ABSTRACT
Infections of domestic and wild birds with low-pathogenic avian influenza viruses (LPAIVs) have been associated with protective immunity to subsequent infection. However, the degree and duration of immunity in wild birds from previous LPAIV infection, by the same or a different subtype, are poorly understood. Therefore, we inoculated H13N2 (A/black-headed gull/Netherlands/7/2009) and H16N3 (A/black-headed gull/Netherlands/26/2009) LPAIVs into black-headed gulls (Chroicocephalus ridibundus), their natural host species, and measured the long-term immune response and protection against one or two reinfections over a period of >1 year. This is the typical interval between LPAIV epizootics in wild birds. Reinfection with the same virus resulted in progressively less virus excretion, with complete abrogation of virus excretion after two infections for H13 but not H16. However, reinfection with the other virus affected neither the level nor duration of virus excretion. Virus excretion by immunologically naive birds did not differ in total levels of excreted H13 or H16 virus between first- and second-year birds, but the duration of H13 excretion was shorter for second-year birds. Furthermore, serum antibody levels did not correlate with protection against LPAIV infection. LPAIV-infected gulls showed no clinical signs of disease. These results imply that the epidemiological cycles of H13 and H16 in black-headed gulls are relatively independent from each other and depend mainly on infection of first-year birds.
IMPORTANCE Low-pathogenic avian influenza viruses (LPAIVs) circulate mainly in wild water birds but are occasionally transmitted to other species, including humans, where they cause subclinical to fatal disease. To date, the effect of LPAIV-specific immunity on the epidemiology of LPAIV in wild birds is poorly understood. In this study, we investigated the effect of H13 and H16 LPAIV infection in black-headed gulls on susceptibility and virus excretion of subsequent infection with the same or the other virus within the same breeding season and between breeding seasons. These are the only two LPAIV hemagglutinin subtypes predominating in this species. The findings suggest that H13 and H16 LPAIV cycles in black-headed gull populations are independent of each other, indicate the importance of first-year birds in LPAIV epidemiology, and emphasize the need for alternatives to avian influenza virus (AIV)-specific serum antibodies as evidence of past LPAIV infection and correlates of protection against LPAIV infection in wild birds.
INTRODUCTION
Wild aquatic birds of the orders Anseriformes (mainly ducks, geese, and swans) and Charadriiformes (mainly gulls and waders) play a major role in the epidemiology of low-pathogenic avian influenza viruses (LPAIVs). Evidence to date indicates that LPAIV infection in these species is mainly a digestive tract infection and causes no clinical disease (1). LPAIVs are categorized into so-called subtypes based on their surface proteins hemagglutinin (HA) (H1 to H16) and neuraminidase (NA) (N1 to N9). From wild birds, these viruses may be transmitted occasionally to domestic animals and sporadically (usually indirectly via poultry) to humans, in which they can cause infections ranging from subclinical infection to fatal disease. For the epidemiology of most LPAIV subtypes, a major role is played by ducks, in which epizootics occur each fall (2, 3). However, there are two subtypes, H13 and H16, for which gulls, such as black-headed gulls (BHGU) (Chroicocephalus ridibundus), are the major reservoir (4–6). BHGU are furthermore special in that they are not commonly infected with other LPAIV subtypes, and epidemics are known to occur annually at the end of each breeding season at colony sites (6). This makes BHGU particularly suited to study the effect of multiple homologous and heterologous LPAIV infections on immunity. Despite numerous studies on the epidemiology of LPAIV in wild birds, the effect of immunity on the epidemiology of LPAIV in wild bird populations is poorly understood.
