Abstract
Background
Keratoconus is a progressive degenerative corneal disorder of children and young adults that is traditionally managed by refractive error correction, with corneal transplantation reserved for the most severe cases. UVA collagen crosslinking is a novel procedure that aims to prevent disease progression, currently being considered for use in the UK NHS. We assess whether it might be a cost-effective alternative to standard management for patients with progressive keratoconus.
Methods
We constructed a Markov model in which we estimated disease progression from prospective follow-up studies, derived costs derived from the NHS National Tariff, and calculated utilities from linear regression models of visual acuity in the better-seeing eye. We performed deterministic and probabilistic sensitivity analyses to assess the impact of possible variations in the model parameters.
Results
Collagen crosslinking is cost effective compared with standard management at an incremental cost of £3174 per QALY in the base case. Deterministic sensitivity analysis shows that this could rise above £33 263 per QALY if the duration of treatment efficacy is limited to 5 years. Other model parameters are not decision significant. Collagen crosslinking is cost effective in 85% of simulations at a willingness-to-pay threshold of £30 000 per QALY.
Conclusion
UVA collagen crosslinking is very likely to be cost effective, compared with standard management, for the treatment of progressive keratoconus. However, further research to explore its efficacy beyond 5 years is desirable.
Introduction
Keratoconus is a progressive degenerative corneal disorder that affects ∼1/2000 of the general population.1 The typical cone-like deformity1 usually initiates during teenage or early adult years.1, 2 Progression may however attenuate after the age of 30 years2, 3 with complete cessation usually expected sometime between age 40 and 50 years.1, 2 Approximately 90% of all patients3 will eventually be bilaterally affected,2 with establishment in the second eye delayed by ∼5 years.2, 3 This may partly account for the characteristic asymmetry of disease severity between the eyes.2, 3, 4
Patients typically experience visual aberration with astigmatic features5 for which visual acuity measurements are considered a somewhat unsatisfactory5 summary measure. Optical correction using spectacles or contact lenses aims therefore to regularise the corneal curvature (measured in dioptres (D)).5 Progression in some younger patients and those from some ethnic groups may be uncharacteristically swift.6, 7 This means that conservative management may require supplementing in some instances with minor surgical procedures, or as a last resort, corneal transplantation.1 Corneal transplantation occurs most frequently within 10 years of diagnosis,2, 8, 9 affecting ∼10–20% of patients overall.1, 10
UVA collagen crosslinking is a recent innovation that aims to restore the underlying integrity of the corneal matrix and hence resist progression of the disease.11 There have been many small-scale demonstrations of its efficacy in both randomised controlled trials11, 12, 13 and a number of uncontrolled prospective evaluations.14, 15, 16, 17 Follow-up of treated patients is presently limited to ∼5 years.15, 16
Quality of life in patients with chronic visual deterioration,18 irrespective of cause,19 is found to correlate most strongly with the visual acuity of their better-seeing18 eye. Therefore, patients with progressive bilateral keratoconus may have severely reduced quality of life, further compromised by considerable pain.20 In addition, although corneal transplantation is traditionally viewed as being successful in restoring vision, it consumes considerable resources in terms of surgical time and follow-up, and requires constant access to donor tissue. It also demands considerable ongoing patient motivation to maintain graft integrity, as failure may have catastrophic visual consequences. The potential benefits of collagen crosslinking are therefore the early arrest of the visual deterioration and the avoidance of surgery.
Collagen crosslinking is currently being considered for use in the UK NHS, although there is not at present, to our knowledge, any published information about its cost effectiveness when compared with traditional management. In this paper we present the results of our simulation model that assesses the cost effectiveness of collagen crosslinking from an NHS perspective, using as a basis a hypothetical population of young adults (aged 21 years at disease onset)2 with progressive early-stage keratoconus. We define ‘progressive' (∼1 D or more per year increase in corneal curvature) in accordance with the inclusion11, 12, 13, 14, 15, 16, 17 and intervention15 criteria of the most recent clinical evaluations.
