| Patent Application Title: |
Isothiazole Derivatives as GPR120 Agonists for The Treatment of Type 2 Diabetes |
| Patent Application Number: |
WO 2015/134039 A1 |
Publication date: |
11 September 2015 |
| Priority Application: |
None given |
| Inventors: |
Illig, C. R.; Player, M. R.; Zhang, X. |
| Assignee Company: |
Janssen Pharmaceutica NV; Turnhoutseweg 30, B-2340 Beerse (BE) |
| Disease Area: |
Type 2 Diabetes and obesity-related disorders |
Biological Target: |
G-protein coupled receptor 120 (GPR120) |
| Summary: |
The invention in this patent application relates to isothiazole and thiophene derivatives represented generally by formula (I), which are GPR120 agonists and may potentially be useful for the treatment of Type 2 diabetes mellitus, obesity, obesity-related disorders, impaired oral glucose tolerance, and insulin resistance. |
| Statistics have shown that current drug therapies for Type 2 diabetes are lacking durable efficacy. More than half of patients on current oral medications fail to reach the targeted blood glucose control after 5 years of treatment. Thus, there is an urgent need for new drug therapies to treat Type 2 diabetes. |
| Glucagon-like peptide-1 receptor (GLP-1) is a member of the glucagon receptor family of G protein-coupled receptors. It is a key regulator of glucose homeostasis, which is secreted by the L-cells in the colon following meals. It is an incretin hormone that potentiates insulin secretion, reduces glucagon secretion, preserves β-cell function, and improves satiety. GLP-1 has been a therapeutic target for several of the recently approved Type 2 diabetes drugs including Januvia (Merck) and Galvus (Novartis), which act by prolonging the half-life of GLP-1, and Byetta (Amylin), which acts by activating the GLP-1 receptor. |
| The complex pathology of free fatty acids (FFAs) plays a key role in the progression of diabetes. While the acute exposure of FFAs in the pancreas and the colon stimulates glucose-dependent insulin secretion and GLP-1 release, chronic exposure of FFAs impairs insulin secretion and becomes toxic to β-cells. The accumulation of FFAs in insulin responsive tissues such as muscles and liver causes tissue insulin resistance. Hyperinsulinemia in the liver has been linked to increased accumulation of fatty acids and hepatic glucose output, which cause impaired insulin resistance and create a vicious cycle of disease progression. Currently available Type 2 diabetes drugs can only treat some of the damaging effects of FFAs on the progression of diabetes. Therefore, researchers are aiming to develop effective new therapies that can address all or most of these effects to efficiently potentiate the release of GLP-1, significantly improve blood glucose control, maintain β-cells function, and may additionally be capable of treating obesity. |
