Abstract
Purpose
To evaluate the association between polymorphisms of RASGRF1 rs8027411 and high myopia in Chinese and Japanese populations.
Methods
All eligible studies investigating the association between the RASGRF1 gene and high myopia listed in PubMed, EMBASE, the Cochrane Library, Web of Science, and the China Biologic Medical Database were retrieved. The effects were assessed with the pooled odds ratio (OR) and 95% confidence interval (CI). Heterogeneity between studies was evaluated with the Q-statistic test. Publication bias was tested with Begg’s and Egger’s linear regression tests. Subgroup analysis and sensitivity analysis were performed to identify the sources of heterogeneity.
Results
In the present meta-analysis, 2,529 individuals with high myopia and 3,127 controls from four studies were included and divided into seven groups. The results indicated that RASGRF1 rs8027411 was significantly associated with high myopia in Chinese and Japanese populations. Carriers of the rs8027411 G allele had a lower risk of high myopia compared to carriers with the T allele (G versus T, OR=0.83, 95% CI=0.77−0.89; p<0.001). Low but not significant heterogeneity was found in a recessive model. No heterogeneity was found in other genetic models. The subgroup analysis indicated that the protective effect of rs8027411 variants was more prominent in Chinese populations (G versus T, OR=0.80 in Chinese and OR=0.86 in Japanese; GG versus TT, OR=0.65 in Chinese and OR=0.77 in Japanese; GT versus TT, OR=0.76 in Chinese and OR=0.81 in Japanese; (GG+GT) versus TT, OR=0.73 in Chinese and OR=0.80 in Japanese; and GG versus (GT+TT), OR=0.77 in Chinese and OR=0.87 in Japanese). Sensitivity analysis indicated that the study results were stable in allelic, homozygote, heterozygote, and dominant models but were not stable in the recessive model. No evidence of publication bias was found.
Conclusions
Carriers of the rs8027411 G allele in the RASGRF1 gene may be at a lower risk of high myopia in Chinese and Japanese populations. The RASGRF1 gene may play a role in the development of high myopia, especially in Asians. Additional studies are required to validate these results.
Introduction
Myopia is the most common eye disease worldwide [1]. The condition may be classified as high myopia or common myopia based on a refractive error more severe than −6.00 diopter (D) or an axial length greater than or equal to 26 mm [2,3]. High myopia can result in extreme complications such as retinal detachment, glaucoma, cataract, or myopia maculopathy [2,3] and is the fourth most common cause of irreversible blindness [4,5]. The prevalence of high myopia ranges from 0.7% to 10.1% [6-10]. A higher prevalence of high myopia has been reported in Chinese and Japanese populations [11]. Although the exact pathogenesis of high myopia is unknown, it is commonly acknowledged that high myopia is a multifactorial disease with genetic and environment factors [2]. The increasing intensity of education, near work, urbanization, and higher individual monthly income are the risk factors proposed for high myopia [12-15]. Additionally, a variety of high-susceptibility genes have been discovered, including transforming growth factor beta 1 (TGFB1; gene ID 7040, OMIM 190180) [16-18], collagen type I alpha 1 (COL1A1; gene ID 1277, OMIM 120150) [19-21], lumican (LUM; gene ID: 4060, OMIM 600616) [5,22], paired box gene 6 (PAX6; gene ID: 5080, OMIM 607108) [23-25], Ras protein-specific guanine nucleotide-releasing factor 1 (RASGRF1; gene ID: 5923, OMIM 606600) [26-29], and gap junction protein delta 2 (GJD2; gene ID: 57,369, OMIM 607058) [30-32].
RASGRF1 was one of the first two genes reported in the earliest published genome-wide association studies (GWASs) for myopia in Europeans in 2010 [33]. The association between RASGRF1 and myopia was identified in other GWASs or GWAS meta-analyses in European, American, Australian, and Asian populations as well [30,31]. However, a GWAS meta-analysis conducted in a Han Chinese population did not find an association between RASGRF1 and high myopia [34]. Similarly, another GWAS meta-analysis also did not identify an association between RASGRF1 and refractive error in Caucasians and Asians [35]. The low power of some studies, ethnic and location differences, and different recruitment criteria may explain these inconsistent results at least to some extent. Some genetic studies reported that the same loci were associated with common and high myopia [29,36,37]; however, it is still undetermined whether common myopia and high myopia share the exact same genetic background. Several studies have been conducted to investigate the relationship between high myopia and the RASGRF1 gene [1,26,28,38]; unfortunately, the results were inconsistent and inconclusive. Thus, we performed this meta-analysis with all available studies to shed light on the effect of RASGRF1 rs8027411 on high myopia.
Methods
Search strategy
A comprehensive literature research was conducted via the online databases PubMed, EMBASE, the Cochrane Library, Web of Science, and the China Biologic Medical Database (up to February 2015) for all relevant studies. The three main search terms were as follows: (1) myopia OR “high myopia” OR “near sight” OR nearsighted OR “refractive error”; (2) allele OR genotype OR SNP OR mutation(s) OR variant(s) OR polymorphism(s) OR single nucleotide polymorphism; and (3) the RASGRF1 gene: RASGRF1 OR Ras protein-specific guanine nucleotide-releasing factor 1 OR GNRP OR CDC25 OR GRF55 OR CDC25L OR H-GRF55 OR ras-GRF1 OR 15q25. References in the retrieved articles were also reviewed.
