Abstract
Despite Coxiella burnetii being an obligate intracellular bacterial pathogen, our recent study demonstrated that B cells play a critical role in vaccine-induced immunity to C. burnetii infection by producing protective antibodies. However, the role of B cells in host defense against primary C. burnetii infection remains unclear. In this study, we investigated whether B cells play an important role in host defense against primary C. burnetii infection. The results showed that peritoneal B cells were able to phagocytose virulent C. burnetii bacteria and form Coxiella-containing vacuoles (CCVs) and that C. burnetii can infect and replicate in peritoneal B1a subset B cells in vitro, demonstrating a potential role for peritoneal B cells in host defense against C. burnetii infection in vivo. In addition, the results showing that B1a cells secreted a high level of interleukin-10 (IL-10) in response to C. burnetii infection in vitro suggest that B1a cells may play an important role in inhibiting the C. burnetii infection-induced inflammatory response. The observation that adoptive transfer of peritoneal B cells did not significantly affect the severity of C. burnetii infection-induced diseases in both severe combined immunity-deficient (SCID) and μMT mice indicates that peritoneal B cells alone may not be able to control C. burnetii infection. In contrast, our finding that C. burnetii infection induced more-severe splenomegaly and a higher bacterial burden in the spleens of B1a cell-deficient Bruton's tyrosine kinase x-linked immunity-deficient (BTKxid) mice than in their wild-type counterparts further suggests that B1a cells play an important role in host defense against primary C. burnetii infection.
INTRODUCTION
Coxiella burnetii is an obligate intracellular Gram-negative bacterium that causes acute and chronic Q fever in humans (1). Acute infections result in a self-limiting illness characterized by pneumonia, high fever, malaise, and headache. Chronic infection arises in about 5% of patients and often results in Q fever endocarditis, which requires 18 months to 3 years of antibiotic treatment to resolve. C. burnetii typically spreads by transmission of infected aerosols from ruminants to humans or through the consumption of unpasteurized milk (2–5). Such infections are considered an occupational hazard among livestock workers, veterinarians, and research laboratory personnel. A recent outbreak in the Netherlands from 2007 to 2010 resulted in more than 3,500 reported clinical Q fever cases (6), highlighting that this worldwide zoonotic pathogen remains a significant threat to public health. Although formalin-inactivated C. burnetii phase I vaccine (PIV) provides nearly complete protection in animal models as well as in human vaccinees, it can induce severe local and systemic adverse reactions when administered to individuals with prior immunity to the agent (7, 8). Due to these side effects, this vaccine is not licensed in the United States, and there is an urgent need to develop a safe and effective vaccine for the prevention of human Q fever. However, the mechanism of protective immunity to C. burnetii infection is not well studied. Understanding the mechanism of host immune responses to C. burnetii infection is a critical step toward developing a safe and effective vaccine against Q fever.
C. burnetii undergoes a lipopolysaccharide (LPS) phase variation in which its virulent smooth LPS phase, phase I (PI) (virulent), converts to an avirulent rough LPS phase, phase II (PII) (avirulent), upon serial passage in eggs and tissue cultures (9, 10). PI is able to replicate in wild-type animals and cause disease in humans, while PII can be rapidly cleared in animals and does not cause disease in humans (11, 12). It has been shown that C. burnetii can proliferate within a large replication vacuole in an acidic environment with a low rate of intracellular multiplication (1, 13, 14). Although C. burnetii can infect a wide range of host cells during infection in humans and animals (15–17), it remains unknown whether virulent C. burnetii can infect B cells and replicate inside the infected B cells.
Both humoral and cell-mediated immune responses are considered to be important for host defense against C. burnetii infection. An earlier study by Humphres and Hinrichs found that treatment of athymic mice with immune sera 24 h before challenge with C. burnetii had no effect on bacterial multiplication within the spleens of the T-cell-deficient animals (18), suggesting that T-cell-mediated immunity plays a critical role in the elimination of C. burnetii. A recent study by Andoh et al. (19) demonstrated that C. burnetii can induce a lethal infection in T cell- or IFN-γ-deficient mice. In addition, Read et al. also showed that CD4+ and CD8+ T cells are crucial for clearance of C. burnetii following primary infection (20). These studies suggest that T cell-mediated immunity may be the primary protective mechanism against C. burnetii infection. However, two recent studies (21, 22) demonstrated that antibodies (Abs) play an important role in vaccine-induced protective immunity to C. burnetii infection. Interestingly, our recent study (23) demonstrated that PIV-vaccinated B cell-deficient mice were unable to control C. burnetii replication and the inflammatory response to C. burnetii challenge in the spleen, suggesting that B cells may play an important role in the clearance of C. burnetii and in regulating inflammatory responses. In addition, a previous study by Andoh et al. (19) also found that B cell deficiency in mice increased the severity of histopathological changes during C. burnetii infection. These findings suggest that B cells may play an important role in controlling bacterial replication and regulating inflammatory responses to C. burnetii infection. Further understanding of the mechanisms of B cell-mediated protective immunity to C. burnetii infection may provide novel information for designing vaccines and immunotherapeutic strategies against Q fever.
B cells, with two main populations, referred to as B-1 and B-2 cells, are the major effectors of humoral immunity (24). B-1 cells are considered to be the major cell population responsible for the production of T cell-independent type 1, T cell-independent type 2, and natural Abs, whereas B-2 cells require T cell help for antigen-specific proliferation and Ab production. B-2 cells predominate in secondary lymphoid tissue, while B-1 cells are most common in the peritoneal and pleural cavities and are self-replenishing. Based on the differential levels of expression of CD5, B-1 cells can be further subdivided into B-1a (CD5+) and B-1b (CD5−) cells. B-1a and B-1b cells are complementary, with distinct functions; B-1a cells are required for production of natural Abs, whereas B-1b cells are involved in adaptive immune responses to certain bacterial infections (25). Interestingly, a recent study (26) demonstrated that peritoneal B-1 cells have phagocytic and microbicidal capacities. Additionally, Horikawa et al. demonstrated that B10 cells play a critical role in the immune response to Listeria monocytogenes by suppressing both innate and adaptive immune responses, allowing the bacteria to thrive (27). In contrast, these cells help to dampen potentially harmful responses during infection with Schistosoma mansoni (28) and Brugia pahangi (29). Thus, new roles for B cells in host immune responses to microbial infections have been described. However, it remains unclear what roles B cells play in host defense against C. burnetii infection.
In this study, we investigated the role of B cells in host defense against C. burnetii infection using both in vitro and in vivo assays. The results demonstrated that B1a cells play an important role in clearance of C. burnetii and in regulating inflammatory responses to C. burnetii infection. This is the first evidence to support the idea that B1a cells play an important role in the host defense against C. burnetii infection.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free, 6-to-8-week-old female C57BL/6, μMT, BALB/c, CBA, Bruton's tyrosine kinase x-linked immunity-deficient (BTKxid), and Cbysmm.CB17-prkdcSCID/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in sterile microisolator cages in a conventional animal facility or in an animal biosafety level 3 (ABSL3) facility at the University of Missouri Laboratory for Infectious Disease Research (MU-LIDR). Animals were provided food and water ad libitum. BTKxid, Cbysmm.CB17-prkdcSCID/J, and μMT mice were provided with autoclaved food and water. All research involving animals was conducted in accordance with the Animal Care and Use guidelines, and all animal use protocols were approved by the Animal Care and Use Committee at the University of Missouri. All C. burnetii Nine Mile phase I (NMI) infection experiments were conducted in the ABSL3 facility at MU-LIDR. For in vivo experiments, each group consisted of four mice.
