Abstract
Whereas DNA provides the information to design life and proteins provide the materials to construct it, the metabolome can be viewed as the physiology that powers it. As such, metabolomics, the field charged with the study of the dynamic small-molecule fluctuations that occur in response to changing biology, is now being used to study the basis of disease. Here, we describe a comprehensive metabolomic analysis of a systemic bacterial infection using Bacillus anthracis, the etiological agent of anthrax disease, as the model pathogen. An organ and blood analysis identified approximately 400 metabolites, including several key classes of lipids involved in inflammation, as being suppressed by B. anthracis. Metabolite changes were detected as early as 1 day postinfection, well before the onset of disease or the spread of bacteria to organs, which testifies to the sensitivity of this methodology. Functional studies using pharmacologic inhibition of host phospholipases support the idea of a role of these key enzymes and lipid mediators in host survival during anthrax disease. Finally, the results are integrated to provide a comprehensive picture of how B. anthracis alters host physiology. Collectively, the results of this study provide a blueprint for using metabolomics as a platform to identify and study novel host-pathogen interactions that shape the outcome of an infection.
INTRODUCTION
Metabolomics, a quickly emerging “omics” field in systems biology, is the global analysis of small molecules in a biological sample (1). Since the 1950s, the central dogma of biological information has been the transition from genes to transcript to protein (2). Only recently has the use of high-throughput systems biology been used to study the products of protein activity, the metabolites. The metabolome represents all endogenous and exogenous low-molecular-mass (<1-kDa) molecules present in a biological state, providing an instantaneous “snapshot” of the cell's metabolic and physiological activity (3). Applications of global metabolomic analysis fall into three broad categories: (i) disease diagnosis, (ii) biomarker and drug discovery, and (iii) study of metabolic pathways and their perturbations due to external factors (1). The analysis of altered metabolites has the potential for discovery of new biomarkers, thus providing the possibility of earlier intervention and insights into the mechanisms of diseases (4).
The diagnosis of a bacterial infection encompasses an assessment of clinical symptoms, positive culture of an organism from tissues or blood, and/or reliance on often expensive, outsourced molecular methods (5). Metabolomics provides a unique perspective on bacterial infections as it is able to comprehensively characterize a vast number of metabolic changes in response to a biological perturbation within the host (2). In addition to the potential for biomarker discovery, the metabolic profiles obtained from this analysis can give insight into the identity and nature of molecules involved in the immune response, detect alterations in host physiology, and identify novel pathways altered during infection (6). The uses of metabolomics are quickly emerging in both clinical and basic research settings to address fundamental questions of bacterial pathogenesis. Efforts in clinical research have focused on biomarker discovery and refining diagnostic methods, as these endeavors serve as the beginning steps toward personalized medicine (7, 8). In particular, for sepsis infections, metabolomics has been used to understand the dynamic physiological changes in individual patient metabolic profiles as an alternative to treating them as homogeneous populations (7–9). On the other hand, basic research studies focused on identifying key metabolic pathways have given insight into physiological changes in specific tissues and/or host systems (10–12). However, a comprehensive analysis of major host organs and tissues has not yet been performed.
In this study, we utilized Bacillus anthracis as a model pathogen to investigate the metabolic changes present during various stages of infection. B. anthracis is a spore-forming bacterium that is the etiological agent of anthrax and a weapon of bioterrorism (13, 14). Upon exposure to the host, spores are engulfed by local macrophages, where they germinate into vegetative cells and replicate as the macrophages travel to lymph nodes (15–18). Vegetative bacilli then escape the cell and produce key virulence factors that contribute to the manifestation of disease. These include anthrax toxin, a tripartite toxin system that consists of one receptor binding component, protective antigen (PA), and two catalytic subunits, lethal factor and edema factor (LF and EF) (19–21). LF exhibits metalloprotease activity, cleaving mitogen-activated protein kinase kinases (MAPKKs) of the MAPK signaling pathway and suppressing subsequent proinflammatory responses (20–23). EF acts as an adenylyl cyclase that converts ATP to cyclic AMP (cAMP), thus increasing intracellular cAMP concentrations, which contribute to disrupting cytokine production and mediating tissue destruction (24). Vegetative bacteria quickly spread into the lymphatic and circulatory systems, initiating a systemic infection (25). The propensity of this bacterium to transition from an inactive spore to a disseminated infection with multiorgan involvement that culminates in massive bacteremia and toxemia offers a unique opportunity to assess the small-molecule metabolome during a developing infection (26). Here, we report the first whole-organism metabolomic analysis of mice infected with B. anthracis, a bacterial pathogen that causes systemic disease, using both spore and vegetative cell infection. A global analysis of whole blood and organs revealed bacillus-induced alterations of energy production and lipid mediators, the latter of which are largely suppressed. Functional studies using pharmacological inhibition of host phospholipase A2 (PLA2) enzymes further invoked a disruption of lipid mediators during anthrax disease. This work demonstrates the usefulness of metabolomics in the identification and analysis of small molecules involved in the innate host response to a bacterial infection.
MATERIALS AND METHODS
Mouse infections.
A global metabolomic analysis of mice was used to assess the effect of B. anthracis infection on host metabolism. Female A/J mice (6 to 8 weeks old) (n = 5 per experimental group) were purchased from Jackson Laboratories. The Sterne 34F2 strain was used for each metabolomics experiment. This strain lacks the pOX2 plasmid and does not produce capsule (15). Spores were made from vegetative cells according to the protocol described by Kim and Goepfert (27). Vegetative cells were generated from freezer stocks of strain 34F2. First, the 50% lethal dose (LD50) was determined by administering phosphate-buffered saline (PBS) suspensions of B. anthracis Sterne 34F2 vegetative cells or spores subcutaneously into the left hind leg of mice. All subcutaneous inoculations in this study were performed as 50-μl injections into the subcutaneous fatty layer of the ventral side of the right hind leg near the last mammary gland of female mice. Inoculum doses ranged from 1 × 103 to 1 × 105 (vegetative cells) and from 2 × 101 to 2 × 104 (spores) bacilli per inoculum. Mice were monitored at 12-h intervals for 1 week. The LD50s were calculated by the Reed and Muench method and were determined to be 1.5 × 103 for vegetative cells and 1 × 102 for spores (see Fig. S1A and B in the supplemental material) (28). The median times to death (MTD) were calculated from the shortest survival time in which percent survival was less than or equal to 50%. Once the LD50 was determined, infections (performed using either vegetative cells or spores) were repeated using this dose, and either blood samples (both vegetative and spore infections) or organ samples (spores only) were harvested at 1 and 3 days postinfection (dpi).
Ethics statement.
All animal protocols for these studies were approved by the Baylor College of Medicine Institutional Animal Use and Care Committee (BCM-IACUC; Animal Assurance no. AN-5177; protocol no. AN-5177:1; IACUC protocol no. D1485 and D1491) and complied with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the regulations under the Animal Welfare Act (USDA registration no. A3823-01).
Sample collection and preparation for metabolomics studies.
Global metabolomic analyses were conducted using whole blood and organs of A/J mice infected with B. anthracis. Specifically, the vegetative cell infection analysis utilized whole blood collected via cardiac puncture (with 4.5 mM EDTA added to prevent clotting) from control and infected mice at 1 and 3 dpi (n = 8 per experimental group). One of the mice in each group was analyzed by the investigators in a blind manner to determine whether the metabolomic changes that were identified were predictive of the type and relative stage of infection. The spore infection utilized whole blood and organ tissues from lungs, liver, kidneys, and spleen harvested from animals at 3 dpi. A total of 12 mice, 6 infected and 6 control, were used. To reduce the effect of the presence of metabolites originating from blood in confounding metabolic profiles of organ tissues, transcardiac perfusions were performed on anesthetized mice to flush out blood from the vasculature, as described in reference 29. Briefly, animals were first anesthetized by intraperitoneal (i.p.) administration of a lethal dose of a 1.2% solution of 2,2,2-tribromoethanol–2-methyl-2-butanol–0.9% NaCl at a final concentration of 0.2 ml/0.01 kg body weight and transcardiac perfusions were performed. To ensure that animals were properly sedated before perfusion procedures were performed, each mouse was tested for physical responsiveness by pedal reflex determination. Sedated animals were placed on operating platforms located in a biosafety cabinet. A horizontal incision was made below the ribcage, and two vertical incisions were made into the skin, each above the ends of the ribcage, leaving the thoracic cavity exposed. Using a peristaltic pump, a butterfly needle with a Luer-lok attachment was secured to the efflux end of the pump. The influx end was placed in a reservoir of cold sterile PBS with 5 mM EDTA. A needle was placed into the left ventricle of the heart, a small, 3-mm-long incision was made at the right atrium, and the pump was allowed to run for 5 min per animal to flush out circulating blood from tissues. In addition, the heart was excised to ensure exsanguination. Perfused organs were harvested after perfusions were completed. Perfusion efficiencies were analyzed as a proxy for the amount of whole blood still present in tissue vasculature after perfusions by determining the average optical density (OD) at 403 nm per gram of perfused tissues in comparison to nonperfused tissues, a value that is directly proportional to the amount of hemoglobin present (see Fig. S3 in the supplemental material) (30).
Metabolomic profiling.
A global metabolomics approach was performed using a pipeline developed by Metabolon Inc. Samples for analysis, either from blood or organs, were prepared using a solvent extraction method (MicroLab Star system from the Hamilton Company) as previously described (31). Briefly, the resulting extract was split into equal parts and applied to gas chromatography/mass spectrometry (GC/MS) and liquid chromatography tandem MS (LC/MS/MS) platforms. The LC/MS portion of the platform is based on a Waters Acquity ultraperformance LC (UPLC) and Thermo-Finnigan linear trap quadrupole (LTQ) mass spectrometer, consisting of an electrospray ionization source and linear ion trap mass analyzer. For GC/MS, samples were derivatized and separated using a 5% phenyl column with a temperature ramp from 40° to 300°C in a 16-min period. Samples were then analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer using electron impact ionization. All compounds were identified by their retention time and mass after comparison to library entries of purified standards in Metabolon's database of ∼5,000 molecules.
