Abstract
Campylobacter jejuni is the most common cause of bacterium-induced gastroenteritis, and while typically self-limiting, C. jejuni infections are associated with postinfectious intestinal disorders, including flares in patients with inflammatory bowel disease and postinfectious irritable bowel syndrome (PI-IBS), via mechanisms that remain obscure. Based on the hypothesis that acute campylobacteriosis may cause pathogenic microbiota dysbiosis, we investigated whether C. jejuni may activate dormant virulence genes in noninvasive Escherichia coli and examined the epithelial pathophysiological consequences of these alterations. Microarray and quantitative real-time PCR analyses revealed that E. coli adhesin, flagellum, and hemolysin gene expression were increased when E. coli was exposed to C. jejuni-conditioned medium. Increased development of bacterial flagella upon exposure to live C. jejuni or C. jejuni-conditioned medium was observed under transmission electron microscopy. Atomic force microscopy demonstrated that the forces of bacterial adhesion to colonic T84 enterocytes, and the work required to rupture this adhesion, were significantly increased in E. coli exposed to C. jejuni-conditioned media. Finally, C. jejuni-modified E. coli disrupted TLR4 gene expression and induced proinflammatory CXCL-8 gene expression in colonic enterocytes. Together, these data suggest that exposure to live C. jejuni, and/or to its secretory-excretory products, may activate latent virulence genes in noninvasive E. coli and that these alterations may directly trigger proinflammatory signaling in intestinal epithelia. These observations shed new light on mechanisms that may contribute, at least in part, to postcampylobacteriosis inflammatory disorders.
INTRODUCTION
Campylobacter jejuni-induced diarrheal disease (i.e., campylobacteriosis) is a major cause of morbidity worldwide (1). Within the United States alone, more than 800,000 cases of campylobacteriosis are domestically acquired each year, and the annual financial burden of the infection is US$6.9 million (2, 3). Campylobacter is a microaerophilic commensal bacterium of the gastrointestinal tract of food-producing animals such as poultry and cattle, and zoonotic transmission of this pathogen is well established; campylobacteriosis is commonly acquired through ingestion of contaminated water, food, or milk (4, 5). Upon infection in the human host, Campylobacter induces an inflammatory response, which in turn leads to the development of acute symptoms, including diarrhea, abdominal pain, and fever (6, 7). Although Campylobacter infections are generally self-limiting, campylobacteriosis may lead to serious long-term complications via mechanisms that remain obscure. Postinfectious intestinal and extraintestinal sequelae include Guillain-Barré paralysis, reactive arthritis, postinfectious irritable bowel syndrome (PI-IBS), or flares in patients with inflammatory bowel disease (IBD) (8–14). PI-IBS is a functional disorder of the gastrointestinal tract that is characterized by symptoms of abdominal pain, bloating, and disturbed bowel movements (i.e., diarrhea and/or constipation) (15). IBD, which comprises Crohn's disease and ulcerative colitis, is characterized by overt intestinal inflammation and arises from genetic, immune, and environmental disturbances, including microbial factors that have yet to be defined (16, 17). Although IBS and IBD are distinct intestinal disorders, it is interesting that a number of IBD patients in clinical remission experience IBS (18, 19) and that in both instances postinfectious events may cause disease activation (20, 21). Research into the microbial mechanisms responsible for postinfectious inflammatory sequelae in the gut will shed new light on the pathophysiology of both IBS and IBD.
It is well established that the intestinal microbiota has an important role in human health and disease, and while research over the past decade has failed to define a specific cause-and-effect relationship for a single pathogen, several research groups have suggested individuals with IBD or IBS have lower proportions of protective, anti-inflammatory bacteria and higher proportions of aggressive, proinflammatory bacteria (22–24). Specifically, the abundance of Faecalibacterium prausnitzii, which is a member of the Firmicutes phylum and has been shown to have anti-inflammatory effects, is reduced in IBD (25, 26). Conversely, Proteobacteria prevalence, and particularly Escherichia coli abundance, is increased in Crohn's disease patients (24, 27, 28).
Intestinal epithelial cells maintain a selective barrier that allows for the transport of ions, nutrients, and water, while separating luminal antigens from underlying immune cells and host tissues. Tight-junction proteins connect epithelial cells and limit solute flux by exerting size and charge selectivity (29). Disruptions of epithelial tight junctions, and the resulting facilitation of paracellular transport of large solutes (e.g., macromolecules, bacterial products, or food antigens) across the epithelium have been implicated in the pathophysiology of numerous infectious gastrointestinal disorders, including campylobacteriosis (30). Recent research has shown that C. jejuni can disrupt tight-junction proteins, including occludin and claudin-4, which in turn facilitates the paracellular translocation of noninvasive E. coli (31, 32). C. jejuni can also promote the transcellular uptake of noninvasive bacteria via lipid-raft-mediated endocytosis or by highjacking the host physiological processes of antigen sampling via M cells (microfold cells) (33, 34). Therefore, we hypothesize that C. jejuni may induce inflammation directed toward invading commensal bacteria, after the acute C. jejuni infection has been cleared.
Intestinal epithelial cells express pattern-recognition receptors (PRRs) that recognize evolutionarily conserved microbe-associated molecular patterns and initiate signaling cascades that promote host antimicrobial defenses (35, 36). There are several classes of PRRs, including transmembrane proteins, such as Toll-like receptors (TLRs) and C-type lectin receptors, as well as cytoplasmic proteins, including retinoic acid-inducible gene (RIG)-I-like receptors and NOD-like (37). TLR dysfunction has been implicated in IBD pathogenesis whereby aberrant TLR signaling may contribute to chronic intestinal inflammation (38). Differential TLR4 expression has been identified in biopsy specimens from IBD patients, and TLR9 polymorphisms have been associated with an increased risk of IBS and IBD (39–42). TLR4 recognizes Gram-negative bacterial lipopolysaccharide, while TLR9 recognizes unmethylated bacterial cytosine-phosphate-guanine (CpG) DNA (43–45). Aberrant TLR signaling may have a genetic origin, but recent research also found that C. jejuni reduces surface TLR9 expression, which in turn predisposes the gut to more severe inflammation upon a mild proinflammatory stimulus in a murine model of colitis (46).
Although the findings described above have provided new insight into the mechanisms of C. jejuni-induced postinfectious intestinal inflammation, little is known of the effects that enteric pathogens may have on commensal microbiota. In a process termed quorum sensing, bacteria are able to sense chemical signals from other organisms within the environment and can consequently modulate their gene expression (47). Bacteria can optimize the expression of genes related to virulence, whereby bacterial survival is maximized and energy cost is minimized. It is also interesting that bacteria can respond to signals within or between species and even between kingdoms (48–50). This observation has great implications for the pathophysiology of postinfectious intestinal inflammation as quorum sensing provides a viable mechanism whereby an enteric pathogen may directly modulate virulence in commensal microbes. Moreover, inflammation induced by an enteric pathogen may subsequently alter virulence in commensal microbes. Indeed, it was recently shown that intestinal inflammation promotes horizontal gene transfer between pathogenic and commensal Enterobacteriaceae (51), raising the possibility that commensal E. coli may not always be innocuous, and may actually become an opportunistic pathobiont upon exposure to environmental stimuli.
