Background: Dietary n-6 polyunsaturated fats (n-6 PUFA) like linoleic acid (LA) may worsen cardiac remodeling after injury.
Results: Excess LA increased cardiac collagen I/III ratio and lysyl oxidase causing early diastolic dysfunction. In vitro experiments in fibroblasts with genetic manipulation confirmed such mechanisms.
Conclusion: LA promotes noncompliant collagen and cardiac stiffening.
Significance: This study demonstrates a novel, cardiac-specific lipotoxic pathway of n-6 PUFAs.
Keywords: collagen, fatty acid metabolism, heart failure, lysyl oxidase, polyunsaturated fatty acid (PUFA)
Abstract
Controversy exists on the benefits versus harms of n-6 polyunsaturated fatty acids (n-6 PUFA). Although n-6 PUFA demonstrates anti-atherosclerotic properties, survival following cardiac remodeling may be compromised. We hypothesized that n-6 PUFA like linoleic acid (LA) or other downstream PUFAs like γ-linolenic acid or arachidonic acid alter the transforming growth factor-β (TGFβ)-collagen axis in the heart. Excess dietary LA increased the collagen I/III ratio in the mouse myocardium, leading to cardiac “stiffening” characterized by impaired transmitral flow indicative of early diastolic dysfunction within 5 weeks. In vitro, LA under TGFβ1 stimulation increased collagen I and lysyl oxidase (LOX), the enzyme that cross-links soluble collagen resulting in deposited collagen. Overexpression of fatty acid desaturase 2 (fads2), which metabolizes LA to downstream PUFAs, reduced collagen deposits, LOX maturation, and activity with LA, whereas overexpressing fads1, unrelated to LA desaturation, did not. Furthermore, fads2 knockdown by RNAi elevated LOX activity and collagen deposits in fibroblasts with LA but not oleic acid, implying a buildup of LA for aggravating such pro-fibrotic effects. As direct incubation with γ-linolenic acid or arachidonic acid also attenuated collagen deposits and LOX activity, we concluded that LA itself, independent of other downstream PUFAs, promotes the pro-fibrotic effects of n-6 PUFA. Overall, these results attempt to reconcile opposing views of n-6 PUFA on the cardiovascular system and present evidence supporting a cardiac muscle-specific effect of n-6 PUFAs. Therefore, aggravation of the collagen I/III ratio and cardiac stiffening by excess n-6 PUFA represent a novel pathway of cardiac lipotoxicity caused by high n-6 PUFA diets.
Introduction
Our understanding of cardiolipotoxicity is undergoing a paradigm shift (1). Instead of saturated fats, recent analyses point toward a possible detrimental effect of n-6 polyunsaturated fatty acids (PUFAs)6 on cardiovascular health (2, 3). This is surprising, as n-6 PUFA prevents atherosclerosis and attenuates mortality from vascular disorders by lowering cholesterol (4). Thus, potential cardiotoxicity, if present, may exist within the heart muscle itself. Work from our group and others has demonstrated inflammation and oxidative stress with excess n-6 PUFA in the myocardium in animal models of diet-induced obesity and diabetes (5–7). More recently, Galvao et al. (8) demonstrated earlier death with dietary n-6 PUFA in a hamster model of cardiomyopathy, which was independent of changes in heart function. Although adverse cardiac remodeling was suspected, the mechanisms remained elusive.
The role of individual n-6 PUFA on cardiac remodeling remains unresolved. Under in vivo conditions, linoleic acid (LA, C18:2), the parent dietary n-6 PUFA, undergoes desaturation/elongation to longer, more unsaturated n-6 PUFAs like γ-linolenic acid (GLA, C18:3) and arachidonic acid (AA, C20:4) in mammals (9); the role of individual n-6 PUFAs on fibrotic processes remains unclear. The aims of this study were as follows: (i) to identify whether excess n-6 PUFA altered TGFβ and collagen dynamics within the murine heart and (ii) to identify which fatty acid was responsible for such alterations. We present evidence that LA, but not downstream PUFA like GLA/AA, induces specific TGFβ isoforms and increases the collagen I/III ratio and lysyl oxidase “stiffening” effects in vivo, leads to impaired cardiac transmitral flow, without altering systolic function, and thus represents a novel pathway for cardiolipotoxicity induced by n-6 PUFA.
Materials and Methods
Animal Protocols and Diet
All protocols were completed in accordance with the University of British Columbia Animal Care Committee Guidelines. Eight-week-old male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in a temperature-controlled room (26 °C) on a 12-h light/dark cycle with free access to food and water. These mice were randomly assigned into three diet groups as follows: chow (low fat control) or either of two high fat (HF) groups composed of olive oil (OO; monounsaturated fatty acid (MUFA)) or corn oil (CO; n-6 PUFA). Both HF diets were commercially prepared. Sucrose was also added to the HF diets to mimic the current dietary trend of high simple sugar intakes. However, sucrose content was similar between the two HF diets, and both the HF diets were isocaloric and isonitrogenous (Harlan Teklad, TD88232). The HF diets were supplemented with 20% w/w of either olive oil or corn oil (40% energy from fats) as described previously (10). The carbohydrate and protein contents were 39 and 19% energy, respectively. The final diet composition is given in Tables 1 and 2. The chow diet (14% energy from fat; Lab Diet-5P76) was utilized to compare the effects of HF diets per se. All diets were fed ad libitum for 5 weeks. At the end of all experiments, mice were euthanized with isoflurane-CO2 and hearts excised. A section of the left ventricle (LV) was put in RNALaterTM and formalin for mRNA quantification and histology, respectively. The remainder was flash-frozen and stored at −80 °C for biochemical assays.
TABLE 1.
Detailed composition of high fat diets
| Ingredients | Olive oil diet | Corn oil diet |
|---|---|---|
| g/kg | g/kg | |
| Casein | 240 | 240 |
| dl-Methionine | 3.6 | 3.6 |
| Corn starch | 150 | 150 |
| Sucrose | 298.8 | 298.8 |
| Cellulose | 50 | 50 |
| Calcium carbonate | 3.6 | 3.6 |
| Mineral mixa | 42 | 42 |
| Vitamin mixb | 12 | 12 |
| Oils | ||
| Soybean oil | 10 | 10 |
| Corn oil | 0 | 190 |
| Olive oil | 190 | 0 |
| Total | 1000 | 1000 |
a Mineral mix (mg/g) contains the following: di-calcium phosphate 500; magnesium oxide 24; potassium citrate 220; potassium sulfate 52; sodium chloride 74; chromium KSO4 12H2O 0.55; cupric carbonate 0.3; potassium iodate 0.01; ferric citrate 6; manganous carbonate 3.5; sodium selenite 0.01; zinc carbonate 1.6; sucrose 118.03.
b Vitamin Mix (mg/g) contains the following: vitamin A 0.8; vitamin D3 1; vitamin E 10; menadione sodium bisulfite 0.08; nicotinic acid 3; calcium pantothenate 1.6; pyridoxine HCl 0.7; riboflavin 0.6; thiamin 0.6; sucrose 978.42.
TABLE 2.
Macronutrient composition of experimental diets
| Macronutrients | % w/w | % energy |
|---|---|---|
| High fat diet | ||
| Protein | 21.2 | 19 |
| Carbohydrate | 44.4 | 39 |
| Fat | 20.0 | 40 |
| Total | 4.53 kcal/g diet | |
| Normal chowa | ||
| Protein | 22.6 | 26.4 |
| Carbohydrate | 51.2 | 60.1 |
| Fat | 5.2 | 13.7 |
| Total | 3.41 kcal/g diet | |
a Note: Normal chow ingredients are variable as with any semi-purified diet and have not been listed.
Echocardiography
At the end of the dietary protocols, Vevo® 2100 system (Fujifilm Visualsonics, Ontario, Canada) was used to examine LV function in mice by noninvasive transthoracic echocardiography. Mice were anesthetized with 2% isoflurane and maintained under anesthesia with 1% isoflurane. Body temperature was kept at 37 ± 0.5 °C by placing mice on a warming platform. Two dimensional M-mode and parasternal short and long axis scans were made to assess changes in left ventricle fractional shortening, ejection fraction, and cardiac output. In addition, transmitral pulsed wave Doppler echocardiography was performed. An apical four-chamber view was acquired by positioning the transducer as parallel to the mitral inflow as possible. From this view, mitral valve early (E) and late (A) flow velocities, E/A ratio, isovolumetric contraction, and relaxation times were determined (11). Deceleration time of early left ventricular filling peak (EDT was calculated as a surrogate of LV wall stiffness, where LV stiffness was inversely proportional to EDT (12).
Gas Chromatographic Analysis
Composition of high fat diets was analyzed using gas chromatography (GC) by NP Analytical Laboratories (St. Louis, MO) on behalf of Harlan Teklad. For GC analysis of fatty acids in heart muscle or cell culture studies, evaluation of fatty acids was done using a combined extraction and methylation protocol (13). Briefly, 400 μl of media was added to 1.5 ml of hexane and 1.5 ml of BF3/MeOH. Samples were heated between 90 and 110 °C for 1 h. Next, 3 ml of water was added to the samples and centrifuged for 1 min, and the top hexane layer was removed. Fatty acid methyl esters were analyzed on a Trace 1300 gas chromatograph with flame ionization detector (FID) using a Supelco Famewax column (30 m × 0.32 mm inner diameter × 0.5 μm). For the analysis of relative ratios of 16:0, 16:1n-9, and 16:1n-10, a shorter, more efficient column from Agilent (DB-WAX, part number 123-7011; 15 m × 0.32 mm inner diameter × 0.15 μm) was utilized with authentic standards of 16:0, 16:1n-9 (Sigma), and 16:1n-10 (Matreya LLC). For all methods, the GC peak area percentage of each fatty acid methyl ester was calculated as a percent of the total of all fatty acid esters.
Western Blotting
Western blotting was performed as described previously (14). In brief, protein was extracted from flash-frozen mouse hearts. 25 μg of protein was separated using SDS-PAGE (10%). Following transfer to a nitrocellulose membrane and blocking, membranes were incubated with primary antibodies for collagen I and III (Santa Cruz Biotechnology, Dallas, TX) at a 1:250 dilution or with LOX (Novus Biological) at a 1:1000 dilution. Membranes were then probed with horseradish peroxidase-conjugated secondary antibodies against rabbit and goat IgGs (Applied Biological Materials Inc., British Columbia, Canada) at a 1:500 dilution. Signals were detected using C-DiGit Blot Scanner (LI-COR) with Image Studio DiGit software (version 3.1). Band density was quantified and expressed relative to total protein loaded, as determined via Ponceau stain, in arbitrary units for collagen.
Masson Trichrome Stain and Immunolocalization of Collagen I and III
Masson trichrome stain was used to evaluate total collagen in the heart as described previously (15). In brief, deparaffinized heart sections were rehydrated and mordanted in Bouin's solution for 1 h at 56 °C. Then they were stained with Weigert's iron hematoxylin and Biebrich Scarlet-Acid Fuchsin followed by incubation in phosphomolybdic-phosphotungstic acid solution. Finally, sections were stained with aniline blue, differentiated in acetic acid, mounted, and visualized using light microscopy. Double immunolabeling was performed as described previously (14). Briefly, deparaffinized sections of mouse hearts were blocked and incubated overnight at a 1:50 dilution with a rabbit polyclonal antibody against collagen I (Santa Cruz Biotechnology) and then with a goat polyclonal antibody against collagen III (Santa Cruz Biotechnology). Secondary anti-rabbit DyLight 488 (BioLegend) and anti-goat DyLight 594 (Santa Cruz Biotechnology) antibodies were used at 1:200 dilution. Sections were imaged with Olympus IX81 microscope.