ELISA for the detection of B. anthracis lethal factor.
An indirect enzyme-linked immunosorbent assay (ELISA) for the detection and quantification of lethal factor was adapted from past studies (32–34). Serum samples from control and infected A/J mice were normalized to 3 mg/ml total protein of bovine serum albumin (BSA) by a Bradford assay. Briefly, a standard curve was generated with known concentrations of BSA and used to determine the total concentration of samples. In doing so, a consistent serum concentration normalized to 3 mg/ml was maintained across all samples. Samples were added in duplicate to Immulon (Thermo-Scientific) plates and incubated overnight at 4°C. LF serially diluted in carbonate coating buffer (0.06 M Na2CO3, 0.14 M NaHCO3, 100 ml distilled H2O [pH 9.6]) was used to generate a standard curve. Wells were blocked with 2% nonfat milk and 100 μg/ml BSA, and the reaction mixture was washed three times with 0.05% Tween 20–PBS (wash buffer) and incubated overnight with anti-B. anthracis LF (bD-17; Santa Cruz) at a 1:100 dilution in PBS overnight at 4°C. Wells were treated with horseradish peroxidase-conjugated donkey anti-goat IgG (Santa Cruz) and washed, and a colorimetric reaction was allowed to develop using 1-Step Ultra TMB (3,3′,5′5′-tetramethylbenzidine)-ELISA substrate solution (Thermo Scientific). Average absorbance values were measured at 450 nm and normalized by subtracting these values from the average absorbance of blank PBS wells. The negative control was 3 mg/ml BSA–PBS, and the positive controls were supernatants of B. anthracis overnight cultures grown in Ristroph media, a toxin-inducing media (35). One-way analysis of variance (ANOVA) followed by Dunnett's test for multiple comparisons was used to compare negative-control groups to the respective positive-control and infected groups.
Data normalization, statistical analysis, and bioinformatics.
The analysis of the metabolomic data was performed in the Maresso laboratory. Raw spectral counts were log transformed and normalized by median centering. Missing values were inputted using the lowest value detected for a particular metabolite. Statistical analysis of fold changes by two-way ANOVA and Welch's two-sample t test were used to determine metabolites with significant results (P ≤ 0.05) for samples obtained from the vegetative cell and spore infections, respectively. ANOVA and Welch's test were used to compare the average spectral counts from the mock-infected negative-control group with those from the experimental group for each metabolite. Bioinformatics analyses such as partial least-squares discriminant analysis (PLS-DA), random forest (RF) analysis, and hierarchical clustering by heat map analysis were performed using the Metaboanalyst web portal (www.metaboanalyst.ca). PLS-DA was used to identify initial trends and clusters in data sets. Principal components denote the percentage of the contribution that can explain the original data set after linear transformation. Component 1 is defined as representing the highest possible variability in the data set after transformation is applied. Component 2 represents the second highest variability after transformation (36). The RF algorithm was used to classify metabolite importance based on the computed mean decrease accuracy (or prediction accuracy), which is determined by random permutation of variables and running observed values through decision trees. For each run, the prediction accuracy is reassessed for random noise or decreases in value if the variable is important to the classification. Hierarchical clustering by heat map analysis was used to demonstrate fold changes of metabolites in comparison to control group results.
Pharmacological inhibition of PLA2 during infection.
Female A/J mice (6 to 8 weeks old) were given intraperitoneal (i.p.) injections in the right hind leg with 200 μl of 80 mg of quinacrine (QC)–PBS (broad-spectrum PLA2 inhibitor) or a 100 μM solution of PACOCF3 (3% dimethyl sulfoxide [DMSO]–saline solution) (cytosolic PLA2 [cPLA2]-specific inhibitor) as a single or repeated infection, followed by subcutaneous injection of the left hind leg with B. anthracis spores (n = 5 per group). Several doses of QC administered to mice were assessed to determine an appropriate concentration that did not yield negative physiological side effects (data not shown). Mice given multiple doses of inhibitor were administered the drug 24 h after the last injection. Disease sequelae and mortality were monitored at 12-h intervals. The effects of inhibitor and infection on disease severity were evaluated using Kaplan-Meir survival curves and a log rank test (P < 0.05) and GraphPad Prism 6.0 software (GraphPad Software, Inc.). A disease index was developed to evaluate visible disease symptoms and physiological health conditions using the following five criteria: ruffled fur, lethargy, hunched posture, edema, and eschar formation (scoring range, 0.00 to 1.00, with 1.00 representing the greatest degree of visible disease progression for a particular criterion). Kruskal-Wallis nonparametric ANOVA was used to compare the area under the curve (AUC) data for the average daily health score values for each animal (P < 0.05).
RESULTS
Establishing a vegetative cell infection model for a metabolomic analysis.
We used a well-characterized murine model of anthrax disease to examine metabolomic changes during bacterial infection. This model was chosen because (i) B. anthracis can initiate a progressive infection from a low dose from either a spore or vegetative cell form, thereby allowing one to monitor early-stage alterations in the host during a developing infection, (ii) the experiments can be performed under biosafety level 2 (BSL2) conditions, thereby enabling the timely completion and experimental manipulation of the conditions, and (iii) the course of the disease models anthrax disease in humans (37, 38). A/J mice were infected (by subcutaneous injection) with B. anthracis vegetative cells or spores to first determine the LD50 for these forms of bacilli. The LD50 for the vegetative cell infection was determined to be 1.5 × 103 cells, while that of the spore infection was determined to be 1.0 × 102 spores (see Fig. S1A and B in the supplemental material). For the first metabolomic analysis, the vegetative form was chosen and mice were mock infected (1% saline solution) or infected with one LD50 of B. anthracis. Following infection, blood was collected at 1 and 3 dpi (experimental design shown in Fig. 1A); indeed, very few or, in most cases, no bacilli were detected in the organs, as determined by a CFU estimation (data not shown).
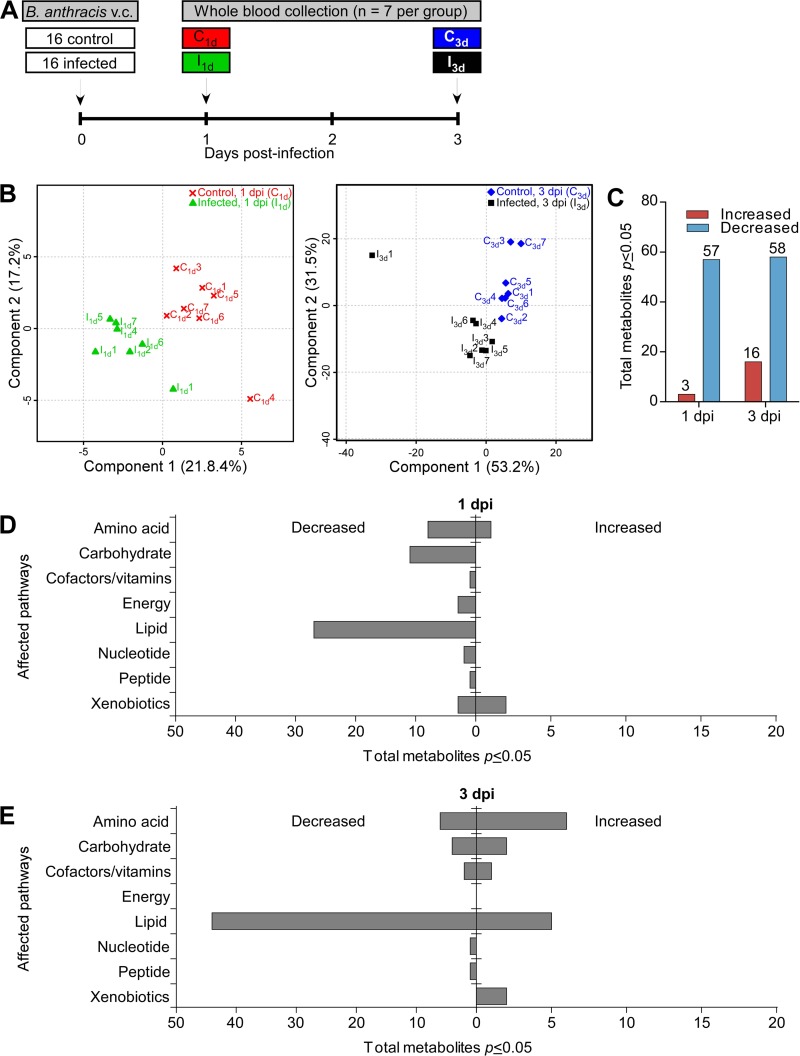
FIG 1.
Global metabolomic analysis of blood from mice infected with B. anthracis vegetative cells. (A) Infection timeline. Mice were infected with vegetative cells (v.c.) via a subcutaneous route (see Materials and Methods), and whole blood from infected and control A/J mice that were separated into 5 groups was collected at 1 dpi (control, C1d; infected, I1d) and 3 dpi (control; C3d; infected, I3d) (n = 7 per group). The fifth group consisted of one mouse from each of the four groups described above; the mice in this group were analyzed in a blind manner (see Fig. S4 in the supplemental material). (B) PLS-DA analysis of each group, demonstrating a clear separation of controls and infected samples from both time points. (C) Identification of statistically significant metabolites by two-way ANOVA t test (P ≤ 0.05) of normalized metabolomics data. (D and E) Categorization of metabolites involved in glucose metabolism, including gluconeogenesis, glycolysis, oxidative phosphorylation, and the Krebs cycle, at 1 dpi (D) and 3 dpi (E). Note the decrease in levels of lipids relative to the other metabolites.