Bacterial adhesins are filamentous appendages expressed on the surface of most commensal and pathogenic bacteria, which facilitate mucosal colonization, a key precursor to cell invasion by pathogens (52, 53). Flagella promote bacterial motility, helping the bacteria swim through the protective mucus layer that lines intestinal epithelial cells, and this promotes host cell adhesion and invasion (54, 55). Bacterial secreted toxins, such as pore-forming hemolysins, can promote host cell damage and subsequent cell invasion or suppression of host inflammatory response (56). The aims of the present study were (i) to investigate the effects of C. jejuni on latent virulence factors, including adhesins, flagella, and hemolysins, in noninvasive Escherichia coli and (ii) to investigate the pathophysiological effects of C. jejuni-modified-E. coli on human colonic epithelial cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Experiments were performed using Campylobacter jejuni strain 81-176 and Escherichia coli strain HB101 as previously described (33). C. jejuni 81-176, which was originally isolated from a case of campylobacteriosis associated with drinking unpasteurized milk, is a pathogenic strain capable of inducing disease (6, 57). E. coli HB101 is a common laboratory strain derived from E. coli K-12, originally isolated from a convalescent diphtheria patient (58, 59). E. coli K-12 is typically regarded as commensal, or nonpathogenic, since it does not functionally express common E. coli virulence factors (60). C. jejuni strain 81-176 was grown in Difco Columbia broth (Becton Dickinson [BD], Mississauga, Ontario, Canada) for 14 to 16 h (37°C, 125 rpm) in a microaerobic gas generating system (Oxoid, Nepean, Ontario, Canada). C. jejuni conditioned medium (C. jejuni-CM) was prepared by filtering C. jejuni inoculum with 0.1-μm-pore-size polyethersulfone membranes (Fisher Scientific, Toronto, Ontario, Canada). E. coli HB101 was grown in three different conditions: (i) as a monoculture (i.e., E. coli) in Columbia broth, (ii) as a coculture with C. jejuni 81-176 in Columbia broth, or (iii) in C. jejuni-conditioned Columbia broth media (CM). Cultures were grown for 14 to 16 h (37°C, 125 rpm).
Microarray gene expression analysis.
Since genome-wide gene expression profiles provide a broad view of virulence factors that are involved in human infections (61), Affymetrix GeneChip E. coli Genome 2.0 arrays (Santa Clara, CA) were used to assess changes in E. coli gene expression upon exposure to C. jejuni-CM. The E. coli microarrays detect changes in 20,366 genes from 10,208 probe sets. E. coli was grown in Columbia broth or C. jejuni-conditioned Columbia broth (i.e., C. jejuni-CM) and normalized to 5 × 108 CFU. Total RNA isolation, cDNA synthesis, cDNA fragmentation, and terminal labeling were performed according to the manufacturer's recommendations for prokaryotic target preparation (Affymetrix). Briefly, total RNA was isolated from E. coli using an RNeasy mini spin kit (Qiagen, Toronto, Ontario, Canada), and the RNA concentration/quality was assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). RNA was considered acceptable quality if the A260/A280 ratio was between 1.8 and 2.1. Subsequent cDNA synthesis was performed on 10 μg of total RNA, using random hexamer primers, 10 mM deoxynucleoside triphosphate mix (2′-deoxynucleoside-5′triphosphate), SuperScript II reverse transcriptase, SUPERase-In RNase inhibitor (all from Life Technologies, Burlington, Ontario, Canada). Poly(A) RNA controls (Affymetrix) were used in the reaction mixture as a downstream indicator of labeling reaction efficiency. RNA was then depleted from cDNA synthesis product using 1 N sodium hydroxide, and this reaction mixture was neutralized with 1 N hydrochloride. A MinElute PCR kit (Qiagen) was used to purify the cDNA, and the purified cDNA product was eluted with 12 μl of elution buffer (buffer EB; 10 mM Tris-Cl [pH 8.5]). Purified cDNA was quantified to ensure adequate levels of cDNA product were present. The entire cDNA product (≥1.5 μg) was fragmented using 10× DNase I buffer (Affymetrix). Terminal labeling of the fragmented cDNA product was performed using GeneChip DNA labeling reagent (Affymetrix) and terminal deoxynucleotidyltransferase (Promega, Madison, WI). Target hybridization, washing, staining, and scanning were performed at the Southern Alberta Cancer Research Institute Microarray and Genomics Facility (Calgary, Alberta, Canada) according to manufacturer's recommendations for a 169 format array.
Genespring GX9 (Agilent Technologies, Santa Clara, CA) was used to analyze Affymetrix CEL files. A robust multiarray average summarization algorithm and a baseline transformation to the median of all samples were used to normalize each CEL file. Using a moderated t test without multiple testing correction, the fold change and P values were calculated for all probe sets for E. coli grown in C. jejuni-CM compared to C. jejuni grown in Columbia broth. Genes related to virulence (i.e., adhesin, flagella, hemolysin, or antibiotic/stress resistance) were identified, and a hypergeometric test was applied to the total number of genes whose expression had a fold change (FC) of >1.7 and a P value of <0.05 (t test).
Although only two gene chips were used for each experimental group, given the high cost of microarrays and relative insensitivity to small gene expression changes (62), additional biological replicates were not included. Rather, differential expression profiles were corroborated by alternative methodologies, including real-time PCR (RT-PCR), transmission electron microscopy (TEM), and atomic force microscopy (AFM).
Epithelial cell culture.
Human colonic T84 cells (American Type Culture Collection, Manassas, VA) were grown in Dulbecco modified Eagle medium (DMEM)/F-12 supplemented with 10% (vol/vol) fetal bovine serum (Mediatech, Inc., Manassas, VA), 200 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (all from Sigma-Aldrich, Oakville, Ontario, Canada). Cells were incubated at 37°C and 5% CO2. Culture medium was replenished every 2 to 3 days, and confluent monolayers were passaged with 0.25% trypsin-EDTA (Life Technologies). Trypsinized cells were seeded at a density of 105 cells/ml into 6-well (2 ml) or 12-well (1 ml) tissue-treated plates (Corning Life Sciences, Kennebunk, MA). Cells were also seeded onto a 25-mm round glass coverslip (VWR, Radnor, PA) that had been precoated with 0.2% gelatin (Sigma). Cells were used between passages 63 and 73.
Cell inoculation protocol.
Bacterial inoculum was normalized to 109 CFU and subsequently pelleted (i.e., sedimented) by centrifugation (8,000 × g for 3 min). Bacterial pellets were rinsed twice with Dulbecco phosphate-buffered saline (Dulbecco PBS; Sigma), and resuspended in PBS. Unless otherwise stated, confluent monolayers were rinsed twice with prewarmed (37°C) Dulbecco PBS and replaced with antibiotic/supplement-free DMEM/F-12. Monolayers were inoculated with bacterial culture to achieve a multiplicity of infection of 100 bacterial cells per enterocyte. Control monolayers received an equivalent volume of Dulbecco PBS. Inoculated cells were incubated at 37°C and 5% CO2 for 3 h.
Real-time PCR.