Quantification of mRNA by Quantitative PCR
mRNA levels were quantified using the real time PCR ΔΔCT method as described recently (14). RNA from hearts were purified using RNeasy kits (Qiagen, Ontario, Canada). cDNA was synthesized with Superscript II reverse transcriptase and oligo(dT)12–16-mer (Invitrogen) followed by quantitative PCR on CFX96 real time PCR system (Bio-Rad) using Ssofast Evagreen Supermix (Bio-Rad). Primer sequences of analyzed genes are given in Table 3. The reference gene used was 18S ribosomal RNA. Each quantitative PCR plate contained no template controls to check for DNA contamination.
TABLE 3.
Primer sequences used for quantification of mRNA levels by real time PCR
Primers were used to determine gene expression. Both the forward primer (F) and reverse (R) primers are indicated.
| Gene | Primer sequences (5′–3′) | GenBank accession no. |
|---|---|---|
| tgfb1 | F: GTCACTGGAGTTGTACGGCA | NM_011577.1 |
| R: AGCCCTGTATTCCGTCTCCT | ||
| tgfb3 | F: CAGCCTACATAGGTGGCAAGAAT | NM_009368.3 |
| R: ACCCAAGTTGGACTCTCTCTTCAA | ||
| col1a1 | F: ACCTGTGTGTTCCCTACTCA | NM_007742.3 |
| R: GACTGTTGCCTTCGCCTCTG | ||
| col3a1 | F: AATGGTGGTTTTCAGTTCAGC | NM_009930.2 |
| R: TGGGGTTTCAGAGAGTTTGGC | ||
| lox | F: CACACACACAGGGATTGAG | NM_001286182.1 |
| R: AATCCACTGGCAGTCTATGTC | ||
| loxl1 | F: GAGATGCAACATCCACTACAC | NM_010729.3 |
| R: CACTCAGATCAGGACTGGAC | ||
| fads2 | F: CGGCTACCACATCCAAGGAA | NR_003278 |
| R: GCTGGAATTACCGCGGCT | ||
| 18S ribosomal RNA (18S rRNA) | F: CGGCTACCACATCCAAGGAA | NR_003278 |
| R: GCTGGAATTACCGCGGCT |
Fibroblast Culture and Treatment
Mouse-derived fibroblast cells NIH/3T3 (catalog no. CRL-1685) were purchased from American Type Culture Collection (ATCC, Manassas, VA). NIH/3T3 cells were cultured in DMEM-LG media supplemented with 2 mm sodium pyruvate, 4 mm gluta-GRO, 10% FBS, and 2% penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2. Cells were passaged at 70–80% confluency, and media were replaced every 3–4 days. Fibroblasts were seeded in 48-well plates at a density of 15,000 cells/well. Wells were treated with various fatty acids for 24 h and were treated with chemokines such as TGFβ1 and TGFβ3 (10 ng/ml).
Soluble and Deposited Collagen Assay
Both soluble and deposited collagen were measured from cell media extracts using Sirius Red staining, adapted from Tullberg-Reinert and Jundt (16). Briefly, supernatants from cell cultures were incubated with Sirius Red for 30 min, and soluble collagen was collected by centrifugation. Following a wash with 0.01 n HCl, soluble collagen was resuspended in 0.1 n NaOH and transferred to a 48-well plate. Absorbance was read at 540 nm. Next, cells were stained with Sirius Red and centrifuged at 10,000 × g for 5 min to pellet the collagen, and the supernatant was carefully discarded. To the pellet, 1 ml of HCl was gently added to wash off any excess/unbound dye. To dissolve the stain, 250 μl of 0.1 n NaOH was added. The dye was transferred to a new 48-well plate, from which the absorbance intensity was read at 540 nm using the GloMax Multi+ detection system. Treated cells were compared with control untreated cells, and values were expressed in relative absorbance units). Deposited collagen within cells was visualized using bright field microscopy.
LOX Activity
LOX activity was assessed using the AmpliteTM fluorimetric lysyl oxidase assay kit (AAT Bioquest, CA) according to the manufacturer's instructions and expressed in relative fluorescence units per mg of protein as determined by Bradford assay (Bio-Rad).
Overexpression of fads Isoforms in Fibroblasts
Fatty acid desaturase 2 (fads2) is a gene involved in the desaturation pathways of n-6 and n-3 fatty acids (17). fads2 was overexpressed in NIH/3T3 cells using the mammalian expression vector pTarget (Promega). Briefly, DH10β Escherichia coli containing pCMV-SPORT6:FADS2 was obtained from ATCC (ATCC catalog no. 10470049). The 1508-bp fads2 cDNA fragment was isolated from the vector by digestion with MluI (Invitrogen), gel-purified using the QIAquick gel extraction kit (Qiagen), and ligated into pTarget vector (previously linearized with MluI and dephosphorylated with calf intestinal alkaline phosphatase) using T4 DNA ligase (Invitrogen, Ontario, Canada). Recombinant plasmids were transformed into DH5α E. coli, and plasmids were isolated using QIAprep spin miniprep (Qiagen). Clones of correct orientation were identified via SmaI (Invitrogen) digestion, made possible by the unique SmaI site found at position 236 within the fads2 cDNA. NIH/3T3 cells were transfected with pTARGET-fads2 using Attractene transfection reagent (Qiagen), according to the manufacturer's protocols. Following transfection, stable-transfected cells were maintained using antibiotic selection with G418 (Invitrogen). Overexpression of fads1 or pCMV-Sport6 empty vector control (Transomic Technologies, Inc.) was achieved using Attractene (Qiagen) following the manufacturer's instructions. After a 48-h recovery, cells were treated with TGFβ1 or TGFβ3 and various fatty acids for 24 h as indicated.
Knockdown of fads2 in Fibroblasts
NIH/3T3 fibroblasts were plated and incubated overnight in complete DMEM. Knockdown of fads2 was achieved by using siRNA (ABMGood, Richmond, Canada; catalog no. i048001) and DharmaFECT 1 transfection reagent (ThermoScientific, catalog no. T-2001-01) following the manufacturer's instructions. Scrambled RNA was used for control transfections (ABMGood, catalog no. LV015). After 48 h, cells were treated with various fatty acids as indicated for respective experiments for an additional 24 h. At 72 h post-transfection, cells were collected for analysis.
Statistical Analysis
Results are expressed as mean ± S.E. Data were analyzed by either one-way or two-way ANOVA with Tukey post-tests as appropriate, to determine the level of significance. Level of significance was set at p < 0.05. GraphPad Prism (version 5) software was used to conduct statistical analyses.
Results
Short Term Fat Feeding Does Not Alter Body Weight in Mice
Fatty acid analysis of experimental OO and CO diets revealed that CO had higher LA (18:2n-6), whereas the OO diet had higher oleic acid (OA, 18:1n-9) (Fig. 1a). Other major classes of fatty acids like saturated (palmitic (16:0) and stearic (18:0) or n-3 PUFA (α-linolenic acid (18:3n-3)) were similar between both diets (Fig. 1a). Long chain n-3 PUFA like docosahexaenoic acid or eicosapentaenoic acid were low in both HF diets. Despite having sucrose, with a higher energy density (4.53 kcal/g versus 3.41 kcal/g), mice on both HF diets consumed fewer grams of food compared with the chow diet (Fig. 1b). However, there was no difference in food intake among mice eating the two HF diets (Fig. 1b). This adjustment of caloric intake among rodents on a HF diet is known (18) and could have led to similar weight gain compared with chow-fed mice over 5 weeks (Fig. 1c). Evaluation of total collagen in the perivascular or interstitial regions using Masson trichrome stain also did not reveal any differences after 5 weeks of HF feeding in either CO- or OO-fed mouse hearts (Fig. 1d). Therefore, any differential outcomes in these HF-fed mice were not related to either sucrose (similar in both HF diets, Tables 1 and 2) or variable weight gain or total cardiac fibrosis but rather due to LA or OA content of their diets.
FIGURE 1.
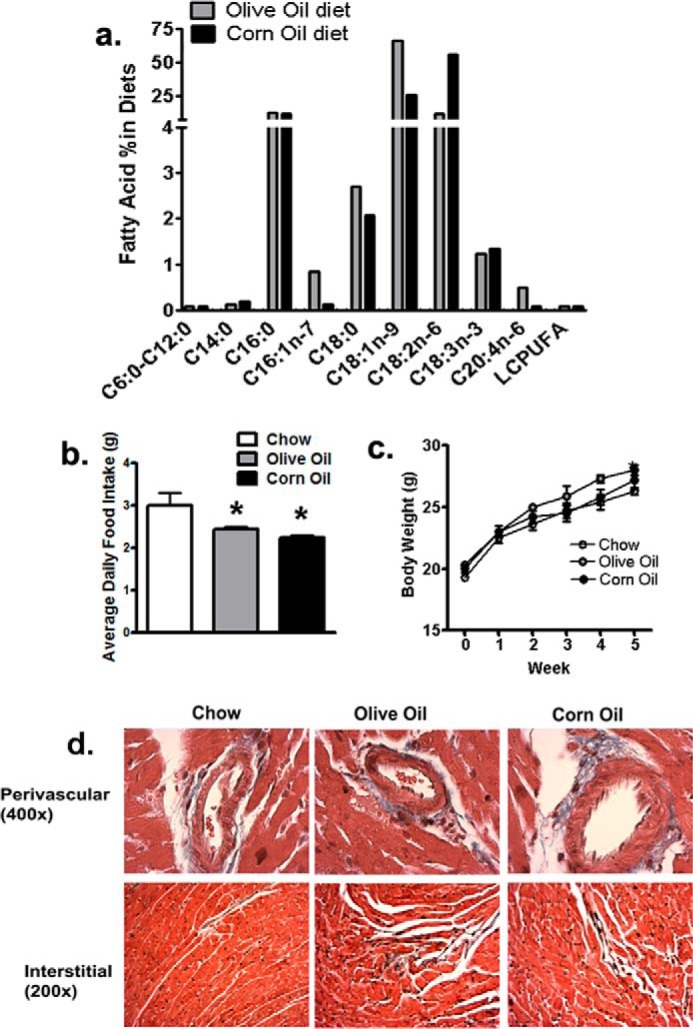
Various diets lead to similar weight gains and no overt fibrotic changes over 5 weeks. a, fatty acid composition of high fat diet groups. Oleic acid (C18:1) and linoleic acid (C18:2n-6) were significantly higher in olive oil and linoleic acid groups, respectively. b, average daily food intake over 5 weeks. Mice on high fat diets consumed less food on a per g basis than chow diets. c, body weight of mice over 5 weeks of diet regimens. Body weight was not significantly different among any group of mice. Bottom panel, Masson trichrome stains of LV depicting perivascular and interstitial regions of LV at ×400 and 200 magnifications, respectively. No difference in total collagen staining (blue) in either areas were observed with any diet. Data were analyzed using one-way ANOVA with Tukey post hoc tests (n = 6 mice). *, p < 0.05 versus the chow-fed group.
Corn Oil Diet Induces Changes to Cardiac Fatty Acids and Causes Early Diastolic Dysfunction without Altering Systolic Function
Table 4 depicts total cardiac fatty acids as measured by GC from the flash-frozen left ventricle. CO feeding led to a 3-fold increase in cardiac LA over 5 weeks compared with OO-fed hearts (Table 4). n-3 PUFAs like eicosapentaenoic acid (C20:5n3) and docosapentaenoic acid (C22:5n3) were both lower in the CO-fed compared with the OO-fed hearts (Table 4). In parallel, the OO diet induced a significant increase in OA (C18:1n9) and GLA (C18:3n6), compared with the CO-fed hearts. The similarity in the relative levels of fatty acids like LA and OA between both the diets and cardiac muscle indicates rapid tissue incorporation of these fatty acids in as early as 5 weeks. Table 5 depicts echocardiographic estimations of cardiac function and flow characteristics from mice fed various diets. No differences in either LV mass or parameters of systolic function like ejection fraction, fractional shortening, and cardiac output were observed among any group after 5 weeks of HF feeding. However, changes in mitral flow characteristics were observed, which are known markers of abnormal ventricular filling (19). Ratio of E/A waves was significantly reduced in CO-fed compared with OO-fed hearts, which suggests diastolic dysfunction and increased LV “stiffness,” especially that EDT was also significantly reduced in CO-fed mice (12). Impaired LV filling without systolic dysfunction indicative of early diastolic dysfunction is associated with altered collagen subtypes (20).
