Abstract
Interleukin-10 (IL-10) has been implicated in susceptibility to genital chlamydial infection and the development of tubal pathologies. IL-10 limitation also resulted in the rapid elicitation of immune responses against Chlamydia, and decreased levels of IL-10 correlated with protective anti-Chlamydia immunity. To investigate the molecular basis for these effects, we compared the reproductive pathologies and fertility rates in Chlamydia-infected wild-type (WT) and IL-10-knockout (IL-10−/−) mice; we also analyzed the expression of the Toll-like receptor (TLR)/interleukin-1 receptor (IL-1R) superfamily, IL-1β production, NLRP3 inflammasome assembly and activation, and the immunostimulatory capacity and apoptotic predilection of Chlamydia-exposed dendritic cells (DCs) from WT and IL-10−/− mice. Our results revealed that, in addition to the rapid clearance of infection, genitally infected IL-10−/− mice were protected from tubal pathologies and infertility, whereas WT (IL-10+/+) mice were not. Chlamydia-pulsed IL-10−/− DCs expressed larger numbers of TLR4/IL-1R molecules and had enhanced IL-1β production. In addition, NLRP3 inflammasome assembly was suppressed in IL-10−/− DCs through the inhibition of the P2X purinoceptor 7 (P2X7) receptor (P2X7R), an ATP-gated ion channel, and a decrease in intracellular Ca2+ levels, which inhibited DC apoptosis. Thus, the potent immunostimulatory capacity of IL-10-deficient DCs is due, at least in part, to the suppression of the intracellular inflammasome assembly, which prevents DC apoptosis, allowing efficient antigen presentation. The results indicate that IL-10 deficiency enables efficient antigen presentation by DCs for rapid and enhanced immune activation against Chlamydia, which results in rapid microbial clearance, which prevents tubal pathologies during infection. Our finding has important implications for the induction of protective immunity against Chlamydia and other infectious and noninfectious diseases by vaccines.
INTRODUCTION
Chlamydia trachomatis is a major infectious bacterial agent of sexually transmitted disease (STD) worldwide (1). Complications in women include pelvic inflammatory disease (PID), ectopic pregnancy, and involuntary tubal factor infertility (TFI) (2). It has been suggested that host inflammatory and antimicrobial immune responses to infection involving cytokines are the key determinants of Chlamydia-induced tubal pathology (3). Therefore, the balance between protective antimicrobial and pathological immune responses during Chlamydia infection becomes a major challenge for controlling genital Chlamydia infections and preventing the sequelae. Evidence of a role for interleukin-10 (IL-10) in Chlamydia-induced tubal pathologies has been obtained clinically in humans and in experimental animal models (4, 5). IL-10 is produced during chlamydial infection and may affect the induction of protective immunity and, thus, the development of pathologies. There was an increased frequency of IL-10 detection in endocervical secretions from women with Neisseria gonorrhoeae, C. trachomatis, and bacterial vaginosis compared with that in women without these infections (5). The absence of IL-10 has been shown to skew the antichlamydial immune response to a predominantly Th1 type and prevent Chlamydia-induced pathologies, whereas the presence of IL-10 results in pathologies (4, 6). However, as a known immunosuppressive cytokine, the ability of IL-10 to potentiate immunopathogenic responses has remained an enigma. We previously reported that the shorter duration of Chlamydia infection and reduced bacterial burden in IL-10-knockout (IL-10−/−) mice was attributed to the ability of IL-10−/− dendritic cells (DCs) to rapidly and preferentially activate a strong Th1 response against chlamydial antigens (7). Thus, studying the role of IL-10−/− DCs in Chlamydia infection may be important for understanding the immunologic mechanisms governing the protective and pathogenic responses to Chlamydia infection. Such information will have significant implications for controlling and preventing genital Chlamydia infections and the associated tubal damage, as well as for vaccine design.
IL-10 was originally identified to be a cytokine synthesis inhibitor (8). In recent years, renewed interest in IL-10 as a key modulator of the immune response to pathogenic microbes has been garnered. IL-10 regulates innate immunity by directly acting on antigen-presenting cells (APCs), such as macrophages and DCs (9), and by downregulating major histocompatibility complex class II proteins as well as costimulatory molecules, such as CD80 and CD86, on the surface of DCs (10). IL-10 can also inhibit phagocytosis and microbial killing through limiting the production of reactive oxygen and nitrogen intermediates in response to interferon gamma (IFN-γ), all of which are essential in mediating immunity to intracellular pathogens such as Chlamydia (11). Moreover, the effects of IL-10 can also enhance the differentiation of IL-10-producing regulatory T cells (Treg) and regulatory B cells (Breg) that promote the survival of microorganisms and contribute to persistent disease (12–14). IL-10-producing DCs in the lower genital tract of mice have been linked to poor Th1 immunity following infection with Chlamydia trachomatis (15). These studies highlight the fact that IL-10 acts as a negative-feedback molecule on Th1 immunity in the absence of IL-10, resulting in robust protective immunity against certain microbes via enhanced DC maturation and antigen-presenting functions. However, the ability of IL-10 to directly affect DC survival during antigen presentation has been unknown.
Nod-like receptors (NLRs), a subset of pattern recognition receptors (PRRs) found in the cytosol, are essential for the detection of invading pathogens and the initiation of the innate immune response. NLRs detect and respond to a variety of pathogenic microbes that contain pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), which include lipopolysaccharide, flagellin, peptidoglycan, uric acid, DNA, IL-1, and IL-18 (16). NLR inflammasomes are multiprotein complexes of oligomerized NLRs and associated proteins which are assembled around a set of core components that include a sensor protein, an adaptor protein (apoptosis-associated speck-like protein containing a caspase activation and recruitment domain [ASC]), and an inflammatory caspase. The inflammasomes serve as platforms for clustering and cellular activation of the caspase pathway, which plays a critical role in innate immunity and also influences the determination of acquired immunity (17). Inflammasomes are involved in DC maturation after antigen uptake and during the control of antigen availability to T cells; thus, any inflammasome-related alteration in DC activation and ultimate death has a major effect on the antigen-specific immune response, inflammation, and immune tolerance (18). Recent studies revealed that NLRP3-dependent cellular activation and apoptosis in DCs play a crucial role in host immunity against microbial infection due to the control of DC homeostasis (19).
To investigate the role of IL-10 in the protective and immunopathogenic responses during chlamydial infection, we tested the hypothesis that IL-10 regulates inflammasome activation in DCs exposed to Chlamydia, thereby influencing the APC function and the induction of immunity that controls inflammatory tubal pathology and infertility. The results of this study suggest that IL-10 deficiency limits tubal damage and infertility in Chlamydia-infected mice and it enhances the antigen-handling function of DCs while suppressing caspase-induced DC apoptosis. The capacity to assemble the NLRP3 inflammasome was also diminished in Chlamydia-pulsed IL-10−/− DCs. This study describes the regulatory role of IL-10 in DC inflammasome assembly, which might be part of the mechanism that forms the basis for the rapid clearance of Chlamydia, retention of a normal tissue architecture, and essentially normal fertility rates in Chlamydia-infected IL-10−/− mice.
MATERIALS AND METHODS
Ethics statement.