Bacteria.
C. burnetii Nine Mile phase I (NMI) clone 7 (RSA493) bacteria were propagated in L929 cell lines and purified by density gradient centrifugation as previously described (30). NMI bacteria were handled under biosafety level 3 (BSL3) conditions at the MU-LIDR.
Peritoneal cell isolation.
Peritoneal cells were isolated from 6-to-8-week-old female BALB/c mice using peritoneal lavage as described previously (31). Briefly, mice were injected intraperitoneally (i.p.) with 10 ml of RPMI 1640 supplemented with 12 mM HEPES and 10% fetal bovine serum (FBS). The peritoneal cavity was massaged vigorously for 30 s to dislodge cells. Cells were then harvested using a fresh 25-gauge needle (BD Biosciences). Purification of total B cells was performed using MACS CD19 microbeads and LS columns according to specifications of the manufacturer (Miltenyi Biotec Inc., CA). B1a B cells were purified using a B1a B cell isolation kit with LS and MS columns according to the specifications of the manufacturer (Miltenyi). Levels of purity were confirmed using flow cytometry performed with a Beckman Coulter CyAN ADP analyzer and analyzed using Summit V5.3 software. The levels of purity of purified B cells and B1a cells were >95% and >90%, respectively. Cells (5 × 105) were plated in RPMI 1640–l-glutamine–10% FBS–12 mM HEPES before experiments were performed.
Indirect immunofluorescence assay (IFA).
Peritoneal cells were isolated as described above and allowed to adhere to poly-d-lysine-coated coverslips (Neuvitro) for 15 min at room temperature prior to infection. At 24 h following infection, the supernatant was removed and the cells were washed twice with RPMI 1640–10% FBS to remove extracellular bacteria. At 1 and 3 days postinfection, cells were blocked with mouse BD Fc Block (BD Biosciences) for 15 min at 4°C and stained with CD19–R-phycoerythrin (CD19-RPE) (eBiosciences) for 1 h at 4°C. Following staining, cells were fixed and permeabilized using a BD Cytofix/Cytoperm Plus kit (BD Biosciences) according to manufacturer specifications. Cells were stained intracellularly with C. burnetii-immunized rabbit serum followed by goat anti-rabbit IgG (Southern Biotech) for 1 h at 4°C. Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) for 5 min at 4°C. Coverslips were mounted in Prolong gold antifade reagent (Invitrogen) and allowed to cure overnight at room temperature. The edges of the slides were sealed using nail polish, and the slides were stored at 4°C. Microscopic analysis was performed using an Olympus IX70 inverted microscope.
Confocal microscopy.
A total of 5 × 105 peritoneal B cells were infected with NMI at a multiplicity of infection (MOI) of 100. At 24 h following infection, the supernatant was removed and the cells were washed twice with RPMI 1640–10% FBS to remove extracellular bacteria. At 1 and 3 days postinfection, cells were fixed with 2% paraformaldehyde at room temperature for 15 min and then permeabilized with −20°C methanol at 4°C for 10 min. Blocking was performed with 5% normal goat serum for 1 h at room temperature. Cells were stained with NMI-immunized rabbit serum for 1 h at room temperature and then either with CD19-Alexa Fluor 647, CD107a-phycoerythrin (CD107a-PE), and goat anti-rabbit IgG-fluorescein isothiocyanate (FITC) for 1 h at room temperature or with CD5-Alexa Fluor 647, CD19-PE, and goat anti-rabbit IgG-FITC for 1 h at room temperature. Finally, cells were stained with DAPI (Invitrogen) for 5 min at room temperature. Coverslips were mounted in Prolong gold antifade reagent (Invitrogen) and allowed to cure overnight at room temperature. The edges of the slides were sealed using nail polish, and the slides were stored at 4°C. Microscopic analysis was performed using a Leica TCP SP8 MP inverted spectral confocal microscope.
Flow cytometry.
Cells were blocked with mouse BD Fc Block (BD Biosciences) for 15 min at 4°C and then stained with CD19 (eBioscience), CD5, and CD11b (BD Biosciences) for 40 min at 4°C in phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA), 2 mM EDTA, and 0.1% sodium azide. Cells were then fixed with 2% paraformaldehyde–PBS for 15 min at 4°C. Cells were analyzed using a Beckman Coulter Cyan ADP analyzer, and data were analyzed using Summit V5.3 software.
Adoptive transfer.
Peritoneal B cells were isolated and purified from C57BL/6 or BALB/c mice as described above. Splenic B cells were also purified from C57BL/6 mice. Briefly, spleens were removed and homogenized using a glass tissue homogenizer. The cell suspension was passed through a 70-μM-pore-size nylon mesh, and red blood cells were lysed using ACK lysis buffer for 5 min at room temperature. B cells were purified from the suspension as described above using CD19 microbeads (Miltenyi). Purified cells were injected i.p. at 1 × 106 into μMT or severe combined immunity-deficient (SCID) mice. Control mice were injected i.p. with sterile PBS. At 24 h following the transfers, mice were challenged i.p. with 1 × 107 NMI bacteria. Weights were measured throughout infection and compared as a ratio to the body weight at day 0. Mice were sacrificed at the desired time points, and spleens and livers were removed. Serum was also collected for cytokine and antibody production. Splenomegaly was calculated and bacterial burden in the spleen was determined using real-time quantitative PCR (qPCR) as described below.
ELISA.
Cell supernatants from NMI-infected B cells were analyzed for interleukin-10 (IL-10), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) using a mouse IL-10 enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go! second-generation kit, a mouse TNF-α ELISA Ready-SET-Go! kit, and a mouse IFN-gamma ELISA Ready-SET-Go! kit (eBioscience) according to manufacturer specifications. ELISAs for detection of C. burnetii-specific antibodies were performed as described previously (32). Briefly, plates were coated with 100 μl of inactivated NMI antigen at 0.5 mg/ml in 0.05 M carbonate/bicarbonate coating buffer (pH 9.6) for 48 h at 4°C. For the standard curve, wells were coated with unconjugated goat anti-mouse IgG or IgM for 24 h at 4°C. Plates were blocked with 1% BSA–PBS-T buffer (0.05% Tween 20–PBS) and then incubated either with 100 μl of diluted sample serum or with purified mouse IgM or IgG for 2 h at 37°C. Plates were washed five times with PBS-T buffer and then incubated with 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgM or IgG at 37°C for 1 h. Plates were again washed five times with PBS-T buffer, and then a Sigma Fast O-phenylenediamine dihydrochloride tablet set (Sigma) was used as a substrate. The absorbance was measured at 492 nm using Tecan F50 and Magellan software.
Real-time PCR.
At 24 h following infection, the supernatant was removed and the cells were washed twice with RPMI 1640–10% FBS to remove extracellular bacteria. NMI-infected B cells were scraped and lysed with 200 μl lysis buffer (1 M Tris, 0.5 M EDTA, 7 mg/ml glucose, 28 mg/ml lysozyme) and 10 μl proteinase K (20 mg/ml) overnight at 60°C. Spleen tissue was lysed with 200 μl lysis buffer (1 M Tris, 0.5 M EDTA, 7 mg/ml glucose, 28 mg/ml lysozyme) and 10 μl proteinase K (20 mg/ml) and incubated for 18 h at 60°C. Following the addition of 21 μl 10% sodium dodecyl sulfate (SDS), samples were incubated at room temperature for 1 h. C. burnetii genomic DNA was extracted from NMI-infected B cells and NMI-infected mouse spleen tissue using a High Pure PCR template preparation kit (Roche) according to manufacturer specifications. The com1 gene copy number was quantified using a standard curve with SYBR green (Applied Biosciences) on an Applied Biosystems 7300 real-time PCR system. Recombinant plasmid DNA (com1 gene ligated into PET23a vector) was used as a standard to quantify com1 gene copy numbers.
