ABSTRACT
The HIV-1 accessory protein Vpr displays different activities potentially impacting viral replication, including the arrest of the cell cycle in the G2 phase and the stimulation of apoptosis and DNA damage response pathways. Vpr also modulates cytokine production by infected cells, but this property remains partly characterized. Here, we investigated the effect of Vpr on the production of the proinflammatory cytokine tumor necrosis factor (TNF). We report that Vpr significantly increases TNF secretion by infected lymphocytes. De novo production of Vpr is required for this effect. Vpr mutants known to be defective for G2 cell cycle arrest induce lower levels of TNF secretion, suggesting a link between these two functions. Silencing experiments and the use of chemical inhibitors further implicated the cellular proteins DDB1 and TAK1 in this activity of Vpr. TNF secreted by HIV-1-infected cells triggers NF-κB activity in bystander cells and allows viral reactivation in a model of latently infected cells. Thus, the stimulation of the proinflammatory pathway by Vpr may impact HIV-1 replication in vivo.
IMPORTANCE The role of the HIV-1 accessory protein Vpr remains only partially characterized. This protein is important for viral pathogenesis in infected individuals but is dispensable for viral replication in most cell culture systems. Some of the functions described for Vpr remain controversial. In particular, it remains unclear whether Vpr promotes or instead prevents proinflammatory and antiviral immune responses. In this report, we show that Vpr promotes the release of TNF, a proinflammatory cytokine associated with rapid disease progression. Using Vpr mutants or inhibiting selected cellular genes, we show that the cellular proteins DDB1 and TAK1 are involved in the release of TNF by HIV-infected cells. This report provides novel insights into how Vpr manipulates TNF production and helps clarify the role of Vpr in innate immune responses and inflammation.
INTRODUCTION
Chronic immune activation is a hallmark of HIV-1 infection in humans and is a good predictor of disease progression (1, 2). HIV-1 activates the immune system through indirect mechanisms (3) and through direct virus-mediated effects, including the stimulation of innate host responses (4) and triggering of pyroptotic cell death (5, 6). This constant activation of the immune system is associated with exacerbated production of proinflammatory and antiviral cytokines (7). Understanding how viral proteins manipulate this proinflammatory state is crucial to design better therapeutic strategies.
In addition to structural and enzymatic proteins, HIV encodes accessory proteins that often antagonize cellular antiviral restriction factors (8). For instance, Vpu counteracts tetherin (9) and Vif degrades APOBEC3 proteins (10). Some accessory proteins regulate the intensity of innate immune responses. Vpu, by degrading tetherin, avoids the stimulation of the NF-κB pathway by tethered virions (11–14). In contrast, Nef enhances NF-κB activation (14) and HIV-2 Vpx, by degrading SAMHD1 (15, 16), promotes infection and subsequent innate sensing in myeloid cells (17–19). The role of HIV-1 Vpr in the modulation of innate responses remains partly characterized. Vpr is closely related to Vpx. These two proteins have a common evolutionary origin (20, 21), share similar amino acid sequences and three-dimensional (3D) structures (22), and are both incorporated in virions (23, 24). Despite these similarities, the two proteins display different activities (25). HIV-1 Vpr does not degrade SAMHD1, and its effect on viral replication remains unclear. Indeed, Vpr is not required for infection of most cell lines and primary CD4+ T cells (26–28). A replication defect for vpr-deleted viruses has been reported in dendritic cells and macrophages, with important donor-to-donor variability (27, 29–32). It was recently suggested that Vpr favors infection of macrophages by counteracting a restriction factor targeting Env expression and viral release (33). Vpr is also necessary for efficient cell-to-cell spread of HIV-1 from macrophages to CD4+ T lymphocytes (34). Vpr plays an important role in vivo. Rhesus macaque simian immunodeficiency virus (SIVMAC) Δvpr viruses rapidly revert to a wild-type (WT) version when injected in rhesus macaques (35). A similar reversion was observed in a laboratory worker accidentally contaminated with a vpr-deficient strain of HIV-1 (36, 37). Several researchers also reported mutations in the vpr gene in patients who were long-term nonprogressors (LTNP) (38–41).
Many activities have been described for Vpr. It induces G2 cell cycle arrest (42–45), stimulates the DNA damage response (DDR) and apoptosis pathways (46–52), and may facilitate several steps of the viral cycle such as nuclear import and transcription (29, 53, 54). Vpr localizes to the nuclear envelope (30) and/or inside the nucleus, where it may form foci and colocalize with DNA damage proteins (55). Vpr arrests the cell cycle in the G2 phase by hijacking the DCAF1-DDB1-Cul4A ubiquitin-ligase complex (56–61). It has also been reported that the premature activation of the structure-specific endonuclease regulator SLX4 complex (SLX4com) by Vpr, through its interaction with DCAF1, mediates G2 cell cycle arrest (62, 63). The SLX4com is involved in the Fanconi anemia DNA repair pathway, thus linking the DDR with the effect of Vpr on the cell cycle. How G2 arrest may affect viral replication and pathogenicity is not fully understood. It was suggested previously that viral transcription is favored in the G2 phase of the cell cycle (37, 64). In HIV-infected humanized mice, T regulatory lymphocytes are arrested in the G2 phase of the cell cycle upon infection and undergo apoptosis in a vpr-dependent fashion, leading to enhanced replication (65). Besides the SLX4com, Vpr interacts with numerous cellular proteins, but the relevance of these interactions is not always clear (66). For instance, Vpr degrades UNG2, a cellular protein involved in the removal of misincorporated uracil in DNA and for which a role during the HIV-1 life cycle remains a subject of debate (67–69). Vpr also binds to TAK1, a kinase involved in NF-κB signaling (70, 71). Vpr binding to TAK1 activates the IκB kinase (IKK) complex, triggering the degradation of IκB, the NF-κB inhibitory subunit (71).
Vpr influences cellular responses to infection, with various outcomes. Vpr has been reported to inhibit the secretion of type I interferon (IFN) (33, 62, 72, 73) and of other proinflammatory cytokines such as tumor necrosis factor (TNF), MIP-1α, MIP-1β, and RANTES (74, 75). In contrast, other studies suggested that Vpr stimulates type I IFN production in astrocytes (76) and induces interferon-stimulated genes (ISGs) in macrophages and monocytes (77, 78) and the proinflammatory cytokines interleukin-1β (IL-1β), IL-8, and TNF in various cells (70, 71, 79–82). These opposing results may reflect cell type differences or the use of different methods to express Vpr (infection, overexpression of Vpr, and treatment with Vpr recombinant protein).
TNF is a proinflammatory cytokine induced in HIV-infected individuals, especially during the acute phase of infection (83, 84). High TNF levels have been associated with poor virus control and disease progression (85, 86). TNF signaling induces NF-κB translocation to the nucleus, where it may bind to regulatory sequences in the HIV-1 long terminal repeat (LTR) promoter to stimulate viral transcription (87). This phenomenon is especially relevant during early transcription, when Tat is absent, and in latently infected T cells, in which TNF is an efficient activator of viral replication (88, 89). In addition to lymphocytes, TNF enhances HIV-1 infection in macrophages, dendritic cells, and Langerhans cells (32, 90, 91). Therefore, understanding how viral proteins influence the secretion of TNF will provide new insights into the mechanisms of HIV replication in these key cell types in vivo.
Here, we examined the impact of Vpr on TNF release by HIV-1-infected T cells. We report that Vpr increases the secretion of the proinflammatory cytokine by infected MT4 lymphoid cells and primary CD4+ T lymphocytes. We further characterized the effect of various point mutations in Vpr and demonstrate the involvement of the cellular proteins DDB1 and TAK1 in this activity of Vpr.
MATERIALS AND METHODS
Cells, reagents, and viruses.
MT4C5 (92), HEK293T, and J-Lat cells and primary CD4+ T cells were grown in RPMI 1640 with GlutaMAX or Dulbecco modified Eagle medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics. Primary CD4+ T cells were purified from human peripheral blood mononuclear cells by Ficoll centrifugation, followed by immunomagnetic selection (Miltenyi Biotec). The blood was provided by the EFS (Etablissement Francais du Sang [the French Official Blood Bank]). About 98% of the cells were CD4+ CD3+ cells after purification. CD4+ T cells were activated with phytohemagglutinin (PHA; Remel Europe Ltd.) (1 mg/ml) for 24 h and then cultured with interleukin 2 (IL-2; Abcys) (50 U/ml). 293T CD4+ CXCR4+ cells (92) and J-Lat 10.6 cells (93, 94) were described previously. J-Lat 10.6 cells are Jurkat cells carrying a latent, integrated, and env-deleted copy of the HIV-1 genome, encoding green fluorescent protein (GFP) instead of Nef. Nevirapine (NVP) (used at 25 nM) and raltegravir (used at 1 μM) were from the NIH AIDS Reagents Program. The TAK1 inhibitor (5Z)-7-oxozeaenol (Sigma) was diluted in dimethyl sulfoxide (DMSO) and used at the indicated concentrations (5 to 50 nM). Viability stainings were performed before fixation using Aqua Vivid reagent (Live/Dead fixable Aqua Dead Cell stain kit from Life Technologies). Phorbol myristate acetate (PMA) and ionomycin (Sigma) were used at doses ranging from 1 to 50 ng/ml and from 20 to 1,000 ng/ml, respectively. The HIV Δvpr provirus was a kind gift of F. Margottin-Goguet. vpr-mutated proviruses were generated by introducing point mutations in HIV-1 NL4-3 provirus using a QuikChange XL site-directed mutagenesis kit (Stratagene). The Δnef and Δnef Δvpr proviruses were generated as previously described (95). The primers used are indicated in Table S1 in the supplemental material. The NL4-3 Vpr S79A provirus was a kind gift of C. Ramirez. The anti-IL-1β blocking antibody (Ab) was a kind gift of E. Laplantine. The NIH45-46 anti-HIV1 broadly neutralizing Ab (used at 50 nM) was a kind gift of Hugo Mouquet.
