Abstract
Invasive nontyphoidal Salmonella (NTS) infections constitute a major health problem among infants and toddlers in sub-Saharan Africa; these infections also occur in infants and the elderly in developed countries. We genetically engineered a Salmonella enterica serovar Typhimurium strain of multilocus sequence type 313, the predominant genotype circulating in sub-Saharan Africa. We evaluated the capacities of S. Typhimurium and Salmonella enterica serovar Enteritidis ΔguaBA ΔclpX live oral vaccines to protect mice against a highly lethal challenge dose of the homologous serovar and determined protection against other group B and D serovars circulating in sub-Saharan Africa. The vaccines S. Typhimurium CVD 1931 and S. Enteritidis CVD 1944 were immunogenic and protected BALB/c mice against 10,000 50% lethal doses (LD50) of S. Typhimurium or S. Enteritidis, respectively. S. Typhimurium CVD 1931 protected mice against the group B serovar Salmonella enterica serovar Stanleyville (91% vaccine efficacy), and S. Enteritidis CVD 1944 protected mice against the group D serovar Salmonella enterica serovar Dublin (85% vaccine efficacy). High rates of survival were observed when mice were infected 12 weeks postimmunization, indicating that the vaccines elicited long-lived protective immunity. Whereas CVD 1931 did not protect against S. Enteritidis R11, CVD 1944 did mediate protection against S. Typhimurium D65 (81% efficacy). These findings suggest that a bivalent (S. Typhimurium and S. Enteritidis) vaccine would provide broad protection against the majority of invasive NTS infections in sub-Saharan Africa.
INTRODUCTION
Non-typhoidal Salmonella (NTS) is a leading cause of bacterial bloodstream infections in febrile children and immunocompromised individuals in sub-Saharan Africa and has been associated with a high case fatality rate of 20 to 25% (1). Although severe malarial anemia and human immunodeficiency virus (HIV) are important risk factors for invasive NTS infection, the disease is also common in low-HIV-prevalence areas (1–4).
There are >2,500 Salmonella serovars that can be differentiated on the basis of the O polysaccharide (OPS) antigens of their lipopolysaccharide (LPS) and their H flagellum antigens, using the Kauffman-White typing scheme (5). For example, Salmonella enterica serovar Typhimurium has O antigens 1, 4, 12, and occasionally 5. Epitope 12 is formed by trisaccharide repeats of mannose, rhamnose, and galactose; glucosylation of the galactose residue forms epitope 1. An abequose linked to each mannose defines it as a serovar within group B and constitutes the immunodominant O4 epitope; epitope 5 results from a phage conversion that introduces an O-acetyl moiety on the abequose. S. Typhimurium also has separate H antigens, i (phase 1) and 1 and 2 (phase 2), expressed alternatively via a process called phase variation. In contrast, Salmonella enterica serovar Enteritidis has O antigen epitope 9, which identifies it as a member of group D. Epitope 9 is formed by a tyvelose residue that is linked to the mannose of the same trisaccharide OPS backbone as group B strains, which is also glucosylated at galactose. S. Enteritidis produces only phase 1 H antigens g and m (i.e., it does not make phase 2 flagella).
In sub-Saharan Africa, S. Typhimurium and S. Enteritidis account for 75 to 90% of all the NTS strains isolated from blood and other normally sterile compartments (6–14). In blood culture surveillance studies conducted by our group in febrile pediatric patients in Bamako, Mali, since 2002, we found that S. Typhimurium and its variants were the NTS bacteria most commonly isolated from blood (69%), followed by Salmonella enterica serovar Dublin (group D, 11%), S. Enteritidis (10%), and Salmonella enterica serovar Stanleyville (group B, 8%) (15, 16). The remaining 2% of NTS strains belonged to other serovars. However, other African sites have reported the isolation of rare serovars, such as the group C1 serovars Salmonella enterica serovar Isangi in South Africa (17) and Salmonella enterica serovar Concord in Ethiopia (18).
A novel genotype of S. Typhimurium, sequence type 313 (ST313), was identified in Malawi and Kenya by multilocus sequence typing (MLST) and associated with invasive disease (19, 20). In contrast, S. Typhimurium strains associated with gastroenteritis and commonly found throughout the world are ST19. There are multiple sequence types of S. Typhimurium circulating in Africa that can cause invasive disease, though ST313 seems to be the most common. In comparison to S. Typhimurium ST19 strains, the prototypic ST313 strain D23580 exhibits many pseudogenes and gene deletions (19). We and others have recently shown that S. Typhimurium ST313 is also phenotypically different from ST19 (21–24). Despite observable differences between S. Typhimurium ST19 and ST313 isolates, it is also feasible that the morbidity and mortality associated with these strains in sub-Saharan Africa is due to other factors, such as comorbidities (e.g., malnutrition, HIV, and malaria), antibiotic resistance, or genetic predisposition (25).
We previously developed live oral S. Typhimurium and S. Enteritidis vaccines with deletion mutations in guaBA and clpP. Mutation of guaBA alone increased the oral 50% lethal dose (LD50) in BALB/c mice by ∼5 log units (26). The clpPX genes encode a protease that normally degrades the master flagellum regulator FlhDC (27, 28). In the absence of ClpPX, FlhDC accumulates, resulting in increased FliC production. Deletion of clpP, an independently attenuating mutation, resulted in increased expression of flagella (26). Our prototypic S. Typhimurium and S. Enteritidis vaccine strains, CVD 1921 and CVD 1941, with mutations in guaBA and clpP, were immunogenic in BALB/c mice and protected the mice against homologous oral challenge with 100 LD50 of wild-type S. Typhimurium or S. Enteritidis, respectively (26). Importantly, the S. Typhimurium vaccine CVD 1921 was well tolerated by orally immunized, simian immunodeficiency virus (SIV)-infected rhesus macaques (29). Murine and macaque antibodies induced by these vaccine strains were functional in vitro, mediating bactericidal and opsonophagocytic activities (26, 29). Robust enhancement of opsonophagocytic uptake was noted for other invasive serovars of homologous serogroups, but the uptake was variable and generally less robust for strains from heterologous serogroups (26). These data suggest that serogroup-specific cross-protection may be possible and that NTS live attenuated vaccines may offer partial protection against heterologous serogroups. A live Salmonella enterica serovar Paratyphi A vaccine, CVD 1902, with deletions in guaBA and clpX, was tested in a phase 1 clinical trial and found to be safe in volunteers immunized orally with up to 1010 CFU (K. K. Kotloff and D. A. Shirley, personal communication).
Here, we describe refinements of the prototypic live attenuated NTS vaccines and direct assessment of cross-protection mediated by these advanced vaccine candidates against homologous and heterologous serogroups.
MATERIALS AND METHODS
Bacterial strains, medium, and chemicals.
The bacterial strains used in this study (shown in Table 1) were grown in HS bacteriological medium (5 g sodium chloride, 10 g soytone [Teknova, Hollister, CA], 5 g Hy yest [Sigma-Aldrich, St. Louis, MO] in 1 liter distilled water) at 37°C. All guaBA mutants were grown on media containing 0.005% (wt/vol) guanine. When required, antibiotics were used at a final concentration of 50 μg/ml carbenicillin, 50 μg/ml kanamycin, or 20 μg/ml chloramphenicol. Chemically defined medium was prepared as described previously (26). NTS serovars were verified by agglutination of bacteria with O-grouping and H-typing antisera (Denka Seiken Co. Ltd., Japan). Phase switching was performed by preparing swarm agar (nutrient broth containing 0.5% agar) and dropping H:i or H:2 antiserum on the surface, followed by stab inoculation of the center of the medium. Following incubation at 37°C for 20 h, the bacteria were agglutinated with H-typing antiserum.
