Abstract
Background and Aims:
Dexmedetomidine (DMT), as intrathecal adjuvant has been shown to successfully prolong duration of analgesia but delay the motor recovery. Hence, this study was designed to find out the dose of DMT which can provide satisfactory analgesia without prolonging motor block.
Methods:
A total of 50 patients scheduled for elective perianal surgeries were randomly allocated to Groups C or D (n = 25). Group D received hyperbaric bupivacaine 0.5% 4 mg + DMT 5 μg and Group C received hyperbaric bupivacaine 0.5% 4 mg + DMT 3 μg intrathecally. Onset and duration of sensory and motor blockade, duration of analgesia, time for ambulation and first urination were recorded. Adverse effects if any were noted.
Results:
Demographic characters, duration of surgery were comparable. The onset of sensory block to S1 was 9.61 ± 5.53 min in Group C compared to 7.69 ± 4.80 min in Group D (P = 0.35). Duration of sensory (145.28 ± 83.17 min – C, 167.85 ± 93.75 min – D, P = 0.5) and motor block (170.53 ± 73.44 min – C, 196.14 ± 84.28 min, P = 0.39) were comparable. Duration of analgesia (337.86 ± 105.11 min – C, 340.78 ± 101.81 min – D, P = 0.9) and time for ambulation (252.46 ± 93.72 min – C, 253.64 ± 88.04 min – D, P = 0.97) were also comparable. One patient in each group had urinary retention requiring catheterization. No other side effects were observed.
Conclusion:
Intrathecal DMT 3 μg dose does not produce faster ambulation compared to intrathecal DMT 5 μg though it produces comparable duration of analgesia for perianal surgeries.
Keywords: Ambulatory, dexmedetomidine, intrathecal, motor block, perianal surgeries
INTRODUCTION
Spinal anaesthesia is the most commonly used technique for lower abdominal and perineal surgeries.[1] It is important to limit the block level to minimise haemodynamic changes during spinal anaesthesia. Low-dose local anaesthetics can limit the block level and induce rapid recovery from anaesthesia with minimal haemodynamic changes.[2,3] Local anaesthetics-when used alone have a relatively short duration of action. Thus early analgesic intervention is needed in the post-operative period. A number of adjuvants have been used to prolong the post-operative analgesia. The addition of opioids to local anaesthetic produces good post-operative analgesia but has disadvantages, such as pruritus, post-operative nausea vomiting and respiratory depression.[4] Dexmedetomidine (DMT), a highly selective α2-agonist, is under evaluation as a neuraxial adjuvant. It provides stable haemodynamic conditions, good quality of intra-operative analgesia and prolonged post-operative analgesia with minimal side effects. However, there may be dose-related prolongation of the duration of motor blockade thus delaying discharge from post-anaesthesia care unit in day case surgeries.[5]
This trial was conducted to investigate if reduction in dose of intrathecal DMT added to a low dose hyperbaric bupivacaine would produce clinically relevant analgesia without prolonging motor blockade in patients undergoing ambulatory perianal surgeries. Time to ambulation and duration of analgesia were the primary outcomes studied and sensory regression, haemodynamic changes and effect on sedation were secondary outcomes observed.
METHODS
After obtaining approval from the Institutional Ethics Committee, 50 adult patients of American Society of Anesthesiologists physical Status I and II undergoing elective perianal surgeries were enrolled in this prospective randomised double-blinded study. Patients were randomly allocated to two groups of 25 each using a sealed envelope method. Group C received DMT 3 μg intrathecally and Group D DMT 5 μg along with the local anaesthetic agent.
Patients with past history of spine surgery, coagulation disorders and infection at site of lumbar puncture, neurological and psychiatric disturbances, diabetes and other endocrine disorders, hypertension and other cardiac disorders, and those on beta blockers, calcium channel blockers, α2 agonists and patients with history of drug allergy were excluded from the study.
Written informed consent was obtained from all the patients who fulfilled the inclusion criteria and were willing to participate in the study. Premedication with oral ranitidine hydrochloride 150 mg and alprazolam 0.25 mg were given the night before the surgery and they were kept nil per oral for 8 h before induction of anaesthesia. During the pre-operative evaluation, patients were given instructions to use a 10-point visual analogue scale (VAS), with 0 indicating no pain and 10 indicating the worst imaginable pain.
On the morning of surgery, patients were premedicated with Inj. Ranitidine 50 mg intravenous (IV). On shifting to operation theatre, electrocardiogram, peripheral oxygen saturation (SpO2), non-invasive blood pressure monitors were connected and baseline values were recorded.