Infection and viral production.
MT4C5 and primary cells were infected with the indicated viruses, pseudotyped with the vesicular stomatitis virus type G (VSV-G) envelope (0.4 to 400 ng Gag p24/ml for 106 cells). Gag levels were monitored at 24 or 48 h. Cells were fixed in phosphate-buffered saline (PBS)–4% paraformaldehyde (PFA) for 5 min, permeabilized and stained with anti-Gag antibody (clone KC57-PE; Beckman Coulter) (1/500), and analyzed by flow cytometry on a FacsCanto II system (Becton Dickinson). HIV-1 strains were produced by calcium-phosphate transfection of 293T cells. VSV-G-pseudotyped viruses were obtained by cotransfection of HEK293T cells with the NL4-3 provirus and VSV-G expression plasmid (5:2 ratio). Hemagglutinin-Vpr (HA-Vpr)-complemented virions were obtained by cotransfection of the NL4-3 Δvpr provirus and the HA-Vpr expression plasmid (2:1 ratio). Lentivectors encoding short hairpin RNAs (shRNAs) were produced by cotransfection of HEK293T cells by the packaging plasmid (R8-2), the DDB1 GipZ shRNA lentiviral plasmid (DDB1 no. 1, V3LHS_646157; DDB1 no. 2, V3LHS_646437; Dharmacon), and VSV-G expression plasmid (5:5:1 ratio).
NF-κB activation assay.
293T CD4+ CXCR4+ cells were plated in 48-well plates (4 × 104 cells per well). After 24 h, cells were cotransfected using FuGENE 6 (Roche Diagnostics) with 100 ng of NF-κB–luciferase reporter plasmid (provided by R. Weil and J. Hiscott) and 20 ng of pRSV–β-galactosidase to control DNA uptake and expression. After 24 h, cells were cocultured with HIV-infected MT4C5 cells at a 1:1 ratio for 16 h. In some experiments, donor cells were preincubated with anti-TNF blocking antibodies (1 μg/ml) for 30 min at room temperature and incubated with 293T CD4+ CXCR4+ cells. Cells were lysed and processed as previously reported (92). Results are expressed as relative luciferase units (RLU) normalized to β-galactosidase activity. Results were normalized using HIV results (set as 100%).
TNF quantification.
MT4C5 and primary cells were infected as previously described. Medium was changed every day, and supernatants were collected and stored at −20°C without detergent. TNF secretion was determined using ProcartaPlex immunoassay kits with magnetic beads (eBiosciences). Samples were acquired using a MagPix System (Life Technology). In some experiments, TNF secretion was monitored by enzyme-linked immunosorbent assay (ELISA), using an anti-TNF human DuoSet kit (R&D Systems). The method of detection of TNF did not impact the results obtained.
Vpr incorporation in virions.
To verify the incorporation of HA-tagged Vpr, viral stocks were lysed in PBS–1% Triton X-100 and analyzed by Western blotting. Gag p24 (20 ng) was loaded into each lane. HA-Vpr was detected using anti-HA monoclonal antibody (MAb) (clone 12CA5; Abcam) (1:1,000). Gag-p24 levels were detected using the anti-Gag MAb 183-H12-5C (NIH AIDS Reagent Program) (1:1,000). To assess incorporation of Vpr mutants, virions were concentrated and purified as described previously (96). Virions were filtered and ultracentrifuged (100,000 × g, 2 h at 4°C) through a cushion of 6% iodixanol (Optiprep; Gibco) diluted in PBS. Gag p24 (60 ng) was loaded into each lane. Endogenous Vpr was detected using the anti-Vpr MAb (clone 8D1; Cosmo Bio) (1:200). The band intensity was measured using Image Studio Lite software (Li-COR Biosciences).
RESULTS
Vpr enhances TNF secretion by infected cells.
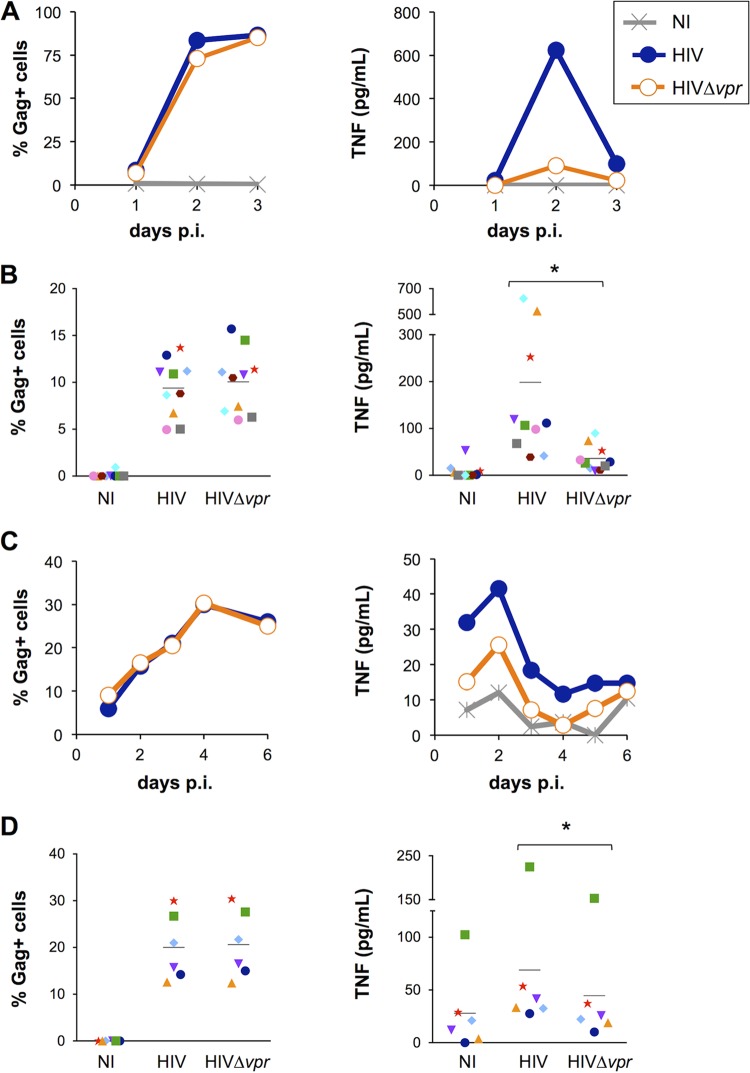
TNF is a proinflammatory cytokine influencing HIV replication in numerous cell types (32, 90, 91). We first asked whether the accessory protein Vpr modulates TNF production by HIV-1-infected lymphocytes. With that aim, we exposed MT4C5 cells and primary CD4+ T lymphocytes to HIV or Δvpr HIV from the NL4-3 strain (pseudotyped with the VSV-G envelope) and measured TNF levels in the supernatants for several days postinfection (p.i.). We did not observe notable differences in viral replication in the absence of Vpr in either cell type, as indicated by the detection of equivalent percentages of infected cells across the conditions (Fig. 1). In MT4C5 cells, we detected a significant production of TNF peaking at day 2 p.i. (Fig. 1A). After 2 to 3 days, nearly 100% of the cells were infected. Noninfected, HIV-infected, and HIV Δvpr mutant-infected cells displayed similar viability levels at 48 h p.i. (see Fig. S1 in the supplemental material), and significant cell death was usually observed in productively infected cells at day 3 p.i. (data not shown). At the peak of production (2 days p.i.), TNF levels were consistently 3-fold to 7-fold higher in the presence of Vpr, regardless of the amounts of TNF produced by wild-type HIV-infected cells (Fig. 1B). This increase of TNF release was also visible when infections were performed with HIV-1 NL4-3 or NLAD8 strains not pseudotyped with VSV-G (data not shown). In primary T cells, TNF release peaked after 2 days and then decreased, probably because of cell death or loss of cellular activation (Fig. 1C). Vpr significantly enhanced TNF release by primary CD4+ T lymphocytes from several donors, although this effect was less marked than in MT4C5 cells (Fig. 1D). Therefore, Vpr enhances TNF secretion by HIV-1-infected MT4C5 cells and primary lymphocytes.
FIG 1.
