Abstract
The coagulase-negative species Staphylococcus lugdunensis is an emerging cause of serious and potentially life-threatening infections, such as infective endocarditis. The pathogenesis of these infections is characterized by the ability of S. lugdunensis to form biofilms on either biotic or abiotic surfaces. To elucidate the genetic basis of biofilm formation in S. lugdunensis, we performed transposon (Tn917) mutagenesis. One mutant had a significantly reduced biofilm-forming capacity and carried a Tn917 insertion within the competence gene comEB. Site-directed mutagenesis and subsequent complementation with a functional copy of comEB verified the importance of comEB in biofilm formation. In several bacterial species, natural competence stimulates DNA release via lysis-dependent or -independent mechanisms. Extracellular DNA (eDNA) has been demonstrated to be an important structural component of many bacterial biofilms. Therefore, we quantified the eDNA in the biofilms and found diminished eDNA amounts in the comEB mutant biofilm. High-resolution images and three-dimensional data obtained via confocal laser scanning microscopy (CSLM) visualized the impact of the comEB mutation on biofilm integrity. The comEB mutant did not show reduced expression of autolysin genes, decreased autolytic activities, or increased cell viability, suggesting a cell lysis-independent mechanism of DNA release. Furthermore, reduced amounts of eDNA in the comEB mutant biofilms did not result from elevated levels or activity of the S. lugdunensis thermonuclease NucI. In conclusion, we defined here, for the first time, a role for the competence gene comEB in staphylococcal biofilm formation. Our findings indicate that comEB stimulates biofilm formation via a lysis-independent mechanism of DNA release.
INTRODUCTION
The first published record of Staphylococcus lugdunensis dates back to 1988, when this species was identified and described as an infectious coagulase-negative Staphylococcus (CoNS) (1). Since then, many reports describing clinical cases of S. lugdunensis have emerged, underlining its significance as an important human pathogen and its special position among all other CoNS species (2, 3). This species can cause a variety of infections, ranging from mild skin abscess to aggressive, life-threatening infective endocarditis (4–6). Native valve endocarditis and device-related infections associated with catheters, prosthetic joints, or heart valves reflect the ability of S. lugdunensis to colonize biotic and abiotic surfaces, respectively, and produce biofilms (7, 8). Inside the host, biofilms offer protection against the host immune system and antibiotics (9). Distinct phases of biofilm formation include the attachment of the bacterial cells to a suitable surface, proliferation, and secretion of adhesive polymeric substances that glue the cells together, resulting in the formation of a multilayered biofilm (3, 9, 10).
While several factors involved in biofilm formation of the species Staphylococcus aureus and Staphylococcus epidermidis have been identified and extensively characterized during the past 2 decades, very little has been known about the mechanisms underlying S. lugdunensis biofilm formation (3, 10, 11). Available studies on the pathogenesis of S. lugdunensis focus on the characterization of adhesins and other protein factors like the von Willebrand factor (vWF)-binding protein (12), fibrinogen (Fg)-binding protein Fbl (13), autolysin AtlL (14), iron-regulated surface determinant (Isd) proteins (15), and sortase A (16). Some of these proteins have homologs in other staphylococci, whose functions are well established (3, 10). Frank and Patel reported that 15 different clinical isolates of S. lugdunensis predominantly formed a protein-dependent and polysaccharide intercellular adhesin (PIA)-independent biofilm despite the presence of the icaADBC operon (17). The icaADBC operon confers the production of PIA (also referred to as poly-N-acetylglucosamine; PNAG) on S. epidermidis and S. aureus as an essential component in PIA/PNAG-dependent biofilms (18, 19). A recent study also demonstrated that clinical S. lugdunensis isolates formed biofilms in a PIA-independent and protein-dependent manner and identified the surface protein IsdC as a mediator of biofilm formation under iron-restricted growth conditions (20).
In many bacterial species, including S. aureus and S. epidermidis, extracellular DNA (eDNA) has been demonstrated to be another important structural component of the biofilm matrix (21–25). Here, we used a random mutagenesis approach and identified a novel genetic locus, the competence gene comEB, involved in S. lugdunensis biofilm formation. Our further experiments suggested that comEB stimulates biofilm formation via a lysis-independent mechanism of DNA release representing a source of biofilm eDNA.
MATERIALS AND METHODS
Bacterial strains and media.
The clinical S. lugdunensis isolates a19263 and w701 from the Institute of Medical Microbiology, University Hospital of Münster, were used for transposon and site-directed mutagenesis, respectively. The clinical strain S. epidermidis RP62A (18, 26), predominantly forms a PIA-dependent biofilm, and the biofilm-negative Staphylococcus carnosus TM300 (27) served as controls in studying the eDNA dependency of biofilm formation. S. epidermidis O-47 (28) and S. aureus SA113 (29), carrying the empty vector pCU1, were used as controls in detecting NucI activity. For cloning, Escherichia coli strains XL1 Blue or TG1 were used. S. lugdunensis was grown in either tryptic soy broth (TSB) or on tryptic soy agar, and E. coli was grown in Luria-Bertani (LB) broth or on LB agar. To analyze NucI activity, DNase test agar (Oxoid) was used according to the instructions of the manufacturer. Antibiotics were added to the growth media when appropriate.
Genetic manipulation, plasmids, complementation, and DNA sequencing.
The plasmid pTV1ts (30), used for transposon Tn917 mutagenesis, was introduced into strain a19263 by protoplast transformation (31) with slight modifications: early exponential a19263 cells were treated with lysostaphin at 37°C until the optical density at 578 nm (OD578) dropped to 25% of the initial value. To determine the Tn917 insertion site, an arbitrary PCR was performed as described previously (32). Primers were synthesized and DNA was sequenced by Eurofins MWG Operon (Ebersberg, Germany).
For site-directed mutagenesis, the replacement cassette (comEB::ermB) first was constructed in the shuttle plasmid pCU1 (33). The ermB cassette and recombination sites were amplified directly from the Tn917 mutant using two separate PCRs: PCR1 (primer pair 599CAKnockfor and 599EryKnockrev) (Table 1) to obtain the 2,457-bp comEA-comEB-ermB fragment and PCR2 (primer pair 599tn917for and 599CCKnockrev) (Table 1) to obtain the 1,607-bp orf3-comEB-comEC fragment. Both fragments were sequentially ligated with pCU1, excised as a single 4,072-bp fragment, and then cloned into the EcoRI and PstI sites of the vector pBT2 (34), generating plasmid pBT2comEB::ermB. Subsequently, pBT2comEB::ermB was introduced into the strain w701 by protoplast transformation to disrupt the comEB gene by homologous recombination essentially as described previously (35). For complementation, DNA fragments encoding either comEB (883 bp) or comEB-comEC (3,147 bp), which were previously amplified from the genomic DNA of strain w701 using primer pairs ComAF and ComBR or ComAF and ComER, respectively (Table 1), were cloned into the EcoRI and BamHI sites of the shuttle vector pRB473 (36), generating pRBcomEB or pRBcomEB/EC, respectively.
TABLE 1.
