Abstract
Batrachochytrium dendrobatidis is a fungal pathogen in the phylum Chytridiomycota that causes the skin disease chytridiomycosis. Chytridiomycosis is considered an emerging infectious disease linked to worldwide amphibian declines and extinctions. Although amphibians have well-developed immune defenses, clearance of this pathogen from the skin is often impaired. Previously, we showed that the adaptive immune system is involved in the control of the pathogen, but B. dendrobatidis releases factors that inhibit in vitro and in vivo lymphocyte responses and induce lymphocyte apoptosis. Little is known about the nature of the inhibitory factors released by this fungus. Here, we describe the isolation and characterization of three fungal metabolites produced by B. dendrobatidis but not by the closely related nonpathogenic chytrid Homolaphlyctis polyrhiza. These metabolites are methylthioadenosine (MTA), tryptophan, and an oxidized product of tryptophan, kynurenine (Kyn). Independently, both MTA and Kyn inhibit the survival and proliferation of amphibian lymphocytes and the Jurkat human T cell leukemia cell line. However, working together, they become effective at much lower concentrations. We hypothesize that B. dendrobatidis can adapt its metabolism to release products that alter the local environment in the skin to inhibit immunity and enhance the survival of the pathogen.
INTRODUCTION
In recent years, amphibian populations around the world have declined due, in part, to the emerging fungal disease chytridiomycosis, caused by Batrachochytrium dendrobatidis (1–6). Heavily infected frogs show lethargy, loss of appetite, and behavioral changes (1, 3, 7, 8), and they die due to a failure to maintain a balance of essential ions across the damaged skin (9). Although amphibians have robust and complex immune defenses (10), immune responses against this pathogen are impaired (11–13). Our previous studies showed that sublethal X irradiation of South African clawed frogs (Xenopus laevis) resulted in decreased lymphocyte numbers in the spleen and increased fungal burdens, demonstrating that lymphocyte-mediated adaptive immune responses are important for control of the infection (14). Further studies demonstrated that B. dendrobatidis cells release molecules that inhibit proliferation of lymphocytes and transformed cell lines and induce apoptosis of lymphocytes (11). Enriched fungal supernatants also interfere with the development of a delayed-type hypersensitivity (DTH)-like response to injected phytohemagglutinin (PHA) in X. laevis (12). Thus, the inhibitory factors can act locally in the skin environment. The nature of these inhibitory molecules has not previously been determined. Using liquid chromatography coupled with mass spectrometry (LC-MS) and UV-visible (UV-Vis) detection, we isolated three small-molecule metabolites from B. dendrobatidis supernatants (B. dendrobatidis Sup) with putative immunomodulatory activity. These metabolites are methylthioadenosine (MTA), tryptophan, and an oxidized product of tryptophan, kynurenine (Kyn). The pure molecules were tested for their effects on cultured lymphocytes. Independently, MTA and Kyn inhibited lymphocyte survival and proliferation. Kyn was active only at supraphysiological concentrations, but the addition of a suboptimal concentration of MTA greatly enhanced the activity of Kyn, bringing its range of activity much closer to physiologically relevant concentrations. These results support the hypothesis that B. dendrobatidis can adapt its metabolism to release products that alter the local environment of the skin to inhibit immunity and enhance survival of the pathogen.
MATERIALS AND METHODS
Culture of B. dendrobatidis and H. polyrhiza and preparation of aqueous supernatants.
B. dendrobatidis isolate JEL197 (2) was cultured and maintained in 1% tryptone broth, as previously described (11). The nonpathogenic chytrid fungus Homolaphlyctis polyrhiza (isolate JEL142) (15) was cultured in medium containing 0.1% peptonized milk, 0.1% tryptone, and 0.5% glucose. We chose to use mixed cultures of zoospores and maturing zoosporangia because they produced highly active supernatant factors in previous studies. Pure zoospores lacked the capacity to produce lymphocyte-inhibitory factors (11), and a preliminary high-performance liquid chromatography (HPLC) analysis of supernatants from zoospores showed an absence of peaks for MTA and tryptophan. Cultures were incubated at 21°C and subcultured twice weekly. After 6 to 7 days of culture, B. dendrobatidis or H. polyrhiza cells were centrifuged, washed with sterile glass-distilled water, resuspended at 107 cells/ml in sterile distilled water, and incubated at 21°C for 24 h in large flasks. Cells were centrifuged, and supernatants were passed through 0.2-μm filters (Fisher, Waltham, MA, USA) to remove any cells. Supernatants were then frozen and lyophilized (11).