We chose 1 and 3 dpi for sample collection because these were time points considered before the onset of robust anthrax disease. In doing so, we wanted to avoid assessing nonspecific changes in metabolites that result from the initiation of death. Thus, by sampling animals well before death, we could observe significant changes during early stages of infection before the onset of substantial bacterial dissemination (data not shown). Samples were harvested on day 1 because it represents an early and convenient time point—exactly 24 h after infection. Day 3 was chosen because it is 1 or 2 days before the mice develop full-blown anthrax disease in this model.
Whole blood was then subjected to an untargeted metabolomic analysis using a combination of gas and liquid chromatography and mass spectrometry, an approach that identified 271 total metabolites in whole blood. There were clear differences between infected and uninfected mice at both 1 and 3 dpi, as revealed by partial least-squares regression (PLS-DA), with a greater separation observed later in infection, indicating considerable variation in the metabolite composition as the infection progressed (Fig. 1B). Analysis of statistically significant metabolites identified by two-way ANOVA (P ≤ 0.05) revealed that more metabolites had decreased in abundance than had increased in abundance at both time points compared to the control group results (Fig. 1C). Of the 60 significant metabolites whose abundance had changed in the 1 dpi groups, 57 metabolites decreased in abundance, whereas only 3 metabolites exhibited increased levels. Similar trends were observed 3 dpi; 58 metabolites showed significant decreases in levels whereas 16 metabolites were increased in abundance in response to infection (Fig. 1C). All altered metabolites were next segmented by metabolic pathways, which revealed major changes in lipid, amino acid, and carbohydrate pathways (Fig. 1D and E). Of these, the changes in lipid levels, the majority of which had decreased by 3 dpi, were the most dramatic. These findings indicate that during the early stages of anthrax disease, well before any observable signs of disease or detectable bacilli in major organ systems, the host demonstrates dramatic alterations in lipid levels. It should be noted that anthrax toxin was detected in blood of these mice, which indicates that the mice were indeed infected (see Fig. S4 in the supplemental material).
Effect of B. anthracis vegetative infection on the levels of polyunsaturated fatty acids (PUFAs) and lysophospholipids (lysolipids).
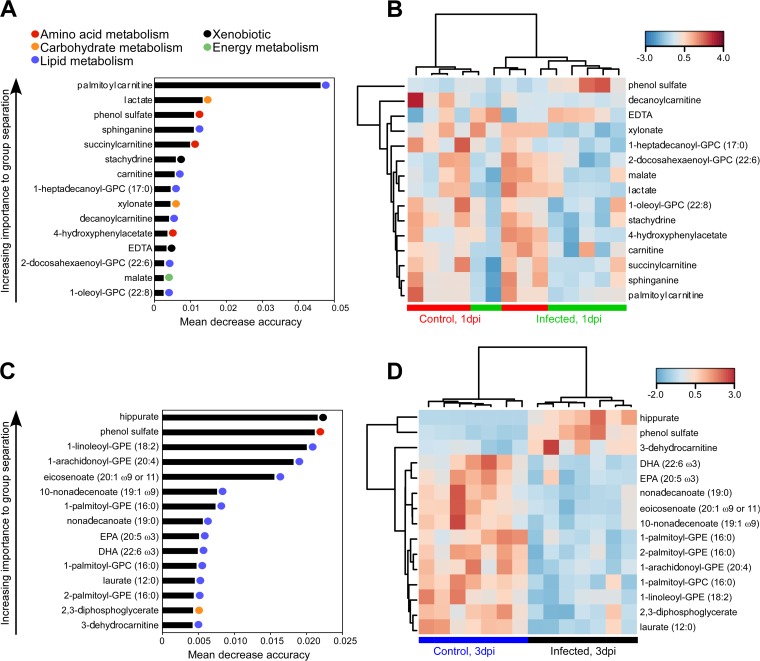
We wondered if there were metabolites that were predictive of anthrax disease. A comparison of (1 dpi) infected and uninfected groups by random forest (RF) analysis yielded a predictive accuracy of 64.3% (Fig. 2A), which is only slightly better than what is expected by chance (50% accuracy). However, distinct trends were present even after 1 dpi. Among the top 15 most important metabolites found by RF analysis, 7 were associated with lipid pathways, the majority belonging to carnitine-derived molecules. Carnitine and its fatty acid derivative, palmitoylcarnitine, have been shown to modulate the activity of caspases involved in apoptosis, suggesting that early stages of B. anthracis infection can alter the apoptotic/survival status of host cells (39, 40). In addition, sphinganine, a precursor in sphingosine biosynthesis, showed reduced levels at 1 dpi compared to control group results, which may indicate altered ceramide signaling in apoptotic events in response to infection (Fig. 2B) (41). Carnitine-conjugated fatty acids such as decanoylcarnitine and succinylcarnitine showed lower levels in the infected group than in the control group (Fig. 2B).
FIG 2.
Random forest (RF) analysis of metabolite changes in blood of animals infected with B. anthracis vegetative cells. Mice were infected with vegetative cells via a subcutaneous route (see Materials and Methods). Data represent mean decrease accuracy and hierarchical clustering by heat map analysis of control and infected blood for the top 15 metabolites at 1 dpi (A and B) and 3 dpi (C and D) determined using Pearson rank correlation distance and complete linkage. Heat map colors reflect relative abundances of metabolites identified by random forest analysis. Red or blue is used to show that the abundance of the indicated metabolite was higher or lower than the mean metabolite abundance, respectively. GPC, glycerophosphocholine; GPE, glycerophosphoethanolamine.
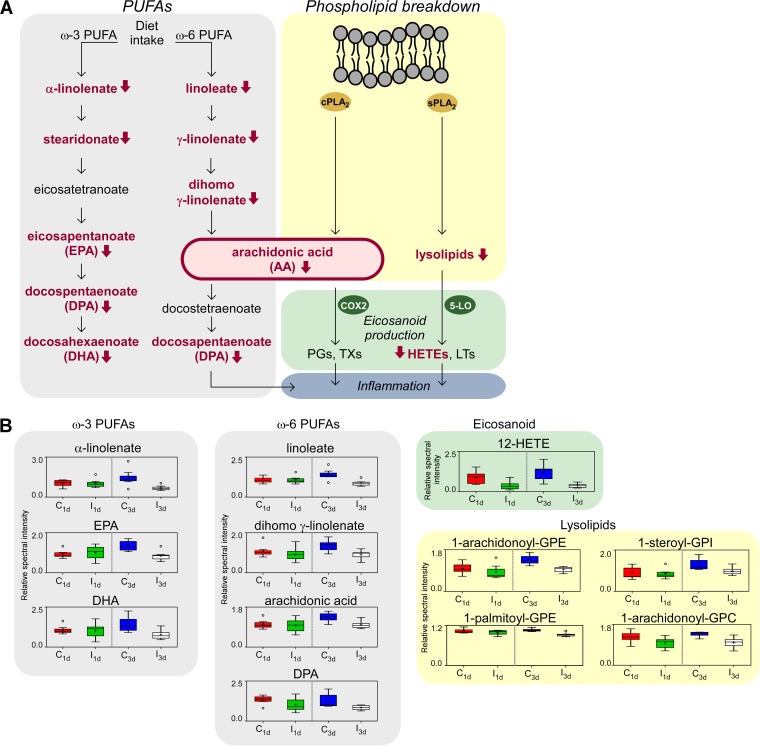
An RF analysis of the 3 dpi group yielded a different story, showing 92.9% accuracy of infection prediction compared to the control and indicating that the changes in metabolic profiles between the two groups are substantial (Fig. 2C). PUFAs, a class of lipid molecules important in inflammation signaling (Fig. 3A), showed alterations at both time points in response to infection. PUFAs of the ω-3 family, which includes eicosapentaenoate (EPA; 20:5 ω-3) and docosahexaenoate (DHA; 22:6 ω-3), identified by RF analysis were shown to be correlated with the decreased levels of essential fatty acids α-linolenate (18:3 ω-3) and linoleate (18:2 ω-6) (Fig. 2B and 3B). Other members of the ω-3 and ω-6 pathways such as dihomo-γ-linolenate (18:3 ω-6) and arachidonic acid (AA) also showed significant decreases in abundance at 3 dpi (Fig. 3). AA is the key intermediate of the arachidonic acid cascade which stems into multiple cell signaling pathways with diverse functions, including production of eicosanoids, considered to be key mediators of inflammation (Fig. 3A). This notion is reflected by the decreased levels of 12-hydroxyeicosatetraenoic acid (12-HETE), an eicosanoid derived from arachidonic acid in response to the vegetative cell infection at both time points (Fig. 3B). A second source for AA operates through the breakdown of phospholipid membranes (Fig. 3A). Products of membrane digestion called lysophospholipids (lysolipids) also showed reduced levels in infected blood at both time points (Fig. 3B). Eicosanoids and lysolipids are both bioactive lipids produced by AA metabolism that contribute to the recruitment of immune cells and initiation of inflammation. In particular, lysophosphatidylcholines (lysoPCs) such as 1-arachidonoyl–glycerophosphocholine (1-arachidonoyl–GPC) are molecules that can accumulate in blood and are considered neutrophil-activating factors, and their levels were found to be reduced in the infected groups at both time points (Fig. 2D) (7, 8). Taken together, these data suggest that vegetative cell infection by B. anthracis generates an overall reduction of levels of circulating lipids, several of which are reportedly involved in the innate immune response during infection.
FIG 3.
