Abstract

Three different types of drug delivery platforms based on imidazolium ionic liquids (ILs) were synthesized in high preparative yields, namely, the models involving (i) ionic binding of drug and IL; (ii) covalent binding of drug and IL; and (iii) dual binding using both ionic and covalent approaches. Seven ionic liquids containing salicylic acid (SA-ILs) in the cation or/and in the anion were prepared, and their cytotoxicity toward the human cell lines CaCo-2 (colorectal adenocarcinoma) and 3215 LS (normal fibroblasts) was evaluated. Cytotoxicity of SA-ILs was significantly higher than that of conventional imidazolium-based ILs and was comparable to the pure salicylic acid. It is important to note that the obtained SA-ILs dissolved in water more readily than salicylic acid, suggesting benefits of possible usage of traditional nonsoluble active pharmaceutical ingredients in an ionic liquid form.
Keywords: Drug development, ionic liquids, active pharmaceutical ingredients, salicylic acid, API-IL, cytotoxicity
Most of active pharmaceutical ingredients (API) currently used in modern medicine are solid substances suffering from several significant disadvantages. Being crystalline compounds, API may exist as various polymorphs or pseudopolymorphs with different solubility and bioavailability, and the determination of a particular form of an API in the drug composition is a difficult and important task.1,2 One of the most common strategies for overcoming this issue is the conversion of neutral API into salts with sodium, chloride, phosphate, citrate, and other counterions. Ionic-core micelles were also proposed to be used for targeted drug delivery.3−5 Recently, it has been suggested that ionic liquids, or liquid salts, also can be used as ionizing agents in pharmaceutics.2,6
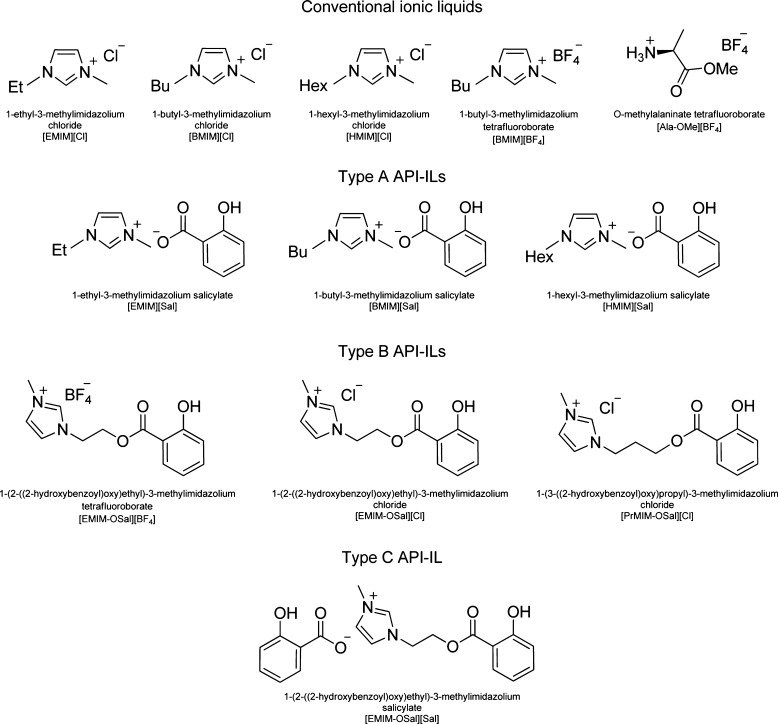
Ionic liquids (ILs) comprise an outstanding class of chemicals, which during the last several decades have found application in such diverse scientific and industrial fields as organic synthesis,7 nanoparticle research,8 catalysis,9 biomass conversion,10 electrochemistry,11 enzymatic reactions,12 extraction,13 and medicine.14,15 Initially ILs, being nonvolatile and nonflammable substances, were considered environmentally benign, but results of intense studies proved otherwise.14 As biological activity of ILs has become apparent, their potential application in medicine has attracted much attention. First, ILs were explored as alternative reaction media for synthesis of various drugs.16 Then it became evident that ILs might be introduced into the drug formula to relieve administration of insoluble preparations into the organism in the form of active pharmaceutical ingredient ionic liquids, or API-ILs.15−18 Being unique tunable chemicals, ILs are ideal candidates for incorporating various functional groups,19 including biologically active substances.20 One of the latest areas of IL application in pharmaceutics involves using them as functionalization agents for nonionizable active substances.6,16,20Figure 1 shows most remarkable structural features, which make ILs valuable potential candidates for new-age drug delivery platforms.