Previous studies reported variable levels of protection by the immune system against reinfection with LPAIV in domestic and wild birds. For instance, LPAIV infections followed by exposure to the same (i.e., homologous) LPAIV HA subtype have been shown to induce strong protection in chickens (7) and mallards (Anas platyrhynchos) (8) but weak protection in Pekin ducks (7). LPAIV infections followed by exposure to a different (i.e., heterologous) LPAIV HA subtype have been shown to induce no protection in chickens (7) and weak protection in Pekin ducks (7), mallards (8, 9), and quails (10). Susceptibility of birds to LPAIV infection is suggested to vary by age, with, in most cases, decreased virus replication with increasing age, but this has been investigated mainly in very young birds (11, 12). In naturally and experimentally infected mallards, avian influenza virus (AIV)-specific serum antibodies have been detected for a long period of time after infection (13, 14), but little is known about their protective effect.
To clarify the role of immunity in the epidemiology of LPAIV subtypes in wild birds, we investigated the protective effect of LPAIV infection on subsequent infections with the homologous or a heterologous virus in a natural host species over a period of >1 year. Clinical effects of infection were also investigated. This study addresses the following questions. (i) What is the protective effect of LPAIV infection on subsequent exposure to the homologous virus? (ii) What is the protective effect of LPAIV infection on subsequent exposure to a heterologous virus? (iii) Are first-year birds equally susceptible to LPAIV infection as second-year birds? (iv) Does LPAIV cause disease in BHGU? To answer these questions, 2-month-old BHGU were inoculated with either LPAIV H13N2 or H16N3, and inoculation was repeated with one of these viruses after 1 month and after 1 year. The results of experimental infections showed that there was a protective effect after previous infection with the homologous virus but not after previous infection with a heterologous virus. In addition, there was no effect of age on susceptibility to LPAIV infection, and neither H13 nor H16 caused clinical signs in experimentally infected BHGU.
MATERIALS AND METHODS
Ethics statement.
This study was carried out in accordance with European guidelines (European Union directive on animal testing 2010/63/EU) and Dutch legislation (Experiments on Animals Act). The protocol was approved by the Animal Ethics Committee of the Dutch National Vaccine Institute of the National Institute for Public Health and the Environment (RIVM) (project number 2012-139). The capture of birds prior to the experiment was approved by the Dutch Ministry of Economic Affairs in compliance with the Flora and Fauna Act (permit number FF/75A/2010/039).
Collection, housing, and feeding of birds.
Fifty BHGU chicks between 1 and 7 days of age were captured by hand at a BHGU breeding colony site on an island at Blauwe Stad (53°10′15″N, 7°00′43″E), in the Netherlands, on 22 May 2012. Birds were hand-raised indoors at the RIVM in Bilthoven, the Netherlands. Prior to the experiment and outside the infectious period (i.e., period from the day when cloaca samples of all birds tested negative for viral RNA until 1 week prior to [the next] virus inoculation, equal to May until June 2012, September 2012 until June 2013, and August until October 2013), birds were housed in two animal rooms. During the infectious period (July to August 2012 and July 2013), birds were housed in groups of six birds per glove box. Birds had continuous access to water (water areas of 2 m2/animal room with 20 to 24 birds and ∼0.30 m2/glove box with a maximum of 6 birds) for bathing and drinking. Perches and shelves were available to roost and rest. The room temperature varied between 20°C and 22°C, and light was on between 6:30 a.m. and 6:30 p.m. Animal rooms or glove boxes were cleaned, and water was changed daily.
The diet consisted of sand eel (Ammodytes tobianus) with additional vitamins (Akwavit; Twilmij BV, Stroe, the Netherlands), ferret pellets (Arie Blok BV, Woerden, the Netherlands), and live earthworms and mealworms (Firma Van der Neut, Groenekan, the Netherlands). Ground shells were available to provide additional calcium.
Experimental design.