TABLE 1.
Bacterial strains used in this study
| O group | O antigens | H antigens | Serovar | Strain | Source and characteristics | Reference |
|---|---|---|---|---|---|---|
| B | 1, 4, [5], 12a | i; 1, 2 | Typhimurium | D65 | Clinical isolate from blood culture, Mali; ST313; antibiotic sensitiveb | 15 |
| CVD 1930 | S. Typhimurium D65 ΔguaBA | This work | ||||
| CVD 1931 | S. Typhimurium D65 ΔguaBA ΔclpX | This work | ||||
| 1, 4, [5], 12, [27] | z4, z23; [1, 2] | Stanleyville | J65 | Clinical isolate from blood culture, Mali | 15 | |
| 1, 4, [5], 12 | e, h; 1, 5 | Reading | 26A | Clinical isolate from blood culture, Chile | CVD collection | |
| 1, 4, [5], 12 | b; 1, 2 | Paratyphi B var. Java | S78 | Clinical isolate from blood culture, Mali | 15 | |
| D | 1, 9, 12 | g, m; −c | Enteritidis | R11 | Clinical isolate from blood culture, Mali; antibiotic sensitiveb | 15 |
| CVD 1940 | S. Enteritidis R11 ΔguaBA | 26 | ||||
| CVD 1944 | S. Enteritidis R11 ΔguaBA ΔclpX | 26 | ||||
| 1, 9, 12, [Vi] | g, p; − | Dublin | R17 | Clinical isolate from blood culture, Mali | 15 | |
| P10 | Clinical isolate from blood culture, Mali | 15 | ||||
| 1, 9, 12 | l, v; 1, 5 | Panama | 107 | Clinical isolate from blood culture, Chile | CVD collection |
The brackets indicate that the O or H factor may be present or absent.
Sensitive to ampicillin, ceftriaxone, chloramphenicol, gentamicin, ciprofloxacin, and trimethoprim-sulfamethoxazole.
The minus symbol indicates that phase 2 H antigens are absent.
DNA methods.
Plasmid extraction and gel purification of DNA fragments were performed using Wizard (Promega, Madison, WI, USA) and QIAquick Gel Extraction (Qiagen, Valencia, CA, USA) kits, respectively, as directed by the manufacturer. Restriction enzymes were purchased from New England BioLabs (Ipswich, MA, USA). PCR amplifications were routinely performed with 1 to 2.5 U Taq DNA polymerase (Genscript, Piscataway, NJ, USA) and 1× PCR buffer containing 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate (dNTP), and 1 μM each primer in a reaction volume of 20 to 50 μl in an Eppendorf Mastercycler. For PCRs using long primers (>25 bp), the amount of MgCl2 was increased as necessary. When error-free and/or blunt-end PCR products were required, Vent DNA polymerase (New England BioLabs) was used according to the manufacturer's instructions.
Construction of attenuated Salmonella strains.
Deletion of guaBA and clpX in S. Typhimurium D65 was achieved by Lambda Red-mediated mutagenesis as described previously (26). Plasmids pKD46, pKD13, and pCP20 were used for chromosomal deletions (30). Plasmid pCR-Blunt II-TOPO (Invitrogen, Carlsbad, CA) was used to clone blunt-ended PCR products. The deletions were verified genotypically by PCR using primers external to the deletion and by sequencing at least 500 bp both upstream and downstream of the deletion.
Challenge experiments.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. BALB/c mice were acclimated for 7 days after arrival before starting the experiments. For LD50 determinations, both attenuated and wild-type Salmonella strains were grown by incubation at 37°C in HS medium for 20 h without shaking. Bacteria were pelleted by centrifugation and resuspended in phosphate-buffered saline (PBS) at the appropriate concentration. Tenfold dilutions (generally 103 to 108 CFU) of wild-type and attenuated NTS strains were administered to five 7-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA, USA). The mice were infected orally with 200 μl of bacterial suspension using a 1.5-in curved gavage needle with a 2.25-mm ball (Braintree Scientific, Braintree, MA, USA) or intraperitoneally (i.p.) using a 25-gauge needle. The exact number of organisms administered was determined by viable counts. Mice were weighed and monitored daily after infection for 14 (i.p.) or 28 (oral) days. Any mouse that lost >20% of its body weight (compared to its weight at the time of challenge) or that showed signs of extreme morbidity (e.g., shallow breathing or hunched posture) was euthanized and scored as a death. The 50% lethal dose of each strain was calculated by linear regression analysis. For challenge with additional serovars, mice were infected with one of the following strains: 3.2 × 107 CFU Salmonella enterica serovar Reading 26A, 6 × 108 CFU S. Stanleyville J65, 9.2 × 108 CFU Salmonella enterica serovar Java S78, 4.6 × 107 CFU Salmonella enterica serovar Panama Chile 107, 3.6 × 108 CFU S. Dublin Mali R17, or 3.6 × 108 CFU S. Dublin Mali P10.
Immunization, serological analysis, and protection against challenge.
Female BALB/c mice 6 to 8 weeks of age were immunized via oral gavage with 200 μl PBS containing 109 CFU of live attenuated Salmonella or PBS alone. The immunization schedule consisted of three spaced doses administered on days 0, 28, and 56. Serum was obtained on days −1, 27, 55, and 83, and anti-LPS and anti-FliC serum IgG titers were determined as described previously (26). The homologous LPS or flagella (i.e., S. Typhimurium LPS and FliC for mice immunized with CVD 1931 and S. Enteritidis LPS and FliC for mice immunized with CVD 1944) were used as coating antigens in the enzyme-linked immunosorbent assays (ELISAs), as previously described (31). On day 83, the mice were challenged orally or i.p. with wild-type Salmonella, as described above.
Statistical methods.
Data were analyzed using Fisher's exact test. A P value of ≤0.05 (two tail) was considered significant.
RESULTS
Construction of refined live attenuated invasive NTS vaccines.
The first-generation S. Typhimurium vaccine, CVD 1921 (ΔguaBA ΔclpP), was made using S. Typhimurium I77 as the parental strain, which is from ST19, a minor endemic genotype in sub-Saharan Africa (26). Here, we describe attenuation of an S. Typhimurium strain of the ST313 genotype, the predominant circulating sequence type in the sub-Saharan region, which is responsible for a high burden of disease (19, 20). Our second refinement was in the secondary attenuating mutation within clpPX. Instead of deleting clpP, we deleted the second gene in the operon, clpX, as this combination of mutations resulted in a live attenuated S. Paratyphi A vaccine that was well tolerated in humans. We constructed the live oral S. Typhimurium vaccine strain CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX). We confirmed the phenotypes of CVD 1931 and the previously constructed S. Enteritidis vaccine strain CVD 1944 (ΔguaBA ΔclpX) (26) and showed that both were unable to grow on minimal medium lacking guanine and were more motile than the parental strains (reference 26 and data not shown).
