Abstract
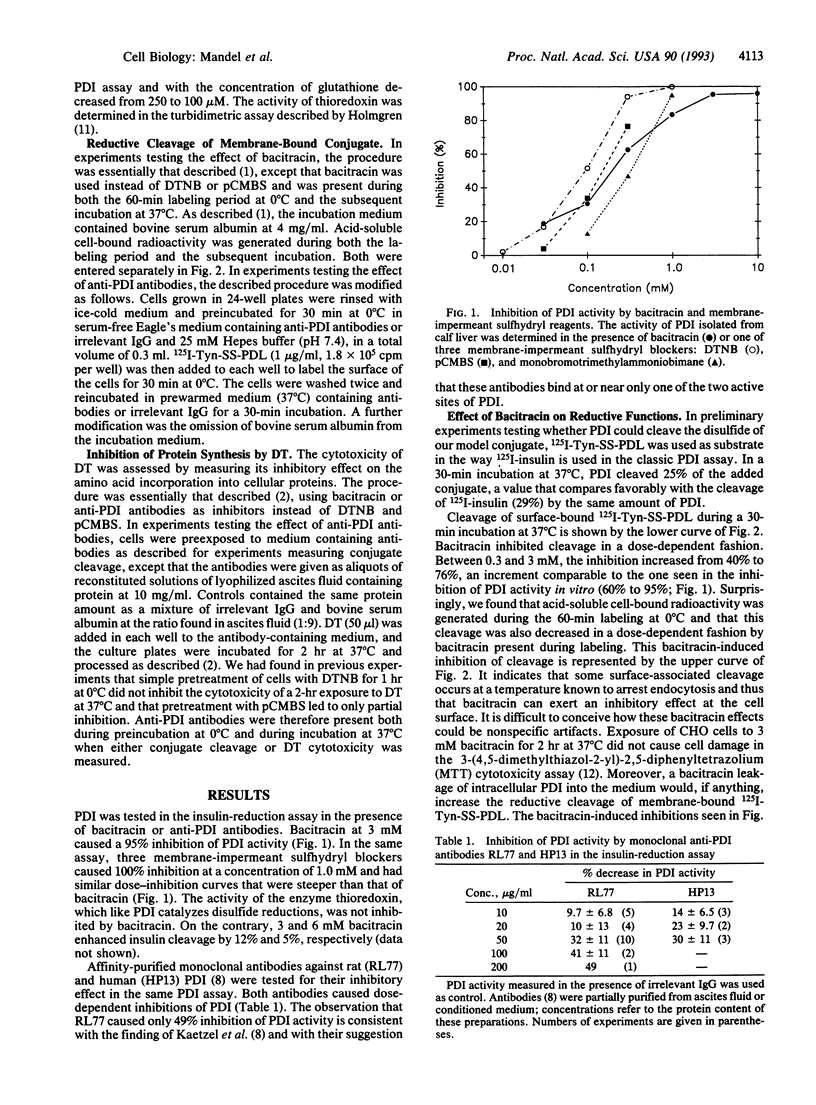
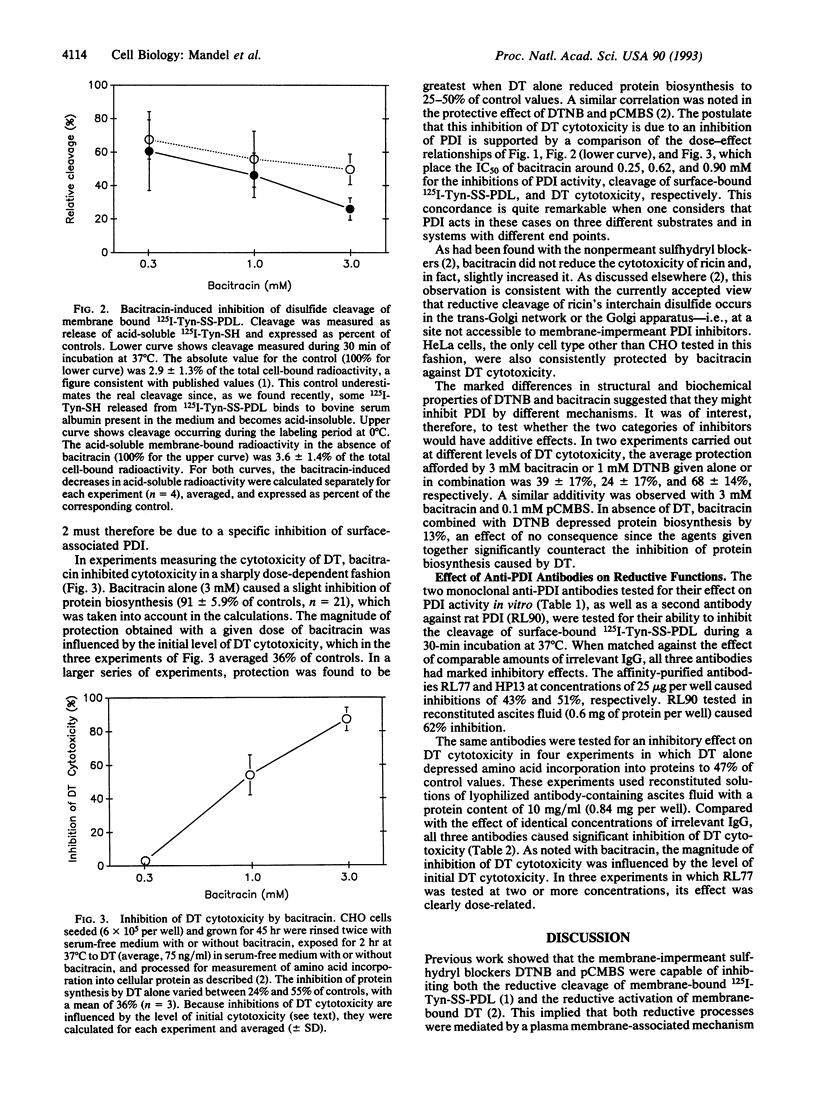
Evidence had been provided that a disulfide-linked [125I]iodotyramine/poly(D-lysine) conjugate was reductively cleaved when bound nonspecifically to the surface of Chinese hamster ovary (CHO) cells and that this cleavage was abolished by membrane-impermeant sulfhydryl blockers. The same blockers were subsequently found to inhibit the cytotoxicity of diphtheria toxin, a disulfide-linked heterodimer that binds to a specific surface receptor and must undergo chain separation to exert its cytotoxicity. This suggested that the disulfides of both macromolecules might be cleaved by a thiol-disulfide interchange reaction, possibly mediated by protein disulfide-isomerase (PDI, EC 5.3.4.1). We tested whether inhibitors of PDI--in particular, bacitracin and anti-PDI antibodies--might mimic the two effects of sulfhydryl blockers. Both bacitracin and anti-PDI antibodies were effective in inhibiting both reductive processes. This strongly suggests that the disulfide cleavage in the two membrane-bound macromolecules is mediated by PDI and that this enzyme, besides its known retention in the endoplasmic reticulum, must also be exposed at the plasma membrane. This paper points to other potentially important disulfide reductions that might be catalyzed by surface-associated PDI. It thereby broadens the known functions of an enzyme already known for its multifunctional properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992 Dec;6(15):3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- Borth W., Scheer B., Urbansky A., Luger T. A., Sottrup-Jensen L. Binding of IL-1 beta to alpha-macroglobulins and release by thioredoxin. J Immunol. 1990 Dec 1;145(11):3747–3754. [PubMed] [Google Scholar]
- Carmichael D. F., Morin J. E., Dixon J. E. Purification and characterization of a thiol:protein disulfide oxidoreductase from bovine liver. J Biol Chem. 1977 Oct 25;252(20):7163–7167. [PubMed] [Google Scholar]