We chose BHGU as the study species because BHGU are abundant, H13 and H16 epizootics occur in BHGU every year, and LPAIV infections occurring enzootically in BHGU are restricted to these two subtypes. The timing of the inoculations was chosen to be synchronous with the breeding season of BHGU in July and August and to reflect a reasonable interval between exposures during a breeding season (i.e., 1 month between the first inoculation in July 2012 and the second inoculation in August 2012) and between breeding seasons (i.e., 1 year between the second inoculation in August 2012 and the third inoculation in July 2013). A total of 48 birds (28 males and 20 females) were distributed randomly into 8 groups of 6 birds. Each group followed a different schedule of three intraesophageal inoculations (Table 1). This route of inoculation was chosen because virus replication was limited to the intestinal tract of BHGU naturally infected with LPAIVs H13 and H16 (15). The inoculum was egg allantoic fluid containing either 106 median egg infectious doses (EID50) of LPAIV H13 or H16 (virus-inoculated birds) or no virus (sham-inoculated birds), diluted with phosphate-buffered saline (PBS) to a volume of 1.5 ml. Birds were weighed and sampled for virus detection daily from day 0 until day 7 and on days 9, 11, 13, 14, 21, and 28 postinoculation. Birds were sampled for antibody detection on days 0, 7, 14, 21, and 28 postinoculation. From 28 days postinoculation (dpi) onwards, birds were weighed and sampled monthly for virus and antibody detection.
TABLE 1.
Experimental design
| Group | No. of birds | LPAIV subtype at inoculation: |
||
|---|---|---|---|---|
| I (6 July 2012, 2 mo of age) | II (3 August 2012, 3 mo of age) | III (15 July 2013, 14 mo of age) | ||
| 1 | 6 | H13 | H16 | H16 |
| 2 | 6 | H16 | H16 | H16 |
| 3 | 6 | Sham | H16 | H16 |
| 4 | 6 | Sham | Sham | H16 |
| 5 | 6 | H16 | H13 | H13 |
| 6 | 6 | H13 | H13 | H13 |
| 7 | 6 | Sham | H13 | H13 |
| 8 | 6 | Sham | Sham | H13 |
Cloacal and oropharyngeal swabs were collected from all 48 gulls at two time points (31 May 2012 and 16 June 2012) prior to the first inoculation, and all swabs tested negative by matrix-specific reverse transcription-PCR (M-RT-PCR). Also, sera from all 48 gulls were collected on the same day but just prior to the first inoculation (6 July 2012) and tested negative for NP-, H13-, and H16-specific antibodies. The exception was the serum of one BHGU from group sham-sham-H13, which tested positive for NP-specific antibodies and negative for H13- and H16-specific antibodies on 16 June 2012, 29 June 2012, 13 July 2012, and 20 July 2012. Ths BHGU also tested negative for H1- to H12-specific antibodies on 6 July 2012. Therefore, this BHGU was retained in the study.
Virus preparation.
Two virus stocks of influenza virus, A/black-headed Gull/Netherlands/7/2009 (H13N2) (collected on 2 July 2009) and A/black-headed Gull/Netherlands/26/2009 (H16N3) (collected on 22 July 2009), were used in this study. Both of these viruses originated from a BHGU breeding colony site at the island of Griend (53°15′07″N, 5°15′14″E), located in the Wadden Sea in the north of the Netherlands. The viruses were isolated from combined oropharyngeal-cloacal swab samples from first-year BHGU and passaged twice in 11-day-old embryonated chicken eggs. Viral titers of stock solutions were 108 EID50/ml. Prior to inoculation, virus stocks were diluted with PBS to 106 EID50/1.5 ml. These viruses were selected as they originated from the same season and colony site and therefore were considered to be good candidates to simulate a natural pair of LPAIV infections on a colony site. The internal gene segments of these two virus isolates showed high levels of sequence identity (for PB2, 97% of 2,322 nucleotides [nt] were identical; for PB1, 100% of 2,314 nt were identical; for PA, 98% of 2,222 nt were identical; for NP, 99% identical of 1,538 nt were identical; and for MA, 93% of 1,017 nt were identical), except for NS (87% of 866 nt).
Sampling for virus detection.