Articles meeting the following criteria were included: (1) A case–control or cohort study compared high myopia and non-high myopia in a Chinese or Japanese population, (2) high myopia was defined as refractive error more severe than −6.00 D or an axial length greater than or equal to 26 mm, (3) the study assessed the association between RASGRF1 rs8027411 and high myopia, and (4) the study provided sufficient data to estimate the odds ratio (OR) and the 95% confidence interval (CI). If one of the following criteria was met, a study was excluded: (1) The study was a review, case series, conference, or sibling or family study, (2) the study was not a full-text article, or (3) the study was an animal study. If more than one cohort or ethnic population was reported in a study, each cohort or ethnic population was treated individually. When two or more of the same populations were used among the studies, the largest or the most recent one was selected.
Data extraction and quality assessment
Data extraction and quality assessment were performed independently by two investigators (TC, JM). The extracted information included the first author’s name, year of publication, ethnicity, sample size, definition of high myopia, mean age, source of controls, and genotyping method. The Newcastle–Ottawa scale (NOS) was used in the quality assessment [39]. Any disagreements were resolved by consensus.
Statistical analysis
Hardy–Weinberg equilibrium (HWE) in the controls was tested with a chi-square test. The association between RASGRF1 rs8027411 and high myopia was evaluated with a pooled OR and 95% CI in five models: allelic (G versus T), homozygote (GG versus TT), heterozygote (GT versus TT), dominant ((GG+CT) versus TT), and recessive (GG versus (GT+TT)) models. Heterogeneity between studies was explored with the Q-statistic test, and the inconsistency index I2 was used to evaluate the extent [40]. I2 ranged from 0% to 100%, with 0% representing no heterogeneity, and the larger value the higher heterogeneity (I2 values of 25%, 50%, and 75% indicate low, moderate, and high heterogeneity, respectively) [41]. A p value of less than 0.05 was defined as significant in the Q-statistic test. When there was significant heterogeneity, a random-effects model was used; otherwise, a fixed-effects model was selected. Egger’s test and Begg’s test were performed to assess publication bias. Sensitivity analysis was performed by removing one study at a time to assess the effect of the individual data on the combined results. Statistical analyses were performed with Stata 11.0 software (Stata Corporation, College Station, TX). Two-sided p<0.05 was considered statistically significant. A power calculation showed that this study had 92.8%, 99.5%, 98.6%, 99.8%, and 80.5% power to detect the associations in the allelic, homozygote, heterozygote, dominant, and recessive models, respectively.
Results
Literature search and study characteristics
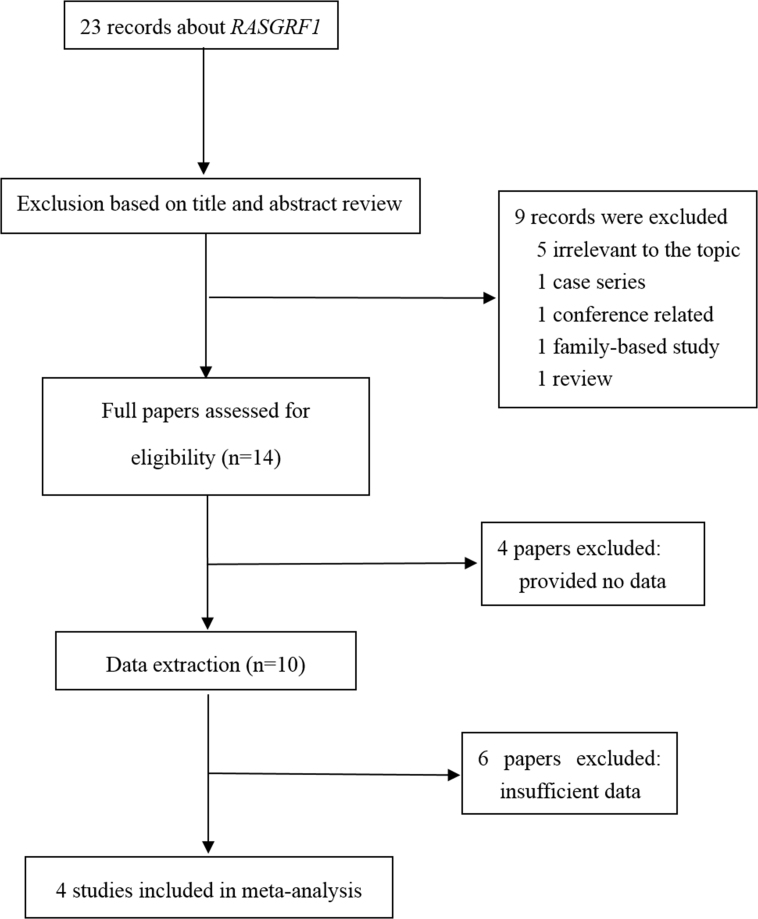
Twenty-three publications about RASGRF1 and high myopia were retrieved. The detailed search process is shown in Figure 1. Nine studies were excluded after the titles and abstracts were reviewed for the following reasons: irrelevant to the topic or a conference, review, or family study. The remaining fourteen articles were retrieved for full-text reading, and another ten studies were excluded. Among them, six studies had insufficient data, and four studies provided no data about rs8027411 and high myopia. Finally, four case–control studies were included in this meta-analysis [26-29]. Two different cohorts were included in studies published by Zhu et al. [27], Jiao et al. [28], and Hayashi et al. [29], and the cohorts were treated separately. The detailed information of these studies is shown in Table 1. Three studies were conducted in China, and one was performed in Japan. A total of 2,529 patients with high myopia and 3,127 controls were included in this meta-analysis. The genetic distributions of all control groups were consistent with HWE. The average NOS score was 7.25 (range: 7 to 8).