Lipid pathways affected by B. anthracis vegetative cell infection. (A) Schematic model of the two inflammatory lipid pathways that were altered by infection: the polyunsaturated fatty acid (PUFA) biochemical pathway and the pathway involved in membrane breakdown by host PLA2 enzymes (cytosolic and secreted PLA2 classes cPLA2 and sPLA2 are shown). These sources merge in the production of arachidonic acid (AA), which feeds into the eicosanoid biosynthesis pathways catalyzed by cyclooxygenase (COX), which yields prostaglandins (PGs) and thromboxanes (TXs), and by lipoxygenase (LOX) enzymes, which yield leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs). Eicosanoid production and lysolipid production can both promote inflammation. Lipid metabolites whose levels were lower following infection are denoted in red with red arrows. (B) Representative lipids from the PUFA and phospholipid breakdown pathways, comparing levels at 1 and 3 dpi. Box-and-whisker plots show metabolite minimum, lower quartile, median, upper quartile, median (middle line), mean (+), and extreme (open circle) values and sample maximums. Metabolites involved in PUFA pathways are in gray, eicosanoids are in red, and lysolipids are in yellow.
Whole-organ metabolomics during B. anthracis spore infection.
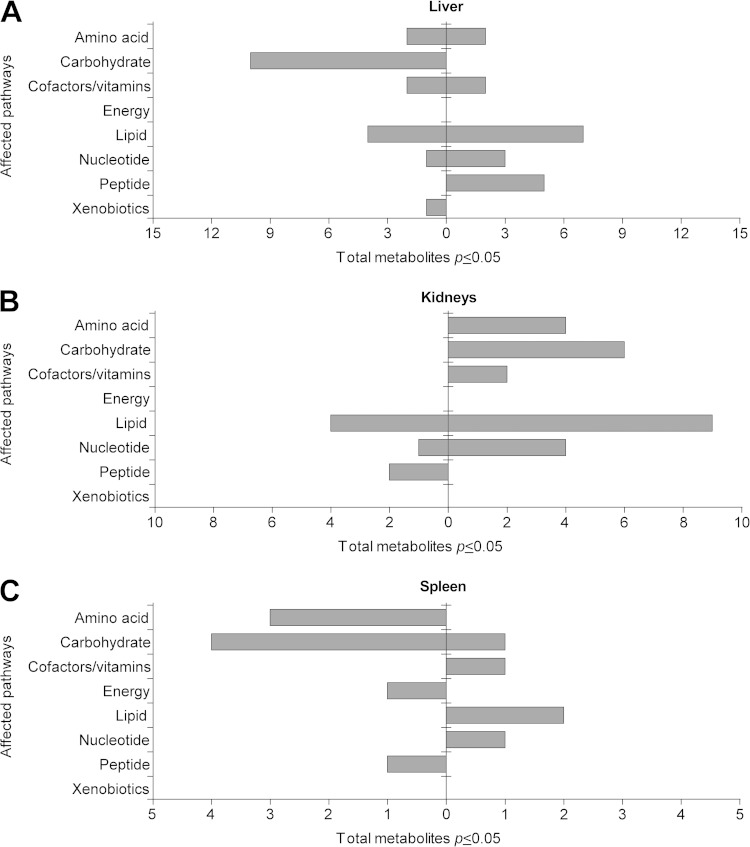
During systemic anthrax, bacilli spread to the lungs, liver, kidneys, and spleen. Having established that there are global changes in lipids in blood as an outcome of B. anthracis infection, we next assessed which metabolites were altered in major organ systems during infection. Spores were used for this arm of our untargeted metabolomic analysis because they represent the infectious form observed in nature. This also allowed us to compare the metabolomes of spore versus vegetative forms of the infection. Mice were mock infected with 1% saline solution or infected with 1.0 × 102 spores (determined to be the LD50 of B. anthracis) (see Fig. S1B in the supplemental material), and samples of lungs, liver, kidneys, spleen, and whole blood were collected at 3 dpi from control and infected mice (n = 6 per experimental group) (Fig. 4A). Of note, to eliminate any contamination of these tissues with blood, each organ was perfused (see Materials and Methods), a process that resulted in a substantial reduction in the levels of hemoglobin in these organs, signifying that the blood had been removed (see Fig. S3). The examination of blood in a different context also provides a control by which to determine how consistent these findings are between two very different modes of infection. Following gas/liquid chromatography and mass spectrometry of organ samples, metabolomic profiling identified a total of 339 metabolites in the lung, 387 metabolites in the liver, 384 metabolites in the kidneys, 378 metabolites in the spleen, and 319 metabolites in the blood. Analysis by Welch's two-sample t test (P ≤ 0.05) revealed 39 statistically significant metabolites between infected and uninfected mice from liver tissues, 32 metabolites from the kidneys, 14 metabolites from the spleen, and 43 metabolites from blood (Fig. 4B). Similarly to the first metabolomic analysis, the abundances of the vast majority of altered metabolites (40 metabolites) in the blood were decreased, which provided confidence in the consistency of our infections and this analysis (Fig. 4B). Furthermore, large changes were again associated with lipids, with more than 17 decreased in abundance (Fig. 4C). Only 7 metabolites in the lungs (all of which had increased in abundance) had changed compared to the control, the lowest number of changes among all of the organ comparisons. The liver demonstrated ∼20 metabolites whose abundance increased or decreased following spore infection (Fig. 4B). In contrast, the kidneys displayed a net increase in levels of metabolites whereas the spleen showed more metabolites whose abundances were reduced in response to infection (9 metabolites). Taken together, these results indicate that the host undergoes substantial changes in metabolite levels in major organ systems in response to B. anthracis infection. In addition, many of the same changes, especially decreases in levels of lipids, were again observed in the blood, regardless of whether the spore or vegetative form was analyzed.
FIG 4.
Metabolomics analysis of blood and organs of animals infected with B. anthracis spores. (A) Infection timeline. Mice were infected with spores via a subcutaneous route (see Materials and Methods). Whole blood (WB) and organ (lung, liver, kidney, and spleen) tissues from infected and control A/J mice were collected at 3 dpi. (B) Identification of statistically significant metabolites by Welch's two-sample t test (P ≤ 0.05). (C) Classification of altered metabolites into categories of affected pathways. (D) Hierarchical clustering by heat map analysis (Person rank correlation distance and complete linkage) of the top 20 metabolites identified by RF analysis of whole blood. Red or blue is used to show that the abundance of the indicated metabolite was higher or lower than the mean metabolite abundance, respectively. GSSG, glutathione disulfide.
Effect of B. anthracis spore infection on lipid metabolism.
Lipids and amino acids identified in whole blood of mice infected with B. anthracis spores were the most abundant metabolites that showed decreased levels, reflecting trends similar to those observed with the vegetative cell infection (Fig. 4C). RF analysis revealed that 10 of 20 of the most important metabolites were lipids (Fig. 4D). Similarly to the vegetative cell infection analysis, the RF analysis identified that the levels of lipids involved in inflammation were decreased after spore infection. Consistent with the vegetative cell analysis, the levels of lipids involved in PUFA metabolism and lysolipid production were decreased, indicating they may play an important role during both stages of B. anthracis infection. Among these lipids were PUFAs such as linoleate (18:2 ω-6) and dihomo-γ-linolenate, both of which were found to be decreased in abundance compared to the control (Fig. 4D). This notion is reflected in decreased levels of downstream products in lysolipids such as 1-linoleoyl-glycerophosphoethanolamine (1-linoleoyl-GPE) (18:2) and 1-linolenoyl-GPC (18:3 ω-3) (Fig. 4D). The changes seen in whole blood from the spore infection at 3 dpi were less significant than those found with the vegetative cell infection (Fig. 4B). No new group or class of metabolites with distinct relationships, aside from similar (albeit smaller) changes in lipids with inflammatory properties found in the vegetative cell infection, was identified by bioinformatics analysis of the spore infection. Taken together, these data suggest that B. anthracis infection suppresses small-lipid mediators prior to the onset of anthrax disease.
Effect of B. anthracis spore infection on the energy status in the spleen and liver.