Vpr enhances TNF secretion by HIV-1-infected T cells. (A) HIV-1 replication and TNF secretion in MT4C5 cells. MT4C5 cells were infected with HIV or Δvpr HIV (0.4 to 4 ng of Gag p24/ml/106 cells). Noninfected cells (NI) were used as a control. Infection was monitored by measuring the percentages of Gag+ cells by flow cytometry, and TNF was quantified in the supernatants by Luminex assay or ELISA at the indicated days postinfection. Data represent the results of one kinetic experiment of viral replication (left panel) and TNF production (right panel) of four performed. (B) Infection levels and TNF production in six independent experiments. MT4C5 cells were infected and analyzed as described for panel A. Data represent the means ± standard deviations (SD) of the results of 10 independent experiments, with Gag-positive (Gag+) cells measured at 24 h and TNF at 48 h postinfection. Individual experiments are depicted with matching symbols.*, P < 0.05 (Wilcoxon test). (C) HIV-1 replication and TNF secretion in primary CD4+ T cells. The cells were infected with HIV or Δvpr HIV (40 to 400 ng of Gag p24/ml/106 cells). Infection was monitored by measuring the percentages of Gag+ cells by flow cytometry, and TNF was quantified in the supernatants by Luminex assay or ELISA at the indicated days postinfection. Data represent the results of one kinetic experiment of viral replication (left panel) and TNF production (right panel) of six performed. (D) Infection levels and TNF production in primary CD4+ T cells in six independent donors. The cells were infected and analyzed as described for panel C. Data represent means ± SD of the results of 6 experiments, with Gag+ cells measured at 24 h and TNF at 48 h postinfection. Individual donors are depicted with matching symbols. *, P < 0.05 (Wilcoxon test).
Previous studies in 293T cells and quiescent CD4 T cells suggested a role for Nef in TNF production through its effects on exosomes (97, 98). Therefore, we assessed the contribution of Nef to TNF release by infecting MT4C5 cells with viruses deleted for vpr or for nef or deleted for both genes (see Fig. S2 in the supplemental material). We did not observe any effect of nef deletion on TNF production by cells infected with WT or Δvpr HIV. This suggests that in activated CD4 T cells, Vpr, and not Nef, stimulates TNF production.
De novo synthesis of Vpr is required to trigger TNF secretion.
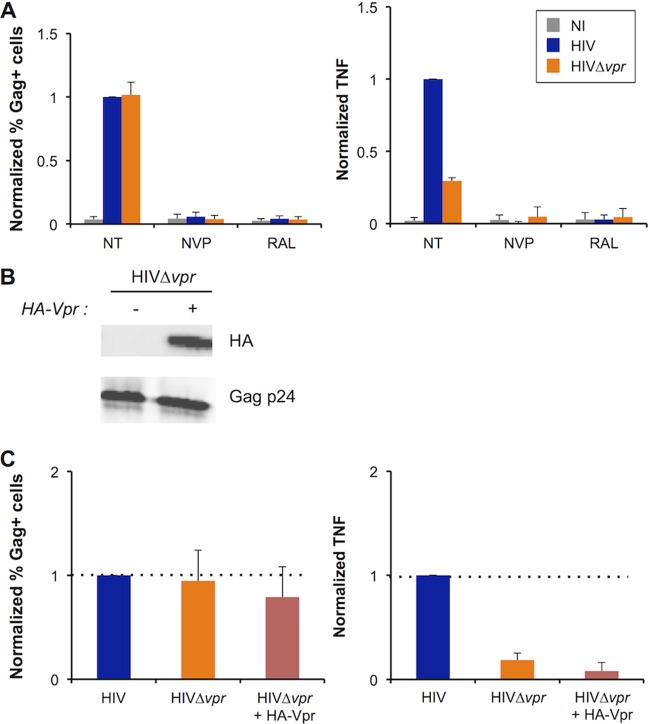
Vpr is packaged into virions by interaction with the p6 domain of Gag (23, 24). In infected cells, Vpr proteins can thus originate from incoming viral particles or from the pool of newly synthesized viral proteins. Virion-associated Vpr was previously described to promote apoptosis and G2 cell cycle arrest, although to a lower extent than newly synthesized Vpr (99–101). We determined which step of the viral cycle is associated with Vpr-induced TNF secretion and asked whether incoming Vpr proteins are sufficient to promote this phenomenon. We infected MT4C5 cells in the presence of inhibitors of reverse transcription (nevirapine [NVP]) or integration (raltegravir [RAL]). The two molecules inhibited TNF production (Fig. 2A; see also Fig. S3A in the supplemental material), suggesting that infection has to proceed until viral integration to trigger the release of the cytokine. We then infected MT4C5 cells with HIV, Δvpr HIV, or with a Δvpr HIV mutant complemented in trans with a N-terminally HA-tagged Vpr (HA-Vpr). Western blot experiments demonstrated that HA-Vpr was incorporated into virions (Fig. 2B). The three virus strains infected MT4C5 cells to similar levels (Fig. 2C; see also Fig. S3B). However, trans-complementation of the HIV Δvpr mutant with HA-Vpr did not rescue TNF production. trans-complementation with another Vpr construct (Flag-Vpr) also had no effect on TNF production (data not shown). Together, these results show that Vpr stimulates TNF production during or after viral integration and suggest that the presence of virion-incorporated Vpr is not sufficient to promote this phenomenon.
FIG 2.
TNF secretion by infected T cells requires viral integration and Vpr neosynthesis. (A) Effect of antiretroviral drugs on TNF release by infected cells. MT4C5 cells were infected with HIV or Δvpr HIV in the presence or absence of the reverse transcriptase inhibitor nevirapine (NVP; 25 nM) or the integrase inhibitor raltegravir (RAL; 1 μM). Nontreated cells (NT) were used as a control. Infection levels at 24 h and TNF release at 48 h were measured as described for Fig. 1. TNF levels obtained with wild-type HIV and no antiretroviral drugs were set at 1. Results represent means ± SD of the results of 3 independent experiments. (B) trans-complementation of Δvpr HIV. Δvpr HIV was complemented in trans with HA-Vpr by cotransfection of virus-producing 293T cells. The indicated viruses were analyzed by Western blotting for HA-Vpr incorporation (upper panel). A 20-ng volume of Gag p24 was loaded in each lane. Levels of Gag p24 were assessed as a control (lower panel). Data represent the results of one representative experiment. (C) Effect of Vpr trans-complementation on TNF release. MT4C5 cells were infected with HIV, Δvpr HIV, or Δvpr HIV plus HA-Vpr. Infection levels at 24 h and TNF release at 48 h were measured as described for Fig. 1. Levels obtained with wild-type HIV were set at 1. Data represent means ± SD of the results of 3 independent experiments.
Vpr mutants differentially activate TNF synthesis.
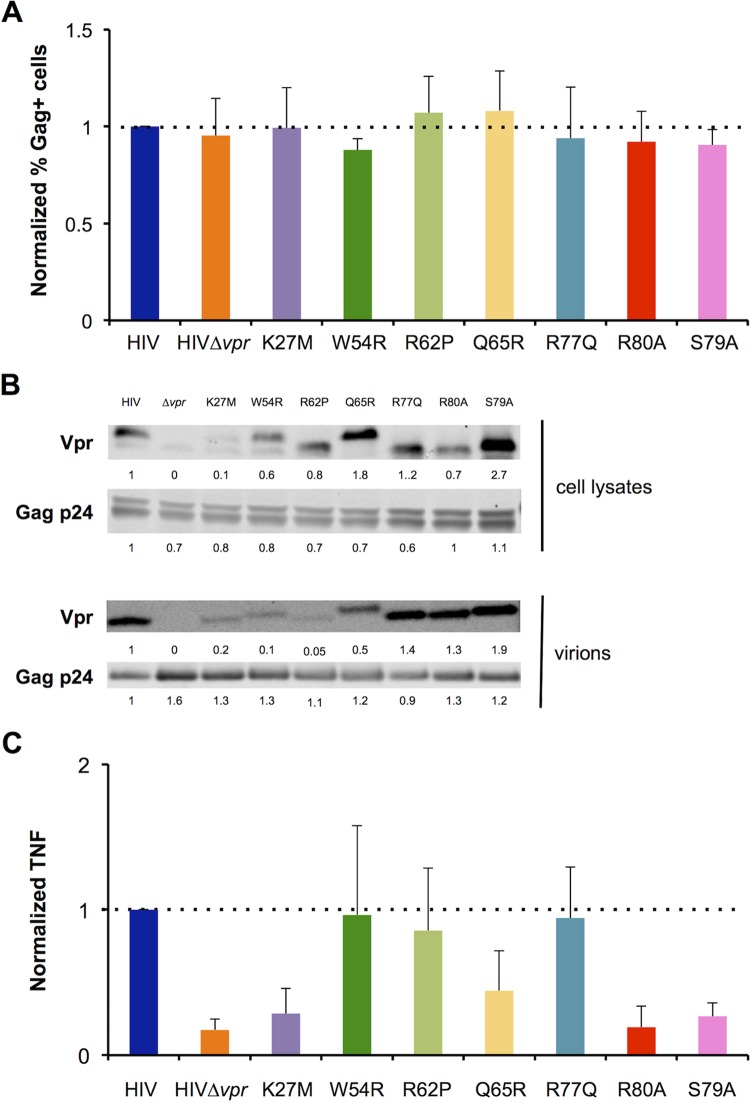
To determine which activities of Vpr are important for stimulation of TNF production, we analyzed the behavior of a panel of Vpr mutants. We selected mutants previously described as impaired in their ability to induce G2 cell cycle arrest (K27M, Q65R, S79A, and R80A) (59, 102) or apoptosis stimulation (Q65R, R77Q, and R80A) (48, 102, 103) or to localize at the nucleus/nuclear envelope (K27M, S79A, R62P, and Q65R) (30, 40, 55, 104). The R62P mutant is unable to form nuclear foci (55). We also used mutants unable to bind proteins known to interact with Vpr, such as UNG2 (W54R) (30, 105), TAK1 (S79A) (71), the nuclear envelope protein hCG1 (K27M) (30), and members of the SLX4com (Q65R, R80A) (62, 63). We introduced the corresponding mutations into the HIV-1 NL4-3 provirus and measured infection and TNF levels as previously described. The Vpr-mutated HIV-1 strain infected MT4C5 cells as efficiently as the WT virus (Fig. 3A; see also Fig. S4A in the supplemental material). All Vpr mutants were expressed and packaged into viral particles, albeit at different levels (Fig. 3B). The G2 arrest-defective mutants K27M, R80A, and (to a lesser extent) Q65R were impaired in their ability to stimulate TNF secretion (Fig. 3C). Interestingly, the ability of S79A Vpr to induce TNF was also reduced (Fig. 3C). This mutant is unable to arrest the cell cycle (106) or to bind to TAK1, a kinase involved in NF-κB signaling (70, 71). The other Vpr mutants tested (W54R, R62P, and R77Q) efficiently triggered TNF release (Fig. 3C). The characteristics of the Vpr mutants as well as our TNF production results are summarized in Table 1. Taken together, our results suggest that the previously reported G2 cell cycle arrest activity of Vpr correlates at the molecular level with its ability to stimulate TNF production.