The Institutional Animal Care and Use Committee of Morehouse School of Medicine (MSM-IACUC) approved the animal care and use protocol (number 12-17) which we followed in this study. MSM-IACUC adheres to the NIH guidelines for the care and use of laboratory animals, PHS policy, and the guidelines of the Animal Welfare Act.
Chlamydia stocks.
Stocks of Chlamydia muridarum (the agent of mouse pneumonitis; Division of Scientific Research, Centers for Disease Control and Prevention, Atlanta, GA) used for infections were prepared by propagating elementary bodies (EBs) in McCoy or HeLa cells (Division of Scientific Research, Centers for Disease Control and Prevention, Atlanta, GA), according to standard procedures. Chlamydia stock titers were expressed as the number of inclusion-forming units (IFU) per milliliter (20).
Animals.
Female IL-10−/− and wild-type (WT) mice on a C57BL/6 J background (age, 6 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME), fed food and provided water ad libitum, and maintained in laminar-flow racks under pathogen-free conditions with a 12-h light and a 12-h dark cycle. The protocols involving mice were approved by MSM-IACUC.
DC isolation and culture.
Bone marrow dendritic cells (BMDCs) were isolated from the bone marrow of normal and IL-10−/− mice by the standard method and differentiated by in vitro culture with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF), as previously described (7). After 5 days in culture, the cells were characterized to be loosely adherent mononuclear cells and were further selected using a CD11c column from Miltenyi Biotec (San Diego, CA). DCs were then washed and pulsed with live Chlamydia EBs.
T cell activation by Chlamydia-pulsed DCs.
Nylon wool-purified splenic T cells were isolated from Chlamydia-infected mice after 45 days of infection. Purified splenic T cells contained at least 97% CD3+ cells, as determined by fluorescence-activated cell sorting. To assess the antigen-presenting function of DCs from either IL-10−/− or WT mice, 1 × 104 or 1 × 105 cells were cocultured with 2 × 105 nylon wool-purified T cells in the presence or absence of chlamydial antigen (i.e., UV-inactivated EBs from mice with pneumonitis at 10 μg/ml) in 96-well tissue culture plates for 5 days. The amounts of IL-2, IL-10, IL-12, IFN-γ, and GM-CSF in the culture supernatants were measured by a Luminex assay. The concentration of cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values ± standard deviations (SDs) for triplicate cultures from each experiment. The results were derived from at least three independent experiments.
Histopathology and fertility assay.
The pathologies and fertility outcomes associated with Chlamydia infection were investigated. Six female WT and IL-10−/− mice were infected intravaginally with C. muridarum (1 × 105 IFU per mouse) approximately 7 days after intramuscular administration of 2.5 μg/mouse Depo-Provera (medroxyprogesterone acetate; Pfizer Inc., New York, NY). The infection was done twice at 4-week intervals to ensure repeated infection that enhanced infertility (3). The entire genital tract was collected and fixed in 4% formaldehyde at 4 weeks after a second infection. The samples were embedded in paraffin, cut longitudinally into 4-μm sections, and stained with hematoxylin and eosin. The uteri, oviducts, and ovaries were individually evaluated for the presence of inflammation and pathological changes.
To assess Chlamydia-induced infertility, the Chlamydia-infected WT and IL-10−/− mice were mated with males demonstrated to be fertile, followed by monitoring of weight gain daily for approximately 21 days. A constant 3 days of weight gain by the mice was considered evidence of pregnancy (3). The numbers of pregnant mice were determined, and the mean number of pups in the different groups was calculated.
Adoptive transfer experiment.
DCs isolated from female IL-10−/− mice were pulsed or not pulsed with live C. muridarum EBs for 2 h and adoptively transferred into 6- to 8-week-old female C57BL/6 mice (2.5 × 107 cells per mouse) by intravenous infusion into the retro-orbital sinus in 0.2 ml of phosphate-buffered saline. Treated mice were maintained in a laminar-flow hood, fed, and maintained under a 12-h light and 12-h dark cycle. After 1 week, the mice were infected intravaginally with 1 × 105 IFU of live C. muridarum bacteria per mouse. The status of the infection was monitored by periodic cervicovaginal swabbing of individual animals and the isolation of chlamydiae in tissue culture. Experiments were repeated twice to give 10 to 12 mice per group.
Proteomic analysis.
WT and IL-10−/− DCs were each pulsed with C. muridarum for 0, 2, 4, and 8 h. Proteins were extracted from the Chlamydia-pulsed cells with a Bio-Rad protein extraction kit and cleaned with a 2-D cleanup kit (GE Healthcare, Piscataway, NJ), according to the manufacturers' protocols. The protein concentration was determined by use of a 2-D Quant kit from GE Healthcare (Piscataway, NJ). Samples (80 μg) were labeled with Cy5 (red, IL-10−/− DCs) and Cy3 (green, WT DCs) and then mixed together in rehydration buffer and subjected to two-dimensional fluorescence differential gel electrophoresis (2D-DIGE) analysis. Yellow spots indicate similar levels of expression of protein by both WT DCs and IL-10−/− DCs. Green spots indicate proteins expressed at a higher level by WT DCs than IL-10−/− DCs. Red spots indicate proteins overexpressed by IL-10−/− DCs compared to their level of expression by WT DCs. The spots corresponding to differentially expressed proteins were digested and analyzed by nanocapillary liquid chromatography-tandem mass spectrometry (LC-MS/MS; Xevo G2 Tof; Waters, Milford, MA). Protein candidates were identified by use of an automated search of the sequences in the NCBI database using MASCOT Daemon software (Matrix Sciences, Boston, MA).
Western blotting.
DC lysates were prepared by homogenization in radioimmunoprecipitation assay lysis buffer supplemented with 1 mmol/liter phenylmethylsulfonyl fluoride and protease inhibitor cocktail on ice. Equal amounts of WT and IL-10−/− DC lysates and supernatants from the cell culture from each sample were loaded onto the same 4% to 20% TGX gradient gel (Bio-Rad) and run for 1 h. Proteins were then transferred onto nitrocellulose paper (Bio-Rad). After 1 h, the blots were washed, blocked with 5% milk, and then incubated with primary antibody for IL-18, IL-1β, P2X purinoceptor 7 (P2X7R), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Santa Cruz and R&D Systems) overnight at 4°C. Horseradish peroxidase (HRP)-conjugated secondary antibodies (R&D Systems) were added for 1 h at room temperature, and then the blots were developed using Clarity Western enhanced chemiluminescence (ECL) substrate (Bio-Rad) and viewed in a Bio-Rad Gel Doc XR+ system. The images were saved as Tiff files, and the blots for WT and IL-10−/− cells were spliced and are presented separately for better clarity.
Confocal microscopy.
DCs were isolated and purified using magnetically activated cell sorting separation with CD11c microbeads (Miltenyi Biotec, San Diego, CA). The cells were fixed after 1 and 2 h of Chlamydia infection and then labeled with antibodies specific for IL-1β, Toll-like receptor 2 (TLR2), TLR4, IL-1 receptor (IL-1R), NLRP3, ASC, and procaspase-1 (Santa Cruz, TX). Fluorescent conjugated secondary antibodies (Jackson ImmunoResearch Labs, PA) were used to tag the DCs, while the DNA was stained with DRAQ5. Cells were examined and images were captured using a Zeiss LSM 510 visible confocal microscope (Carl Zeiss Microscopy GmbH). Images were taken from different fields on each plate, and colocalization was observed as a different additive color. Quantitative colocalization analysis (QCA) was performed by counting the number of positively stained cells and dividing by the total number of cells (ImageJ software; NIH, USA).