Figure 1.
Flow diagram outlining the selection process for studies in the systematic review and the meta-analysis.
Table 1. Characteristics of include studies in this meta-analysis.
| Study (year) | Ethnicity | Sample size | High myopia | Mean age (cases;control) | Genotyping method | Control | NOS score |
|---|---|---|---|---|---|---|---|
| Qiang, Y (2014) |
Chinese |
1,461 |
≤ −6.00 |
36±14.95;42.5±13.3/31±10.66 |
Taqman |
Free of myopia and
fundus diseases |
7 |
| Zhu, J (a; 2014) |
Chinese |
741 |
≤ −8.00 and AL>26 mm |
39.32±16.94; 71.14±8.52 |
PCR -MassArray |
Normal |
7 |
| Zhu, J (b; 2014) |
Chinese |
234 |
≤ −8.00 and AL>26 mm |
40.96±17.63; 70.17±7.37 |
PCR -MassArray |
Normal |
|
| Jiao, X (a; 2012) |
Chinese |
192 |
≤ −6.00 |
21.80±1.27;
21.68±1.30 |
PCR-DS |
Without refractive error |
8 |
| Jiao, X (b; 2012) |
Chinese |
608 |
≤ −6.00 |
22.19±1.67; 21.66±1.54 |
PCR-DS |
Without refractive error |
|
| Hayashi, H (a; 2011) |
Japanese |
1491 |
AL>26.1 |
57.57±14.75; 74.40±8.37 |
Taqman |
AL<25.0 mm |
7 |
| Hayashi, H (b; 2011) | Japanese | 2054 | AL>26.1 | 57.57±14.75; 38.81±11.83 | Taqman | No history of ocular disease |
AL: axial lengths; DS: directional sequencing; NOS: Newcastle –Ottawa scale
Main results of the meta-analysis
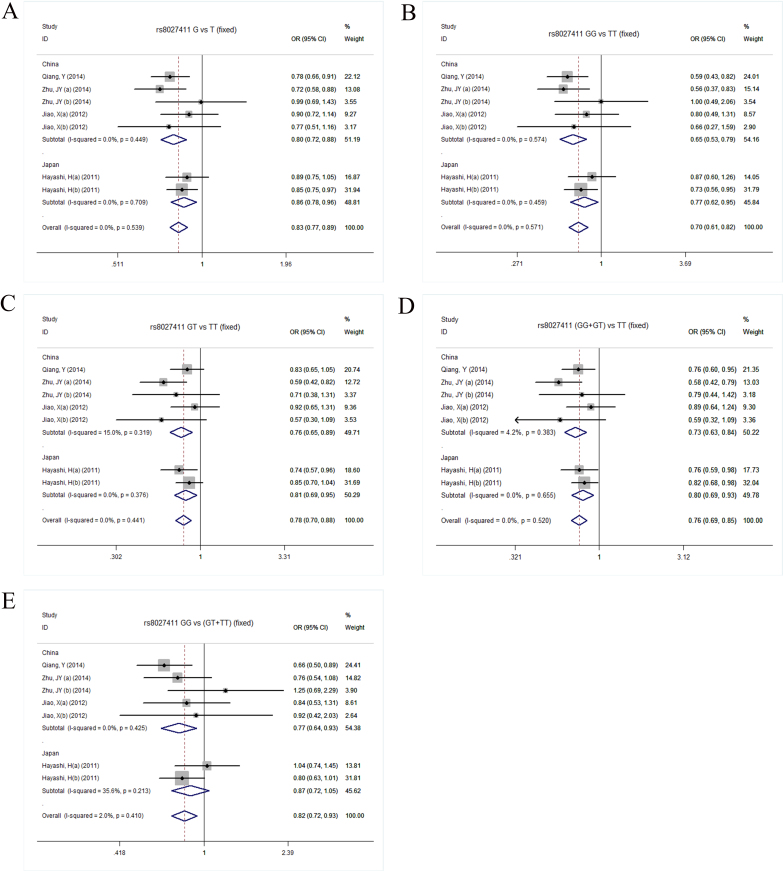
There was no heterogeneity across the studies (Table 2); thus, we used fixed-effects models with the Mantel–Haenzel method. The meta-analysis indicated that the rs8027411 polymorphism was significantly associated with high myopia in all genetic models (G versus T, OR=0.83, 95% CI=0.77−0.89; p<0.001; GG versus TT, OR=0.70, 95% CI=0.61–0.82; p<0.001; GT versus TT, OR=0.78, 95% CI=0.70–0.88; p<0.001; (GG+GT) versus TT, OR=0.76, 95% CI=0.69–0.85; p<0.001; GG versus (GT+TT), OR=0.82, 95% CI=0.72–0.93; p=0.003). The Q-statistic test and I2 showed only low but not significant heterogeneity in the recessive model (Q=6.12, p=0.41, I2=2.0%). No heterogeneity was found in the other genetic models (Table 2). The subgroup analysis was based on ethnicity. In the Chinese populations, a significant association between rs8027411 and high myopia was found in all genetic models (Figure 2A-E). Similarly, in the Japanese population, except the recessive model (GG versus (GT+TT), OR=0.87, 95% CI=0.72–1.05), there was a significant association between rs8027411 and high myopia in all other genetic models (Figure 2A-E).