Oligonucleotide primers used in this study
| Name and purpose | Sequencea (5′→3′) | Gene(s) |
|---|---|---|
| Disruption of comEB | ||
| 599CAKnockfor | CTGAATTCTCAGATTCACAAAAGGGAACAG (EcoRI) | |
| 599EryKnockrev | CAGGATCCTAGGGACCTCTTTAGCTCCTTG (BamHI) | |
| 599tn917for | CAGGATCCCGAAGGATCACTCATGGACTAA (BamHI) | |
| 599CCKnockrev | GCACTGCAGATCTTAGTGCACTTGGTGCAAA (PstI) | |
| Complementation | ||
| ComAF | CAGGATCCGAACAGCACGGCCAATTTAC (BamHI) | comEB |
| ComBR | CTGAATTCTGCAATATAAAACACTTAAATCACGA (EcoRI) | |
| ComAF | CAGGATCCGAACAGCACGGCCAATTTAC (BamHI) | comEB, comEC |
| ComER | CTGAATTCTTTTGAGGCATGCTTATAACTCC (EcoRI) | |
| Real-time PCR | ||
| aroE1F | AACGAGCAGCAATCAATACC | aroE |
| aroE1R | TTCAATAATTCCCCCACCTG | |
| gyrBF | CACACGTATGAAGGCGGAAC | gyrB |
| gyrBR | CTACGGCCGTTAAACCTTCTC | |
| holAF | AAATTGGATTCAGCAACAATTACA | holA |
| holAR | GGTCTATCCCCTATGAACAACATT | |
| atlF | TGCATTTGTACATGCGTTTG | atlL |
| atlR | GCGCAAATGAAGCATAGTCA | |
| aalF | CCTTTACCAATAGCTGCACGA | aal |
| aalR | ACTGGCACAGCTTCAGGTTC | |
| nucF | AAACACGTCCATAGCGATCA | nucI |
| nucR | CGTTGATACGCCTGAAACAG |
Restriction sites are underlined.
Quantitative biofilm assay.
Microtiter plate biofilm assays were performed as described earlier (28). In some experiments, biofilms were grown in the presence of DNase I (0.1 mg/ml). In the controls, the enzyme was replaced by the same volume of phosphate-buffered saline. Each assay was performed at least in triplicate.
Quantification of eDNA.
Biofilm eDNA from 24-h biofilms was isolated as described previously (14) and quantified by UV-visible (Vis) spectrophotometry using a NanoPhotometer P330 (Implen) according to the instructions of the manufacturer. Each assay was performed in triplicate.
Confocal laser scanning microscopy (CLSM).
Both 24-h and 48-h biofilms were grown on coverslips at 37°C. For the cultivation of 48-h biofilms, spent medium was exchanged for fresh medium thrice during growth. Afterwards, the biofilms were washed twice with 0.85% NaCl, stained with the live/dead staining kit (Invitrogen) according to the manufacturer's recommendations, and fixed with 10% neutral buffered formalin. The coverslips were mounted onto glass slides with Mowiol and observed with an LSM700 CLS microscope (Zeiss). Images were acquired with a 63× objective and a Z interval of 0.3 μm for 24-h biofilms and 1 μm for 48-h biofilms. Image analysis and processing was carried out with either the Zen Imaging software (Zeiss) or Fiji (37). Three-dimensional (3D) reconstructions were generated with Fiji.
Triton X-100-induced autolysis.
For the autolysis assay, overnight cultures of S. lugdunensis were diluted in 25 ml fresh TSB medium to give a starting OD578 of 0.05. The cultures were shaken at 37°C and 160 rpm until an OD578 of 0.7 to 0.8 was reached. Five milliliters of each sample was pelleted. The cells were washed once with cold double-distilled water and resuspended in an equal volume of 0.05 M Tris-HCl containing 0.05% Triton X-100 (pH 7.2). The samples were incubated at 30°C for 3 h, and the OD578 was measured every 30 min.
Zymographical analysis of bacteriolytic activity.
Surface-associated proteins for zymographical analysis were prepared essentially as described previously, except that overnight cultures of the bacteria were grown in TSB at 37°C and 160 rpm (38). One-milliliter samples were harvested at different time points of growth, and the bacterial cells were resuspended in 1 volume of SDS sample buffer. The samples were heated at 95°C for 10 min and centrifuged, and 10 μl of the supernatant containing the surface-associated autolysins was loaded on SDS gels (10% separation gel) and on corresponding zymogram gels (10% SDS gels containing 0.05% heat-inactivated S. lugdunensis cells in the separation gel). After electrophoresis, the zymogram gels were incubated overnight at 37°C in 25 mM Tris-HCl (pH 8) containing 1% (vol/vol) Triton X-100. The SDS and zymogram gels were stained with Coomassie brilliant blue R250 and methylene blue, respectively.
Viability assay.
Overnight-grown cultures of the S. lugdunensis strains were diluted in 150 ml fresh TSB to give a starting OD578 of 0.05. The cultures then were incubated at 37°C and 160 rpm. Serial dilutions of samples collected at different time points of growth were spread on blood agar plates, and the corresponding CFU were determined. Prior to dilution, the OD578 of each sample was measured.
Real-time PCR-based gene expression analysis.
RNA was isolated from S. lugdunensis cells grown planktonically for 3 or 6 h or in a biofilm for 24 h using an RNeasy minikit (Qiagen) according to the manufacturer's protocol. One microgram of RNA was reverse transcribed to cDNA using the QuantiTect reverse transcription kit and then applied to SYBR green dye-based real-time PCR (SYBR green supermix; Bio-Rad) using the primers listed in Table 1. Melting curve analysis was performed for each reaction to ensure specificity, and the fold change values were calculated with the Bio-Rad iQ5 software using the housekeeping genes aroE and gyrB for normalization.
Statistical analysis.
Data are given as means ± standard errors of the means (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA) and a Bonferroni's posttest; P ≤ 0.05 was considered to indicate statistically significant differences.
Nucleotide sequence accession number.
The GenBank accession number of the comE operon DNA sequence of strain S. lugdunensis w701 is KM232909.
RESULTS
Isolation of a Tn917 insertion mutant producing decreased levels of biofilm.