Immunoprecipitation.
WT and IL-10−/− DC lysates were mixed with anti-ASC antibody, incubated with protein A beads overnight, and then spun down. The pellet and supernatant were run on TGX gels (Bio-Rad) for 1 h. Proteins were then transferred onto nitrocellulose paper (Bio-Rad). After 1 h the blots were washed, blocked with 5% milk, and then incubated with primary antibody for NLRP3, caspase-1, and GAPDH (Santa Cruz and R&D Systems) overnight at 4°C. HRP-conjugated secondary antibodies (R&D Systems) were added for 1 h at room temperature, and then the blots were developed using Western ECL substrate and viewed in a Bio-Rad Gel Doc XR+ system. The images were saved as Tiff files, and the blots for WT and IL-10−/− cells are presented separately.
cAMP ELISA.
Cyclic AMP (cAMP) expression in WT and IL-10−/− DCs was determined by enzyme-linked immunosorbent assay (ELISA; Cell Biolabs). The cells were lysed with the lysis buffer provided in the ELISA kit and spun down at 14,000 rpm. The supernatant was separated and assayed directly for cAMP following the manufacturer's instructions.
Calcium imaging.
Fluorescent Ca2+ imaging was performed on the basis of a revision of a previously described method (45). DCs were incubated with 2 μM Fura-2 for 20 min at room temperature in 1.5-ml tubes and then centrifuged at 1,500 rpm for 15 min. After being washed in extracellular fluid (ECF), cells were placed on poly-l-ornithine-coated coverslips and transferred to a perfusion chamber on an inverted microscope (Nikon TE2000-U; Nikon). Cells were illuminated using a xenon lamp and observed with a 40× objective lens (numerical aperture = 0.90; Super Fluor X40; Nikon). Images were obtained using a cooled charge-coupled-device camera (Cool Snap ES2; Photometrics). Digitized images were acquired, stored, and analyzed by Axon Imaging Workbench software (INDEC BioSystems). The shutter and filter wheel (Lambda 10-3; Sutter Instruments) were also controlled by the software to allow timed illumination of cells at either 340- or 380-nm excitation wavelengths. Fluorescence was detected at an emission wavelength of 510 nm. Images were analyzed by averaging the ratio of the number of pixels obtained at 340 nm/number of pixels obtained at 380 nm in circumscribed regions of cells within the field of view.
Apoptosis assay.
The level of apoptosis in WT and IL-10−/− DCs before and after infection with Chlamydia was determined using annexin V and 7-aminoactinomycin D (7-AAD) flow cytometry (BioLegend, San Diego, CA). The cells were then collected at time zero, 12, and 24 h after infection, washed twice with cold BioLegend cell staining buffer, and then resuspended in annexin V binding buffer. Five microliters of fluorescein isothiocyanate (FITC)-annexin V and 5 μl of 7-AAD viability staining solution were added to 100-μl cell suspensions, and the cells were incubated in the dark at room temperature for 15 min. Four hundred microliters of binding buffer was added to the sample, and the mixture was vortexed and then analyzed by flow cytometry using a FACSAria flow cytometer (BD Biosciences, San Jose, CA).
Statistical analysis.
The data derived from different experiments were analyzed and compared by performing a 1- or 2-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance (ANOVA) (GraphPad Prism software; GraphPad Software, La Jolla, CA). Statistical significance was judged at a P value of <0.05.
RESULTS
Chlamydia-infected IL-10-deficient mice suffered limited inflammatory tubal pathology and infertility.
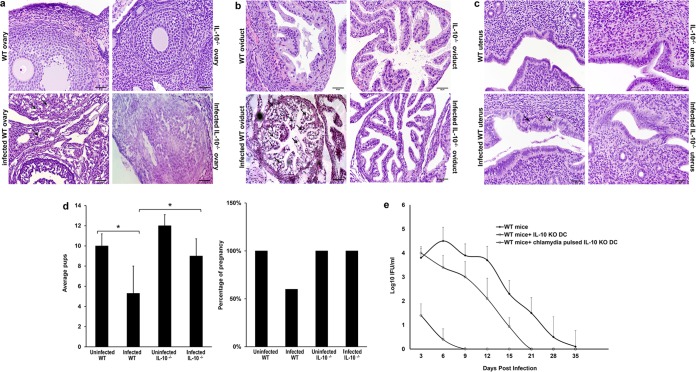
We first assessed the role of IL-10 in inflammatory tubal pathologies and fertility in mice infected twice with C. muridarum (repeated infection). The reproductive systems, consisting of the ovaries, oviducts, and uteri, of WT and IL-10−/− mice were evaluated histopathologically for evidence of tissue pathologies. Significant changes in tissue morphology were detected in the ovaries, oviducts, and uteri of WT mice only. The findings consisted of vacuolar degeneration of oviduct epithelia and ovarian stromal cells, a swollen oviduct, and widespread apoptotic necrosis of the endometrium. However, the ovaries, oviducts, and uteri of Chlamydia-infected IL-10−/− mice did not show pathological changes (Fig. 1a to c). Fertility studies revealed that noninfected WT or IL-10−/− mice and Chlamydia-infected IL-10−/− mice were 100% fertile, whereas infected WT mice suffered significant infertility (60% fertile). There was also a significantly higher number of pups in pregnant noninfected WT mice and Chlamydia-infected IL-10−/− mice than Chlamydia-infected WT mice (P = 0.04 and P = 0.048, respectively) (Fig. 1d).
FIG 1.
Histological assessment of genital tract pathology and fertility in Chlamydia-infected mice. (a) Representative hematoxylin and eosin-stained sections of the ovaries of genitally infected WT and IL-10−/− mice and their controls. Changes in tissue morphology were detected in WT mice only. Arrows, examples of vacuolar degeneration of ovarian stromal cells. Bars, 50 μm. (b) Oviduct sections from infected or noninfected WT and IL-10−/− mice. Arrows, regions with vacuolar degeneration of oviductal epithelia. Bars, 50 μm. (c) Representative H&E staining of uteri from infected or noninfected WT and IL-10−/− mice. Arrows, widespread apoptotic necrosis of the endometrium. Bars, 50 μm. (d) Female Chlamydia-infected WT and IL-10−/− mice (n = 10) and their uninfected controls (n = 10) were mated with male mice. The percentage of pregnant mice was determined, and the mean number of pups in the different groups was calculated. Data are presented as means ± SDs. *, P ≤ 0.05 using Student's t test to compare Chlamydia-infected WT and IL-10−/− mice. (e) DCs isolated from IL-10−/− mice were pulsed or not pulsed with live C. muridarum EBs for 2 h and adoptively transferred into female C57BL/6 mice (2.5 × 107 cells per mouse). After 1 week, each mouse was infected intravaginally with 1 × 105 IFU of C. muridarum. The status of the infection was monitored by periodic cervicovaginal swabbing of individual animals and isolation of Chlamydiae in tissue culture. Experiments were repeated twice to give 10 to 12 mice per group. KO, knockout.