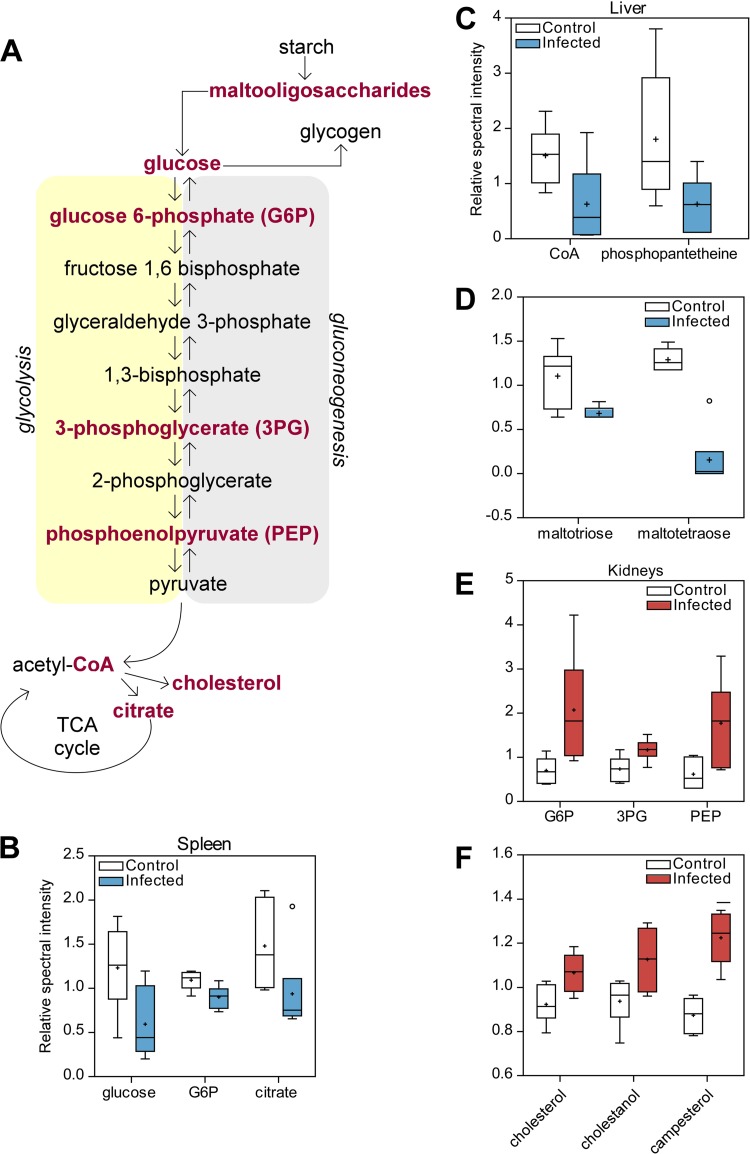
Aside from their roles in the immune response and signaling, fatty acids can be used as an energy source (42–45). For example, it is known that fatty acid oxidation can drive ATP production in rat heart tissues (46). Up to 50% of the ATP produced in myocardial tissues can be a result of fatty acid contributions, suggesting that energy demands cannot be met solely by glucose supplies (46). It is possible that lipids produced in organ systems can be released into the vasculature and affect the composition of metabolites found in whole blood. In particular, fatty acid production can occur in the liver and the fatty acids can be released into circulation (44). Liver tissues showed significant decreases in the levels of lipid metabolites associated with fatty acid biosynthesis (Fig. 5A). Specifically, the levels of coenzyme A (CoA) and 4′phosphopantetheine, a metabolite in the CoA biosynthesis pathway, were decreased in infected liver tissues relative to the control, suggesting alterations in normal hepatocellular energy metabolism (Fig. 6C). In addition, carbohydrates participating in energy metabolism were also affected (Fig. 5A). Levels of glycolysis intermediates in both the spleen and liver were decreased, suggesting additional decreases in host energy consumption due to infection (Fig. 5C and 6B and C). In the liver, levels of maltooligosaccharides, carbohydrates involved in glycogen production that can be converted into glucose, were also decreased during infection, suggesting that the stored glycogen that had been depleted had not been replenished (Fig. 6D). These results suggest that both arms of energy production, carbohydrates and lipids, in normal liver function are disrupted in response to spore infection.
FIG 5.
Summary of the altered pathways in organs following infection with B. anthracis spores. Metabolites were identified by Welch's two-sample t test (P < 0.05), comparing control and infected samples from the liver (A), kidneys (B), and spleen (C) following infection of A/J mice with B. anthracis strain Sterne 34F2 (as outlined in Materials and Methods).
FIG 6.
The analysis of organ tissues as it pertains to energy metabolism following infection with B. anthracis spores. (A) Changes in metabolites in energy production, consumption, and storage pathways that were significantly altered following infection of A/J mice with B. anthracis strain Sterne 34F2 (spores). (B to F) Representative metabolite data, comparing control and infected spleens (B), livers (C and D), and kidneys (E and F).
Effect of B. anthracis spore infection on glucose metabolism in the kidney.
Further separation of significant metabolites into affected pathways revealed the increases in the levels of lipid and carbohydrate-derived metabolites to be the most abundant (Fig. 5B). Prominent increases in levels of lipid metabolites associated with increased oxidative stress were of particular interest. Specifically, a higher abundance of cholesterol and cholestanol, a derivative of cholesterol metabolism, was observed in response to infection (Fig. 6F). In addition, plant sterol-derived campesterol, a marker of cholesterol absorption, also displayed increased levels relative to the uninfected kidney samples (Fig. 6F) (47). The levels of several metabolites involved in glucose metabolism were revealed to be significantly increased in response to infection (Fig. 6E). Intermediates participating in gluconeogenesis, including glucose-6-phosphate (G6P), phosphoenolpyruvate (PEP), and 3-phosphoglycerate (3PG) (Fig. 6E), were identified. G6P and PEP levels increased almost 3-fold in infected kidneys relative to the control.
The role of PLA2 in the systemic response to B. anthracis infection and anthrax disease.
Lysolipids are produced by the activities of phospholipase A enzymes, which catalyze the cleavage of membrane phospholipids. There was no obvious preference for the sn-1 position or the sn-2 position among the lysolipids altered in the vegetative cell or spore infection. This may indicate both PLA1 and/or PLA2 may be inhibited or somehow downmodulated as the infection develops. However, AA production and subsequent lysolipid release are due only to the action of PLA2 breakdown of membranes. The latter finding invokes a more prominent role for PLA2 than for the members of other classes of phospholipases in this response (48). Products of PLA2 activity can act as direct mediators of inflammation and can also initiate multiple downstream inflammatory signaling cascades in response to an infection. The levels of several of these lipids were found to be decreased in blood in both the vegetative cell and spore infections, perhaps as a mechanism directed by B. anthracis to dampen the innate immune response.
Along these lines, we tested the hypothesis that PLA2 was important in the host response to B. anthracis infection by assessing the progression to anthrax disease in mice administered PLA2 inhibitors. Mice were injected intraperitoneally (i.p.) with quinacrine (QC) or PACOCF3, each of which is an inhibitor of PLA2, and then immediately inoculated with B. anthracis spores, followed by another dose of inhibitor every 24 h. Single-blind experiments were conducted to assess disease severity and mortality by health index scores, monitored at 12-h intervals (Fig. 7 and Table 1). Briefly, before scores were given, research personnel removed identifying information from each experimental group and randomized the placement order from the previous day on the shelves in which the cages were housed. Different research personnel then recorded the scores without having knowledge of which experimental group was being assessed. The same researcher personnel were then used to access health scores for all time points to eliminate technical variation. Infected mice given quinacrine, a broad-spectrum PLA2 inhibitor, displayed a more rapid and severe appearance of visible disease symptoms than animals given QC or spores alone (Table 1). Disease severity was correlated to increased mortality and resulted in decreased MTD in infected mice receiving the inhibitor (Fig. 7A and B) (Table 1). No lethal effects were observed in mice given QC alone. As expected, increasing the spore inoculum from 5 × 102 to 2 × 103 spores augmented disease severity and decreased the MTD even further compared to lower spore doses. Similar results were observed with mice given PACOCF3, an inhibitor that specifically targets the cytosolic form of PLA2 (cPLA2) (Fig. 7C) (Table 1). Since lipid inflammatory cascades can be amplified by the activities of PLA2 and since the inhibition of PLA2 under three different conditions worsens the outcome of B. anthracis infection, the findings from these functional studies suggest that B. anthracis may inhibit inflammatory lipid production (as directly determined by the metabolomics studies) as a means to dampen the host immune response. These results also suggest that inhibition of phospholipases and their lipid products is important for host survival to B. anthracis.
FIG 7.
Effects of PLA2 inhibition on the lethality of B. anthracis spore infection. (A to C) Kaplan-Meier survival curves of A/J mice given i.p. injections of a broad-range PLA2 inhibitor, quinacrine (QC), or a cPLA2-specific inhibitor, PACOCF3 (C), followed by a subsequent subcutaneous infection with B. anthracis spores. Animals were inoculated with inhibitor only, with inhibitor immediately followed by spores, with inhibitor at multiple doses (M) and then spores, or with spores only at 5 × 102 spores/dose (A) or 2 × 103 spores/dose (B and C). All differences in groups were statistically significant by the log-rank test (P < 0.0001).
TABLE 1.
Average heath index scores and median times to death following PLA2 inhibition in B. anthracis-infected micea
| Treatment | Avg heath index score |
MTD | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | ||
| QC ± 5 × 102 spores* | ||||||
| QC only | 0.25 ± 0.00 | 0 | 0 | 0 | 0 | NA |
| 5 × 102 spores only | 0 | 0.20 ± 0.27 | 1.62 ± 0.85 | 3.08 ± 2.45 | 0.50 | 4.0 |
| QC + 5 × 102 spores | 0.70 ± 0.21 | 0.99 ± 0.36 | 2.32 ± 0.29 | 4.38 ± 0.88 | 3.8 | |
| QC(M) + 5 × 102 spores | 1.20 ± 0.21 | 1.59 ± 0.29 | 3.12 ± 0.22 | 4.75 | 3.6 | |
| QC ± 2 × 103 spores* | ||||||
| QC only | 0 | 0 | 0 | 0 | 0 | NA |
| 2 × 103 spores only | 0 | 1.80 ± 0.89 | 3.50 ± 0.90 | 3.25 | 5 | 3.2 |
| QC + 2 × 103 spores | 0.70 ± 0.27 | 2.63 ± 1.30 | 3.25 | 2.2 | ||
| QC(M) + 2 × 103 spores | 0.75 + 0.35 | 2.81 ± 1.60 | 2.3 | |||
| PA ± 2 × 103 spores* | ||||||
| PA only | 0 | 0.20 ± 0.00 | 0.20 ± 0.00 | 0 | 0 | NA |
| 2 × 103 spores only | 0 | 0.37 ± 0.16 | 2.59 ± 0.19 | 3.5 | ||
| PA + 2 × 103 spores | 0 | 0.86 ± 0.12 | 2.57 ± 0.25 | 4.0 | ||
| PA(M) + 2 × 103 spores | 0 | 1.04 ± 0.55 | 2.71 ± 0.14 | 3.5 | ||
The table shows average daily health index scores for surviving mice of each group ± standard deviations (SD). *, P < 0.05 by Kruskal-Wallis test comparing the area under the curve (AUC) of daily average health score values for each animal. (M), multiple doses; MTD, median time to death; NA, not applicable.