TABLE 4.
Proportion of total cardiac fatty acids in mice fed various diets over 5 weeks
Data are means ± S.E. (n = 4 mice in each group). Differences between CO- and OO-fed hearts were analyzed by Mann-Whitney test.
| Fatty acid | Chow | OO | CO |
|---|---|---|---|
| C14:0 | 0.66 ± 0.13 | 0.62 ± 0.05 | 0.87 ± 0.05 |
| C14:1 | 0.73 ± 0.12 | 0.40 ± 0.04 | 0.29 ± 0.03 |
| C16:0 | 19.26 ± 0.73 | 17.19 ± 2.39 | 14.99 ± 0.27 |
| C16:1 | 0.93 ± 0.19 | 0.77 ± 0.11 | 0.32 ± 0.17 |
| C18:0 | 0.27 ± 0.17 | 0.22 ± 0.13 | 0.35 ± 0.04 |
| C18:1n9 | 34.30 ± 1.24 | 45.63 ± 3.00 | 31.79 ± 1.92a |
| C18:2n6 | 21.88 ± 1.19 | 10.84 ± 1.71 | 30.12 ± 0.86a |
| C18:3n3 | 0.18 ± 0.08 | 0.08 ± 0.06 | 0.12 ± 0.01 |
| C18:3n6 | 0.34 ± 0.07 | 0.75 ± 0.17 | 0.33 ± 0.03a |
| C20:4n6 | 10.16 ± 0.59 | 9.36 ± 0.41 | 9.40 ± 0.91 |
| C20:4n3 | 0.42 ± 0.29 | 1.20 ± 1.15 | NDb |
| C20:5n3 | 0.15 ± 0.11 | 0.28 ± 0.02 | 0.13 ± 0.070a |
| C22:5n3 | 0.97 ± 0.36 | 3.64 ± 0.14 | 1.99 ± 0.16a |
| C22:5n6 | 0.26 ± 0.09 | 0.63 ± 0.53 | 0.12 ± 0.01 |
| C22:6n3 | 10.76 ± 1.55 | 7.07 ± 0.84 | 6.45 ± 0.65 |
a p < 0.05 versus CO-fed mice.
b ND means not detectable.
TABLE 5.
Cardiac function as estimated by echocardiography in mice fed various diets
Data are means ± S.E. (n = 6). Differences between CO and OO groups were analyzed by two-tailed Mann-Whitney tests. NC, normal chow; MV, mitral valve; MV A, A wave; MV E, E wave; DT, deceleration time; IVCT, isovolumetric contraction time; IVRT, isovolumetric relaxation time; NFT, no flow time; ms, millisecond. Significant p values are italicized.
| Parameter | Chow | OO | CO | p value |
|---|---|---|---|---|
| LV mass (mg) | 96.97 ± 6.1 | 113.78 ± 12.6 | 107.10 ± 11.7 | 0.59 |
| Diameter (mm), systole | 1.57 ± 0.1 | 1.58 ± 0.1 | 1.60 ± 0.2 | 0.91 |
| Diameter (mm), diastole | 3.59 ± 0.1 | 3.70 ± 0.1 | 3.66 ± 0.2 | 0.88 |
| Ejection fraction (%) | 85.9 ± 0.9 | 1.581 ± 0.121 | 1.596 ± 0.145 | 0.70 |
| Fractional shortening (%) | 54.74 ± 1.1 | 57.51 ± 2.7 | 56.59 ± 2.8 | 0.69 |
| Cardiac output (ml/min) | 24.32 ± 1.3 | 26.78 ± 1.3 | 26.25 ± 2.7 | 1.00 |
| MV A (mm/s) | 536.72 ± 28.93 | 462.50 ± 21.11 | 526.46 ± 44.10 | 0.13 |
| MV E (mm/s) | 714.23 ± 39.4 | 762.72 ± 33.89 | 740.68 ± 63.05 | 0.73 |
| MV E/A | 1.53 ± 0.06 | 1.68 ± 0.14 | 1.40 ± 0.07a | 0.041 |
| DT (EDT, ms) | 29.44 ± 2.7 | 31.45 ± 1.7 | 26.56 ± 1.3a | 0.030 |
| IVCT (ms) | 14.17 ± 1.02 | 14.81 ± 1.53 | 16.30 ± 0.53 | 0.39 |
| IVRT (ms) | 16.88 ± 1.61 | 20.55 ± 1.44 | 16.14 ± 0.53 | 0.16 |
| NFT (ms) | 81.32 ± 4.93 | 83.92 ± 3.53 | 84.12 ± 3.37 | 1.00 |
a p < 0.05 versus CO-fed mice.
Corn Oil Increases Cardiac Collagen I/III Ratio without Altering Total Fibrosis
Double immunolabeling for collagen I and III was performed in LV sections (Fig. 2a). Out of the two HF diets, OO- and CO-fed hearts demonstrated less and more punctate collagen I immunopositivity, respectively. In contrast, compared with chow and CO, OO-fed hearts demonstrated increased collagen III, at least across some parts of the myocardium (Fig. 2a, red block, arrow). Collagen production is under the direct control of TGFβ in the heart. With respect to tgfβ subtypes, gene expression of tgfβ1 was decreased, whereas tgfβ3 levels was increased in OO-fed hearts (Fig. 2b). tgfβ subtypes in CO-fed hearts remained similar to chow-fed hearts (Fig. 2b). To quantify changes in collagen subtypes, immunoblotting revealed a global decrease of both collagen I and III in both HF diet groups compared with chow-fed mice. However, between the two HF diets, CO-fed mouse hearts demonstrated increased collagen I and decreased collagen III compared with OO-fed mouse hearts (Fig. 2, c and d). In contrast, collagen III was attenuated in the CO-fed mouse hearts but not in OO-fed mouse hearts (Fig. 2, c and d). Taken together, such differences between the two HF diets led to a 3-fold increase in collagen I to III ratio in CO compared with OO-fed hearts (Fig. 2e).
FIGURE 2.
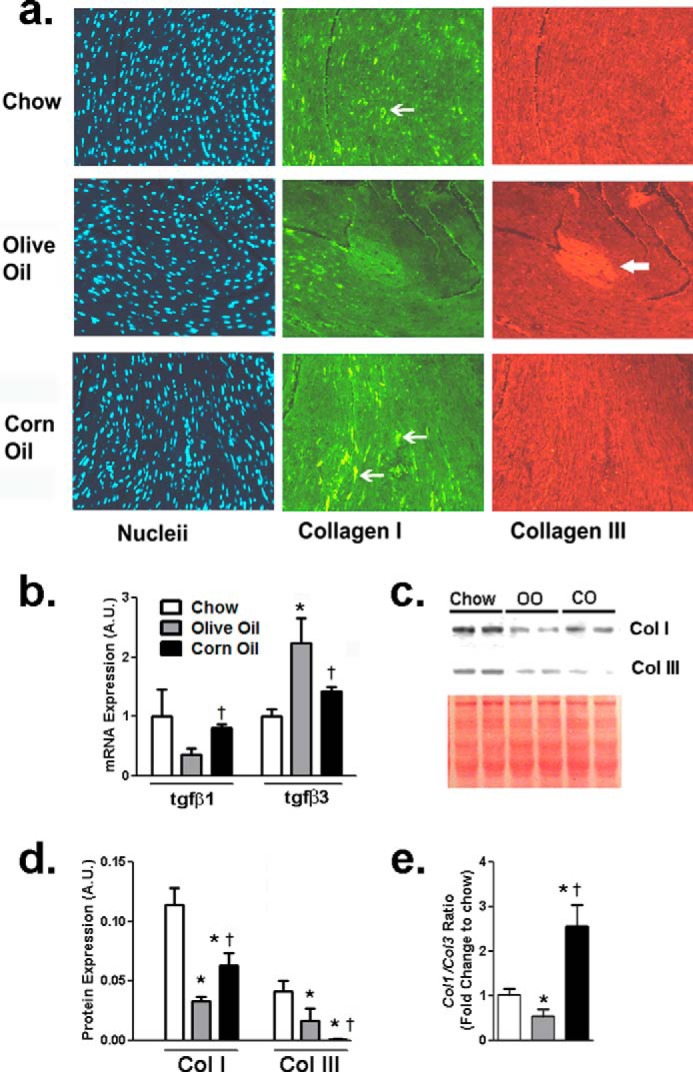
Corn oil alters collagen subtypes in the mouse heart. a, distribution and localization of collagen I and III in the heart across diet groups via double immunolabeling. Chow mouse hearts depicted low collagen I (fine arrow) with negligible collagen III positivity. OO increased diffuse collagen III across the myocardium (block arrow) and reduced punctate collagen I stains. CO hearts demonstrated the highest collagen I (fine arrows) with negligible collagen III stains. Magnification ×200. b, cardiac mRNA expression of tgfβ1 and tgfβ3 across diet groups. c, cardiac mRNA expression of col1α1 and col3α1. d, representative bands from Western blots for collagen (Col) I and III with total protein depicted by Ponceau stain. e, Western blot analysis of collagen I and III in mouse hearts normalized to total protein under various diets. Data were analyzed using one-way ANOVA with Tukey post hoc tests (n = 6 mice). *, p < 0.05 versus chow fed group. †, p < 0.05 versus corresponding olive oil fed group. A.U., arbitrary units; tgfβ, gene of tissue growth factor β.
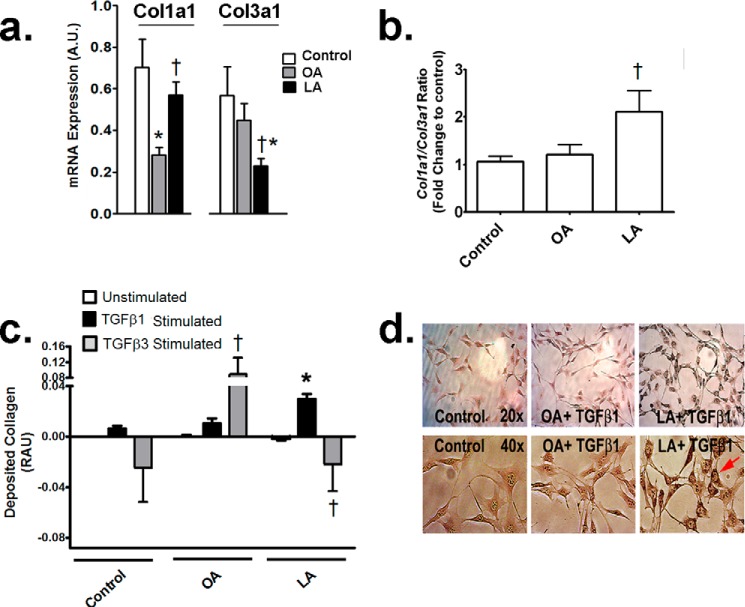
Linoleic Acid Increases Collagen in Response to TGFβ1 but Not TGFβ3 in Fibroblasts
To decipher underlying mechanisms of differential cardiac collagen subtypes induced by various diets in vivo, mouse NIH/3T3 fibroblasts were incubated with LA or OA, which represent the major fatty acids in CO- and OO-fed diets, respectively (Fig. 1a). In a high fat (40% energy) fed C57/Bl6 mouse, plasma levels of nonesterified LA and OA are∼0.5 and 1.2 mm/liter, respectively (21). In preliminary studies, 0.5 mm fatty acids induced toxicity in NIH/3T3 fibroblasts (data not shown), and only 0.1 mm fatty acid was used instead in all incubation experiments. 0.1 mm LA caused an increase in total col1a1 and a decrease in col3a1 within 24 h in fibroblasts (Fig. 3a). As a consequence, akin to in vivo, the ratio of collagen I to III gene expression was increased specifically in LA-treated fibroblasts within 24 h (Fig. 3b). Sirius Red staining can differentiate between soluble and deposited collagen and assess their interconversion in culture (22). With TGFβ1 stimulation, fibroblasts deposited more collagen with LA than OA-treated cells (Fig. 3c). To confirm these results, light microscopy revealed increased intracellular collagen deposits within fibroblasts treated with LA but not OA in response to TGFβ1 (Fig. 3d). In contrast, TGFβ3 stimulated collagen production in OA-treated cells only (Fig. 3c). Interestingly, with TGFβ3, LA induced even lower collagen deposition compared with untreated fibroblasts (Fig. 3c).