For virus detection, samples were taken from the cloaca of birds by using sterile cotton swabs. After sampling, the swab was submerged in 1.2 ml virus transport medium (VTM) (16). Within 2 h, the sample was frozen at −80°C until analysis.
Sampling for antibody detection.
For antibody detection, a blood sample of at most 1 ml from the jugular vein was collected. Blood was collected in gel tubes (MiniCollect, Z serum separator tubes; Greiner Bio-One, Kremsmünster, Austria) and centrifuged at 3,000 rpm for 10 min within 2 h of sampling. Serum was stored at −20°C until analysis.
Detection of viruses: RNA isolation and M-RT-PCR.
RNA was isolated from 200 μl of sample in VTM by using a MagnaPure LC system with a MagnaPure LC total nucleic acid isolation kit (Roche Diagnostics, Almere, the Netherlands). Subsequently, RNA was tested for the presence of the highly conserved matrix segment by M-RT-PCR. Amplification and detection were performed by using an ABI 7700 machine (Applied Biosystems, Foster City, CA, USA) with a TaqMan Fast Virus 1-Step master mix (Applied Biosystems, Nieuwerkerk aan den IJssel, the Netherlands) and 20 μl of RNA eluate in a total volume of 30 μl. Oligonucleotides (5′-CTT-CTR-ACC-GAG-GTC-GAA-ACG-TA-3′ and 5′-TCT-TGT-CTT-TAG-CCA-YTC-CAT-GAG-3′) and labeled probes (5′-FAM [6-carboxyfluorescein]-TCA-GGC-CCC-CTC-AAA-GCC-GAG-A-black hole quencher [BHQ]-3′ and 5′-FAM-TCA-GGC-CCC-CTC-AAA-GCC-GAA-A-BHQ-3′) were used for the detection of the M segment. Samples were considered positive if the cycle threshold (CT) value was <40.
Virus isolation and titration.
In all specimens, the presence or absence of infectious virus was detected by inoculating an aliquot of 100 μl of VTM into 11-day-old embryonated chicken eggs (4 eggs/specimen). For a subset of specimens, namely, the original specimens after the first inoculation with H13 (i.e., inoculation group H13-H16-H16) and after the first inoculation with H16 (i.e., inoculation group H16-H16-H16), the virus titer was measured. To do so, we made a 10-fold dilution series of VTM in a volume of 100 μl and used these dilutions to inoculate 11-day-old embryonated chicken eggs (4 eggs/dilution). Eggs were incubated at 37°C for 2 days before allantoic fluid was harvested. Next, allantoic fluid was tested in a hemagglutination test for the presence of AIV (16).
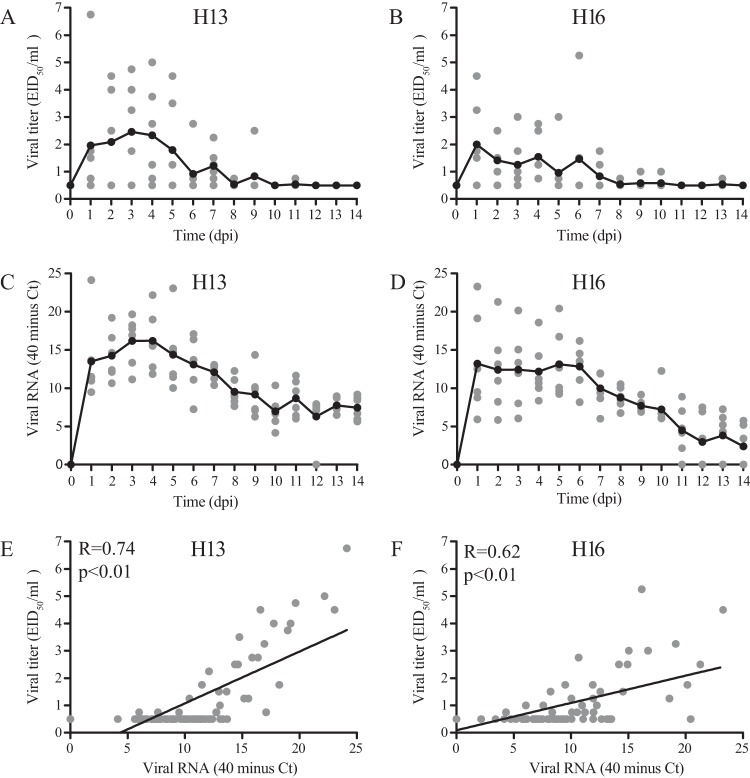
The use of the CT value as a proxy for viral titer was based on comparison of CT values and viral titers of identical cloacal samples collected daily after the first inoculation with H13 and H16 viruses. Despite the strong overall correlation between the CT value and viral titer (P < 0.01 by the Pearson correlation test) (Fig. 1), the use of the CT value as a proxy for viral titer needs to be used with caution, as viral RNA is detectable longer after inoculation than infectious virus.