DISCUSSION
Metabolomics is becoming increasingly important for the analysis of host-pathogen interactions, particularly in the discovery of biomarkers of disease (11). Recent studies have shown that metabolomics can be used to identify metabolites whose levels are altered in response to an infection (10, 49–51). In particular, with respect to bacterial infections, the rapid emergence of multidrug-resistant Gram-positive and Gram-negative bacteria fuels the need for novel strategies in diagnosis and treatment (11). Diagnosis primarily relies on nonmolecular tests that are time- and labor-intensive. Here, we report the first global metabolomic analysis of multiple organ systems following a systemic bacterial infection. Our results reveal (i) that there are clear differences between infected and uninfected animals in the small-molecule metabolome of blood and organs, (ii) that these differences highlight major changes in amino acid, energy, and lipid metabolism as early as 1 dpi, (iii) that major alterations in two different arms of inflammation-related lipid metabolism suggest that B. anthracis infection leads to decreased levels of signaling molecules that function in the innate immune response to pathogens, and, finally, (vi) that the targeted inhibition of phospholipases accelerated anthrax disease, suggesting a novel role for these enzymes in host survival during B. anthracis infection. In addition to the points discussed above, since similar results were observed for the spore and vegetative forms of the infection, for different mouse groups, and, generally, for blood and organs, the findings presented in this report suggest that B. anthracis infection induces a pronounced reduction in the steady-state levels of small-lipid mediators, some of which are important in activating an innate immune response, during the onset of anthrax disease.
In this study, we chose to use B. anthracis as a model organism to identify metabolomics changes during blood-borne bacterial infections. We choose to use a murine infection model and the Sterne strain to facilitate these studies. Some limitations to using this strain (versus a fully virulent pXO1- and pXO2-containing strain) include the fact that anthrax toxin is primarily thought to be the main factor driving overall disease severity. Therefore, based on our LD50 determination, we were careful in selecting time points at 1 and 3 dpi, which are likely before the onset of significant toxemia.
Due to the sensitivity of the metabolomics platform, selection of an animal model can result in various outcomes depending on the species and strain used for infection. We were aware that there is no ideal animal model capable of perfectly recapitulating human anthrax disease. Past studies have used guinea, rabbits, nonhuman primates, and mice to study anthrax in mammals (52). While the guinea pig, rabbit, and nonhuman primate models are relevant for inhalational studies, the mouse models are generally accepted for studying early stages of infection (52). Since this was the primary focus of our study, we chose to use the A/J mouse model due to the feasibility of handling a larger sample size in an untargeted metabolomics platform.
The LD50 was chosen as the infectious dose so that the metabolomics analysis would be performed on mice that were undergoing a developing infection. By doing so, we are able to determine if our metabolomics platform was sensitive enough to detect metabolites during an early state in an infection that was not yet robust in nature. Even if the infection did not progress, it was expected that the bacterial inoculation would produce a host response and corresponding changes in host metabolites. As such, this approach was meant to also avoid nonspecific changes in physiology that might be associated with the process of dying. No mortalities were observed at either day in the mice used for this study, which is consistent with the idea that we were measuring early changes in the development of the infection. Attempts by the host defense to mitigate early stages of B. anthracis infection rely heavily on the activity of innate immune cells, which can induce activation of host-defense mechanisms that initiate inflammatory pathways (21). Expression of secreted and cytosolic families of the PLA2 enzymes is one common host defense strategy employed by macrophages to hydrolyze membrane lipids (48, 53). These enzymes can act as a bactericidal component and/or simultaneously produce lysolipids which can activate immune cells (54–57). B. anthracis lethal and edema toxins decrease the expression of the secreted phospholipase sPLA2-IIA in alveolar macrophages (56, 58, 59). However, it is unclear whether this downregulation can affect lysis of B. anthracis membranes and, more importantly, whether it can impair lipid mediator production or subsequent inflammatory status. Some preliminary data determined using a targeted metabolomic approach with toxin-deficient strains indicate that the levels of PUFAs are still reduced following infection (data not shown). This may indicate that other virulence factors play a more prominent role in this response. In addition, on the host side, it is also unknown whether other subclasses of PLA2 enzymes are actively participating in the response during early infections. Animal studies using a broad-spectrum PLA2 inhibitor, QC, in conjunction with spore infection resulted in increased disease severity and mortality. Interestingly, previous studies showed that addition of QC (in a culture setting) inhibits lethal toxin cytotoxicity in mouse peritoneal macrophages (60). These findings are somewhat at odds with the in vivo results presented here, which show that administration of QC increases disease severity and mortality in B. anthracis-infected mice. However, these two distinctions together may suggest that PLA2 enzymes play an important role during B. anthracis infection. PLA2 enzymes could have a protective effect in guarding against systemic infection but may be deleterious to the host in local tissue environments. More-specific forms of PLA2 such as cPLA2 are known to target membranes that contain AA, suggesting a more prominent role for cPLA2 than for sPLA2 in promoting inflammatory pathways (61). The finding that inhibition of cPLA2 by PACOCF3 also leads to increased mortality suggests that although cPLA2 does not have a direct role in producing deleterious effects on B. anthracis, it is still important in the response to this type of infection.
Our analysis also revealed vast differences in metabolic networks, illustrating the complexity and interconnection of biological systems due to infection. Hence, no single metabolome can be completely identical to the next, and each represents a unique profile exclusive to each organism (3). Despite this variation, we were able to successfully assign blind blood samples to their respective infection groups based on the results of PLS-DA clustering analysis. This finding supports the idea of the potential use of metabolomics in the diagnosis of infections before the onset of symptoms (see Fig. S4 in the supplemental material). Additionally, the use of different animals in our study instead of the same one for each time point resulted in sufficient resolution to detect minor but distinct changes in entirely different subjects. In this analysis, the use of metabolomics analysis also led to the successful identification of new biochemical pathways altered by B. anthracis that had not been previously reported. Studies of innate immune responses to infection by bacilli have focused on cytokines and chemokines, proteins that can evoke inflammation (21, 24, 62). However, the metabolomic approach described here has identified lipids and associated pathways involved in inflammation, implicating a new class of molecules that could be equally important to host defenses during B. anthracis infection.
The profiles from infected whole blood from B. anthracis vegetative cell and spore infections provided us with a connection to infection status and the life cycle of B. anthracis. Comparisons of blood metabolites from the two analyses revealed similar trends to perturbed metabolic pathways, providing a link to infection progression. The spore infection is more representative of natural infections, as it is the form of B. anthracis that the host encounters, whereas the vegetative cell form represents a later stage of infection. These changes to the pathogen's life cycle are correlated to the differences seen in our results. There were fewer significant changes found in the spore infection, and the changes were not as pronounced as those found in the vegetative cell infection. Aside from the inflammatory lipids, no other class of metabolites displayed significant trends corresponding to spore infection alone, suggesting a steady accumulation of metabolic perturbations induced by the transition from the bacillus spore to the vegetative cell. Similar trends in decreased levels of PUFAs and lysolipids were found in the two analyses; however, fewer of these lipids were identified in the spore infection whereas the decrease was most pronounced during the vegetative cell infection at 3 dpi. This suggests that the vegetative cell is the more disruptive form of B. anthracis or, perhaps more intriguing, that the infection by the spore form is stealthier.
Our results showed that the decreases in the levels of lipid biomolecules with links to inflammation originated from two major pathways that converge in the production of AA. The eicosanoid biosynthesis pathway centers on the metabolism of PUFA precursors and is thus important in modulating host inflammatory status. We choose to further explore the link between these inflammatory lipids and their relationship to infection. The second pathway involves the PLA2 breakdown of phospholipid membranes, which can also produce AA and, in addition, release lysolipids. Lysolipids are not related to eicosanoids but can serve functions similar to those of inflammatory mediators. The metabolomics findings presented here support the idea of the importance of AA, since decreases in levels remained constant for both time points of vegetative cell infection, suggesting a contribution of both pathways to AA production. Decreases observed in levels of ω-6 PUFAs and lysolipids from infected blood during 3 dpi suggests that both arms of AA production were disrupted. These results are consistent with other metabolomics studies of infection whose results have indicated the importance of eicosanoids and lysolipids during infection (45, 50, 63). However, it appears that decreases in levels of eicosanoids and lysolipids are not universal traits during bacterial infections. Serum profiles of patients infected with Mycobacterium leprae revealed increased levels of anti-inflammatory PUFAs and accumulation of phospholipids (12, 45). Feces and liver profiles from mice subjected to infection with Salmonella enterica serovar Typhimurium showed increases in levels of eicosanoid biosynthesis products (50). Not all types of infections evoke the activity of PLA2 enzymes as an immune defense mechanism against invading pathogens. It has been shown that PLA2 is a crucial factor in hepatitis C virus (HCV) virion core protein production and envelopment, as well as for the secretion of particles from host cells (64). Ultimately, PLA2 is an important prerequisite host component needed for improving the infectivity of HCV. This suggests that downmodulation of eicosanoids and lysolipids is specific to B. anthracis and thus may be used as a biomarker to differentiate B. anthracis infections from other types of infection. While we chose to focus on possible mechanisms of B. anthracis targeting host lipid production, an equally plausible alternative hypothesis is that decreases seen in levels of lipids may be due to lipid consumption by the host and/or bacteria. Perhaps the host needs to consume more lipids to compensate for the energy losses observed in other areas of metabolism. Additional experimentation would be needed to address these issues.