FIGURE 3.
Linoleic acid induces specific TGFβ and collagen isoforms in fibroblasts. a, col1a 1 and col3a1 expression following 24 h of treatment with 0.1 mm fatty acids. b, ratio of col1a1/col3a1 mRNA expression in fibroblasts with 0.1 mm fatty acids over 24 h. c, deposited collagen over 24 h following incubations with 0.1 mm fatty acids with or without 10 ng/ml TGFβ1 and TGFβ3. LA treatment increased collagen deposition following TGFβ1 but not TGFβ3 treatment. Conversely, TGFβ3 reduced collagen deposition with LA but increased with OA. RAU, relative absorbance units. d, representative micrographs of fibroblasts demonstrating collagen deposits with 0.1 mm fatty acids and 10 ng/ml TGFβ1. Upper panel magnification, ×200; lower panel magnification, ×400. LA increased collagen in fibroblasts under TGFβ1 stimulation. Experiments were repeated at least twice in triplicate. Data were analyzed using two-way ANOVA with Tukey post hoc tests, p < 0.05. *, p < 0.05 versus control cells. †, p < 0.05 versus corresponding treatments with OA. A.U., arbitrary units.
Overexpressing fads2 but Not fads1 Abolishes Collagen Deposition in LA-treated Fibroblasts
Having identified specific increases in collagen I and deposited collagen with LA both in vivo and in vitro so far, our next goal was to identify the n-6 PUFA moiety that drives such responses. In this regard, LA begins to undergo bioconversion to GLA and ultimately ARA using Δ6-desaturase encoded by the fatty acid desaturase 2 (fads2) gene (17). The ratio of LA to AA is used to study clinical outcomes associated with FADS activities (9). Table 6 depicts fatty acid composition of cells following fads2 overexpression. fads2 overexpression did not alter major fatty acids in fibroblasts either in BSA- or OA-treated fibroblasts compared with nontransfected controls. As expected, overexpression of fads2 significantly lowered LA and GLA, while increasing ARA within fibroblasts treated with LA only. This lowered the ratio of LA/ARA by ∼50% in fibroblasts treated with LA.
TABLE 6.
Fibroblast fatty acid composition with fads2 overexpression
Either nontransfected or stably transfected cells were incubated with BSA, 0.1 mm OA, or LA for 24 h. Data represent mean ± S.E. of relative peak area in gas chromatograms from three different experiments. Differences between cells with or without fads2 overexpression were analyzed by t tests.
| BSA | BSA + fads2 | OA | OA + fads2 | LA | LA + fads2 | |
|---|---|---|---|---|---|---|
| C16:0 | 27.8 ± 1.6 | 34.0 ± 0.7 | 22.4 ± 1.1 | 20.1 ± 6.6 | 29.2 ± 1.3 | 26.5 ± 0.8 |
| C16:1n10 | 3.3 ± 0.8 | 4.4 ± 0.1 | 4.0 ± 0.1 | 3.9 ± 1.8 | 3.1 ± 0.3 | 4.1 ± 0.6 |
| C16:1n9 | 2.8 ± 1.1 | 3.4 ± 0.1 | 2.9 ± 0.1 | 2.5 ± 0.9 | 3.1 ± 0.1 | 4.4 ± 0.5 |
| C18:1n9 | 23.9 ± 4.7 | 22.1 ± 1.3 | 49.2 ± 2.1 | 59.3 ± 9.8 | 17.2 ± 1.1 | 16.2 ± 0.2 |
| C18:2n6 | 2.2 ± 0.7 | 1.7 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.2 | 18.2 ± 0.9 | 12.6 ± 0.2a |
| C18:3n6 | ND | 0.6 ± 0.1 | 1.0 ± 0.1 | 1.6 ± 0.4 | 1.3 ± 0.2 | 0.8 ± 0.1 |
| C20:4n6 | 12.9 ± 1.5 | 10.9 ± 0.4 | 5.9 ± 0.2 | 4.4 ± 1.6 | 10.3 ± 1.2 | 16.2 ± 0.4 |
| C18:2/C20:4 ratio | 0.17 ± 0.1 | 0.16 ± 0.0 | 0.16 ± 0.1 | 0.23 ± 0.2 | 1.29 ± 0.4 | 0.70 ± 0.1a |
a p < 0.05 versus nontransfected cells with the same fatty acid treatment. ND, not detectable.
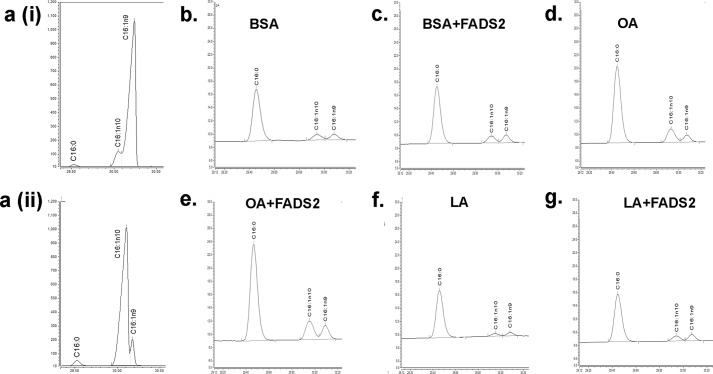
Some earlier studies had suggested that Δ6-desaturase, the enzyme encoded by fads2, can lead to increased bioconversion of palmitate to sapienic acid (C16:1n10) in the human sebaceous gland (23) and even in COS-7 cells (24). However, in this study, cells treated with BSA (Fig. 4, b and c), OA (Fig. 4, d and e), or LA (Fig. 4, f and g) had identical levels of both palmitate and total 16:1 (both 16:1n-9 and 16:1n-10) with or without fads2 overexpression (also Table 6). Therefore, any effects observed with fads2 overexpression is unlikely to be due to either a loss of palmitate or an increase in sapienic acids in fibroblasts.
FIGURE 4.
Overexpression of fads2 does not alter 16:1n-10 in fibroblasts. a, characterization of gas chromatographic separations for 16:1n9 and 16:1n10. Panel i, representative chromatogram of a spiked fibroblast cell extract with 1 μg of 16:1n9. Panel ii, representative chromatogram of a spiked fibroblast cell extract with 1 μg of 16:1n10. Palmitate (C16:0) is also depicted in panels i and ii for positional reference. Representative chromatograms of C16:0, C16:1n9, and C16:1n10 in untransfected NIH/3T3 cell extracts treated with BSA (b), OA (d) and LA (f). Impact of fads2 overexpression in NIH/3T3 cells treated with BSA (c), OA (e) and LA (g). Overexpression of fads2 did not affect either C16:0, C16:1n9, or 16:1n10 content in any group. Experiments were repeated at least twice in triplicate. Table 6 depicts numerical values for these data. fads2, fatty acid desaturase 2.
Regarding collagen, overexpression of fads2 prevented collagen deposition with and without TGFβ1 stimulation in LA- but not with OA-treated fibroblasts (Fig. 5a). Interestingly, fads2 overexpression lowered collagen deposition with both OA and LA under TGFβ3 stimulation as well (Fig. 5b). To negate nonspecific effects of fads2 overexpression, we overexpressed fads1, which is not directly involved in LA desaturation, in fibroblasts (17). Overexpression of fads1 did not affect collagen deposition in fibroblasts with either fatty acid (Fig. 5c), thus indicating that the increased collagen deposition with LA was due to LA itself and not to other downstream n-6 PUFAs.
FIGURE 5.
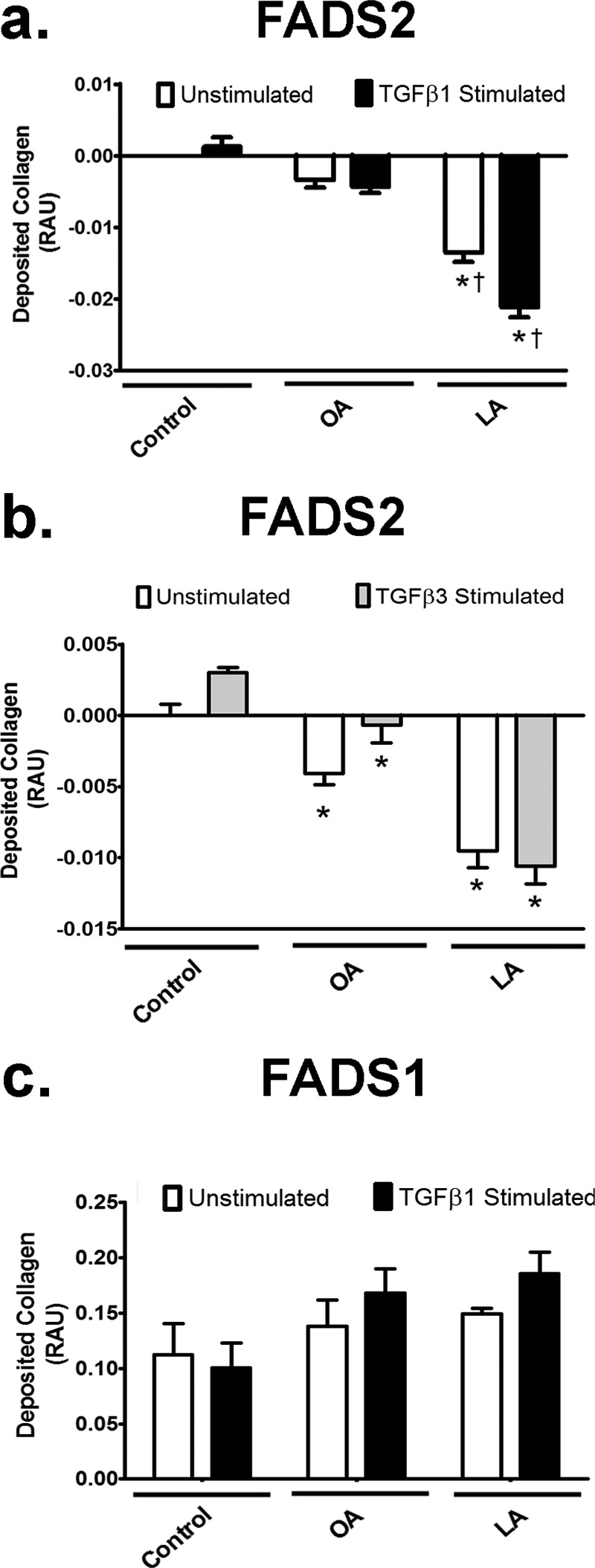
Overexpression of fads2 but not fads1 reduces fibroblast collagen deposition with LA. a, impact of fads2 overexpression in fatty acid-treated cells with or without 10 ng/ml TGFβ1 on collagen deposition. fads2 overexpression lowers collagen deposition with and without TGFβ1 with LA but not OA. b, impact of fads2 overexpression in NIH/3T3 cells with or without 10 ng/ml TGFβ3 on collagen deposition. fads2 overexpression lowers collagen deposition with both OA and LA, with or without TGFβ3. c, impact of fads2 overexpression in NIH/3T3 cells with or without 10 ng/ml TGFβ1 on collagen deposition. Unlike fads2, overexpression of fads1 does not affect collagen deposition in cells treated with either OA or LA, with or without TGFβ1. Experiments were repeated at least twice in triplicate. Data are presented relative to untreated control. Data were analyzed using two-way ANOVA with Tukey post hoc tests, p < 0.05. *, p < 0.05 versus control cells. †, p < 0.05 versus corresponding OA-treated cells. RAU, relative absorbance units; fads1, fatty acid desaturase 1; fads2, fatty acid desaturase 2.