To assess changes in gene expression, real-time PCR was performed on bacterial RNA and T84 cell RNA. T84 monolayers were grown in 6-well or 12-well plates and inoculated with bacterial culture or PBS as described above. As a positive control for interleukin-8 (IL-8, or CXCL-8) expression, 100 ng of tumor necrosis factor alpha (TNF-α)/ml was added to the monolayer. After incubation, bacterial RNA and T84 cell RNA were collected. Bacterial RNA was stabilized using RNAprotect bacterial reagent (Qiagen). RNA was extracted from bacterial cells using an RNeasy mini spin kit (Qiagen) according the manufacturer's directions for enzymatic lysis and proteinase K digestion of bacteria. To collect T84 cell RNA, cells were lysed with RLT buffer (Qiagen), and T84 cells were subsequently homogenized using QIAshredder (Qiagen) spin columns. Cell RNA was purified using the RNeasy mini spin kit according to the manufacturer's recommendations. Total RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) and 1 μg of total RNA was reverse transcribed to cDNA using the QuantiTect reverse transcription kit (Qiagen). A QuantiFast SYBR green PCR kit (Qiagen) was used to amplify the cDNA on a Rotor Gene Q (Qiagen). PCRs were carried out in 25-μl volumes with forward and reverse primers (0.5 μM each; E. coli [Table 1] and T84 [Table 2]) and 1 μl of cDNA. If primers were not found in the literature, primers were designed using Primer3 software and specifying an amplicon size of 100 to 150 bp (63, 64). Primer specificity was checked using the NCBI (Bethesda, MD) Primer-BLAST database. Primers were only used if their efficiency was ca. 100%. Amplification conditions were as follows: 1 cycle at 95°C for 5 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. Melting curve analysis was conducted over a range of 55 to 95°C to assess the specificity of amplification.
TABLE 1.
Oligonucleotide primer sequences used for real-time PCR on E. coli cDNA
| Gene | Product | Primera |
Source or reference | |
|---|---|---|---|---|
| Orientationb | Sequence (5′-3′) | |||
| fimA | Major type 1 fimbrial subunit | F | GTT/GAT/GCA/GGC/TCT/GTT/GA | This study |
| R | AGC/GGC/TTT/AGA/TGC/AAC/AT | |||
| sfmF | Fimbria-like adhesion protein | F | TGC/ACG/GTA/ACG/TTG/TTG/AT | This study |
| R | ATC/GCC/AGC/GTT/CAG/TAG/TT | |||
| fliD | Flagellar cap protein | F | TTC/AGA/CGC/AGT/TGA/AAT/CG | This study |
| R | GAG/TTT/GTC/GGC/ATC/CAG/TT | |||
| hlyE | Hemolysin E | F | CCT/GGC/AGA/CCT/TTG/ATG/AAA/CC | 87 |
| R | CTG/GAA/TCA/GTT/TTC/CTT/CAA/CTA/C | |||
| cysG | Siroheme synthase (internal control) | F | GAA/AGC/CTT/CTC/GAC/ACC/TG | This study |
| R | CGT/TAC/AGA/AGA/TGC/GAC/GA | |||
Primers were ordered from the University Core DNA Services at the University of Calgary.
F, forward; R, reverse.
TABLE 2.
Oligonucleotide primer sequences used for real-time PCR on T84 cDNA
| Gene | Product | Primera |
Source or reference | |
|---|---|---|---|---|
| Orientationb | Sequence (5′–3′) | |||
| TLR4 | Toll-like receptor 4 (recognizes bacterial lipopolysaccharide) | F | CGG/AGG/CCA/TTA/TGC/TAT/GT | This study |
| R | TCC/CTT/CCT/CCT/TTT/CCC/TA | |||
| CXCL-8 | Interleukin-8 (inflammatory mediator) | F | ATG/ACT/TCC/AAG/CTG/GCC/GTG/GCT | 88 |
| R | TCT/CAG/CCC/TCT/TCA/AAA/ACT/TCT | |||
| β-Actin | Cytoskeletal protein (internal control) | F | CCT/GGC/ACC/CAG/CAC/AAT | 88 |
| R | GCC/GAT/CCA/CAC/GGA/GTA/CT | |||
Primers were ordered from the University Core DNA Services at the University of Calgary.
F, forward; R, reverse.
Quantitative RT-PCR results were analyzed using the comparative threshold cycle (CT) (65). Briefly, the relative gene expression was calculated by using the formula 2−ΔCT, where ΔCT is equivalent to the CT of the internal control subtracted from the CT of the gene of interest. Genes related to E. coli virulence were normalized to the cysG gene, which encodes a metabolic enzyme involved in siroheme synthesis. The cysG gene was recently identified to be a reliable reference gene in RT-PCR (65). Genes monitoring the host response (i.e., proinflammatory cytokines and host ligand receptors) were normalized to the housekeeping gene β-actin.
Transmission electron microscopy.
The bacterial inoculum was normalized to 108 CFU and subsequently pelleted by centrifugation (8,000 × g for 3 min). Bacterial pellets were rinsed twice with Dulbecco PBS (Sigma) and resuspended in 1 ml of PBS. A drop of bacterial suspension was placed on a carbon coated copper grid (Electron Microscopy Sciences, Hatfield, PA) for 10 min. Bacteria were fixed for 5 min in 10 mM cacodylate buffer (pH 7.5) containing 2.5% glutaraldehyde (Electron Microscopy Sciences). Grids were washed twice with water and negatively stained for 10 min with 1% phosphotungstic acid (Electron Microscopy Sciences). Grids were air dried and viewed using a Hitachi H-7650 transmission electron microscope operated at 60 kV. Grids with 30 to 130 E. coli organisms were assessed for the presence of flagella.
Atomic force microscopy.
Force spectroscopy measurements were performed using a Nanowizard II atomic force microscope equipped with a CellHesion module (JPK Instruments, Berlin, Germany). The AFM unit was mounted on a Zeiss Axiovert 200 inverted light microscope (Carl Zeiss, Thornwood, NY). Force spectroscopy measurements were conducted in a chamber maintained at 37°C and 5% CO2. To immobilize E. coli on an AFM cantilever, 20 μl of 0.01% poly-l-lysine (Sigma) was added to Arrow TL1 probes (Nanoworld, Neuchâtel, Switzerland), followed by incubation for 20 min at room temperature. After incubation, 2 × 107 E. coli CFU were added to the tip of the cantilever, followed by incubation at room temperature for 20 min.
To assess the efficiency of E. coli adherence to the AFM cantilever, scanning electron microscopy (SEM) was used. After E. coli was immobilized on the AFM cantilever, the AFM cantilever was incubated in DMEM/F-12. After incubation, the cantilever was fixed with 10 mM cacodylate buffer (pH 7.5) containing 2.5% glutaraldehyde (Electron Microscopy Sciences) for 10 min. Cantilevers were coated with gold in a sputter coater and visualized on an FEI XL30 SEM.
In the AFM experiments, each E. coli-coated AFM cantilever was calibrated in antibiotic/supplement-free DMEM/F-12 immediately prior to force spectroscopy measurements. Force curves were conducted with a set point of 1 nN, extend/retract rates of 5 μm/s, a Z length of 10 μm, and a sample rate of 512 Hz. All force measurements were conducted within 30 min of immobilization of E. coli cells on an AFM cantilever. Analysis of force curves was performed using software provided by JPK Instruments.
Statistical analysis.
All experiments were repeated at least three times on at least two separate occasions. The data are presented as means ± the standard errors of the mean. Statistical analyses were performed using GraphPad Prism version 5.0 software (GraphPad Software, Inc., La Jolla CA). A one-way analysis of variance with a Tukey multiple comparison test was used to compare the means of the control to the bacterium-treated samples. An unpaired Student t test was used to compare the means of the E. coli to those of the C. jejuni-treated E. coli samples. A P value of <0.05 was considered significant.