Figure 1.

Three types of drug development platforms based on ILs: API-IL containing ionic API as its anion (A), covalently linked API within its cation (B), or both binding options (C).
Presently, there are two main strategies of combining API and ILs: (a) using API as an anion or a cation (ionic transport, Figure 1A) and (b) covalently linking API to IL (covalent transport, Figure 1B). Moreover, the cationic–anionic structure of IL allows combining API with different properties within one molecule (both cation an anion may bear different or same API, Figure 1C).21 Poorly soluble API may be transformed into readily soluble API-ILs.2,22 Synthesis and activity of several drugs, such as ampicillin,23,24 aspirin,25 and salicylic acid,26 in the IL form have been investigated. Particularly, ampicillin ILs demonstrated dramatically higher antibiotic activity than conventional sodium ampicillin.23
In this work, we used salicylic acid (SA) as a model drug. SA is a relatively simple molecule, and its biological activity has been studied thoroughly.27 We synthesized seven imidazolium-based ionic liquids (SA-ILs), which corresponded to the three API-IL classes stated in Figure 1: ILs bearing SA in their anion ([EMIM][Sal], [BMIM][Sal], [HMIM][Sal]), ILs with SA covalently linked to the cation ([EMIM-OSal][Cl], [PrMIM-OSal][Cl], [EMIM-OSal][BF4]), and an IL bearing SA both in the anion and cation ([EMIM-OSal][Sal]). Formulas of SA-ILs and conventional ILs used in the study are shown in Figure 2.
Figure 2.
Ionic liquids with various combinations of anions and cations utilized in the present study (see Figure 1 for types A–C).
Synthesis of [EMIM][Sal]26,28 and [BMIM][Sal]28,29 has been described earlier, whereas preparation of [HMIM][Sal], [EMIM-OSal][Cl], [PrMIM-OSal][Cl], [EMIM-OSal][BF4], and [EMIM-OSal][Sal] have, to our knowledge, not been published before. As a representative example, the scheme of synthesis of [EMIM-OSal][Sal] is shown in Figure 3. The preparation procedure involves three stages: (1) synthesis of chloroalkyl ethers of salicylic acid; (2) incorporation of imidazole core; and (3) anion exchange reaction with sodium salicylate. Structures and purity of the synthesized ILs were confirmed using 1H and 13C NMR spectroscopy and mass spectrometry; the melting temperature was measured for solid substances. The corresponding experimental details and characterization data for all synthesized compounds are provided in the Supporting Information.
Figure 3.
Synthetic procedure for the preparation of [EMIM-OSal][Sal].
The molecular structure of [PrMIM-OSal][Cl] was established by X-ray analysis (Figure 4). The geometry of the imidazolium cation in this salt is comparable to that observed in other reported analogous compounds. The imidazolium ring is connected with the salicylic fragment via the alkane bridge, which adopts the t-g(+)-t-g(−) (t, trans; g, gauche) conformation. It is well-known that, in ionic liquids, a 2-position imidazolyl [NC(H)N] proton possesses the highly acidic nature and is able to compete with active hydrogen atoms for the formation of hydrogen bonding interactions with halogenide anions.30 In the case of [PrMIM-OSal][Cl], however, the hydrogen atom of the hydroxyl group in the side arm functionalized with salicylic acid forms a strong O3–H3···Cl1 hydrogen bond with a chloride anion [O···Cl 2.969(2), H···Cl 2.14(4) Å, ∠O–H···Cl 172(3)°], whereas the acidic imidazolyl proton links two O1 and O3 oxygen atoms of a neighboring cation by the bifurcate hydrogen bond (see the Supporting Information). Thus, cations and anions of [PrMIM-OSal][Cl] are bound by the above hydrogen bonds into zigzag chains toward [010] (Figure S19, see the Supporting Information). The chains are packed in stacks along the a axis.
Figure 4.