Following infusion of 500 ml Ringers lactate solution, lumbar puncture was performed at L3-L4 interspace or L4-L5 interspace with the patient in the sitting position using 27 gauge Quincke needle. Group C received hyperbaric bupivacaine 0.5% (0.8 ml) (4 mg) + DMT 3 μg intrathecally and Group D received hyperbaric bupivacaine 0.5% (0.8 ml) (4 mg) + DMT 5 µg intrathecally. The required dose of dexmedetomidine was drawn using a tuberculine syringe (0.03 ml for 3 µg and 0.05 ml for 5 µg). The total volume of solution to be injected intrathecally was made to one ml using normal saline. The drug solutions were prepared under sterile precautions by an anaesthesiologist as per instructions in the envelope, who was not involved in administration and further monitoring of the patient. The anaesthesiologist performing the spinal block and monitoring the patient was unaware of the drug administered. After the spinal block, the patients were made to sit for 5 min and then made supine. All the patients received injection midazolam 1 mg IV after being made supine. The level of sensory block, defined as the dermatomal segment with total loss of sensation to cold swab was assessed every 2 min after intrathecal injection till 10 min and every 5 min thereafter until maximum level of sensory block level stabilised. The maximum level of sensory block was considered when the loss of sensation was recorded at the same dermatomal level for 2 consecutive time intervals. The degree of motor block was assessed using Breens modification of Bromage scale.[6] (Appendix) Onset of sensory block, defined as the time required for a sensory block to reach S1 was noted. Blood pressure and heart rate, was recorded every 2 min for initial 10 min and then every 5 min until the end of surgery and then at 30 min intervals in post-operative period until regression of block. Sedation was monitored using Ramsay sedation scale[7] and time for sensory and motor regression to S1 and B6, respectively, were noted and were considered as the duration of sensory and motor blockade, respectively. If patient complained of pain during surgery, it was considered as failure of subarachnoid block and inj fentanyl 2 μg/kg was given as bolus and if necessary, supplementation with general anaesthesia was done. Patients requiring fentanyl supplementation or general anaesthesia were not included for statistical analysis.
Post-operatively, pain intensity was assessed using VAS scale and Inj diclofenac 75 mg administered intramuscularly as rescue analgesic, once the VAS score was more than three. Duration of post-operative analgesia was noted from the time of SAB to the first demand for rescue analgesic. Patient was made ambulant when the following criteria were achieved (1) Able to perform partial knee bend (Bromage scale 6) (2) recovery of proprioception of great toe (3) return of perianal sensation (4) no postural hypotension on making the patient stand. Time for ambulation was recorded from the time of SAB to the time when patient was made ambulant. Time for urination, sedation and side effects if any were also observed in the post-operative period. Side effects such as hypotension (defined as a decrease in mean arterial pressure >25% of the baseline value) was treated with IV boluses of 6 mg ephedrine. Bradycardia defined as a pulse rate of <50 beat/min was treated with bolus of 0.6 mg atropine IV, Respiratory depression (RR <8 or SpO2 <95%) was treated with oxygen supplementation and respiratory support if required, vomiting and others if any were noted.
Sample size calculation was done based on a pilot study. We hypothesised that reduction in dose of DMT would reduce the time required for ambulation. Keeping (1 – β) error at 80% and two sided significance level (α error) at 0.05, to detect at least a 10% difference in time for ambulation between the two groups, the minimum sample size required was 21 in each group. We included 25 patients in each group for better validation of results. Categorical data between study groups were compared using the Chi-square test. Independent sample t-test was used to compare parametric and continuous data, and Mann–Whitney U-test applied if variables were not uniformly distributed. Level of statistical significance was fixed at P < 0.05.
RESULTS
Fifty patients were enrolled for the study. One patient in each group was excluded from analysis due to the failure of the subarachnoid block. Hence, 24 patients in each group were analysed. Demographic characteristics such as age, weight, height, sex and duration of surgery were comparable. Surgeries performed were haemorrhoidectomy, fistulectomy and sphincterotomy. All the surgeries were uniformly distributed in both groups [Table 1].
Table 1.
Demographic characteristics
Duration of post-operative analgesia was 337.86 ± 105.11 min in Group C whereas 340.78 ± 101.81 min in Group D (P = 0.9) and time to ambulation in Group C was 252.46 ± 93.72 min and 253.64 ± 88.04 min in Group D (P = 0.97). The onset of sensory block to S1 was 9.61 ± 5.53 min in Group C compared to 7.69 ± 4.80 min in Group D (P = 0.35). Median level of cephalad spread of sensory block was L1 (T11 – S1 interquartile range) in Group C and T11 (T10 – S1 interquartile range) in Group D. Duration of sensory (145.28 ± 83.17 min – C, 167.85 ± 93.75 min – D, P = 0.5) and motor block (170.53 ± 73.44 min – C, 196.14 ± 84.28 min, P = 0.39) were comparable.