FIG 3.
Characterization of Vpr mutants. (A) Infection of MT4C5 cells with Vpr-mutated viruses. The indicated vpr mutations were introduced into HIV NL4-3. Infection levels were analyzed by flow cytometry at 24 h as described for Fig. 1. Infection with wild-type HIV-1 was set at 1. Data represent means ± SD of the results of 4 independent experiments. (B) Expression and incorporation of Vpr mutants. Cell lysates were obtained from transfected 293T cells. Viral particles were purified by ultracentrifugation on an iodixanol (Optiprep) cushion. Vpr levels were assessed by Western blot analysis performed with an anti-Vpr monoclonal antibody. For cell lysates and purified virions, 45 ng and 100 ng of Gag p24 were loaded in each lane, respectively (upper panel). Levels of Gag p24 were assessed as a control (lower panel). Relative band intensities were calculated using ImageStudio Lite and are indicated for each lane. Data represent the results of one representative experiment. (C) TNF induction in MT4C5 cells infected by the Vpr-mutated viruses. TNF was measured in the supernatants of infected cells at 48 h p.i. as described for Fig. 1, with the levels obtained with wild-type HIV set at 1. Data represent means ± SD of the results of 4 independent experiments.
TABLE 1.
The ability of Vpr to induce TNF correlates with its previously reported cell cycle arrest activitya
| Activity | Vpr WT or mutant result(s) (reference[s]) |
|||||||
|---|---|---|---|---|---|---|---|---|
| WT | K27M | W54R | R62P | Q65R | R77Q | R80A | S79A | |
| Cell cycle arrest | + | − (102) | + (56) | ± (55, 104) | − (59) | + (103) | − (56) | − (102) |
| Apoptosis | + | + (102) | ND | ND | − (102) | − (48) | − (48) | + (102) |
| Nuclear localization | + | − (30) | + (30) | − (55, 104) | − (40) | + (40) | + (30) | − (55) |
| Disrupted interaction(s) | hCG1 (30) | UNG2 (105) | DDB1 (57), DCAF1 (59), SLX4 (62, 63), MUS81 (62), PLK1 (62) | MUS81 (62), PLK1 (62) | TAK1 (71) | |||
| TNF induction | + | − | + | + | ± | + | − | − |
Summary of the main properties of the Vpr mutants used in this study. The ability of Vpr mutants to induce cell cycle arrest and apoptosis, to localize to the nucleus/nuclear envelope, and to bind known interactants, as described in the literature, is shown. The corresponding references are indicated in parentheses. ND, not determined. Our TNF production results are presented in bold. +, wild-type activity; ±, intermediate phenotype; −, decreased or absent activity.
DDB1 and TAK1 are required for TNF induction.
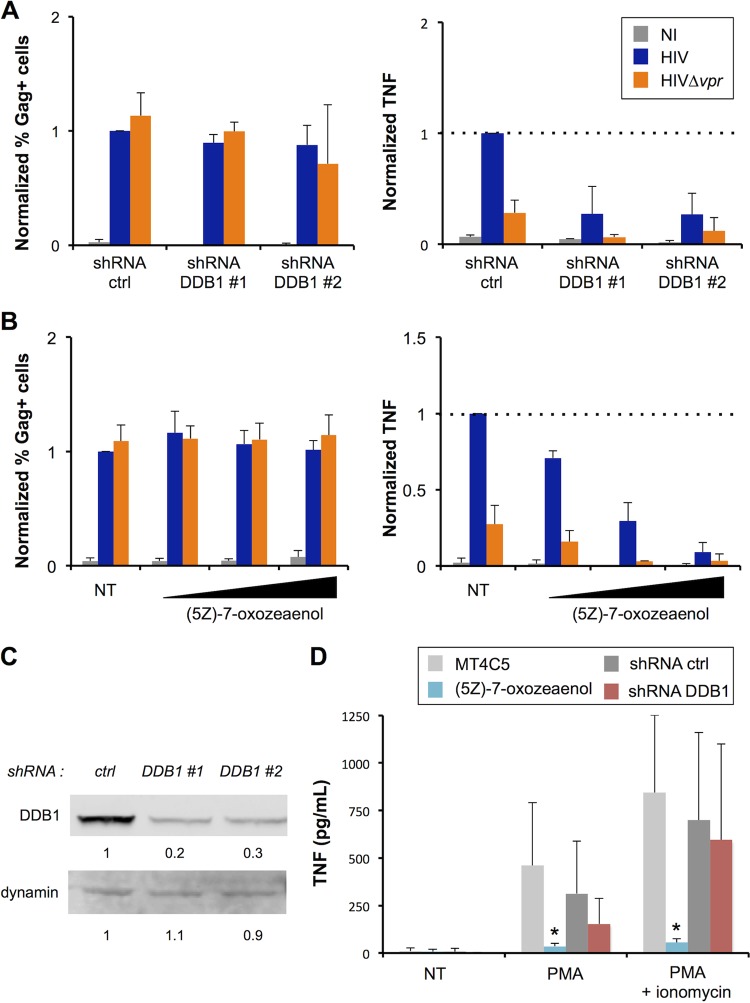
We then analyzed the role of selected cellular proteins that may be involved in the induction of TNF by Vpr. From our mutant analysis, we determined that the previously reported cell cycle arrest activity of Vpr correlates with upregulation of TNF. Thus, we first investigated the role of DDB1, a protein critical for several Vpr activities such as DNA damage and cell cycle arrest (55, 59, 62, 63). MT4C5 cells were transduced with control or DDB1-specific shRNA lentivectors and selected with puromycin. Western blot analysis indicated that about 70% of DDB1 was silenced in these cells, with two different shRNAs (Fig. 4C). Silenced cells were then infected with WT and Δvpr viruses, and levels of TNF were measured in the supernatants. DDB1 silencing impacted neither infection (Fig. 4A) nor cell viability (data not shown). We observed a 5-fold reduction of TNF synthesis when DDB1 was silenced (Fig. 4A). It is noteworthy that the amounts of TNF produced by HIV Δvpr mutant-infected cells were also reduced in the absence of DDB1, suggesting that DDB1 enhances TNF synthesis independently of Vpr (Fig. 4A).
FIG 4.
DDB1 and TAK1 mediate TNF release by infected cells. (A) DDB1 is required for TNF release by infected cells. Control or DDB1-silenced MT4C5 cells were infected with HIV or Δvpr HIV. Infection levels at 24 h and TNF alpha (TNF-α) release at 48 h were measured as described for Fig. 1. Levels obtained with wild-type HIV and control cells were set at 1. Data represent means ± SD of the results of 3 independent experiments. (B) TAK1 is required for TNF release by infected cells. MT4C5 cells were infected with HIV or Δvpr HIV in the presence of increasing concentrations (5, 15, and 50 nM) of (5Z)-7-oxozeaenol, a previously described TAK1 inhibitor. Infection levels at 24 h and TNF release at 48 h were measured as described for Fig. 1. Levels obtained with wild-type HIV and nontreated (NT) cells were set at 1. Data represent means ± SD of the results of 3 independent experiments. (C) DDB1 silencing efficiency. MT4C5 cells were transduced with lentiviral vectors expressing control or anti-DDB1 shRNAs and selected by puromycin. DDB1 levels were assessed by Western blot analysis (upper panel), with dynamin used as a loading control (lower panel). Relative band intensities were calculated using ImageStudio Lite and are indicated for each lane. Data represent the results of one representative experiment. (D) Effect of TAK1 inhibition and DDB1 depletion on PMA-induced TNF production. Control, DDB1-silenced, and (5Z)-7-oxozeaenol (50 nM)-treated cells were stimulated with PMA (10 ng/ml) alone or in combination with ionomycin (200 ng/ml). TNF levels were quantified after 20 h. Data represent means ± SD of the results of 5 independent experiments. *, P < 0.05 (Mann-Whitney test).
