Abstract
Leishmania infantum is a protozoan parasite that causes visceral leishmaniasis (VL). This infection triggers dendritic cell (DC) activation through the recognition of microbial products by Toll-like receptors (TLRs). Among the TLRs, TLR9 is required for DC activation by different Leishmania species. We demonstrated that TLR9 is upregulated in vitro and in vivo during infection. We show that C57BL/6 mice deficient in TLR9 expression (TLR9−/− mice) are more susceptible to infection and display higher parasite numbers in the spleen and liver. The increased susceptibility of TLR9−/− mice was due to the impaired recruitment of neutrophils to the infection foci associated with reduced levels of neutrophil chemoattractants released by DCs in the target organs. Moreover, both Th1 and Th17 cells were also committed in TLR9−/− mice. TLR9-dependent neutrophil recruitment is mediated via the MyD88 signaling pathway but is TIR domain-containing adapter-inducing interferon beta (TRIF) independent. Furthermore, L. infantum failed to activate both plasmacytoid and myeloid DCs from TLR9−/− mice, which presented reduced surface costimulatory molecule expression and chemokine release. Interestingly, neutrophil chemotaxis was affected both in vitro and in vivo when DCs were derived from TLR9−/− mice. Our results suggest that TLR9 plays a critical role in neutrophil recruitment during the protective response against L. infantum infection that could be associated with DC activation.
INTRODUCTION
Leishmania intracellular protozoan parasites cause a heterogeneous disease that can range from cutaneous lesions to visceral disease. Visceral disease can be caused by both Leishmania donovani and Leishmania infantum and is the most severe manifestation of this type of parasite infection. Therefore, Leishmania infections remain an important cause of human mortality and morbidity around the world (www.paho.org/english/ad/dpc/cd/res-dch-leish-priorities.pdf).
Host defenses against Leishmania spp. depend on the activation of an inflammatory response initiated by the innate immune system (1), followed by specific immune responses mediated by gamma interferon (IFN-γ)- or interleukin-17 (IL-17)-producing CD4+ T lymphocytes (2, 3). The innate immune system has specific mechanisms to rapidly recognize the parasite. A major component of pathogen recognition comprises a family of Toll-like receptors (TLRs) that detect common pathogen-associated molecular patterns (PAMPs) of various groups of microorganisms (4–7), including Leishmania spp. (8, 9).
The role of TLRs in Leishmania infection control has been supported by the observations that mice lacking MyD88, an adaptor molecule required for TLR signaling, display enhanced susceptibility to infection (10, 11). It has been described that resistance to Leishmania species infection is dependent on parasite lipophosphoglycan (LPG) recognition by TLR2 (8), the induction of IL-12, and the development of a Th1 immune response (12) together with NO production (13). Previous studies demonstrated a role for TLR2 and TLR3 in L. donovani recognition and killing by macrophages (14). Moreover, the absence of TLR4 increased the number of lesions during Leishmania major infection (15).
It has been shown that TLR9 recognizes CpG DNA sequences of many protozoans, including Leishmania (16–18). The recognition of L. major DNA by TLR9 promotes dendritic cell (DC) activation, which induces a Th1-dominant response that resolves the lesions (16). Furthermore, it has been described that TLR9 is related to draining lymph node hypertrophy, indicating that TLR9 is associated with cellular recruitment (17). During Leishmania braziliensis infection, TLR9 is important for the early control of lesion development and parasite burden but is dispensable for the differentiation of a Th1 response (18), suggesting that there are other mechanisms related to TLR9 protection.
Regarding visceral leishmaniasis disease, it has been demonstrated that L. infantum activates CD11chigh DCs via TLR9 for the generation of IL-12 that subsequently triggers NK cell cytotoxicity and IFN-γ production (19). However, the relevance of TLR9 during in vivo parasite control has not yet been evaluated. Similar to cutaneous leishmaniasis disease, it is possible that during L. infantum infection, there are additional protective mechanisms related to TLR9 activation.
Therefore, the aim of this study was to evaluate the role of and the mechanism by which TLR9 acts to control L. infantum infection. Here, we demonstrated that mice lacking TLR9 displayed impairment in neutrophil recruitment to inflammatory foci during parasite infection. Specifically, neutrophils are not recruited to inflammatory foci, at least in part because neutrophil chemotactic mediators are not produced by plasmacytoid and myeloid dendritic cells, leading to parasite resilience.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (wild type [WT]), TLR9−/− (Tlr9tm1Aki; nonfunctional leucine-rich repeat region), MyD88−/−, and TRIF−/− mice weighing between 18 and 22 g were housed in the animal facility of the Department of Biochemistry and Immunology, School of Medicine of Ribeirão Preto, University of São Paulo (Brazil), in temperature-controlled rooms (22°C to 25°C) and received water and food ad libitum. All animals are of the C57BL/6 background. All experiments were conducted in accordance with National Institutes of Health (NIH) guidelines on the welfare of experimental animals and with the approval of the Ethics Committee of the School of Medicine of Ribeirão Preto.
Parasite cultures, infection, and parasite load estimation.
L. infantum (isolate HU-UFS14) promastigotes were cultured in Schneider medium with 20% heat-inactivated fetal bovine serum (FBS), 5% penicillin-streptomycin (both from Sigma-Aldrich, St. Louis, MO, USA), and 2% male human urine. Parasite virulence was maintained by serial passages in BALB/c mice. Mice were injected in their retro-orbital plexus with 107 stationary-phase L. infantum promastigotes in 100 μl phosphate-buffered saline (PBS). Hepatic and splenic parasite burdens were determined by using a quantitative limiting-dilution assay (LDA).
DC generation, stimulation, and sorting.
Generation of bone marrow-derived cells (BMDCs) was performed as previously described (20). For activation, BMDCs (1 × 106 cells/ml) cultured in RPMI 1640 supplemented with 10% FBS were infected with L. infantum promastigotes at a 1:5 ratio (cells/parasites) or stimulated with CpG oligodeoxynucleotide (ODN) 1668 (10 μg/ml) (Thermo Electron, Ulm, Germany) for 24 h. The supernatants were collected to measure CXCL1 and CXCL2 production by an enzyme-linked immunosorbent assay (ELISA). The cells were harvested, and their surface expression was characterized by flow cytometry using antibodies against CD11c, TLR9, major histocompatibility complex class II (MHC-II), CD80, and CD40 conjugated to Alexa 700, phycoerythrin (PE), PE-Cy7, peridinin chlorophyll protein (PerCP), and allophycocyanin (APC), respectively, as well as control isotypes. For TLR9 expression, cells were permeabilized with a Cytofix/Cytoperm kit (BD Bioscience) according to the manufacturer's guidelines and stained with an antibody against TLR9 conjugated to fluorescein isothiocyanate (FITC) (eBioscience).
Myeloid DC (mDC) and plasmacytoid DC (pDC) subsets were characterized based on CD11c and B220 expression (21). Both DC populations were isolated from single-cell suspensions prepared from the spleens of naive or infected WT and TLR9−/− mice at 6 weeks postinfection (wpi) by using a BD FACSAria III instrument (BD Biosciences).
Phenotypes of inflammatory cells.
Splenic inflammatory cells were gated based on their characteristic size (forward scatter [FSC]) and granularity (side scatter [SSC]), and T lymphocytes (CD4+ CD3+, CD8+ CD3+, and CD4+ CD25+), B lymphocytes (CD19+), dendritic cells (CD11c+ CD11b+ MHC-II+), macrophages (F4/80+CD11c− MHC-II+), and neutrophils (LY6G+ MHC-II−) (BD Biosciences) were identified individually. The isotype control used was rat IgG2b or rat IgG2a. Cell acquisition was performed by using a FACSort flow cytometer. Data were plotted and analyzed by using FlowJo software (Tree Star, Ashland, OR).
Immunohistochemistry analysis.
Frozen liver sections from WT or TLR9−/− mice at different time points after infection were incubated with anti-mouse antibody 7/4, which recognizes Ly6B, which is expressed in low levels in neutrophils (Abcam PLC, Cambridge, United Kingdom), or control isotype antibodies. Afterwards, the sections were incubated with an avidin-biotin-peroxidase complex (Vector Laboratories, Ontario, Canada), and the color was developed by using 3,3′-diaminobenzidine (Vector Laboratories). The slides were counterstained with Mayer's hematoxylin.
Quantification of cytokine production.