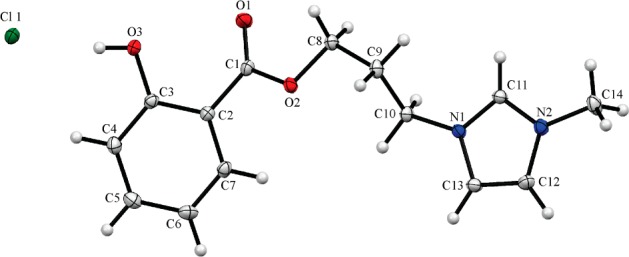
Molecular structure of [PrMIM-OSal][Cl] determined by X-ray analysis (N1–C11 1.329(3) Å, N1–C13 1.377(3) Å, N2–C11 1.330(3) Å, N2–C12 1.372(3) Å, C12–C13 1.357(4) Å, N1–C11–N2 108.2(2)°, C1–O2–C8–C9 –179.5(2)°, O2–C8–C9–C10 64.0(3)°, C8–C9–C10–N1 166.7(2)°, C9–C10–N1–C11 –55.8(4)°).
Salicylic acid is a known cytotoxin with anticancer effects.31,32 Therefore, to investigate whether SA retains its activity upon incorporation into an IL, we used the MTT assay in two human cell lines, CaCo-2 (colorectal adenocarcinoma) and 3215 LS (normal fibroblasts). The resultant data on cytotoxicity of the SA-ILs, as well as conventional imidazolium-based ILs and pure salicylic acid, are shown in Table 1 (expressed as 24 h IC50, half maximal inhibitory concentration after 24 h exposure). Details on experimental procedures and statistical analysis are provided in the Supporting Information.
Table 1. Cytotoxicity of Studied ILs Towards Cell Lines 3215 LS and CaCo-2.
| IL | 3215 LS (24 h IC50, mM)a | CaCo-2 (24 h IC50, mM)a | |
|---|---|---|---|
| 1 | [EMIM][Cl] | 46.15 (45.70–46.59) | 32.1033 |
| 2 | [BMIM][Cl] | 30.08 (19.47–40.70) | 18.8533 |
| 3 | [HMIM][Cl] | 7.54 (2.55–12.54) | 6.2133 |
| 4 | [BMIM][BF4] | 4.88 (4.27–5.50) | 11.1933 |
| 5 | [Ala-OMe][BF4] | 2.63 (2.32–2.94) | 6.24 (4.32–8.17) |
| 6 | [EMIM][Sal] | 1.39 (0.64–2.14) | 5.97 (4.51–7.91) |
| 7 | [BMIM][Sal] | 0.64 (0.19–1.09) | 4.34 (2.48–7.61) |
| 8 | [HMIM][Sal] | 2.00 (0.69–3.31) | 1.96 (0.79–3.14) |
| 9 | [EMIM-O][Sal][BF4] | 4.12 (2.51–5.73) | 4.77 (3.27–6.27) |
| 10 | [EMIM-OSal][Cl] | 2.85 (1.30–4.40) | 3.82 (3.34–4.31) |
| 11 | [PrMIM-OSal][Cl] | 3.19 (0.84–5.54) | 2.55 (2.08–3.13) |
| 12 | [EMIM-OSal][Sal] | 0.71 (0.58–0.84) | b |
| 13 | salicylic acid | 1.14 (0.67–1.94) | ∼5–7c |
95% confidence interval is shown in parentheses.
No data available.
Approximate value (see the text).
Our first observation was that the studied ILs, both conventional and newly synthesized ones, demonstrated similar toxicity in the cancer and normal cell lines. Several ILs, such as [BMIM][BF4], [Ala-OMe][BF4], [EMIM][Sal], and [BMIM][Sal], exhibited significantly higher toxicity toward normal fibroblasts, as compared with cells of colorectal adenocarcinoma (Table 1, entries 4–7). Salicylic acid also did not distinguish between the two cell lines and was even less toxic toward the cancerous one; thus, we could not determine IC50 in CaCo-2 because the saturated solution of SA did not kill 100% cells (Table 1, entry 13).
Introduction of salicylic acid into imidazolium-based ILs dramatically increased their cytotoxicity. The replacement of the chloride anion with salicylate led to a significant decrease of 24 h IC50 (Table 1, entries 1–3 and 6–8). [BMIM][Sal] was even more cytotoxic than [BMIM][BF4], for which rather high toxicity had been demonstrated in this and other33 studies (Table 1, entries 4 and 7). Introduction of salicylic acid into the cation resulted in a similar increase of cytotoxicity (Table 1, entries 9–11).