The Vpr S79A mutant, which no longer binds TAK1 (71), was impaired in its ability to trigger TNF activity (Fig. 3C). To further investigate the role of TAK1, we used (5Z)-7-oxozeanol, a chemical inhibitor of this kinase (71, 107). (5Z)-7-oxozeanol was not toxic at the doses employed (data not shown) and did not impact viral replication but reduced dramatically the production of TNF by infected cells (Fig. 4B). The residual production of TNF by HIV Δvpr mutant-infected cells was also decreased in the presence of the TAK1 inhibitor. Together, these results suggest that DDB1 and TAK1 are implicated in the enhanced production of TNF by infected cells but also play a role when Vpr is absent.
We assessed the role of DDB1 and TAK in TNF production in the absence of infection, using PMA or PMA plus ionomycin as stimuli (Fig. 4D). DDB1 silencing did not significantly affect TNF production induced by treatment with PMA or PMA plus ionomycin. In contrast, the TAK1 inhibitor almost completely abrogated TNF production by PMA-treated cells. This was expected given the implication of TAK1 in the NF-κB pathway that controls TNF production. These results strongly suggest that the induction of TNF by HIV-infected cells involves a canonical pathway of synthesis of this cytokine.
TNF secreted by infected cells enhances NF-κB activity and viral reactivation in bystander cells.
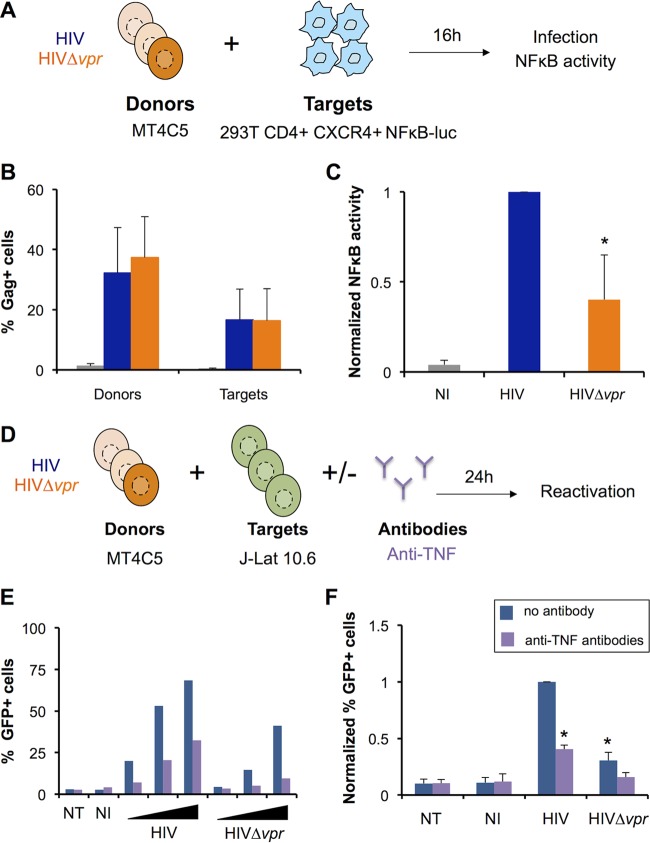
TNF signaling induces the nuclear translocation of NF-κB, which may activate the LTR promoter as well as a proinflammatory innate response (87, 108). To assess whether Vpr-induced TNF can signal in bystander cells, we cocultivated infected MT4C5 cells with 293T CD4+ CXCR4+ cells carrying a NF-κB–luciferase reporter plasmid (Fig. 5A). In these cells, TNF signaling would lead to NF-κB-mediated expression of luciferase. The absence of Vpr did not impact viral cell-to-cell transmission from MT4C5 cells to target 293T cells (Fig. 5B). Coculture with HIV-1-infected cells triggered NF-κB activity in 293T cells (Fig. 5C). Consistent with our previous results determined on the basis of TNF production, there was a 2-fold to 3-fold decrease in luciferase levels in the absence of Vpr (Fig. 5C). To determine if this increased NF-κB activity was due to TNF signaling, we performed coculture experiments in the presence of antibodies blocking TNF. As expected, anti-TNF antibodies neutralized the action of recombinant TNF (data not shown). In the presence of these antibodies, NF-κB activation mediated by HIV-1 was reduced (see Fig. S5A in the supplemental material). Thus, Vpr-induced TNF can stimulate the NF-κB pathway in bystander cells.
FIG 5.
TNF produced by HIV-1-infected cells stimulates NF-κB and viral reactivation in bystander cells. (A) NF-κB activity in bystander cells (experimental outline). 293T CD4+ CXCR4+ cells were transiently transfected with a NF-κB–luciferase reporter plasmid and cocultivated for 16 h with MT4C5 cells infected with either HIV or Δvpr HIV. The percentage of Gag+ cells was quantified by flow cytometry, and luciferase levels were measured with a luminometer. (B) Gag levels in MT4C5 donor and 293T CD4+ CXCR4+ target cells. Results represent means ± SD of the results of 7 independent experiments. (C) NF-κB activity in the coculture. The values obtained with wild-type HIV were set at 1. Results represent means ± SD of the results of 6 independent experiments. *, P < 0.05 (Wilcoxon test). (D) Viral reactivation in latently infected bystander cells (experimental outline). MT4C5 cells were infected with HIV or Δvpr HIV and cocultivated with J-Lat 10.6 cells at a 1:1 ratio. Prior to and during coculture, donor cells were treated with anti-TNF antibodies (1 μg/ml). Viral reactivation was quantified after 24 h by measuring the percentages of GFP+ cells by flow cytometry. (E) Dose-response analysis of viral reactivation in J-Lat cells. MT4C5 cells infected at increasing levels (from 5% to 25% of Gag+ cells, for both HIV and Δvpr HIV) were used as donors. As a control, J-Lat cells were cultured alone (NT) or with noninfected MT4C5 (NI). Data represent the results of one representative experiment. (F) Viral reactivation in bystander cells is enhanced by Vpr. The result obtained with wild-type HIV was set at 1. Data represent means ± SD of the results of 4 independent experiments. *, P < 0.05 (Wilcoxon test).
TNF promotes viral reactivation in different models of latently infected T cells (89). This prompted us to examine whether HIV-1-infected cells could trigger viral reactivation in bystander cells through the effect of Vpr on TNF production. To this end, we used J-Lat 10.6 cells, a Jurkat T cell derivative carrying a latent, integrated viral genome where env is deleted and nef is replaced by gfp (94). Without cell activation, GFP is not produced, but treatment with TNF, PMA, or other molecules induces HIV-1 reactivation and GFP expression (93, 94). MT4C5 cells were infected with HIV or the HIV Δvpr mutant and cocultivated with J-Lat cells (Fig. 5D). GFP levels were monitored by flow cytometry 24 h after stimulation. In this short-term coculture system, direct activation of the LTR by incoming Tat contributed only modestly to reactivation, since anti-Env broadly neutralizing antibodies successfully blocked infection of Jurkat T cells but not reactivation of J-Lat cells (see Fig. S5B and C in the supplemental material). Consistent with increased TNF production, reactivation was stronger when J-Lat cells were cocultivated with MT4C5 cells infected with WT HIV-1 (Fig. 5E). This effect of Vpr was visible using various viral inputs (Fig. 5E) and was inhibited by anti-TNF antibodies (Fig. 5F). Therefore, by increasing TNF levels produced by infected cells, Vpr may favor viral reactivation in bystander cells.
DISCUSSION
We have characterized the effect of Vpr on the release of TNF by HIV-1-infected T cells. Contradictory results regarding the role of Vpr in the modulation of cytokine production have been reported. Some studies indicated that Vpr stimulates the IFN pathway, whereas others indicated that Vpr decreased IFN production (33, 62, 72, 73). The effect of Vpr on the NF-κB pathway remains controversial as well. It has been proposed that Vpr may impair NF-κB nuclear translocation by inducing and/or stabilizing the IκBα inhibitory subunit (14, 74, 109). In contrast, it has been suggested that Vpr interaction with TAK1 leads to IKKα/β phosphorylation and ultimately results in IκBα degradation (70, 71). Vpr may also activate NF-κB through other pathways such as the Jun N-terminal protein kinase (JNK) pathway or AP1 pathway (80). These discrepancies may be explained by the different cell types used and by the method of Vpr expression. Overexpression of Vpr might be deleterious to the cells, due to its proapoptotic properties. The use of recombinant Vpr proteins may introduce bacterial contaminants, triggering immune side effects. Vpr expressed alone or in the context of infected cells may also exert different effects. Thus, using a relevant system to study the effect of Vpr on TNF is critical. Here, we infected CD4+ T cells with wild-type or vpr-deleted viruses and observed that Vpr stimulates the secretion of TNF in productively infected cells. Our results confirm and extend earlier studies showing that CD4+ T lymphocytes, dendritic cells, and macrophages infected by HIV produce higher levels of TNF in the presence of Vpr (79, 81, 82). Using inhibitors of reverse transcription and integration, as well as Δvpr viruses complemented in trans with HA-Vpr, we found that de novo synthesis of Vpr was required for this effect. Additionally, some Vpr mutants (W54R and R62P) are less extensively incorporated than wild-type Vpr but still trigger TNF release. This could suggest that TNF stimulation and G2 cell cycle arrest require different levels of Vpr expression.