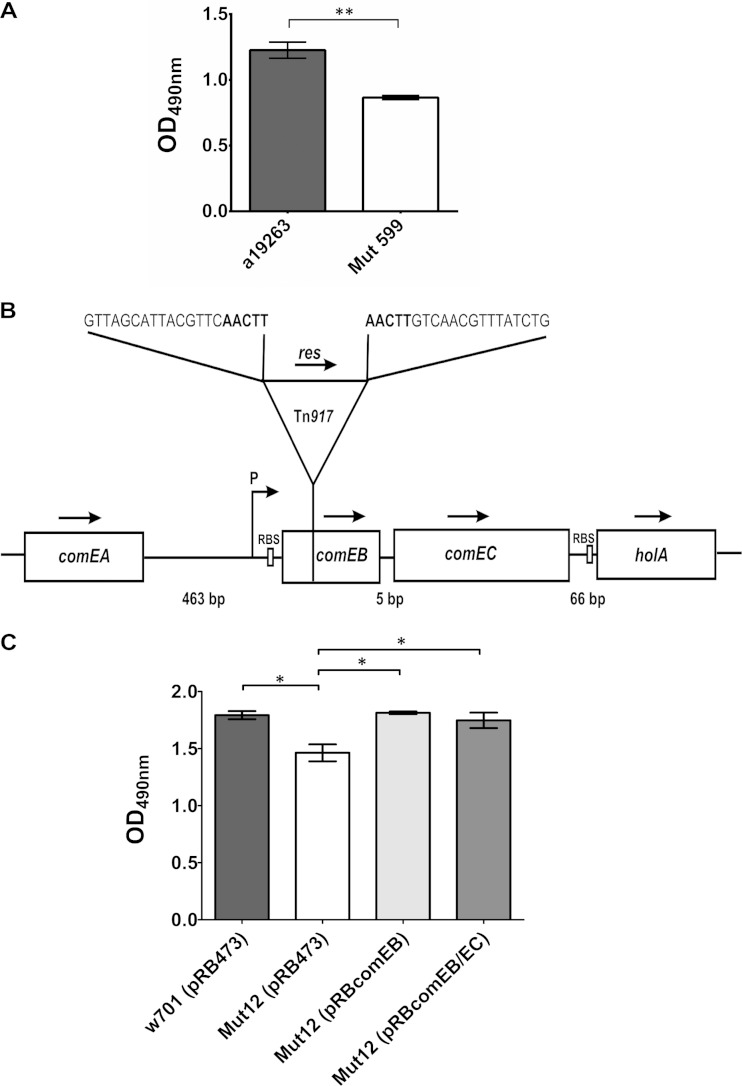
Approximately 5,000 transposon insertion mutants generated by Tn917 mutagenesis of the biofilm-forming clinical isolate S. lugdunensis a19263 in a previously described procedure (28) were screened for an altered biofilm-forming phenotype using the quantitative biofilm assay. The mutant Mut599 formed less biofilm (average OD490, 0.89 ± 0.02) compared to its wild type (average OD490, 1.23 ± 0.06) (Fig. 1A). By means of arbitrary PCR and subsequent DNA sequencing, the Tn917 insertion site was mapped to the competence gene comEB, which is part of an operon. Downstream of comEB, the comE operon contains the competence gene comEC and holA, encoding the putative DNA polymerase PolC δ subunit (Fig. 1B). Analysis of the DNA sequence suggested that the comEC gene is translationally coupled to the comEB gene, because comEC lacks its own ribosomal binding site (RBS).
FIG 1.
comEB is involved in biofilm formation. (A) Quantitative analysis of 24-h biofilms produced by the Tn917 mutant Mut599 and its wild type. The OD490 values ± SEM are the averages from three independent experiments. **, P ≤ 0.01. (B) The Tn917 insertion site in Mut599 is located 64 bp downstream of the comEB start codon, which is part of a putative operon containing the comEB, comEC, and holA genes. The arrows mark the direction of transcription. P, putative promoter; RBS, ribosomal binding site; res, resolvase gene. The nucleotide sequences correspond to the regions flanking the transposon, with the nucleotides in boldface representing the characteristic 5-bp duplication. comEB, comEC, and holA putatively are transcribed from the common promoter upstream of comEB. (C) Quantitative analysis of 24-h biofilms produced by the clinical isolate S. lugdunensis w701 harboring the empty vector pRB473, its site-directed comEB mutant Mut12 containing pRB473, and the complemented mutants. For complementation, the vector pRB473 carrying comEB-comEC (pRBcomEB/EC) or comEB alone (pRBcomEB) was introduced into Mut12. The OD490 values ± SEM are averages from three independent assays. *, P ≤ 0.05. The data were analyzed with one-way ANOVA and a Bonferroni's posttest.
Upon site-directed mutagenesis, the comEB disruption and the associated biofilm phenotype were successfully verified in a different genetic background, the clinical isolate S. lugdunensis w701. The resulting mutant was designated Mut12. Although the difference in biofilm formation between the wild type, w701(pRB473) (OD490, 1.79 ± 0.04), and Mut12(pRB473) (OD490, 1.46 ± 0.07) was not drastic, it was statistically significant (Fig. 1C). The Mut12 phenotype could be fully restored to the wild-type level of biofilm formation by complementation with either the comEB gene alone [Mut12(pRBcomEB); OD490, 1.81 ± 0.01] or with comEB together with comEC [Mut12(pRBcomEB/EC); OD490, 1.75 ± 0.07] (Fig. 1C).
comEB disruption decreases biofilm eDNA.
Because competence has been correlated with DNA release in different bacterial species (39–41) and eDNA is an important structural component in many bacterial biofilms (21–25, 42), we addressed the questions of whether eDNA is also an important structural component of S. lugdunensis biofilms and if the level of eDNA within the biofilms differs among Mut12 and its wild type. To analyze the eDNA dependency of the biofilm formation of the clinical S. lugdunensis isolates a19263 and w701, we performed biofilm assays and observed the influence of DNase I. Biofilm formation of S. lugdunensis a19263 and w701 in the presence of DNase I was almost completely abolished, clearly demonstrating that eDNA is an essential component of their biofilms (Fig. 2A). The same observation was made when preformed biofilms of S. lugdunensis a19263 and w701 were treated with DNase I (data not shown). In contrast, biofilm formation of the control strain S. epidermidis RP62A, predominantly forming a PIA-dependent biofilm (18), was largely unaffected by DNase I (Fig. 2A). Similarly, another recent study demonstrated that the biofilms of all 9 clinical S. lugdunensis strains tested were sensitive to DNase I treatment (20). We next quantified the eDNA content of 24-h biofilms. The biofilm eDNA concentration of mutant Mut12(pRB473) (30.8 ± 1.3 ng/μl) was significantly lower than that of the wild type, w701(pRB473) (41.7 ± 1.9 ng/μl) (P ≤ 0.05), and the complemented mutants Mut12(pRBcomEB) (48.5 ± 6.3 ng/μl) (P ≤ 0.01) and Mut12(pRBcomEB/EC) (44.7 ± 2.8 ng/μl) (P ≤ 0.05) (Fig. 2B). The observed differences clearly indicated that the defect in biofilm formation was due to an eDNA loss mediated by comEB disruption.
FIG 2.