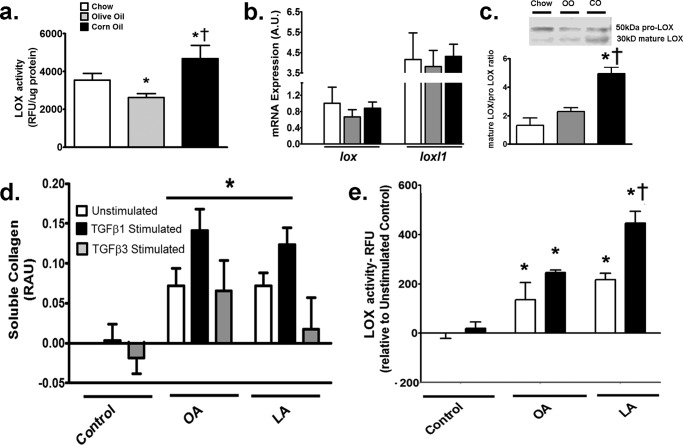
LOX Activity Is Augmented with LA Both in Vivo and in Vitro
LOX is a copper-dependent amine oxidase that promotes cross-linking of collagen fibrils and therefore the deposition of insoluble collagen fibers from soluble collagen (25). First, we sought to determine LOX activity in the hearts of various fat-fed mice. In vivo, LOX activity was higher in CO-fed than in OO-fed hearts (Fig. 6a). To identify the mechanism of LOX induction in vivo, gene expression of lox and LOX-like protein-1 (loxl1), the major genes contributing to LOX activity in the myocardium (26), were measured in mouse hearts. Gene expression of either protein was unaltered with dietary intervention in any group of mice (Fig. 6b). As LOX proteins can also undergo post-translational modifications whereby prolysyl oxidase (50 kDa) is cleaved to the active, mature LOX (30 kDa), Western blotting was performed to identify these two fractions (26). CO feeding led to the highest increase in mature LOX to prolysyl oxidase ratio in mouse hearts (Fig. 6c).
FIGURE 6.
LOX activity is increased with LA both in vivo and in vitro. a, LOX activity in hearts of mice fed various diets. Corn oil increased whereas olive oil decreased LOX activity compared with chow-fed mice. b, mRNA expression of lox and loxl1 in hearts of mice. No change in either gene was noted with any diet. c, protein expression of prolysyl oxidase and mature LOX from Western blot of LOX in vivo. Inset, representative bands of prolysyl oxidase (50 kDa) and mature LOX (30 kDa). Corn oil-fed hearts demonstrated higher mature 30-kDa fraction of LOX compared with other groups. d, soluble collagen after 24-h incubations with 0.1 mm fatty acids with or without 10 ng/ml TGFβ1 and TGFβ3. Soluble collagen was increased with TGFβ1 in both LA- and OA-treated cells, whereas TGFβ3 only increased soluble collagen in the presence of OA. e, LOX activity in fibroblasts treated with 0.1 mm fatty acids for 24 h with or without TGFβ1. LOX activity was the highest in LA-treated fibroblasts with TGFβ1. In vitro experiments were repeated at least twice in triplicate. Data were analyzed using two-way ANOVA with Tukey post hoc tests, p < 0.05. *, p < 0.05 versus corresponding control cells. †, p < 0.05 versus corresponding OA-treated cells. lox, gene for LOX; loxl1, gene for lysyl oxidase like 1; RFU, relative fluorescence units; fads2, fatty acid desaturase 2.
In vitro, to identify LOX activity, its substrate, i.e. soluble collagen, was assessed in fibroblasts incubated with fatty acids. Soluble collagen increased similarly with both OA and LA under TGFβ1 stimulation (Fig. 6d). As described earlier with deposited collagen (Fig. 3c), TGFβ3 also failed to increase soluble collagen (Fig. 6d) in response to LA, and it was not included in further studies to assess collagen dynamics with LA or other n-6 PUFAs. With regard to LOX, as LA increases collagen deposition under TGFβ1 (Fig. 3c), we hypothesized an increase in LOX activity in response to LA and TGFβ1, which would generate the insoluble (i.e. deposited) collagen. Without stimulation, LOX activity was increased with both OA and LA incubation (Fig. 6e). However, as predicted, TGFβ1 led to a further increase in LOX activity in LA-treated fibroblasts only (Fig. 6e).
To verify the specific impact of LA and its relationship to LOX, fads2-overexpressing fibroblasts were again utilized. OA-treated cells did not demonstrate any change in LOX activity under increasing doses of this fatty acid or with fads2 overexpression (Fig. 7a). In contrast, increasing doses of LA from 0.05 to 0.2 mm led to a dose-dependent increase in LOX activity in LA-treated cells (Fig. 7b). In contrast, cells overexpressing fads2 lowered and completely abolished LOX activities when incubated with 0.2 mm for 24 h (Fig. 7b). Western blotting of prolysyl oxidase and mature LOX in nontransfected and fads2-transfected fibroblasts revealed higher mature LOX in nontransfected fibroblasts treated with LA (Fig. 7c), followed by a significant down-regulation of mature LOX with fads2 overexpression. In contrast, OA-treated cells did not demonstrate any change in either prolysyl oxidase or mature LOX with fads2 overexpression. In conjunction with Table 6, which demonstrates a specific down-regulation of LA and an increase in ARA with fads2 overexpression, these data suggest that LOX activity and maturation is directly related to cellular LA levels in fibroblasts.
FIGURE 7.
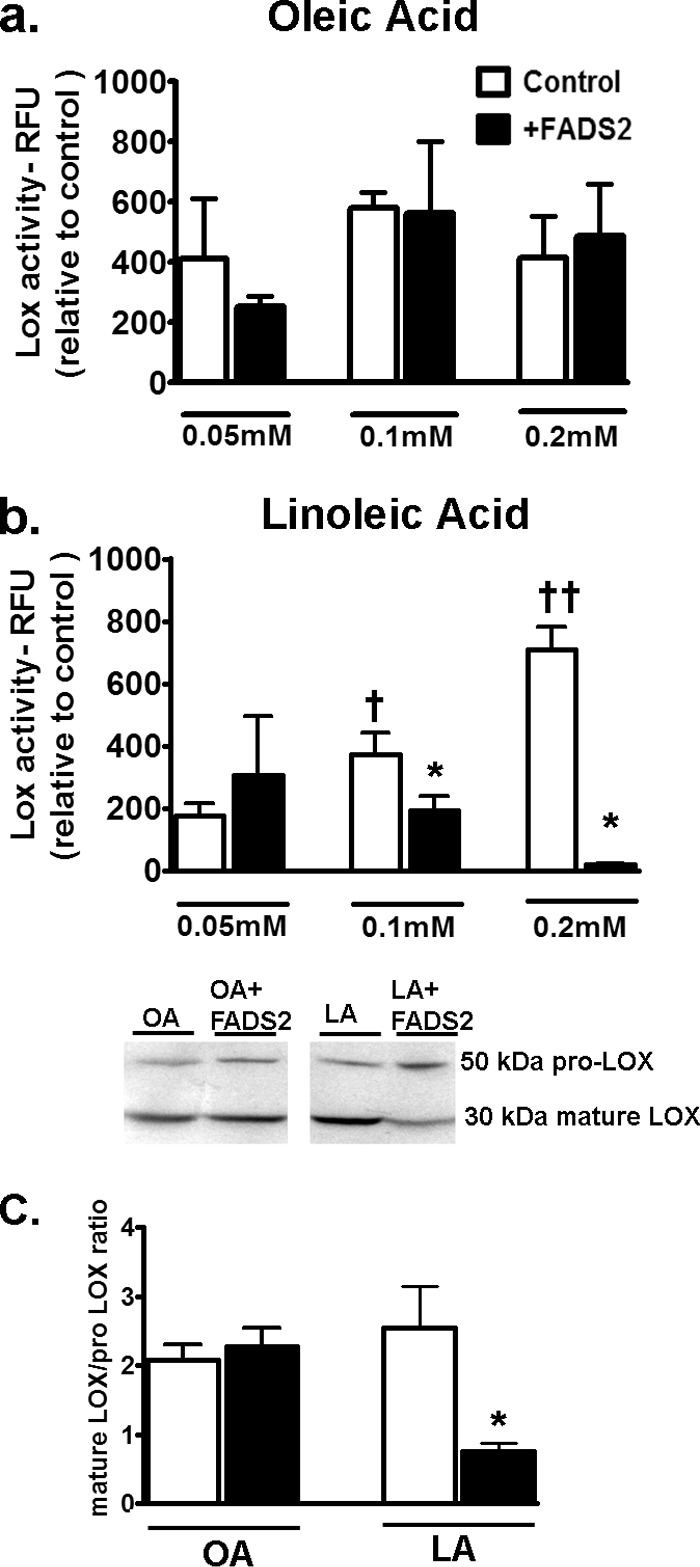
Effect of fads2 overexpression on LOX activity in fibroblasts. Control or stably fads2 transfected fibroblasts were treated with 0.05, 0.1, or 0.2 mm of OA (a) and LA (b) for 24 h. Experiments were repeated at least twice in triplicate. Overexpression of fads2 abolished LOX activity in LA-treated fibroblasts. c, representative Western blots for LOX across 0.2 mm fatty acid treatments. Mature 30-kDa fraction of LOX was profoundly inhibited by fads2 overexpression with LA. Data were analyzed using two-way ANOVA with Tukey post hoc tests, p < 0.05. *, p < 0.05 versus corresponding untransfected cells. †, p < 0.05 versus corresponding 0.05 mm cells.††, p < 0.05 versus corresponding 0.1 mm cells. RFU, relative fluorescence units; fads2, fatty acid desaturase 2.
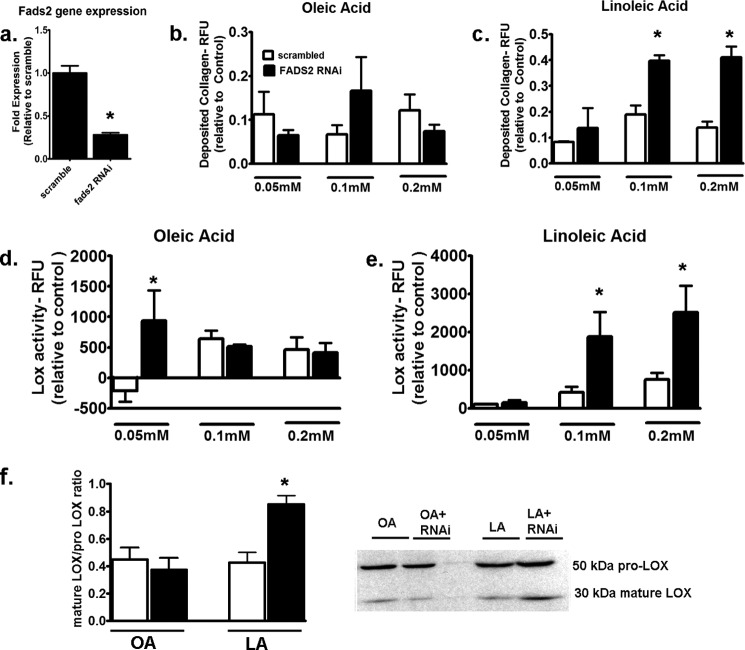
Knockdown of fads2 Exacerbates LA-mediated Fibrogenesis
To confirm the role of fads2 in LA-mediated induction of collagen deposition and LOX activity, we performed RNAi-mediated knockdown of fads2 in fibroblasts. fads2 gene levels were reduced by ∼70% with RNAi (Fig. 8a). Incubation with incremental doses of OA did not change collagen deposition when stimulated with TGFβ1 (Fig. 8b). In contrast, fads2 RNAi led to almost doubling of collagen deposition when incubated with either 0.1 or 0.2 mm LA (Fig. 8c). Similar effects were noted with LOX activity with OA in the presence of fads2 RNAi where only the lowest concentration of OA at 0.05 mm led to an increase in LOX activity (Fig. 8d). However, at higher concentrations of fatty acids (i.e. at 0.1 and 0.2 mm), LA demonstrated augmented LOX activity with fads2 RNAi (Fig. 8e). Western blotting of these fibroblasts revealed a significant up-regulation of the mature form when fads2 RNAi-treated fibroblasts were incubated with 0.2 mm LA but not with OA (Fig. 8f). These data suggest that inhibition of fads2 directly increases both maturation of LOX and its enzymatic activity and results in augmented collagen deposition with LA.