We previously showed that the rapid induction of a specific antichlamydial T cell response in IL-10−/− mice was due to the capacity of Chlamydia-pulsed IL-10−/− DCs to be highly efficient APCs that activated a rapid T cell response in vivo and in vitro (7). To further support the hypothesis that the decreased histopathologic changes observed in the genital tract of IL-10−/− mice were related to the efficient immunostimulatory action of IL-10−/− DCs, Chlamydia-pulsed and nonpulsed IL-10−/− DCs were adoptively transferred into WT mice. The mice were then infected intravaginally with 105 IFU of C. muridarum 1 week after adoptive transfer. The course of infection and bacterial load were monitored. The results showed that the mice that received the IL-10−/− DCs were immune to a challenge infection and cleared their infections within 9 and 21 days for Chlamydia-infected WT mice and mice into which IL-10−/− DCs were transferred, respectively (Fig. 1e). As with previous studies (21), we focused on the shedding of chlamydiae into the cervicovaginal vault as the main assay for determining the acquisition of immunity in the infected mice. Thus, these results as well as previously published infectivity data (7) and confirmation of ascending infection (21) suggest that the efficient immunomodulatory function of IL-10−/− DCs is responsible for the relative protection of the mice from chlamydial infection and associated reproductive system pathologies.
IL-10-deficient DCs activate an enhanced innate immune response that promotes adaptive immunity.
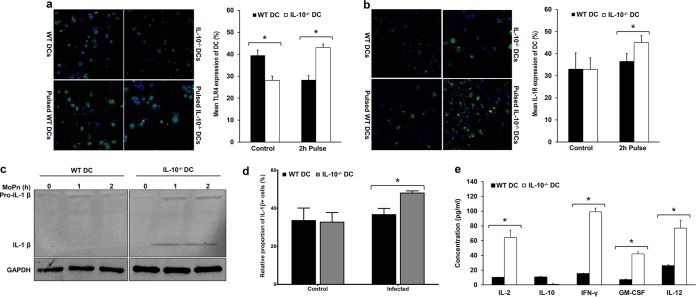
To further investigate the molecular and biochemical mechanisms underlying the ability of IL-10−/− DCs to activate an elevated protective immune response against Chlamydia, we evaluated the innate immune response to Chlamydia. We compared the expression of TLR4, IL-1R, and IL-1β in Chlamydia-pulsed WT and IL-10−/− DCs using confocal microscopy and Western blot analysis. The results showed that TLR4 and IL-1R expression by Chlamydia-pulsed IL-10−/− DCs was significantly increased compared to that by Chlamydia-pulsed WT DCs (P = 0.0005 and 0.034, respectively) (Fig. 2a and b). In addition, IL-1β production was upregulated in the supernatants from the Chlamydia-pulsed IL-10−/− DC culture (Fig. 2c), and there was a significant difference between the relative proportion of IL-1β-positive cells in Chlamydia-pulsed IL-10−/− DCs and that in Chlamydia-pulsed WT DCs (Fig. 2d). Furthermore, the levels of cytokines (IL-2, IL-10, IL-12, IFN-γ, and GM-CSF) secreted by activated CD4+ T cells that were cocultured with either Chlamydia-pulsed WT DCs or IL-10 −/− DCs were also measured using a Luminex assay. The results showed that the secretion of IL-2, IL-12, IFN-γ, and GM-CSF was significantly higher in the supernatants of T cell cultures activated by Chlamydia-pulsed IL-10−/− DCs than in the supernatants of T cell cultures activated by Chlamydia-pulsed WT DCs (P < 0.0001) (Fig. 2e). These results indicate that, in the absence of IL-10, the antigen-handling function of Chlamydia-exposed DCs along with the secretion of the costimulatory cytokine IL-1β was boosted and that this actively triggered the T cell response. This affirms our previous findings that the endogenous IL-10 produced by the DCs is a crucial regulatory factor in the rate and magnitude of T cell activation (7).
FIG 2.
IL-10 upregulates both innate and adaptive immunity during Chlamydia infection in vitro. (a and b) (Left) Immunofluorescence showing the expression of TLR4 (a) and IL-1R (b) in Chlamydia-pulsed and nonpulsed WT and IL-10−/− DCs. (Right) The graphs shows the percentage of TLR4- and IL-1R-positive DCs ± SD. *, significant difference (P ≤ 0.05) between WT and IL-10−/− DCs. The results are representative of those from three independent experiments. (c) Representative Western blot of pro-IL-1β and active IL-1β expression in supernatants collected from WT and IL-10−/− DCs pulsed with Chlamydia for 0, 1, and 2 h. Note that all samples were run on the same gel and that the gel figure was spliced for better presentation. MoPn, Chlamydia muridarum. (d) Graph showing the percentage of IL-1β-positive Chlamydia-pulsed and nonpulsed WT and IL-10−/− DCs. *, significant difference (P ≤ 0.05) between WT and IL-10−/− DCs. The results are representative of those from three independent experiments. (e) The concentrations of IL-2, IL-10, IL-12, IFN-γ, and GM-CSF secreted from CD4+ T cells cocultured with Chlamydia-pulsed WT or IL-10−/− DCs were measured by a Luminex assay. Data are presented as means ± SDs. *, significant difference (P ≤ 0.05) in the concentration of cytokines from WT and IL-10−/− DCs. The results are representative of those from three independent experiments.
IL-10 deficiency altered inflammasome activation in IL-10−/− DCs.