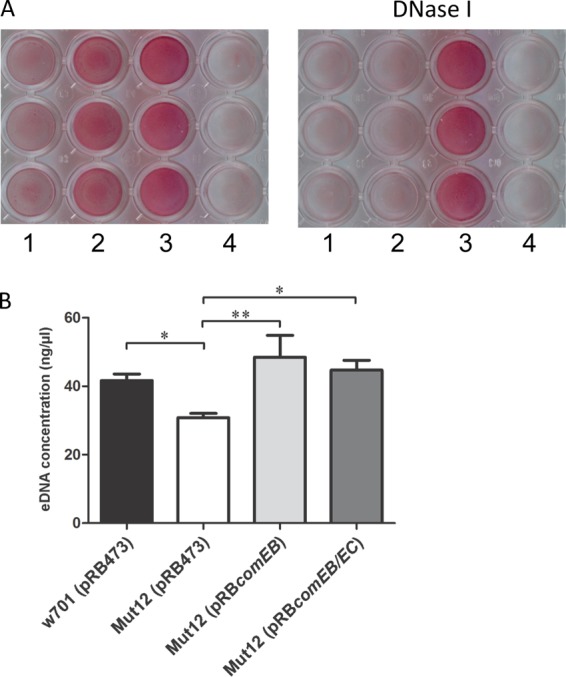
S. lugdunensis biofilm formation is eDNA dependent and comEB modulates eDNA levels in S. lugdunensis biofilms. (A) Biofilm formation in TSB in polystyrene microtiter plates in the presence (0.1 mg/ml) (right) or absence (left) of DNase I. Biofilms were stained with safranin. Lanes: 1, S. lugdunensis a19263; 2, S. lugdunensis w701; 3, S. epidermidis RP62A; 4, S. carnosus TM300. (B) Quantification of eDNA in biofilms. eDNA from 24-h biofilms was isolated and quantified by UV-Vis spectrophotometry. Mut12(pRB473) contained significantly less eDNA in the biofilm than its wild type, w701(pRB473), and the complemented mutants Mut12(pRBcomEB) and Mut12(pRBcomEB/EC). The values represented here are the averages from three independent experiments, and the error bars represent SEM. *, P ≤ 0.05; **, P ≤ 0.01. Statistical analysis was performed with one-way ANOVA and a Bonferroni's posttest.
Visualization of eDNA loss and its effect on biofilm integrity.
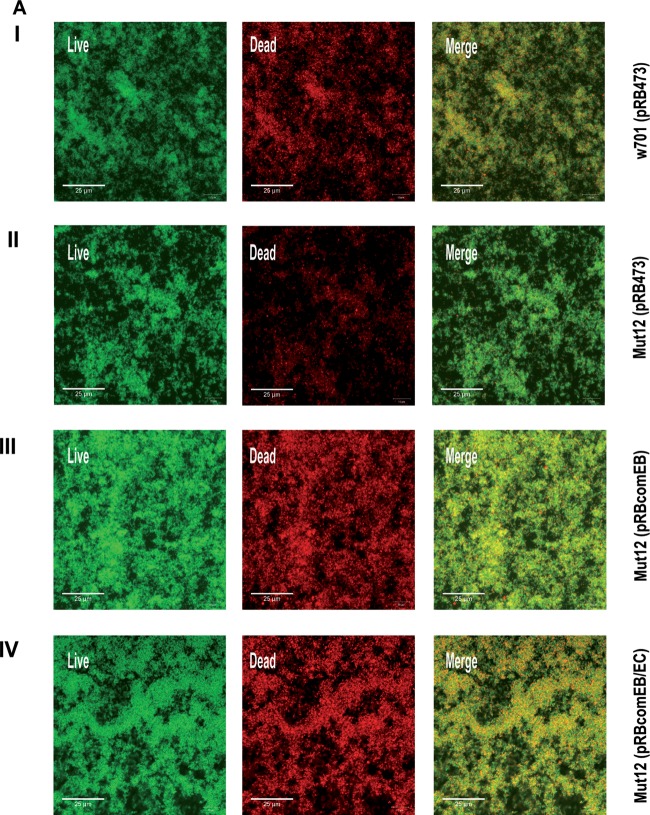
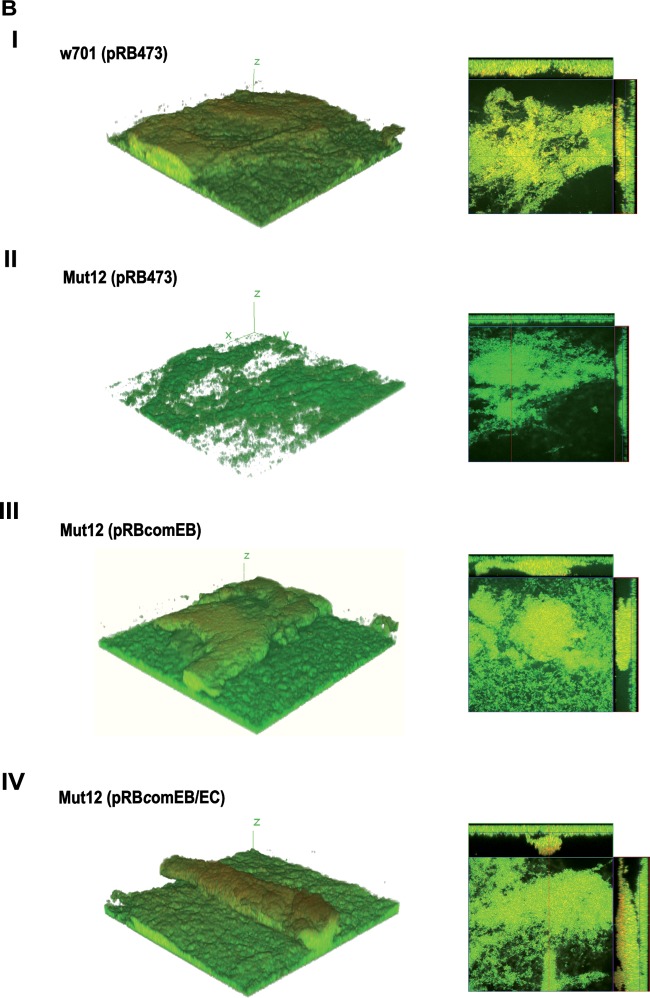
To further qualitatively and quantitatively validate the decrease in the eDNA level, 24-h biofilms were stained with the live/dead staining kit and observed by CLSM. The nucleic acid stain SYTO9 (green fluorescence) penetrates membranes and stains the bacteria green, while the membrane-impermeable propidium iodide (PI; red fluorescence) stains membrane-compromised bacteria and extracellular nucleic acids red. By using both stains, it is possible to distinguish between live bacterial cells and dead bacteria/eDNA. This assay has been used to observe cell death and eDNA content of biofilms formed by various bacteria (21, 23, 25). Mut12(pRB473) (Fig. 3A, row II) showed diminished PI (red) staining compared to the wild type, w701(pRB473) (row I), and the complemented mutants Mut12(pRBcomEB) (row III) and Mut12(pRBcomEB/EC) (row IV). Since PI does not effectively distinguish between dead cells and eDNA, the lowered PI staining observed in the Mut12(pRB473) biofilm could be indicative of a decrease in both eDNA and dead cells. However, the viability of Mut12(pRB473) was not altered (see Fig. 4D), suggesting a decreased eDNA level.
FIG 3.
CLSM analysis of biofilm. (A) To visualize eDNA, mature 24-h biofilms grown on coverslips were stained with SYTO9 (live; green fluorescent) and PI (dead; red fluorescent) and are presented as maximum intensity projections. Colocalization occurs when live cells are closely associated with dead cells or are covered with eDNA, appearing yellow in the merged image (Merge). (B) To obtain 3D data, 48-h biofilms grown on coverslips were stained with the live/dead staining kit and 3D reconstructions were generated with z-stack images obtained with a z-slice interval of 1 μM. The images are representative of three independent experiments.