RESULTS
C. jejuni-CM activates latent virulence genes in E. coli.
Using Affymetrix (E. coli Genome 2.0) microarrays, studies assessed the effect C. jejuni-CM has on virulence gene expression of nonpathogenic E. coli. Genes encoding adhesins (e.g., fimH), flagella (fliD), and hemolysins (e.g., hlyE) were significantly upregulated (FC > 1.7, P < 0.05) in E. coli grown in C. jejuni-CM compared to E. coli that had not been exposed to C. jejuni-CM (Table 3). Genes related to biofilm formation (e.g., pgaA) and antibiotic/stress resistance (e.g., mdtH) were also significantly upregulated (see Table S1 in the supplemental material). Although there were genes related to E. coli virulence that were downregulated (see Table S2 in the supplemental material), a hypergeometric analysis of significant gene modification (see Table S3 in the supplemental material) indicated there were more virulence genes upregulated in E. coli grown in C. jejuni-CM, compared to E. coli that had been grown as a monoculture in Columbia broth, than would be expected by chance alone (P = 0.008).
TABLE 3.
Microarray analysis of genes related to E. coli adhesion, motility, and cell lysis that were significantly upregulated upon exposure to C. jejuni-CM
| Type | Gene | Product | Fold change | P |
|---|---|---|---|---|
| Adhesin | ECs4480 | Putative adhesin | 2.01 | 0.005 |
| manY | Mannose-specific PTS enzyme IIC | 1.94 | 0.013 | |
| nlpE | Lipoprotein involved with copper homeostasis and adhesion | 1.89 | 0.013 | |
| fimH | d-Mannose-specific adhesin | 1.70 | 0.037 | |
| Flagellum | fliD | Flagellar capping protein | 1.73 | 0.009 |
| Hemolysin | yqfA | Inner membrane protein, hemolysin III family HlyIIII | 2.80 | 0.004 |
| hlyE | Hemolysin E | 1.88 | 0.019 |
C. jejuni-CM promotes the expression of genes related to E. coli virulence in the presence of enterocytes in vitro.
To validate changes in gene expression, RT-PCR analysis was performed on genes encoding E. coli adhesin (fimA and sfmF), flagella (fliD), and hemolysin (hlyE) in the presence of T84 colonic epithelial cells. Expression of E. coli fimA, the major subunit of type 1 fimbriae, was increased ∼4-fold when E. coli was grown in C. jejuni-CM compared to untreated E. coli (P = 0.0341; Fig. 1A). Expression of the E. coli adhesin gene sfmF was also significantly increased upon exposure to C. jejuni-CM (P = 0.0212; Fig. 1B). The E. coli flagellin fliD gene exhibited an ∼1.5-fold increase in gene expression when E. coli was grown in C. jejuni-CM, versus untreated E. coli; however, this change failed to reach statistical significance (P = 0.066; Fig. 1C). The hemolysin gene (hlyE) expression was increased ∼2-fold when E. coli was grown in C. jejuni-CM compared to control E. coli (P = 0.0284; Fig. 1D).
FIG 1.
Exposure to C. jejuni-CM increased the expression of virulence genes in E. coli upon exposure to human colonic T84 monolayers. Changes in E. coli fimA (A), sfmF (B), fliD (C), and hlyE (D) gene expression were assessed. The results were analyzed using the comparative CT method of analysis. E. coli cysG was used as an internal reference gene. The data are represented as means ± the standard errors of the mean (n = 5 or 6). *, P < 0.05.
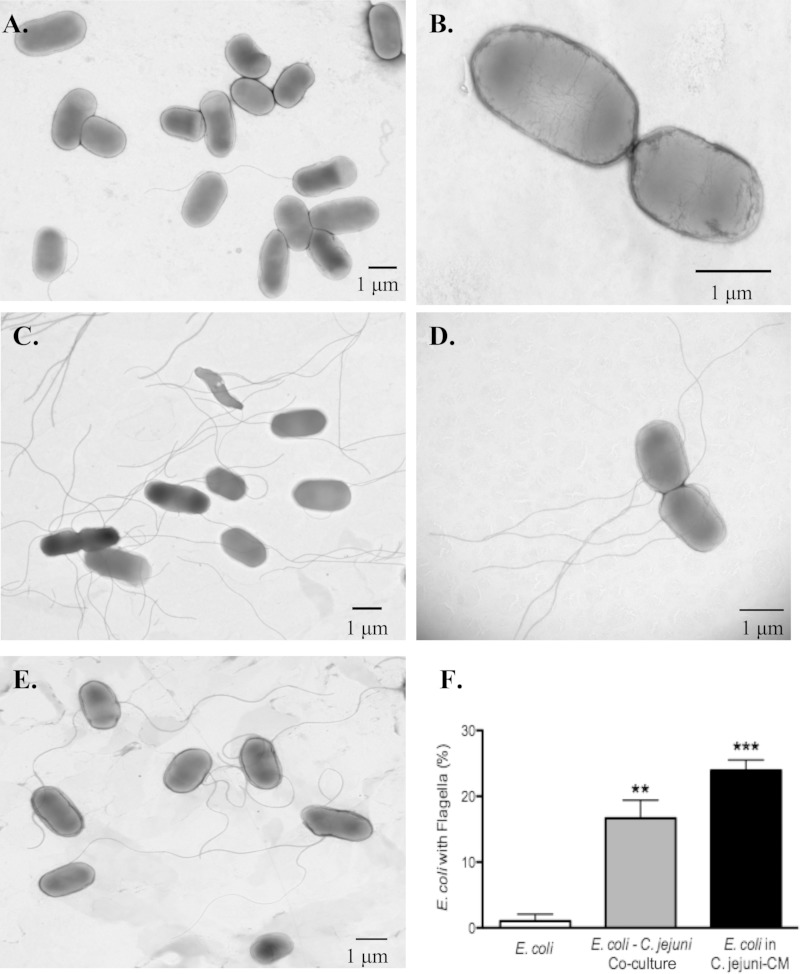
C. jejuni and C. jejuni-CM induce E. coli flagellar expression.
TEM was used to examine whether changes in gene expression corresponded to phenotypic changes in E. coli flagellar expression. When E. coli was grown as a monoculture in Columbia broth, most of the E. coli organisms were nonflagellated; however, a significant increase in flagellated bacteria was seen when E. coli was cocultured with C. jejuni or exposed to C. jejuni-CM (P < 0.05; Fig. 2).
FIG 2.
Exposure to C. jejuni and C. jejuni-CM promotes expression of E. coli flagella. E. coli strain HB101 was grown as a monoculture (A and B), as a coculture with C. jejuni (C and D), or in C. jejuni-CM (E). The spiral-shaped C. jejuni (see panel C) could also be observed in the E. coli-C. jejuni coculture. (F) E. coli with flagella were enumerated at ×60,000 magnification. The data are represented as means ± the standard errors of the mean (n = 3). **, P < 0.01; ***, P < 0.001 compared to an E. coli monoculture that had not been exposed to C. jejuni-CM.
Exposure of E. coli to C. jejuni-CM increases adhesion forces to human enterocytes.
To assess the biological significance of increased adhesin gene expression in E. coli exposed to C. jejuni, in vitro experiments using AFM measured the force of adhesion between E. coli and human enterocytes. Since this represented the first attempt at obtaining such measurements between enteric bacteria and epithelial cells, preliminary experiments first determined that poly-l-lysine was an adequate adhesive for immobilization of E. coli to an AFM cantilever. Light microscopy was initially used to visualize bacterial adherence to the AFM cantilever, and alternative adhesives were excluded. Furthermore, SEM was used to visualize E. coli attached to the AFM cantilever after incubation in DMEM/F-12 to confirm that E. coli was not washed off the cantilever upon submersion in cell culture medium. SEM images showed similar numbers of E. coli organisms attached to the AFM cantilever, and E. coli remain glued to the AFM cantilever after a 60-min incubation in cell culture medium, regardless of E. coli growth condition (Fig. 3).