FIG 1.
Comparison of viral titers with viral RNA in cloacal swabs after the first LPAIV H13N2 and H16N3 inoculations of black-headed gulls. (A, C, and E) H13; (B, D, and F) H16. Black lines indicate means per sampling day (A to D), and gray dots indicate values for individual birds (n = 6 birds per day) (A to F). Correlation analyses for H13 and H16 based on viral titers and viral RNA from days 0 to 14 postinoculation resulted in R values of 0.74 for H13 (P < 0.01) and 0.62 for H16 (P < 0.01) (Pearson correlation test).
Detection of antibodies.
Serum samples were tested for the presence of H13-specific, H16-specific, and NP-specific antibodies. H13- and H16-specific antibodies were detected by using a hemagglutination inhibition (HI) test with H13N2 and H16N3 virus isolates used for inoculation as reference antigens (17). The starting serum dilution in the HI test was 1:6; thus, the minimal detectable antibody titer was 3. Phosphate-buffered saline was included as a serum control. NP-specific antibodies were detected by using a commercial blocking enzyme-linked immunosorbent assay (bELISA) (Idexx FlockChek* AI MultiS-Screen; Idexx Laboratories BV, Hoofddorp, the Netherlands). Samples were tested according to the manufacturer's instructions. A sample was considered NP positive when the signal-to-noise ratio (i.e., ratio of the mean optical density [ODx] of the sample/ODx of the negative control) was ≤0.5.
Clinical signs of infection.
Body mass was monitored daily from day 0 to day 7 and on days 9, 11, 13, and 14 postinoculation. After inoculation, each morning, each group was scored qualitatively during 5-min observations for signs of ruffled feathers or decreased movement, feeding, or bathing activity for all individuals. Fecal water content was monitored daily on day 0 until day 7 postinoculation. Per inoculation group, birds were kept for 1 h in a box measuring 45 cm long by 67 cm wide by 20 cm high directly after sampling. Feces fell through a wire mesh grid in the bottom of the box onto a removable polyester sheet (Melinex). After release of the birds into the glove box, the sheet, including feces, was removed and weighed before and after autoclaving in a dry cycle (134°C for 3 min) to evaporate the water in the feces. The mass loss during autoclaving was considered the fecal water content.
As additional methods to measure clinical signs of infection, head movements were measured after the second inoculation, and activity levels were measured after the third inoculation. Head movements (as a proxy for activity) were videotaped for 10 min daily on days 1 to 6 after the second inoculation on 3 August 2012. Activity levels were scored at 3-min intervals during daily observations of 15 min from days −1 to 7 after the third inoculation on 15 July 2013. Activity levels were categorized as active (walking, feeding, preening, and bathing) or passive (standing, sleeping, and sitting).
Statistical analyses.