Single-cell suspensions of spleens from infected WT or TLR9−/− mice were prepared aseptically, diluted to a concentration of 2 × 106 cells/ml, and dispensed into 48-well plates in a total volume of 500 μl of complete RPMI 1640 medium. The cells were restimulated with Leishmania antigen (50 μg/ml) in vitro at 37°C in 5% CO2, and culture supernatants were harvested after 48 h for the detection of CXCL1 (keratinocyte chemoattractant [KC]) and CXCL2 (macrophage inflammatory protein 2 [MIP-2]) levels and after 72 h for the detection of IFN-γ and IL-17 levels by an ELISA (R&D Systems, Minneapolis, MN, USA). For intracellular staining, the cells were cultured with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (500 ng/ml) in the presence of GolgiPlug (BD Biosciences) for 4 h, permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's guidelines, and stained with antibodies against IFN-γ, IL-17, CD3, and CD4 conjugated to APC, PE, APC-Cy7, and FITC, respectively. The isotype controls used were rat IgG2b and rat IgG2a. All of the antibodies were obtained from BD Biosciences and eBioscience (San Diego, CA, USA).
In vivo depletion of neutrophils.
WT mice were administered 20 μg of purified anti-mouse LY6G (clone 1A8) (BioLegend, San Diego, CA, USA) or normal rat IgG control antibody intraperitoneally 1 day before infection with L. infantum (day 1) and 10 μg of the respective antibody every 3 days for 3 weeks thereafter. Spleens and livers were harvested, and the parasite load was determined by an LDA at 6 weeks postinfection.
In vitro and in vivo neutrophil migration.
Neutrophil migration was evaluated by a chemotaxis assay in vitro. Chemotaxis was assayed with a 48-well microchamber (Boyden chamber; Neuro Probe, Gaithersburg, MD), using a 5-μm-pore-size polycarbonate membrane (28- by 50-mm filter; Neuro Probe). Neutrophils (5 × 105 cells/ml) from naive WT mice were allowed to migrate toward BMDC supernatants from infected WT or TLR9−/− mice in culture at 37°C in 5% CO2 for 24 h. CXCL2 (30 ng/ml) or medium was added to the wells as positive and negative controls, respectively. After 1 h, the membrane was removed, and the cells were fixed and stained. The neutrophils that had migrated through the membrane were counted in five randomly selected fields (at a magnification of ×1,000) by using a light microscope.
To analyze neutrophil recruitment in vivo, 107 L. infantum parasites were injected intraperitoneally into WT and TLR9−/− mice. After 24 h, peritoneal exudate cells (PECs) were collected and harvested for quantification of neutrophils by flow cytometry. Air pouches were prepared by injecting 3 ml of sterile air into the dorsal surface of anesthetized WT and TLR9−/− mice twice within a 3-day interval. In total, 800 μl of the BMDC supernatant from naive WT or TLR9−/− mice or from WT or TLR9−/− mice infected with L. infantum at a 1:5 ratio (cells/parasites) for 24 h was injected into the air pouches. In some experiments, the supernatant from naive or infected WT or TLR9−/− mice was filtered with a 0.22-μm filter before injection into the air pouch cavity. Control mice were injected with 800 μl of endotoxin-free saline (negative control) or lipopolysaccharide (LPS) (1 μg/pouch; positive control). Six hours after intrapouch inoculation, five animals per experimental group were lethally anesthetized, and the pouch was washed with a total of 2 ml of endotoxin-free saline to collect neutrophils from the exudates. Lavage fluids were centrifuged at 100 × g for 10 min at 4°C. The pellets were then resuspended in saline, stained with a trypan blue solution to exclude death cells, and counted in a Neubauer chamber. Neutrophils were identified as being LY6G+ MHC− in exudates by flow cytometry using specific antibodies.
Quantitative reverse transcription-PCR.
Total RNA was isolated from the livers and spleens of WT and TLR9−/− mice at 4 and 6 weeks postinfection by using the Illustra RNAspin minikit (GE Healthcare, Buckinghamshire, United Kingdom). Gene expression was normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression for spleen and liver samples and to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression for sorted DCs. The primer sequences used were as follows: GAPDH forward primer 5′-TGCAGTGGC AAAGTGGAGAT-3′ and reverse primer 5′-CGTGAGTGGAGTCATACTGGAA-3′, hypoxanthine phosphoribosyltransferase (HPRT) forward primer 5′-TGGAAAAGCCAAATACAAAGC-3′ and reverse primer 5′-CAACATCAACAGGACTCCTCG-3′, and TLR9 forward primer 5′-GTGGAAAAACCTCGTCCAGA-3′ and reverse primer 5′-GCTCGGCTTCCAGTATTGAG-3′.
Statistical analysis.
Data are expressed as the means ± standard errors of the means (SEM) and are representative of data from 2 to 4 independent experiments. The results from individual experiments were not combined because they were analyzed individually. The means from the different groups were compared by analysis of variance (ANOVA) followed by Tukey's honestly significant difference (HSD) test. Comparisons between two groups were determined by using Student's t test. Analyses were performed by using Prism 5.0 software (GraphPad). Statistical significance was set at a P value of <0.05.
RESULTS
L. infantum upregulates TLR9 expression during infection.
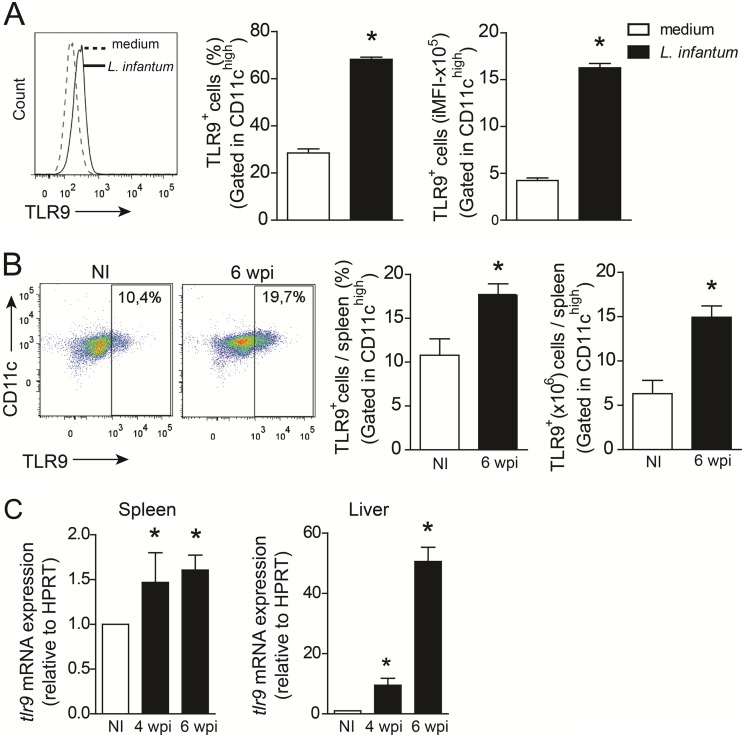
To determine whether L. infantum infection affects TLR9 expression, bone marrow-derived dendritic cells (BMDCs) were infected with L. infantum for 24 h in vitro, and TLR9 expression was evaluated. We found that infection of these cells with the parasite resulted in an induction of TLR9 expression in the CD11chigh subset (Fig. 1A). This response was confirmed by infection in vivo. A significant number of CD11chigh cells expressing TLR9 were observed in the spleen at 6 wpi compared to the numbers in uninfected mice. L. infantum infection enhanced the percentage and absolute numbers of TLR9+ CD11chigh cells by 2- and 3-fold, respectively (Fig. 1B). In addition, mRNA expression of TLR9 was significantly induced in target organs, such as the spleen, in which the mRNA expression level of TLR9 was increased at 4 and 6 wpi, and the liver, in which the expression level was increased at 4 wpi and pronounced at 6 wpi (Fig. 1C). These data suggest that TLR9 expression is altered during L. infantum infection.
FIG 1.
L. infantum infection induces TLR9 expression in DCs. (A and B) TLR9 expression levels in WT BMDCs infected with L. infantum (1:5 ratio) or medium for 24 h (A) and splenic DCs from WT mice at 6 wpi and uninfected (NI) control mice (B) were determined in vitro. The percentages and integrated mean fluorescence intensity (iMFI) of CD11chigh cells expressing TLR9 were determined by flow cytometry. (C) tlr9 mRNA expression levels in the spleens and livers of WT mice at 4 and 6 wpi were determined by real-time PCR and normalized to the level of the constitutively expressed HPRT gene. mRNA expression levels were calculated as the fold change compared to values for uninfected littermate controls. Data are expressed as the means ± SEM. Representative data from two independent experiments performed in quadruplicate (A) are shown (n = 4 to 5 [B and C]). *, P < 0.05 (determined by Student's t test [A and B] or two-way ANOVA with Tukey's post hoc test [C]) relative to medium (A) or uninfected controls (B and C).
TLR9 participates in the control of L. infantum infection.
Next, we addressed the importance of TLR9 during infection. TLR9−/− and WT mice were intravenously infected with 107 promastigotes of L. infantum, and the course of infection was monitored by quantification of parasites in organs. We observed that WT mice presented increasing parasite titers in the spleen (Fig. 2A) and liver (Fig. 2B) over time. TLR9−/− mice were more susceptible to infection and harbored more parasites in both target organs than WT animals for all periods analyzed. Thus, these results demonstrate that TLR9 is required for control of VL infection.