To gain further insight into the viral and cellular components required to trigger TNF production in CD4+ T cells, we analyzed the behavior of a panel of Vpr mutants and investigated the role of host factors DDB1 and TAK1. Vpr mutants previously reported to be defective for cell cycle arrest were unable to stimulate TNF production. This is consistent with our observation that silencing of DDB1, a protein critical for Vpr-mediated G2 cell cycle arrest, also decreased TNF release. Whether TNF production is a downstream event of the cell cycle arrest remains to be determined. It is noteworthy that only the Q65R mutant was defective for both TNF stimulation and DCAF1 binding. This suggests that binding to DCAF1 by Vpr is necessary but not sufficient to enhance TNF production. Q65R and R80A, both unable to stimulate TNF production, do not bind the SLX4com (62, 63). Thus, it will be worth examining whether the untimely activation of the SLX4com by Vpr is mediating TNF release.
By using the Vpr S79A mutant, which is unable to bind to the TAK1 kinase, and (5Z)-7-oxozeanol, a chemical inhibitor specific for this kinase (71, 107), we found that TAK1 is required to trigger TNF release. This suggests that HIV-induced TNF production occurs mainly through the NF-κB pathway. The TAK1 inhibitor almost completely abrogated TNF production by both WT and Δvpr mutant-infected cells, whereas Vpr S79A had only a partial phenotype. Additionally, both DDB1-silenced and HIV Δvpr mutant-infected cells produced low but detectable amounts of TNF. Even if the residual DDB1 could still interact with Vpr through DCAF1 to induce TNF, these results may indicate that the requirement for Vpr and G2 arrest for TNF induction is not absolute. Thus, TNF production by infected cells might be triggered by a Vpr-independent TNF pathway involving TAK1 and DDB1, a phenomenon that would be enhanced by the presence of Vpr. TNF production is known to be additionally triggered by other viral proteins such as Tat or gp41 (110–112). Nef has also been reported to increase exosomal release by infected cells, leading to a processing of pro-TNF to TNF (97, 98). We did not observe an impact of Nef on TNF release in our system. This result might reflect cell type-specific differences, since previous studies used quiescent CD4 T cells and 293T cells. Future work will help to clarify whether Vpr, by hijacking the ubiquitin ligase complex DCAF1-DDB1-Cul4, enhances the effect of other HIV proteins such as Tat and gp41 or acts by enhancing TNF production triggered by viral reverse transcription, integration, or another step of the viral life cycle. It will also be of great interest to determine precisely whether Vpr acts at the transcriptional level or by stimulating maturation and/or release of TNF.
What could be the biological consequences of this increased release of TNF by infected cells? TNF signaling has a significant impact on the HIV-1 life cycle, facilitating viral replication in a variety of cell types (87, 90, 91) as well as reactivation from the reservoir (88, 89, 98). Our experiments using NF-κB reporter cells and a model of latently infected Jurkat-derived cells suggest that the variations of TNF levels may modulate NF-κB activation and viral reactivation in bystander cells. Moreover, in addition to direct effects on the virus, these enhanced levels of TNF will likely impact survival, apoptosis, and activation of bystander cells and host inflammation (113, 114), which are key to the progression to AIDS.
In summary, our work shows that Vpr displays proinflammatory features by potentiating TNF release by infected cells, a phenomenon that likely impacts HIV replication and pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
O.S. was supported by grants from the ANRS, SIDACTION, AREVA Foundation, the Labex IBEID program, the FP7 program HIT Hidden HIV (Health-F3-2012-305762), and the Vaccine Research Institute, Investissements d'Avenir program managed by the ANR-10-LABX77.
We thank members of the Virus & Immunity Unit for discussions and Lise Chauveau, Diana Ayinde, Molly OhAinle, and Oliver Fregoso for critical reading of the manuscript. We thank Delphine Golinelli for helping with experiments. We thank Eric Cohen for helpful comments. We thank Florence Margottin-Goguet, Emmanuel Laplantine, Cécilia Ramirez, John Hiscott, Robert Weil, and the NIH AIDS reagents program for the gift of reagents.
We declare that we have no competing financial interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02098-15.
REFERENCES
- 1.Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 2.Paiardini M, Muller-Trutwin M. 2013. HIV-associated chronic immune activation. Immunol Rev 254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miedema F, Hazenberg MD, Tesselaar K, van Baarle D, de Boer RJ, Borghans JA. 2013. Immune activation and collateral damage in AIDS pathogenesis. Front Immunol 4:298. doi: 10.3389/fimmu.2013.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luban J. 2012. Innate immune sensing of HIV-1 by dendritic cells. Cell Host Microbe 12:408–418. doi: 10.1016/j.chom.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuter MA, Pombo C, Betts MR. 2012. Cytokine production and dysregulation in HIV pathogenesis: lessons for development of therapeutics and vaccines. Cytokine Growth Factor Rev 23:181–191. doi: 10.1016/j.cytogfr.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malim MH, Emerman M. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 10.Sheehy AM, Gaddis NC, Choi JD, Malim MH. 2002. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 11.Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. 2012. Innate sensing of HIV-1 assembly by Tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe 12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galão RP, Pickering S, Curnock R, Neil SJ. 2014. Retroviral retention activates a Syk-dependent HemITAM in human tetherin. Cell Host Microbe 16:291–303. doi: 10.1016/j.chom.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. 2013. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J Virol 87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauter D, Hotter D, Van Driessche B, Sturzel CM, Kluge SF, Wildum S, Yu H, Baumann B, Wirth T, Plantier JC, Leoz M, Hahn BH, Van Lint C, Kirchhoff F. 22 January 2015, posting date. Differential regulation of NF-kappaB-mediated proviral and antiviral host gene expression by primate lentiviral Nef and Vpu Proteins. Cell Rep doi: 10.1016/j.celrep.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. 2011. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puigdomènech I, Casartelli N, Porrot F, Schwartz O. 2013. SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells. J Virol 87:2846–2856. doi: 10.1128/JVI.02514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. 2013. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Sharp PM, Bailes E, Stevenson M, Emerman M, Hahn BH. 1996. Gene acquisition in HIV and SIV. Nature 383:586–587. doi: 10.1038/383586a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tristem M, Marshall C, Karpas A, Hill F. 1992. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J 11:3405–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khamsri B, Murao F, Yoshida A, Sakurai A, Uchiyama T, Shirai H, Matsuo Y, Fujita M, Adachi A. 2006. Comparative study on the structure and cytopathogenic activity of HIV Vpr/Vpx proteins. Microbes Infect 8:10–15. doi: 10.1016/j.micinf.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Checroune F, Yao XJ, Gottlinger HG, Bergeron D, Cohen EA. 1995. Incorporation of Vpr into human immunodeficiency virus type 1: role of conserved regions within the P6 domain of Pr55gag. J Acquir Immune Defic Syndr Hum Retrovirol 10:1–7. [PubMed] [Google Scholar]
- 24.Selig L, Pages JC, Tanchou V, Preveral S, Berlioz-Torrent C, Liu LX, Erdtmann L, Darlix J, Benarous R, Benichou S. 1999. Interaction with the p6 domain of the gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J Virol 73:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayinde D, Maudet C, Transy C, Margottin-Goguet F. 2010. Limelight on two HIV/SIV accessory proteins in macrophage infection: is Vpx overshadowing Vpr? Retrovirology 7:35. doi: 10.1186/1742-4690-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dedera D, Hu W, Vander Heyden N, Ratner L. 1989. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol 63:3205–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 28.Eckstein DA, Sherman MP, Penn ML, Chin PS, De Noronha CM, Greene WC, Goldsmith MA. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J Exp Med 194:1407–1419. doi: 10.1084/jem.194.10.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connor RI, Chen BK, Choe S, Landau NR. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 30.Jacquot G, Le Rouzic E, David A, Mazzolini J, Bouchet J, Bouaziz S, Niedergang F, Pancino G, Benichou S. 2007. Localization of HIV-1 Vpr to the nuclear envelope: impact on Vpr functions and virus replication in macrophages. Retrovirology 4:84. doi: 10.1186/1742-4690-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zander K, Sherman MP, Tessmer U, Bruns K, Wray V, Prechtel AT, Schubert E, Henklein P, Luban J, Neidleman J, Greene WC, Schubert U. 2003. Cyclophilin A interacts with HIV-1 Vpr and is required for its functional expression. J Biol Chem 278:43202–43213. doi: 10.1074/jbc.M305414200. [DOI] [PubMed] [Google Scholar]