Materials and methods
The model
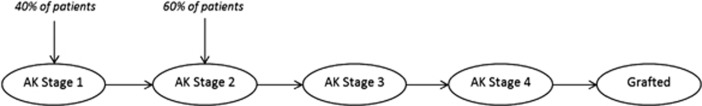
We used a Markov model structure21 that consists of descriptive states that highlight relevant features of patients' health at a given time. The model has a 25-year time horizon and updates in 4-weekly cycles. These values respectively represent both the expected period of active disease and a suitable scaling of ongoing time based on typical clinical interventions and recovery from them. We divided patients in the model into two cohorts of 1000 patients each, all aged 21 years2 and with early-stage keratoconus.22 One cohort receives standard management, and the other receives collagen crosslinking around the time of diagnosis. Patients then progress through the model according to predicted changes in their keratoconus. As they do so, cost and utility values accrue in accordance with disease severity and accumulated interventions that are then discounted and compared between the cohorts. We use the Amsler–Krumeich (AK stage) classification22 to represent the severity of keratoconus. This scales keratoconus from the least severe AK stage 1 to the most severe AK stage 4. We model each eye separately. In the model we assume that all patients present in AK stage 1 (40% of first eyes and all fellow eyes) or stage 2 (60% of first eyes).7 Figure 1 provides an overview of the health states and the potential transitions between them.
Figure 1.
Health states through which patients progress in the model.
Disease progression
Progression at ~1 D a year or more, measured at for example 3–6-month intervals, features commonly11, 12, 13, 14, 15, 16 in the recent literature and appears to be regarded as a benchmark of progressive keratoconus. The literature is not however clear as to the extended clinical course of such patients. Specifically, although we can assume that such patients' disease will either self-limit or require a corneal transplant,2 neither the time course of such end points nor the proportion of patients requiring surgery are explicit.
In order to model disease progression therefore, we made use of data from epidemiological studies in which the marked levels of progression in some patients considered as ‘progressors', for example, 1.01 D per year over 5 years23 and 1.37 D per half-year24 over 3 years, contrasted with larger groups of ‘non progressors' showing advancement at ~0.2–0.3 D per year.23, 24
Although keratoconus is chiefly a bilateral disorder,2 there is very little information about how disease in fellow eyes might progress. Both the well-documented asymmetry2, 4 of keratoconus and the fact that only ~6% of patients eventually require bilateral transplantation10 suggest that this might be very slow compared with the first eye. However, sustained and rapid progression of the disease in fellow eyes15 has been observed. This may indicate that rapid progression in fellow eyes is a feature of ‘progressor' subgroups.
Although this literature on progressor subgroups does not provide sufficient follow-up to indicate what proportion of these patients might eventually require surgery, statistical modelling8, 9 indicates that disease severity is a central risk factor, and also suggests that transplantation is strongly associated with younger age groups and very much less likely more than 10 years from diagnosis. From this and the levels of deformity typically seen in explanted corneas,25 we conclude that highly progressive disease is very strongly associated with eventual transplantation.
We therefore assumed that patients in the model would have an initial constant progression rate, in the first eye diagnosed, of 1.01 D per year.23 Progression commences in the second eye at AK stage 1 after 5 years,2 and at the same initial rate as the first. All diseased eyes in the model then progress at this same rate for 10 years, or until they reach AK stage 3. As all patients enter the model at age 21 years, this enabled us to represent the well-documented tendency of the disease to begin stabilising after age 30 years2 as well as allow that a proportion of patients in the model may halt progression before surgery.2
Management
All patients in the model receive an initial assessment including keratometry to assess their disease status. Patients in the standard management cohort then receive contact lens correction, with new lenses every 2 years (value based on local clinical opinion). A proportion of patients in the model, who reach AK stage 4,8, 9 undergo corneal transplantation (1.3% per year).2 We did not model any postoperative correction of astigmatism. Following surgery, they undergo intensive outpatient follow-up consisting of six visits in first year, five in the second year, and every 6 months thereafter (value based on local clinical opinion). Systemic immunosuppression and monitoring of haematological parameters for three and a half years is required in 7.5% of these patients (value based on local clinical opinion).
Following their initial assessment, patients in the collagen crosslinking cohort undergo further keratometry to assess their progression. Both affected and fellow eyes then receive crosslinking, followed by further keratometry to assess efficacy. Crosslinking is not carried out in eyes with AK stage 4 disease because of safety issues11 and poor efficacy.26 Contact lens correction is still required for all patients in this cohort,2 and we assume the same replacement schedules as for the standard management cohort.