- Cheng S. Y. Characterization of binding and uptake of 3,3',5-triido-L-thyronine in cultured mouse fibroblasts. Endocrinology. 1983 May;112(5):1754–1762. doi: 10.1210/endo-112-5-1754. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Hasumura S., Willingham M. C., Pastan I. Purification and characterization of a membrane-associated 3,3',5-triiodo-L-thyronine binding protein from a human carcinoma cell line. Proc Natl Acad Sci U S A. 1986 Feb;83(4):947–951. doi: 10.1073/pnas.83.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Harrison L. C. Disulfide exchange between insulin and its receptor. A possible post-binding step in insulin action. J Biol Chem. 1983 Oct 10;258(19):11434–11437. [PubMed] [Google Scholar]
- Feener E. P., Shen W. C., Ryser H. J. Cleavage of disulfide bonds in endocytosed macromolecules. A processing not associated with lysosomes or endosomes. J Biol Chem. 1990 Nov 5;265(31):18780–18785. [PubMed] [Google Scholar]
- Freedman R. B., Bulleid N. J., Hawkins H. C., Paver J. L. Role of protein disulphide-isomerase in the expression of native proteins. Biochem Soc Symp. 1989;55:167–192. [PubMed] [Google Scholar]
- Freedman R. B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989 Jun 30;57(7):1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Ghetie M. A., Uhr J. W., Vitetta E. S. Covalent binding of human alpha 2-macroglobulin to deglycosylated ricin A chain and its immunotoxins. Cancer Res. 1991 Mar 1;51(5):1482–1487. [PubMed] [Google Scholar]
- Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979 Oct 10;254(19):9627–9632. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Horiuchi R., Cheng S. Y., Willingham M., Pastan I. Inhibition of the nuclear entry of 3,3',5'-triiodo-L-thyronine by monodansylcadaverine in GH3 cells. J Biol Chem. 1982 Mar 25;257(6):3139–3144. [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Specific covalent binding of platelet-derived growth factor to human plasma alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1984 Jan;81(2):342–346. doi: 10.1073/pnas.81.