FIGURE 8.
Effect of fads2 RNAi on collagen deposition and LOX activity in fibroblasts. a, fads2 gene levels after incubation with scrambled or fads2 RNAi in fibroblasts. b, deposited collagen in fibroblasts treated with incremental doses of OA (b) or LA (c) in the presence of either scrambled or fads2 RNAi after 24 h. No effect of fads2 RNAi was noted with OA, but it increased collagen deposition with both 0.1 and 0.2 mm LA. LOX activity in fibroblasts treated with incremental doses of OA (d) or LA (e) in the presence of either scrambled or fads2 RNAi after 24 h. OA demonstrated increased LOX activity at 0.05 mm, whereas LA demonstrated increased LOX activity at 0.1 and 0.2 mm under fads2 RNAi. f, Western blots for LOX from fibroblasts with fads2 RNAi with 0.2 mm fatty acids. fads2 RNAi induced significant increase in mature/prolysyl oxidase levels with LA but not OA. Experiments were repeated at least twice in triplicate. Data were analyzed using two-way ANOVA with Tukey post hoc tests, p < 0.05. *, p < 0.05 versus corresponding scrambled controls. RFU, relative fluorescence units; fads2, fatty acid desaturase 2.
Downstream n-6 PUFA Reduces Deposited Collagen in the Presence of TGFβ1
To confirm our results with fads2 overexpression, fibroblasts were directly incubated with 0.1 mm GLA or ARA, which are the downstream n-6 PUFAs of LA. Compared with LA, GLA and ARA induced lower deposited collagen (Fig. 9a). Furthermore, unlike LA, TGFβ1 stimulation had no effect on collagen deposition in GLA- or ARA-treated cells (Fig. 9a). Overexpression of fads2 inhibited collagen following TGFβ1 stimulation in LA-treated cells but had no effect on GLA- or ARA-treated cells (Fig. 9b). Measurement of LOX activity revealed lower activity in GLA and an almost 3-fold reduction in activity in AA-treated fibroblasts compared with LA (Fig. 9c). Overexpression of fads2 also lowered LOX activity in LA-treated cells alone and had no effect on either GLA- or AA-treated fibroblasts (Fig. 9c). These results confirmed that downstream n-6 PUFAs like GLA or ARA reduce collagen deposition in fibroblasts compared with LA and reinforce the predominant role of LA, the parent n-6 PUFA, in driving collagen deposition under TGFβ1 stimulation.
FIGURE 9.
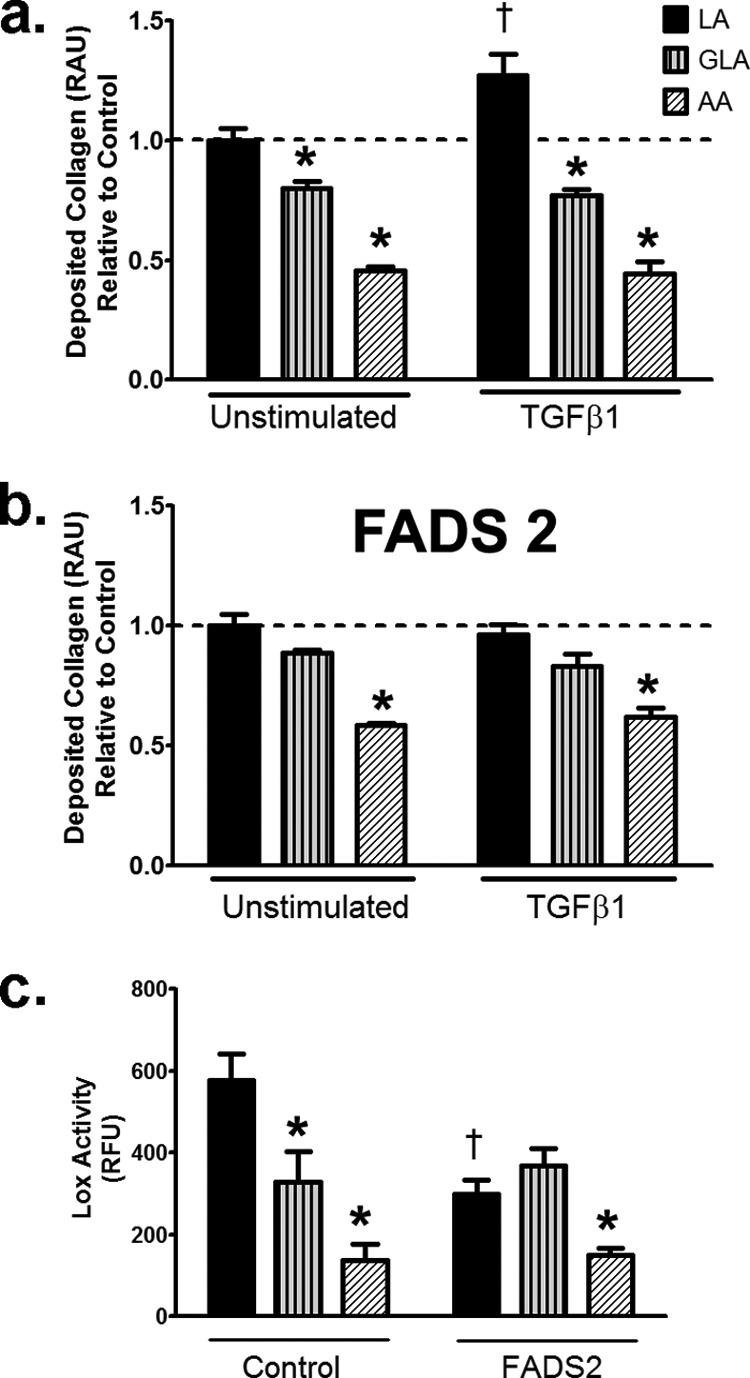
n-6 PUFAs, GLA, and AA does not induce collagen deposition in fibroblasts. a, deposited collagen with 0.1 mm fatty acids with or without TGFβ1 for 24 h. b, deposited collagen in fads2 overexpressing fibroblasts with 0.1 mm fatty acids with or without TGFβ1 for 24 h. c, LOX activity in normal and fads2 overexpressing fibroblasts with 0.1 mm fatty acids and TGFβ1 for 24 h. Experiments were repeated at least twice in triplicate. Data are expressed as a percentage of control. Data were analyzed by two-way ANOVA with Tukey post hoc tests, p < 0.05. *, p < 0.05 versus corresponding LA treated cells. †, p < 0.05 versus corresponding unstimulated cells. RAU, relative absorbance units, RFU, relative fluorescence units, fads2, fatty acid desaturase 2.
Discussion
Effects of lipids on heart disease have been extensively studied in animal models. The usual mechanism for lipotoxicity involves generation of ceramide and reactive oxygen species by palmitic acid (or other saturated fatty acids) leading to ectopic lipid deposition and heart failure (27). However, these pathways, based on saturated fats, may only partially explain the current heart disease pandemic in the Western world (1). This is because dietary saturated fats are extensively substituted by n-6 PUFA in the Western diet, and the intake of saturated fats has been relatively constant in the Western population for some time (28). In this regard, the intake of n-6 PUFA has increased from 2.79 to 7.21% energy intake in North America over the last century (28). However, unlike saturated fats, the cardiotoxic potential of n-6 PUFA remains less well defined. We and others have demonstrated that n-6 PUFA overload induces oxidative stress (6, 29, 30), inflammation (7), and mitochondrial damage (31, 32) in endothelial cells and cardiomyocytes. Although it is conceivable that any of the above could alter cardiac remodeling, n-6 PUFA-mediated effects on cardiac fibrosis remain unclear. Here, we present evidence that LA, but not other downstream PUFAs, induce specific TGFβ isoforms and increase the collagen I/III ratio and LOX activity both in vivo and in vitro. Such changes in collagen subtypes induce cardiac stiffening in vivo, leading to early diastolic dysfunction within 5 weeks, without altering systolic function.
In recent years, several clinical reports profess a paradoxical association between increased dietary n-6 PUFA and either reduced survival or no benefit following cardiac injury (2, 3, 33). These reports are in disagreement with other analyses showing that n-6 PUFA lowers coronary heart disease by lowering cholesterol, atherosclerosis progression, and coronary heart disease mortality (4). As shown in this study, LA-mediated cardiac changes may be mediated by alterations of the collagen I/III ratio in the heart muscle itself and not atherosclerotic properties. This is because the C57Bl6 mice as used in this study are resistant to atherosclerosis/fatty streaks with a short term HF diet (34). It is also curious that a negative impact of n-6 PUFA on cardiac function has not been reported earlier. This could be because mitral valve flow measurements are not the standard practice during echocardiography in animal studies (35), and they are difficult to assess using in situ working heart preparations. It has been known for some time that changes in the collagen I/III ratio could lead to early diastolic dysfunction in the absence of changes in commonly assessed systolic or diastolic parameters (20). Clinically, such occurrence is steadily increasing among heart failure patients. It is predicted that heart failure with preserved ejection fraction will be a leading type of heart failure within a decade (36).
Type I and III collagens include ∼90% of all collagen within the myocardium (37). Physically, in a single fiber, collagen I is around 37% stiffer than collagen III (38). It is considered that collagen I provides rigidity, whereas collagen III promotes elasticity of the cardiac muscle (39). Therefore, instead of total collagen, collagen subtypes dictated by TGFβ isoforms dictate cardiac stiffness (40). Although TGF-β1 stimulates collagen I production (41), little is known about the role of TGF-β3 in the post-natal heart. Taking cues from its role in the skin, where TGF-β3 attenuates TGF-β1 and collagen I production (42–44) and appears up to 48 h after TGF-β1 expression (45), it is tempting to speculate that TGF-β3 might act to “balance” the production of the noncompliant collagen I expression with the flexible collagen III production or prevent further collagen deposition altogether. Indeed, in avian hearts, specific TGF-β3 stimulation reduces compaction of the extracellular matrix (46).
LOX is an extracellular amine oxidase that plays a crucial role in cross-linking collagen fibrils and deposition of insoluble collagen (25). Excess LOX, fibrillar collagen cross-linking, and fiber deposition are characteristics of heart failure (47). Moreover, TGFβ1 and LOX are known to cross-enhance each other's activities (48). Soluble collagen is nonaggregated, whereas deposited collagen represents aggregated collagen fibers. LOX catalyzes the oxidation of the amino groups in lysine or hydroxylysine residues, resulting in the formation of aldehydes, which react with each other or with another amino group resulting in cross-linking two polypeptide chains.
