer M., Nick H. P., Schnebli H. P. Different susceptibility of elastase inhibitors to inactivation by proteinases from Staphylococcus aureus and Pseudomonas aeruginosa. Biol Chem Hoppe Seyler. 1991 Nov;372(11):963–970. doi: 10.1515/bchm3.1991.372.2.963. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Burnett D. Proteinases in chronic lung infection. Ann N Y Acad Sci. 1991;624:257–266. doi: 10.1111/j.1749-6632.1991.tb17024.x. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Hill S. L., Morrison H. M., Starkie C. M. Elastolytic activity of sputum and its relation to purulence and to lung function in patients with bronchiectasis. Thorax. 1984 Jun;39(6):408–413. doi: 10.1136/thx.39.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Tegner H., Ohlsson K., Desgrandchamps D., Waldvogel F. A. Levels of free granulocyte elastase in bronchial secretions from patients with cystic fibrosis: effect of antimicrobial treatment against Pseudomonas aeruginosa. J Infect Dis. 1986 May;153(5):902–909. doi: 10.1093/infdis/153.5.902. [DOI] [PubMed] [Google Scholar]
- Tetley T. D. Emphysema revisited. Respir Med. 1992 May;86(3):187–193. doi: 10.1016/s0954-6111(06)80053-3. [DOI] [PubMed] [Google Scholar]
- Tetley T. D., Smith S. F., Winning A. J., Foxall J. M., Cooke N. T., Burton G. H., Harris E., Guz A. The acute effect of cigarette smoking on the neutrophil elastase inhibitory capacity of peripheral lung lavage from asymptomatic volunteers. Eur Respir J. 1989 Oct;2(9):802–810. [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W., D'Amato D. A., Sulavik S. B. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982 Sep;126(3):488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- Tournier J. M., Jacquot J., Puchelle E., Bieth J. G. Evidence that Pseudomonas aeruginosa elastase does not inactivate the bronchial inhibitor in the presence of leukocyte elastase. Studies with cystic fibrosis sputum and with pure proteins. Am Rev Respir Dis. 1985 Sep;132(3):524–528. doi: 10.1164/arrd.1985.132.3.524. [DOI] [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Weiland J. E., Garcia J. G., Davis W. B., Gadek J. E. Neutrophil collagenase in rheumatoid interstitial lung disease. J Appl Physiol (1985) 1987 Feb;62(2):628–633. doi: 10.1152/jappl.1987.62.2.628. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Weitz J. I., Silverman E. K., Thong B., Campbell E. J. Plasma levels of elastase-specific fibrinopeptides correlate with proteinase inhibitor phenotype. Evidence for increased elastase activity in subjects with homozygous and heterozygous deficiency of alpha 1-proteinase inhibitor. J Clin Invest. 1992 Mar;89(3):766–773. doi: 10.1172/JCI115654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Clausbruch U. C., Tschesche H. Cathepsin D from human leukocytes. Purification by affinity chromatography and properties of the enzyme. Biol Chem Hoppe Seyler. 1988 Aug;369(8):683–691. doi: 10.1515/bchm3.1988.369.2.683. [DOI] [PubMed] [Google Scholar]