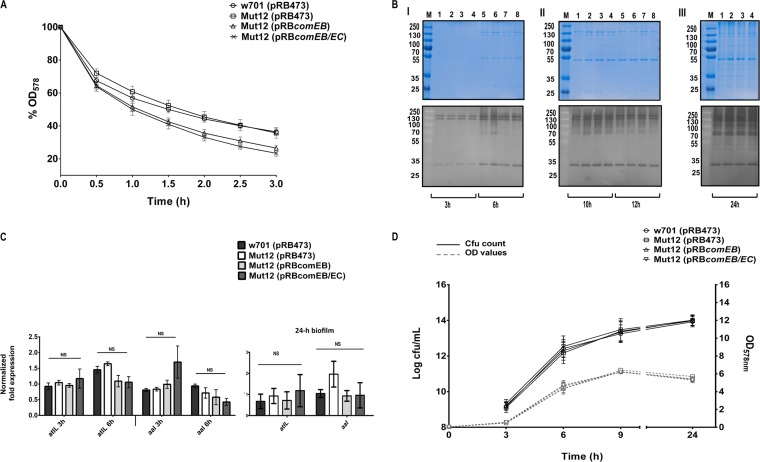
FIG 4.
comEB does not impact the expression of autolytic activities. (A) Mid-log-phase cells were treated with Triton X-100, and autolysis was monitored by the drop in OD578. The OD values are expressed in percentages, with the initial OD set to 100%. n = 3. Error bars indicate SEM. (B) Surface-associated proteins prepared from bacterial strains that were harvested at different time points of growth (3 h, 6 h [I], 10 h, 12 h [II], and 24 h [III]) were separated by SDS-PAGE on SDS gels and corresponding zymogram gels. Zymogram gels contained heat-inactivated S. lugdunensis cells in the separation gel. Bacteriolytic activities were observed as clear zones after overnight incubation in buffer. (Upper) SDS gels; (lower) corresponding zymogram gels. Molecular masses (in kilodaltons) of marker proteins (M) are indicated on the left. Lanes 1 and 5, w701(pRB473); 2 and 6, Mut12(pRB473); 3 and 7, Mut12(pRBcomEB); 4 and 8, Mut12(pRBcomEB/EC). SDS and zymogram gels are representative of three independent experiments. (C) The expression of the S. lugdunensis homologs of two major staphylococcal autolysin genes, atlL and aal, were analyzed by real-time PCR from cultures grown to mid-logarithmic (3 h) or stationary phase (6 h) or in 24-h biofilms. The values represent the averages from three independent experiments, and the error bars represent the SEM. The data were analyzed with one-way ANOVA and a Bonferroni's posttest. (D) Growth was initiated at a starting OD578 of 0.05 in TSB (37°C, 160 rpm) and monitored. At the indicated time points, the cultures were sampled, serially diluted, and plated on blood agar. Resulting colonies were counted after 24 h of incubation. The viability was expressed as log CFU/ml. n = 3. Error bars indicate SEM. NS, not significant.
eDNA is known to act as a cohesive agent that sticks the bacterial cells together and stabilizes the structure of a bacterial biofilm (21, 24, 43, 44). Consequently, the eDNA loss in Mut12 should negatively impact biofilm integrity. To detect potential structural alterations, 48-h biofilms grown on coverslips were stained and imaged by CLSM. The 3D reconstructions obtained from the image stacks illustrated a pronounced architectural weakness in Mut12. The biofilm produced by Mut12(pRB473) (Fig. 3B, row II) lacked the thickness that clearly defined the biofilms of w701(pRB473) (row I) and the complemented mutants Mut12(pRBcomEB) (row III) and Mut12(pRBcomEB/EC) (row IV). Using the COMSTAT program (45), this difference in thickness was quantified. The average thickness of the Mut12(pRB473) biofilm was 10.67 ± 1.12 μm, while that of w701(pRB473) was 23.89 ± 3.29 μm. The complemented mutants exhibited a biofilm thickness comparable to that of the wild type: 23.19 ± 1.81 μm for Mut12(pRBcomEB) and 25.97 ± 1.90 μm for Mut12(pRBcomEB/EC). Thus, it became evident that the comEB mutation resulted in decreased eDNA availability, which severely influenced biofilm integrity.
Autolytic activity in the comEB mutant is unaffected.
DNA release by bacteria can occur via both lysis-dependent and lysis-independent mechanisms (21, 39, 41, 42, 46, 47). Lysis of bacterial cells is mediated by peptidoglycan hydrolases, also referred to as autolysins (48). To determine if comEB influences cell lysis, the expression of autolytic activities was analyzed. No marked differences were observed among Mut12(pRB473), its wild type, w701(pRB473), and the complemented mutants in both Triton X-100-induced autolysis assays (Fig. 4A) and zymographical analysis of autolytic activities (Fig. 4B, lower). In analogy to S. aureus and S. epidermidis (see below), clearing zones ranging from approximately 70 to 150 kDa probably represent AtlL-associated amidase and glucosaminidase activities, while the clearing zone around 35 kDa presumably is caused by Aal-associated bacteriolytic activity (Fig. 4B, lower). Moreover, no significantly differential expression of the atlL (SLUG_18190; available at the KEGG database [http://www.genome.ad.jp/kegg/]) and aal (SLUG_01420; available at the KEGG database) genes, encoding the autolysins AtlL and Aal, which are homologous to the major autolysins AtlA and Aaa from S. aureus (49, 50) and AtlE and Aae from S. epidermidis (38, 51), respectively, was detected by real-time PCR (Fig. 4C). Additionally, we analyzed the viability of Mut12(pRB473) compared to that of its wild type, w701(pRB473), and the complemented mutants by determining the CFU counts during growth, which did not indicate an altered cell viability (Fig. 4D).
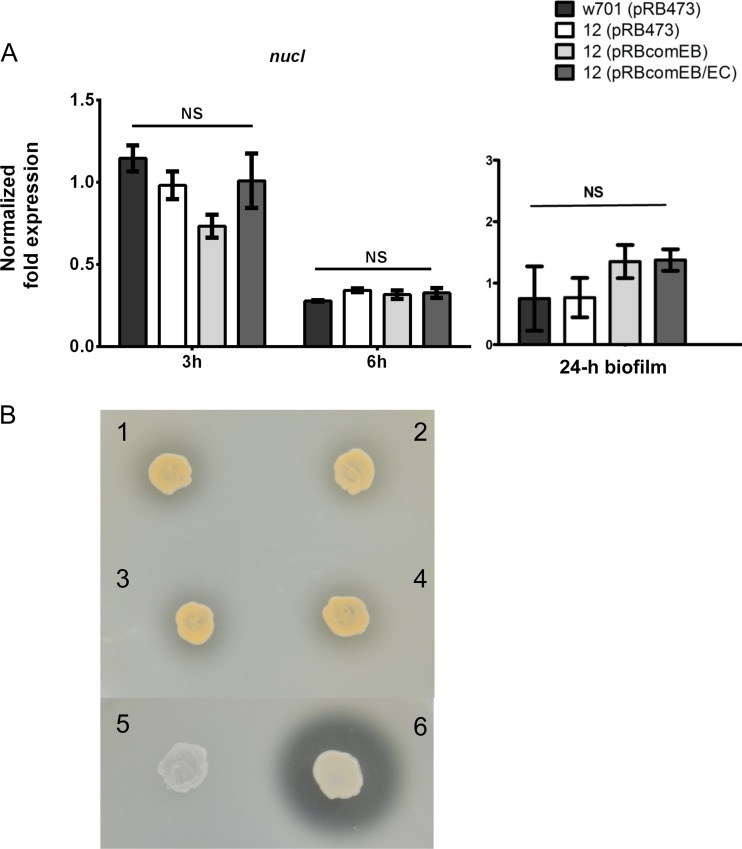
nucI gene expression is not impaired by the comEB disruption.