Histopathology.
Spleens were collected from mice at different time points postinfection with C. burnetii, fixed in 10% formalin–PBS for at least 72 h, prepared as 5-μm-thick paraffin-embedded sections by standard methods, and sliced. Slides were stained with hematoxylin and eosin and examined in a blind fashion for evaluation of histopathology by a trained pathologist.
Statistical analysis.
Statistical analysis was performed using Prism 5.0 (Graphpad Software Inc., San Diego, CA). Results were compared using a two-sample Student's t test. Differences were considered significant if the P value was ≤0.05.
RESULTS
C. burnetii NMI can infect peritoneal B cells.
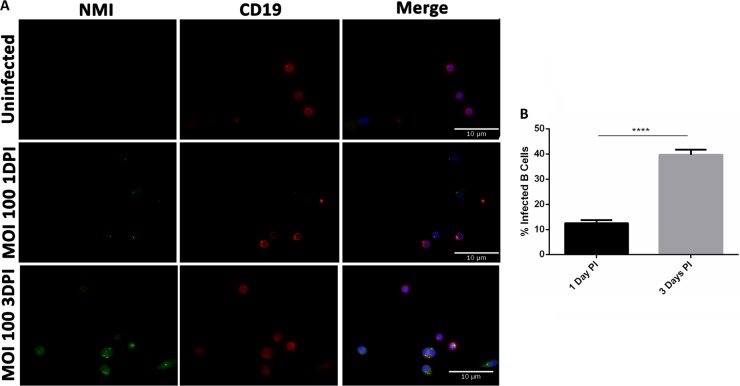
IFA was used to determine whether C. burnetii can infect peritoneal B cells. Purified peritoneal B cells were infected with NMI at an MOI of 100 for 1 and 3 days and stained with anti-C. burnetii and anti-CD19 antibodies. As shown in Fig. 1A, colocalization of B cell marker CD19 and C. burnetii was observed in NMI-infected peritoneal B cells at 1 and 3 days postinfection. The numbers of C. burnetii per cell appeared to be different between 1 and 3 days postinfection, with more C. burnetii observed in each infected cell at 3 days postinfection. In addition, compared to NMI-infected B cells at 1 day postinfection, numbers of C. burnetii-infected B cells were significantly increased at 3 days postinfection. Approximately 12.5% of the B cells were infected at 1 day postinfection, while 39.6% of the B cells were infected at 3 days postinfection (Fig. 1B). These results indicate that virulent C. burnetii can infect peritoneal B cells. The observation that the infection rate was significantly increased at 3 days postinfection suggests that C. burnetii was able to replicate in the infected B cells.
FIG 1.
C. burnetii NMI is taken up by CD19+ peritoneal B cells. Peritoneal cells were infected at an MOI of 100 with NMI. At 1 day and 3 days postinfection, cells were stained extracellularly with CD19, fixed, permeabilized with saponin, and then stained intracellularly with rabbit anti-NMI IgG. The NMI IgG was stained with goat anti-rabbit IgG-FITC, and the nuclei were stained with DAPI. DPI, days postinfection. (A) Microscopic analysis shows NMI in CD19+ cells. (B) Infection rates in B cells were 10% to 15% at 1 day postinfection and 30% to 40% at 3 days postinfection (PI). These data indicate that NMI is taken up by CD19+ B cells. ****, P < 0.0001.
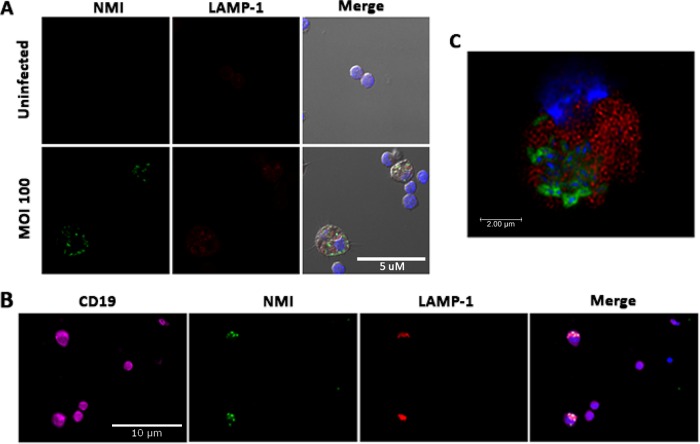
Peritoneal B cells can take up NMI bacteria into LAMP-1-positive vacuoles.
It has been shown that NMI can proliferate within Coxiella-containing vacuoles (CCVs) of infected cells and that CCVs can be visualized by staining the lysosome-associated membrane protein 1 (LAMP-1) lysosomal marker (1, 13, 14). To determine whether peritoneal B cells can take up C. burnetii and form CCVs for C. burnetii replication, NMI-infected peritoneal B cells were intracellularly stained with anti-C. burnetii and anti-LAMP-1 antibodies and observed by confocal microscopy. As shown in Fig. 2A, colocalization between intracellular C. burnetii and LAMP-1 was observed in NMI-infected B cells at 1 day postinfection. Figure 2B shows infected LAMP-1-positive vacuoles within CD19-positive cells, confirming that the NMI-infected cells were B cells. Figure 2C represents an image of a deconvoluted slice of an infected B cell taken by confocal microscopy. This image is shown 2 μm into the cell, where NMI (green) is visible in a CD19-positive cell (red). These data further indicate that C. burnetii resides within a vacuole in the NMI-infected B cells. This observation indicates that peritoneal B cells are able to take up C. burnetii and form CCVs for C. burnetii replication.
FIG 2.
C. burnetii NMI is taken up into LAMP-1-positive vacuoles in purified peritoneal B cells. Peritoneal B cells were purified using MACS and infected at an MOI of 100 with NMI. Cells were then fixed and stained with anti-C. burnetii IgG-FITC and LAMP-1–PE. (A) At 1 day postinfection, NMI bacteria were visible inside LAMP-1-positive vacuoles in B cells. (B) Purified peritoneal B cells were stained with CD19-Alexa Fluor 647, LAMP-1–PE, anti-C. burnetii IgG-FITC, and DAPI. (C) Cells for confocal microscopy were stained with CD19-PE and anti-C. burnetii IgG-FITC. A deconvolved image of a CD19 (red)-positive B cell containing NMI (green) is shown. This image is of a slice taken 2 μm into the cell. These data indicate that peritoneal B cells take up NMI into LAMP-1-positive vacuoles.