Extraction of B. dendrobatidis supernatants and HPLC isolation of fractions.
A lyophilized, cell-free supernatant of B. dendrobatidis (JEL197) (B. dendrobatidis Sup) (2) was reconstituted in 1.0 ml of HPLC-grade water and analyzed by LC-MS (Shimadzu LC-20 liquid chromatograph equipped with an ACE C18 column [3 μm; 150 by 4.6 mm], a Shimadzu SPD-M20A diode array detector, and an Applied Biosystems Sciex API 2000 triple-quadrupole mass spectrometer). After elution (ramping from acidified H2O to acidified acetonitrile, as described previously by Umile et al. [16]), the chromatogram was compared to that for lyophilized water and the H. polyrhiza supernatant. Compounds unique to B. dendrobatidis were isolated by semipreparative HPLC (ACE C18 column [5 μm; 250 by 10 mm]).
Identification of metabolites by NMR.
Tryptophan was identified on the basis of 1H nuclear magnetic resonance (NMR), HRMS-ESI (high-resolution mass spectrometry–electrospray ionization), and UV-Vis spectroscopy. MTA was characterized on the basis of HRMS-ESI and UV-Vis spectroscopy. Although the isolated compound was too dilute for complete 1H NMR characterization, the 1H NMR spectrum showed singlet peaks at 8.345 and 8.232 ppm, corroborating the postulated adenine chromophore. Kyn was identified on the basis of its characteristic UV-Vis chromophore (257 nm; 362 nm). For all compounds, authentic standards were purchased and shown to have identical retention times under the LC-MS conditions used in their initial discovery.
For tryptophan, 1H NMR (500 MHz, CD3OD, δ) 7.70 (d, J = 7.9 Hz, 1H), 7.36 (d, J = 7.4 Hz, 1H), 7.20 (s, 1H), 7.12 (t, J = 8.0 Hz, 1H), 7.05 (t, J = 8.0 Hz, 1H), 3.86 (dd, J = 9 Hz, 4 Hz, 1H), 3.51 (dd, J = 15 Hz, 4 Hz, 1 H), 3.16 (m, 1H). HRMS-ESI (m/z) [M + H]+ calculated for C11H13N2O2, 205.0977; found, 205.0969; UV λmax, 278 nm.
For MTA, 1H NMR (500 MHz, CD3OD, δ) 8.345 (s), 8.232 (s), other peaks obscured. HRMS-ESI (m/z) [M + H]+ calculated for C11H16N5O3S, 298.0974; found, 298.0971; UV λmax, 257 nm.
For kynurenine, UV λmax, 257, 362 nm.
Assays of lymphocyte inhibition.
Spleen cells from X. laevis were isolated and cultured with PHA and increasing concentrations of purified metabolites, as previously described (11). Splenocytes were incubated at 26°C in an atmosphere of 5% CO2–95% air for 3 days before harvesting, as previously described (11). All wells were pulsed with 0.5 μCi [3H]thymidine (5 μCi/ml; specific activity, 2 Ci/mmol) (PerkinElmer, Waltham, MA, USA) during the last 24 h prior to harvesting.