FIG 3.
Scanning electron microscopy of atomic force microscopy cantilevers confirms adhesion of E. coli to the cantilevers. Approximately 2 × 107 E. coli CFU were added to the tip of the cantilever precoated with poly-l-lysine. After a 20-min incubation in PBS, cantilevers were incubated in DMEM/F-12 for another hour (or not, to determine potential of the bacteria to wash off). Cantilevers were fixed with 2.5% glutaraldehyde, sputter coated with gold, and visualized on an FEI XL30 SEM device. Equivalent numbers of E. coli organisms grown alone (A) and E. coli organisms grown in C. jejuni-CM (B) were observed. Furthermore, cell culture medium did not rinse the E. coli off the cantilever, since equivalent numbers of E. coli organisms grown alone (C) and E. coli organisms grown in C. jejuni-CM (D) were still observed after a 60-min incubation in DMEM/F-12.
Force spectroscopy was used to measure the force of adhesion between E. coli and human colonic T84 cells. The maximum force of adhesion between the E. coli and T84 epithelial cells was significantly greater for E. coli grown in C. jejuni-CM than E. coli grown in untreated media (P < 0.0001; Fig. 4A). Moreover, the work required to disrupt the adhesion between E. coli and T84 cells was significantly greater (2-fold increase) for E. coli exposed to C. jejuni-CM (P = 0.0002; Fig. 4B).
FIG 4.
C. jejuni-CM increases the force of adhesion of E. coli to human enterocytes. The maximum force of adhesion (A) and the work required to disrupt the adhesive bond (B) were determined for E. coli grown in C. jejuni-CM, compared to E. coli that had not been exposed to C. jejuni-CM. The data are represented as means ± the standard errors of the mean (n = 12 to 15). *, P < 0.05 versus the control.
C. jejuni-modified E. coli reduces TLR4 and increases CXCL-8 expression in enterocytes.
In an attempt to identify host proinflammatory consequences in colonic enterocytes exposed to C. jejuni-modified E. coli, studies assessed changes in TLR4 and CXCL-8 gene expression in T84 epithelial cells. E. coli grown as a coculture with C. jejuni, or E. coli treated with C. jejuni-CM, significantly reduced TLR4 gene expression relative to the controls (P < 0.05; Fig. 5A). TLR4 expression in T84 cells that were incubated with an E. coli monoculture (that had not been exposed to C. jejuni-CM) was comparable to that of the controls (i.e., PBS-treated T84 cells). Conversely, E. coli treated with live C. jejuni, or with C. jejuni-CM, significantly increased CXCL-8 expression in T84 cells (P < 0.05; Fig. 5B). Untreated E. coli did not alter CXCL-8 expression relative to the controls. Used as a positive control, recombinant human TNF-α also increased CXCL-8 expression.
FIG 5.
C. jejuni-modified E. coli reduces TLR4 expression and increases CXCL-8 expression in colonic enterocytes. The relative TLR4 (A) and CXCL-8 (B) gene expression when confluent T84 monolayers were exposed to sterile PBS (Control), bacterial cultures, or proinflammatory stimuli (TNF-α) was determined. E. coli HB101 was grown as a monoculture, as a coculture with C. jejuni, or in C. jejuni-CM. The results were analyzed using the comparative CT method of analysis, and β-actin was used as an internal reference gene. The data are represented as means ± the standard errors of the mean (n = 3 to 9 per group). There was no significant (NS) difference in gene expression between various E. coli cultures. There was no significant difference in E. coli monoculture compared to control (i.e., PBS-treated T84 cells). *, P < 0.05; **, P < 0.01 versus the control.
DISCUSSION
Results from the present study demonstrate that live C. jejuni and C. jejuni secretory-excretory products activate latent virulence genes in noninvasive E. coli. These C. jejuni-induced alterations promote E. coli adhesion to enterocytes. In turn, the altered E. coli reduces TLR4 gene expression and increases proinflammatory CXCL-8 gene expression in colonic enterocytes. Microarray analyses indicated that genes related to E. coli virulence, including adhesin, flagellum, hemolysin, biofilm, and antibiotic/stress resistance genes, were upregulated in noninvasive E. coli exposed to C. jejuni-CM. Furthermore, in focusing on adhesins, flagella, and hemolysins, these changes were confirmed via quantitative PCR, since two adhesin genes (fimA and sfmF) and the latent hemolysis gene (hlyE) were significantly upregulated when E. coli was grown in C. jejuni-CM and exposed to human enterocytes. Another set of studies determined that phenotypic changes were indeed associated with altered E. coli gene expression profiles. TEM studies revealed that exposure to live C. jejuni or to C. jejuni-CM significantly increased the number of E. coli organisms expressing flagellar structures. To measure the functional consequence of the altered adhesin gene expression, atomic force microscopy assessed the effects of C. jejuni on forces of E. coli adhesion to enterocytes. These experiments provide for the first time data on actual adhesive forces between enteric bacteria and intestinal epithelial cells. The results indicate that the force of adhesion between E. coli and human colonic epithelial cells, as well as the work required to disrupt this bond, was greater for E. coli grown in C. jejuni-CM than for untreated E. coli. Finally, E. coli exposed to live C. jejuni or C. jejuni-CM reduced enterocytic TLR4 gene expression and increased the expression of proinflammatory CXCL-8. Together, these findings shed new light on mechanisms that may contribute to postinfectious inflammatory disorders of the gut.
C. jejuni is the most common cause of bacterial gastroenteritis. The mechanisms remain incompletely understood, and direct effects of C. jejuni on commensal microbes within the intestine have yet to be uncovered. We hypothesized that a gastrointestinal pathogen, such as C. jejuni, may disrupt intestinal homeostasis and contribute to postinfectious intestinal inflammation by inducing virulence factors in commensal E. coli.
Adherent-invasive Proteobacteria, and specifically adherent-invasive E. coli (AIEC), have repeatedly been associated with the pathogenesis of IBD (66, 67). AIEC uses type 1 fimbriae and long polar fimbriae, which interact with CEACAM6 and Peyer's patches, to adhere to and invade intestinal epithelial cells (68, 69). AIEC induces the secretion of proinflammatory CXCL-8 in T84 colonic epithelial cells, which leads to the recruitment and activation of neutrophils, a hallmark of the inflamed intestine (70, 71). Interestingly, unlike other bacterial pathogens, AIEC is not found in the environment. This observation incited the hypothesis that instead of being an acquired pathogen, AIEC may in fact represent an opportunistic pathobiont locally induced by stimuli that have yet to be identified. The findings reported here illustrate that exposure to an active enteropathogen such as C. jejuni induces genotypic, phenotypic, and functional alterations in noninvasive E. coli that resemble these reported for AIEC.
We first examined microarray gene expression profiles of E. coli grown in C. jejuni-CM, compared to control E. coli, to get an overview of which E. coli genes may be changed upon exposure to C. jejuni secretory-excretory products. We identified several genes related to bacterial adherence and motility (adhesins and flagella), secreted toxins (hemolysins), and resistance (biofilm formation and antimicrobial/stress resistance) that were differentially expressed in E. coli exposed to C. jejuni-CM. We used these data to focus our experimental efforts on E. coli adhesins, flagella, and hemolysins, which are well-defined virulence factors and have been associated with AIEC.