FIG 2.
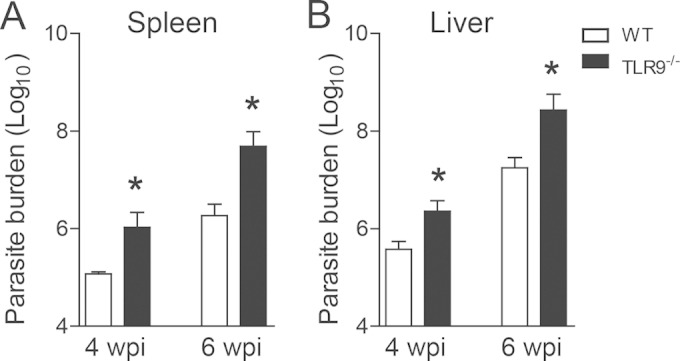
TLR9 participates in the control of L. infantum infection. Parasite burdens in the spleens (A) and livers (B) of WT and TLR9−/− mice at 4 and 6 wpi were determined by an LDA. Data are expressed as the means ± SEM and are representative of data from three independent experiments performed separately (n = 4 to 5). *, P < 0.05 (by Student's t test) relative to the WT group.
Neutrophil recruitment during L. infantum infection is dependent on TLR9 signaling and participates in parasite restriction.
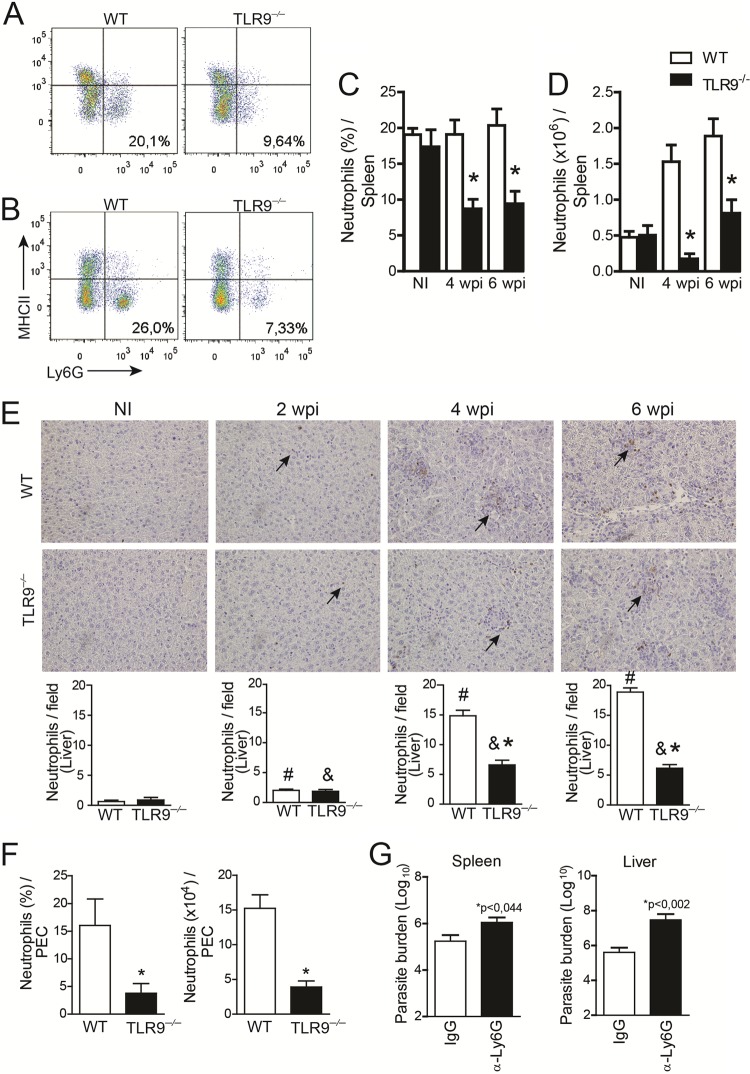
Having determined that TLR9 displays a protective role during VL infection, we investigated the composition of inflammatory leukocytes in organs that were infected with L. infantum to uncover the mechanism of parasite restriction. Leukocyte subsets were analyzed by flow cytometry at 4 and 6 wpi. L. infantum infection induced significant LY6G+ MHC-II− cell (neutrophil) recruitment in infected WT mice over time. Conversely, the number of LY6G+ MHC-II− cells that migrated to the spleens of the TLR9−/− mice was dramatically decreased at 4 and 6 wpi (Fig. 3A and B). The percent reductions in the number of neutrophils were ∼75% at 4 weeks and 50% at 6 wpi, compared to the number of neutrophils in WT mice (Fig. 3C and D). Numbers of other leukocyte subtypes were unchanged (see Table S1 in the supplemental material), except for B cells, which presented a significantly reduced number independent of TLR9 signaling, as evidenced by the similar decline in the numbers of B cells between infected WT and TLR9−/− mice. Such phenomena are relatively common during Leishmania infection and may be related to the chronic phase of the disease (22, 23).
FIG 3.
TLR9 promotes neutrophil recruitment to infection foci. (A and B) Dot plots representing neutrophils in the spleens of WT and TLR9−/− mice at 4 wpi (A) and 6 wpi (B) as determined by flow cytometry. (C and D) Bar graphs displaying the percentages (C) and the absolute numbers (D) of cells characterized as a LY6G+ MHC-II− population in spleens of WT and TLR9−/− mice at 4 and 6 wpi or uninfected mice (NI). (E) Kinetics of neutrophil migration to the liver assessed by immunohistochemistry with anti-mouse antibody 7/4 in tissues of WT and TLR9−/− mice at different times postinfection. WT and TLR9−/− mice were intraperitoneally infected with 1 × 107 cells/ml of L. infantum. (F) PECs were harvested 24 h after parasite infection, and the neutrophil population was phenotyped as being LY6G+ MHC-II− by flow cytometry. (G) Parasite burden in the spleen and liver after neutrophil depletion in infected WT mice. Data are expressed as the means ± SEM. Representative data from three (A to D) or two (E to G) independent experiments are shown (n = 4 to 5). *, P < 0.05 (determined by Student's t test [E to G] or two-way ANOVA with Tukey's post hoc test [C and D]) compared with the WT (A to F) or IgG (G) group. #, P < 0.05 (by Student's test) compared to naive WT mice; &, P < 0.05 (by Student's test) compared with naive TLR9−/− mice.
Neutrophil recruitment to the liver was assessed by immunohistochemistry. Thus, we performed a kinetic analysis by 7/4 staining of liver sections from WT and TLR9−/− mice. Our data demonstrated an increase in neutrophil infiltration into the liver 6 weeks after infection. As shown in Fig. 3E, microscopic analyses visually revealed that parasite infection promoted the recruitment of neutrophils to the livers of WT and TLR9−/− mice at all time points evaluated (2, 4, and 6 wpi) compared with noninfected mice (Fig. 3E). Notably, neutrophil recruitment induced by infection was localized to the liver of TLR9−/− mice even at later time points (6 wpi). To validate that neutrophil migration is dependent on TLR9, we performed a migration assay. First, WT and TLR9−/− mice were infected intraperitoneally with L. infantum (107 parasites/mice). Peritoneal exudate cells (PECs) were then harvested 24 h later, and neutrophils were phenotyped by using flow cytometry. We observed a similar failure in neutrophil migration to the peritoneal cavity in the absence of TLR9 in terms of cell percentages as well as absolute numbers (Fig. 3F). Altogether, these findings suggest that TLR9 participates in the recruitment of neutrophils to inflammatory foci and may be required to reduce parasite burden during L. infantum infection. Supporting our hypothesis, the depletion of neutrophils using anti-LY6G antibodies resulted in a lack of parasite control, as observed in the increased numbers of parasites present in spleens and livers from infected mice (Fig. 3G). To confirm the effectiveness of neutrophil depletion, we analyzed the neutrophil population. Based on their characteristic size (FSC) and granularity (SSC), we observed a significant reduction in the number of cells in the granulocyte gate in anti-LY6G-treated mice. By phenotyping the cells, we found that the LY6G+ MHC-II population was significantly reduced in anti-LY6G-treated mice, which showed reductions of ∼60% in percentages and 75% in terms of absolute numbers (see Fig. S1 in the supplemental material) compared with control treatment, demonstrating the effectiveness of neutrophil depletion.
The absence of TLR9 on neutrophils does not affect their activation and migration ability.