In this study, both high fat diets did not induce a change in gene expression of either lox or loxl1 genes in mouse hearts, which are the primary proteins contributing to LOX activities in the adult heart muscle (49). In contrast, corn oil-based diets did induce augmented LOX maturation from the proenzyme form (50 kDa) to the mature form (30 kDa). Such results were then repeated in vitro in fibroblasts, where gene expressions of either LOX or LOXL1 did not change (data not shown), but increased maturation of LOX was noted with conditions that promoted LA (either direct incubation of LA or RNAi mediated inhibition of fads2 with LA in the incubation medium). These results are supported by an earlier study, where a high fat, high sucrose diet induced LOX activity in mouse hearts by promoting LOX maturation, independent of its mRNA levels, via T cell activation (26). Although unknown in the heart, we recently demonstrated T cell recruitment in the gut with an LA-rich diet (10). In an earlier study, the increased dietary n-6 to n-3 PUFA ratio was shown to aggravate cross-linking of collagen in the bone during osteoarthritis (50). Despite such evidence, the direct effect of LA or other n-6 PUFA on cardiac LOX remains unclear. As LOX was also activated in fibroblasts with direct incubations with LA, this indicates that LA, rather than any other component of corn oil, might be directly responsible for up-regulation of LOX activity. In this regard, it was demonstrated earlier that oxidized LA promotes cross-linking of collagen in rat tail tendons (51). In contrast, ARA-derived prostaglandin E2 or prostacyclin can down-regulate lysyl oxidase expression or collagen production in lung (52) and cardiac fibroblasts (53), respectively. These opposing effects of LA and ARA on LOX activities are supported in our study as overexpression of FADS2-lowered LA, increased ARA, and simultaneously reduced LOX activity in fibroblasts. Interestingly, in an earlier study, ARA directly inhibited TGFβ activity as well (54).
Unlike PUFA, the role of OA in fibrosis is clearer. Similar to this study, olive oil reduces both TGFβ1 and collagen I in the liver after injury (55). Collagen III mRNA levels were also increased in olive oil-fed mouse hearts in this study. This result is in agreement with an earlier study describing the same in MUFA-fed BALB/c mouse skin following wounding (56). We also show that olive oil did not induce gene levels of LOX proteins in mouse hearts. Moreover, we found that OA up to 0.2 mm did not affect LOX processing in fibroblasts. In an earlier study, higher levels of oleate (0.8 mm) were required for LOX activity with collagen in vitro (57). Therefore, up-regulation of collagen III mRNA by olive oil/OA, reduction of collagen I/III ratio, and inhibition of LOX activities are novel cardioprotective pathways induced by the OO-rich Mediterranean diets (55, 56).
In this study, both fads1 and fads2 overexpression strategies were incorporated to clarify the role of various PUFAs on collagen dynamics in vitro. Although single nucleotide polymorphisms (SNPs) that alter fads activities do alter plasma free fatty acid profiles (58), the role of fads on cardiac events remain unclear. The reported effects with fads SNPs range from nil (59) to detrimental (9) to beneficial (60). Usually, increased desaturase activities that increase AA are blamed for coronary artery diseases and atherosclerosis through pro-inflammatory pathways (9). However, lower activity of fads2 can also induce higher oxidative stress markers in healthy nonobese middle aged men (61), which is in line with earlier studies showing pro-oxidant effects in the myocardium with an LA-rich diet (5–7). In this study, RNAi-mediated inhibition of fads2 also augmented LOX maturation and activity under incubation with LA. In a recent study, cardiac collagen production was diminished by increased activation of fads1/2 and elevated ARA accumulation in a rat model of hypertension. Not surprisingly, such effects were also down-regulated by knockdown of fads1 or fads2 in cultured cardiac fibroblasts in the same study (53). Based on such results, investigations of fads SNPs in clinical populations may reveal significant associations with cardiac fibrosis and remodeling.
In summary, we demonstrate that n-6 PUFA-rich corn oil diets alter the collagen I to III ratio and LOX activity, which can have a functional consequence in the heart within 5 weeks (Fig. 10). Although the nutritional requirement of n-6 PUFA is only 1% energy (62), current clinical recommendations propose 5–10% energy intake. Thus, these findings represent a novel pathway of cardiolipotoxicity associated with LA, a fatty acid increasingly present in Western diets (28). If this mechanism holds true in humans, decreasing dietary LA and increasing OA (as in traditional Mediterranean diets) could be an effective preventative strategy to limit adverse cardiac remodeling. Alternatively, pharmacologically increasing the TGFβ3/collagen III axis represents an attractive therapeutic target to confer a more “elastic” phenotype to the failing myocardium. Beyond the heart, increased collagen I/III ratio can induce adverse clinical outcomes in fibrotic diseases like cirrhosis of the liver (63), pancreatitis (64), and respiratory distress in the lungs (65). Whether soaring dietary LA influences the progression of such diseases as well remains an attractive but unexplored area of research.
FIGURE 10.
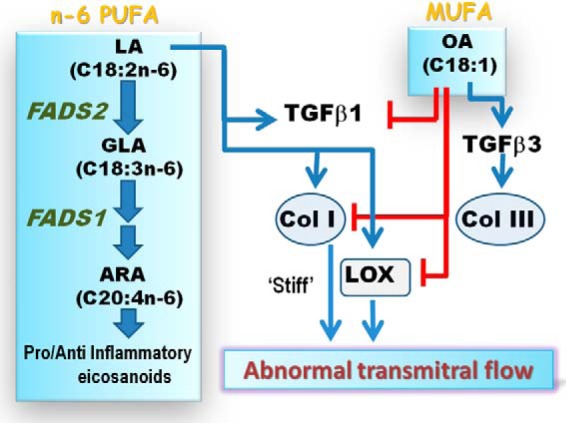
Proposed schematic of the opposing roles of linoleic and oleic acids on TGFβ-collagen axis. The parent n-6 polyunsaturated fatty acid, linoleic acid (LA, blue arrows), activates TGFβ1 and induces collagen I and promotes LOX maturation and activity. Together, LOX forms insoluble collagen fibers and induces a stiff myocardium that may induce abnormal transmitral flow, indicative of early diastolic dysfunction. Downstream PUFAs of LA, generated by fads2 or fads1 action, were not found to have significant roles in this axis. Oleic acid (blue arrow), a monounsaturated fatty acid, induces TGFβ3 and collagen III as a direct effect. However, OA can also block LA action (red lines) by directly inhibiting TGFβ1, collagen I, and LOX activity, thereby promoting an elastic myocardial phenotype.
Author Contributions
This project was primarily carried out by J. B., who also wrote the first draft. Animal work and revisions were carried out by A. B., who also contributed to the writing of the revised version. J. Y. performed the RNAi experiments. H. S. performed echocardiography and analyzed data. B. J. M. performed gene expression and Western blotting. M. F. constructed the fads2-overexpressing cell line. K. M. .M. critically reviewed the data and the manuscript. The core idea, funding, coordination, and writing of the final draft of the manuscript was performed by S. G.
This work was supported by operating grants from Canadian Diabetes Association (to S. G. and K. M. M.). The authors declare that they have no conflicts of interest with the contents of this article.
- PUFA
- polyunsaturated fat
- GLA
- γ-linolenic acid
- ARA
- arachidonic acid
- LOX
- lysyl oxidase
- LA
- linoleic acid
- OA
- oleic acid
- HF
- high fat
- CO
- corn oil
- OO
- olive oil
- MUFA
- monounsaturated fatty acid
- AA
- arachidonic acid
- ANOVA
- analysis of variance
- LV
- left ventricle
- FADS
- fatty acid desaturase
- SNP
- single nucleotide polymorphism.
References
- 1. Siri-Tarino P. W., Sun Q., Hu F. B., Krauss R. M. (2010) Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 91, 502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramsden C. E., Hibbeln J. R., Majchrzak S. F., Davis J. M. (2010) n-6 fatty acid-specific and mixed polyunsaturated dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br. J. Nutr. 104, 1586–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramsden C. E., Zamora D., Leelarthaepin B., Majchrzak-Hong S. F., Faurot K. R., Suchindran C. M., Ringel A., Davis J. M., Hibbeln J. R. (2013) Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 346, e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris W. S., Poston W. C., Haddock C. K. (2007) Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 193, 1–10 [DOI] [PubMed] [Google Scholar]
- 5. Ochoa J. J., Quiles J. L., Huertas J. R., Mataix J. (2005) Coenzyme Q10 protects from aging-related oxidative stress and improves mitochondrial function in heart of rats fed a polyunsaturated fatty acid (PUFA)-rich diet. J. Gerontol. A Biol. Sci. Med. Sci. 60, 970–975 [DOI] [PubMed] [Google Scholar]
- 6. Ghosh S., Kewalramani G., Yuen G., Pulinilkunnil T., An D., Innis S. M., Allard M. F., Wambolt R. B., Qi D., Abrahani A., Rodrigues B. (2006) Induction of mitochondrial nitrative damage and cardiac dysfunction by chronic provision of dietary ω-6 polyunsaturated fatty acids. Free Radic. Biol. Med. 41, 1413–1424 [DOI] [PubMed] [Google Scholar]
- 7. Ghosh S., Novak E. M., Innis S. M. (2007) Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 α-linolenic acid ratios in normal, fat-fed pigs. Am. J. Physiol. Heart Circ. Physiol. 293, H2919–H2927 [DOI] [PubMed] [Google Scholar]
- 8. Galvao T. F., Brown B. H., Hecker P. A., O'Connell K. A., O'Shea K. M., Sabbah H. N., Rastogi S., Daneault C., Des Rosiers C., Stanley W. C. (2012) High intake of saturated fat, but not polyunsaturated fat, improves survival in heart failure despite persistent mitochondrial defects. Cardiovasc. Res. 93, 24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinelli N., Girelli D., Malerba G., Guarini P., Illig T., Trabetti E., Sandri M., Friso S., Pizzolo F., Schaeffer L., Heinrich J., Pignatti P. F., Corrocher R., Olivieri O. (2008) FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am. J. Clin. Nutr. 88, 941–949 [DOI] [PubMed] [Google Scholar]
- 10. Ghosh S., Molcan E., Decoffe D., Dai C., Gibson D. L. (2013) Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br. J. Nutr. 2013; 110, 515–523 [DOI] [PubMed] [Google Scholar]
- 11. Ram R., Mickelsen D. M., Theodoropoulos C., Blaxall B. C. (2011) New approaches in small animal echocardiography: imaging the sounds of silence. Am. J. Physiol. Heart Circ. Physiol. 301, H1765–H1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Little W. C., Ohno M., Kitzman D. W., Thomas J. D., Cheng C. P. (1995) Determination of left ventricular chamber stiffness from the time for deceleration of early left ventricular filling. Circulation 92, 1933–1939 [DOI] [PubMed] [Google Scholar]
- 13. Kang J. X., Wang J. (2005) A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem. 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Botta A., Laher I., Beam J., Decoffe D., Brown K., Halder S., Devlin A., Gibson D. L., Ghosh S. (2013) Short term exercise induces PGC-1α, ameliorates inflammation and increases mitochondrial membrane proteins but fails to increase respiratory enzymes in aging diabetic hearts. PLoS ONE 8, e70248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laher I., Beam J., Botta A., Barendregt R., Sulistyoningrum D., Devlin A., Rheault M., Ghosh S. (2013) Short-term exercise worsens cardiac oxidative stress and fibrosis in 8-month-old db/db mice by depleting cardiac glutathione. Free Radic. Res. 47, 44–54 [DOI] [PubMed] [Google Scholar]