RT-PCR validated changes in E. coli adhesin, flagellum, and hemolysin gene expression upon exposure to C. jejuni-CM. We found that expression of the fimA gene, which encodes FimA, the major subunit of type 1 fimbriae, was significantly increased in E. coli exposed to C. jejuni-CM (72). Similarly, expression of the sfmF gene, which encodes a cryptic yet functional adhesin in E. coli K-12, was also increased in E. coli that had been exposed to C. jejuni-CM (73). Adhesins encoded by the sfmF gene do not have any affinity for d-mannose glycoproteins (73), and thus the present findings uncover alterations in genes encoding two distinct E. coli adhesins when the noninvasive bacteria are exposed to C. jejuni-CM. Consistent with the microarray data, RT-PCR analysis revealed a trend for upregulation of fliD gene expression, but these alterations failed to reach statistical significance. The FliD protein is a necessary component of functioning flagella; the FliD protein functions as a flagellar cap, which prevents flagellin (i.e., main structural component of flagella) monomers from leaking out of the filament during assembly (74). Finally, RT-PCR revealed an increase in hlyE gene expression in C. jejuni-treated E. coli. Hemolysin E (HlyE), which is also known as cytolysin A (ClyA) or silent hemolysin (SheA), is a latent pore-forming toxin found in E. coli K-12 (75–77). Like other cytotoxins, purified HlyE and hlyE-expressing E. coli K-12 can lyse erythrocytes and induce apoptosis in human and murine macrophages (78). Interestingly, AIEC has been shown to have hemolytic activity (66).
In an attempt to clarify the significance of the fliD gene alterations seen in our microarray and RT-PCR analyses, transmission electron microscopy was used to assess the effects C. jejuni has on E. coli flagellar structures. TEM results indicated a significant increase in the proportion of flagellated E. coli organisms when E. coli was grown as a coculture with live C. jejuni or when E. coli was grown in C. jejuni-CM. This increase in proportion of flagellated E. coli organisms may promote E. coli host cell colonization and subsequent host invasion and immune cell activation.
Given the importance of filamentous appendages in host cell adhesion, AFM was used to quantify the force of adhesion between E. coli and human colonic T84 epithelial cells. AFM is a novel technique capable of detecting piconewton changes in force of adhesion. AFM has successfully been used to investigate dendritic cell activation and, most recently, adhesion of Plasmodium falciparum-infected red blood cells to endothelial cells (79, 80). Moreover, until the present study, to the best of our knowledge, this technique has not yet been successfully applied to characterize interactions between enteropathogens and epithelial cells. The findings presented here reveal a significant increase in the force of adhesion between E. coli and T84 cells when E. coli is exposed to C. jejuni-CM, further highlighting the pathophysiological significance of these alterations.
A final set of experiments investigated whether these C. jejuni-modified E. coli organisms may affect key factors involved in host proinflammatory responses of the gut. Bacterial recognition by host TLRs plays a crucial role in regulating inflammation. The present results indicate that while untreated E. coli failed to change TLR4 expression, TLR4 gene expression is decreased when colonic epithelial cells are infected with E. coli that had been exposed to C. jejuni or C. jejuni-CM, in comparison to controls. This finding may seem counterintuitive when considering that intestinal TLR4 expression appears to be increased in IBD patients (39); however, TLRs also have a role in commensal microbiota tolerance and protection from intestinal epithelial injury (81). Therefore, reduced epithelial TLR4 expression in enterocytes exposed to C. jejuni-modified E. coli may disrupt host homeostasis, thereby promoting a proinflammatory response. Moreover, RT-PCR results indicated C. jejuni and C. jejuni-CM, but not untreated E. coli, significantly increased epithelial CXCL-8 expression, in comparison to controls; CXCL-8 is a well-established proinflammatory chemokine. Overall, these results demonstrate that E. coli exposed to C. jejuni or to C. jejuni secretory/excretory products may modulate host proinflammatory responses, and this may contribute to postinfectious complications that can arise following a gastrointestinal infection.
To our knowledge, this study represents the first report in which an enteric bacterial pathogen may actively induce pathogenicity in noninvasive E. coli. While the presence of virulence genes as a prediction of bacterial virulence has been questioned (82), results corroborating this in the present study can be found in the literature. In Toxoplasma gondii-induced models of murine colitis, researchers have shown that nonpathogenic E. coli K-12 and probiotic E. coli Nissle 1917 incite a proinflammatory, postinfectious response (83). Recent research has also shown that the parasite Giardia duodenalis alters human mucosal microbiota composition (J. K. Beatty, S. V. Akierman, J. P. Motta, S. Muise, A. Bhargava, P. L. Beck, K. P. Rioux, G. W. McKnight, J. L. Wallace, and A. G. Buret, unpublished data), and these Giardia-induced alterations of commensal bacteria are toxic to Caenorhabditis elegans (84). Furthermore, G. duodenalis was shown to restore virulence in attenuated mutants of Citrobacter rodentium in C. elegans infection models (84). Researchers have reported the presence of virulence genes in probiotic E. coli (Symbioflor2; DSM 17252) despite the absence of adverse effects during long-term commercial use (85), but these findings were based on only five healthy volunteers. Disease-inciting consequences of commensal/probiotic E. coli have been documented; administration of the probiotic E. coli Nissle 1917, which was given in response to gastroenteritis induced by a coinfection of rotavirus and adenovirus, resulted in severe sepsis in a preterm infant (86).
The present study shows that C. jejuni and C. jejuni-CM are capable of inducing virulence in noninvasive E. coli, and this altered E. coli phenotype may act as a pathobiont with human enterocytes. These findings also demonstrate changes in noninvasive E. coli that resemble the genotypic and phenotypic traits of AIEC. More research is needed to uncover how these novel observations may translate into pathogenicity-modulatory effects on gut microbiota in the human intestine. In turn, this type of research may help identify new mechanisms through which acute enteric infection may lead to PI-IBS, as well as to the initiation/exacerbation of symptoms in patients with IBD.
Supplementary Material
ACKNOWLEDGMENTS
We thank Xiuling Wang of the Southern Alberta Cancer Research Institute Microarray and Genomics Facility and Wei-Xiang Dong and Michael Schoel of the Microscopy and Imaging Facility at the University of Calgary for their technical assistance with the prokaryotic target hybridization, transmission electron microscopy, and scanning electron microscopy, respectively.
This study was supported by grants from Crohn's and Colitis Canada and the Natural Sciences and Engineering Research Council of Canada. K.L.R. was the recipient of a graduate student scholarship from the Alberta Scholarship Programs.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00970-15.