Treatment effect
In the collagen crosslinking cohort, we modify the rate of progression of keratoconus according to the expected efficacy of collagen crosslinking. In what is presently the longest and largest follow-up study,15 patients showed sustained mean improvements in both corneal power and visual acuity, based on 44/363 (12%) patients at 5-year follow-up. The results of the remaining patients are unreported, limiting further analysis. Other reports suggest that there is an initial failure rate associated with individual treatments of ∼7.6%.26 We cannot be certain about the efficacy of treatment after 5 years, or whether retreatment, if required, would be successful.
We chose therefore to assume that, for patients in the collagen crosslinking cohort, collagen crosslinking halts progression without leading to disease improvement, and that 7.6% of modelled treatments fail.26 We model a retreatment after this point, as patients in the model will not have reached disease stability.2, 26 We assumed this has a similar efficacy to initial treatments.
Adverse events
The majority of adverse events associated with collagen crosslinking are typically transient or do not require treatment.26 We assume that comprehensive antibiotic and antiviral cover effectively prevents infection.26 Therefore, we do not model any adverse events associated with collagen crosslinking.
Adverse events associated with corneal transplantation include graft rejection, raised intraocular pressure (IOP), cataract, and infection.27, 28 We assumed that 3% of grafted patients develop cataract. We extrapolated the findings of Lim et al28 with regard to revision procedures (1% required over 46.5 months) to cover the model time horizon.
Raised IOP is a common complication, incident in ∼20% of patients in the first year after the procedure (value based on local clinical opinion), although in 80% of patients it resolves within a year. In the model, we assume that all incidences of raised IOP follow this pattern. In general, we assume that all these adverse events incur only costs in the model, although the onset of prolonged raised IOP may result in some quality of life reduction, for which we have no data.
Utilities
Each health state has an associated utility value, quantifying the expected quality of life for a patient in that state of health, anchored on the scale of 0 (dead) to 1 (perfect health).29 As the model runs, these values weight the time spent in each cycle and, when summed across the whole time frame, express the number of QALYs accruing to the alternatives being evaluated.
Direct measurements of utility in keratoconus, based on disease severity, are not available. Therefore, we estimated the impact of keratoconus on quality of life according to the expected visual acuity of each disease stage.
We chose mean visual acuity values measured in association with AK stages 1, 2, and 4 from a study with sizeable groups and good coverage of disease states.30 We imputed, by weighted regression on the group sizes, an estimate of visual acuity for AK stage 3. We assumed that corneal transplantation confers a postoperative visual acuity of 0.1 logMAR.31 We calculated utility values (Table 1) using data from linear regression models based on the time tradeoff (TTO) method.18 We assigned these values to the bilateral disease states according to the visual function in the better-seeing eye that is most strongly correlated with utility.18 For unilaterally affected patients, however, we chose those values associated with the worse-seeing eye.18 This was because, first, the preferred values were derived from patients whose vision in one eye was worse than 0.3 logMAR, and this does not apply to all the patients in our model. Second, there would otherwise be no gradation in utility throughout our unilateral disease states.
Table 1. Utility values used for patients in the model with bilateral disease 18 (column 3) or unilateral disease (column 4) 18 .
| AK stage | Visual acuity (logMAR) | Utility in bilateral disease based on better-seeing eye18 | Utility in unilateral disease based on affected eye18 |
|---|---|---|---|
| N | 0 | 0.920 | N/A |
| 1 | 0.16 | 0.852 | 0.920 |
| 2 | 0.30 | 0.800 | 0.860 |
| 3 | 0.40 | 0.770 | 0.860 |
| 4 | 0.50 | 0.749 | 0.860 |
| GRAFTED (graft is the better-seeing eye if bilateral) | 0.10 | 0.870 | 0.920 |
| GRAFTED (graft is the worse-seeing eye if bilateral) | — | 0.920 |
Costs
We derived treatment and monitoring costs in the model (Table 2) from the NHS National Tariff (NHS National Tariff 2012–2013). Discounting was applied to both utilities and costs at 3.5% per year in accordance with the National Institute for Health and Care Excellence (NICE) guidelines.32
Table 2. Costs applied for any simulated treatment of patients in the model.