While changes associated with lipids found in whole blood were the primary focus of this study, there were also many biochemicals from whole organs that were substantially changed in response to infection. We chose to analyze the lung, liver, kidneys, and spleen for three main reasons. First, we wanted to understand how infection altered the host's metabolism on a systematic level. Therefore, we chose to analyze these major organs due to their important physiological functions. Second, our metabolomics experimental design was intended to be untargeted so as to cast a wide net. Surveying other tissues would have been beyond what was feasible to perform in this current study. That said, it will be interesting to determine what metabolites are altered in other tissues and cells relevant to anthrax disease, including regional lymph nodes and macrophages, respectively.
We discovered that organ metabolites involved in energy metabolism showed differential levels among the experimental groups, which could correspond to changes to the inflammatory status observed in blood. Global analysis of A/J mice infected with B. anthracis spores showed that the metabolic profiles of the kidneys, liver, and, to a lesser extent, spleen revealed complex networks involved in energy metabolism. Lung tissues from infected mice were considered relatively unchanged compared to the control and were excluded from any further analysis. Given that subcutaneous sublethal infections were used, this lack of change suggests that host responses were not yet active in the lungs. Renal glucose metabolism plays a significant role in the energy production and storage involved in maintaining homeostatic functions and responding to pathological alterations (65, 66). Increased levels of metabolites involved in gluconeogenesis in infected kidneys could reflect the host's growing energy consumption needs in response to the invading pathogen. Although the liver is considered the more prominent site of gluconeogenesis, renal glucose release can contribute to ∼20% of the total glucose released into the circulation (65).
Energy production, consumption, and storage are split between the use of carbohydrates and the use of lipids. CoA, an important coenzyme present in both arms of energy production, was found to be decreased in abundance in infected liver tissues relative to the control. CoA plays a role in β-oxidation and in addition feeds into the first step of the tricarboxylic acid (TCA) cycle (Fig. 6) (42). Alterations in its levels are consistent with decreased free fatty acid levels in blood later during infection, supporting the notion of compromised liver function and interconnection between major metabolic pathways and tissues. Additionally, decreases in levels of starch byproducts (maltooligosaccharides) indicate alterations in glycogen metabolism, suggesting that the depleted liver glycogen stores were not replenished, as levels of glucose and gluconeogenesis intermediates were also decreased during infection. Our results are in agreement with those of other studies that show that systemic infection can lead to decreased liver gluconeogenic function, suggesting that B. anthracis acts similarly during the course of spore infection (67–69).
Although we chose to focus our interpretation on energy metabolism in these organ systems, there were many other pathways found in our analysis that exceed the scope of this paper. It is possible that the complex metabolic networks that span organs and whole blood are interconnected. To our knowledge, this concept has not been investigated extensively during a B. anthracis infection. Organ-specific effects, such as in microenvironments in certain organ tissues, and global effects may be occurring simultaneously, but it is possible that these organ-specific effects can translate to global changes. Functional studies would be required to confirm which of these scenarios plays the more prominent role during infection.
In summary, we provide the first systemic, multiorgan metabolomic analysis of an infected mammal, using B. anthracis as a model bacterial pathogen. Downmodulation of lipid mediators of the host immune response was observed and was functionally confirmed to result in enhanced disease when the enzymes that generate these lipids were inhibited. We propose the use of metabolomics to identify new pathogenic processes and biomarkers for infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health to A.W.M. (AI097167 and AI096314) and seed funds from Baylor College of Medicine's Alkek Center for Metagenomics and Microbiome Research.
Animal studies were performed with the assistance of Sabrina Green. B. anthracis Sterne 7702 and toxin-deficient mutant strains were generously given by Shauna McGillivray of Texas Christian University.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00947-15.
REFERENCES
- 1.Hollywood K, Brison DR, Goodacre R. 2006. Metabolomics: current technologies and future trends. Proteomics 6:4716–4723. doi: 10.1002/pmic.200600106. [DOI] [PubMed] [Google Scholar]
- 2.Fontana JM, Alexander E, Salvatore M. 2012. Translational research in infectious disease: current paradigms and challenges ahead. Transl Res 159:430–453. doi: 10.1016/j.trsl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang A, Sun H, Wang P, Han Y, Wang X. 2012. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics 75:1079–1088. doi: 10.1016/j.jprot.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Jansen JJ, Szymańska E, Hoefsloot HCJ, Smilde AK. 2012. Individual differences in metabolomics: individualised responses and between-metabolite relationships. Metabolomics 8:94–104. doi: 10.1007/s11306-012-0414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockerill FR III, Smith TF. 2004. Response of the clinical microbiology laboratory to emerging (new) and reemerging infectious diseases. J Clin Microbiol 42:2359–2365. doi: 10.1128/JCM.42.6.2359-2365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maertzdorf J, Weiner J, Kaufmann SHE. 2012. Enabling biomarkers for tuberculosis control. Int J Tuberc Lung Dis 16:1140–1148. doi: 10.5588/ijtld.12.0246. [DOI] [PubMed] [Google Scholar]
- 7.Christaki E, Giamarellos-Bourboulis EJ. 2014. The beginning of personalized medicine in sepsis: small steps to a bright future. Clin Genet 86:56–61. doi: 10.1111/cge.12368. [DOI] [PubMed] [Google Scholar]
- 8.Schmerler D, Neugebauer S, Ludewig K, Bremer-Streck S, Brunkhorst FM, Kiehntopf M. 2012. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J Lipid Res 53:1369–1375. doi: 10.1194/jlr.P023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seymour CW, Yende S, Scott MJ, Pribis J, Mohney RP, Bell LN, Chen YF, Zuckerbraun BS, Bigbee WL, Yealy DM, Weissfeld L, Kellum JA, Angus DC. 2013. Metabolomics in pneumonia and sepsis: an analysis of the GenIMS cohort study. Intensive Care Med 39:1423–1434. doi: 10.1007/s00134-013-2935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller C, Dietz I, Tziotis D, Moritz F, Rupp J, Schmitt-Kopplin P. 2013. Molecular cartography in acute Chlamydia pneumoniae infections—a non-targeted metabolomics approach. Anal Bioanal Chem 405:5119–5131. doi: 10.1007/s00216-013-6732-5. [DOI] [PubMed] [Google Scholar]
- 11.Hoerr V, Zbytnuik L, Leger C, Tam PPC, Kubes P, Vogel HJ. 2012. Gram-negative and gram-positive bacterial infections give rise to a different metabolic response in a mouse model. J Proteome Res 11:3231–3245. doi: 10.1021/pr201274r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Mubarak R, Vander Heiden J, Broeckling CD, Balagon M, Brennan PJ, Vissa VD. 2011. Serum metabolomics reveals higher levels of polyunsaturated fatty acids in lepromatous leprosy: potential markers for susceptibility and pathogenesis. PLoS Negl Trop Dis 5:e1303. doi: 10.1371/journal.pntd.0001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidi-Rontani C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol 10:405–409. doi: 10.1016/S0966-842X(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 14.Koehler TM. 2009. Bacillus anthracis physiology and genetics. Mol Aspects Med 30:386–396. doi: 10.1016/j.mam.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolstø A-B, Tourasse NJ, Økstad OA. 2009. What sets Bacillus anthracis apart from other Bacillus species? Annu Rev Microbiol 63:451–476. doi: 10.1146/annurev.micro.091208.073255. [DOI] [PubMed] [Google Scholar]
- 16.Duong S, Chiaraviglio L, Kirby JE. 2006. Histopathology in a murine model of anthrax. Int J Exp Pathol 87:131–137. doi: 10.1111/j.0959-9673.2006.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidi-Rontani C, Weber-Levy M, Labruyère E, Mock M. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol Microbiol 31:9–17. doi: 10.1046/j.1365-2958.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz M. 2009. Dr. Jekyll and Mr. Hyde: a short history of anthrax. Mol Aspects Med 30:347–355. [DOI] [PubMed] [Google Scholar]
- 19.Guichard A, Nizet V, Bier E. 2012. New insights into the biological effects of anthrax toxins: linking cellular to organismal responses. Microbes Infect 14:97–118. doi: 10.1016/j.micinf.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tournier J-N, Rossi Paccani S, Quesnel-Hellmann A, Baldari CT. 2009. Anthrax toxins: a weapon to systematically dismantle the host immune defenses. Mol Aspects Med 30:456–466. doi: 10.1016/j.mam.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Ribot WJ, Panchal RG, Brittingham KC, Ruthel G, Kenny TA, Lane D, Curry B, Hoover TA, Friedlander AM, Bavari S. 2006. Anthrax lethal toxin impairs innate immune functions of alveolar macrophages and facilitates Bacillus anthracis survival. Infect Immun 74:5029–5034. doi: 10.1128/IAI.00275-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. 1999. Anthrax lethal factor cleaves the N-terminus of MAPKKS and induces tyrosine/threonine phosphorylation of MAPKS in cultured macrophages. J Appl Microbiol 87:288. doi: 10.1046/j.1365-2672.1999.00893.x. [DOI] [PubMed] [Google Scholar]