| G-protein coupled receptor 120 (GPR120) is a member of the rhodopsin family of G protein-coupled receptors (GPCRs), which also includes GPR40, GPR41, and GPR43. GPR120 is expressed predominantly in the intestine and adipose tissue and functions as a receptor for long chain FFAs. It is activated by unsaturated long chain FFAs, which stimulate the secretion of GLP-1. It is believed that GPR120 signaling activates Ca2+ flux as well as protein kinase C (PKC), which may explain how FFAs contribute to the release of GLP-1 in the L-cells. While GPR120 is not yet very well studied, available data suggest that GPR120 agonists would potentiate insulin secretion and reduce glucagon indirectly via GLP-1 release. The beneficial effects of elevating GLP-1 levels are already well documented in clinical studies. Thus, GPR120 presents a potentially viable therapeutic target to develop novel treatments for Type 2 diabetes, obesity, and insulin resistance. GPR120 agonists such as the compounds described in this patent application may be effective in improving glucose homeostasis and can potentially treat obesity. They might additionally act as complementary treatments to existing diabetes therapies that affect liver insulin sensitivity and those that preserve β-cells function. |
| Important Compound Classes: |
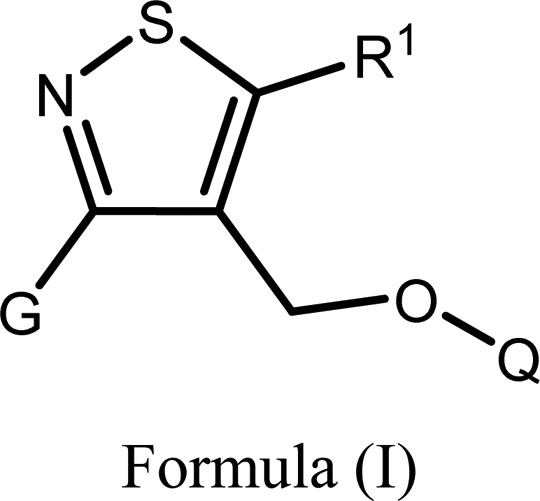 |
| Key Structures: |
The inventors listed the structures of 279 examples of formula (I) including the following compounds: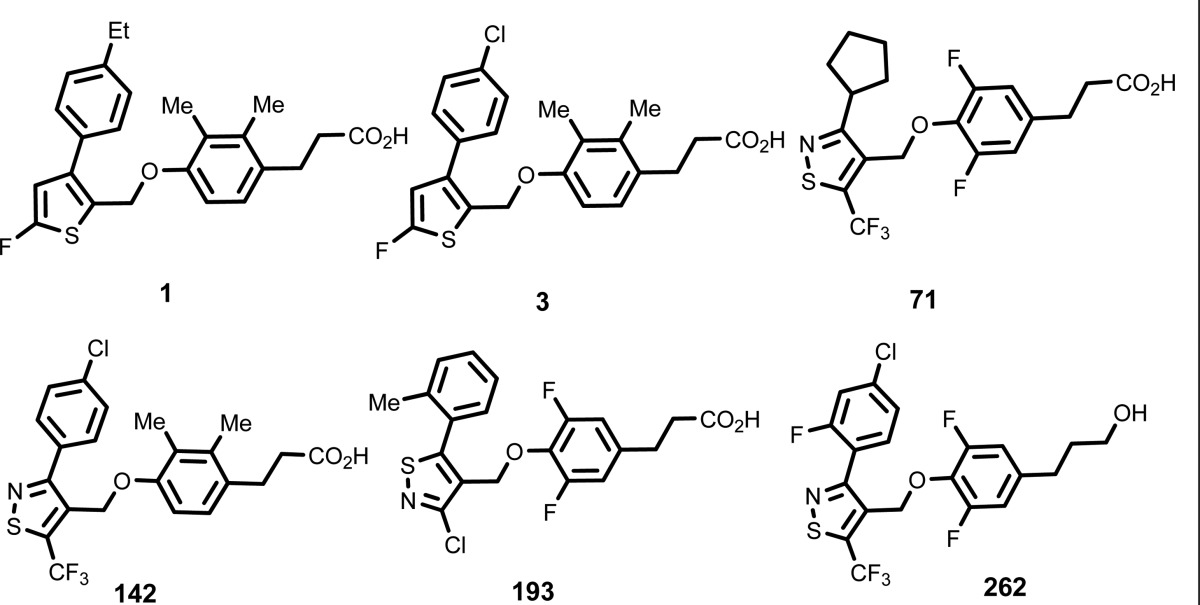
|
| Biological Assay: |
In Vitro Assays: |
|
| In Vivo Assays: |
|
| A: GPR120 C57bl6Mouse IPGTT |
| B: C57bl6 mouse OGTT |
| Biological Data: |
The biological data obtained from testing the above represented examples are listed in the following tables: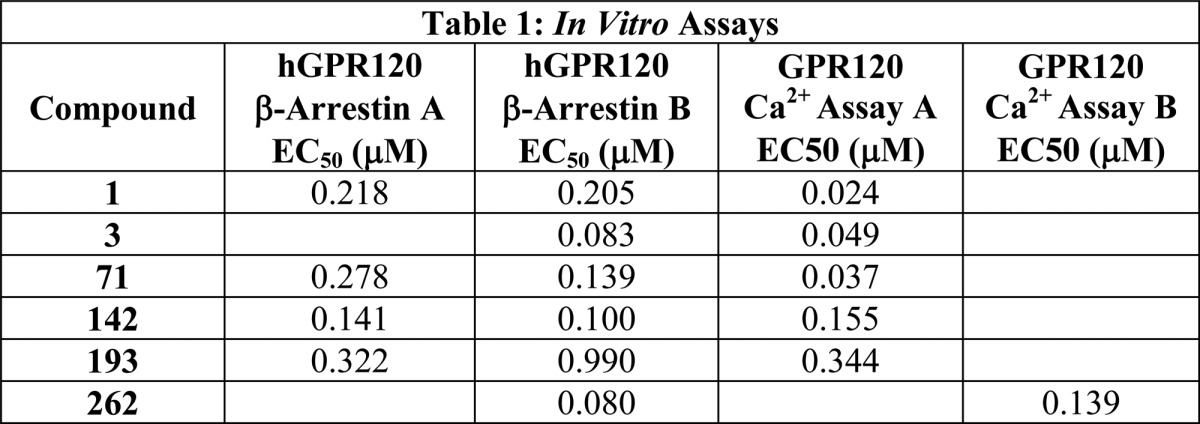 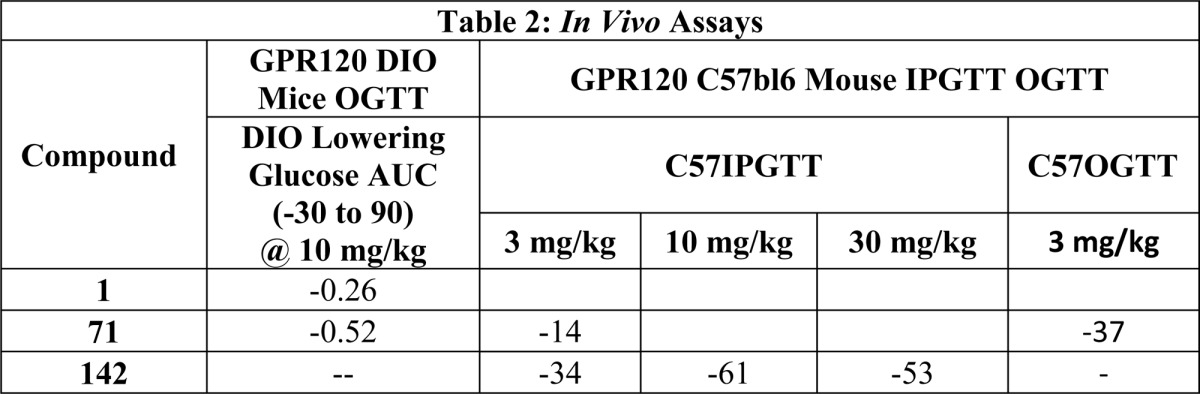
|
| Recent Review Articles: |
1. Li A.; Li Y.; Du L.. Future Med. Chem. 2015, 7 ( (11), ), 1457–1468. |
| 2. Cornall L.M.; Mathai M. L.; Hryciw D. H.; McAinch A. J.. Drug Discovery Today 2014, 19 ( (5), ), 670–679. |
| 3. Halder S.; Kumar S.; Sharma R.. Expert Opin. Ther. Pat. 2013, 23 ( (12), ), 1581–1590. |