Of note, cytotoxicity of the ILs with SA in the cation was comparable to that of the amino acid-based IL [Ala-OMe][BF4] (Table 1, entries 5 and 9–11). In our previous work, we demonstrated that the presence of an amino acid led to unexpectedly high cytotoxicity of ILs with the tetrafluoroborate anion, possibly due to specific interactions between the amino acid and transporter proteins on the surface of the cell.33 In the case of salicylic acid, the anion had insignificant influence on the cytotoxicity. In contrast to the conventional [BMIM][Cl] and [BMIM][BF4], [EMIM-OSal][BF4] and [EMIM-OSal][Cl] possessed similar toxicity (Table 1, entries 2 and 4, 9 and 10), suggesting the inherent toxicity of the anion to be overwhelmed by that of the cation.
We also studied the cytotoxicity of [EMIM-OSal][Sal], which contained salicylic acid both in its cation (covalent linkage) and anion (ionic interaction) (Table 1, entry 12). Its IC50 was 0.71 mM and did not differ significantly from the SA-ILs bearing SA in the anion only (Table 1, entries 6–8). This observation suggests that, in the case of imidazolium-based ILs, one part of salicylic acid per IL molecule is enough for reaching the maximum possible cytotoxicity and that increasing the SA content does not lead to higher biological activity. In general, IC50 of SA-ILs belonging to the three structural API-ILs types did not differ significantly, though SA-IL bearing covalently linked salicylic acid within their cation tended to demonstrate lower toxicity (IC50 2.85–4.12 mM) than SA-ILs with salicylate anions (IC50 0.64–2.00 mM). This observation may be possibly explained by known allosteric activity of SA.34 The covalent linkage between SA and IL may hinder binding of the former to its cell targets; however, the ionic linkage allows for more flexible interactions.
Interestingly, in most cases the cytotoxicity of SA-ILs did not differ significantly from that of the pure salicylic acid (Table 1, entries 6–13). We were unable to determine the exact 24 h IC50 for SA in CaCo-2 cells (at maximum concentration, SA killed about 95% cells) and estimated it as 5–7 mM; in 3215 LS, 24 h IC50 for SA was 1.14 mM. Therefore, the introduction of SA into an ionic liquid did not impede its cytotoxic action, but significantly increased its solubility. For [EMIM-OSal][Cl], solubility in water was 267 g/L (r.t.), as compared to 2.20 g/L (r.t.)35 for pure salicylic acid. All SA-ILs were readily dissolved in water in the concentration range used for the testing (see Table S1 in the Supporting Information).
Salicylic acid is known to cause apoptosis in vitro and in vivo,31,32,36 but the exact mechanisms of its action just begin to emerge. SA was shown to impact mitochondrial activity37 and to inhibit protective catalase in malignant cells directly, therefore activating the signaling mediated by reactive oxygen species (ROS), which triggers apoptosis in the cell.32 SA decreased glucose consumption by breast cancer cells MCF-738 and increased caspase activity in CaCo-2 cells when applied at such low concentration as 10 μM, the latter effect being dependent on the oxygen availability.31
The fact that SA retains its ability to cause cell death upon covalent or ionic linking to an IL suggests that its mechanism of action remains unperturbed and does not include metabolic modifications.
In summary, we investigated cytotoxicity of salicylic acid-containing imidazolium-based ILs (SA-ILs) belonging to three currently available structural classes of API-ILs: API-ILs with ionic API as their anion (type A); API-ILs with covalently linked API within their cation (type B); and API-ILs bearing API both in the anion and cation (type C). Cytotoxicity of the studied SA-ILs was significantly higher than that of conventional imidazolium-based ILs and was comparable to that of the pure salicylic acid. In contrast to salicylic acid, SA-ILs possessed relatively high water solubility. According to our results, API-ILs of types A and C demonstrated similar biological activity, which was higher than that of type B. Nevertheless, all three API-IL types proposed in this work are promising candidates for future studies. The obtained results confirm the possibility of using IL advantages, such as ionic linkage and improved solubility, in pharmaceutics.