| Description of cost | Total cost | Unit costs | Source |
|---|---|---|---|
| Collagen crosslinking treatment | £928 | Procedure: £212 | NICE OPCS 51.8 |
| Riboflavin (1 ml solution): £75 | Kestrel Opthalmics Ltd | ||
| Four outpatient visits: 1 × £115 (initial) and 3 × £67 each | NHS National Tariff 2012–2013 WF01B/WF01A | ||
| Two keratometry measurements: £145 each | HRG BZ13 | ||
| Follow-up medication for 1 month (acyclovir, chloramphenicol, fluorometholone, gabapentin, proxymetacaine, co-codamol): £35 | BNF 64 | ||
| Corneal transplantation | £2902 | Procedure (weighted average of 40% DALK procedure (deep anterior lamellar keratoplasty) and 60% PK procedure34 (penetrating keratoplasty)): £1765.6 | HRG BZ11 (DALK) HRG BZ12 (PK) |
| Initial assessment (£115) and 11 outpatient follow-ups (£67 each) required in first 2 years; 1–2 per year thereafter | National Tariff 2012–2013 WF01B/WF01A | ||
| Mycophenolate for 3.5 years for 7.5% of patients: £222 | BNF 64 | ||
| Monthly full blood count monitoring for 3.5 years for those taking mycophenolate: £24.92 per month (first year), £17.59 per month (thereafter) | |||
| Adverse event: Cataracts | £997 | Initial assessment: £67 | National Tariff 2012–2013 WF01A |
| Listing of procedure: £115 | WF01B | ||
| Phaecoemulsification and cataract extraction: £748 | HRG BZ02Z | ||
| Follow-up: £67 | National Tariff 2012-13_WF01A BNF 66 | ||
| Raised IOP | £1.47 per 5 ml | Timolol eye drops, twice daily. A 5 ml bottle lasts 2 months (Boots Company Plc.) | |
| Follow-up is included with follow-up for transplant procedure | |||
| Visual correction for keratoconus patients | £52 | Contact lenses (ROSE K) for standard visual correction: £52 per eye | Royal Devon and Exeter NHS Foundation Trust Optometry dept |
| £99 | Contact lenses suitable for post-graft patients: £99 per eye |
Sensitivity analyses
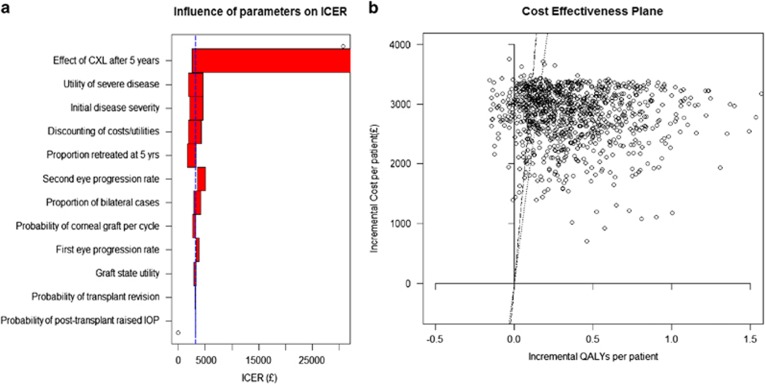
Parameter values used for modelling carry with them uncertainty, because the parameterisations typically represent estimates of an average. Sensitivity analysis allows us to assess the potential impact of parameter inaccuracies on the results of the model, thereby indicating how ‘sensitive' the model results are to each potential inaccuracy. We varied the value of every parameter in the model in turn using ranges of plausible values for each one (Table 3 and Figure 2a), and then applied a probabilistic sensitivity analysis (PSA) using Monte Carlo simulation. This technique draws every parameter value randomly from a statistical distribution. We generated 1000 such combinations of parameter values (Figure 2b), using standard probability distributions (Supplementary Appendix A). We used these results to infer the probability of collagen crosslinking being cost effective when compared with a willingness-to-pay (WTP) range of £20 000 to £30 000 per QALY gained. This is the range used by NICE in assessing what constitutes a cost-effective use of resources in the UK NHS.32
Table 3. Variations in the incremental cost-effectiveness ratio (ICER) of collagen crosslinking, resulting from changes to individual model parameters.