Jurkat T cells were cultured in RPMI medium (supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin) at 37°C with 5% CO2–95% air and passaged twice weekly. Cells were centrifuged at ∼200 × g for 10 min and resuspended in RPMI medium. Five or more replicates of cells (104 cells/well) in 50 μl were cultured for 3 days with or without increasing concentrations of purified MTA or l-kynurenine or with 25 μg/ml etoposide (negative control for growth) in 50 μl of RPMI medium (final concentration in culture, 12.5 μg/ml). After incubation, 100 μl of MTT (thiazolyl blue tetrazolium bromide) (500 μg/ml) was added to each sample, and cells were incubated for another 2 to 4 h at 37°C. The samples were then centrifuged for 30 min at ∼2,200 × g, culture supernatants were removed, and the contents of each well were resuspended in 100 μl of dimethyl sulfoxide (DMSO). The plate was incubated 37°C for 10 min, and the absorbance was read with a BioTek ELx808 plate reader at 570 nm after 2 min of fast shaking.
Percent inhibition of PHA-induced splenocyte proliferation was determined by subtracting the average reduced counts per minute divided by the average control PHA-induced counts per minute from 1 and multiplying this value by 100 {% inhibition = [1 − (avg. reduced cpm/avg. PHA-only cpm)] × 100%}. Percent inhibition of Jurkat growth was determined by subtracting the average reduced MTT absorbance divided by the average absorbance of untreated Jurkat cells from 1 and multiplying this value by 100 {% inhibition = [1 − (avg. reduced MTT abs./avg. MTT abs. of untreated cells] × 100%}.
RESULTS
B. dendrobatidis metabolites and their lymphotoxic activity.
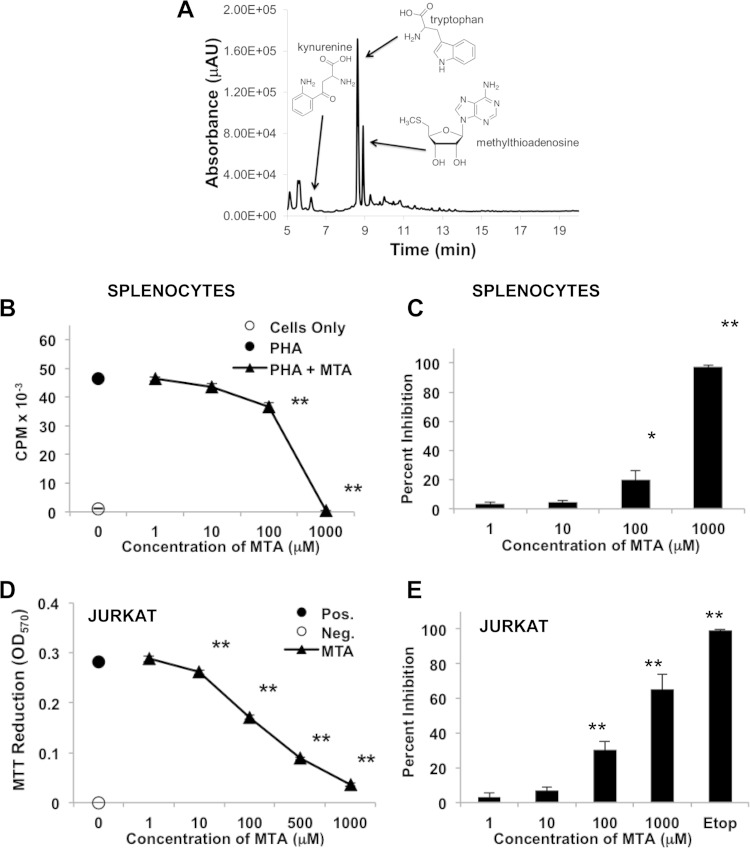
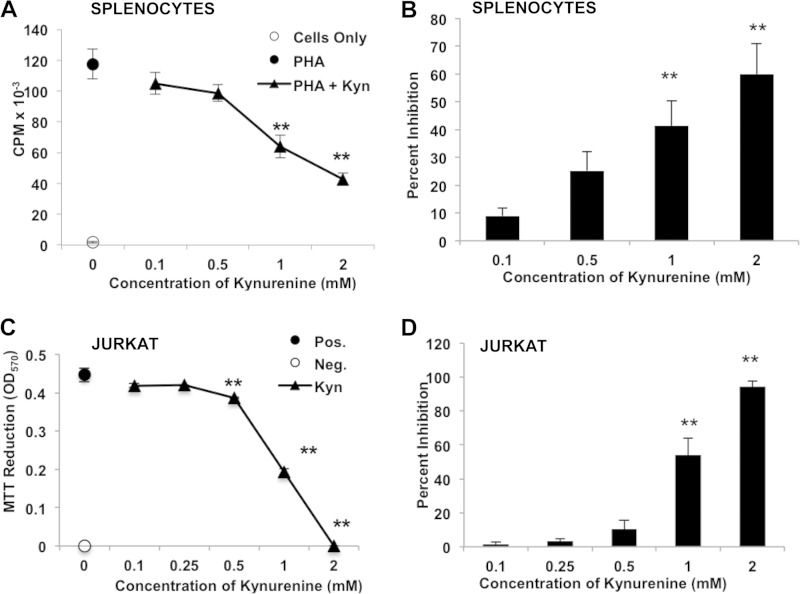
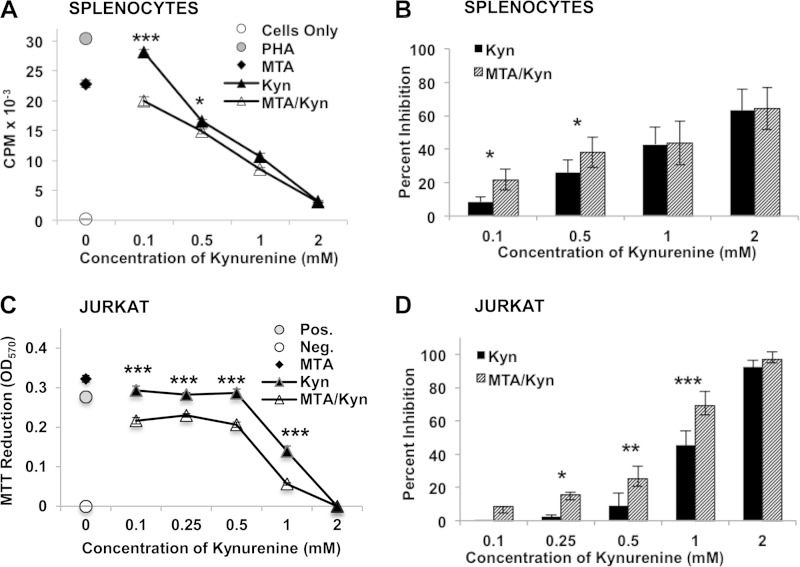