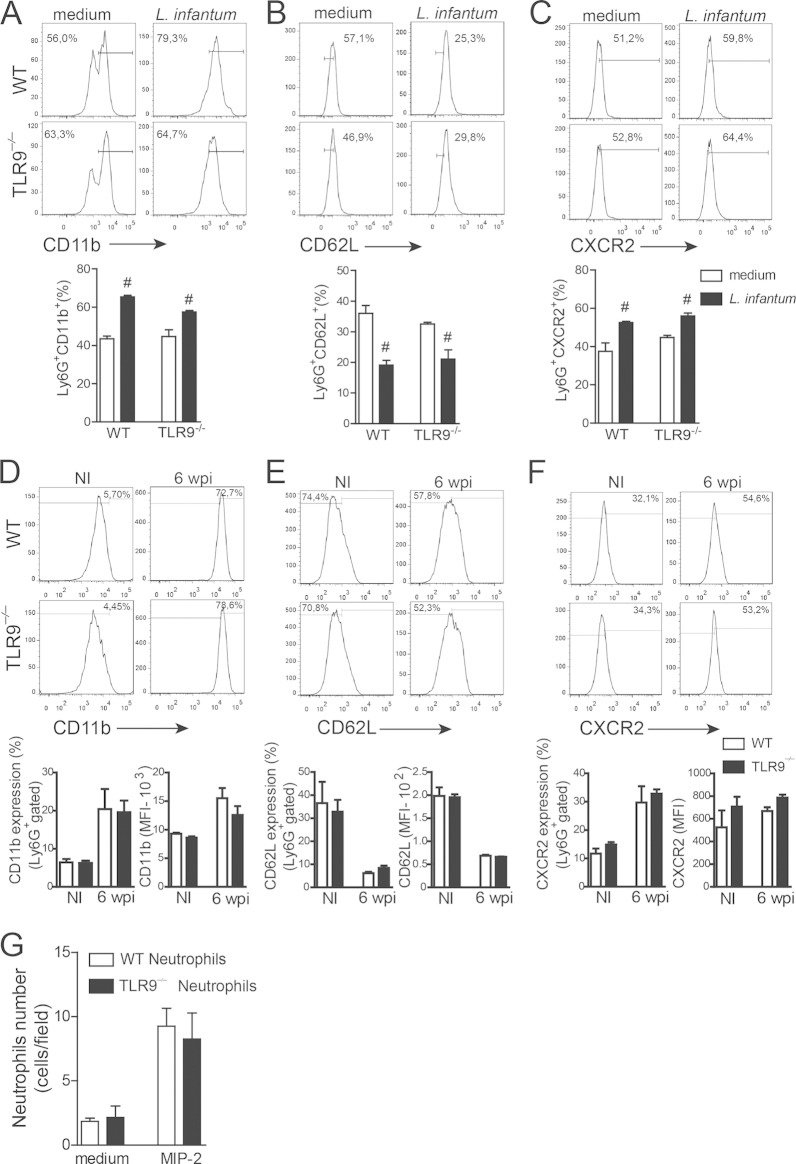
It is well established that TLR agonists modulate neutrophil activation by promoting the upregulation of selectins, integrins, and chemokine receptors, which allow neutrophils to roll, adhere, and subsequently transmigrate across endothelial walls to reach the site of infection or injury (24, 25). Thus, we examined neutrophil activation to determine whether differences in CD11b expression, shedding of CD62L, and CXCR2 expression could account for the failure of neutrophil migration observed in infected TLR9−/− mice. Parasite infection induced neutrophil activation and promoted the upregulation of CD11b expression, shedding of CD62L, and upregulation of CXCR2 expression (Fig. 4A to C). To determine the in vivo activation profile of the neutrophils, blood-derived neutrophils from WT and TLR9−/− mice at 6 wpi were evaluated. Similar to the in vitro data, circulating neutrophils isolated from WT and TLR9−/− mice also showed similar activation profiles (Fig. 4D to F).
FIG 4.
Neutrophil activation is not altered in TLR9−/− mice infected with L. infantum. CD11bhigh and CXCR2 expression as well as CD62L shedding were used to characterize the neutrophil activation profile in vitro following L. infantum infection. (A to C) Bone marrow-derived neutrophils from WT and TLR9−/− mice were cultured at a ratio of 5:1 (Leishmania parasites to neutrophils) with Leishmania or medium for 12 h for in vitro activation assays. (D to F) For in vivo activation assays, blood neutrophils from WT and TLR9−/− mice at 6 wpi were used. The LY6G+ MHC-II− population was analyzed by flow cytometry. Histograms represent data for infected and noninfected (NI) WT and TLR9−/− mice. MFI, mean fluorescence intensity. (G) A migration assay was performed by using WT and TLR9−/− neutrophils with MIP-2 (30 ng/ml) or medium as a chemotactic factor for 1 h. Data are expressed as the means ± SEM. Representative data from two (A to F) and three (G) independent experiments performed in quadruplicate (A to C) and quintuplicate (G) are shown (n = 4 to 5 [D to F]). #, P < 0.05 (by two-way ANOVA with Tukey's post hoc test) compared with medium.
Because chemotaxis is crucial for neutrophil migration, the migratory ability of bone marrow-isolated neutrophils from naive WT and TLR9−/− mice was assessed by a Boyden chamber assay using MIP-2 or medium as the chemotaxis stimulus or negative control, respectively. The MIP-2 stimulus promoted WT neutrophil migration compared to that with medium alone. Similarly, TLR9−/− neutrophils also migrated toward the MIP-2 chemoattractant factor (Fig. 4G). These results demonstrate that the failure of neutrophils to migrate to the site of infection in the absence of TLR9 expression is not related to an intrinsic defect in the neutrophil that would interfere with it becoming active and migrating toward inflammatory foci during L. infantum infection.
Infected TLR9−/− mice present with a defect in the production of neutrophil chemotactic factors.
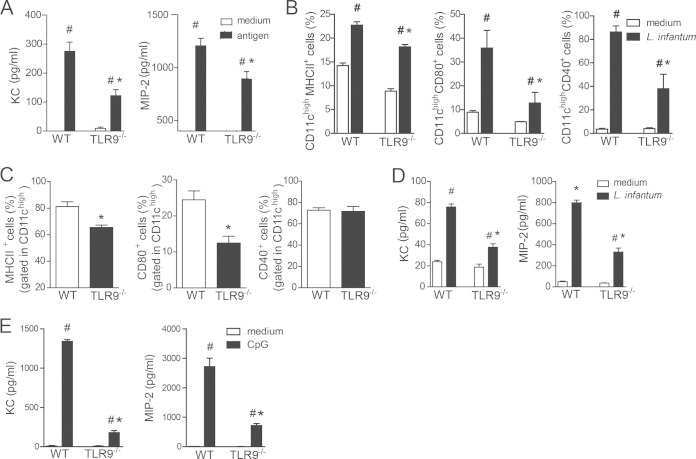
KC (CXCL1) and MIP-2 (CXCL2) are ligands for the chemotactic receptor CXCR2, which is present on circulating neutrophils. Because neutrophils from TLR9−/− mice are able to migrate when given the proper stimuli in vitro, we evaluated the ability of these chemotactic mediators to be released during infection. Spleen cells from infected WT and TLR9−/− mice at 6 wpi were isolated and restimulated in vitro with either L. infantum antigen or medium. Supernatants were collected 48 h later, and KC and MIP-2 levels were measured. Antigen-specific stimulation of spleen cells from infected WT and TLR9−/− mice induced the production of both chemokines compared to medium alone. However, stimulated TLR9−/− spleen cells showed decreased release of KC and MIP-2 (Fig. 5A) compared to that in stimulated WT cells. These results indicate that the impairment of neutrophils in recruitment to inflammatory foci during L. infantum infection observed for TLR9−/− mice may be related to the production of chemotactic mediators.
FIG 5.
TLR9 is required for DC maturation and production of neutrophil chemotactic factors. (A) Spleen cells from WT and TLR9−/− mice were collected at 6 wpi and in vitro stimulated with L. infantum antigen (50 μg/ml) or medium. The levels of KC and MIP-2 in culture supernatants were then measured by an ELISA. (B) WT and TLR9−/− BMDCs were infected with L. infantum (5:1) or medium for 24 h. BMDCs were harvested, and costimulatory molecule expression was evaluated by flow cytometry. (C) Levels of surface markers on DCs from infected WT and TLR9−/− mice at 6 wpi were determined. All analyses were performed on the CD11chigh population. (D and E) KC and MIP-2 levels in the supernatants from WT and TLR9−/− BMDCs cultured with medium, L. infantum (D), or CpG for 24 h (E) were measured by an ELISA. Data are expressed as the means ± SEM. Shown are data from one representative of two (E) and three (A to D) independent experiments performed in quadruplicate (B, D, and E) (n = 4 to 5 [A and C]). #, P < 0.05 (by two-way ANOVA with Tukey's post hoc test) compared with medium (A, B, D, and E); *, P < 0.05 (by two-way ANOVA with Tukey's post hoc test [B to E] or Student's test [A, C]) compared with the WT group.
Release of neutrophil chemoattractants by DCs is dependent on TLR9 signaling.