| Parameter | Range explored | Low end of ICER range (£ per QALY) | High end of ICER range (£ per QALY) |
|---|---|---|---|
| Treatment efficacy | Treatment is completely effective for time horizon vs no efficacy beyond 5 years | 1450 | 33 263 |
| Severe disease (AK stage 3 or 4) utility | From 10% lower to same value as AK stage 2 | 1871 | 4661 |
| Initial disease severity | From all starting in AK stage 1 vs all start in AK stage 2 | 2170 | 4607 |
| Discount rate | From 0 to 6%. | 1939 | 4251 |
| Costs of repeat treatments at 5 years | From no treatments repeated to all treatments repeated | 1701 | 3377 |
| Second eye progression rate | From 0.5 D to 0.2 D per year | 3513 | 5090 |
| Proportion of bilateral cases | From 100 to 60% | 2900 | 4210 |
| Probability of corneal graft per year | From 0.4 to 4% per year | 2683 | 3214 |
| First eye progression rate | From 2 D per year to 0.5 D per year | 3376 | 3830 |
| Graft state utility | From 0.8 (same as AK stage 2 disease) to 0.92 (comparable to baseline vision) | 2930 | 3357 |
| Probability of transplant revision | 1% over time horizon to 10% over time horizon | 3153 | 3177 |
| Probability of post-transplant raised IOP | From 10 to 50% | 3174.2 | 3174.5 |
Figure 2.
(a) ‘Tornado' plot showing the variation in the ICER because of changes in individual parameters. The broken line indicates base case. CXL, collagen crosslinking. (b) Cost-effectiveness plane showing the cost/QALY pairs arising from 1000 Monte Carlo simulations.
Software
The model was built using Microsoft EXCEL 2010. Additional statistical analyses were performed in STATA 13 (StataCorp, College Station, TX, USA).
Results
In the base case, the incremental cost-effectiveness ratio (ICER) was £3174 per QALY. Uncertainty around the expected duration of treatment benefit would have the most influence upon this result (Table 3 and Figure 2a). Specifically, the ICER may rise to £33 263 per QALY if there is no treatment benefit beyond 5 years. Potential variations in other parameters do not bring the ICER near to the WTP thresholds. We note that the ICER tends to increase because of any variation in disease progression using the values we applied.
Our PSA generates a cost-effectiveness plane (Figure 2b) that shows potential variations in the incremental costs and QALYs because of the present overall state of uncertainty over the complete set of model parameters. The mean ICER is £6316 per QALY, compared with the base case of £3174 per QALY. The flat shape of the distribution is because modelled costs do not generally vary with disease severity. Points situated below the added lines (dotted=£20 000 per QALY, dashed=£30 000 per QALY) show the simulations in which collagen crosslinking is cost effective compared with the respective NICE WTP thresholds.32 This accounts for 79% and 85% of simulations respectively.
Discussion
Our base case result shows that UVA collagen crosslinking is cost effective for progressive keratoconus, compared with standard management, at an incremental cost of £3174 per additional QALY over a 25-year time horizon. This value may rise to over £33 263 if the treatment can only provide a one-off benefit of 5 years of halted progression. However, the results of our sensitivity analysis suggest that our results are relatively insensitive to inaccuracies in most of our parameterisation. Therefore, we are confident that UVA collagen crosslinking would be a cost-effective treatment for progressive keratoconus compared against the NICE WTP range of £20 000 to £30 000 per QALY gained.32
The analysis of overall uncertainty provided by our PSA predicts that collagen crosslinking would be cost effective compared with standard management in 79 and 85% of simulations. The difference between the mean estimate of our PSA, at £6316 per QALY, and the deterministic result may be attributable to the properties of the statistical distributions applied.