Table 2. Pooled results of the associations between rs8027411 and high myopia.
| Models | cases | controls | Associations |
Heterogeneity |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | Q | PQ | I2, % | |||
| G versus T |
1966/3058 |
2721/3491 |
0.83 |
0.77–0.89 |
<0.001 |
5.04 |
0.54 |
0 |
| GG versus TT |
400/946 |
593/978 |
0.70 |
0.61–0.82 |
<0.001 |
4.79 |
0.57 |
0 |
| GT versus TT |
1166/946 |
1535/978 |
0.78 |
0.70–0.88 |
<0.001 |
5.84 |
0.44 |
0 |
| (GG+GT) versus TT |
1566/946 |
2128/978 |
0.76 |
0.69–0.85 |
<0.001 |
5.18 |
0.52 |
0 |
| GG versus (GT+TT) | 400/2112 | 593/2513 | 0.82 | 0.72–0.93 | 0.003 | 6.12 | 0.41 | 2.0 |
CI=confidence interval; OR=odds ratio.
Figure 2.
Forest plots of RASGRF1 rs8027411 polymorphism and high myopia in overall and subgroup analysis. A: Allelic model (G versus T). B: Homozygote model (GG versus TT). C: Heterozygote model (GT versus TT). D: Dominant model ((GG+GT) versus TT). E: Recessive model (GG versus (GT+TT)). CI=confidence interval; OR=odds ratio.
Sensitivity analysis
Sensitivity analysis showed that when Qiang’s study [26] was excluded, the observed estimates between RASGRF1 rs8027411 and high myopia were still significant in the allelic, homozygote, heterozygote, and dominant genetic models (Table 3). However, in the recessive model, the association was not significant (OR=0.87, 95% CI=0.75–1.01). When the possible causes were investigated, the OR in Qiang’s study [26] was distinct from that in the other studies in the recessive model (Figure 2E). Thus, once Qiang’s study was excluded, the result was no longer significant. However, excluding Qiang’s study did not change the results of the four other genetic comparison models (Table 3). When other six single studies were excluded, the associations between RASGRF1 rs8027411 and high myopia remained significant in all genetic models (Table 3).
Table 3. Results of leave-one-out sensitivity analysis.
| Study excluded |
OR, 95% CI |
||||
|---|---|---|---|---|---|
| G versus T* | GG versus TT* | GT versus TT* | (GG+GT) versus TT* | GG versus (GT+TT)* | |
| Qiang, Y, 2004 |
0.85 (0.78–0.92) |
0.74 (0.62–0.87) |
0.77 (0.68–0.88) |
0.76 (0.68–0.86) |
0.87 (0.75–1.01) |
| Zhu, JY(a), 2014 |
0.85 (0.79–0.92) |
0.73 (0.62–0.86) |
0.81 (0.73–0.92) |
0.79 (0.70–0.88) |
0.83 (0.72–0.95) |
| Zhu, JY(b), 2014 |
0.83 (0.77–0.89) |
0.69 (0.60–0.81) |
0.79 (0.70–0.88) |
0.76 (0.69–0.85) |
0.80 (0.70–0.92) |
| Jiao, X(a), 2012 |
0.82 (0.76–0.89) |
0.69 (0.59–0.81) |
0.77 (0.69–0.87) |
0.75 (0.67–0.84) |
0.81 (0.71–0.94) |
| Jiao, X(b), 2012 |
0.83 (0.77–0.90) |
0.70 (0.61–0.82) |
0.79 (0.71–0.89) |
0.77 (0.69–0.86) |
0.81 (0.71–0.93) |
| Hayashi, H(a), 2011 |
0.82 (0.76–0.89) |
0.68 (0.58–0.80) |
0.80 (0.70–0.90) |
0.76 (0.68–0.86) |
0.78 (0.68–0.90) |
| Hayashi, H(b), 2011 | 0.82 (0.75–0.90) | 0.69 (0.58–0.83) | 0.75 (0.66–0.86) | 0.74 (0.65–0.84) | 0.83 (0.70–0.97) |
CI=confidence interval; OR=odds ratio. * All the analysis was in fixed-effects models.
Publication bias
When publication bias was quantitatively evaluated with Begg’s and Egger’s tests, no significant publication bias was found in any genetic model as well (G versus T: Begg p=0.76 and Egger p=0.85; GG versus TT: Begg p=1.00 and Egger p=0.62; GT versus TT: Begg p=0.23 and Egger p=0.21; (GG+GT) versus TT: Begg p=0.76 and Egger p=0.41; GG versus (GT+TT): Begg p=0.37 and Egger p=0.25).