Given that DCs are the primary cells involved in orchestrating immune responses during Leishmania species infection through cytokine release and leukocyte chemoattractants (26–28), we hypothesized that the activation of TLR9 signaling in DCs would produce chemokines to recruit neutrophils to the site of infection. To test this hypothesis, we first determined the TLR9-dependent activation profile of DCs induced by L. infantum. Bone marrow-derived DCs from WT mice infected with parasites enhanced the surface expression of MHC-II, CD40, and CD80 compared to that with medium alone. In contrast, DCs from TLR9−/− mice infected with L. infantum were unable to be activated and presented reduced expression levels of these surface markers (Fig. 5B). Likewise, during in vivo L. infantum infection, splenic DCs from TLR9−/− mice at 6 wpi presented a committed state of maturation and had reduced surface expression levels of MHC-II and CD80 compared to those in the infected WT group (Fig. 5C).
Next, we evaluated the release of neutrophil chemoattractants by DCs. Thus, the levels of KC and MIP-2 in the supernatants from WT and TLR9−/− BMDCs cultured with L. infantum parasites or medium were determined. Consistent with the in vivo data, parasite infection induced significant production of KC and MIP-2 by DCs from WT mice compared to that in the respective control group. Interestingly, parasite infection also promoted a significant amount of chemokine release from TLR9−/− DCs compared to that in TLR9−/− DCs stimulated with medium alone. However, chemokine release from TLR9−/− DCs was significantly decreased compared to that in infected WT DCs. The reduction in chemokine release in the absence of TLR9 was 2-fold compared to that in WT DCs (Fig. 5D). To confirm the participation of TLR9 signaling in neutrophil migration, we stimulated TLR9−/− BMDCs with CpG and observed a marked reduction in the amounts of released KC and MIP-2 compared with those in WT BMDCs (Fig. 5E). The remaining chemokine factors observed in TLR9−/− DCs may be released due to the recognition of CpG by others receptors, such as AIM2 and STING (29).
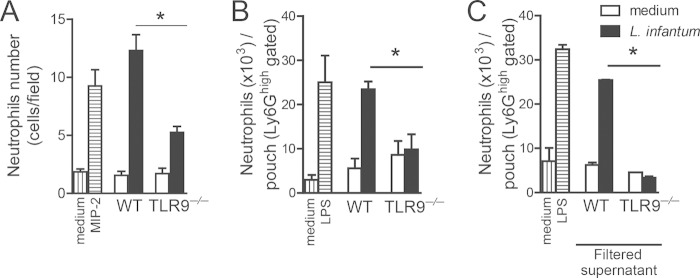
To assess the influence of TLR9 signaling in dendritic cells on neutrophil recruitment to infection foci, we performed a chemotaxis assay using a Boyden chamber with the supernatant from WT or TLR9−/− BMDCs stimulated with L. infantum or medium as the chemotaxis factor for WT neutrophils. The supernatant from infected WT BMDCs induced significant neutrophil migration compared to that of WT BMDCs stimulated with medium. In the absence of TLR9 signaling, the ability of neutrophils to migrate toward the supernatant was reduced. Although the supernatant of infected WT BMDCs induced the migration of 12.3 neutrophils/field, infected TLR9−/− BMDCs induced the migration of 5.25 neutrophils/field, which was approximately 2-fold less (Fig. 6A). To confirm these data in vivo, we performed an air pouch experiment using recipient naive WT mice that were injected in their air pouch cavity with the supernatant from WT or TLR9−/− BMDCs previously infected with L. infantum or stimulated with medium. Afterwards, neutrophil recruitment was determined by flow cytometry using specific antibodies. Consistent with data from the Boyden chamber assay, the supernatant from TLR9−/− BMDCs failed to recruit neutrophils to the air pouch cavity compared with infected WT BMDCs (Fig. 6B). To exclude a direct effect on L. infantum on neutrophil migration, we performed an air pouch assay using the filtered supernatant recovered from infected or uninfected WT and TLR9−/− DCs (Fig. 6C). The filtration of the supernatant did not interfere with neutrophil migration because similar rates of chemotaxis by filtered and nonfiltered supernatants from infected WT DCs were observed. Moreover, the filtered supernatant from TLR9−/− DCs also reduced neutrophil chemotaxis compared with that in the nonfiltered supernatant from TLR9−/− DCs, supporting the role of the mediators released by DCs in a TLR9-dependent pathway. Taken together, these data suggest that TLR9 signaling in DCs induced by L. infantum infection promotes the release of chemoattractants to recruit neutrophils to infection foci.
FIG 6.
TLR9 is important for neutrophil recruitment mediated by the production of chemoattractants by DCs. (A) In vitro migration assays using a Boyden chamber were performed with WT bone marrow-derived neutrophils, using supernatants from WT or TLR9−/− BMDCs cultured with L. infantum or medium as a chemoattractant. MIP-2 (30 ng/ml) or medium was used as the positive or negative control, respectively. (B) Air pouches were created on the dorsal side of WT mice injected with the supernatant from WT or TLR9−/− BMDCs cultured with L. infantum or medium. LPS (1 μg/pouch) or medium was used as the positive or negative control, respectively. (C) Filtered supernatants from WT or TLR9−/− BMDCs cultured with L. infantum or medium were injected into air pouches. Exudate cells were harvested and stained for neutrophil phenotype. Data are expressed as the means ± SEM. Shown are representative data from three (A and B) independent experiments performed in quintuplicate (A) (n = 5 [B and C]). *, P < 0.05 (by two-way ANOVA with Tukey's post hoc test) for TLR9−/− mice compared with WT mice.
Because both IFN-γ and IL-17 play a key role in neutrophil recruitment (30, 31), we analyzed both Th1 and Th17 T cells in the spleens of infected WT and TLR9−/− mice. Spleen cells from naive WT and TLR9−/− mice at 6 wpi were restimulated in vitro with polyclonal PMA plus ionomycin, and intracellular cytokine production was analyzed. Significant reductions in the frequencies and absolute numbers of both IL-17- and IFN-γ-producing CD4+ T cells in infected TLR9−/− mice compared with those in infected WT mice were observed (see Fig. S2A to S2E in the supplemental material). In addition, mice infected with the L. infantum antigen that lacked TLR9 also exhibited reductions in the amounts of IL-17 and IFN-γ released in restimulated spleen cells (see Fig. S2F and S2G in the supplemental material), suggesting that TLR9 signaling in DCs is required for the development of Th1 and Th17 cells. Taken together, our results show that neutrophil recruitment to inflammatory sites may be due to a direct effect of TLR9 signaling on dendritic cells, promoting the release of neutrophil chemoattractant mediators and the modulation of both the Th1 and Th17 patterns of immune responses.
The MyD88 signaling pathway is involved in the release of neutrophil chemotactic mediators.
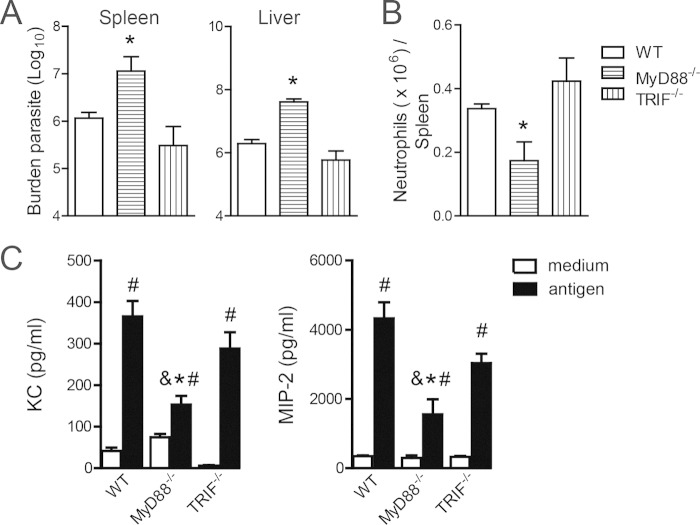
Because pathogen recognition through TLR9 signaling may be activated by the MyD88 or TIR domain-containing adapter-inducing interferon beta (TRIF) signaling pathway (32), we investigated which adaptor molecule was essential for anti-Leishmania effector function. We observed a higher number of parasites in both target organs of infected MyD88−/− mice at 6 wpi than in the WT control group (Fig. 7A). Furthermore, there was a lower number of neutrophils in the spleens of the MyD88−/− mice, which was accompanied by a reduction in the release of neutrophil chemoattractants, than in WT mice (Fig. 7B and C). We excluded the TRIF signaling pathway because the parasite burden was unaltered in infected TRIF−/− mice. Additionally, neutrophils were observed in high numbers in the spleens of infected TRIF−/− mice, which correlated with high levels of neutrophil chemoattractants. Taken together, these results suggest that TLR9/MyD88 signaling participates in the induction of an inflammatory response against parasites.