Determination of the LD50 for invasive Salmonella serovars.
The oral LD50 of S. Typhimurium ST313 strain D65 was found to be 2 × 104 CFU. This is the same oral LD50 that was determined for the ST19 strain S. Typhimurium I77 (26). In preparation for cross-protection studies, we assessed the LD50 of several additional Salmonella serovars isolated from blood or other normally sterile sites. Mice (3/group) were infected orally with >107 CFU of one of the following group B or D Salmonella strains: S. Reading Chile 26A (group B), S. Stanleyville Mali J65 (group B), S. Java Mali S78 (group B), S. Panama Chile 107 (group D), or S. Dublin Mali R17 or Mali P10 (group D). Only S. Dublin Mali R17 and S. Stanleyville Mali J65 showed lethality—100% and 67%, respectively. We then performed LD50 experiments by infecting BALB/c mice with six 10-fold dilutions of bacteria. Linear regression was used to calculate the LD50.The oral LD50 for S. Dublin R17 was 9.1 × 104 CFU. The oral LD50 for S. Stanleyville J65 was >109 CFU. For the last strain, since a dose of ∼100 LD50 would require ∼1011 CFU and cannot be practically given, we elected to pursue i.p. infection; the i.p. LD50 of S. Stanleyville J65 was experimentally determined to be ∼1.4 × 105 CFU. Intraperitoneal LD50 were not determined for the other strains.
S. Typhimurium CVD 1931 and S. Enteritidis CVD 1944 protect against a highly lethal homologous challenge.
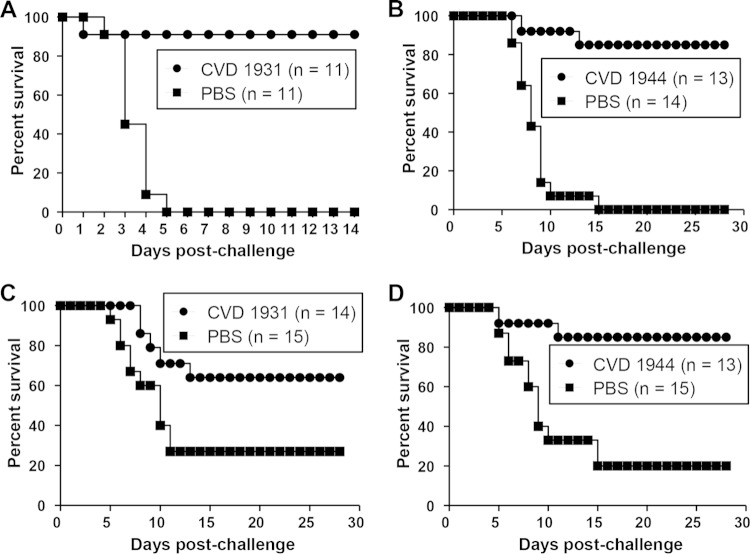
We immunized BALB/c mice orally three times at 28-day intervals with 109 CFU of CVD 1931 or CVD 1944 and challenged them orally with ∼10,000 LD50 of S. Typhimurium D65 or S. Enteritidis R11 1 month after the last immunization. BALB/c mice, which are NRamp negative and cannot control disseminated Salmonella, were chosen as an infection model because they can be vaccinated orally, and a lethal infection is induced when they are challenged with wild-type organisms by the same route, thus providing a model to assess protective efficacy (32). Likewise, oral immunization and infection routes are relevant for humans. All control mice that received PBS and were challenged with virulent S. Typhimurium D65 succumbed to the infection, whereas none of the CVD 1931-immunized mice died (Table 2 and Fig. 1). For mice challenged with S. Enteritidis, we observed 83% vaccine efficacy against a highly fatal infection that killed all the control mice (Table 2 and Fig. 1). High anti-LPS and anti-FliC serum IgG titers were elicited after three immunizations (Fig. 2). CVD 1931 attained 100% anti-LPS seroconversion after only two doses, whereas CVD 1944 achieved 92% anti-LPS seroconversion after three immunizations.
TABLE 2.
Vaccine efficacy of live attenuated S. Typhimurium CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX)a
| Immunization | Challenge | Challenge dose (LD50) | Mortality rate | Vaccine efficacy (%) | P valueb |
|---|---|---|---|---|---|
| PBS | S. Typhimurium D65 | ∼10,000 | 12/12 | ||
| CVD 1931 | 0/12 | 100 | <0.001 | ||
| PBS | S. Enteritidis R11 | ∼10,000 | 12/12 | ||
| CVD 1944 | 2/12 | 83 | <0.001 |
Mice were immunized orally with 109 CFU three times, with 1 month between immunizations, and orally challenged 1 month later with 2.6 × 108 CFU of S. Typhimurium D65 and 1.5 × 108 CFU of S. Enteritidis R11. The mice were monitored daily for 29 days.
Fisher's exact test (two tailed).
FIG 1.
Percent survival of mice immunized with live attenuated NTS strains and challenged with the homologous serovar. The mice were immunized orally with 109 CFU of CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX), CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX), or PBS three times, with 1 month between immunizations. One month after the last immunization, they were orally challenged with 10,000 LD50 of S. Typhimurium D65 (A) or S. Enteritidis R11 (B).
FIG 2.
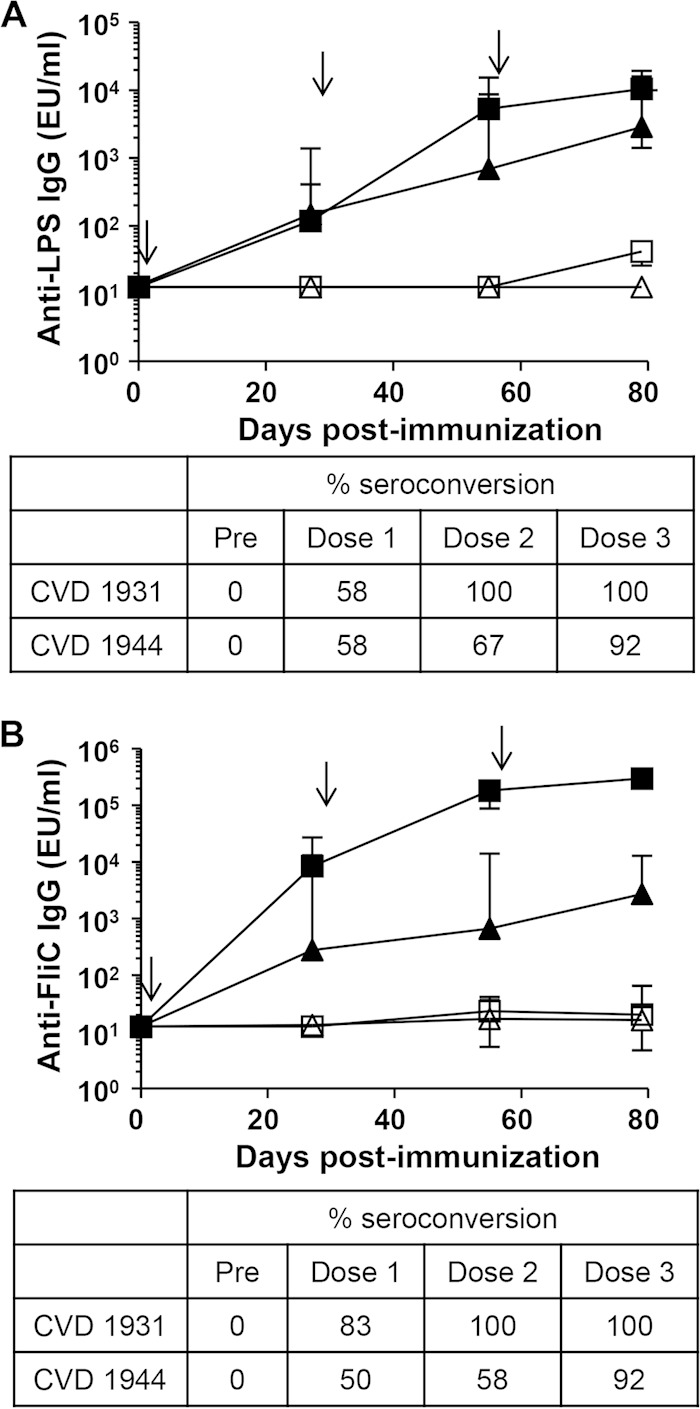
Seroconversion and anti-LPS and anti-FliC IgG geometric mean titers (GMTs) elicited by live oral vaccines CVD 1931 and CVD 1944. Mice (n = 12) were immunized orally with 109 CFU in a 200-μl volume three times (on days 0, 28, and 56 [indicated by arrows]), with 1 month between immunizations. Anti-LPS (A) and anti-FliC (B) serum IgG titers were measured by ELISA pre- and postimmunization (squares, CVD 1931; triangles, CVD 1944; solid symbols, immunized; open symbols, PBS). Titers are expressed as GMTs ± standard errors of the mean (SEM). The percent seroconversion (4-fold increase in titer versus preimmune) is shown. EU, ELISA units.