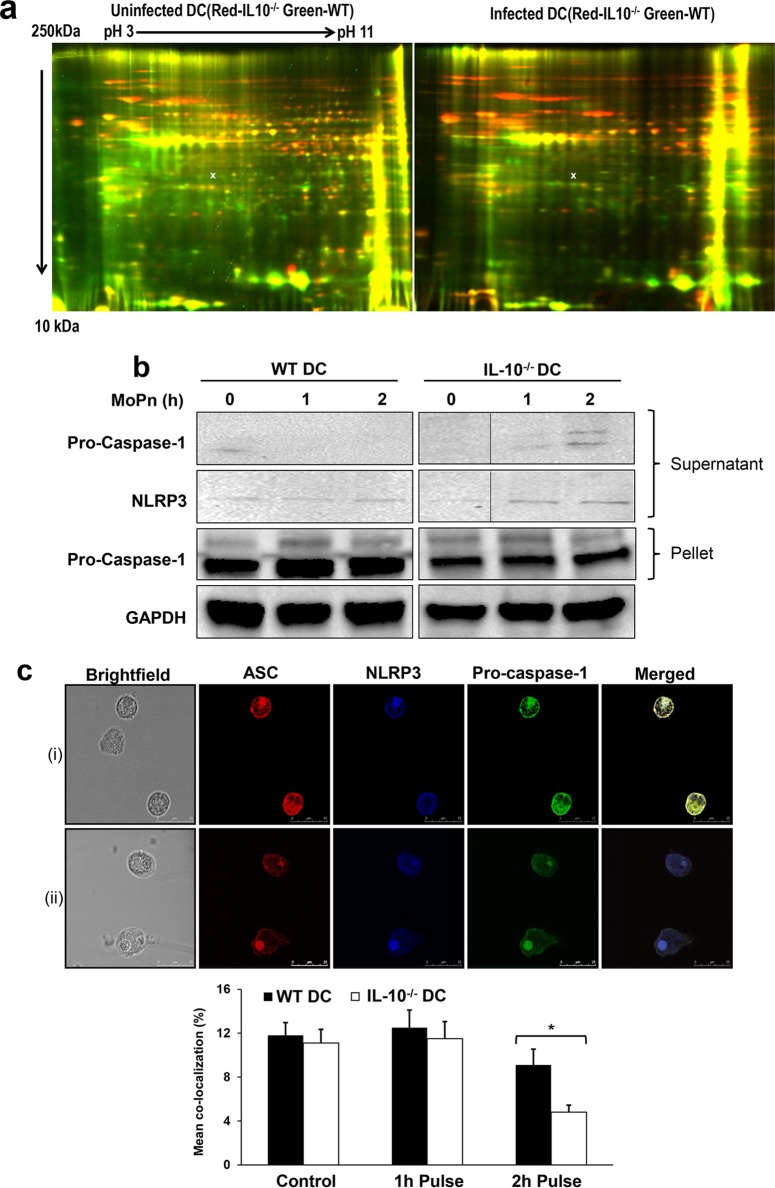
Both the in vivo and in vitro data suggested that IL-10 deficiency protects the host against Chlamydia infection and its resulting sequelae by enhancing the immune-stimulatory function of DCs. We therefore hypothesized that IL-10−/− DCs would express key immunomodulatory proteins that can boost the immune activation of DCs but that are normally suppressed in the presence of IL-10. We compared the total proteins expressed in Chlamydia-pulsed WT and IL-10−/− DCs using 2D-DIGE and LC-MS/MS to identify differentially expressed molecules. The apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), a key component of the NALP3 inflammasome complex that activates caspase-1 and IL-1β production (22), was downregulated 3.5-fold in IL-10−/− DCs compared to its level of regulation in WT DCs (Fig. 3a). To investigate the consequence of ASC downregulation in IL-10−/− DCs, we explored the possibility that IL-10 deficiency affects inflammasome activation by inhibiting the assembly of the components that are required for caspase activation and cellular apoptosis. We therefore analyzed NLRP3 assembly in Chlamydia-pulsed WT and IL-10−/− DCs using immunoprecipitation and immunofluorescence assays. Supernatants recovered after immunoprecipitation using anti-ASC antibody were probed with anti-NLRP3 and anti-procaspase-1 antibodies. We observed that the amount of NLRP3 and procaspase-1 in the supernatants from IL-10−/− DCs was higher than that in the supernatants from WT DCs, suggesting that NLRP3 assembly was inhibited in Chlamydia-pulsed IL-10−/− DCs. There was also a smaller amount of procaspase-1 in the precipitated pellet (Fig. 3b). We confirmed this result using confocal microscopy with immunofluorescence labeling, which provided clear visual evidence of colocalization in labeled cells. Cells were stained with antibodies specific for NLRP3, ASC, and procaspase-1. Colocalization was observed as a different additive color (Fig. 3Ci). After quantitative colocalization analysis (QCA), the results indicated that colocalization was significantly decreased in Chlamydia-pulsed IL-10−/− DCs compared to the level of colocalization in WT DCs (P < 0.05), suggesting that IL-10 regulates the assembly of the NLRP3 inflammasome.
FIG 3.
IL-10 regulates inflammasome activation in DCs. (a) Total protein expression in Chlamydia-pulsed and nonpulsed WT and IL-10−/− DCs was assessed by 2D-DIGE and LC-MS/MS. x, the position where ASC, which was differentially regulated, was found to be expressed in Chlamydia-pulsed DCs. (b) Immunoprecipitation with an anti-ASC antibody was performed on lysates collected at 0, 1, and 2 h from Chlamydia-pulsed and nonpulsed WT and IL-10−/− DCs. The supernatant and pellet were analyzed by Western blotting. Note that all samples were run on the same gel and that the figure showing the gel was spliced for better presentation. (c) Immunofluorescence showing the colocalization of NLRP3, ASC, and caspase-1 in Chlamydia-pulsed and nonpulsed WT and IL-10−/− DCs. (i) The merged cells depict the presence of the colocalization of the proteins in the DCs. (ii) The merged cells depict the absence of colocalization of the proteins in the DCs. The graph at the bottom shows the percent colocalization (mean ± SD) of NLRP3, ASC, and caspase-1 in DCs. IL-10−/− DCs have less colocalization, indicating that IL-10 regulates NLRP3 activation through inflammasome assembly. Data from three independent experiments were pooled.
IL-10 deficiency inhibited P2X7R expression, decreased the intracellular Ca2+ concentration, and increased the cAMP concentration in Chlamydia-exposed DCs.
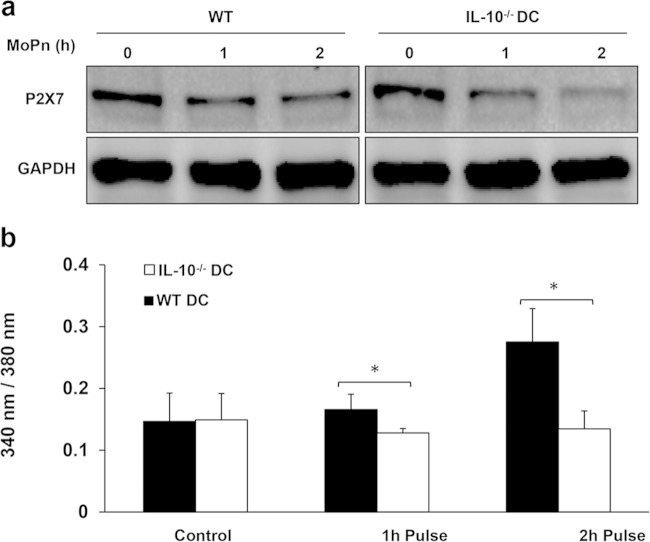
Previous studies have reported that P2X7R, intracellular Ca2+, and cAMP are key molecular regulators of NLRP3 inflammasome activation (23, 24). P2X7Rs are members of the family of ionotropic ATP-gated receptors and are channels for calcium influx into cells. They have been shown to regulate the processing and release of IL-1β through pannexin-1- mediated pore formation, which activates NLRP3 (25). Calcium is known to increase the assembly and activation of the NLRP3 inflammasome (26), while high levels of cAMP have been shown to reduce the activity of the NLRP3 inflammasome (27). We investigated the expression of the ATP-gated P2X7 ion channels and intracellular cAMP and Ca2+ concentrations in Chlamydia-pulsed WT and IL-10−/− DCs using Western blotting, ELISA, and fluorescent Ca2+ imaging analyses. We observed that the amount of P2X7R decreased with time in Chlamydia-pulsed IL-10−/− DCs compared to the amount in WT DCs, whose protein expression remained unchanged (Fig. 4). In addition, there was a significant increase in the cytoplasmic Ca2+ concentration only in Chlamydia-pulsed WT DCs (P = 0.001 and 0.002) (Fig. 4). However, there was a nonsignificant increase in the cAMP concentration in Chlamydia-pulsed IL-10−/− DCs compared to that in WT DCs (data not shown). These results indicate that in Chlamydia-pulsed IL-10−/− DCs, NLRP3 inflammasome activation is incomplete due to repression of P2X7 ion channels and the cytoplasmic Ca2+ concentration.
FIG 4.