Recently, it was shown that the S. aureus thermonuclease Nuc has a negative impact on biofilm formation, because it degrades the eDNA that is necessary for maintaining the biofilm architecture (52). Thus, another possible cause of comEB-dependent, eDNA-based differential biofilm formation between Mut12 and its wild type is differentially expressed nuclease activities. While the S. aureus genome encodes two nucleases (53), analysis of the published S. lugdunensis genomes (54, 55) indicated the presence of a single gene (nucI) encoding the thermonuclease NucI (SLUG_15760; available at the KEGG database [http://www.genome.ad.jp/kegg/]). By using real-time PCR, no significant differences were observed in the nucI gene expression among the wild type, w701(pRB473), Mut12(pRB473), and the complemented mutants (Fig. 5A), suggesting that no altered nuclease expression accounted for the observed differences in biofilm formation. Moreover, the NucI activity observed as distinct clearing zones around bacterial growth on DNase agar was comparable among the wild type, w701(pRB473) (Fig. 5B, image 2), Mut12(pRB473) (image 1), and the complemented mutants Mut12(pRBcomEB) (image 3) and Mut12(pRBcomEB/EC) (image 4). For comparison, the positive-control S. aureus SA113(pCU1) (6), known to produce large amounts of nuclease activity, showed a pronounced clearing zone, while the negative-control S. epidermidis O-47(pCU1), which produces a nuclease in very small quantities, did not reveal a clearing zone (Fig. 5B).
FIG 5.
Expression (A) and activity (B) of the thermonuclease NucI is unchanged. (A) nucI gene expression was measured during the mid-logarithmic (3 h) and stationary (6 h) phases of growth or in 24-h-grown biofilms. The mRNA levels were quantified by real-time PCR and normalized against the relative quantities of the aroE and gyrB housekeeping gene transcripts. The data were analyzed with one-way ANOVA and a Bonferroni's posttest. (B) NucI activity, observed as clearing zones around bacterial growth on DNase agar, was similar among wild-type w701(pRB473) (2), Mut12(pRB473) (1), and the complemented mutants Mut12(pRBcomEB) (3) and Mut12(pRBcomEB/EC) (4). S. aureus SA113(pCU1) (6) and S. epidermidis O-47(pCU1) (5) served as positive and negative controls, respectively.
DISCUSSION
S. lugdunensis is a human opportunistic pathogen (3, 56). Being part of the microbiota of the nasal cavity and of other skin areas particularly the lower abdomen and extremities (57, 58), it is not surprising that this species causes a wide range of foreign body-associated infections characterized by the formation of biofilms (2). However, factors contributing to S. lugdunensis biofilm formation have remained largely unknown. To identify genes involved in S. lugdunensis biofilm formation, we performed transposon mutagenesis and identified a mutant (Mut599) with a Tn917 insertion in the competence gene comEB that produced less biofilm than the wild-type strain. Upon site-directed mutagenesis of the comEB gene (generating Mut12), the same biofilm phenotype was reproduced in a different genetic background, verifying that impaired biofilm formation is indeed due to the inactivation of the comEB gene and not due to secondary mutations. Moreover, complementation with comEB was sufficient to restore biofilm formation to wild-type levels, further verifying the implication of comEB.
comEB is part of the comE competence operon, which in S. aureus consists of the comEA, comEB, and comEC genes (59); thus, it largely resembles the organization of the Bacillus subtilis comE operon (60). In contrast, in S. lugdunensis, comEB and comEC form a transcriptional unit with holA, while comEA is encoded independently upstream of comEB (Fig. 1B). The holA gene putatively encodes the DNA polymerase PolC δ subunit, which is essential for DNA replication (61). Because (i) Mut12 exhibited the same growth characteristics as its wild type (Fig. 4D), (ii) comEB was sufficient to complement Mut12, and (iii) real-time PCR analysis indicated unaltered holA expression (see Fig. S1 in the supplemental material), we can rule out a potential involvement of holA in the observed biofilm phenotype.
Natural competence for DNA uptake has only recently been demonstrated in S. aureus, where it depends on the expression of a cryptic gene encoding the alternative σ factor SigH (62). In S. aureus, SigH is produced only under certain circumstances in a minor subpopulation and formerly was reported to induce the transcription of the comG and comE competence operons (59, 62). However, the situation seems to differ in S. lugdunensis, because in preliminary RT-PCR experiments we were able to detect comEB and comEC transcripts in the S. lugdunensis w701 wild type during mid-log and stationary growth phases (data not shown).
While in staphylococci the components required for the development of competence and the functions of comEB and comEC are currently largely undefined, such components and their regulation have been well studied in other bacterial species. In B. subtilis, both comEB and comEC belong to the late competence genes, and their gene products form the DNA uptake machinery (63). ComEC is the membrane channel protein that delivers the DNA into the cytosol, while ComEB has no clear role in the whole process of DNA uptake, although it is known to localize at the cell poles where the DNA uptake machinery assembles (60, 64). Analysis of the S. lugdunensis ComEC amino acid sequence at HMMTOP (http://www.enzim.hu/hmmtop/) revealed the presence of 12 potential transmembrane helices, indicating its location within the membrane analogous to B. subtilis ComEC. However, B. subtilis ComEC has only 7 transmembrane helices (64).
The fact that comEB was sufficient to complement Mut12 indicates that either Mut12 still was able to translate comEC and to produce ComEC independently of comEB translation or that the function of ComEC in S. lugdunensis biofilm formation is dispensable. In order to determine a potential involvement of ComEC, we sought to analyze if Mut12 still produces ComEC. For this, we aimed to raise antibodies against ComEC. However, we were not successful in the expression and purification of ComEC from E. coli (data not shown), indicating that ComEC is toxic. Likewise, ComEC from B. subtilis has been found to be toxic to E. coli (60).
Because the induction of competence stimulates DNA release essential for biofilm formation in Streptococcus pneumoniae and B. subtilis (41, 46), we aimed to identify a possible correlation of the eDNA content of S. lugdunensis biofilms with the competence gene comEB. For this, we quantified the eDNA from mature biofilms, and indeed we did observe a significant reduction of biofilm eDNA in Mut12 compared to the level of its wild type. Furthermore, by staining the eDNA with PI, we were able to visualize the shortage of eDNA in the biofilm matrix of Mut12. Additionally, 3D images from 48-h biofilms were obtained, which confirmed the structural weakness of the Mut12 biofilm. Mutants with the inability to release DNA or factors that disturb the integrity of eDNA have been used in staphylococci before to demonstrate the negative outcome of a biofilm that is not supported by the eDNA scaffold (21, 22, 43, 52).