Acknowledgments
The authors thank E. Kopantsev (M. M. Shemyakin and Yu. A. Ovchinnikov Institute of Bioorganic Chemistry RAS) for the 3215 LS cell culture.
Glossary
ABBREVIATIONS
- IL
ionic liquid
- API-IL
active pharmaceutical ingredient ionic liquid
- SA-IL
salicylic acid-containing ionic liquid
- IC50
half maximal inhibitory concentration
Biographies
Valentine Ananikov received his Ph.D. in 1999, Habilitation in 2003, and in 2005 became Professor and Laboratory Head of the Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences. In 2008, he was elected as a Member of Russian Academy of Sciences. He was a recipient of Russian State Prize for Outstanding Achievements in Science and Technology (2004), an Award of Science Support Foundation (2005), a Medal of Russian Academy of Sciences (2000), Liebig Lecturer by German Chemical Society (2010), and Balandin Prize for outstanding achievements in catalysis (2010). His scientific interests are focused on molecular complexity and transformations.
Ksenia Egorova graduated from Lomonosov Moscow State University as a Master of Science in Biochemistry. She received her Ph.D. in Molecular Biology while working at the Institute of Molecular Genetics of Russian Academy of Sciences. Since 2012, she has been a research fellow at the Zelinsky Institute of Organic Chemistry. Her major scientific interests include biological activity, natural products, cancer proteomics, ionic liquids, and carbohydrate databases. She is an award recipient of the Grant of the President of the Russian Federation (2015−2016).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00258.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by the Russian Foundation for Basic Research (project 14-03-31478) and by the Grant of the President of the Russian Federation (project MK-5053.2015.3). Mechanistic studies were supported by the Russian Science Foundation (RSF grant 14-50-00126).
The authors declare no competing financial interest.
Supplementary Material
References
- Pasquali I.; Bettini R.; Giordano F. Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals. Adv. Drug Delivery Rev. 2008, 60, 399–410. 10.1016/j.addr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Shamshina J. L.; Barber P. S.; Rogers R. D. Ionic liquids in drug delivery. Expert Opin. Drug Delivery 2013, 10, 1367–1381. 10.1517/17425247.2013.808185. [DOI] [PubMed] [Google Scholar]
- Bontha S.; Kabanov A. V.; Bronich T. K. Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J. Controlled Release 2006, 114, 163–174. 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Kim J. O.; Sahay G.; Kabanov A. V.; Bronich T. K. Polymeric micelles with ionic cores containing biodegradable cross-links for delivery of chemotherapeutic agents. Biomacromolecules 2010, 11, 919–926. 10.1021/bm9013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura M.; Kim J. O.; Kabanov A. V.; Bronich T. K.; Nagasaki Y. Block ionomer complexes of PEG-block-poly(4-vinylbenzylphosphonate) and cationic surfactants as highly stable, pH responsive drug delivery system. J. Controlled Release 2012, 160, 486–494. 10.1016/j.jconrel.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Cojocaru O. A.; Bica K.; Gurau G.; Narita A.; McCrary P. D.; Shamshina J. L.; Barber P. S.; Rogers R. D. Prodrug ionic liquids: functionalizing neutral active pharmaceutical ingredients to take advantage of the ionic liquid form. MedChemComm 2013, 4, 559–563. 10.1039/c3md20359j. [DOI] [Google Scholar]
- Plechkova N. V.; Seddon K. R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. 10.1039/B006677J. [DOI] [PubMed] [Google Scholar]
- Scholten J. D.; Leal B. C.; Dupont J. Transition metal nanoparticle catalysis in ionic liquids. ACS Catal. 2012, 2, 184–200. 10.1021/cs200525e. [DOI] [Google Scholar]