- 32.de Silva S, Planelles V, Wu L. 2012. Differential effects of Vpr on single-cycle and spreading HIV-1 infections in CD4+ T-cells and dendritic cells. PLoS One 7:e35385. doi: 10.1371/journal.pone.0035385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mashiba M, Collins DR, Terry VH, Collins KL. 2014. Vpr overcomes macrophage-specific restriction of HIV-1 Env expression and virion production. Cell Host Microbe 16:722–735. doi: 10.1016/j.chom.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins DR, Lubow J, Lukic Z, Mashiba M, Collins KL. 2015. Vpr promotes macrophage-dependent HIV-1 infection of CD4+ T lymphocytes. PLoS Pathog 11:e1005054. doi: 10.1371/journal.ppat.1005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang SM, Weeger M, Stahl-Hennig C, Coulibaly C, Hunsmann G, Muller J, Muller-Hermelink H, Fuchs D, Wachter H, Daniel MM, Desrosiers RC, Fleckenstein B. 1993. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol 67:902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaumont T, van Nuenen A, Broersen S, Blattner WA, Lukashov VV, Schuitemaker H. 2001. Reversal of human immunodeficiency virus type 1 IIIB to a neutralization-resistant phenotype in an accidentally infected laboratory worker with a progressive clinical course. J Virol 75:2246–2252. doi: 10.1128/JVI.75.5.2246-2252.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med 4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 38.Caly L, Saksena NK, Piller SC, Jans DA. 2008. Impaired nuclear import and viral incorporation of Vpr derived from a HIV long-term non-progressor. Retrovirology 5:67. doi: 10.1186/1742-4690-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadi K, Walker LA, Guha D, Murali R, Watkins SC, Tarwater P, Srinivasan A, Ayyavoo V. 2014. Human immunodeficiency virus type 1 Vpr polymorphisms associated with progressor and nonprogressor individuals alter Vpr-associated functions. J Gen Virol 95:700–711. doi: 10.1099/vir.0.059576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacquot G, Le Rouzic E, Maidou-Peindara P, Maizy M, Lefrere JJ, Daneluzzi V, Monteiro-Filho CM, Hong D, Planelles V, Morand-Joubert L, Benichou S. 2009. Characterization of the molecular determinants of primary HIV-1 Vpr proteins: impact of the Q65R and R77Q substitutions on Vpr functions. PLoS One 4:e7514. doi: 10.1371/journal.pone.0007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzitzivacos DB, Tiemessen CT, Stevens WS, Papathanasopoulos MA. 2009. Viral genetic determinants of nonprogressive HIV type 1 subtype C infection in antiretroviral drug-naive children. AIDS Res Hum Retroviruses 25:1141–1148. doi: 10.1089/aid.2009.0080. [DOI] [PubMed] [Google Scholar]
- 42.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau NR. 1995. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol 69:7909–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol 69:6705–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jowett JB, Planelles V, Poon B, Shah NP, Chen ML, Chen IS. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol 69:6304–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Re F, Braaten D, Franke EK, Luban J. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol 69:6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arokium H, Kamata M, Chen I. 2009. Virion-associated Vpr of human immunodeficiency virus type 1 triggers activation of apoptotic events and enhances fas-induced apoptosis in human T cells. J Virol 83:11283–11297. doi: 10.1128/JVI.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muthumani K, Hwang DS, Desai BM, Zhang D, Dayes N, Green DR, Weiner DB. 2002. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J Biol Chem 277:37820–37831. doi: 10.1074/jbc.M205313200. [DOI] [PubMed] [Google Scholar]
- 48.Rajan D, Wildum S, Rucker E, Schindler M, Kirchhoff F. 2006. Effect of R77Q, R77A and R80A changes in Vpr on HIV-1 replication and CD4 T cell depletion in human lymphoid tissue ex vivo. AIDS 20:831–836. doi: 10.1097/01.aids.0000218546.31716.7f. [DOI] [PubMed] [Google Scholar]
- 49.Belzile JP, Richard J, Rougeau N, Xiao Y, Cohen EA. 2010. HIV-1 Vpr induces the K48-linked polyubiquitination and proteasomal degradation of target cellular proteins to activate ATR and promote G2 arrest. J Virol 84:3320–3330. doi: 10.1128/JVI.02590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai M, Zimmerman ES, Planelles V, Chen J. 2005. Activation of the ATR pathway by human immunodeficiency virus type 1 Vpr involves its direct binding to chromatin in vivo. J Virol 79:15443–15451. doi: 10.1128/JVI.79.24.15443-15451.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. 2003. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem 278:25879–25886. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- 52.Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. 2009. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog 5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hogan TH, Nonnemacher MR, Krebs FC, Henderson A, Wigdahl B. 2003. HIV-1 Vpr binding to HIV-1 LTR C/EBP cis-acting elements and adjacent regions is sequence-specific. Biomed Pharmacother 57:41–48. doi: 10.1016/S0753-3322(02)00333-5. [DOI] [PubMed] [Google Scholar]
- 54.Mansky LM. 1996. The mutation rate of human immunodeficiency virus type 1 is influenced by the vpr gene. Virology 222:391–400. doi: 10.1006/viro.1996.0436. [DOI] [PubMed] [Google Scholar]
- 55.Belzile JP, Abrahamyan LG, Gerard FC, Rougeau N, Cohen EA. 2010. Formation of mobile chromatin-associated nuclear foci containing HIV-1 Vpr and VPRBP is critical for the induction of G2 cell cycle arrest. PLoS Pathog 6:e1001080. doi: 10.1371/journal.ppat.1001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belzile JP, Duisit G, Rougeau N, Mercier J, Finzi A, Cohen EA. 2007. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog 3:e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeHart JL, Zimmerman ES, Ardon O, Monteiro-Filho CM, Arganaraz ER, Planelles V. 2007. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol J 4:57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, Washburn MP, Skowronski J. 2007. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A 104:11778–11783. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, Margottin-Goguet F. 2007. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell Cycle 6:182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- 60.Tan L, Ehrlich E, Yu XF. 2007. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J Virol 81:10822–10830. doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen X, Duus KM, Friedrich TD, de Noronha CM. 2007. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem 282:27046–27057. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- 62.Laguette N, Bregnard C, Hue P, Basbous J, Yatim A, Larroque M, Kirchhoff F, Constantinou A, Sobhian B, Benkirane M. 2014. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell 156:134–145. doi: 10.1016/j.cell.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 63.Berger G, Lawrence M, Hue S, Neil SJ. 2015. G2/M cell cycle arrest correlates with primate lentiviral Vpr interaction with the SLX4 complex. J Virol 89:230–240. doi: 10.1128/JVI.02307-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gummuluru S, Emerman M. 1999. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J Virol 73:5422–5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato K, Misawa N, Iwami S, Satou Y, Matsuoka M, Ishizaka Y, Ito M, Aihara K, An DS, Koyanagi Y. 2013. HIV-1 Vpr accelerates viral replication during acute infection by exploitation of proliferating CD4+ T cells in vivo. PLoS Pathog 9:e1003812. doi: 10.1371/journal.ppat.1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guenzel CA, Herate C, Benichou S. 2014. HIV-1 Vpr—a still “enigmatic multitasker”. Front Microbiol 5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wen X, Casey Klockow L, Nekorchuk M, Sharifi HJ, de Noronha CM. 2012. The HIV1 protein Vpr acts to enhance constitutive DCAF1-dependent UNG2 turnover. PLoS One 7:e30939. doi: 10.1371/journal.pone.0030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen R, Le Rouzic E, Kearney JA, Mansky LM, Benichou S. 2004. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J Biol Chem 279:28419–28425. doi: 10.1074/jbc.M403875200. [DOI] [PubMed] [Google Scholar]
- 69.Guenzel CA, Herate C, Le Rouzic E, Maidou-Peindara P, Sadler HA, Rouyez MC, Mansky LM, Benichou S. 2012. Recruitment of the nuclear form of uracil DNA glycosylase into virus particles participates in the full infectivity of HIV-1. J Virol 86:2533–2544. doi: 10.1128/JVI.05163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu R, Tan J, Lin Y, Jia R, Yang W, Liang C, Geng Y, Qiao W. 2013. HIV-1 Vpr activates both canonical and noncanonical NF-kappaB pathway by enhancing the phosphorylation of IKKalpha/beta. Virology 439:47–56. doi: 10.1016/j.virol.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 71.Liu R, Lin Y, Jia R, Geng Y, Liang C, Tan J, Qiao W. 2014. HIV-1 Vpr stimulates NF-kappaB and AP-1 signaling by activating TAK1. Retrovirology 11:45. doi: 10.1186/1742-4690-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong HS, Bhatnagar N, Ballmaier M, Schubert U, Henklein P, Volgmann T, Heiken H, Schmidt RE, Meyer-Olson D. 2009. Exogenous HIV-1 Vpr disrupts IFN-alpha response by plasmacytoid dendritic cells (pDCs) and subsequent pDC/NK interplay. Immunol Lett 125:100–104. doi: 10.1016/j.imlet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Harman AN, Lai J, Turville S, Samarajiwa S, Gray L, Marsden V, Mercier SK, Jones K, Nasr N, Rustagi A, Cumming H, Donaghy H, Mak J, Gale M Jr, Churchill M, Hertzog P, Cunningham AL. 2011. HIV infection of dendritic cells subverts the IFN induction pathway via IRF-1 and inhibits type 1 IFN production. Blood 118:298–308. doi: 10.1182/blood-2010-07-297721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med 3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 75.Muthumani K, Kudchodkar S, Papasavvas E, Montaner LJ, Weiner DB, Ayyavoo V. 2000. HIV-1 Vpr regulates expression of beta chemokines in human primary lymphocytes and macrophages. J Leukoc Biol 68:366–372. [PubMed] [Google Scholar]