Abilities of live oral vaccines CVD 1931 and CVD 1944 to offer cross-serovar protection against other group B and D Salmonella serovars circulating in sub-Saharan Africa.
We investigated in vivo whether our live oral vaccines would protect against Salmonella strains representative of heterologous group B and D serovars. For this, mice were immunized orally, on three occasions 1 month apart, with S. Typhimurium vaccine CVD 1931 and challenged i.p. with 3 LD50 of S. Stanleyville J65. This challenge dose was selected to optimize the kinetics of infection, as we found that higher i.p. challenge doses resulted in ∼100% mortality after 24 h, presumably due to overwhelming endotoxemia. CVD 1931 exhibited 91% vaccine efficacy in preventing death from an S. Stanleyville J65 i.p. challenge that led to the deaths of all the PBS control mice (Fig. 3A).
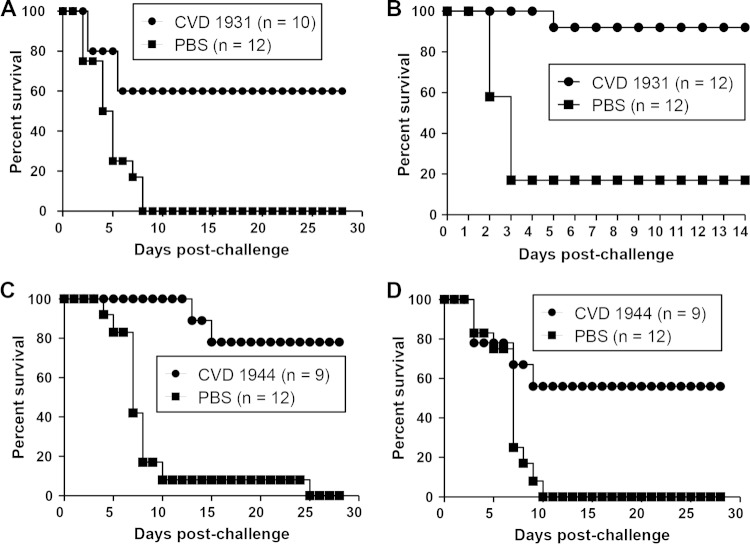
FIG 3.
Percent survival of mice immunized with live attenuated NTS strains and challenged with other group B and D serovars. Mice were immunized orally with 109 CFU of CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX), CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX), or PBS three times, with 1 month between immunizations, and challenged 1 month after the last immunization. (A) CVD 1931-immunized mice were intraperitoneally challenged with 3 LD50 of S. Stanleyville J65. (B) CVD 1944-immunized mice were orally challenged with 800 LD50 of S. Dublin R17. (C) CVD 1931-immunized mice were orally challenged with 50 LD50 of S. Enteritidis R11. (D) CVD 1944-immunized mice were orally challenged with 200 LD50 of S. Typhimurium D65.
We also determined the ability of the S. Enteritidis vaccine CVD 1944 to protect mice against the group D serovar S. Dublin (H:g, p). Mice were similarly orally immunized and challenged with 800 LD50 of S. Dublin R17. As shown in Fig. 3B, CVD 1944 manifested 85% vaccine efficacy in the face of a virulent S. Dublin challenge that caused 100% mortality in the PBS control mice. These data clearly show that both our S. Typhimurium CVD 1931 and S. Enteritidis CVD 1944 vaccines can protect against other group B and D serovars (Table 3).
TABLE 3.
Vaccine efficacies of live attenuated S. Typhimurium CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX) against other group B or D serovarsa
| Immunization | Challenge | Challenge dose (LD50) | Mortality rate | Vaccine efficacy (%) | P valueb |
|---|---|---|---|---|---|
| PBS | S. Stanleyville J65 | ∼3 | 11/11 | ||
| CVD 1931 | 1/11 | 91 | <0.001 | ||
| PBS | S. Dublin R17 | ∼800 | 14/14 | ||
| CVD 1944 | 2/13 | 85 | <0.001 |
Mice were immunized orally with 109 CFU three times, with 1 month between immunizations, and challenged 1 month later with 4.5 × 105 CFU of S. Stanleyville J65 (i.p.) or 7.9 × 107 CFU of S. Dublin R17 (orally). The mice were monitored daily for 14 (S. Stanleyville infection) or 28 (S. Dublin infection) days.
Fisher's exact test (two tailed).
Live oral vaccines CVD 1931 and CVD 1944 mediate cross-protection.
First, we tested the ability of the live attenuated S. Typhimurium vaccine CVD 1931 (group B) to mediate protection against the heterologous group D serovar S. Enteritidis. Mice were immunized orally with each of the vaccine strains three times, with 1 month between immunizations, and challenged orally with 50 LD50 of S. Enteritidis R11 or with 200 LD50 of S. Typhimurium D65. When CVD 1931-immunized mice were challenged with S. Enteritidis R11, we observed a vaccine efficacy of 50.7% (36% mortality for vaccinated mice and 73% mortality for the PBS control [Fig. 3C]). In contrast, 81.3% vaccine efficacy was observed for CVD 1944 against challenge with virulent S. Typhimurium D65 (15% of immunized mice and 80% of PBS control mice succumbed [Fig. 3D]). These data indicate that while the S. Typhimurium live vaccine CVD 1931 was not effective in cross-protecting against a lethal S. Enteritidis infection (Table 4), the S. Enteritidis vaccine CVD 1944 (group D) afforded cross-protection against the group B serovar S. Typhimurium (Table 4).
TABLE 4.