Discussion
RASGRF1, a large gene with 28 exons, is expressed at high levels in the retina [42]. A proposed mechanism has indicated that RASGFR1 has significant influence on myopia. One suggestion is that this gene encodes Ras protein-specific guanine nucleotide-releasing factor-1, which is highly expressed in the retina and neurons, and then activates Ras [33]. In addition, RASGRF1 is a nuclear exchange factor that promotes GDP/GTP exchange on the Ras family GTPases and is related to synaptic transmission of the photoreceptor responses [43]. Moreover, muscarinic receptors and retinoic acid can regulate RASGRF1 expression [33,44]. Animal models and human interventional studies showed that muscarinic inhibitors prevent the development of myopia [45,46], and reduction synthesis of choroidal retinoic acid was detected in myopia animal models [47]. Therefore, RASGRF1 was presumed to be a strong candidate gene in association with high myopia. To date, the single nucleotide polymorphism (SNP) rs8027411, located in the transcription initiation site of RASGRF1, has been widely investigated for an association with high myopia, but the results are controversial [26-29].
In the present meta-analysis, it was surprising that rs8027411 was associated with high myopia in all five genetic models. These results indicated that the G allele of rs8027411 had a strong protective effect on high myopia (OR=0.83). Individuals with the GG (OR=0.70) or GT (OR=0.78) genotype also had a lower risk of high myopia than those with the TT genotype. Heterogeneity should be considered a potential factor that may influence the interpretation of the results. In this meta-analysis, only low but not significant heterogeneity was found in the recessive model, and no heterogeneity was found in the four other genetic models. In the subgroup analysis, the results showed that this association was more prominent in the Chinese population. This indicated that the protective effect of rs8027411 variants was different among races, and stronger in Chinese populations. Sensitivity analysis indicated that although the association between RASGRF1 rs8027411 and high myopia was not robust in the recessive model, the association was highly consistent in the allelic, homozygote, heterozygote, and dominant models. No evidence of publication bias was detected among the included studies. These results indicated the reliability of the combined results.
The limitations of this meta-analysis should be noted. First, the results were based on Chinese and Japanese populations. Therefore, the meta-analysis may be applicable only to these specific populations. Second, since the association with only one SNP rs8027411 in RASGRF1 was confirmed, other polymorphisms in RASGRF1 may also be important in other populations that were not studied in this analysis. Third, the association between RASGRF1 rs8027411 and high myopia in the recessive model was inconclusive. Additional studies are needed to confirm this association. Last, since high myopia is a multifactorial disease, many factors such as age, levels of education, lifestyle, and other polymorphisms of susceptible genes may play roles in the pathogenesis. However, insufficient information impeded a more precise analysis.
In conclusion, despite these limitations, our meta-analysis delineated the association between the RASGRF1 polymorphism and high myopia in Chinese and Japanese populations. In these populations, carriers of the rs8027411 G allele have a lower risk of high myopia. However, large-scale studies are required for further confirmation. Gene–gene and gene–environment interactions also must be considered in future studies.
References
- 1.Oishi M, Yamashiro K, Miyake M, Akagi-Kurashige Y, Kumagai K, Nakata I, Nakanishi H, Yoshikawa M, Oishi A, Gotoh N, Tsujikawa A, Yamada R, Matsuda F, Yoshimura N. Association between ZIC2, RASGRF1, and SHISA6 genes and high myopia in Japanese subjects. Invest Ophthalmol Vis Sci. 2013;54:7492–7. doi: 10.1167/iovs.13-12825. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–48. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 3.Xiang X, Wang T, Tong P, Li Y, Guo H, Wan A, Xia L, Liu Y, Li Y, Tian Q, Shen L, Cai X, Tian L, Jin X, Xia K, Hu Z. New ZNF644 mutations identified in patients with high myopia. Mol Vis. 2014;20:939–46. [PMC free article] [PubMed] [Google Scholar]