REFERENCES
- 1.World Health Organization. 2013. The global view of campylobacteriosis: report of an expert consultation. World Health Organization, Utrecht, Netherlands. [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharff RL. 2012. Economic burden from health losses due to foodborne illness in the United States. J Food Prot 75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- 4.Acheson D, Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis 32:1201–1206. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- 5.Humphrey T, O'Brien S, Madsen M. 2007. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J Infect Dis 157:472–479 doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 7.Blaser MJ, Berkowitz ID, LaForce FM, Cravens J, Reller LB, Wang W-LL. 1979. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med 91:179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- 8.Garcia Rodriguez L, Ruigomez A, Panes J. 2006. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 130:1588–1594. doi: 10.1053/j.gastro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H. 2009. Increased short- and long-term risk of inflammatory bowel disease after Salmonella or Campylobacter gastroenteritis. Gastroenterology 137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Hannu T, Mattila L, Rautelin H, Pelkonen P, Lahdenne P, Siitonen A, Leirisalo-Repo M. 2002. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology 41:312–318. doi: 10.1093/rheumatology/41.3.312. [DOI] [PubMed] [Google Scholar]
- 11.Marshall J, Thabane M, Garg A, Clark W, Salvadori M, Collins S. 2006. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 131:445–450. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Rees JH, Soudain SE, Gregson NA, Hughes RAC. 1995. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med 333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes KM, Tattersfield AE. 1982. Guillain-Barre syndrome associated with Campylobacter infection. Br Med J 285:173–174. doi: 10.1136/bmj.285.6336.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thabane M, Kottachchi DT, Marshall JK. 2007. Systematic review and meta-analysis: the incidence and prognosis of postinfectious irritable bowel syndrome. Aliment Pharmacol Ther 26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 15.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. 1999. Functional bowel disorders and functional abdominal pain. Gut 45:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor RB. 2011. Key questions to guide a better understanding of host-commensal microbiota interactions in intestinal inflammation. Mucosal Immunol 4:127–132. doi: 10.1038/mi.2010.87. [DOI] [PubMed] [Google Scholar]
- 17.Vermeire S, Van Assche G, Rutgeerts P. 2012. Classification of inflammatory bowel disease: the old and the new. Curr Opin Gastroenterol 28:321–326. doi: 10.1097/MOG.0b013e328354be1e. [DOI] [PubMed] [Google Scholar]
- 18.Fukuba N, Ishihara S, Tada Y, Oshima N, Moriyama I, Yuki T, Kawashima K, Kushiyama Y, Fujishiro H, Kinoshita Y. 2014. Prevalence of irritable bowel syndrome-like symptoms in ulcerative colitis patients with clinical and endoscopic evidence of remission: prospective multicenter study. Scand J Gastroenterol 49:674–680. doi: 10.1007/s00535-013-0829-7. [DOI] [PubMed] [Google Scholar]
- 19.Minderhoud I, Oldenburg B, Wismeijer J, Van Berge Henegouwen G, Smout AJPM. 2004. IBS-like symptoms in patients with inflammatory bowel disease in remission: relationships with quality of life and coping behavior. Dig Dis Sci 49:469–474. doi: 10.1023/B:DDAS.0000020506.84248.f9. [DOI] [PubMed] [Google Scholar]
- 20.Buret AG, Reti K. 2014. Acute enteric infections alter commensal microbiota: new mechanisms in postinfectious intestinal inflammatory disorders, p 87–106. In Heidt PJ, Rusch V, Walker RI, Lange D, Riddle MS (ed), Persisting consequences of intestinal infection. Seminar 27 Old Herborn University, Herborn, Germany. [Google Scholar]
- 21.Kalischuk LD, Buret AG. 2009. A role for Campylobacter jejuni-induced enteritis in inflammatory bowel disease? Am J Physiol Gastrointest Liver Physiol 298:G1–G9. doi: 10.1152/ajpgi.00193.2009. [DOI] [PubMed] [Google Scholar]
- 22.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EMM, Simrén M. 2012. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 24.Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J. 2003. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux J-J, Blugeon S, Bridonneau C, Furet J-P, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. 2009. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Siles M, Martinez-Medina M, Busquets D, Sabat-Mir M, Duncan SH, Flint HJ, Aldeguer X, Garcia-Gil LJ. 2014. Mucosa-associated Faecalibacterium prausnitzii and Escherichia coli co-abundance can distinguish irritable bowel syndrome and inflammatory bowel disease phenotypes. Int J Med Microbiol 304:464–475. doi: 10.1016/j.ijmm.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil JL. 2006. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 12:1136–1145. doi: 10.1097/01.mib.0000235828.09305.0c. [DOI] [PubMed] [Google Scholar]
- 29.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. 2011. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hara J, Buret A. 2008. Mechanisms of intestinal tight junctional disruption during infection. Front Biosci 1:7008–7021. [DOI] [PubMed] [Google Scholar]
- 31.MacCallum A, Hardy SP, Everest PH. 2005. Campylobacter jejuni inhibits the absorptive transport functions of Caco-2 cells and disrupts cellular tight junctions. Microbiology 151:2451–2458. doi: 10.1099/mic.0.27950-0. [DOI] [PubMed] [Google Scholar]
- 32.Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG. 2008. Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun 76:3390–3398. doi: 10.1128/IAI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalischuk L, Inglis GD, Buret A. 2009. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog 1:2. doi: 10.1186/1757-4749-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalischuk L, Leggett F, Inglis GD. 2010. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog 2:14. doi: 10.1186/1757-4749-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Cario E. 2010. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis 16:1583–1597. doi: 10.1002/ibd.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cario E, Podolsky DK. 2000. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun 68:7010–7017. doi: 10.1128/IAI.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuse K, Katakura K, Sakamoto N, Ohira H. 2010. Toll-like receptor 9 gene mutations and polymorphisms in Japanese ulcerative colitis patients. World J Gastroenterol 16:5815–5821. doi: 10.3748/wjg.v16.i46.5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torok HP, Glas J, Tonenchi L, Bruennler G, Folwaczny M, Folwaczny C. 2004. Crohn's disease is associated with a Toll-like receptor-9 polymorphism. Gastroenterology 127:365–366. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 42.Villani AC, Lemire M, Thabane M, Belisle A, Geneau G, Garg AX, Clark WF, Moayyedi P, Collins SM, Franchimont D, Marshall JK. 2010. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology 138:1502–1513. doi: 10.1053/j.gastro.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 43.Poltorak A, He X, Smirnova I, Liu M-Y, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4. Gene Sci 282:2085–2088. http://dx.doi.org/10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 44.Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 46.O'Hara JR, Feener TD, Fischer CD, Buret AG. 2012. Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice. Infect Immun 80:1563–1571. doi: 10.1128/IAI.06066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Njoroge J, Sperandio V. 2009. Jamming bacterial communication: new approaches for the treatment of infectious diseases. EMBO Mol Med 1:201–210. doi: 10.1002/emmm.200900032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperandio V, Mellies JL, Nguyen W, Shin S, Kaper JB. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc Natl Acad Sci U S A 96:15196–15201. doi: 10.1073/pnas.96.26.15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 50.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. 