Intraoperative and post-operative haemodynamic parameters were comparable between the two groups [Figures 1 and 2]. One patient in each group had urinary retention requiring catheterization. No other side effects were observed. The mean sedation score (average of mean sedation score of each patient) was 2.008 ± 0.03 in Group D compared to 2 ± 0.05 in Group C (P = 0.5), and average post-operative sedation scores were two in both groups. None of the patients had sedation scores more than four in either group at any point of time during the study period. None of the patients in either group developed hypotension or bradycardia either in intraoperative period or post-operative period. Post-operative VAS scores were comparable in both groups [Figure 3].
Figure 1.
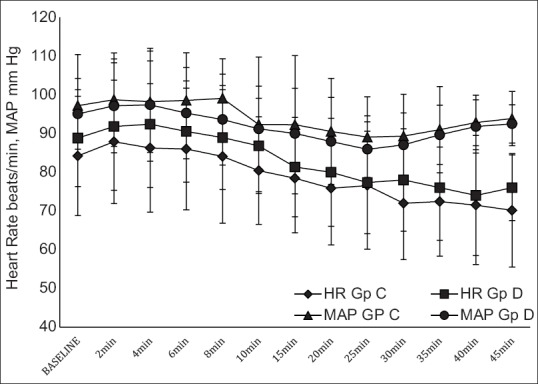
Intraoperative haemodynamics
Figure 2.
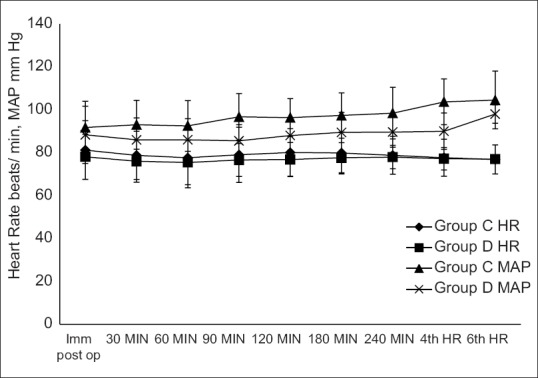
Post-operative haemodynamic parameters
Figure 3.
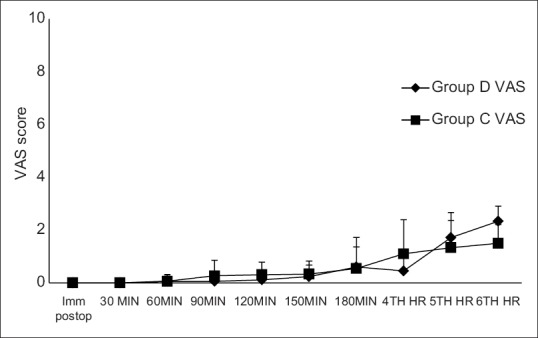
Post-operative visual analogue scale scores
DISCUSSION
The present study showed that DMT 3 μg administered as intrathecal adjuvant to a low dose hyperbaric bupivacaine provided similar duration of analgesia compared to 5 μg of DMT as intrathecal adjuvant, but did not shorten the time required for ambulation. Though the onset of sensory block was slightly shortened in Group D, this was not statistically significant. The cephalad spread of sensory block was comparable.
Doses varying from 3 to 15 μg of DMT have been added as an adjuvant to intrathecal bupivacaine.[2,8,9,10,11] The present study tried to assess the effects of lower doses of DMT in ambulatory surgeries, hence doses of 3 and 5 μg were compared.
Low-dose local anaesthetic for the subarachnoid block is commonly administered to limit the block level to minimise the haemodynamic changes. A low dose of hyperbaric bupivacaine has been successfully used for ambulatory arthroscopic knee surgeries and also in perianal surgeries. The addition of an adjuvant should further potentiate analgesia without prolonging motor blockade or causing other side effects. DMT when administered intrathecally, prolonged the duration of motor and sensory blockade.[2,8,10,11] The prolongation of sensory effect may result from synergism between local anaesthetic and α2-adrenoceptor agonist, while the prolongation of the motor block of spinal anaesthetics may result from the binding of α2-adrenoceptor agonists to motor neurons in the dorsal horn.[12] Perineural administration of DMT also is reported to prolong the duration of the motor blockade in rats.[13]
Kanazi et al.[8] observed faster onset of sensory and motor block with addition of DMT, but the present study did not reveal any statistically significant difference in onset of sensory and motor block with 3 and 5 μg of DMT. The shorter duration of motor block in the present study compared to Kanazi et al. may be attributed to the lower dose of local anaesthetic used. Making the patient sit for 5 min may also limit the spread of drug further limiting the motor blockade.