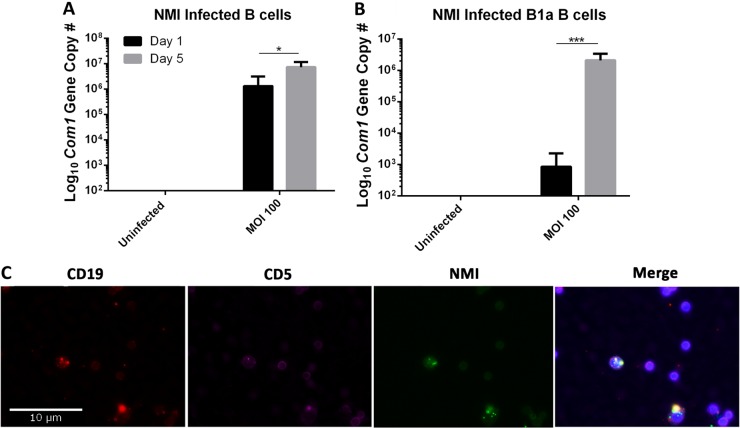
C. burnetii can replicate in peritoneal B cells and B1a subset cells.
To determine whether C. burnetii can replicate within B cells, real-time quantitative PCR was used to measure the numbers of genomic copies of C. burnetii in NMI-infected peritoneal B cells at 1 and 5 days postinfection. As shown in Fig. 3A, compared to NMI-infected B cells at 1 day postinfection, the numbers of genomic copies of C. burnetii were significantly increased in NMI-infected B cells at 5 days postinfection, suggesting that C. burnetii was able to replicate within peritoneal B cells. It has been shown that peritoneal B-1 cells have phagocytic and microbicidal capacities (33). To determine whether peritoneal B-1 cells can take up and kill C. burnetii, we examined if NMI bacteria can infect and replicate in peritoneal B1a subset B cells. Purified peritoneal B1a cells were infected with NMI bacteria at an MOI of 100 and cultured for 1 or 5 days. Compared to NMI-infected B1a cells at 1 day postinfection, the numbers of genomic copies of C. burnetii were significantly increased in NMI-infected B1a cells at 5 days postinfection (Fig. 3B). In addition, to confirm whether C. burnetii can infect B1a cells, purified B1a cells were infected with NMI bacteria and stained with CD5, CD19, anti-NMI IgG, and DAPI. As shown in Fig. 3C, C. burnetii was visible within CD5 and CD19 double-stained cells, indicating that C. burnetii infects B1a B cells. These results indicate that C. burnetii is able to infect and replicate in peritoneal B1a subset B cells in vitro, suggesting that peritoneal B1a cells may play an important role in the host defense against C. burnetii infection.
FIG 3.
NMI replicates in peritoneal B cells and B cell subsets. Peritoneal B cells and B cell subsets were infected with NMI at an MOI of 100. At 1 and 5 days postinfection, cells were lysed and DNA was purified to enumerate C. burnetii com1 gene copy numbers. (A) Purified peritoneal B cells were infected with NMI at an MOI of 100. (B) Purified peritoneal B1a B cells were infected with NMI at an MOI of 100. (C) Purified peritoneal B1a cells were stained with CD5-Alexa Fluor 647, CD19-PE, anti-C. burnetii IgG-FITC, and DAPI at 3 days postinfection. *, P < 0.05; ***, P < 0.001. These data indicate that NMI is taken up by peritoneal B cells and B cell subsets and replicates in these cells.
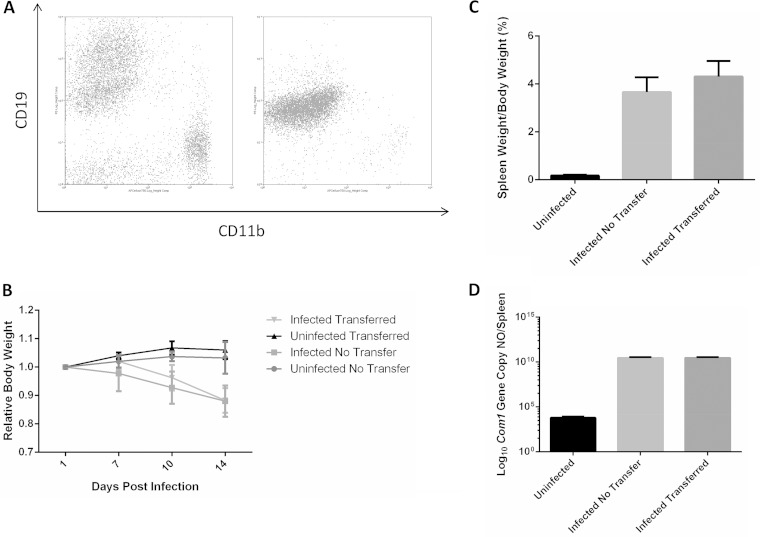
Adoptive transfer of peritoneal B cells does not impact disease progression in SCID mice.
The observation that peritoneal B cells can take up NMI bacteria in vitro suggests a potential role for peritoneal B cells in host defense against C. burnetii infection in vivo. To determine whether peritoneal B cells play an important role in host defense against C. burnetii infection in vivo, we examined whether adoptive transfer of peritoneal B cells to recipient SCID mice would provide significant protection against C. burnetii infection. Peritoneal B cells were purified from wild-type BALB/c mice. The purity of peritoneal B cells was confirmed by flow cytometry (Fig. 4A), and B cells were used at >95% purity for transfer to naive SCID mice by i.p. injection 24 h prior to infection with NMI bacteria. The severity of the disease induced by the C. burnetii infection in peritoneal B cell recipient and control mice was evaluated by comparing levels of body weight loss, splenomegaly, and bacterial burden in the spleen. As shown in Fig. 4B, compared to uninfected mice, regardless of receiving peritoneal B cells, all NMI-infected mice started losing body weight at 7 days postinfection. In addition, there was no difference in the levels of splenomegaly and bacterial burden in the spleen between peritoneal B cell-recipient and control mice at 14 days postinfection with NMI bacteria (Fig. 4C and D). These results indicate that peritoneal B cells alone are not sufficient to protect SCID mice against C. burnetii infection, suggesting that B cells may not play an essential function in directly controlling C. burnetii replication.
FIG 4.
B cell adoptive transfer does not improve disease during infection with NMI in SCID mice. (A) Purity of peritoneal B cells before and after MACS separation. B cell purity was >95% when measured by flow cytometry following purification. (B) Relative body weight as a ratio versus day 0. At 14 days postinfection, mice were subjected to necropsy. (C) Splenomegaly in mice that received adoptively transferred B cells versus mice that were not adoptively transferred. (D) Bacterial burden in the spleens of infected animals. These data indicate that B cells alone do not improve disease in SCID mice.
Adoptive transfer of B cells protects μMT mice from weight loss and reduces serum IFN-γ levels during NMI infection.