- 4.Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157:9–25. doi: 10.1016/j.ajo.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 5.He M, Wang W, Ragoonundun D, Huang W. Meta-Analysis of the Association between Lumican Gene Polymorphisms and Susceptibility to High Myopia. PLoS ONE. 2014;9:e98748. doi: 10.1371/journal.pone.0098748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durkin SR, Tan EW, Casson RJ, Selva D, Newland HS. Distance refractive error among Aboriginal people attending eye clinics in remote South Australia. Clin Experiment Ophthalmol. 2007;35:621–6. doi: 10.1111/j.1442-9071.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- 7.Wolfram C, Hohn R, Kottler U, Wild P, Blettner M, Buhren J, Pfeiffer N, Mirshahi A. Prevalence of refractive errors in the European adult population: the Gutenberg Health Study (GHS). Br J Ophthalmol. 2014;98:857–61. doi: 10.1136/bjophthalmol-2013-304228. [DOI] [PubMed] [Google Scholar]
- 8.Ziaei H, Katibeh M, Solaimanizad R, Hosseini S, Gilasi HR, Golbafian F, Javadi MA. Prevalence of refractive errors; the yazd eye study. J Ophthalmic Vis Res. 2013;8:227–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Allison CL. Proportion of refractive errors in a Polish immigrant population in Chicago. Optom Vis Sci. 2010;87:588–92. doi: 10.1097/OPX.0b013e3181e61beb. [DOI] [PubMed] [Google Scholar]
- 10.Liang YB, Wong TY, Sun LP, Tao QS, Wang JJ, Yang XH, Xiong Y, Wang NL, Friedman DS. Refractive errors in a rural Chinese adult population the Handan eye study. Ophthalmology. 2009;116:2119–27. doi: 10.1016/j.ophtha.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Zhang HX. Polymorphism in the 11q24.1 genomic region is associated with myopia: a comprehensive genetic study in Chinese and Japanese populations. Mol Vis. 2014;20:352–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Li J, Cui T, Hu A, Fan G, Zhang R, Yang H, Sun B, Jonas JB. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005;112:1676–83. doi: 10.1016/j.ophtha.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Krishnaiah S, Srinivas M, Khanna RC, Rao GN. Prevalence and risk factors for refractive errors in the South Indian adult population: The Andhra Pradesh Eye disease study. Clin Ophthalmol. 2009;3:17–27. [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TY, Foster PJ, Hee J, Ng TP, Tielsch JM, Chew SJ, Johnson GJ, Seah SK. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Invest Ophthalmol Vis Sci. 2000;41:2486–94. [PubMed] [Google Scholar]
- 15.Tarczy-Hornoch K, Ying-Lai M, Varma R. Myopic refractive error in adult Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2006;47:1845–52. doi: 10.1167/iovs.05-1153. [DOI] [PubMed] [Google Scholar]
- 16.Rasool S, Ahmed I, Dar R, Ayub SG, Rashid S, Jan T, Ahmed T, Naikoo NA, Andrabi KI. Contribution of TGFbeta1 codon 10 polymorphism to high myopia in an ethnic Kashmiri population from India. Biochem Genet. 2013;51:323–33. doi: 10.1007/s10528-012-9565-6. [DOI] [PubMed] [Google Scholar]
- 17.Khor CC, Fan Q, Goh L, Tan D, Young TL, Li YJ, Seielstad M, Goh DL, Saw SM. Support for TGFB1 as a susceptibility gene for high myopia in individuals of Chinese descent. Arch Ophthalmol. 2010;128:1081–4. doi: 10.1001/archophthalmol.2010.149. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Inoko H, Nishizaki R, Ohno S, Mizuki N. Exclusion of transforming growth factor-beta1 as a candidate gene for myopia in the Japanese. Jpn J Ophthalmol. 2007;51:96–9. doi: 10.1007/s10384-006-0417-y. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Shi Y, Gong B, He F, Lu F, Lin H, Wu Z, Cheng J, Chen B, Liao S, Ma S, Hu J, Yang Z. An association study of the COL1A1 gene and high myopia in a Han Chinese population. Mol Vis. 2011;17:3379–83. [PMC free article] [PubMed] [Google Scholar]
- 20.Nakanishi H, Yamada R, Gotoh N, Hayashi H, Otani A, Tsujikawa A, Yamashiro K, Shimada N, Ohno-Matsui K, Mochizuki M, Saito M, Saito K, Iida T, Matsuda F, Yoshimura N. Absence of association between COL1A1 polymorphisms and high myopia in the Japanese population. Invest Ophthalmol Vis Sci. 2009;50:544–50. doi: 10.1167/iovs.08-2425. [DOI] [PubMed] [Google Scholar]
- 21.Metlapally R, Li YJ, Tran-Viet KN, Abbott D, Czaja GR, Malecaze F, Calvas P, Mackey D, Rosenberg T, Paget S, Zayats T, Owen MJ, Guggenheim JA, Young TL. COL1A1 and COL2A1 genes and myopia susceptibility: evidence of association and suggestive linkage to the COL2A1 locus. Invest Ophthalmol Vis Sci. 2009;50:4080–6. doi: 10.1167/iovs.08-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, Zhai L, Zeng S, Peng Q, Wang J, Deng Y, Xie L, He Y, Li T. Lack of Association Between LUM rs3759223 Polymorphism and High Myopia. Optom Vis Sci. 2014;91:707–12. doi: 10.1097/OPX.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 23.Miyake M, Yamashiro K, Nakanishi H, Nakata I, Akagi-Kurashige Y, Tsujikawa A, Moriyama M, Ohno-Matsui K, Mochizuki M, Yamada R, Matsuda F, Yoshimura N. Association of paired box 6 with high myopia in Japanese. Mol Vis. 2012;18:2726–35. [PMC free article] [PubMed] [Google Scholar]