FIG 7.
TLR9-dependent neutrophil recruitment is mediated by the MyD88 signaling pathway. (A) Parasite burdens in spleens and livers from WT, MyD88−/−, and TRIF−/− mice were determined at 6 wpi by an LDA. (B) Numbers of neutrophils in spleens of WT, MyD88−/−, and TRIF−/− mice at 6 wpi were determined by flow cytometry. (C) Spleen cells from WT, MyD88−/−, and TRIF−/− mice at 6 wpi were in vitro stimulated with L. infantum antigen (50 μg/ml) or medium, and the levels of KC and MIP-2 in culture supernatants were measured by an ELISA. Representative data from three independent experiments are shown (n = 5). #, P < 0.05 compared with the NI group; *, P < 0.05 for MyD88−/− mice compared with WT mice; &, P < 0.05 for MyD88−/− mice compared with TRIF−/− mice (determined by two-way ANOVA with Tukey's post hoc test).
TLR9 signaling in pDCs and mDCs is required for neutrophil recruitment in vivo during VL.
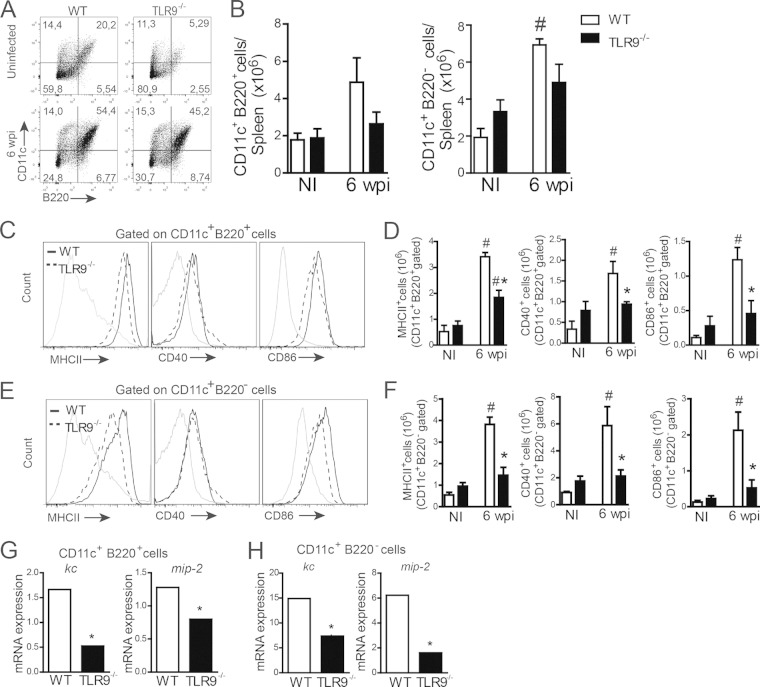
To identify which specific DC subsets are involved in TLR-dependent neutrophil recruitment, we phenotyped plasmacytoid or myeloid DCs by characterizing them as a CD11c+ B220+ or CD11c+ B220− population (21), respectively, in the spleen of WT and TLR9−/− mice at 6 wpi. Naive WT or TLR9−/− mice were used as a littermate control group. No difference in the absolute numbers of pDCs and mDCs between infected WT and TLR9−/− mice was observed (Fig. 8A and B). In addition, both WT DC subsets presented similar rates of activation, with a significant increase in the expression of costimulatory molecules such as MHC-II, CD80, and CD86 (Fig. 8C to F) compared with that in the naive WT group. However, the ablation of TLR9 affected the maturation profiles of both pDCs and mDCs, exhibiting significant reductions in MHC-II, CD40, and CD86 expression that reached basal levels similar to those in the naive TLR9−/− group (Fig. 8C to F). Both pDCs (CD11c+ B220+) and mDCs (CD11c+ B220−) from the spleens of WT and TLR9−/− mice at 6 wpi were sorted to evaluate KC and MIP-2 mRNA expression by quantitative PCR (qPCR). Interestingly, despite both WT DC subtypes being able to produce neutrophil chemotactic mediators, the most measured levels of KC and MIP-2 came from mDCs, presenting levels 8- to 10-fold higher than those produced by pDCs, suggesting that myeloid DCs are the primary source of neutrophil chemokines during VL (Fig. 8G and H). According to the above-described results, the absence of TLR9 affects the expression of neutrophil chemokines in both DC subsets, supporting the idea that TLR9 signaling in pDCs and mDCs is required for in vivo neutrophil recruitment during disease.
FIG 8.
Activation and neutrophil chemoattractant expression in pDCs and mDCs are dependent on TLR9 signaling during L. infantum infection. (A and B) Spleen cells from uninfected WT or TLR9−/− mice at 6 wpi were isolated and harvested for flow cytometry analysis. Dot plots represent the frequencies of CD11c+ B220+ and CD11c+ B220− cells (A), and graphs represent the absolute numbers of these cells (B). (C to F) Costimulatory molecule expression was evaluated by gating the CD11c+ B220+ (C and D) or CD11c+ B220− (E and F) population. The histograms represent data from infected WT and TLR9−/− mice (C and E). (G and H) KC and MIP-2 mRNA expression levels in sorted CD11c+ B220+ or CD11c+ B220− cells from WT and TLR9−/− mice at 6 wpi were determined by real-time PCR and normalized to the expression level of the constitutively expressed GAPDH gene. Data are expressed as the means ± SEM. Representative data from two independent experiments (A to F) are shown (n = 4 to 5 [A to H]). #, P < 0.05 compared with the NI group; *, P < 0.05 compared with infected WT mice (two-way ANOVA with Tukey's post hoc test [B, D, and F] or Student's t test [G and H]).
DISCUSSION
In the present study, we elucidated the mechanism by which L. infantum infection is controlled by TLR9 activation. We demonstrated that TLR9 mediates a protective immune response through the recruitment of neutrophils to the inflammatory site. This recruitment is dependent, at least in part, on the activation of plasmacytoid and myeloid DCs and the subsequent release of neutrophil chemotactic mediators through a MyD88-dependent signaling pathway. By understanding the mechanism by which Leishmania infection is controlled, new vaccines can be developed to protect the host against parasitic infections.
TLRs play a central role in pathogen recognition and the development of effector immune responses during various infections, including leishmaniasis. Previous studies showed that Leishmania spp. presented the ability to regulate TLR expression (33). Here, we found that L. infantum infection positively modulated the expression of TLR9 on the surface of DCs, suggesting that this receptor may be involved in the recognition of parasites and may orchestrate the immune response against this parasite. In this context, the key factor involved in the control of parasite replication is the cellular production of IFN-γ, and TLR9 may be required for this differentiation pattern (16). Furthermore, it has been demonstrated that TLR9 is essential for IL-12 production and the subsequent activation of NK cells that secrete IFN-γ during L. major or L. infantum infection (19, 34). Conversely, Weinkopff et al. showed that TLR9−/− mice infected with L. braziliensis presented with larger lesions and higher numbers of parasite, even in the presence of large amounts of IFN-γ in the draining lymph node and IL-12p40 mRNA at the site of infection (18), suggesting that mechanisms other than those related to adaptive immunity are involved in the susceptibility of animals deficient in TLR9 to infection. Although we verified that IFN-γ production is also dependent on TLR9 signaling, our results show that neutrophil migration to inflammatory foci during L. infantum infection is important for controlling parasite replication in the spleen and liver.
Using TLR9-deficient mice, we observed a decrease in neutrophil recruitment in the spleen and liver, establishing a relationship between TLR9 signaling, the recruitment of neutrophils, and consequent protection against parasites. Various studies have reported the involvement of TLRs in modulating neutrophil recruitment. Johnson et al., using TLR9−/− mice, demonstrated that during experimental keratitis, TLR9 and MyD88 signaling pathways were essential for neutrophil recruitment to the cornea (35). Furthermore, it was demonstrated that small interfering RNA (siRNA) silencing of TLR9 signaling on Langerhans cells reduces neutrophil migration to inflammatory sites during Pseudomonas aeruginosa infection and increases bacterial loads (36). Additionally, in a severe sepsis model, it was demonstrated that TLR9−/− mice exhibit decreased neutrophil migration to inflammatory sites because TLR9 activation under these pathological conditions promotes CXCR2 receptor desensitization, and therefore, it failed to recruit neutrophils to infection foci (37).