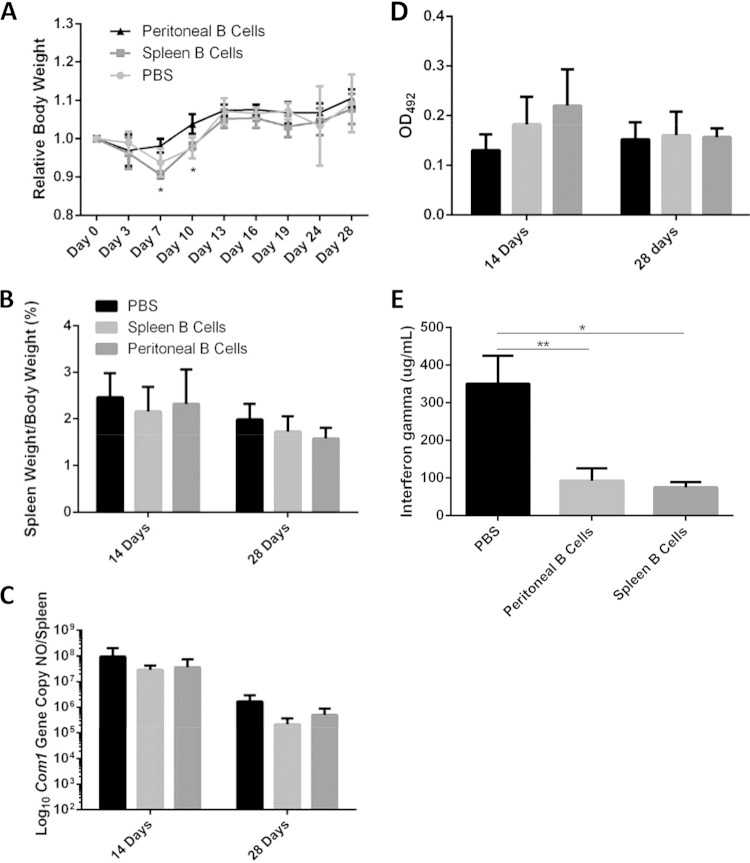
The observation that adoptive transfer of peritoneal B cells alone did not affect the severity of C. burnetii-induced diseases in mice lacking both functional T cells and functional B cells suggests that T cells may be required for peritoneal B cell-mediated protection against C. burnetii infection. To test this hypothesis and further determine the role of B cells in host defense against C. burnetii primary infection, we examined if adoptive transfer of peritoneal or splenic B cells to B cell-deficient μMT mice would significantly affect their ability to control C. burnetii infection. B cell-deficient μMT mice lack all subsets of B cells, but their T cell populations remain intact. As shown in Fig. 5A, peritoneal B cell recipient mice had significantly higher body weights on day 7 and day 10 than splenic B cell or PBS recipient mice. This result suggests that peritoneal B cells may be involved in protecting against C. burnetii-induced clinical disease at early stages of infection. However, the levels of splenomegaly and bacterial burden in peritoneal or splenic B cell recipient mice and control mice were similar at 14 and 28 days postinfection (Fig. 5B and C). These results suggest that peritoneal and splenic B cells may not play a major role in controlling C. burnetii infection. Additionally, we also examined whether the antibody responses of peritoneal or splenic B cell recipient mice and control mice to C. burnetii infection are different. The results indicated that anti-C. burnetii IgG levels were not significantly different between peritoneal or splenic B cell recipient mice and control mice (Fig. 5D). Interestingly, serum levels of the IFN-γ proinflammatory cytokine were significantly lower in both peritoneal and splenic B cell recipient mice (Fig. 5E). This finding suggests that both peritoneal and splenic B cells may play an important role in regulating T cell-mediated immunity during C. burnetii infection. Collectively, these data suggest that although B cells may not play a major role in directly controlling C. burnetii replication, they may play an important role in regulating the host inflammatory response to C. burnetii infection.
FIG 5.
Adoptive transfer of peritoneal B cells protects μMT mice from weight loss during infection with NMI and reduces serum IFN-γ levels. μMT mice were subjected to adoptive i.p. transfer of 1 × 106 purified splenic or peritoneal B cells. PBS was injected as a control. At 24 h following transfer, mice were challenged i.p. with 1 × 107 NMI cells. (A) Body weight was measured throughout the course of infection. (B) Splenomegaly was measured at 14 and 28 days postinfection. (C) Bacterial burden was measured at 14 and 28 days postinfection. (D) C. burnetii-specific IgG responses were measured at day 14 and day 28. OD492, optical density at 492 nm. (E) Serum IFN-γ levels were measured using ELISA at day 14 postinfection. *, P < 0.05; **, P < 0.01. These data indicate that μMT mice that were subjected to adoptive transfer of peritoneal B cells were protected from weight loss during early infection and that adoptive transfer of B cells reduces circulating IFN-γ levels in NMI-infected mice. This indicates a role for B cells in the early infection response and in controlling inflammation levels during infection.
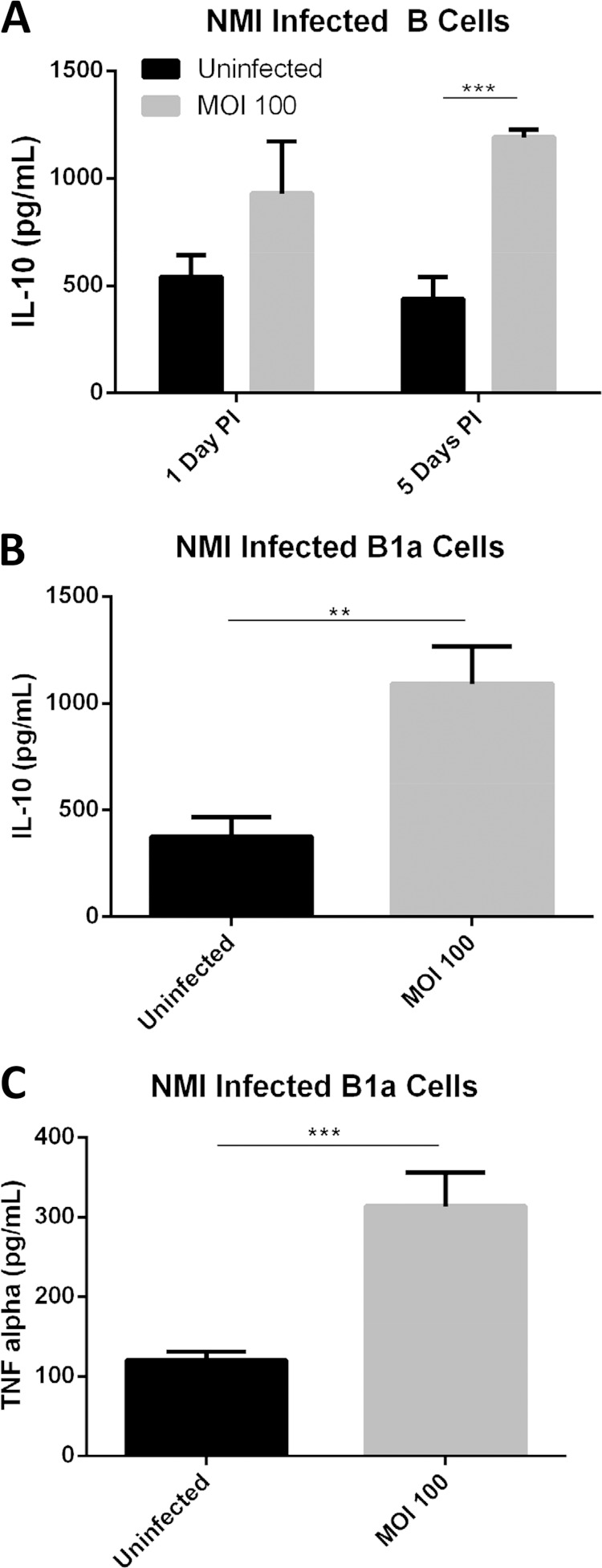
C. burnetii-infected B1a cells can produce both pro- and anti-inflammatory cytokines in vitro.
To determine whether B cells can regulate host inflammatory responses during C. burnetii infection, we examined if NMI-infected B cells can produce pro- and anti-inflammatory cytokines in vitro. As shown in Fig. 6A, compared to uninfected control cell results, a high level of IL-10 was detected in C. burnetii-infected peritoneal B cells at both 1 day and 5 days postinfection, suggesting that peritoneal B cells can produce anti-inflammatory cytokines during C. burnetii infection in vitro. To further determine whether B1a B cells are responsible for producing pro- and/or anti-inflammatory cytokines, we measured the concentrations of IL-10 and TNF-α in the culture supernatant from C. burnetii-infected B1a cells at 1 day postinfection. Interestingly, compared to uninfected control cells, high levels of IL-10 and TNF-α were detected in C. burnetii-infected B1a cells (Fig. 6B and C). These results indicate that B1a cells are responsible for production of pro- and anti-inflammatory cytokines in response to C. burnetii infection in vitro and suggest that B1a cells may play an important role in regulating the host inflammatory response to C. burnetii infection in vivo.