- 24.Han W, Leung KH, Fung WY, Mak JY, Li YM, Yap MK, Yip SP. Association of PAX6 polymorphisms with high myopia in Han Chinese nuclear families. Invest Ophthalmol Vis Sci. 2009;50:47–56. doi: 10.1167/iovs.07-0813. [DOI] [PubMed] [Google Scholar]
- 25.Ng TK, Lam CY, Lam DS, Chiang SW, Tam PO, Wang DY, Fan BJ, Yam GH, Fan DS, Pang CP. AC.and AG dinucleotide repeats in the PAX6 P1 promoter are associated with high myopiaMol Vis 2009152239–48. [PMC free article] [PubMed] [Google Scholar]
- 26.Qiang Y, Li W, Wang Q, He K, Li Z, Chen J, Song Z, Qu J, Zhou X, Qin S, Shen J, Wen Z, Ji J, Shi Y. Association study of 15q14 and 15q25 with high myopia in the Han Chinese population. BMC Genet. 2014;15:51. doi: 10.1186/1471-2156-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu JY, Rong WN, Jia Q, Zhuang WJ, Li ZL, Li HP, Liu YN, Wang XP, Sheng XL. Associations of single nucleotide polymorphisms of chromosomes 15q14, 15q25 and 13q12.12 regions with high myopic eyes in Hui and Han population of Chinese Ningxia area. Chin J Exp Ophthalmol. 2014;32:354–8. [Google Scholar]
- 28.Jiao X, Wang P, Li S, Li A, Guo X, Zhang Q, Hejtmancik JF. Association of markers at chromosome 15q14 in Chinese patients with moderate to high myopia. Mol Vis. 2012;18:2633–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi H, Yamashiro K, Nakanishi H, Nakata I, Kurashige Y, Tsujikawa A, Moriyama M, Ohno-Matsui K, Mochizuki M, Ozaki M, Yamada R, Matsuda F, Yoshimura N. Association of 15q14 and 15q25 with high myopia in Japanese. Invest Ophthalmol Vis Sci. 2011;52:4853–8. doi: 10.1167/iovs.11-7311. [DOI] [PubMed] [Google Scholar]
- 30.Verhoeven VJ, Hysi PG, Wojciechowski R, Fan Q, Guggenheim JA, Hohn R, MacGregor S, Hewitt AW, Nag A, Cheng CY, Yonova-Doing E, Zhou X, Ikram MK, Buitendijk GH, McMahon G, Kemp JP, Pourcain BS, Simpson CL, Makela KM, Lehtimaki T, Kahonen M, Paterson AD, Hosseini SM, Wong HS, Xu L, Jonas JB, Parssinen O, Wedenoja J, Yip SP, Ho DW, Pang CP, Chen LJ, Burdon KP, Craig JE, Klein BE, Klein R, Haller T, Metspalu A, Khor CC, Tai ES, Aung T, Vithana E, Tay WT, Barathi VA, Chen P, Li R, Liao J, Zheng Y, Ong RT, Doring A, Evans DM, Timpson NJ, Verkerk AJ, Meitinger T, Raitakari O, Hawthorne F, Spector TD, Karssen LC, Pirastu M, Murgia F, Ang W, Mishra A, Montgomery GW, Pennell CE, Cumberland PM, Cotlarciuc I, Mitchell P, Wang JJ, Schache M, Janmahasatian S, Igo RJ, Lass JH, Chew E, Iyengar SK, Gorgels TG, Rudan I, Hayward C, Wright AF, Polasek O, Vatavuk Z, Wilson JF, Fleck B, Zeller T, Mirshahi A, Muller C, Uitterlinden AG, Rivadeneira F, Vingerling JR, Hofman A, Oostra BA, Amin N, Bergen AA, Teo YY, Rahi JS, Vitart V, Williams C, Baird PN, Wong TY, Oexle K, Pfeiffer N, Mackey DA, Young TL, van Duijn CM, Saw SM, Bailey-Wilson JE, Stambolian D, Klaver CC, Hammond CJ. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–8. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiefer AK, Tung JY, Do CB, Hinds DA, Mountain JL, Francke U, Eriksson N. Genome-wide analysis points to roles for extracellular matrix remodeling, the visual cycle, and neuronal development in myopia. PLoS Genet. 2013;9:e1003299. doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solouki AM, Verhoeven VJ, van Duijn CM, Verkerk AJ, Ikram MK, Hysi PG, Despriet DD, van Koolwijk LM, Ho L, Ramdas WD, Czudowska M, Kuijpers RW, Amin N, Struchalin M, Aulchenko YS, van Rij G, Riemslag FC, Young TL, Mackey DA, Spector TD, Gorgels TG, Willemse-Assink JJ, Isaacs A, Kramer R, Swagemakers SM, Bergen AA, van Oosterhout AA, Oostra BA, Rivadeneira F, Uitterlinden AG, Hofman A, de Jong PT, Hammond CJ, Vingerling JR, Klaver CC. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2010;42:897–901. doi: 10.1038/ng.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hysi PG, Young TL, Mackey DA, Andrew T, Fernandez-Medarde A, Solouki AM, Hewitt AW, Macgregor S, Vingerling JR, Li YJ, Ikram MK, Fai LY, Sham PC, Manyes L, Porteros A, Lopes MC, Carbonaro F, Fahy SJ, Martin NG, van Duijn CM, Spector TD, Rahi JS, Santos E, Klaver CC, Hammond CJ. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–5. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Gong B, Chen L, Zuo X, Liu X, Tam PO, Zhou X, Zhao P, Lu F, Qu J, Sun L, Zhao F, Chen H, Zhang Y, Zhang D, Lin Y, Lin H, Ma S, Cheng J, Yang J, Huang L, Zhang M, Zhang X, Pang CP, Yang Z. A genome-wide meta-analysis identifies two novel loci associated with high myopia in the Han Chinese population. Hum Mol Genet. 2013;22:2325–33. doi: 10.1093/hmg/ddt066. [DOI] [PubMed] [Google Scholar]