Because preliminary studies suggested that lymphotoxic activity could be detected in low-molecular-weight fractions derived from the B. dendrobatidis Sup, we used LC-MS with UV-Vis detection to investigate small molecules produced by the original type strain of B. dendrobatidis (isolate JEL197) (2) but not by the closely related nonpathogenic chytrid H. polyrhiza (15, 17). The B. dendrobatidis Sup from cultures of maturing zoosporangia grown overnight in water (11) consisted of a mixture of two major light-absorbing components as well as a number of less abundant components not present in the H. polyrhiza supernatant (Fig. 1A). One major metabolite was identified as MTA, which is a compound known to have immunomodulatory effects in mammalian systems (18, 19). This compound inhibited the proliferation of X. laevis splenocytes at concentrations of ≥100 μM (Fig. 1B and C) and inhibited the division and survival of Jurkat T cells at concentrations of ≥10 μM (Fig. 1D and E). Another major component was identified as tryptophan. While tryptophan itself is not inhibitory to lymphocytes, an oxidized tryptophan metabolite, Kyn, was also detected in the B. dendrobatidis Sup and is known to have immunosuppressive effects (20–22). Kynurenine was inhibitory for frog splenocytes (X. laevis) at supraphysiological concentrations of 1 to 2 mM (Fig. 2A and B) and for Jurkat cells at concentrations of >0.5 mM (Fig. 2C and D), well above the concentrations found in human or mouse serum (2 to 6 μM) (23, 24). Although Kyn alone was inhibitory only at micromolar concentrations, the addition of 10 μM MTA (a suboptimal concentration) significantly enhanced the inhibitory activity of Kyn at much lower concentrations (0.1 to 0.5 mM) in PHA-stimulated frog splenocytes (Fig. 3A and B) and Jurkat cells (Fig. 3C and D), suggesting a synergistic inhibition of T cell proliferation. Preliminary HPLC analysis of supernatants of purified B. dendrobatidis zoospores showed an absence of the UV-visible peaks for MTA and tryptophan.