VL is classically described as a chronic disease, and thus, the observed increase in the number of neutrophils in the target organs of WT mice was surprising. However, there is a broad body of literature that has reported that neutrophils are the first cells to migrate to inflammatory sites during infection caused by L. major and L. infantum (38, 39). Furthermore, in mouse strains resistant to L. major, neutrophils are recruited to the site within hours of inoculation, and their numbers are decreased by day 3 after infection (40). Thus, it is reasonable that during VL, the dynamic recruitment of polymorphonuclear cells is different from that during cutaneous leishmaniasis. In this case, the replication of amastigote forms in target organs acts as a stimulus for neutrophil migration during infection. In fact, neutrophil influx correlated with the number of Leishmania infantum parasites present at the infection site. We do not rule out the possible role of adaptive immunity cytokines such as IFN-γ and IL-17 in TLR9-dependent neutrophil recruitment because both are potent neutrophil modulators (30, 31). In fact, levels of both Th1 and Th17 subtypes were reduced in the spleens of infected TLR9−/− mice. Likewise, lower levels of IFN-γ and IL-17 were detected in the supernatant of spleen cultures from TLR9−/− mice after specific antigen stimulation. Taken together, our results show that neutrophil recruitment to inflammatory sites may be due to a direct effect of TLR9 signaling on dendritic cells, which promote the release of neutrophil chemoattractant mediators and modulate both the Th1 and Th17 patterns of immune response.
The role of neutrophils during Leishmania infection is still controversial because of the heterogeneity of the experimental models used to characterize their function. In our study, depletion of polymorphonuclear neutrophils by using specific antibodies demonstrated that neutrophils contributed to the control of parasite replication during VL. Supporting our data, it was demonstrated previously that during infection induced by L. infantum and L. donovani, neutrophil depletion causes increased parasites numbers in the spleen and liver, suggesting their protective role during infection (41–43). However, in a model of cutaneous leishmaniasis induced by L. major, it was demonstrated that neutrophils play a protective role in a strain-dependent manner, because in BALB/c mice genetically susceptible to disease, neutrophil depletion reduces the parasitic load, but depletion in genetically resistant C57BL/6 mice promotes increased infection (44–46).
TLR signaling regulates several neutrophil functions, such as activation, migration, and apoptosis (47). In general, TLR ligands such as LPG, zymosan, R484 (TLR7 ligands), and LPS are able to stimulate the expression of receptors such as CXCR2 on the surface of neutrophils to induce their activation. Therefore, we hypothesized that the observed failure to recruit neutrophils to the inflammatory site in infected TLR9−/− mice could be related to factors involved in neutrophil activation. However, our data demonstrate that the absence of TLR9 signaling does not interfere with neutrophil activation and CXCR2 expression in vitro or in vivo, suggesting that other TLRs present on neutrophils may promote their activation. Interestingly, neutrophils failed to migrate because of deficiencies in the levels of neutrophil chemotactic factors, such as MIP-2 and KC, at the infection site. Using an experimental model of cutaneous leishmaniasis, Müller et al. demonstrated that efficient neutrophil migration to the skin of animals infected with L. major correlates with the production of MIP-2 and KC (38). Furthermore, in humans, the migration of neutrophils following L. major infection is dependent on CXCL8/IL-8, which is a chemokine homologous to CXCL1 in mice (48, 49). Regarding VL, there are no studies demonstrating the mechanisms involved in neutrophil recruitment to the infection site thus far. Therefore, we demonstrated for the first time that the failure of neutrophil migration in animals genetically deficient for TLR9 is not due to an intrinsic defect of the neutrophil but to an inefficient production of chemoattractant mediators.
Although a small leukocyte population, DCs play an important role in the initiation of immune responses because they are the main cells involved in pathogen recognition and trigger several proinflammatory mechanisms that bridge the innate and adaptive immune responses (26, 50). During Leishmania species infection, DCs present at the inflammation site capture parasites and acquire a mature phenotype by increasing their secretion of MHC-II, costimulatory molecules (CD40, CD80, and CD86), and IL-12p40 (51, 52), a response dependent on MyD88 signaling (8). This effect reflects a protective immune response against parasites that is dependent on increased levels of production of IFN-γ, suggesting the involvement of TLRs in this process. In the present study, we demonstrated that the activation of DCs during infection, at least in part, might be dependent on TLR9 signaling because DCs from TLR9−/− mice showed an immature phenotype and presented with lower expression levels of MHC-II and costimulatory molecules. Interestingly, TLR9 activation mediated the secretion of neutrophil chemoattractant factors during infection. BMDCs from TLR9−/− mice produced lower levels of KC and MIP-2 when cultured with L. infantum and, therefore, did not induce neutrophil migration in an efficient manner in vitro or in vivo.
Different DC populations have the ability to produce chemotactic mediators in response to stimulation of TLR7 (R848) and TLR9 (CpG) ligands (53), demonstrating that the secretion of chemoattractants is TLR dependent. Interestingly, the absence of TLR9 affects the maturation stage and the secretion of neutrophil chemokines in both plasmacytoid and myeloid DCs, supporting the idea that TLR9 signaling on pDCs and mDCs is required for in vivo neutrophil recruitment during disease. However, the participation of DC subsets during visceral leishmaniasis is poorly explored. In a cutaneous leishmaniasis model, pDC is related to the recruitment of monocytes, macrophages, and neutrophils to the site of L. major infection (54), and it has been suggested that different DC subsets participate in the development of the Th1 immune response during cutaneous leishmaniasis (55). In vitro, mDCs are essential for NK cell cytotoxicity induction in a TLR9-dependent manner, whereas this effect is TLR9 independent in pDCs during L. infantum infection (19). In this study, we explored for the first time the in vivo role of pDCs and mDCs in the promotion of direct neutrophil recruitment during LV and demonstrated that both DC subsets are important for neutrophil migration in a TLR9 signaling-dependent manner.
We do not rule out the possibility that other cells are also sources of such mediators. It has been shown that resident peritoneal cells, such as macrophages and mast cells, also participate in the recruitment of neutrophils in a TLR4- and MyD88-dependent manner (56). Interestingly, in our study, we found that bone marrow-derived macrophages infected with L. infantum were able to secrete KC and MIP-2 but at a concentration 10 times lower than that in DCs (data not shown), demonstrating that DCs play a central role in the migration of neutrophils during infection.
In summary, this study demonstrated that TLR9 contributes to the development of the innate immune response to L. infantum infection. This protective mechanism is related to the recruitment of neutrophils to the appropriate inflammatory site. This mechanism is directly coordinated by DCs, which, when activated via TLR9, induce the release of chemotactic mediators to recruit neutrophils to infection foci and thus control parasite replication. Therefore, we believe that understanding the mechanisms that control the infection process caused by L. infantum may contribute to the generation of new vaccine strategies to prevent the establishment of infection in vertebrate hosts.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to CAPES-PRODOC, FAPESP, CNPq, INCTV, and FAEPA for their financial support. The research leading to these results has received funding from the São Paulo Research Foundation (FAPESP) under grant agreement no. 2012/14524-9 and no. 2013/08216-2 (Center for Research in Inflammatory Disease).
We have no conflicting financial interests.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00975-15.