Vaccine efficacies of live attenuated S. Typhimurium CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX) against heterologous serogroupsa
| Immunization | Challenge | Challenge dose (LD50) | Mortality rate | Vaccine efficacy (%) | P valueb |
|---|---|---|---|---|---|
| PBS | S. Enteritidis R11 | ∼50 | 11/15 | ||
| CVD 1931 | 5/14 | 51 | 0.07 | ||
| PBS | S. Typhimurium D65 | ∼200 | 12/15 | ||
| CVD 1944 | 2/13 | 81 | <0.001 |
Mice were immunized orally with 109 CFU three times, with 1 month between immunizations, and orally challenged 1 month later with 1.2 × 106 CFU of S. Enteritidis R11 or 3.9 × 106 CFU of S. Typhimurium D65. The mice were monitored daily for 28 days.
Fisher's exact test (two tailed).
Live oral vaccines CVD 1931 and CVD 1944 elicit long-lasting protective immunity.
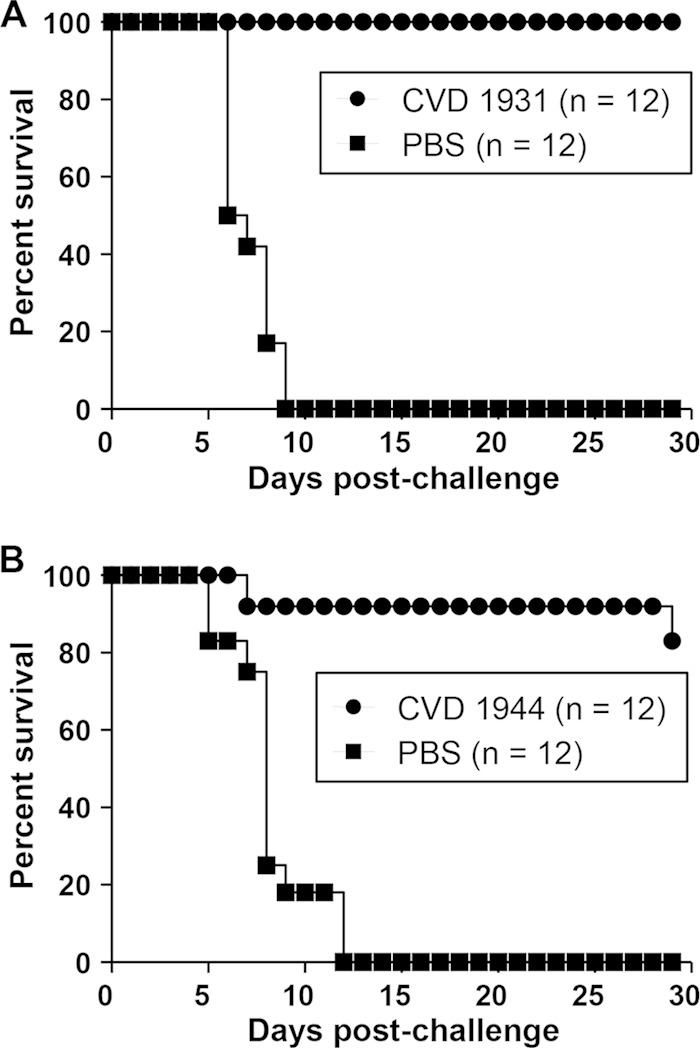
To confirm that the live NTS vaccines CVD 1931 and CVD 1944 can elicit long-lasting immunity, we repeated the immunization experiment but challenged the mice 12 weeks after the last immunization. BALB/c mice were immunized orally with S. Typhimurium CVD 1931 and challenged orally with 5,000 LD50 of S. Typhimurium D65 or i.p. with 6 LD50 of S. Stanleyville J65. CVD 1931 exhibited vaccine efficacies of 60% and 90% against S. Typhimurium and S. Stanleyville, respectively (Table 5 and Fig. 4). Likewise, BALB/c mice were immunized orally with S. Enteritidis CVD 1944 and challenged orally with 5,000 LD50 of S. Enteritidis R11 or 1,000 LD50 of S. Dublin R17. CVD 1944 exhibited vaccine efficacies of 78% and 56% against S. Enteritidis and S. Dublin, respectively (Table 5 and Fig. 4). As shown in Fig. 5, anti-LPS serum IgG titers remained elevated after the last immunization, with 95 to 100% seroconversion.
TABLE 5.
Vaccine efficacies of live attenuated S. Typhimurium CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX) against lethal challenge 12 weeks after the last immunizationa
| Immunization | Challenge | Challenge dose (LD50) | Mortality rate | Vaccine efficacy (%) | P valueb |
|---|---|---|---|---|---|
| PBS | S. Typhimurium D65 | ∼5,000 | 12/12 | ||
| CVD 1931 | 4/10 | 60 | 0.003 | ||
| PBS | S. Stanleyville J65 | ∼6 | 10/12 | ||
| CVD 1931 | 1/12 | 90 | <0.001 | ||
| PBS | S. Enteritidis R11 | ∼5,000 | 12/12 | ||
| CVD 1944 | 2/9 | 78 | <0.001 | ||
| PBS | S. Dublin R17 | ∼1,000 | 12/12 | ||
| CVD 1944 | 4/9 | 56 | 0.006 |
Mice were immunized orally with 109 CFU three times, with 1 month between immunizations, and challenged 3 months later with 9.3 × 107 CFU of S. Typhimurium D65 (oral), 1.19 × 108 CFU of S. Enteritidis R11 (oral), 8.2 × 105 CFU of S. Stanleyville J65 (i.p.), or 8.0 × 107 CFU of S. Dublin R17 (oral). The mice were monitored daily for 14 days (i.p.) or 28 days (oral).
Fisher's exact test (two tailed).
FIG 4.
Percent survival of mice immunized with live attenuated NTS strains and challenged with other group B and D serovars. The mice were immunized orally with 109 CFU of CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX), CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX), or PBS three times, with 1 month between immunizations, and challenged 3 months after the last immunization. (A) CVD 1931-immunized mice were orally challenged with 5,000 LD50 of S. Typhimurium D65. (B) CVD 1931-immunized mice were intraperitoneally challenged with 6 LD50 of S. Stanleyville J65. (C) CVD 1944-immunized mice were orally challenged with 5,000 LD50 of S. Enteritidis R11. (D) CVD 1944-immunized mice were orally challenged with 1,000 LD50 of S. Dublin R17.
FIG 5.