Administration of an α2-agonist like DMT via an intrathecal route provides an analgesic effect in the post-operative period without severe sedation. This effect is due to the sparing of supraspinal central nervous system sites from excessive drug exposure, resulting in robust analgesia without heavy sedation. At spinal cord level, activation of both α2-C and α2-A receptors, in the neurons of superficial dorsal horn, especially lamina II, directly reduces pain transmission, by suppressing the release of pro-nociceptive transmitter, substance P and glutamate from primary afferent terminals, and by hyperpolarising spinal interneurons via G-protein-mediated activation of potassium channels. None of the patients in either group had excessive sedation and is comparable with other studies.[8,11]
DMT 5 μg when used as adjuvant to bupivacaine 6 mg prolonged duration of motor block and delayed ambulation in patients undergoing perianal surgeries.[14] In this study, an attempt was made to assess the effect of reduction in doses of both DMT and bupivacaine on ambulation and analgesia.
Haemodynamic parameters were comparable in both the groups and there were no incidences of hypotension and bradycardia in the intraoperative or post-operative period. Eid et al. observed higher incidence of bradycardia with use of higher dose of DMT.[5]
The present study did not observe the effect of these doses of DMT on analgesic consumption in the 24 h period, which may be a limitation. Future studies may be targeted to assess the effect of these doses on analgesic efficacy in ambulatory surgeries.
CONCLUSION
Intrathecal bupivacaine with dexmedetomidine 3 μg does not produce faster ambulation compared to intrathecal bupivacaine with dexmedetomidine 5 μg, though it produces comparable duration of analgesia for perianal surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX: Modified Bromage Score (Breen et al.)[6]

REFERENCES
- 1.Gudaityte J, Marchertiene I, Karbonskiene A, Saladzinskas Z, Tamelis A, Toker I, et al. Low-dose spinal hyperbaric bupivacaine for adult anorectal surgery: A double-blinded, randomized, controlled study. J Clin Anesth. 2009;21:474–81. doi: 10.1016/j.jclinane.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Kim JE, Kim NY, Lee HS, Kil HK. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull. 2013;36:959–65. doi: 10.1248/bpb.b12-01067. [DOI] [PubMed] [Google Scholar]
- 3.Awad IT, Cheung JJ, Al-Allaq Y, Conroy PH, McCartney CJ. Low-dose spinal bupivacaine for total knee arthroplasty facilitates recovery room discharge: A randomized controlled trial. Can J Anaesth. 2013;60:259–65. doi: 10.1007/s12630-012-9867-5. [DOI] [PubMed] [Google Scholar]
- 4.Carr DB, Cousins MJ. Spinal route of analgesia. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, editors. Cousins and Bridenbaugh's Neural Blockade in Clinical Anaesthesia and Pain Medicine. 4th ed. Philadelphia: Lipincott Williams and Wilkins; 2009. pp. 886–95. [Google Scholar]
- 5.Eid HE, Shafie MA, Youssef H. Dose-related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;4:83–95. [Google Scholar]
- 6.Breen TW, Shapiro T, Glass B, Foster-Payne D, Oriol NE. Epidural anesthesia for labor in an ambulatory patient. Anesth Analg. 1993;77:919–24. doi: 10.1213/00000539-199311000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 10.Abdelhamid SA, El-Lakany MH. Intrathecal dexmedetomidine: Useful or not? J Anesth Clin Res. 2013;4:351. [Google Scholar]
- 11.Yektas A, Belli E. The effects of 2 µg and 4 µg doses of dexmedetomidine in combination with intrathecal hyperbaric bupivacaine on spinal anesthesia and its postoperative analgesic characteristics. Pain Res Manag. 2014;19:75–81. doi: 10.1155/2014/956825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada Y, Nishioka K, Kitahata LM, Kishikawa K, Collins JG. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology. 1995;83:344–52. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nethra SS, Sathesha M, Dixit A, Dongare PA, Harsoor SS, Devikarani D. Intrathecal dexmedetomidine as adjuvant for spinal anaesthesia for perianal ambulatory surgeries: A randomised double-blind controlled study. Indian J Anaesth. 2015;59:177–81. doi: 10.4103/0019-5049.153040. [DOI] [PMC free article] [PubMed] [Google Scholar]