- 16. Tullberg-Reinert H., Jundt G. (1999) In situ measurement of collagen synthesis by human bone cells with a Sirius Red-based colorimetric microassay: effects of transforming growth factor β2 and ascorbic acid 2-phosphate. Histochem. Cell Biol. 112, 271–276 [DOI] [PubMed] [Google Scholar]
- 17. Glaser C., Heinrich J., Koletzko B. (2010) Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism 59, 993–999 [DOI] [PubMed] [Google Scholar]
- 18. Lin S., Thomas T. C., Storlien L. H., Huang X. F. (2000) Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. Relat. Metab. Disord. 24, 639–646 [DOI] [PubMed] [Google Scholar]
- 19. Schmidt A. G., Gerst M., Zhai J., Carr A. N., Pater L., Kranias E. G., Hoit B. D. (2002) Evaluation of left ventricular diastolic function from spectral and color M-mode Doppler in genetically altered mice. J. Am. Soc. Echocardiogr. 15, 1065–1073 [DOI] [PubMed] [Google Scholar]
- 20. Kasner M., Westermann D., Lopez B., Gaub R., Escher F., Kühl U., Schultheiss H. P., Tschöpe C. (2011) Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 57, 977–985 [DOI] [PubMed] [Google Scholar]
- 21. Balogun K. A., Albert C. J., Ford D. A., Brown R. J., Cheema S. K. (2013) Dietary ω-3 polyunsaturated fatty acids alter the fatty acid composition of hepatic and plasma bioactive lipids in C57BL/6 mice: a lipidomic approach. PLoS ONE 8, e82399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C., Yang S., Zhang M., Zhang Z., Zhang B., Han D., Ma J., Wang X., Hong J., Guo Y., Okunieff P., Zhang L. (2013) In vitro Sirius Red collagen assay measures the pattern shift from soluble to deposited collagen. Adv. Exp. Med. Biol. 765, 47–53 [DOI] [PubMed] [Google Scholar]
- 23. Ge L., Gordon J. S., Hsuan C., Stenn K., Prouty S. M. (2003) Identification of the Δ-6 desaturase of human sebaceous glands: expression and enzyme activity. J. Invest. Dermatol. 120, 707–714 [DOI] [PubMed] [Google Scholar]
- 24. Guillou H., Rioux V., Catheline D., Thibault J. N., Bouriel M., Jan S., D'Andrea S., Legrand P. (2003) Conversion of hexadecanoic acid to hexadecenoic acid by rat Δ6-desaturase. J. Lipid Res. 44, 450–454 [DOI] [PubMed] [Google Scholar]
- 25. Smith-Mungo L. I., Kagan H. M. (1998) Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 16, 387–398 [DOI] [PubMed] [Google Scholar]
- 26. Zibadi S., Vazquez R., Larson D. F., Watson R. R. (2010) T lymphocyte regulation of lysyl oxidase in diet-induced cardiac fibrosis. Cardiovasc. Toxicol. 10, 190–198 [DOI] [PubMed] [Google Scholar]
- 27. Unger R. H., Orci L. (2000) Lipotoxic diseases of nonadipose tissues in obesity. Int. J. Obes. Relat. Metab. Disord. 24, S28–S32 [DOI] [PubMed] [Google Scholar]
- 28. Blasbalg T. L., Hibbeln J. R., Ramsden C. E., Majchrzak S. F., Rawlings R. R. (2011) Changes in consumption of ω-3 and ω-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 93, 950–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saraswathi V., Wu G., Toborek M., Hennig B. (2004) Linoleic acid-induced endothelial activation: role of calcium and peroxynitrite signaling. J. Lipid Res. 45, 794–804 [DOI] [PubMed] [Google Scholar]
- 30. Toborek M., Barger S. W., Mattson M. P., Barve S., McClain C. J., Hennig B. (1996) Linoleic acid and TNF-α cross-amplify oxidative injury and dysfunction of endothelial cells. J. Lipid Res. 37, 123–135 [PubMed] [Google Scholar]
- 31. Pepe S., Tsuchiya N., Lakatta E. G., Hansford R. G. (1999) PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am. J. Physiol. 276, H149–H158 [DOI] [PubMed] [Google Scholar]
- 32. Ghosh S., Qi D., An D., Pulinilkunnil T., Abrahani A., Kuo K. H., Wambolt R. B., Allard M., Innis S. M., Rodrigues B. (2004) Brief episode of STZ-induced hyperglycemia produces cardiac abnormalities in rats fed a diet rich in n-6 PUFA. Am. J. Physiol. Heart Circ. Physiol. 287, H2518–H2527 [DOI] [PubMed] [Google Scholar]
- 33. Schwingshackl L., Hoffmann G. (2014) Dietary fatty acids in the secondary prevention of coronary heart disease: a systematic review, meta-analysis and meta-regression. BMJ Open 4, e004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schreyer S. A., Wilson D. L., LeBoeuf R. C. (1998) C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 136, 17–24 [DOI] [PubMed] [Google Scholar]
- 35. Brainard R. E., Watson L. J., Demartino A. M., Brittian K. R., Readnower R. D., Boakye A. A., Zhang D., Hoetker J. D., Bhatnagar A., Baba S. P., Jones S. P. (2013) High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PLoS ONE 8, e83174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zouein F. A., de Castro Brás L. E., da Costa D. V., Lindsey M. L., Kurdi M., Booz G. W. (2013) Heart failure with preserved ejection fraction: emerging drug strategies. J. Cardiovasc. Pharmacol. 62, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bosman F. T., Stamenkovic I. (2003) Functional structure and composition of the extracellular matrix. J. Pathol. 200, 423–428 [DOI] [PubMed] [Google Scholar]
- 38. Collier P., Watson C. J., van Es M. H., Phelan D., McGorrian C., Tolan M., Ledwidge M. T., McDonald K. M., Baugh J. A. (2012) Getting to the heart of cardiac remodeling; how collagen subtypes may contribute to phenotype. J. Mol. Cell. Cardiol. 52, 148–153 [DOI] [PubMed] [Google Scholar]
- 39. Marijianowski M. M., van der Loos C. M., Mohrschladt M. F., Becker A. E. (1994) The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J. Am. Coll. Cardiol. 23, 1204–1208 [DOI] [PubMed] [Google Scholar]
- 40. Norton G. R., Tsotetsi J., Trifunovic B., Hartford C., Candy G. P., Woodiwiss A. J. (1997) Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation 96, 1991–1998 [DOI] [PubMed] [Google Scholar]
- 41. Pan X., Chen Z., Huang R., Yao Y., Ma G. (2013) Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PLoS ONE 8, e60335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah M., Foreman D. M., Ferguson M. W. (1994) Neutralising antibody to TGF-β1,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 107, 1137–1157 [DOI] [PubMed] [Google Scholar]
- 43. Shah M., Foreman D. M., Ferguson M. W. (1995) Neutralisation of TGF-β1 and TGF-β2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 108, 985–1002 [DOI] [PubMed] [Google Scholar]
- 44. Hosokawa R., Nonaka K., Morifuji M., Shum L., Ohishi M. (2003) TGF-β3 decreases type I collagen and scarring after labioplasty. J. Dent. Res. 82, 558–564 [DOI] [PubMed] [Google Scholar]
- 45. Briest W., Homagk L., Rassler B., Ziegelhöffer-Mihalovicová B., Meier H., Tannapfel A., Leiblein S., Saalbach A., Deten A., Zimmer H. G. (2004) Norepinephrine-induced changes in cardiac transforming growth factor-β isoform expression pattern of female and male rats. Hypertension 44, 410–418 [DOI] [PubMed] [Google Scholar]
- 46. Buskohl P. R., Sun M. J., Sun M. L., Thompson R. P., Butcher J. T. (2012) Serotonin potentiates transforming growth factor-β3 induced biomechanical remodeling in avian embryonic atrioventricular valves. PLoS ONE 7, e42527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. López B., Querejeta R., González A., Beaumont J., Larman M., Díez J. (2009) Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension 53, 236–242 [DOI] [PubMed] [Google Scholar]
- 48. Shanley C. J., Gharaee-Kermani M., Sarkar R., Welling T. H., Kriegel A., Ford J. W., Stanley J. C., Phan S. H. (1997) Transforming growth factor-β1 increases lysyl oxidase enzyme activity and mRNA in rat aortic smooth muscle cells. J. Vasc. Surg. 25, 446–452 [DOI] [PubMed] [Google Scholar]
- 49. López B., González A., Hermida N., Valencia F., de Teresa E., Díez J. (2010) Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am. J. Physiol. Heart Circ. Physiol. 299, H1–H9 [DOI] [PubMed] [Google Scholar]
- 50. Knott L., Avery N. C., Hollander A. P., Tarlton J. F. (2011) Regulation of osteoarthritis by ω-3 (n-3) polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthritis Cartilage 19, 1150–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hicks M., Delbridge L., Yue D. K., Reeve T. S. (1989) Increase in crosslinking of nonenzymatically glycosylated collagen induced by products of lipid peroxidation. Arch. Biochem. Biophys. 268, 249–254 [DOI] [PubMed] [Google Scholar]
- 52. Roy R., Polgar P., Wang Y., Goldstein R. H., Taylor L., Kagan H. M. (1996) Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: interactions among TGF-β, IL-1β, and prostaglandin E. J. Cell. Biochem. 62, 411–417 [DOI] [PubMed] [Google Scholar]
- 53. Omori Y., Ohtani T., Sakata Y., Mano T., Takeda Y., Tamaki S., Tsukamoto Y., Kamimura D., Aizawa Y., Miwa T., Komuro I., Soga T., Yamamoto K. (2012) l-Carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease. J. Hypertens. 30, 1834–1844 [DOI] [PubMed] [Google Scholar]
- 54. Ling T. Y., Huang Y. H., Lai M. C., Huang S. S., Huang J. S. (2003) Fatty acids modulate transforming growth factor-β activity and plasma clearance. FASEB J. 17, 1559–1561 [DOI] [PubMed] [Google Scholar]
- 55. Tanaka N., Kono H., Ishii K., Hosomura N., Fujii H. (2009) Dietary olive oil prevents carbon tetrachloride-induced hepatic fibrosis in mice. J. Gastroenterol. 44, 983–990 [DOI] [PubMed] [Google Scholar]
- 56. Cardoso C. R., Favoreto S. Jr., Oliveira L. L., Vancim J. O., Barban G. B., Ferraz D. B., Silva J. S. (2011) Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology 216, 409–415 [DOI] [PubMed] [Google Scholar]
- 57. Losty T. A., Miller D. S., O'Dell B. L. (1985) Modulation of lysyl oxidase substrate specificity by the oleate anion. Proc. Soc. Exp. Biol. Med. 179, 155–158 [DOI] [PubMed] [Google Scholar]
- 58. Gillingham L. G., Harding S. V., Rideout T. C., Yurkova N., Cunnane S. C., Eck P. K., Jones P. J. (2013) Dietary oils and FADS1-FADS2 genetic variants modulate [13C]α-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 97, 195–207 [DOI] [PubMed] [Google Scholar]
- 59. Baylin A., Ruiz-Narvaez E., Kraft P., Campos H. (2007) α-Linolenic acid, Δ6-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am. J. Clin. Nutr. 85, 554–560 [DOI] [PubMed] [Google Scholar]
- 60. Das U. N. (2007) A defect in the activity of Δ6 and Δ5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids 76, 251–268 [DOI] [PubMed] [Google Scholar]
- 61. Hong S. H., Kwak J. H., Paik J. K., Chae J. S., Lee J. H. (2013) Association of polymorphisms in FADS gene with age-related changes in serum phospholipid polyunsaturated fatty acids and oxidative stress markers in middle-aged nonobese men. Clin. Interv. Aging 8, 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collins F. D., Sinclair A. J., Royle J. P., Coats D. A., Maynard A. T., Leonard R. F. (1971) Plasma lipids in human linoleic acid deficiency. Nutr. Metab. 13, 150–167 [DOI] [PubMed] [Google Scholar]
- 63. Murata K., Kudo M., Onuma F., Motoyama T. (1984) Changes of collagen types at various stages of human liver cirrhosis. Hepatogastroenterology 31, 158–161 [PubMed] [Google Scholar]
- 64. Suda K., Tsukahara M. (1992) Histopathological and immunohistochemical studies on apparently uninvolved areas of pancreas in patients with acute pancreatitis. Arch. Pathol. Lab. Med. 116, 934–937 [PubMed] [Google Scholar]
- 65. Last J. A., Siefkin A. D., Reiser K. M. (1983) Type I collagen content is increased in lungs of patients with adult respiratory distress syndrome. Thorax 38, 364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]