FIG 6.
Infection with C. burnetii NMI induces production of IL-10 and TNF-α by peritoneal B cells. (A) Purified peritoneal B cells were infected with NMI. At 1 day and 5 days postinfection, the supernatant was collected and IL-10 levels were measured by ELISA. (B) Purified peritoneal B1a B cells were infected with NMI. At 1 day postinfection, the supernatant was collected. IL-10 production was measured by ELISA in NMI-infected cells. (C) Purified peritoneal B1a B cells were also measured for TNF-α production after NMI infection for 24 h. **, P < 0.01; ***, P < 0.001. These data indicate that peritoneal B cells and B1a B cells can produce anti-inflammatory and proinflammatory cytokines when exposed to NMI.
B1a cells play an important role in host defense against C. burnetii infection.
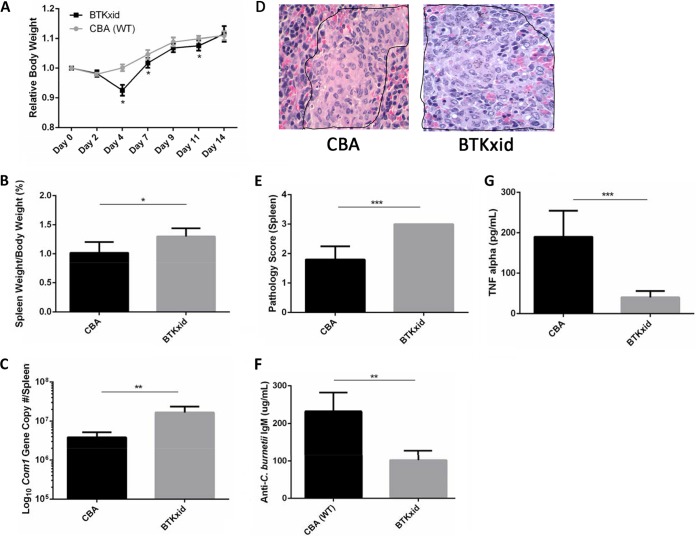
To further understand the role of B1a cells in host defense against C. burnetii infection in vivo, we investigated if B1a cell deficiency in mice would significantly affect their susceptibility to C. burnetii infection. Previous studies demonstrated that Bruton's tyrosine kinase X-linked immunity-deficient (BTKxid) mice are deficient in B1a cells (34, 35). The severity of C. burnetii infection in BTKxid and wild-type (CBA) mice was evaluated by comparing levels of body weight loss, splenomegaly, and bacterial burden and pathological changes in the spleen at 14 days postinfection with NMI bacteria. Compared to CBA mice, BTKxid mice had significant body weight loss at 4, 7, and 11 days postinfection (Fig. 7A), suggesting that B1a cells may play an important role in protecting the host from developing clinical disease at the early stage of the infection. The results also indicate that the levels of splenomegaly and bacterial burden in the spleen were significantly different between CBA and BTKxid mice (Fig. 7B and C), suggesting that B1a cells may be involved in protecting the host from development of severe disease and controlling C. burnetii replication in vivo. In addition, to determine whether B1a cells can regulate the host inflammatory response to C. burnetii infection in vivo, we also compared the histopathological changes in the spleens and TNF-α concentrations in the sera between CBA and BTKxid mice at 14 days postinfection. As shown in Fig. 7D, large numbers of moderate to large accumulations of macrophages (encircled by black border) were present in red pulp of spleens from BTKxid mice, while a few small to moderate accumulations of macrophages (encircled by black border) were observed in red pulp of spleens from CBA mice. The inflammatory scores were significantly different between CBA and BTKxid mice. BTKxid mice had spleen inflammation that was more severe than that seen with CBA mice at 14 days postinfection (Fig. 7E). In addition, compared to that in the sera from CBA mice, a significantly lower level of TNF-α was detected in the sera from BTKxid mice (Fig. 7F). These results indicate that B1a cells play an important role in regulating the host inflammatory response to C. burnetii infection. Furthermore, we also examined if the antibody responses of CBA and BTKxid mice to C. burnetii infection are different. Interestingly, a significantly lower concentration of anti-C. burnetii IgM was detected in the sera from BTKxid mice than in the sera from CBA mice at 14 days postinfection (Fig. 7F), suggesting that B1a cells may play an important role in antibody responses to C. burnetii infection. Collectively, these observations support the idea that B1a cells play an important role in host defense against C. burnetii infection.
FIG 7.
B1a B cell-deficient Bruton's tyrosine kinase X-linked immunity-deficient mice have disease during NMI infection that is more severe than that seen with wild-type mice. Wild-type (CBA) mice and Bruton's tyrosine kinase X-linked immunity-deficient (BTKxid) mice were challenged i.p. with 1 × 107 NMI cells. (A) Body weight was measured throughout infection and compared to the weight at day 0. (B) Splenomegaly was measured at 14 days postinfection. (C) Bacterial burden in the spleen was measured at 14 days postinfection by real-time PCR. (D) Histopathology in the spleen shows that inflammation was more severe in BTKxid mice than in wild-type CBA mice. (E) Pathology scores from spleens demonstrate more inflammatory infiltrates in BTKxid spleens than in wild-type CBA mouse spleens. One section of spleen was evaluated for each mouse. Spleens for groups were scored for histiocytic inflammation in red pulp of spleen using the following scale: 0, no inflammation (no accumulations of macrophages); 1, a few small accumulations of macrophages; 2, a few small-to-moderate accumulations of macrophages; 3, large numbers of moderate-to-large accumulations of macrophages. (F) Anti-C. burnetii-specific IgM titers were significantly higher in wild-type CBA mice than in BTKxid mice. (G) Serum levels of TNF-α were significantly higher in wild-type CBA mice than in BTKxid mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001. These data indicate that B1a B cell-deficient BTKxid mice have disease during NMI infection that is more severe than that seen with CBA mice. B1a B cells may play a role in regulating immune responses and producing IgM during primary infection with NMI.
DISCUSSION
Despite the obligate intracellular lifestyle of C. burnetii, accumulated evidence demonstrates that Ab-mediated humoral immunity is crucial for vaccine-induced protective immunity to C. burnetii infection (22, 23, 36). However, due to T cell-mediated immunity being considered the primary mechanism of protection against C. burnetii infection, there is a fundamental gap in knowledge regarding the mechanisms of B cell-mediated immunity to C. burnetii infection. To fill this knowledge gap, we examined if B cells play roles beyond production of Abs in host defense against C. burnetii infection. The observation that B1a cells were able to secrete both pro- and anti-inflammatory cytokines in response to C. burnetii infection in vitro suggests that B1a cells play an important role in regulating the C. burnetii infection-induced inflammatory response. In addition, the findings that C. burnetii infection induced splenomegaly that was more severe and bacterial burdens that were higher in the spleens of B1a cell-deficient mice than in the spleens of wild-type mice suggest that B1a cells are involved in protecting the host from development of severe disease and controlling C. burnetii replication. Thus, B cells may play an important role in clearance of C. burnetii and in regulating C. burnetii infection-induced inflammatory responses.