- 23.Tonello F, Montecucco C. 2009. The anthrax lethal factor and its MAPK kinase-specific metalloprotease activity. Mol Aspects Med 30:431–438. doi: 10.1016/j.mam.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins JF, McNally EM, Tang W-J, Leppla SH. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol 167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Moayeri M, Leppla SH. 2014. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 22:317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidi-Rontani C, Levy M, Ohayon H, Mock M. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol Microbiol 42:931–938. doi: 10.1046/j.1365-2958.2001.02695.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim HU, Goepfert JM. 1974. A sporulation medium for Bacillus anthracis. J Appl Bacteriol 37:265–267. doi: 10.1111/j.1365-2672.1974.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 28.Reed L, Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27:493–497. [Google Scholar]
- 29.Gage GJ, Kipke DR, Shain W. 2012. Whole animal perfusion fixation for rodents. J Vis Exp 2012:3564. doi: 10.3791/3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balderas MA, Nobles CL, Honsa ES, Alicki ER, Maresso AW. 2012. Hal is a Bacillus anthracis heme acquisition protein. J Bacteriol 194:5513–5521. doi: 10.1128/JB.00685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. 2009. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 32.Watson LE, Kuo S-R, Katki K, Dang T, Park SK, Dostal DE, Tang W-J, Leppla SH, Frankel AE. 2007. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS One 2:e466. doi: 10.1371/journal.pone.0000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbull PC, Broster MG, Carman JA, Manchee RJ, Melling J. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect Immun 52:356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunow R, Porsch-Ozcürümez M, Splettstoesser W, Buckendahl A, Hahn U, Beyer W, Böhm R, Huber M, vd Esche U, Bessler W, Frangoulidis D, Finke EJ. 2007. Monitoring of ELISA-reactive antibodies against anthrax protective antigen (PA), lethal factor (LF), and toxin-neutralising antibodies in serum of individuals vaccinated against anthrax with the PA-based UK anthrax vaccine. Vaccine 25:3679–3683. doi: 10.1016/j.vaccine.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 35.Ristroph JD, Ivins BE. 1983. Elaboration of Bacillus anthracis antigens in a new, defined culture medium. Infect Immun 39:483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wold S, Esbensen K, Geladi P. 1987. Principal component analysis. Chemometrics Intell Lab Syst 2:37–52. doi: 10.1016/0169-7439(87)80084-9. [DOI] [Google Scholar]
- 37.Chand HS, Drysdale M, Lovchik J, Koehler TM, Lipscomb MF, Lyons CR. 2009. Discriminating virulence mechanisms among Bacillus anthracis strains by using a murine subcutaneous infection model. Infect Immun 77:429–435. doi: 10.1128/IAI.00647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cote CK, Welkos SL, Bozue J. 2011. Key aspects of the molecular and cellular basis of inhalational anthrax. Microbes Infect 13:1146–1155. doi: 10.1016/j.micinf.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Vescovo G, Ravara B, Gobbo V, Sandri M, Angelini A, Della Barbera M, Dona M, Peluso G, Calvani M, Mosconi L, Dalla Libera L. 2002. l-Carnitine: a potential treatment for blocking apoptosis and preventing skeletal muscle myopathy in heart failure. Am J Physiol Cell Physiol 283:C802–C810. doi: 10.1152/ajpcell.00046.2002. [DOI] [PubMed] [Google Scholar]
- 40.Mutomba MC, Yuan H, Konyavko M, Adachi S, Yokoyama CB, Esser V, McGarry JD, Babior BM, Gottlieb RA. 2000. Regulation of the activity of caspases by l-carnitine and palmitoylcarnitine. FEBS Lett 478:19–25. doi: 10.1016/S0014-5793(00)01817-2. [DOI] [PubMed] [Google Scholar]
- 41.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. 1997. Ceramide signaling in apoptosis. Br Med Bull 53:539–553. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 42.Stansbie D, Brownsey RW, Crettaz M, Denton RM. 1976. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J 160:413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritsche K. 2006. Fatty acids as modulators of the immune response. Annu Rev Nutr 26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- 44.Frayn KN, Arner P, Yki-Järvinen H. 2006. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem 42:89–103. doi: 10.1042/bse0420089. [DOI] [PubMed] [Google Scholar]
- 45.Amaral JJ, Antunes LCM, de Macedo CS, Mattos KA, Han J, Pan J, Candéa ALP, Henriques MDGMO, Ribeiro-Alves M, Borchers CH, Sarno EN, Bozza PT, Finlay BB, Pessolani MCV. 2013. Metabonomics reveals drastic changes in anti-inflammatory/pro-resolving polyunsaturated fatty acids-derived lipid mediators in leprosy disease. PLoS Negl Trop Dis 7:e2381. doi: 10.1371/journal.pntd.0002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saddik M, Lopaschuk GD. 1991. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266:8162–8170. [PubMed] [Google Scholar]
- 47.Matthan NR, Resteghini N, Robertson M, Ford I, Shepherd J, Packard C, Buckley BM, Jukema JW, Lichtenstein AH, Schaefer EJ. 2010. Cholesterol absorption and synthesis markers in individuals with and without a CHD event during pravastatin therapy: insights from the PROSPER trial. J Lipid Res 51:202–209. doi: 10.1194/jlr.M900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurley BP, McCormick BA. 2008. Multiple roles of phospholipase A2 during lung infection and inflammation. Infect Immun 76:2259–2272. doi: 10.1128/IAI.00059-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demirev PA, Fenselau C. 2008. Mass spectrometry for rapid characterization of microorganisms. Annu Rev Anal Chem (Palo Alto Calif) 1:71–93. doi: 10.1146/annurev.anchem.1.031207.112838. [DOI] [PubMed] [Google Scholar]
- 50.Antunes LCM, Arena ET, Menendez A, Han J, Ferreira RBR, Buckner MMC, Lolic P, Madilao LL, Bohlmann J, Borchers CH, Finlay BB. 2011. Impact of salmonella infection on host hormone metabolism revealed by metabolomics. Infect Immun 79:1759–1769. doi: 10.1128/IAI.01373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin JH, Yang JY, Jeon BY, Yoon YJ, Cho SN, Kang YH, Ryu DH, Hwang GS. 2011. 1H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J Proteome Res 10:2238–2247. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- 52.Twenhafel NA. 2010. Pathology of inhalational anthrax animal models. Vet Pathol 47:819–830. doi: 10.1177/0300985810378112. [DOI] [PubMed] [Google Scholar]
- 53.Ghosh M, Tucker DE, Burchett SA, Leslie CC. 2006. Properties of the group IV phospholipase A 2 family. Prog Lipid Res 45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 54.van der Meer-Janssen YPM, van Galen J, Batenburg JJ, Helms JB. 2010. Lipids in host-pathogen interactions: pathogens exploit the complexity of the host cell lipidome. Prog Lipid Res 49:1–26. doi: 10.1016/j.plipres.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison LM, Balan KV, Babu US. 2013. Dietary fatty acids and immune response to food-borne bacterial infections. Nutrients 5:1801–1822. doi: 10.3390/nu5051801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gimenez AP, Wu Y, Paya M, Delclaux C, Touqui L, Goossens PL. 2004. High bactericidal efficiency of type iia phospholipase A2 against Bacillus anthracis and inhibition of its secretion by the lethal toxin. J Immunol 173:521–530. doi: 10.4049/jimmunol.173.1.521. [DOI] [PubMed] [Google Scholar]
- 57.Kelley DS. 2001. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition 17:669–673. doi: 10.1016/S0899-9007(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Raymond B, Goossens PL, Njamkepo E, Guiso N, Paya M, Touqui L. 2010. Type-IIA secreted phospholipase A 2 is an endogenous antibiotic-like protein of the host. Biochimie 92:583–587. doi: 10.1016/j.biochi.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 59.Leslie CC. 1997. Properties and regulation of cytosolic phospholipase A2. J Biol Chem 272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- 60.Shin S, Kim YB, Hur GH. 1999. Involvement of phospholipase A2 activation in anthrax lethal toxin-induced cytotoxicity. Cell Biol Toxicol 15:19–29. doi: 10.1023/A:1007546505528. [DOI] [PubMed] [Google Scholar]
- 61.Murakami M, Taketomi Y, Sato H, Yamamoto K. 2011. Secreted phospholipase A2 revisited. J Biochem 150:233–255. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- 62.Cui X, Li Y, Li X, Haley M, Moayeri M, Fitz Y, Leppla SH, Eichacker PQ. 2006. Sublethal doses of Bacillus anthracis lethal toxin inhibit inflammation with lipopolysaccharide and Escherichia coli challenge but have opposite effects on survival. J Infect Dis 193:829–840. doi: 10.1086/500468. [DOI] [PubMed] [Google Scholar]
- 63.Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. 2013. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 13:203. doi: 10.1186/1471-2334-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menzel N, Fischl W, Hueging K, Bankwitz D, Frentzen A, Haid S, Gentzsch J, Kaderali L, Bartenschlager R, Pietschmann T. 2012. MAP-kinase regulated cytosolic phospholipase A2 activity is essential for production of infectious Hepatitis C virus particles. PLoS Pathog 8:e1002829. doi: 10.1371/journal.ppat.1002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerich J, Meyer C, Woerle H, Stumvoll M. 2001. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24:382–391. doi: 10.2337/diacare.24.2.382. [DOI] [PubMed] [Google Scholar]
- 66.Ekberg K, Landau BR, Wajngot A, Chandramouli V, Efendic S, Brunengraber H, Wahren J. 1999. Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 48:292–298. doi: 10.2337/diabetes.48.2.292. [DOI] [PubMed] [Google Scholar]
- 67.Guillem JG, Clemens MG, Chaudry IH, McDermott PH, Baue AE. 1982. Hepatic gluconeogenic capability in sepsis is depressed before changes in oxidative capability. J Trauma 22:723–729. doi: 10.1097/00005373-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 68.Jones CG, Titheradge MA. 1993. The effect of treatment of the rat with bacterial endotoxin on gluconeogenesis and pyruvate metabolism in subsequently isolated hepatocytes. Biochem J 289:169–172. doi: 10.1042/bj2890169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kushner I. 1982. The phenomenon of the acute phase response. Ann N Y Acad Sci 389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- 70.Maślanka K, Smoleńska-Sym G, Michur H, Wróbel A, Lachert E, Brojer E. 2012. Lysophosphatidylcholines: bioactive lipids generated during storage of blood components. Arch Immunol Ther Exp (Warsz) 60:55–60. doi: 10.1007/s00005-011-0154-x. [DOI] [PubMed] [Google Scholar]
- 71.Drobnik W, Liebisch G, Audebert F-X, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. 2003. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res 44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.