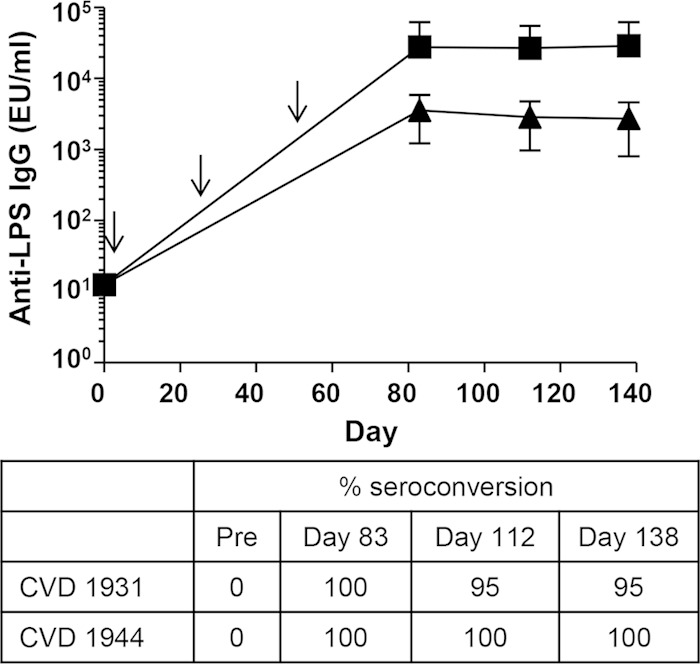
Seroconversion and anti-LPS IgG GMTs elicited by live oral vaccines CVD 1931 and CVD 1944 up to 12 weeks after the last immunization. Mice (n = 24) were immunized orally with 109 CFU in a 200-μl volume three times (on days 0, 28, and 56 [indicated by arrows]), with 1 month between immunizations. Anti-LPS serum IgG titers were measured by ELISA pre- and postimmunization (squares, CVD 1931; triangles, CVD 1944). Titers are expressed as GMTs ± SEM. The percent seroconversion (4-fold increase in titer versus preimmune) is shown.
DISCUSSION
Two subunit parenteral vaccines are in development to prevent invasive NTS infections among young children in sub-Saharan Africa, including a conjugate consisting of core plus O polysaccharide linked to phase 1 flagellin subunits of the homologous serovar and another consisting of outer membrane particles, called Generalized Module for Membrane Antigens (GMMA) (Gendrivax [http://www.gendrivax.org]) (31, 33). Administered parenterally, these vaccines are expected to protect by eliciting functional (likely IgG) antibodies that intercept the invading bacteria during primary bacteremia. Here, we propose an alternative approach to prevent invasive NTS disease in Africa with live oral vaccines that in an optimistic view offer theoretical advantages over the parenteral vaccines. First, oral vaccines elicit secretory IgA mucosal antibodies, thereby providing barrier protection against mucosal invasion, in addition to stimulating serum antibodies and a panoply of cell-mediated immune mechanisms. Second, consequent to mucosal immune responses, the oral vaccines may confer protection against NTS gastroenteritis. It is not known whether Salmonella parenteral vaccines would protect against mucosal colonization. However, IgG induced by parenteral vaccination with Streptococcus pneumoniae capsular polysaccharides protects against invasive infections (34), and it is conceivable that parenteral Salmonella vaccines could have a similar effect. Anti-Salmonella IgG antibodies induced in rabbits by heat-killed vaccines have been shown to accumulate in the gastrointestinal lumen by passive transudation through the mucosal epithelium (35) and to retain functional bactericidal capacity. In addition to functionally active systemic IgG, live vaccines would have the advantage of inducing strong mucosal IgA. Other advantages include their lower cost and easier implementation. Finally, as demonstrated here, the live vaccines stimulate impressive cross-protection against other serovars within the same group and, for the S. Enteritidis vaccine, serovars from another serogroup.
We have previously reported the development of prototypic live attenuated NTS vaccines harboring deletions in guaBA and clpP. Here, we have described the development of live NTS vaccines with deletions in guaBA and clpX. Furthermore, the S. Typhimurium vaccine is derived from a clinically relevant genotype that has been associated with invasive disease and is prevalent in sub-Saharan Africa. There are multiple genetic and phenotypic differences (22, 23) between ST19 and ST313 strains, and a vaccine derived from a genotype that is circulating in the target population is likely to be most effective by affording cross-protection via shared antigens. However, one of the main reasons for developing an ST313-based vaccine is that these strains produce less enteropathogenicity than ST19 strains, which we anticipate will result in less inflammation and possibly reduced shedding (22). One of the barriers for developing live attenuated S. Typhimurium vaccines to date has been their propensity to cause bacterial shedding for long periods in human volunteer studies (36).
The immunogenic capacities of our NTS vaccines S. Typhimurium CVD 1931 (S. Typhimurium D65 ΔguaBA ΔclpX) and S. Enteritidis CVD 1944 (S. Enteritidis R11 ΔguaBA ΔclpX) in mice were comparable with those of our previous prototypic vaccines, CVD 1921 (S. Typhimurium I77 ΔguaBA ΔclpP) and CVD 1941 (S. Enteritidis R11 ΔguaBA ΔclpP) (26) (Fig. 2). The S. Typhimurium CVD 1921 and CVD 1931 vaccines elicited 100% seroconversion for anti-LPS and anti-FliC IgG. The S. Enteritidis vaccine CVD 1941 elicited anti-LPS serum IgG seroconversion in 93% (13/14) of mice and anti-FliC serum IgG seroconversion in 100% (14/14) of mice. Similarly, CVD 1944 elicited anti-LPS and anti-FliC seroconversion in 92% (11/12) of mice. Importantly, the live oral vaccines CVD 1931 and CVD 1944 were able to protect mice against a highly lethal challenge dose of S. Typhimurium and S. Enteritidis strains recently isolated from the blood of febrile children in Mali, indicating that these vaccines can protect against clinically relevant virulent strains. Furthermore, protection against these high challenge doses was observed when mice were infected 12 weeks after the last immunization, indicating that the vaccines can elicit long-lived specific immunity.
We also screened several Salmonella serovars in mice in an attempt to identify the ones that were pathogenic for use in cross-protection experiments. However, only a few serovars were found to cause lethal infection in mice (see Table S1 in the supplemental material), in agreement with previous studies (37–39). It is interesting that despite the existence of scores (if not hundreds) of Salmonella serovars that have been linked to human disease, less than a handful are pathogenic in mice (40). We confirmed that some strains of S. Dublin are lethal and identified the group B serovar S. Stanleyville as another lethal serovar. To our knowledge, this is the first report that S. Stanleyville is pathogenic in a mouse model. S. Stanleyville has been isolated sporadically from clinical specimens, including those from Senegal and Cameroon (41, 42). Interestingly, a recent publication described a case of a urinary tract infection due to S. Stanleyville following enteritis in a healthy child (43). The 26 S. Stanleyville strains that we previously isolated from the blood of children in Mali, West Africa, is the largest known outbreak of invasive S. Stanleyville to date (15).
The ability of live attenuated S. Typhimurium and S. Enteritidis vaccines to confer cross-protection against a heterologous serovar or other Salmonella serovars has been reported. For instance, NTS DNA adenine methylase (Dam) mutants have been evaluated as vaccines in several animal models, including murine, avian, and bovine models (44–47). An S. Enteritidis Dam− mutant protected mice against challenge with S. Dublin or S. Typhimurium at 10,000 LD50 (44). An S. Typhimurium Dam− mutant protected mice against S. Dublin at 10,000 LD50 or S. Enteritidis at 1,000 LD50. More recently, Matulova et al. (48) showed that S. Typhimurium and S. Enteritidis live vaccines with SPI-1 mutations can protect chickens against challenge with either serovar. However, our results are congruent with those of Hormaeche et al. (49), who showed that the S. Typhimurium aroA vaccine SL3261 conferred protection against 10,000 LD50 of S. Typhimurium but no protection against S. Enteritidis. Taken together, the cross-protection data suggest that live attenuated S. Typhimurium vaccines can protect against challenge with S. Typhimurium but that protection against other serovars is variable. On the other hand, live attenuated S. Enteritidis vaccines are highly immunogenic and can protect against homologous and heterologous serogroup challenge.