Interplay between IL-10, P2X7, and Ca2+ in modulating NLRP3 assembly. (a) The expression of ATP-gated P2X7 ion channels was evaluated by Western blotting of lysates collected from Chlamydia-pulsed or nonpulsed WT and IL-10−/− DCs after 0, 1, and 2 h. Note that all samples were run on the same gel and that the figure showing the gel was spliced for better presentation. (b) Intracellular Ca2+ concentrations of Chlamydia-pulsed or nonpulsed WT and IL-10−/− DCs at 0, 1, and 2 h. Data are presented as means ± SDs. *, P ≤ 0.05 using Student's t test to compare the results for Chlamydia-infected WT and IL-10−/− mice. Data from 10 independent experiments were pooled.
IL-10 deficiency suppressed the apoptotic tendency of antigen-activated DCs.
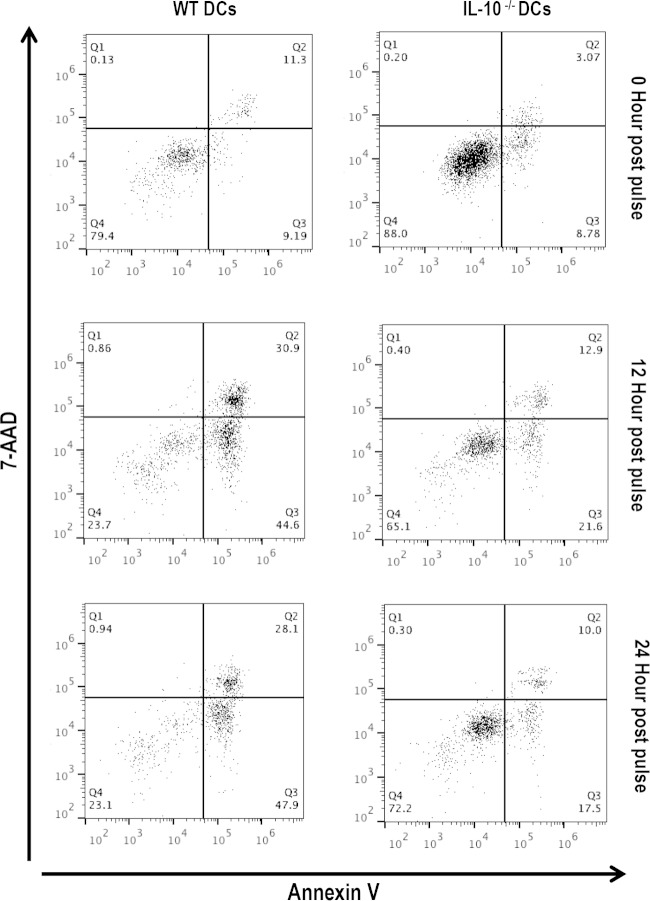
To investigate the consequence of inflammasome inhibition in Chlamydia-pulsed IL-10−/− DCs, we compared the level of apoptosis in WT and IL-10−/− DCs before and after pulsing with C. muridarum using annexin V and 7-AAD flow cytometry. Annexin V staining paired with 7-AAD is widely used to identify apoptotic stages by flow cytometry (28). The results indicated that in the early and late apoptotic stages, there were fewer IL-10−/− DCs than WT DCs in response to Chlamydia infection, suggesting that IL-10−/− DCs were significantly less apoptotic than WT DCs at 12 h and 24 h after exposure to Chlamydia (P ≤ 0.05) (Fig. 5). These results suggest that the IL-10-mediated apoptosis of WT DCs reduces the number of DCs and adversely affects the antigen presentation efficiency of WT DCs. Therefore, the relative resistance of IL-10−/− DCs to activation-driven apoptosis may contribute to APC potency.
FIG 5.
IL-10 deficiency decreases DCs apoptosis. The effect of IL-10 deficiency on the apoptosis of Chlamydia-pulsed and nonpulsed WT and IL-10−/− DCs was determined using annexin V and 7-AAD flow cytometry.
DISCUSSION
We tested the hypothesis that IL-10 regulates inflammasome activation in DCs exposed to Chlamydia, thereby influencing the APC function and the induction of immunity that controls inflammatory tubal pathology and infertility. Our results revealed that (i) in the absence of IL-10 there was very limited tubal damage from inflammation in the reproductive tracts of Chlamydia-infected mice and the fertility rate was similar to that of noninfected wild-type mice, (ii) Chlamydia-pulsed IL-10−/− DCs had increased levels of expression of members of the TLR4/IL-1R superfamily and higher levels of IL-1β compared to the levels for WT DCs, (iii) Chlamydia-pulsed IL-10−/− DCs were less apoptotic than Chlamydia-pulsed IL-10+/+ DCs, and (iv) IL-10−/− DCs modulated NLRP3 inflammasome formation by inhibiting P2X7R expression and the static intracellular Ca2+ concentration in Chlamydia-exposed DCs. The results indicate that IL-10 deficiency promotes efficient antigen presentation by DCs for the rapid and enhanced immune activation against Chlamydia, at least in part through suppression of the intracellular inflammasome assembly that prevented DC apoptosis to allow effective antigen presentation. The rapid and enhanced antichlamydial immune responses resulted in rapid microbial clearance that prevented tubal pathologies during infection.
Clinical observations and experimental data have indicated that the deleterious host immune response to chronic or repeated chlamydial infection leads to permanent tubal damage and infertility. Therefore, controlling the balance between a protective immune response and Chlamydia-induced immunopathology is important to prevent the complications of genital chlamydial infections. Our results showed that IL-10 may contribute to the pathogenesis of chlamydial infection and its complications by limiting the induction of a rapid and robust protective immunity during infections. Thus, the absence of IL-10 in Chlamydia-infected mice was associated with protection from tubal pathologies and the rapid induction of a protective immune response that was associated with microbial clearance. This also meant that the Chlamydia-infected mice without IL-10 were fertile and able to produce offspring at rates similar to those for uninfected WT mice due to the rapid activation of the immune response and the dampening of immunopathogenic responses. Our present results confirm that the ability of IL-10−/− mice to resist Chlamydia-induced pathologies is associated with the antigen-presenting effectiveness of the IL-10-deficient DCs from these mice (7). Thus, we have once more shown that the adoptive transfer of IL-10 deficiency into WT (IL-10+/+) mice results in a reduced bacterial burden and the rapid clearance of chlamydiae from mice challenged with Chlamydia by genital infection. The rapid and robust Th1 response and clearance of infection resulted in normal fertility in infected animals. In addition, Chlamydia-pulsed IL-10−/− DCs induced a predominantly Th1 phenotype when used to activate immune T cell cultures. A Th1-type response is crucial for protective immunity against Chlamydia infection (7, 15). The early activation, the robust costimulatory molecules, and the rapid IL-1β expression by Chlamydia-exposed IL-10−/− DCs are consistent with previous reports on the rapid maturation and acquisition of an antigen presentation capacity by IL-10-deficient DCs. Also, confocal microscopy revealed a rapid increase in the expression of TLR4 and IL-1R by IL-10−/− DCs after a 2-h pulse with C. muridarum. The TLR/IL-1R superfamily plays a fundamental role in innate and acquired immune responses. Once activated by their ligands, IL-1R, the IL-18 receptor, and TLRs engage with one or more adaptor proteins, such as MyD88, MAL/TIRAP, TRIF, and TRAM in various combinations to initiate complex signaling pathways that result in proinflammatory cytokine and chemokine secretion (29, 30) and facilitate the induction of adaptive immunity.