- 35.Verhoeven VJ, Hysi PG, Saw SM, Vitart V, Mirshahi A, Guggenheim JA, Cotch MF, Yamashiro K, Baird PN, Mackey DA, Wojciechowski R, Ikram MK, Hewitt AW, Duggal P, Janmahasatian S, Khor CC, Fan Q, Zhou X, Young TL, Tai ES, Goh LK, Li YJ, Aung T, Vithana E, Teo YY, Tay W, Sim X, Rudan I, Hayward C, Wright AF, Polasek O, Campbell H, Wilson JF, Fleck BW, Nakata I, Yoshimura N, Yamada R, Matsuda F, Ohno-Matsui K, Nag A, McMahon G, St PB, Lu Y, Rahi JS, Cumberland PM, Bhattacharya S, Simpson CL, Atwood LD, Li X, Raffel LJ, Murgia F, Portas L, Despriet DD, van Koolwijk LM, Wolfram C, Lackner KJ, Tonjes A, Magi R, Lehtimaki T, Kahonen M, Esko T, Metspalu A, Rantanen T, Parssinen O, Klein BE, Meitinger T, Spector TD, Oostra BA, Smith AV, de Jong PT, Hofman A, Amin N, Karssen LC, Rivadeneira F, Vingerling JR, Eiriksdottir G, Gudnason V, Doring A, Bettecken T, Uitterlinden AG, Williams C, Zeller T, Castagne R, Oexle K, van Duijn CM, Iyengar SK, Mitchell P, Wang JJ, Hohn R, Pfeiffer N, Bailey-Wilson JE, Stambolian D, Wong TY, Hammond CJ, Klaver CC. Large scale international replication and meta-analysis study confirms association of the 15q14 locus with myopia. The CREAM consortium. Hum Genet. 2012;131:1467–80. doi: 10.1007/s00439-012-1176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu B, Jiang D, Wang P, Gao Y, Sun W, Xiao X, Li S, Jia X, Guo X, Zhang Q. Replication study supports CTNND2 as a susceptibility gene for high myopia. Invest Ophthalmol Vis Sci. 2011;52:8258–61. doi: 10.1167/iovs.11-7914. [DOI] [PubMed] [Google Scholar]
- 37.Fan Q, Barathi VA, Cheng CY, Zhou X, Meguro A, Nakata I, Khor CC, Goh LK, Li YJ, Lim W, Ho CE, Hawthorne F, Zheng Y, Chua D, Inoko H, Yamashiro K, Ohno-Matsui K, Matsuo K, Matsuda F, Vithana E, Seielstad M, Mizuki N, Beuerman RW, Tai ES, Yoshimura N, Aung T, Young TL, Wong TY, Teo YY, Saw SM. Genetic variants on chromosome 1q41 influence ocular axial length and high myopia. PLoS Genet. 2012;8:e1002753. doi: 10.1371/journal.pgen.1002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CD, Yu ZQ, Chen XL, Zhou JQ, Zhou XT, Sun XH, Chu RY. Evaluating the Association between Pathological Myopia and SNPs in RASGRF1. ACTC1 and GJD2 Genes at Chromosome 15q14 and 15q25 in a Chinese Population. Ophthalmic Genet. 2015;36:1–7. doi: 10.3109/13816810.2013.812737. [DOI] [PubMed] [Google Scholar]
- 39.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 40.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 41.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zippel R, Gnesutta N, Matus-Leibovitch N, Mancinelli E, Saya D, Vogel Z, Sturani E. Ras-grf, the activator of ras, is expressed preferentially in mature neurons of the central nervous system. Brain Res Mol Brain Res. 1997;48:140–4. doi: 10.1016/s0169-328x(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 43.Fernández-Medarde A, Barhoum R, Riquelme R, Porteros A, Nunez A, de Luis A, de Las RJ, de la Villa P, Varela-Nieto I, Santos E. RasGRF1 disruption causes retinal photoreception defects and associated transcriptomic alterations. J Neurochem. 2009;110:641–52. doi: 10.1111/j.1471-4159.2009.06162.x. [DOI] [PubMed] [Google Scholar]
- 44.Tonini R, Mancinelli E, Balestrini M, Mazzanti M, Martegani E, Ferroni A, Sturani E, Zippel R. Expression of Ras-GRF in the SK-N-BE neuroblastoma accelerates retinoic-acid-induced neuronal differentiation and increases the functional expression of the IRK1 potassium channel. Eur J Neurosci. 1999;11:959–66. doi: 10.1046/j.1460-9568.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 45.Tigges M, Iuvone PM, Fernandes A, Sugrue MF, Mallorga PJ, Laties AM, Stone RA. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis Sci. 1999;76:397–407. doi: 10.1097/00006324-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–9. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Mertz JR, Wallman J. Choroidal retinoic acid synthesis. a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–27. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]