- Zhang Q.; Zhang S.; Deng Y. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619–2637. 10.1039/c1gc15334j. [DOI] [Google Scholar]
- Khokhlova E. A.; Kachala V. V.; Ananikov V. P. The first molecular level monitoring of carbohydrate conversion to 5-hydroxymethylfurfural in ionic liquids. B2O3--an efficient dual-function metal-free promoter for environmentally benign applications. ChemSusChem 2012, 5, 783–789. 10.1002/cssc.201100670. [DOI] [PubMed] [Google Scholar]
- Liu H.; Liu Y.; Li J. Ionic liquids in surface electrochemistry. Phys. Chem. Chem. Phys. 2010, 12, 1685–1697. 10.1039/b921469k. [DOI] [PubMed] [Google Scholar]
- Moniruzzaman M.; Kamiya N.; Goto M. Activation and stabilization of enzymes in ionic liquids. Org. Biomol. Chem. 2010, 8, 2887–2899. 10.1039/b926130c. [DOI] [PubMed] [Google Scholar]
- Seitkalieva M. M.; Kachala V. V.; Egorova K. S.; Ananikov V. P. Molecular extraction of peptides in ionic liquid systems. ACS Sustainable Chem. Eng. 2015, 3, 357–364. 10.1021/sc500770v. [DOI] [Google Scholar]
- Egorova K. S.; Ananikov V. P. Toxicity of ionic liquids: eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization. ChemSusChem 2014, 7, 336–360. 10.1002/cssc.201300459. [DOI] [PubMed] [Google Scholar]
- Williams H. D.; Sahbaz Y.; Ford L.; Nguyen T. H.; Scammells P. J.; Porter C. J. Ionic liquids provide unique opportunities for oral drug delivery: structure optimization and in vivo evidence of utility. Chem. Commun. (Cambridge, U. K.) 2014, 50, 1688–1690. 10.1039/c3cc48650h. [DOI] [PubMed] [Google Scholar]
- Marrucho I. M.; Branco L. C.; Rebelo L. P. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. 10.1146/annurev-chembioeng-060713-040024. [DOI] [PubMed] [Google Scholar]
- Dobler D.; Schmidts T.; Klingenhofer I.; Runkel F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. (Amsterdam, Neth.) 2013, 441, 620–627. 10.1016/j.ijpharm.2012.10.035. [DOI] [PubMed] [Google Scholar]
- Kumar S. S.; Surianarayanan M.; Vijayaraghavan R.; Mandal A. B.; MacFarlane D. R. Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid--in vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. 10.1016/j.ejps.2013.08.036. [DOI] [PubMed] [Google Scholar]
- Visser A. E.; Swatloski R. P.; Reichert W. M.; Davis J. H. Jr.; Rogers R. D.; Mayton R.; Sheff S.; Wierzbicki A. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 135–136. 10.1039/b008041l. [DOI] [Google Scholar]
- Balk A.; Widmer T.; Wiest J.; Bruhn H.; Rybak J. C.; Matthes P.; Muller-Buschbaum K.; Sakalis A.; Luhmann T.; Berghausen J.; Holzgrabe U.; Galli B.; Meinel L. Ionic liquid versus prodrug strategy to address formulation challenges. Pharm. Res. 2015, 32, 2154–2167. 10.1007/s11095-014-1607-9. [DOI] [PubMed] [Google Scholar]
- Ferraz R.; Branco L. C.; Prudencio C.; Noronha J. P.; Petrovski Z. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. 10.1002/cmdc.201100082. [DOI] [PubMed] [Google Scholar]
- McCrary P. D.; Beasley P. A.; Gurau G.; Narita A.; Barber P. S.; Cojocaru O. A.; Rogers R. D. Drug specific, tuning of an ionic liquid’s hydrophilic–lipophilic balance to improve water solubility of poorly soluble active pharmaceutical ingredients. New J. Chem. 2013, 37, 2196–2202. 10.1039/c3nj00454f. [DOI] [Google Scholar]
- Cole M. R.; Li M.; El-Zahab B.; Janes M. E.; Hayes D.; Warner I. M. Design, synthesis, and biological evaluation of beta-lactam antibiotic-based imidazolium- and pyridinium-type ionic liquids. Chem. Biol. Drug Des. 2011, 78, 33–41. 10.1111/j.1747-0285.2011.01114.x. [DOI] [PubMed] [Google Scholar]