Unique roles for B cells, such as roles in antigen presentation, cytokine production, phagocytosis and killing of pathogens, and killing of effector cells by Fas/FasL interaction, in the immune response have been described (26, 33, 37–41). Peritoneal B cell subset B1a cells have previously been demonstrated to be able to phagocytose both particles and bacteria (33). Popi et al. previously showed that a mononuclear cell derived from peritoneal B1 cells was able to phagocytose avirulent C. burnetii NMII and form CCVs (41). In addition, these B1 cell-derived monocytes were more permissive of NMII infection and replication than bone marrow-derived macrophages. However, it remains unknown whether peritoneal B cells can phagocytose virulent C. burnetii NMI and form CCVs for NMI replication. Our results demonstrated that peritoneal B cells were able to take up NMI and form CCVs for C. burnetii replication and that NMI can infect and replicate in peritoneal B1a subset B cells in vitro. It is also noted that the real-time PCR results showed a 2-fold increase in com1 gene copy numbers in whole peritoneal B cells, but a severalfold replication rate was noted in B1a subset B cells. Since B1a B cells make up only a small (20% to 30%) fraction of the total peritoneal B cell compartment following purification, the remaining B cells are mostly B2 cells, which are not inherently phagocytic. This is the likely explanation for the fact that the real-time PCR results show several logs of com1 gene copy replication in B1a B cells but only a 2 log difference in the total population of peritoneal B cells. These results suggest a potential role for peritoneal B1 cells in the host immune response to C. burnetii infection in vivo. To determine what role peritoneal B cells play against C. burnetii infection in vivo, we examined if adoptive transfer of peritoneal B cells would significantly affect the severity of C. burnetii infection-induced disease in SCID mice. The observation that NMI infection induced similar levels of severe splenomegaly and high bacterial burdens in the spleen in peritoneal B cell recipient and control SCID mice indicates that peritoneal B cells alone are not sufficient to control C. burnetii infection in mice lacking functional B and T cells. This result suggests that peritoneal B cells may play a substantial role in the presence of other B and T cells. To test this hypothesis, we also examined if adoptive transfer of peritoneal B cells to B cell-deficient μMT mice would significantly affect their ability to control C. burnetii infection. The results indicated that, although peritoneal B cells can provide protection from C. burnetii infection-induced body weight loss to μMT mice at an early stage of infection, the levels of splenomegaly and bacterial burden in the spleen were similar between peritoneal B cell recipient and control mice. These data suggest that peritoneal B cells may be involved in protection against C. burnetii infection-induced clinical disease at the early stage of infection but may not play a major role in controlling C. burnetii replication. However, it is also possible that the number of B cells transferred may not have been sufficient to show the effect of one cell subset, as B1a cells make up only a fraction of the peritoneal B cell compartment. The results of the adoptive-transfer experiments were unable to rule out a role of peritoneal B cell subsets in directly controlling C. burnetii replication.
A previous study by Andoh et al. (19) demonstrated that, although there was no difference in the levels of splenomegaly and bacterial burden in the spleen between μMT and wild-type control mice at 28 days postinfection with NMI bacteria, μMT mice developed inflammation that was more severe in hearts, lungs, livers, and spleens. These observations suggest that B cells play an important role in regulation of the host inflammatory response to protect against tissue damage during C. burnetii infection. However, there is no direct evidence to support this hypothesis, and it remains unknown which subset of B cells is responsible for the regulatory role of B cells during C. burnetii infection. Recently, cells composing a regulatory B cell subset, known as B10 cells, have demonstrated the ability to secrete large amounts of IL-10 and to suppress immune responses even with very few cells (33, 42). Sindhava et al. also found that B1 cells from the peritoneal cavity produce high levels of IL-10 and play a critical role in the immune response to Borrelia hermsii infection (43). To determine whether B cells can play a role in regulating the host immune response during C. burnetii infection, we examined if NMI-infected B cells can produce regulatory cytokines. Interestingly, our results indicate that both peritoneal B cells and B1a cells were able to secrete a high level of IL-10 in response to C. burnetii infection in vitro, suggesting that B1a cells may play an important role in inhibiting the host inflammatory response to C. burnetii infection. In addition, the observation that the concentration of IFN-γ in sera from peritoneal B cell recipient μMT mice was significantly lower than the concentration in sera from PBS recipient control mice at 14 days postinfection with NMI bacteria suggests that peritoneal B cells may play an important role in regulating T cell-mediated immunity during C. burnetii infection. Overall, these data suggest that peritoneal B cells play an important role in regulating the host immune response to C. burnetii infection.
It has been shown that B cells can play both helpful and harmful roles during bacterial pathogen infections (44, 45). Crane et al. demonstrated that B1a cells enhance disease susceptibility in mice infected with Francisella tularensis by producing IL-10 and suppressing NK/NKT cell function (44). However, Culkin et al. found that total B cell deficiency had a negative impact on the immune response to F. tularensis (45). Thus, multiple roles for B cells during infection with bacterial pathogens may be attributed to the diverse functions of B cell subsets. It has been shown that B cells can regulate immune functions by producing cytokines, acting as antigen-presenting cells (APCs), producing antibodies, and potentially killing phagocytosed bacteria and other immune cells (26, 33, 37–41). Interestingly, our results demonstrated that virulent C. burnetii induced disease in B1a cell-deficient mice that was more severe than that seen in their wild-type counterparts, suggesting that B1a cells play an important role in controlling C. burnetii infection. Although the mechanisms of B1a cell-mediated protection against primary C. burnetii infection remain undefined, the result showing that the bacterial burden in the spleen from B1a cell-deficient mice was significantly higher than that in the spleen from wild-type mice suggests that B1a cells may play an important role in controlling C. burnetii replication in vivo. In addition, the observations that, compared to wild-type mice, B1a cell-deficient mice had inflammation that was more severe in the spleens and a significant lower level of TNF-α in serum at 14 days postinfection with NMI support the idea that B1a cells play an important role in regulating the host inflammatory response to C. burnetii infection. BTKxid mice also have a functional impairment in their ability to produce reactive nitrogen species and signal through the B cell receptor. We were unable to rule out the possibility that some of the observed differences are due to these defects. However, the finding that the concentration of anti-C. burnetii IgM in serum from B1a cell-deficient mice was significantly lower than the concentration in serum from their wild-type counterparts indicates that B1a cells are critical for production of T cell-independent antibodies in response to C. burnetii infection. Collectively, these results suggest that B1a cells play an important role in host defense against C. burnetii infection through their ability to secrete regulatory cytokines, produce protective antibody, and phagocytose C. burnetii.
ACKNOWLEDGMENTS
This study was funded by NIH public service grant RO1AI083364 from the National Institute of Allergy and Infectious Diseases and a contract from the Defense Threat Reduction Agency (DTRA).
We thank the staff at the MU Laboratory for Infectious Disease Research for their assistance with these experiments. We also thank Aleksandr Jurkevic and the MU Molecular Cytology Core for their assistance with confocal microscopy.