Our data suggest that an S. Enteritidis-based live attenuated vaccine may be sufficient to mediate protection against S. Enteritidis, S. Dublin, and S. Typhimurium, which is particularly important considering their concomitant prevalence, as demonstrated in West Africa. We speculate that our S. Enteritidis vaccine CVD 1944 elicits immunity against an antigen common to S. Enteritidis, S. Dublin, and S. Typhimurium. Cross-protection may be due to humoral immune response cross-reactivity among LPS epitopes or common outer membrane proteins, such as OmpD, which has been shown to be a key target of protective antibodies (50). However, the protection can also be due to cross-reacting cell-mediated immune responses, which live oral Salmonella vaccines potently stimulate (51, 52). There is evidence for cross-protection in humans between certain typhoidal Salmonella serovars. Besides protecting against typhoid fever, live oral Salmonella enterica serovar Typhi vaccine strain Ty21a confers significant cross-protection against S. Paratyphi B disease but not against S. Paratyphi A (53, 54).
Based on our results, we believe that the most effective vaccine strategy to combat invasive NTS would be to implement a bivalent (S. Typhimurium and S. Enteritidis) NTS vaccine in target pediatric populations. If the results of mouse studies can predict the protective activities of these vaccines in humans, not only would the vaccines target the most common invasive NTS serovars, but they would also protect children against other group B and D Salmonella serovars, such as S. Stanleyville and S. Dublin. In the future, one might wish to combine these NTS vaccines with typhoid and paratyphoid vaccines, as well as Salmonella group C vaccines, to target the predominant causes of invasive Salmonella disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sofie Livio for assistance with agglutinations.
This work was supported by the National Institutes of Health (Middle Atlantic RCE Program, grant number 2 U54 AI057168 to M.M.L., and a career development award to S.M.T.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00924-15.
REFERENCES
- 1.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enwere G, Biney E, Cheung YB, Zaman SM, Okoko B, Oluwalana C, Vaughan A, Greenwood B, Adegbola R, Cutts FT. 2006. Epidemiologic and clinical characteristics of community-acquired invasive bacterial infections in children aged 2–29 months in The Gambia. Pediatr Infect Dis J 25:700–705. doi: 10.1097/01.inf.0000226839.30925.a5. [DOI] [PubMed] [Google Scholar]
- 3.Gordon MA. 2008. Salmonella infections in immunocompromised adults. J Infect 56:413–422. doi: 10.1016/j.jinf.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Graham SM. 2002. Salmonellosis in children in developing and developed countries and populations. Curr Opin Infect Dis 15:507–512. doi: 10.1097/00001432-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Grimont PA, Weill FX. 2007. Antigenic formulae of the Salmonella serovars, 9th ed WHO Collaborating Centre for Reference and Research on Salmonella, Paris, France. [Google Scholar]
- 6.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 7.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. 2006. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J 25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 8.Graham SM, Walsh AL, Molyneux EM, Phiri AJ, Molyneux ME. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg 94:310–314. doi: 10.1016/S0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 9.Ikumapayi UN, Antonio M, Sonne-Hansen J, Biney E, Enwere G, Okoko B, Oluwalana C, Vaughan A, Zaman SM, Greenwood BM, Cutts FT, Adegbola RA. 2007. Molecular epidemiology of community-acquired invasive non-typhoidal Salmonella among children aged 2 29 months in rural Gambia and discovery of a new serovar, Salmonella enterica Dingiri. J Med Microbiol 56:1479–1484. doi: 10.1099/jmm.0.47416-0. [DOI] [PubMed] [Google Scholar]
- 10.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. 2006. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol 6:101. doi: 10.1186/1471-2180-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepage P, Bogaerts J, Van Goethem C, Ntahorutaba M, Nsengumuremyi F, Hitimana DG, Vandepitte J, Butzler JP, Levy J. 1987. Community-acquired bacteraemia in African children. Lancet i:1458–1461. [DOI] [PubMed] [Google Scholar]
- 12.Mandomando I, Macete E, Sigauque B, Morais L, Quinto L, Sacarlal J, Espasa M, Valles X, Bassat Q, Aide P, Nhampossa T, Machevo S, Ruiz J, Nhacolo A, Menendez C, Kotloff KL, Roca A, Levine MM, Alonso PL. 2009. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 14:1467–1474. doi: 10.1111/j.1365-3156.2009.02399.x. [DOI] [PubMed] [Google Scholar]
- 13.O'Dempsey TJ, McArdle TF, Lloyd-Evans N, Baldeh I, Laurence BE, Secka O, Greenwood BM. 1994. Importance of enteric bacteria as a cause of pneumonia, meningitis and septicemia among children in a rural community in The Gambia, West Africa. Pediatr Infect Dis J 13:122–128. doi: 10.1097/00006454-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. 2000. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J 19:312–318. doi: 10.1097/00006454-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Lagos R, Nataro JP, Galen JE, Levine MM. 2008. PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol 46:1861–1866. doi: 10.1128/JCM.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennant SM, Diallo S, Levy H, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Nataro JP, Galen JE, Levine MM. 2010. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis 4:e621. doi: 10.1371/journal.pntd.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadula J, von Gottberg A, Kilner D, de Jong G, Cohen C, Khoosal M, Keddy K, Crewe-Brown H. 2006. Nosocomial outbreak of extended-spectrum beta-lactamase-producing Salmonella isangi in pediatric wards. Pediatr Infect Dis J 25:843–844. doi: 10.1097/01.inf.0000233543.78070.a2. [DOI] [PubMed] [Google Scholar]
- 18.Beyene G, Nair S, Asrat D, Mengistu Y, Engers H, Wain J. 2011. Multidrug resistant Salmonella Concord is a major cause of salmonellosis in children in Ethiopia. J Infect Dev Ctries 5:23–33. [DOI] [PubMed] [Google Scholar]
- 19.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G. 2009. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacLennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 44:1215–1221. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carden S, Okoro C, Dougan G, Monack D. 2015. Non-typhoidal Salmonella Typhimurium ST313 isolates that cause bacteremia in humans stimulate less inflammasome activation than ST19 isolates associated with gastroenteritis. Pathog Dis 73:ftu023. doi: 10.1093/femspd/ftu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoro CK, Barquist L, Connor TR, Harris SR, Clare S, Stevens MP, Arends MJ, Hale C, Kane L, Pickard DJ, Hill J, Harcourt K, Parkhill J, Dougan G, Kingsley RA. 2015. Signatures of adaptation in human invasive Salmonella Typhimurium ST313 populations from sub-Saharan Africa. PLoS Negl Trop Dis 9:e0003611. doi: 10.1371/journal.pntd.0003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandran G, Perkins DJ, Schmidlein PJ, Tulapurkar ME, Tennant SM. 2015. Invasive Salmonella Typhimurium ST313 with naturally attenuated flagellin elicits reduced inflammation and replicates within macrophages. PLoS Negl Trop Dis 9:e3394. doi: 10.1371/journal.pntd.0003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Barrila J, Roland KL, Kilbourne J, Ott CM, Forsyth RJ, Nickerson CA. 2015. Characterization of the invasive, multidrug resistant non-typhoidal Salmonella strain D23580 in a urine odel of nfection. PLoS Negl Trop Dis 9:e0003839. doi: 10.1371/journal.pntd.0003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilchrist JJ, MacLennan CA, Hill AV. 2015. Genetic susceptibility to invasive Salmonella disease. Nat Rev Immunol 15:452–463. doi: 10.1038/nri3858. [DOI] [PubMed] [Google Scholar]