The efficiency of antigen presentation by DCs is dependent upon the survival of the DCs after activation through inhibition of apoptosis and the duration of the immunologic synapse formed with T cells (31). The high efficiency with which IL-10-deficient DCs induced robust protective antichlamydial immunity was previously attributed to the rapid maturation, cytoskeletal reorganization, and expression of elevated levels of costimulatory molecules that promote antigen presentation for T cell activation (7, 32). To investigate whether inhibition of DC apoptosis played a role in the potent antigen-presenting ability of IL-10−/− DCs, we studied several inflammatory mechanisms leading to apoptosis in DCs. DCs activate the immune response during an infection by initially sensing the microbe through pattern recognition receptors (PRRs), such as the Toll-like receptors (TLRs) and Nod-like receptors (NLRs) that bind microbial PAMPs. This binding results in DC activation, leading to a range of responses that include the secretion of proinflammatory cytokines and chemokines, the production of antimicrobial factors, and the induction of cell death by apoptosis, autophagy, or pyroptosis (17). In addition, inflammasome activation, leading to activation of proapoptotic molecules, and cellular activation occur. To investigate whether inhibition of DC apoptosis may play a role in the high efficiency of antigen presentation by IL-10−/− DCs, we compared Chlamydia-pulsed WT and IL-10-knockout DCs for molecular signatures of inflammasome activation. Proteomics results showed that ASC, a component of the NALP3 inflammasome, was highly differentially expressed in IL-10−/− versus IL-10+/+ DCs. However, immunoprecipitation and immunofluorescence assays revealed that Chlamydia-pulsed IL-10−/− DCs had significantly lower levels of NLRP3 inflammasome assembly than WT DCs, suggesting that IL-10 might act posttranslationally to regulate NLRP3 inflammasome function. NLRP3 inflammasome activation by extracellular ATP is mediated through the P2X purinoceptor 7 (P2X7) receptor (P2X7R), an ATP-gated ion channel (33, 34). High concentrations of extracellular ATP could perturb the plasma membrane by activation of P2X7 channels and serve as a danger signal to activate inflammasomes (33, 34). Further investigation of the mechanism by which IL-10 controls NLRP3 inflammasome assembly in DCs revealed that IL-10−/− DCs pulsed with Chlamydia inhibited the expression of P2X7. Inhibition of P2X7R in IL-10−/− DCs decreases ATP-dependent NLRP3 inflammasome activation. Ca2+ signaling plays a critical role in activation of the NLRP3 inflammasome (24, 35). Studies have indicated that increased intracellular Ca2+ influx is necessary for NLRP3 inflammasome activation (26, 36), and the blocking of Ca2+ mobilization inhibits the assembly and activation of the NLRP3 inflammasome (37). The signal-dependent influx of Ca2+ from the extracellular space and intracellular Ca2+ stores (e.g., the endoplasmic reticulum [ER]) can be regulated by the transient receptor potential cation channels TRPM7 and TRPV2, by the reactive oxygen species-sensitive TRPM2 channel (38), and the calcium-sensing receptor (CASR) and GPRC6A (24). Our results revealed that IL-10−/− DCs pulsed with Chlamydia have a static (nonincreasing) cytoplasmic Ca2+ concentration. This low cytoplasmic Ca2+ concentration in IL-10−/− DCs might be mediated by the inhibited P2X7 receptor. Intracellular cAMP regulates the NLRP3 inflammasome; studies show that cAMP suppresses NLRP3 inflammasome activation through the inhibition of inflammasome assembly (24, 39). Our results showed an inverse correlation between the levels of cAMP and the presence or absence of IL-10, suggesting that Chlamydia-pulsed IL-10−/− DCs had increased levels of cAMP synthesis, which might repress NLRP3 inflammasome formation. Using a combination of immunological and biochemical approaches, we determined that in the absence of IL-10, Chlamydia-pulsed DCs utilized the TLR/IL-1 signaling pathway, which then impaired NLRP3 inflammasome activation. However, Chlamydia-pulsed WT DCs had no significant changes in NLRP3 activity or the level of mature IL-1β production, suggesting that IL-1β activation is independent of the NLRP3 inflammasome. Another possible pathway of IL-1β production is the IL-10/STAT3 circuit, which is essential for the regulation of cytokine receptor signaling (40, 41). Binding of IL-10 to the extracellular domain of the IL-10 receptor activates phosphorylation of the receptor-associated Janus kinase 1 (JAK1), tyrosine kinase 2 (Tyk2), and specific tyrosine residues on the intracellular domain of the IL-10 receptor, which binds STAT3, which then induces the interleukin-1 receptor antagonist (IL-1RA). IL-1RA binds to IL-1R to prevent IL-1β activation (42). In the absence of IL-10, IL-1RA activity is blocked, which results in elevation of the IL-1β level (22, 43).
Finally, since the recruitment and activation of caspases and the induction of apoptosis are considered intrinsic parts of the NLRP3 inflammasome function (44), we assessed the degree of apoptosis in WT and IL-10−/− DCs before and after exposure to Chlamydia. Compared to the resistance of Chlamydia-pulsed WT DCs to apoptosis, IL-10−/− DCs were relatively resistant to apoptosis. Our results suggest that NLRP3 activation was inhibited in IL-10-deficient DCs, which suppressed DC apoptosis and extended the life span for antigen presentation for the induction of immunity against antigens. This phenomenon may provide at least a partial basis for the increased efficiency of the antigen-presenting function of IL-10-deficient DCs and their ability to induce a robust Chlamydia-specific Th1 response that rapidly clears the infection in vivo and prevents the onset of tubal pathologies. Our findings may lead to effective immunomodulatory vaccines and the design of drugs with activity against infectious and noninfectious diseases, as well as prevent clinical complications.
ACKNOWLEDGMENTS
We thank V. M. Dixit, Department of Physiological Chemistry, Genentech, San Francisco, CA, for providing comments on the data from the study and suggesting new approaches. We thank Andrew Shaw of the Georgia Institute of Technology Microscopy and Microanalysis Core for assistance with the confocal microscopy. We also thank G. J. Leitch, Department of Physiology, Morehouse School of Medicine, for reviewing the manuscript.
We declare that we have no conflict of interest.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This work was supported by NIH grant 8G12MD007602 from NIMHD and 1SC1AII03041-01A1 from NIGMS to Qing He.
Y.O., D.M., K.R., C.K., R.S., K.I., Z.-G.X., and Q.H. performed the experiments; Y.O., D.L., and Q.H. performed the animal work; U.B.-M. and Q.H. performed the histological analysis; Y.O., Q.H., and J.I. assisted with the data analysis; and Q.H., Y.O., R.S., F.E., C.B., and J.I. contributed to the study design and participated in manuscript preparation.