- 76.Na H, Acharjee S, Jones G, Vivithanaporn P, Noorbakhsh F, McFarlane N, Maingat F, Ballanyi K, Pardo CA, Cohen EA, Power C. 2011. Interactions between human immunodeficiency virus (HIV)-1 Vpr expression and innate immunity influence neurovirulence. Retrovirology 8:44. doi: 10.1186/1742-4690-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zahoor MA, Xue G, Sato H, Murakami T, Takeshima SN, Aida Y. 2014. HIV-1 Vpr induces interferon-stimulated genes in human monocyte-derived macrophages. PLoS One 9:e106418. doi: 10.1371/journal.pone.0106418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zahoor MA, Xue G, Sato H, Aida Y. 2015. Genome-wide transcriptional profiling reveals that HIV-1 Vpr differentially regulates interferon-stimulated genes in human monocyte-derived dendritic cells. Virus Res 208:156–163. doi: 10.1016/j.virusres.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 79.Guha D, Nagilla P, Redinger C, Srinivasan A, Schatten GP, Ayyavoo V. 2012. Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells. J Neuroinflammation 9:138. doi: 10.1186/1742-2094-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varin A, Decrion AZ, Sabbah E, Quivy V, Sire J, Van Lint C, Roques BP, Aggarwal BB, Herbein G. 2005. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J Biol Chem 280:42557–42567. doi: 10.1074/jbc.M502211200. [DOI] [PubMed] [Google Scholar]
- 81.Roux P, Alfieri C, Hrimech M, Cohen EA, Tanner JE. 2000. Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol 74:4658–4665. doi: 10.1128/JVI.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Majumder B, Venkatachari NJ, Schafer EA, Janket ML, Ayyavoo V. 2007. Dendritic cells infected with vpr-positive human immunodeficiency virus type 1 induce CD8+ T-cell apoptosis via upregulation of tumor necrosis factor alpha. J Virol 81:7388–7399. doi: 10.1128/JVI.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reddy MM, Sorrell SJ, Lange M, Grieco MH. 1988. Tumor necrosis factor and HIV P24 antigen levels in serum of HIV-infected populations. J Acquir Immune Defic Syndr 1:436–440. [PubMed] [Google Scholar]
- 84.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osmond DH, Shiboski S, Bacchetti P, Winger EE, Moss AR. 1991. Immune activation markers and AIDS prognosis. AIDS 5:505–511. doi: 10.1097/00002030-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 86.Vaidya SA, Korner C, Sirignano MN, Amero M, Bazner S, Rychert J, Allen TM, Rosenberg ES, Bosch RJ, Altfeld M. 2014. Tumor necrosis factor alpha is associated with viral control and early disease progression in patients with HIV type 1 infection. J Infect Dis 210:1042–1046. doi: 10.1093/infdis/jiu206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Israël N, Hazan U, Alcami J, Munier A, Arenzana-Seisdedos F, Bachelerie F, Israel A, Virelizier JL. 1989. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol 143:3956–3960. [PubMed] [Google Scholar]
- 88.Kitano K, Rivas CI, Baldwin GC, Vera JC, Golde DW. 1993. Tumor necrosis factor-dependent production of human immunodeficiency virus 1 in chronically infected HL-60 cells. Blood 82:2742–2748. [PubMed] [Google Scholar]
- 89.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, Greene WC, Kashuba A, Lewin SR, Margolis DM, Mau M, Ruelas D, Saleh S, Shirakawa K, Siliciano RF, Singhania A, Soto PC, Terry VH, Verdin E, Woelk C, Wooden S, Xing S, Planelles V. 2013. An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 9:e1003834. doi: 10.1371/journal.ppat.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Jong MA, de Witte L, Oudhoff MJ, Gringhuis SI, Gallay P, Geijtenbeek TB. 2008. TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. J Clin Invest 118:3440–3452. doi: 10.1172/JCI34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guillemard E, Jacquemot C, Aillet F, Schmitt N, Barre-Sinoussi F, Israel N. 2004. Human immunodeficiency virus 1 favors the persistence of infection by activating macrophages through TNF. Virology 329:371–380. doi: 10.1016/j.virol.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 92.Lepelley A, Louis S, Sourisseau M, Law HK, Pothlichet J, Schilte C, Chaperot L, Plumas J, Randall RE, Si-Tahar M, Mammano F, Albert ML, Schwartz O. 2011. Innate sensing of HIV-infected cells. PLoS Pathog 7:e1001284. doi: 10.1371/journal.ppat.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roesch F, Meziane O, Kula A, Nisole S, Porrot F, Anderson I, Mammano F, Fassati A, Marcello A, Benkirane M, Schwartz O. 2012. Hyperthermia stimulates HIV-1 replication. PLoS Pathog 8:e1002792. doi: 10.1371/journal.ppat.1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jordan A, Bisgrove D, Verdin E. 2003. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schwartz O, Marechal V, Danos O, Heard JM. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol 69:4053–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cavrois M, Neidleman J, Bigos M, Greene WC. 2004. Fluorescence resonance energy transfer-based HIV-1 virion fusion assay. Methods Mol Biol 263:333–344. [DOI] [PubMed] [Google Scholar]
- 97.Lee JH, Wittki S, Brau T, Dreyer FS, Kratzel K, Dindorf J, Johnston IC, Gross S, Kremmer E, Zeidler R, Schlotzer-Schrehardt U, Lichtenheld M, Saksela K, Harrer T, Schuler G, Federico M, Baur AS. 2013. HIV Nef, paxillin, and Pak1/2 regulate activation and secretion of TACE/ADAM10 proteases. Mol Cell 49:668–679. doi: 10.1016/j.molcel.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Affabris E, Baur A, Federico M. 2014. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J Virol 88:11529–11539. doi: 10.1128/JVI.01712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stewart SA, Poon B, Jowett JB, Xie Y, Chen IS. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc Natl Acad Sci U S A 96:12039–12043. doi: 10.1073/pnas.96.21.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poon B, Grovit-Ferbas K, Stewart SA, Chen IS. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266–269. doi: 10.1126/science.281.5374.266. [DOI] [PubMed] [Google Scholar]
- 101.Hrimech M, Yao XJ, Bachand F, Rougeau N, Cohen EA. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J Virol 73:4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maudet C, Bertrand M, Le Rouzic E, Lahouassa H, Ayinde D, Nisole S, Goujon C, Cimarelli A, Margottin-Goguet F, Transy C. 2011. Molecular insight into how HIV-1 Vpr protein impairs cell growth through two genetically distinct pathways. J Biol Chem 286:23742–23752. doi: 10.1074/jbc.M111.220780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lai M, Chen J. 2006. The role of Vpr in HIV-1 disease progression is independent of its G2 arrest induction function. Cell Cycle 5:2275–2280. doi: 10.4161/cc.5.19.3317. [DOI] [PubMed] [Google Scholar]
- 104.Subbramanian RA, Yao XJ, Dilhuydy H, Rougeau N, Bergeron D, Robitaille Y, Cohen EA. 1998. Human immunodeficiency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J Mol Biol 278:13–30. doi: 10.1006/jmbi.1998.1685. [DOI] [PubMed] [Google Scholar]
- 105.Mansky LM, Preveral S, Selig L, Benarous R, Benichou S. 2000. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 in vivo mutation rate. J Virol 74:7039–7047. doi: 10.1128/JVI.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barnitz RA, Chaigne-Delalande B, Bolton DL, Lenardo MJ. 2011. Exposed hydrophobic residues in human immunodeficiency virus type 1 Vpr helix-1 are important for cell cycle arrest and cell death. PLoS One 6:e24924. doi: 10.1371/journal.pone.0024924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. 2003. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 108.Chan JK, Greene WC. 2012. Dynamic roles for NF-kappaB in HTLV-I and HIV-1 retroviral pathogenesis. Immunol Rev 246:286–310. doi: 10.1111/j.1600-065X.2012.01094.x. [DOI] [PubMed] [Google Scholar]
- 109.Kogan M, Deshmane S, Sawaya BE, Gracely EJ, Khalili K, Rappaport J. 2013. Inhibition of NF-kappaB activity by HIV-1 Vpr is dependent on Vpr binding protein. J Cell Physiol 228:781–790. doi: 10.1002/jcp.24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ben Haij N, Leghmari K, Planes R, Thieblemont N, Bahraoui E. 2013. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-alpha and IL-10. Retrovirology 10:123. doi: 10.1186/1742-4690-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bennasser Y, Badou A, Tkaczuk J, Bahraoui E. 2002. Signaling pathways triggered by HIV-1 Tat in human monocytes to induce TNF-alpha. Virology 303:174–180. doi: 10.1006/viro.2002.1676. [DOI] [PubMed] [Google Scholar]
- 112.Postler TS, Desrosiers RC. 2012. The cytoplasmic domain of the HIV-1 glycoprotein gp41 induces NF-kappaB activation through TGF-beta-activated kinase 1. Cell Host Microbe 11:181–193. doi: 10.1016/j.chom.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zelová H, Hošek J. 2013. TNF-alpha signalling and inflammation: interactions between old acquaintances. Inflamm Res 62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 114.Chu WM. 2013. Tumor necrosis factor. Cancer Lett 328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.