- Ferraz R.; Teixeira V.; Rodrigues D.; Fernandes R.; Prudêncio C.; Noronha J. P.; Petrovski Ž.; Branco L. C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. 10.1039/C3RA44286A. [DOI] [Google Scholar]
- Bica K.; Rijksen C.; Nieuwenhuyzen M.; Rogers R. D. In search of pure liquid salt forms of aspirin: ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 2010, 12, 2011–2017. 10.1039/b923855g. [DOI] [PubMed] [Google Scholar]
- Pinto P. C. A. G.; Ribeiro D. M. G. P.; Azevedo A. M. O.; Dela Justina V.; Cunha E.; Bica K.; Vasiloiu M.; Reis S.; Saraiva M. L. M. F. S. Active pharmaceutical ingredients based on salicylate ionic liquids: insights into the evaluation of pharmaceutical profiles. New J. Chem. 2013, 37, 4095–4102. 10.1039/c3nj00731f. [DOI] [Google Scholar]
- Alfonso L.; Ai G.; Spitale R. C.; Bhat G. J. Molecular targets of aspirin and cancer prevention. Br. J. Cancer 2014, 111, 61–67. 10.1038/bjc.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. S.; Yang M.; Pitz D.; Cybinska J.; Mudring A. V. Highly luminescent and color-tunable salicylate ionic liquids. Chem. - Eur. J. 2014, 20, 4704–4712. 10.1002/chem.201301363. [DOI] [PubMed] [Google Scholar]
- Jork C.; Kristen C.; Pieraccini D.; Stark A.; Chiappe C.; Beste Y. A.; Arlt W. Tailor-made ionic liquids. J. Chem. Thermodyn. 2005, 37, 537–558. 10.1016/j.jct.2005.04.013. [DOI] [Google Scholar]
- Holbrey J. D.; Reichert W. M.; Nieuwenhuyzen M.; Johnston S.; Seddon K. R.; Rogers R. D. Crystal polymorphism in 1-butyl-3-methylimidazolium halides: supporting ionic liquid formation by inhibition of crystallization. Chem. Commun. (Cambridge, U. K.) 2003, 1636–1637. 10.1039/b304543a. [DOI] [Google Scholar]
- Zitta K.; Meybohm P.; Bein B.; Huang Y.; Heinrich C.; Scholz J.; Steinfath M.; Albrecht M. Salicylic acid induces apoptosis in colon carcinoma cells grown in-vitro: influence of oxygen and salicylic acid concentration. Exp. Cell Res. 2012, 318, 828–834. 10.1016/j.yexcr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Scheit K.; Bauer G. Direct and indirect inactivation of tumor cell protective catalase by salicylic acid and anthocyanidins reactivates intercellular ROS signaling and allows for synergistic effects. Carcinogenesis 2015, 36, 400–411. 10.1093/carcin/bgv010. [DOI] [PubMed] [Google Scholar]
- Egorova K. S.; Seitkalieva M. M.; Posvyatenko A. V.; Ananikov V. P. An unexpected increase of toxicity of amino acid-containing ionic liquids. Toxicol. Res. (Cambridge, U. K.) 2015, 4, 152–159. 10.1039/C4TX00079J. [DOI] [Google Scholar]
- Hawley S. A.; Fullerton M. D.; Ross F. A.; Schertzer J. D.; Chevtzoff C.; Walker K. J.; Peggie M. W.; Zibrova D.; Green K. A.; Mustard K. J.; Kemp B. E.; Sakamoto K.; Steinberg G. R.; Hardie D. G. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 2012, 336, 918–922. 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalmashi A.; Eliassi A. Solubility of salicylic acid in water, ethanol, carbon tetrachloride, ethyl acetate, and xylene. J. Chem. Eng. Data 2008, 53, 199–200. 10.1021/je7004962. [DOI] [Google Scholar]
- Vejselova D.; Kutlu H. M. Inhibitory effects of salicylic acid on A549 human lung adenocarcinoma cell viability. Turk. J. Biol. 2015, 39, 1–5. 10.3906/biy-1401-7. [DOI] [Google Scholar]
- Kamble P.; Litvinov D.; Aluganti Narasimhulu C.; Jiang X.; Parthasarathy S. Aspirin may influence cellular energy status. Eur. J. Pharmacol. 2015, 749, 12–19. 10.1016/j.ejphar.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz G. A.; Furtado C. M.; Sola-Penna M.; Zancan P. Acetylsalicylic acid and salicylic acid decrease tumor cell viability and glucose metabolism modulating 6-phosphofructo-1-kinase structure and activity. Biochem. Pharmacol. 2009, 77, 46–53. 10.1016/j.bcp.2008.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.