FIG 1.
(A) HPLC chromatogram of the lyophilized cell-free supernatant of B. dendrobatidis cells (JEL197) showing the presence of tryptophan, Kyn, and MTA. Absorbance is reported as micro-absorbance units (μAU). (B and C) Inhibition of PHA-stimulated X. laevis spleen cells by MTA. (B) Data from one representative of five similar experiments. X. laevis splenocytes were cultured alone or with PHA. PHA-stimulated cells were incubated with increasing concentrations of MTA, as shown. (C) Percent inhibition of growth at each concentration (n = 4 or 5 experiments at each concentration). (D and E) Inhibition of Jurkat T cells by MTA. (D) One representative experiment (seven total) showing inhibition of growth measured as the reduction of MTT at 570 nm. The positive control (Pos.) was medium alone, and the negative control (Neg.) was treated with etoposide (Etop). OD570, optical density at 570 nm. (E) Summary of data from seven experiments reported as percent inhibition of growth. For all panels, error bars show standard errors, and the indicated treatments were significantly different from the positive control (PHA alone for splenocytes and medium alone for Jurkat cells). *, P < 0.05; **, P < 0.01 (determined by one-way analysis of variance with a Tukey post hoc test).
FIG 2.
Inhibition of splenocytes and Jurkat cells by Kyn. (A) Inhibition of PHA-stimulated X. laevis splenocytes by Kyn (data from one representative of eight similar experiments). Splenocytes were cultured alone or with PHA. PHA-stimulated cells were incubated with increasing concentrations of Kyn, as shown. (B) Percent inhibition of growth at each concentration (n = 8 experiments). (C) Inhibition of Jurkat T cells by Kyn. Data from one representative experiment of six show inhibition of growth measured as the reduction of MTT at 570 nm. The positive control (Pos.) was medium alone, and the negative control (Neg.) was treated with etoposide. (D) Summary of data from seven experiments shown as percent inhibition of growth. For all panels, error bars show standard errors, and the indicated treatments were significantly different from the positive control (PHA alone for splenocytes and medium alone for Jurkat cells). **, P < 0.01 (determined by one-way analysis of variance with a Tukey post hoc test).
FIG 3.
Synergism of MTA and Kyn. (A) Inhibition of PHA-stimulated X. laevis spleen cells by Kyn alone at the concentrations shown, MTA alone (10 μM), or Kyn plus 10 μM MTA at the concentrations shown (data from one representative of seven similar experiments). (B) Percent inhibition of PHA-stimulated splenocytes induced by Kyn alone or MTA and Kyn in seven replicate experiments. (C) Inhibition of Jurkat T cells by Kyn alone at the dilutions shown, MTA alone (10 μM), or Kyn plus 10 μM MTA at the concentrations shown (representative of data from five similar experiments). The addition of etoposide (12.5 μg/ml) as a negative control (Neg.) for Jurkat cells completely inhibited growth. (D) Percent inhibition of Jurkat cells by Kyn alone or MTA plus Kyn in five replicate experiments. For all panels, error bars show standard errors, and the indicated MTA/Kyr treatments were significantly different from the Kyr-alone treatment. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (determined by two-way analysis of variance with Bonferroni comparisons). For panels B and D, analysis of variance was done with multiple repeats.
DISCUSSION
Possible roles of B. dendrobatidis supernatant factors in B. dendrobatidis metabolism and immune evasion in frogs.