- 26.Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM. 2011. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun 79:4175–4185. doi: 10.1128/IAI.05278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomoyasu T, Ohkishi T, Ukyo Y, Tokumitsu A, Takaya A, Suzuki M, Sekiya K, Matsui H, Kutsukake K, Yamamoto T. 2002. The ClpXP ATP-dependent protease regulates flagellum synthesis in Salmonella enterica serovar Typhimurium. J Bacteriol 184:645–653. doi: 10.1128/JB.184.3.645-653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomoyasu T, Takaya A, Isogai E, Yamamoto T. 2003. Turnover of FlhD and FlhC, master regulator proteins for Salmonella flagellum biogenesis, by the ATP-dependent ClpXP protease. Mol Microbiol 48:443–452. doi: 10.1046/j.1365-2958.2003.03437.x. [DOI] [PubMed] [Google Scholar]
- 29.Ault A, Tennant SM, Gorres JP, Eckhaus M, Sandler NG, Roque A, Livio S, Bao S, Foulds KE, Kao SF, Roederer M, Schmidlein P, Boyd MA, Pasetti MF, Douek DC, Estes JD, Nabel GJ, Levine MM, Rao SS. 2013. Safety and tolerability of a live oral Salmonella Typhimurium vaccine candidate in SIV-infected nonhuman primates. Vaccine 31:5879–5888. doi: 10.1016/j.vaccine.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, Pasetti MF, Galen JE, Levine MM. 2011. Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect Immun 79:4240–4249. doi: 10.1128/IAI.05484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon R, Tennant SM, Galen JE, Levine MM. 2011. Mouse models to assess the efficacy of non-typhoidal Salmonella vaccines: revisiting the role of host innate susceptibility and routes of challenge. Vaccine 29:5094–5106. doi: 10.1016/j.vaccine.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berlanda Scorza F, Colucci AM, Maggiore L, Sanzone S, Rossi O, Ferlenghi I, Pesce I, Caboni M, Norais N, Di Cioccio V, Saul A, Gerke C. 2012. High yield production process for Shigella outer membrane particles. PLoS One 7:e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dagan R, Givon-Lavi N, Fraser D, Lipsitch M, Siber GR, Kohberger R. 2005. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J Infect Dis 192:367–376. doi: 10.1086/431679. [DOI] [PubMed] [Google Scholar]
- 35.Eddie DS, Schulkind ML, Robbins JB. 1971. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium “O” antibodies from rabbit intestinal fluid and colostrum. J Immunol 106:181–190. [PubMed] [Google Scholar]
- 36.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, Everest P, Wu T, Pickard D, Holden DW, Dougan G, Griffin GE, House D, Santangelo JD, Khan SA, Shea JE, Feldman RG, Lewis DJ. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun 70:3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roudier C, Krause M, Fierer J, Guiney DG. 1990. Correlation between the presence of sequences homologous to the vir region of Salmonella dublin plasmid pSDL2 and the virulence of twenty-two Salmonella serotypes in mice. Infect Immun 58:1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suez J, Porwollik S, Dagan A, Marzel A, Schorr YI, Desai PT, Agmon V, McClelland M, Rahav G, Gal-Mor O. 2013. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One 8:e58449. doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swearingen MC, Porwollik S, Desai PT, McClelland M, Ahmer BM. 2012. Virulence of 32 Salmonella strains in mice. PLoS One 7:e36043. doi: 10.1371/journal.pone.0036043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, Medus C, Cronquist A, Angulo FJ. 2008. Salmonellosis outcomes differ substantially by serotype. J Infect Dis 198:109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 41.Lafaix C, Castets M, Denis F, Diop Mar I. 1979. Salmonellosis in Dakar: bacteriological, clinical, epidemiological and therapeutic aspects. Ten years records (author's transl). Med Trop (Mars) 39:369–379. [PubMed] [Google Scholar]
- 42.Faucon R, Ducloux M. 1964. Salmonella Bareilly, Salmonella Durham, Salmonella Stanleyville isolated for the 1st time in man in Cameroun Med Trop (Mars) 24:417–426. [PubMed] [Google Scholar]
- 43.Leung AK, Kao CP, Robson WL. 2005. Urinary tract infection due to Salmonella stanleyville in an otherwise healthy child. J Natl Med Assoc 97:281–283. [PMC free article] [PubMed] [Google Scholar]
- 44.Heithoff DM, Enioutina EY, Daynes RA, Sinsheimer RL, Low DA, Mahan MJ. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect Immun 69:6725–6730. doi: 10.1128/IAI.69.11.6725-6730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dueger EL, House JK, Heithoff DM, Mahan MJ. 2003. Salmonella DNA adenine methylase mutants prevent colonization of newly hatched chickens by homologous and heterologous serovars. Int J Food Microbiol 80:153–159. doi: 10.1016/S0168-1605(02)00152-6. [DOI] [PubMed] [Google Scholar]
- 46.Mohler VL, Heithoff DM, Mahan MJ, Walker KH, Hornitzky MA, McConnell CS, Shum LW, House JK. 2006. Cross-protective immunity in calves conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine. Vaccine 24:1339–1345. doi: 10.1016/j.vaccine.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 47.Mohler VL, Heithoff DM, Mahan MJ, Walker KH, Hornitzky MA, Shum LW, Makin KJ, House JK. 2008. Cross-protective immunity conferred by a DNA adenine methylase deficient Salmonella enterica serovar Typhimurium vaccine in calves challenged with Salmonella serovar Newport. Vaccine 26:1751–1758. doi: 10.1016/j.vaccine.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 48.Matulova M, Havlickova H, Sisak F, Babak V, Rychlik I. 2013. SPI1 defective mutants of Salmonella enterica induce cross-protective immunity in chickens against challenge with serovars Typhimurium and Enteritidis. Vaccine 31:3156–3162. doi: 10.1016/j.vaccine.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Hormaeche CE, Joysey HS, Desilva L, Izhar M, Stocker BA. 1991. Immunity conferred by Aro- Salmonella live vaccines. Microb Pathog 10:149–158. doi: 10.1016/0882-4010(91)90075-L. [DOI] [PubMed] [Google Scholar]
- 50.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, Buckley CD, Dougan G, MacLennan IC, Lopez-Macias C, Cunningham AF. 2009. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A 106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. 2007. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine 25:1416–1425. doi: 10.1016/j.vaccine.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. 2008. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue-homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol 1:389–398. doi: 10.1038/mi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. 2007. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica serovar Paratyphi B. Clin Infect Dis 45(Suppl 1):S24–S28. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- 54.Simanjuntak CH, Paleologo FP, Punjabi NH, Darmowigoto R, Soeprawoto Totosudirjo H, Haryanto P, Suprijanto E, Witham ND, Hoffman SL. 1991. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 338:1055–1059. doi: 10.1016/0140-6736(91)91910-M. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.