Competence triggers a lysis-dependent or lysis-independent DNA release in bacterial biofilms. Thus, possible origins of eDNA in the biofilm matrix may include cell lysis of a subpopulation in the biofilm, as shown for S. epidermidis (22), S. aureus (21), S. pneumoniae (46), and Pseudomonas aeruginosa (42), and specific secretion as identified in Bacillus cereus (25). Active DNA secretion that depends on a type IV secretion system also has been described in Neisseria gonorrhoeae (47). To analyze if the comEB-mediated, eDNA-based mechanism of biofilm formation involves lysis-dependent or lysis-independent mechanisms of DNA release, we determined autolysis, autolytic activities, and cell viability. Autolysis and autolytic activities of Mut12, its wild type, and the complemented mutants were comparable in Triton X-100-induced autolysis assays and zymograms, respectively (Fig. 4A and B). Moreover, we did not observe a change in cell viability (Fig. 4D). In agreement, in our recent study we did not observe significant differences in the eDNA content of preparations from the clinical S. lugdunensis isolate Sl253 and its atlL mutant (14), further supporting our findings that the release of DNA is lysis independent in S. lugdunensis. Consequently, comEB-mediated DNA release and biofilm formation also appear to be independent of the cid-lrg system, which has been reported to contribute to S. aureus cell lysis, genomic DNA release, and biofilm formation in vitro and in vivo (21). Considering further possible factors that may cause the loss of eDNA in Mut12, we also checked the expression and activity of the thermonuclease NucI and again did not observe any differences. Taken together, these results suggest normal cell lysis and nuclease activity in Mut12, indicative of a lysis-independent mechanism of DNA release mediated by comEB.
In conclusion, we propose a novel comEB-dependent and lysis-independent mechanism of DNA release that stimulates eDNA-based biofilm formation in S. lugdunensis. Because the comE operon has not been characterized in other staphylococcal species yet, we further hypothesize that this novel mechanism also represents a new source of biofilm eDNA in other relevant staphylococcal species, such as S. aureus and S. epidermidis. However, further research is necessary to characterize the underlying mechanisms involved in the comEB-dependent DNA release.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the German National Research Foundation (Cluster of Excellence–Cells-in-Motion, EXC 1003–CiM) and in part by the Interdisciplinary Clinical Research Center (IZKF) (project Be2/023/11 to K.B.).
We thank A. Püschel, J. Chiang, and B. Shah for advice and technical help with the CLSM. I. Bleiziffer is acknowledged for her technical guidance in arbitrary PCR.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00775-15.
REFERENCES
- 1.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PAD, Nervi C, Fleurette J. 1988. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol 38:168–172. doi: 10.1099/00207713-38-2-168. [DOI] [Google Scholar]
- 2.Ravaioli S, Selan L, Visai L, Pirini V, Campoccia D, Maso A, Speziale P, Montanaro L, Arciola CR. 2012. Staphylococcus lugdunensis, an aggressive coagulase-negative pathogen not to be underestimated. Int J Artif Organs 35:742–753. [DOI] [PubMed] [Google Scholar]
- 3.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguera I, Del Rio A, Miro JM, Matinez-Lacasa X, Marco F, Guma JR, Quaglio G, Claramonte X, Moreno A, Mestres CA, Mauri E, Azqueta M, Benito N, Garcia-de la Maria C, Almela M, Jimenez-Exposito MJ, Sued O, De Lazzari E, Gatell JM. 2005. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart 91:e10. doi: 10.1136/hrt.2004.040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böcher S, Tonning B, Skov RL, Prag J. 2009. Staphylococcus lugdunensis, a common cause of skin and soft tissue infections in the community. J Clin Microbiol 47:946–950. doi: 10.1128/JCM.01024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, Mueller NJ. 2008. Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 36:314–321. doi: 10.1007/s15010-008-7287-9. [DOI] [PubMed] [Google Scholar]
- 7.Seifert H, Oltmanns D, Becker K, Wisplinghoff H, von Eiff C. 2005. Staphylococcus lugdunensis pacemaker-related infection. Emerg Infect Dis 11:1283–1286. doi: 10.3201/eid1108.041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch LD, Knoll F, Hartmann G, Lhotta K. 2011. Recurrent exit-site infection due to Staphylococcus lugdunensis–a virulent coagulase-negative Staphylococcus. Perit Dial Int 31:372–373. doi: 10.3747/pdi.2010.00272. [DOI] [PubMed] [Google Scholar]
- 9.Kiedrowski MR, Horswill AR. 2011. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C. 2011. Adhesion mechanisms of staphylococci, p 105–123. In Linke D, Goldman A (ed), Bacterial adhesion chemistry, biology and physics: advances in experimental medicine and biology, vol 715 Springer Science and Business Media, New York, NY. [DOI] [PubMed] [Google Scholar]
- 11.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol 12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson M, Bjerketorp J, Wiebensjo A, Ljungh A, Frykberg L, Guss B. 2004. A von Willebrand factor-binding protein from Staphylococcus lugdunensis. FEMS Microbiol Lett 234:155–161. doi: 10.1111/j.1574-6968.2004.tb09527.x. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell J, Tristan A, Foster TJ. 2004. Characterization of the fibrinogen-binding surface protein Fbl of Staphylococcus lugdunensis. Microbiology 150:3831–3841. doi: 10.1099/mic.0.27337-0. [DOI] [PubMed] [Google Scholar]
- 14.Hussain M, Steinbacher T, Peters G, Heilmann C, Becker K. 2015. The adhesive properties of the Staphylococcus lugdunensis multifunctional autolysin AtlL and its role in biofilm formation and internalization. Int J Med Microbiol 305:129–139. doi: 10.1016/j.ijmm.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Haley KP, Janson EM, Heilbronner S, Foster TJ, Skaar EP. 2011. Staphylococcus lugdunensis IsdG liberates iron from host heme. J Bacteriol 193:4749–4757. doi: 10.1128/JB.00436-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heilbronner S, Hanses F, Monk IR, Speziale P, Foster TJ. 2013. Sortase A promotes virulence in experimental Staphylococcus lugdunensis endocarditis. Microbiology 159:2141–2152. [DOI] [PubMed] [Google Scholar]
- 17.Frank KL, Patel R. 2007. Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect Immun 75:4728–4742. doi: 10.1128/IAI.00640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol 20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 19.Cramton SE, Ulrich M, Götz F, Döring G. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun 69:4079–4085. doi: 10.1128/IAI.69.6.4079-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Missineo A, Di Poto A, Geoghegan JA, Rindi S, Heilbronner S, Gianotti V, Arciola CR, Foster TJ, Speziale P, Pietrocola G. 2014. IsdC from Staphylococcus lugdunensis induces biofilm formation under low-iron growth conditions. Infect Immun 82:2448–2459. doi: 10.1128/IAI.01542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083–2092. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 23.Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, Heuser J, Dodson KW, Caparon MG, Hultgren SJ. 2009. Contribution of autolysin and sortase A during Enterococcus faecalis DNA-dependent biofilm development. Infect Immun 77:3626–3638. doi: 10.1128/IAI.00219-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seper A, Fengler VH, Roier S, Wolinski H, Kohlwein SD, Bishop AL, Camilli A, Reidl J, Schild S. 2011. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol Microbiol 82:1015–1037. doi: 10.1111/j.1365-2958.2011.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilain S, Pretorius JM, Theron J, Brozel VS. 2009. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl Environ Microbiol 75:2861–2868. doi: 10.1128/AEM.01317-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen GD, Simpson WA, Bisno AL, Beachey EH. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun 37:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleifer KH, Fischer U. 1982. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int J Syst Bacteriol 32:153–156. doi: 10.1099/00207713-32-2-153. [DOI] [Google Scholar]