REFERENCES
- 1.Liese J, Schleicher U, Bogdan C. 2008. The innate immune response against Leishmania parasites. Immunobiology 213:377–387. doi: 10.1016/j.imbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Engwerda CR, Kaye PM. 2000. Organ-specific immune responses associated with infectious disease. Immunol Today 21:73–78. doi: 10.1016/S0167-5699(99)01549-2. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh K, Sharma G, Saha A, Kar S, Das PK, Ukil A. 2013. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J Infect Dis 207:1016–1025. doi: 10.1093/infdis/jis771. [DOI] [PubMed] [Google Scholar]
- 4.Bafica A, Santiago HC, Goldszmid R, Ropert C, Gazzinelli RT, Sher A. 2006. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol 177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 5.Debierre-Grockiego F, Azzouz N, Schmidt J, Dubremetz J-F, Geyer H, Geyer R, Weingart R, Schmidt RR, Schwarz RT. 2003. Roles of glycosylphosphatidylinositols of Toxoplasma gondii induction of tumor necrosis factor-α production in macrophages. J Biol Chem 278:32987–32993. doi: 10.1074/jbc.M304791200. [DOI] [PubMed] [Google Scholar]
- 6.Shoda LKM, Kegerreis KA, Suarez CE, Roditi I, Corral RS, Bertot GM, Norimine J, Brown WC. 2001. DNA from protozoan parasites Babesia bovis, Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun 69:2162–2171. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos MAS, Almeida IC, Takeuchi O, Akira S, Valente EP, Procópio DO, Travassos LR, Smith JA, Golenbock DT, Gazzinelli RT. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol 167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 8.de Veer MJ, Curtis JM, Baldwin TM, DiDonato JA, Sexton A, McConville MJ, Handman E, Schofield L. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol 33:2822–2831. doi: 10.1002/eji.200324128. [DOI] [PubMed] [Google Scholar]
- 9.McConville MJ, Schnur LF, Jaffe C, Schneider P. 1995. Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species. Biochem J 310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muraille E, De Trez C, Brait M, De Baetselier P, Leo O, Carlier Y. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol 170:4237–4241. doi: 10.4049/jimmunol.170.8.4237. [DOI] [PubMed] [Google Scholar]
- 11.De Trez C, Brait M, Leo O, Aebischer T, Torrentera FA, Carlier Y, Muraille E. 2004. Myd88-dependent in vivo maturation of splenic dendritic cells induced by Leishmania donovani and other Leishmania species. Infect Immun 72:824–832. doi: 10.1128/IAI.72.2.824-832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osanya A, Song EH, Metz K, Shimak RM, Boggiatto PM, Huffman E, Johnson C, Hostetter JM, Pohl NLB, Petersen CA. 2011. Pathogen-derived oligosaccharides improve innate immune response to intracellular parasite infection. Am J Pathol 179:1329–1337. doi: 10.1016/j.ajpath.2011.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya P, Bhattacharjee S, Gupta G, Majumder S, Adhikari A, Mukherjee A, Majumdar SB, Saha B, Majumdar S. 2010. Arabinosylated lipoarabinomannan-mediated protection in visceral leishmaniasis through up-regulation of Toll-like receptor 2 signaling: an immunoprophylactic approach. J Infect Dis 202:145–155. doi: 10.1086/653210. [DOI] [PubMed] [Google Scholar]
- 14.Flandin JF, Chano F, Descoteaux A. 2006. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-γ-primed macrophages. Eur J Immunol 36:411–420. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 15.Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, Antoniazi S, Galanos C, Smith DF, Müller I. 2004. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun 72:1920–1928. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou Fakher FH, Rachinel N, Klimczak M, Louis J, Doyen N. 2009. TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J Immunol 182:1386–1396. doi: 10.4049/jimmunol.182.3.1386. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho LP, Petritus PM, Trochtenberg AL, Zaph C, Hill DA, Artis D, Scott P. 2012. Lymph node hypertrophy following Leishmania major infection is dependent on TLR9. J Immunol 188:1394–1401. doi: 10.4049/jimmunol.1101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinkopff T, Mariotto A, Simon G, Hauyon-La Torre Y, Auderset F, Schuster S, Zangger H, Fasel N, Barral A, Tacchini-Cottier F. 2013. Role of Toll-like receptor 9 signaling in experimental Leishmania braziliensis infection. Infect Immun 81:1575–1584. doi: 10.1128/IAI.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JAA, Weiss S, Kalinke U, Kunz S. 2007. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med 204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carregaro V, Valenzuela JG, Cunha TM, Verri WA, Grespan R, Matsumura G, Ribeiro JMC, Elnaiem DE, Silva JS, Cunha FQ. 2008. Phlebotomine salivas inhibit immune inflammation-induced neutrophil migration via an autocrine DC-derived PGE2/IL-10 sequential pathway. J Leukoc Biol 84:104–114. doi: 10.1189/jlb.1107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colonna M, Trinchieri G, Liu Y-J. 2004. Plasmacytoid dendritic cells in immunity. Nat Immunol 5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 22.Coura-Vital W, Marques M, Giunchetti R, Teixeira-Carvalho A, Moreira N, Vitoriano-Souza J, Vieira P, Carneiro C, Corrêa-Oliveira R, Martins-Filho O. 2011. Humoral and cellular immune responses in dogs with inapparent natural Leishmania infantum infection. Vet J 190:e43–e47. doi: 10.1016/j.tvjl.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues V, Laforge M, Campillo-Gimenez L, Soundaramourty C, Correia-de-Oliveira A, Dinis-Oliveira RJ, Ouaissi A, Cordeiro-da-Silva A, Silvestre R, Estaquier J. 2014. Abortive T follicular helper development is associated with a defective humoral response in Leishmania infantum-infected macaques. PLoS Pathog 10:e1004096. doi: 10.1371/journal.ppat.1004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan J, Malik AB. 2003. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med 9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 25.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MKB. 2003. Selective roles for Toll-like receptor (TLR) 2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 26.Gorak P, Engwerda CR, Kaye PM. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol 28:687–695. [DOI] [PubMed] [Google Scholar]
- 27.Von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med 188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.León B, López-Bravo M, Ardavín C. 2007. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Broz P, Monack DM. 2013. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol 13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- 30.Nandi B, Behar SM. 2011. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med 208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacramento LA, Cunha FQ, de Almeida RP, da Silva JS, Carregaro V. 2014. Protective role of 5-lipoxigenase during Leishmania infantum infection is associated with Th17 subset. Biomed Res Int 2014:264270. doi: 10.1155/2014/264270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, Orabona C, De Luca A, Boon L, Romani L. 2013. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun 4:1852. doi: 10.1038/ncomms2874. [DOI] [PubMed] [Google Scholar]
- 33.Cezário GAG, Oliveira LRC, Peresi E, Nicolete VC, Polettini J, Lima CRG, Gatto M, Calvi SA. 2011. Analysis of the expression of Toll-like receptors 2 and 4 and cytokine production during experimental Leishmania chagasi infection. Mem Inst Oswaldo Cruz 106:573–583. doi: 10.1590/S0074-02762011000500010. [DOI] [PubMed] [Google Scholar]
- 34.Liese J, Schleicher U, Bogdan C. 2007. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol 37:3424–3434. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]
- 35.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. 2005. Activation of Toll-like receptor (TLR) 2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci 46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Barrett RP, McClellan SA, Hazlett LD. 2005. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- 37.Trevelin SC, Alves-Filho JC, Sônego F, Turato W, Nascimento DC, Souto FO, Cunha TM, Gazzinelli RT, Cunha FQ. 2012. Toll-like receptor 9 activation in neutrophils impairs chemotaxis and reduces sepsis outcome. Crit Care Med 40:2631–2637. doi: 10.1097/CCM.0b013e318258fb70. [DOI] [PubMed] [Google Scholar]
- 38.Müller K, Zandbergen G, Hansen B, Laufs H, Jahnke N, Solbach W, Laskay T. 2001. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med Microbiol Immunol 190:73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- 39.Thalhofer CJ, Chen Y, Sudan B, Love-Homan L, Wilson ME. 2011. Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum chagasi. Infect Immun 79:108–117. doi: 10.1128/IAI.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. 2007. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol 82:288–299. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- 41.McFarlane E, Perez C, Charmoy M, Allenbach C, Carter KC, Alexander J, Tacchini-Cottier F. 2008. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infect Immun 76:532–541. doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rousseau D, Demartino S, Ferrua B, Michiels JF, Anjuère F, Fragaki K, Le Fichoux Y, Kubar J. 2001. In vivo involvement of polymorphonuclear neutrophils in Leishmania infantum infection. BMC Microbiol 1:17. doi: 10.1186/1471-2180-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smelt SC, Cotterell SEJ, Engwerda CR, Kaye PM. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol 164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 44.Lima GMAC, Vallochi AL, Silva UR, Bevilacqua EMAF, Kiffer MMF, Abrahamsohn IA. 1998. The role of polymorphonuclear leukocytes in the resistance to cutaneous Leishmaniasis. Immunol Lett 64:145–151. doi: 10.1016/S0165-2478(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 45.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis J. 2000. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol 165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-de-Souza MCA, Cysne-Finkelstein L, Arnholdt AC, Calich VL, Coutinho SG, Lopes MF, DosReis GA. 2004. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol 172:4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 47.Sabroe I, Dower SK, Whyte MKB. 2005. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis 41:S421–S426. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- 48.Badolato R, Sacks DL, Savoia D, Musso T. 1996. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp Parasitol 82:21–26. doi: 10.1006/expr.1996.0003. [DOI] [PubMed] [Google Scholar]
- 49.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. 2004. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol 173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Uzonna JE. 2012. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol 2:83. doi: 10.3389/fcimb.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basu A, Chakrabarti G, Saha A, Bandyopadhyay S. 2001. Modulation of CD11C+ splenic dendritic cell functions in murine visceral leishmaniasis: correlation with parasite replication in the spleen. Immunology 99:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moll H. 2000. The role of dendritic cells at the early stages of Leishmania infection. Adv Exp Med Biol 497:163–173. [DOI] [PubMed] [Google Scholar]
- 53.Proietto AI, O'Keeffe M, Gartlan K, Wright MD, Shortman K, Wu L, Lahoud MH. 2004. Differential production of inflammatory chemokines by murine dendritic cell subsets. Immunobiology 209:163–172. doi: 10.1016/j.imbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Guillerey C, Mouriès J, Polo G, Doyen N, Law HK, Chan S, Kastner P, Leclerc C, Dadaglio G. 2012. Pivotal role of plasmacytoid dendritic cells in inflammation and NK-cell responses after TLR9 triggering in mice. Blood 120:90–99. doi: 10.1182/blood-2012-02-410936. [DOI] [PubMed] [Google Scholar]
- 55.Ashok D, Acha-Orbea H. 2014. Timing is everything: dendritic cell subsets in murine Leishmania infection. Trends Parasitol 30:499–507. doi: 10.1016/j.pt.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 56.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.