MTA is a product of the methionine salvage pathway common to prokaryotes and eukaryotes (25). A search of the B. dendrobatidis genome (Broad Institute Batrachochytrium dendrobatidis Database [http://www.broadinstitute.org/annotation/genome/batrachochytrium_dendrobatidis/MultiHome.html]) shows the presence of enzymes necessary for the synthesis of MTA as well as enzymes that use MTA as a metabolic precursor to adenine and methionine (25). Thus, MTA is a natural product of B. dendrobatidis metabolism. We estimate its concentration in a 10× B. dendrobatidis Sup (previously shown to be strongly inhibitory [11]) to be ∼2 μM. Its abundance in a cell-free B. dendrobatidis Sup may result from the stress of a rapid shift from a rich growth medium (tryptone broth) to water during the preparation of the supernatant shown in Fig. 1A. Alternatively, it may be a natural product of rapidly growing B. dendrobatidis cells that accumulates when fungal cells grow at a high cell density, as occurs in bacterial biofilms (26). Its role in B. dendrobatidis metabolism awaits further study, but its presence in inhibitory supernatants suggests that it may be one factor that contributes to the immune evasion of B. dendrobatidis in vitro and in vivo (11, 12). Although the mechanism of action of MTA in the inhibition of lymphocytes is not well understood, this small molecule can inhibit both T lymphocytes and B lymphocytes in mammalian model systems, and it has been proposed for use in the treatment of melanoma and autoimmune disorders (18, 27). Thus, if it is produced abundantly and released by B. dendrobatidis within the skin, it could contribute to local immune suppression, allowing zoosporangia to mature and release more propagules onto the skin surface to support further colonization.
The concentrations of tryptophan and Kyn released by B. dendrobatidis within the skin compartment are unknown. However, it seems unlikely that Kyn is released at a sufficiently high concentration to directly inhibit lymphocyte viability. Instead, it may have the potential for blunting inflammatory responses in vivo. Tryptophan metabolism, particularly the oxidation product Kyn, promotes regulatory T cell development (20). An analysis of the transcriptional response of X. laevis following exposure to B. dendrobatidis showed that some proinflammatory responses were downregulated (28). Regulatory T cells produce interleukin-10 (IL-10), which suppresses inflammatory responses (29). A recent study showed that IL-10 was upregulated on the skin of Atelopus zeteki frogs during B. dendrobatidis infection (13). Candida albicans, a fungal pathogen of humans, manipulates tryptophan metabolism, promoting the production of 5-hydroxytryptophan to decrease inflammatory type 17 responses, which are typically protective against fungal infections (30). Tryptophan and its oxidation products released by B. dendrobatidis might inhibit inflammation, potentially explaining the lack of leukocyte recruitment during chytridiomycosis (1, 3). The B. dendrobatidis genome (Broad Institute Batrachochytrium dendrobatidis Database [http://www.broadinstitute.org/annotation/genome/batrachochytrium_dendrobatidis/MultiHome.html]) also shows the presence of indoleamine 2,3-dioxygenase, which is involved in the metabolism of tryptophan to Kyn. Cancer cells have been shown to release Kyn, stalling effector lymphocytes (31). Thus, B. dendrobatidis may be actively converting tryptophan to Kyn and releasing it in the local skin environment to create a barrier to effective T cell immunity. The synergism of MTA with Kyn was a novel and unexpected finding. There appear to be no previous reports of these agents acting together to inhibit lymphocyte proliferation. The presence of a very small amount of MTA can greatly facilitate the activity of Kyn, bringing it closer to the perceived physiologically important range of activity. Taken together, our results suggest that rapidly growing B. dendrobatidis cells have the potential to alter the local microenvironment in the skin and inhibit effective T cell-mediated fungal clearance.
Tryptophan and MTA may be considered natural primary metabolites of B. dendrobatidis. Many fungi produce other small molecules, which have been termed secondary metabolites, that are produced at defined and restricted stages of the life cycle and are dispensable for growth (32). In ongoing studies, we plan to look for new secondary metabolites produced by B. dendrobatidis that may have other pharmacological properties.