REFERENCES
- 1.Maurin M, Raoult D. 1999. Q fever. Clin Microbiol Rev 12:518–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tissot-Dupont H, Amadei M, Nezri M, Raoult D. 2004. Wind in November, Q fever in December. Emerg Infect Dis 10:1264–1269. doi: 10.3201/eid1007.030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij MM, Schimmer B, Versteeg B, Schneeberger P, Berends BR, Heederik D, van der Hoek W, Wouters IM. 2012. Risk factors of Coxiella burnetii (Q fever) seropositivity in veterinary medicine students. PLoS One 7:e32108. doi: 10.1371/journal.pone.0032108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuiston JH, Holman RC, McCall CL, Childs JE, Swerdlow DL, Thompson HA. 2006. National surveillance and the epidemiology of human Q fever in the United States, 1978–2004. Am J Trop Med Hyg 75:36–40. [DOI] [PubMed] [Google Scholar]
- 5.Walsh MG. 2012. Assessing Q fever in a representative sample from the United States population: identification of a potential occupational hazard. Epidemiol Infect 140:42–46. doi: 10.1017/S0950268811000227. [DOI] [PubMed] [Google Scholar]
- 6.Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, Wijkmans CJ, Vellema P, Schneeberger PM. 2012. The 2007–2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol 64:3–12. doi: 10.1111/j.1574-695X.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 7.Ormsbee RA, Bell EJ, Lackman DB, Tallent G. 1964. The influence of phase on the protective potentcy of Q fever vaccine. J Immunol 92:404–412. [PubMed] [Google Scholar]
- 8.Marmion BP, Ormsbee RA, Kyrkou M, Wright J, Worswick DA, Izzo AA, Esterman A, Feery B, Shapiro RA. 1990. Vaccine prophylaxis of abattoir-associated Q fever: eight years' experience in Australian abattoirs. Epidemiol Infect 104:275–287. doi: 10.1017/S0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackstadt T, Peacock MG, Hitchcock PJ, Cole RL. 1985. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun 48:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoker MG, Fiset P. 1956. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol 2:310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]
- 11.Moos A, Hackstadt T. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun 55:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vishwanath S, Hackstadt T. 1988. Lipopolysaccharide phase variation determines the complement-mediated serum susceptibility of Coxiella burnetii. Infect Immun 56:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baca OG, Paretsky D. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol Rev 47:127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mege JL, Maurin M, Capo C, Raoult D. 1997. Coxiella burnetii: the ‘query’ fever bacterium. A model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev 19:209–217. [DOI] [PubMed] [Google Scholar]
- 15.Baca OG, Akporiaye ET, Aragon AS, Martinez IL, Robles MV, Warner NL. 1981. Fate of phase I and phase II Coxiella burnetii in several macrophage-like tumor cell lines. Infect Immun 33:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howe D, Melnicakova J, Barak I, Heinzen RA. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell Microbiol 5:469–480. doi: 10.1046/j.1462-5822.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 17.Maurin M, Lepidi H, La Scola B, Feuerstein M, Andre M, Pellissier JF, Raoult D. 1997. Guinea pig model for Staphylococcus aureus native valve endocarditis. Antimicrob Agents Chemother 41:1815–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphres RC, Hinrichs DJ. 1981. Role for antibody in Coxiella burnetii infection. Infect Immun 31:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andoh M, Zhang G, Russell-Lodrigue KE, Shive HR, Weeks BR, Samuel JE. 2007. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect Immun 75:3245–3255. doi: 10.1128/IAI.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read AJ, Erickson S, Harmsen AG. 2010. Role of CD4+ and CD8+ T cells in clearance of primary pulmonary infection with Coxiella burnetii. Infect Immun 78:3019–3026. doi: 10.1128/IAI.00101-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon JG, Cockrell DC, Takahashi K, Stahl GL, Heinzen RA. 2009. Antibody-mediated immunity to the obligate intracellular bacterial pathogen Coxiella burnetii is Fc receptor- and complement-independent. BMC Immunol 10:26. doi: 10.1186/1471-2172-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G, Russell-Lodrigue KE, Andoh M, Zhang Y, Hendrix LR, Samuel JE. 2007. Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice. J Immunol 179:8372–8380. doi: 10.4049/jimmunol.179.12.8372. [DOI] [PubMed] [Google Scholar]
- 23.Zhang G, Peng Y, Schoenlaub L, Elliott A, Mitchell W, Zhang Y. 2013. Formalin-inactivated Coxiella burnetii phase I vaccine-induced protection depends on B cells to produce protective IgM and IgG. Infect Immun 81:2112–2122. doi: 10.1128/IAI.00297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montecino-Rodriguez E, Dorshkind K. 2006. New perspectives in B-1 B cell development and function. Trends Immunol 27:428–433. doi: 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Haas KM, Poe JC, Steeber DA, Tedder TF. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, Barreda DR, Sunyer JO. 2012. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leuk Biol 91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. 2013. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol 190:1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. 2004. Helminth infection protects mice from anaphylaxis via IL-10-producing B Cells. J Immunol 173:6346–6356. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 29.Gillan V, Lawrence RA, Devaney E. 2005. B cells play a regulatory role in mice infected with the L3 of Brugia pahangi. Int Immunol 17:373–382. doi: 10.1093/intimm/dxh217. [DOI] [PubMed] [Google Scholar]
- 30.Ho T, Htwe KK, Yamasaki N, Zhang GQ, Ogawa M, Yamaguchi T, Fukushi H, Hirai K. 1995. Isolation of Coxiella burnetii from dairy cattle and ticks, and some characteristics of the isolates in Japan. Microbiol Immunol 39:663–671. doi: 10.1111/j.1348-0421.1995.tb03254.x. [DOI] [PubMed] [Google Scholar]
- 31.Ray A, Dittel BN. 28 January 2010, posting date Isolation of mouse peritoneal cavity cells. J Vis Exp doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang GQ, Hotta A, Ho T, Yamaguchi T, Fukushi H, Hirai K. 1998. Evaluation of a recombinant 27-kDa outer membrane protein of Coxiella burnetii as an immunodiagnostic reagent. Microbiol Immunol 42:423–428. doi: 10.1111/j.1348-0421.1998.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 33.DiLillo DJ, Mastsushita T, Tedder TF. 2010. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 34.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Müller S, Kantor AB, Herzenberg LA, Rosen FS, Sideras P. 1995. Defective B cell development and function in Btk-deficient mice. Immunity 3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 35.Middendorp S, Dingjan GM, Hendriks RW. 2002. Impaired precursor B cell differentiation in Bruton's tyrosine kinase-deficient mice. J Immunol 168:2695–2703. doi: 10.4049/jimmunol.168.6.2695. [DOI] [PubMed] [Google Scholar]
- 36.Shannon JG, Heinzen RA. 2009. Adaptive immunity to the obligate intracellular pathogen Coxiella burnetii. Immunol Res 43:138–148. doi: 10.1007/s12026-008-8059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouaziz J-D, Yanaba K, Tedder TF. 2008. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 38.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. 2007. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 39.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornand T, Macdonald HR, Tschopp J. 1996. Activated B cells express functional Fas ligand. Eur J Immunol 26:721–724. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 40.Lundy SK. 2009. Killer B lymphocytes: the evidence and the potential. Inflamm Res 58:345–357. doi: 10.1007/s00011-009-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popi AF, Zamboni DS, Mortara RA, Mariano M. 2009. Microbicidal property of B1 cell derived mononuclear phagocyte. Immunobiology 214:664–673. doi: 10.1016/j.imbio.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Carter NA, Rosser EC, Mauri C. 2012. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther 14:R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sindhava V, Woodman ME, Stevenson B, Bondada S. 2010. Interleukin-10 mediated autoregulation of murine B-1 B-cells and its role in Borrelia hermsii infection. PLoS One 5:e11445. doi: 10.1371/journal.pone.0011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crane DD, Griffin AJ, Wehrly TD, Bosio CM. 2013. B1a cells enhance susceptibility to infection with virulent Francisella tularensis via modulation of NK/NKT cell responses. J Immunol 190:2756–2766. doi: 10.4049/jimmunol.1202697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Culkin SJ, Rinehart-Jones T, Elkins KL. 1997. A novel role for B cells in early protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J Immunol 158:3277–3285. [PubMed] [Google Scholar]