- 28.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun 64:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iordanescu S, Surdeanu M. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol 96:277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- 30.Youngman PJ, Perkins JB, Losick R. 1983. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci U S A 80:2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Götz F, Schumacher B. 1987. Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett 40:285–288. doi: 10.1111/j.1574-6968.1987.tb02040.x. [DOI] [Google Scholar]
- 32.Knobloch JK, Nedelmann M, Kiel K, Bartscht K, Horstkotte MA, Dobinsky S, Rohde H, Mack D. 2003. Establishment of an arbitrary PCR for rapid identification of Tn917 insertion sites in Staphylococcus epidermidis: characterization of biofilm-negative and nonmucoid mutants. Appl Environ Microbiol 69:5812–5818. doi: 10.1128/AEM.69.10.5812-5818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozlowski M, Thatte V, Lau PC, Visentin LP, Iyer VN. 1987. Isolation and structure of the replicon of the promiscuous plasmid pCU1. Gene 58:217–228. doi: 10.1016/0378-1119(87)90377-5. [DOI] [PubMed] [Google Scholar]
- 34.Brückner R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett 151:1–8. doi: 10.1016/S0378-1097(97)00116-X. [DOI] [PubMed] [Google Scholar]
- 35.Marlinghaus L, Becker K, Korte M, Neumann S, Gatermann SG, Szabados F. 2012. Construction and characterization of three knockout mutants of the fbl gene in Staphylococcus lugdunensis. APMIS 120:108–116. doi: 10.1111/j.1600-0463.2011.02819.x. [DOI] [PubMed] [Google Scholar]
- 36.Brückner R, Wagner E, Götz F. 1993. Characterization of a sucrase gene from Staphylococcus xylosus. J Bacteriol 175:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilmann C, Hussain M, Peters G, Götz F. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol 24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 39.Steinmoen H, Knutsen E, Havarstein LS. 2002. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc Natl Acad Sci U S A 99:7681–7686. doi: 10.1073/pnas.112464599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz MG, Gerjets D, Wackernagel W. 1991. Release of transforming plasmid and chromosomal DNA from two cultured soil bacteria. Arch Microbiol 156:319–326. doi: 10.1007/BF00263005. [DOI] [PubMed] [Google Scholar]
- 41.Zafra O, Lamprecht-Grandio M, de Figueras CG, Gonzalez-Pastor JE. 2012. Extracellular DNA release by undomesticated Bacillus subtilis is regulated by early competence. PLoS One 7:e48716. doi: 10.1371/journal.pone.0048716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- 43.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bose JL, Lehman MK, Fey PD, Bayles KW. 2012. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146(Part 10):2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 46.Trappetti C, Potter AJ, Paton AW, Oggioni MR, Paton JC. 2011. LuxS mediates iron-dependent biofilm formation, competence, and fratricide in Streptococcus pneumoniae. Infect Immun 79:4550–4558. doi: 10.1128/IAI.05644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton HL, Dominguez NM, Schwartz KJ, Hackett KT, Dillard JP. 2005. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol 55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 48.Rice KC, Bayles KW. 2008. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev 72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirschhausen N, Schlesier T, Peters G, Heilmann C. 2012. Characterization of the modular design of the autolysin/adhesin Aaa from Staphylococcus aureus. PLoS One 7:e40353. doi: 10.1371/journal.pone.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirschhausen N, Schlesier T, Schmidt MA, Götz F, Peters G, Heilmann C. 2010. A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc70 as host cell receptor. Cell Microbiol 12:1746–1764. doi: 10.1111/j.1462-5822.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- 51.Heilmann C, Thumm G, Chhatwal GS, Hartleib J, Uekötter A, Peters G. 2003. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 149:2769–2778. doi: 10.1099/mic.0.26527-0. [DOI] [PubMed] [Google Scholar]
- 52.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang J, Zhou R, Shi X, Kang M, Wang H, Chen H. 2008. Two thermostable nucleases coexisted in Staphylococcus aureus: evidence from mutagenesis and in vitro expression. FEMS Microbiol Lett 284:176–183. doi: 10.1111/j.1574-6968.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 54.Tse H, Tsoi HW, Leung SP, Lau SK, Woo PC, Yuen KY. 2010. Complete genome sequence of Staphylococcus lugdunensis strain HKU09-01. J Bacteriol 192:1471–1472. doi: 10.1128/JB.01627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heilbronner S, Holden MT, van Tonder A, Geoghegan JA, Foster TJ, Parkhill J, Bentley SD. 2011. Genome sequence of Staphylococcus lugdunensis N920143 allows identification of putative colonization and virulence factors. FEMS Microbiol Lett 322:60–67. doi: 10.1111/j.1574-6968.2011.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank KL, Del Pozo JL, Patel R. 2008. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21:111–133. doi: 10.1128/CMR.00036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaspar U, Kriegeskorte A, Schubert T, Peters G, Rudack C, Pieper DH, Wos-Oxley M, Becker K. 28 April 2015. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ Microbiol. [DOI] [PubMed] [Google Scholar]
- 58.Bieber L, Kahlmeter G. 2010. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin Microbiol Infect 16:385–388. doi: 10.1111/j.1469-0691.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 59.Morikawa K, Inose Y, Okamura H, Maruyama A, Hayashi H, Takeyasu K, Ohta T. 2003. A new staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Genes Cells 8:699–712. doi: 10.1046/j.1365-2443.2003.00668.x. [DOI] [PubMed] [Google Scholar]
- 60.Inamine GS, Dubnau D. 1995. ComEA, a Bacillus subtilis integral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J Bacteriol 177:3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruck I, Georgescu RE, O'Donnell M. 2005. Conserved interactions in the Staphylococcus aureus DNA PolC chromosome replication machine. J Biol Chem 280:18152–18162. doi: 10.1074/jbc.M413595200. [DOI] [PubMed] [Google Scholar]
- 62.Morikawa K, Takemura AJ, Inose Y, Tsai M, Nguyen TL, Ohta TT, Msadek T. 2012. Expression of a cryptic secondary sigma factor gene unveils natural competence for DNA transformation in Staphylococcus aureus. PLoS Pathog 8:e1003003. doi: 10.1371/journal.ppat.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dubnau D. 1991. Genetic competence in Bacillus subtilis. Microbiol Rev 55:395–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Draskovic I, Dubnau D. 2005. Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulfide bonds. Mol Microbiol 55:881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.