Future studies of possible roles of Batrachochytrium salamandrivorans supernatant factors.
In addition to B. dendrobatidis, a second species in the genus Batrachochytrium (B. salamandrivorans) was reported in 2013 (33). Unlike B. dendrobatidis, which infects and causes pathogenesis in frogs, toads, and salamanders, B. salamandrivorans appears to be highly pathogenic to salamanders but spares frogs and toads (34). Thus, these two species appear to have distinct pathogenic profiles, and determining the lymphotoxicity and metabolic profile of B. salamandrivorans will be an important area for future research.
ACKNOWLEDGMENTS
This research was supported by National Science Foundation grants IOS-1121758 (to L.A.R.-S.) and DEB-1136662 (to K.P.C.M.).
We acknowledge assistance from the Princeton University MS and NMR facilities.
REFERENCES
- 1.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A 95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227. doi: 10.2307/3761366. [DOI] [Google Scholar]
- 3.Pessier AP, Nichols DK, Longcore JE, Fuller MS. 1999. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J Vet Diagn Invest 11:194–199. doi: 10.1177/104063879901100219. [DOI] [PubMed] [Google Scholar]
- 4.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 5.Collins JP. 2010. Amphibian decline and extinction: what we know and what we need to learn. Dis Aquat Organ 92:93–99. doi: 10.3354/dao02307. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SHM, Carpenter KE, Chanson J, Collen B, Cox NA, Darwell WRT, Dulvy NK, Harrison LR, Katariya V, Pollock CM, Quader S, Richman NI, Rodrigues ASL, Tognelli MF, Vié J-C, Aguiar JM, Allen DJ, Allen GR, Amori G, Ananjeva NB, Andreone F, Andrew P, Aquino Ortiz AL, Baillie JEM, Baldi R, Bell BD, Biju SD, Bird JP, Black-Decima P, Blanc JJ, Bolaños F, Bolivar-GW, Burfield IJ, Burton JA, Capper DR, Castro F, Catullo G, Cavanagh RD, Channing A, Chao NL, Chenery AM, Chiozza F, Clausnitzer V, Collar NJ, Collett LC, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330:1503–1509. doi: 10.1126/science.1194442. [DOI] [PubMed] [Google Scholar]
- 7.Berger L, Hyatt AD, Speare R, Longcore JE. 2005. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Organ 68:51–63. doi: 10.3354/dao068051. [DOI] [PubMed] [Google Scholar]
- 8.Peterson J, Steffen JE, Reinert LK, Cobine PA, Appel A, Rollins-Smith L, Mendonca MT. 2013. Host stress response is important for the pathogenesis of the deadly amphibian disease, chytridiomycosis in Litoria caerulea. PLoS One 8:e62146. doi: 10.1371/journal.pone.0062146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF, Speare R. 2009. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 10.Robert J, Ohta Y. 2009. Comparative and developmental study of the immune system in Xenopus. Dev Dyn 328:1249–1270. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, Gayek AS, Dermody TS, Aune TM, Oswald-Richter K, Rollins-Smith LA. 2013. The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342:366–369. doi: 10.1126/science.1243316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fites JS, Reinert LK, Chappell TM, Rollins-Smith LA. 2014. Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infect Immun 82:4698–4706. doi: 10.1128/IAI.02231-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison AR, Savage AE, DiRenzo GV, Langhammer P, Lips KR, Zamudio KR. 2014. Fighting a losing battle: vigourous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3(Bethesda) 4:1275–1289. doi: 10.1534/g3.114.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. 2010. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect Immun 78:3981–3992. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longcore JE, Letcher PM, James TY. 2011. Homolaphlyctis polyrhiza gen. et sp. nov., a species in the Rhizophydiales (Chytridiomycetes) with multiple rhizoidal axes. Mycotaxon 118:433–440. [Google Scholar]