2003. Bacteria–host communication: the language of hormones. Proc Natl Acad Sci U S A 100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stecher B, Denzler R, Maier L, Bernet F, Sanders MJ, Pickard DJ, Barthel M, Westendorf AM, Krogfelt KA, Walker AW, Ackermann M, Dobrindt U, Thomson NR, Hardt W-D. 2012. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci U S A 109:1269–1274. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juge N. 2012. Microbial adhesins to gastrointestinal mucus. Trends Microbiol 20:30–39. doi: 10.1016/j.tim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Kline KA, Fälker S, Dahlberg S, Normark S, Henriques-Normark B. 2009. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 5:580–592. doi: 10.1016/j.chom.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Ramos HC, Rumbo M, Sirard J-C. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol 12:509–517. doi: 10.1016/j.tim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Konkel ME, Klena JD, Rivera-Amill V, Monteville MR, Biswas D, Raphael B, Mickelson J. 2004. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J Bacteriol 186:3296–3303. doi: 10.1128/JB.186.11.3296-3303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhakal Bijaya K, Mulvey Matthew A. 2012. The UPEC pore-forming toxin α-hemolysin triggers proteolysis of host proteins to disrupt cell adhesion, inflammatory, and survival pathways. Cell Host Microbe 11:58–69. doi: 10.1016/j.chom.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korlath J, Osterholm M, Judy L, Forfang J, Robinson R. 1985. A point-source outbreak of campylobacteriosis associated with consumption of raw milk. J Infect Dis 152:592–596. doi: 10.1093/infdis/152.3.592. [DOI] [PubMed] [Google Scholar]
- 58.Boyer HW, Roulland-dussoix D. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 59.Kuhnert P, Nicolet J, Frey J. 1995. Rapid and accurate identification of Escherichia coli K-12 strains. Appl Environ Microbiol 61:4135–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mühldorfer I, Hacker J. 1994. Genetic aspects of Escherichia coli virulence. Microb Pathog 16:171–181. doi: 10.1006/mpat.1994.1018. [DOI] [PubMed] [Google Scholar]
- 61.Mobley H. 2015. Redefining virulence of bacterial pathogens. Microbe 10:239–246. [Google Scholar]
- 62.Yao B, Rakhade S, Li Q, Ahmed S, Krauss R, Draghici S, Loeb J. 2004. Accuracy of cDNA microarray methods to detect small gene expression changes induced by neuregulin on breast epithelial cells. BMC Bioinform 5:99. doi: 10.1186/1471-2105-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koressaar T, Remm M. 2007. Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 64.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3: new capabilities and interfaces. Nucleic Acids Res 40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 66.Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel J-F. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115:1405–1413. doi: 10.1016/S0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 67.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. 2012. IBD—what role do proteobacteria play? Nat Rev Gastroenterol Hepatol 9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- 68.Boudeau J, Barnich N, Darfeuille-Michaud A. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol 39:1272–1284. doi: 10.1111/j.1365-2958.2001.02315.x. [DOI] [PubMed] [Google Scholar]
- 69.Chassaing B, Rolhion N, de Vallée A, Salim SY, Prorok-Hamon M, Neut C, Campbell BJ, Söderholm JD, Hugot J-P, Colombel J-F, Darfeuille-Michaud A. 2011. Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae. J Clin Invest 121:966–975. doi: 10.1172/JCI44632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eaves-Pyles T, Allen CA, Taormina J, Swidsinski A, Tutt CB, Jezek GE, Islas-Islas M, Torres AG. 2008. Escherichia coli isolated from a Crohn's disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int J Med Microbiol 298:397–409. doi: 10.1016/j.ijmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. 1994. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 56:559–564. [PubMed] [Google Scholar]
- 72.Krogfelt KA, Klemm P. 1988. Investigation of minor components of Escherichia coli type 1 fimbriae: protein chemical and immunological aspects. Microb Pathog 4:231–238. doi: 10.1016/0882-4010(88)90073-3. [DOI] [PubMed] [Google Scholar]
- 73.Korea C-G, Badouraly R, Prevost M-C, Ghigo J-M, Beloin C. 2010. Escherichia coli K-12 possesses multiple cryptic but functional chaperone–usher fimbriae with distinct surface specificities. Environ Microbiol 12:1957–1977. doi: 10.1111/j.1462-2920.2010.02202.x. [DOI] [PubMed] [Google Scholar]
- 74.Maki S, Vonderviszt F, Furukawa Y, Imada K, Namba K. 1998. Plugging interactions of HAP2 pentamer into the distal end of flagellar filament revealed by electron microscopy. J Mol Biol 277:771–777. doi: 10.1006/jmbi.1998.1663. [DOI] [PubMed] [Google Scholar]
- 75.Ludwig A, Tengel C, Bauer S, Bubert A, Benz R, Mollenkopf H-J, Goebel W. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol Gen Genet 249:474–486. doi: 10.1007/BF00290573. [DOI] [PubMed] [Google Scholar]
- 76.Oscarsson J, Mizunoe Y, Uhlin BE, Haydon DJ. 1996. Induction of haemolytic activity in Escherichia coli by the slyA gene product. Mol Microbiol 20:191–199. doi: 10.1111/j.1365-2958.1996.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 77.Ralph ET, Guest JR, Green J. 1998. Altering the anaerobic transcription factor FNR confers a hemolytic phenotype on Escherichia coli K-12. Proc Natl Acad Sci U S A 95:10449–10452. doi: 10.1073/pnas.95.18.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai X-H, Arencibia I, Johansson A, Wai SN, Oscarsson J, Kalfas S, Sundqvist K-G, Mizunoe Y, Sjöstedt A, Uhlin BE. 2000. Cytocidal and apoptotic effects of the ClyA protein from Escherichia coli on primary and cultured monocytes and macrophages. Infect Immun 68:4363–4367. doi: 10.1128/IAI.68.7.4363-4367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis SP, Amrein M, Gillrie MR, Lee K, Muruve DA, Ho M. 2012. Plasmodium falciparum-induced CD36 clustering rapidly strengthens cytoadherence via p130CAS-mediated actin cytoskeletal rearrangement. FASEB J 26:1119–1130. doi: 10.1096/fj.11-196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling C-C, Amrein MW, Shi Y. 2008. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 82.Wassenaar TM, Gunzer F. 2015. The prediction of virulence based on presence of virulence genes in Escherichia coli may not always be accurate. Gut Pathog 7:15. doi: 10.1186/s13099-015-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bereswill S, Fischer A, Dunay IR, Kühl AA, Göbel UB, Liesenfeld O, Heimesaat MM. 2013. Proinflammatory potential of Escherichia coli strains K-12 and Nissle 1917 in a murine model of acute ileitis. Eur J Microbiol Immunol 3:126–134. doi: 10.1556/EuJMI.3.2013.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerbaba TK, Gupta P, Rioux K, Hansen D, Buret AG. 2015. Giardia duodenalis-induced alterations of commensal bacteria kill Caenorhabditis elegans: a new model to study microbial-microbial interactions in the gut. Am J Physiol Gastrointest Liver Physiol 308:G550–G561. doi: 10.1152/ajpgi.00335.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wassenaar TM, Zschüttig A, Beimfohr C, Geske T, Auerbach C, Cook H, Zimmermann K, Gunzer F. 2015. Virulence genes in a probiotic Escherichia coli product with a recorded long history of safe use. Eur J Microbiol Immunol 5:81–93 doi: 10.1556/EuJMI-D-14-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guenther K, Straube E, Pfister W, Guenther A, Huebler A. 2010. Sever sepsis after probiotic treatment with Escherichia coli NISSLE 1917. Pediatr Infect Dis J 29:188–189. doi: 10.1097/INF.0b013e3181c36eb9. [DOI] [PubMed] [Google Scholar]
- 87.Ludwig A, von Rhein C, Mischke A, Brade V. 2008. Release of latent ClyA cytolysin from Escherichia coli mediated by a bacteriophage-associated putative holin (BlyA) from Borrelia burgdorferi. Int J Med Microbiol 298:473–481. doi: 10.1016/j.ijmm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 88.Negroni A, Costanzo M, Vitali R, Superti F, Bertuccini L, Tinari A, Minelli F, Di Nardo G, Nuti F, Pierdomenico M, Cucchiara S, Stronati L. 2012. Characterization of adherent-invasive Escherichia coli isolated from pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 18:913–924. doi: 10.1002/ibd.21899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.