Our study has considerable strengths: we make a comprehensive representation of the clinical course of keratoconus, based on accepted disease staging and a well-defined subpopulation of patients.23 Our costs come from NHS tariffs and therefore reflect actual practice. We based our quality-of-life measurements on clearly demonstrated associations with visual function.18
However, we acknowledge a number of limitations with this study. Clearly, the available data for assessing the potential impact of collagen crosslinking remain limited. Current estimations of efficacy rest on a number of small, mostly single-centre, studies of limited duration relative to the disease time course. As these studies are recent, it will clearly be some years before more substantial data are available. However, our model strongly suggests that provided that efficacy can be robustly demonstrated beyond 5 years, the treatment would be very likely to be cost effective. We were unable to model certain parameters such as the effects of correcting post-transplantation astigmatism because of lack of data. This omission would not however affect our conclusions, as it seems clear that both the net impact of the excess costs of correction and any transient decrease in utility before the correction would reduce the ICER further. We limited the inclusion of patients in the model to those with early-stage disease that may exaggerate the benefits of collagen crosslinking. In reality, however, some patients with later-stage disease may not be safely treatable11 and hence could not be modelled in our treatment cohort. In addition, the clinical course of keratoconus is clearly highly variable between patients, even for those patients with more aggressive disease. Furthermore, we cannot be certain what proportion of these patients will eventually have surgery, the major cost contributor of the standard management cohort. We also had very little information about the likely progression of disease in the fellow eye. By examining the uncertainty around these limitations in our PSA and nevertheless demonstrating a high probability of cost effectiveness, we are confident however that the uncertainty around treatment efficacy remains the key issue to address.
To our knowledge, this is the first report to investigate the cost effectiveness of UVA collagen crosslinking. Therefore, we anticipate that our findings will be of great interest to clinicians working with keratoconus. Our results suggest that there is now a strong case for commissioning UVA collagen crosslinking in the UK NHS. On the present evidence, this would be limited to those patients presenting with progressive disease,11, 23, 24 although the policy with regard to treating fellow eyes could be at clinical discretion. If treatment arrests the disease before substantial progression occurs, the quality of life of patients would approach that of unaffected individuals. In addition, considerable health service resources currently routed into transplantation surgery and follow-up would be available, given that keratoconus is one of the most common indications for this procedure in first world countries.6, 31 It would also reduce the demands on available tissue. At present, the available literature on efficacy comprises many small single-centre studies. Although initial evidence through repeated demonstrations of efficacy in Caucasians11, 12, 13, 14, 15, 16 as well as in Middle East17 and Indian33 populations is promising, there is clearly a need for more widespread reporting and for extended demonstrations of treatment efficacy.
Conclusion
UVA collagen crosslinking is highly likely to be cost effective for management of progressive keratoconus, with an 85% probability of being cost effective at a threshold of £30 000 per QALY. However, there is an ongoing need for reports on the longer-term efficacy of treatment and its widespread applicability.

Acknowledgments
We thank Hilary Pearce and Christopher Roome of the Peninsula Health Technology Commissioning Group for initiating and overseeing the project, and for their assistance and guidance during the project. Also Louise Frost at the West of England Eye Unit, based at the Royal Devon and Exeter NHS Foundation Trust, for help in sourcing costs of optical correction. This research was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
Supplementary Material
References
- Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998; 42(4): 297–319. [DOI] [PubMed] [Google Scholar]
- Weed KH, McGhee CN. Referral patterns, treatment management and visual outcome in keratoconus. Eye 1998; 12(4): 663–668. [DOI] [PubMed] [Google Scholar]
- Weed KH, MacEwen CJ, Giles T, Low J, McGhee CN. The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye 2008; 22(4): 534–541. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Steger-May K, Fink BA, Joslin CE, Nichols JJ, Rosenstiel CE et al. Between-eye asymmetry in keratoconus. Cornea 2002; 21(7): 671–679. [DOI] [PubMed] [Google Scholar]
- Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol 1984; 28(4): 293–322. [DOI] [PubMed] [Google Scholar]
- Al Suhaibani AH, Al-Rajhi AA, Al-Motowa S. Inverse relationship between age and severity and sequelae of acute corneal hydrops associated with keratoconus. Br J Ophthalmol 2007; 91(7): 984–985.17576720 [Google Scholar]
- Leoni-Mesplie S, Mortemousque B, Touboul D, Malet F, Praud D, Mesplie N et al. Scalability and severity of keratoconus in children. Am J Ophthalmol 2012; 154(1): 56–62. [DOI] [PubMed] [Google Scholar]
- Pouliquen Y, Forman MR, Giraud JP. [Evaluation of the rapidity of progression of keratoconus by a study of the relationship between age when first detected and age at operation (author's transl)]. J Fr Ophtalmol 1981; 4(3): 219–221. [PubMed] [Google Scholar]
- Reeves SW, Stinnett S, Adelman RA, Afshari NA. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol 2005; 140(4): 607–611. [DOI] [PubMed] [Google Scholar]
- Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology 1994; 101(3): 439–447. [DOI] [PubMed] [Google Scholar]
- Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg 2008; 24(7): S720–S725. [DOI] [PubMed] [Google Scholar]
- O'Brart DP, Chan E, Samaras K, Patel P, Shah SP. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linkage to halt the progression of keratoconus. Br J Ophthalmol 2011; 95(11): 1519–1524. [DOI] [PubMed] [Google Scholar]
- Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg 2011; 37(1): 149–160. [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Albe E, Frueh B, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol 2012; 154: 520–526. [DOI] [PubMed] [Google Scholar]
- Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol 2010; 149(4): 585–593. [DOI] [PubMed] [Google Scholar]
- Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg 2008; 34(5): 796–801. [DOI] [PubMed] [Google Scholar]
- El-Raggal TM, Riboflavin-Ultraviolet A. Corneal cross-linking for keratoconus. Middle East Afr J Ophthalmol 2009; 16(4): 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc 1999; 97: 473–511. [PMC free article] [PubMed] [Google Scholar]
- Brown MM, Brown GC, Sharma S, Landy J, Bakal J. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol 2002; 120(4): 481–484. [DOI] [PubMed] [Google Scholar]
- Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study: methods and findings to date. Cont Lens Anterior Eye 2007; 30(4): 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenbrink JB, Rutten-van Mölken MPMH, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 2005; 8(1): 32–46. [DOI] [PubMed] [Google Scholar]
- Gómez-Miralles M, Peris-Martínez C, Pastor-Pascual F. Biomechanical corneal response measurement after manual insertion of intrastromal rings in patients with keratoconus. J Emmetropia 2010; 1: 206–212. [Google Scholar]
- Oshika T, Tanabe T, Tomidokoro A, Amano S. Progression of keratoconus assessed by fourier analysis of videokeratography data. Ophthalmology 2002; 109(2): 339–342. [DOI] [PubMed] [Google Scholar]
- Kim H, Joo CK. Measure of keratoconus progression using Orbscan II. J Refract Surg 2008; 24(6): 600–605. [DOI] [PubMed] [Google Scholar]
- Lawless M, Coster DJ, Phillips AJ, Loane M. Keratoconus: diagnosis and management. Aust N Z J Ophthalmol 1989; 17(1): 33–60. [DOI] [PubMed] [Google Scholar]
- Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg 2009; 35(8): 1358–1362. [DOI] [PubMed] [Google Scholar]
- Kirkness CM, Ficker LA, Steele AD, Rice NS. The success of penetrating keratoplasty for keratoconus. Eye 1990; 4(5): 673–688. [DOI] [PubMed] [Google Scholar]
- Lim L, Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: visual outcome and success. Ophthalmology 2000; 107(6): 1125–1131. [DOI] [PubMed] [Google Scholar]
- Brazier J, Ratcliffe J, Salomon J A, Tsuchiya A. Techniques of economic evaluation. In Measuring and Valuing Health Benefits for Economic Evaluation. Oxford University Press: Oxford, UK, 2007, p 11. [Google Scholar]
- Pinero DP, Alio JL, Barraquer RI, Michael R, Jimenez R. Corneal biomechanics, refraction, and corneal aberrometry in keratoconus: an integrated study. Invest Ophthalmol Vis Sci 2010; 51(4): 1948–1955. [DOI] [PubMed] [Google Scholar]
- Olson RJ, Pingree M, Ridges R, Lundergan ML, Alldredge C Jr, Clinch TE. Penetrating keratoplasty for keratoconus: a long-term review of results and complications. J Cataract Refract Surg 2000; 26(7): 987–991. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal 2008. [PubMed]
- Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol 2010; 94(8): 965–970. [DOI] [PubMed] [Google Scholar]
- Keenan TD, Jones MN, Rushton S, Carley FM. Trends in the indications for corneal graft surgery in the United Kingdom: 1999 through 2009. Arch Ophthalmol 2012; 130(5): 621–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.