- 16.Umile TP, McLaughlin PJ, Johnson KR, Honarvar S, Blackman AL, Burzynski EA, Davis RW, Teotonio TL, Hearn GW, Hughey CA, Lagalante AF, Minbiole KPC. 2014. Nonlethal amphibian skin swabbing of cutaneous natural products for HPLC fingerprinting. Anal Methods 6:3277–3284. doi: 10.1039/C4AY00566J. [DOI] [Google Scholar]
- 17.Joneson S, Stajich JE, Shiu S-H, Rosenblum EB. 2011. Genomic transition to pathogenicity in chytrid fungi. PLoS Pathog 7:e1002338. doi: 10.1371/journal.ppat.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Padova R, Di Padova C, Stramentinoli G, Tritapepe R. 1985. Inhibition of lymphocyte function by naturally occurring nucleoside 5′-methylthioadenosine (MTA). Int J Immunopharmacol 7:193–198. doi: 10.1016/0192-0561(85)90026-8. [DOI] [PubMed] [Google Scholar]
- 19.Yang M-L, Gee AJP, Gee RJ, Zurita-Lozez CI, Khare S, Clarke S, Mamula MJ. 2013. Lupus autoimmunity altered by cellular methylation metabolism. Autoimmunity 46:21–31. doi: 10.3109/08916934.2012.732133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. 2010. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. 2010. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A 107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romani L. 2011. Immunity to fungal infections. Nat Rev Immunol 11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 23.Laich A, Neurauter G, Widner B, Fuchs D. 2002. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem 48:579–581. [PubMed] [Google Scholar]
- 24.Wang Y, Liu H, McKenzie G, Witting PK, Stasch J-P, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celemajer DS, Mellow AL, Keaney JF Jr, Hunt NH, Stocker R. 2010. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albers E. 2009. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′methylthioadenosine. IUBMB Life 61:1132–1142. doi: 10.1002/iub.278. [DOI] [PubMed] [Google Scholar]
- 26.Parveen N, Cornell KA. 2011. Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Mol Microbiol 79:7–20. doi: 10.1111/j.1365-2958.2010.07455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreu-Pérez P, Hernandez-Losa J, Moliné T, Gil R, Grueso J, Pujol A, Cortés J, Avila MA, Recio JA. 2010. Methylthioadeonsine (MTA) inhibits melanoma cell proliferation and in vivo tumor growth. BMC Cancer 10:265. doi: 10.1186/1471-2407-10-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribas L, Li M-S, Doddington BJ, Robert J, Seidel JA, Kroll JS, Zimmerman LB, Grassly NC, Garner TWJ, Fisher MC. 2009. Expression profiling the temperature-dependent response to infection by Batrachochytrium dendrobatidis. PLoS One 12:e8408. doi: 10.1371/journal.pone.0008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills KHG. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol 4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 30.Cheng SC, van de Veerdonk F, Smeekens S, Joosten LA, van der Meer JW, Kullberg B, Netea MG. 2010. Candida albicans dampens host defense by downregulating IL-17 production. J Immunol 185:2450–2457. doi: 10.4049/jimmunol.1000756. [DOI] [PubMed] [Google Scholar]
- 31.Siska PJ, Rathmell JC. 2015. T cell metabolic fitness in antitumor immunity. Trends Immunol 36:257–264. doi: 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller NP, Turner G, Bennett JW. 2005. Fungal secondary metabolism—from biochemistry to genomics. Nat Rev Microbiol 3:937–947. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 33.Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci U S A 110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martel A, Blooi M, Adriaensen C, Van Rooij P, Beukema W, Fisher MC, Farrer RA, Schmidt BR, Tobler U, Goka K, Lips KR, Muletz C, Zamudio KR, Bosch J, Lötters S, Wombwell E, Garner TWJ, Cunningham AA, Spitzen-van der Sluijs A, Salvidio S, Ducatelle R, Nishikawa K, Nguyen TT, Kolby JE, Van Bocxlaer I, Bossuyt F, Pasmans F. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630–631. doi: 10.1126/science.1258268. [DOI] [PMC free article] [PubMed] [Google Scholar]