REFERENCES
- 1.Torrone E, Papp J, Weinstock H. 2014. Prevalence of Chlamydia trachomatis genital infection among persons aged 14-39 years—United States, 2007-2012. MMWR Morb Mortal Wkly Rep 63:834–838. [PMC free article] [PubMed] [Google Scholar]
- 2.Darville T, Pelvic Inflammatory Disease Workshop Proceedings Committee. 2013. Pelvic inflammatory disease: identifying research gaps—proceedings of a workshop sponsored by Department of Health and Human Services/National Institutes of Health/National Institute of Allergy and Infectious Diseases, November 3-4, 2011. Sex Transm Dis 40:761–767. doi: 10.1097/OLQ.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Igietseme JU, Omosun Y, Partin J, Goldstein J, He Q, Joseph K, Ellerson D, Ansari U, Eko FO, Bandea C, Zhong G, Black CM. 2013. Prevention of Chlamydia-induced infertility by inhibition of local caspase activity. J Infect Dis 207:1095–1104. doi: 10.1093/infdis/jit009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Gartner J, Zhu L, Wang S, Brunham RC. 1999. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J Immunol 162:1010–1017. [PubMed] [Google Scholar]
- 5.Cohen CR, Plummer FA, Mugo N, Maclean I, Shen C, Bukusi EA, Irungu E, Sinei S, Bwayo J, Brunham RC. 1999. Increased interleukin-10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS 13:327–332. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 6.Yang X. 2003. Role of cytokines in Chlamydia trachomatis protective immunity and immunopathology. Curr Pharm Des 9:67–73. doi: 10.2174/1381612033392486. [DOI] [PubMed] [Google Scholar]
- 7.He Q, Moore TT, Eko FO, Lyn D, Ananaba GA, Martin A, Singh S, James L, Stiles J, Black CM, Igietseme JU. 2005. Molecular basis for the potency of IL-10-deficient dendritic cells as a highly efficient APC system for activating Th1 response. J Immunol 174:4860–4869. doi: 10.4049/jimmunol.174.8.4860. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Marchesi F. 2014. IL-10 and macrophages orchestrate gut homeostasis. Immunity 40:637–639. doi: 10.1016/j.immuni.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. 2003. The dual role of IL-10. Trends Immunol 24:36–43. doi: 10.1016/S1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 10.Rizzuti D, Ang M, Sokollik C, Wu T, Abdullah M, Greenfield L, Fattouh R, Reardon C, Tang M, Diao J, Schindler C, Cattral M, Jones NL. 2015. Helicobacter pylori inhibits dendritic cell maturation via interleukin-10-mediated activation of the signal transducer and activator of transcription 3 pathway. J Innate Immun 7:199–211. doi: 10.1159/000368232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CY, St John AL, Abraham SN. 2013. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 38:349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore-Connors JM, Kim HS, Marshall JS, Stadnyk AW, Halperin SA, Wang J. 2015. CD43−, but not CD43+, IL-10-producing CD1dCD5+ B cells suppress type 1 immune responses during Chlamydia muridarum genital tract infection. Mucosal Immunol 8:94–106. doi: 10.1038/mi.2014.45. [DOI] [PubMed] [Google Scholar]
- 13.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. 2013. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol 4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 15.Marks E, Tam MA, Lycke NY. 2010. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS Pathog 6:e1001179. doi: 10.1371/journal.ppat.1001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. 2012. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donnell H, Pham OH, Li LX, Atif SM, Lee SJ, Ravesloot MM, Stolfi JL, Nuccio SP, Broz P, Monack DM, Baumler AJ, McSorley SJ. 2014. Toll-like receptor and inflammasome signals converge to amplify the innate bactericidal capacity of T helper 1 cells. Immunity 40:213–224. doi: 10.1016/j.immuni.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. 2012. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. 2013. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Igietseme JU, He Q, Joseph K, Eko FO, Lyn D, Ananaba G, Campbell A, Bandea C, Black CM. 2009. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis 200:926–934. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igietseme JU, Ananaba GA, Bolier J, Bowers S, Moore T, Belay T, Eko FO, Lyn D, Black CM. 2000. Suppression of endogenous IL-10 gene expression in dendritic cells enhances antigen presentation for specific Th1 induction: potential for cellular vaccine development. J Immunol 164:4212–4219. doi: 10.4049/jimmunol.164.8.4212. [DOI] [PubMed] [Google Scholar]
- 22.Redpath S, Ghazal P, Gascoigne NR. 2001. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol 9:86–92. doi: 10.1016/S0966-842X(00)01919-3. [DOI] [PubMed] [Google Scholar]
- 23.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. 2011. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity 35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. 2012. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianchi G, Vuerich M, Pellegatti P, Marimpietri D, Emionite L, Marigo I, Bronte V, Di Virgilio F, Pistoia V, Raffaghello L. 2014. ATP/P2X7 axis modulates myeloid-derived suppressor cell functions in neuroblastoma microenvironment. Cell Death Dis 5:e1135. doi: 10.1038/cddis.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schoneberg T, Schaefer M, Krugel U, Smajilovic S, Brauner-Osborne H, Baerwald C, Wagner U. 2012. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun 3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen H, Miao EA, Ting JP. 2013. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann M, Meyer N. 2011. Annexin V/7-AAD staining in keratinocytes. Methods Mol Biol 740:57–63. doi: 10.1007/978-1-61779-108-6_8. [DOI] [PubMed] [Google Scholar]
- 29.Pang IK, Ichinohe T, Iwasaki A. 2013. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol 14:246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kagan JC, Iwasaki A. 2012. Phagosome as the organelle linking innate and adaptive immunity. Traffic 13:1053–1061. doi: 10.1111/j.1600-0854.2012.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Fernandez JL, Riol-Blanco L, Delgado-Martin C. 2010. What is the function of the dendritic cell side of the immunological synapse? Sci Signal 3:re2. doi: 10.1126/scisignal.3105re2. [DOI] [PubMed] [Google Scholar]
- 32.He Q, Eko FO, Lyn D, Ananaba GA, Bandea C, Martinez J, Joseph K, Kellar K, Black CM, Igietseme JU. 2008. Involvement of LEK1 in dendritic cell regulation of T cell immunity against Chlamydia. J Immunol 181:4037–4042. doi: 10.4049/jimmunol.181.6.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR. 2009. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol 182:5052–5062. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubyak GR. 2012. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol 14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horng T. 2014. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol 35:253–261. doi: 10.1016/j.it.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. 2013. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci 126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- 37.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, Horng T. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compan V, Baroja-Mazo A, Bragg L, Verkhratsky A, Perroy J, Pelegrin P. 2012. A genetically encoded IL-1beta bioluminescence resonance energy transfer sensor to monitor inflammasome activity. J Immunol 189:2131–2137. doi: 10.4049/jimmunol.1201349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA, Cronstein BN, Mehal WZ. 2013. Adenosine is required for sustained inflammasome activation via the A(2)A receptor and the HIF-1alpha pathway. Nat Commun 4:2909. doi: 10.1038/ncomms3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. 2011. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, Muise AM, Snapper SB. 2014. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol 122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carey AJ, Tan CK, Ulett GC. 2012. Infection-induced IL-10 and JAK-STAT: a review of the molecular circuitry controlling immune hyperactivity in response to pathogenic microbes. JAKSTAT 1:159–167. doi: 10.4161/jkst.19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. 1993. Interleukin-10. Annu Rev Immunol 11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 44.Rajan JV, Rodriguez D, Miao EA, Aderem A. 2011. The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J Virol 85:4167–4172. doi: 10.1128/JVI.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Kratzer E, Inoue K, Simon RP, Xiong ZG. 2010. Developmental change in the electrophysiological and pharmacological properties of acid-sensing ion channels in CNS neurons. J Physiol 588:3883–3900. doi: 10.1113/jphysiol.2010.192922. [DOI] [PMC free article] [PubMed] [Google Scholar]