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel C. S., Rao C. K., Lamm M. E. Protein disulphide-isomerase from human placenta and rat liver. Purification and immunological characterization with monoclonal antibodies. Biochem J. 1987 Jan 1;241(1):39–47. doi: 10.1042/bj2410039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W. J., Gidwitz S., Ayers V. K., Schoepp R. J., Johnston R. E. Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH. J Virol. 1992 Jun;66(6):3504–3513. doi: 10.1128/jvi.66.6.3504-3513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunaga T., Katakura Y., Miura T., Maruyama Y. Purification and characterization of yeast protein disulfide isomerase. J Biochem. 1990 Nov;108(5):846–851. doi: 10.1093/oxfordjournals.jbchem.a123291. [DOI] [PubMed] [Google Scholar]
- Parkkonen T., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of a multifunctional chicken protein acting as the prolyl 4-hydroxylase beta-subunit, protein disulphide-isomerase and a cellular thyroid-hormone-binding protein. Comparison of cDNA-deduced amino acid sequences with those in other species. Biochem J. 1988 Dec 15;256(3):1005–1011. doi: 10.1042/bj2561005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A. Bacitracin: an inhibitor of the insulin degrading activity of glutathione-insulin transhydrogenase. Biochem Biophys Res Commun. 1981 Jan 30;98(2):431–438. doi: 10.1016/0006-291x(81)90858-5. [DOI] [PubMed] [Google Scholar]
- Ryser H. J., Mandel R., Ghani F. Cell surface sulfhydryls are required for the cytotoxicity of diphtheria toxin but not of ricin in Chinese hamster ovary cells. J Biol Chem. 1991 Oct 5;266(28):18439–18442. [PubMed] [Google Scholar]
- Varandani P. T., Raveed D., Nafz M. A. Insulin degradation. XXIII. Distribution of glutathione-insulin transhydrogenase in isolated rat hepatocytes as studied by immuno-ferritin and electron microscopy. Biochim Biophys Acta. 1978 Jan 18;538(2):343–353. doi: 10.1016/0304-4165(78)90362-8. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Semba T., Takemoto H., Akagi S., Yamamoto A., Tashiro Y. Protein disulfide-isomerase in rat exocrine pancreatic cells is exported from the endoplasmic reticulum despite possessing the retention signal. J Biol Chem. 1990 Sep 15;265(26):15984–15990. [PubMed] [Google Scholar]