To investigate the correlation between virus excretion based on viral RNA and virus excretion based on viral titer, a Pearson correlation test was performed. To compare virus excretion within and between groups, the area under the curve (AUC) of viral RNA (i.e., based on 40 minus the CT value as determined by M-RT-PCR) from days 0 to 14 postinoculation was calculated. The mean quantity of virus excreted from cloacae per group (i.e., mean AUC) was based on the AUCs for all birds in the group. To compare the durations of virus excretion within and between groups, the median maximum day of the presence of infectious virus (i.e., positive virus isolation) was used. The median duration of virus excretion per group was based on values from all birds in the group. To investigate whether differences in virus excretion or duration between two groups or time points were statistically significant, a Mann-Whitney test was performed. To investigate whether differences in virus excretion or duration among three groups or time points were statistically significant, a Kruskal-Wallis test was performed (i.e., for comparisons of H16 virus excretion and durations for three groups of different ages).
To compare the proportions of birds that generated AIV-specific antibodies between groups and between virus subtypes, a Fisher exact test was used. To compare AIV-specific antibody titers within and between groups, the log2 AUC values for the H13- and H16-specific antibody titers measured weekly from 0 to 28 dpi were calculated. The mean quantity of antibodies generated per group (i.e., mean AUC) was based on AUC values for all birds in the group. To investigate whether differences in antibody production between two groups or time points were statistically significant, a Mann-Whitney test was performed. To investigate whether differences in antibody production among three groups or time points were statistically significant, a Kruskal-Wallis test was performed. When a statistically significant value was determined (P < 0.05), the pairwise difference in levels of antibody production at different time points was analyzed by using the Mann-Whitney test.
To investigate the correlation between virus excretion based on viral RNA and water content of feces, a Pearson correlation test was performed. To investigate the protective effect of homologous AIV-specific antibodies generated after previous virus inoculation, the following values were compared by using a Mann-Whitney test: (i) quantity of virus excretion (i.e., AUC for viral RNA based on 40 minus the CT value, from days 0 to 14 postinoculation), (ii) peak of virus excretion (i.e., based on viral RNA based on 40 minus the lowest CT value), (iii) timing of peak of virus excretion (i.e., based on viral RNA, in days postinoculation), and (iv) duration of infectious virus excretion (i.e., based on virus isolation, in days postinoculation) between birds with and those without detectable H16-specific antibody titers on the day of inoculation. Birds that died within 0 to 14 dpi were excluded from analyses.
Nucleotide sequence accession numbers.
Full genome sequences of these viruses are available from GenBank under the following accession numbers: KR087561 to KR087576.
RESULTS
Virus excretion.
The first, second, and third inoculations of the different groups, as shown in Table 1, were successful, with the exception of the second inoculation of group sham-H13-H13 (group 7) and group H16-H13-H13 (group 5) (Table 2). Nevertheless, the results obtained based on the remaining groups are still enough to answer the main questions posed above. The unsuccessful inoculation of group sham-H13-H13 was based on the failure to detect virus by M-RT-PCR except in two of six birds on day 1 and day 2 postinoculation and the failure to isolate virus from any bird at any time point, whereas H13 virus replicated well in immunologically naive groups inoculated with H13 virus at the first and third inoculations. The unsuccessful inoculation of group H16-H13-H13 was based on the failure to detect virus by M-RT-PCR except in one of five birds on day 5 postinoculation and the failure to isolate virus from any bird at any time point.
TABLE 2.
Virus excretion by black-headed gulls after one or more inoculations with LPAIV H13N2, H16N3, or both
| Group | Inoculation schedule | Virus excretion at inoculation: |
|||||
|---|---|---|---|---|---|---|---|
| I |
II |
III |
|||||
| Mean quantity (AUC of viral RNA) ± SEb | Median duration (days) (range)c | Mean quantity (AUC of viral RNA) ± SEb | Median duration (days) (range)c | Mean quantity (AUC of viral RNA) ± SEb | Median duration (days) (range)c | ||
| 1 | H13-H16-H16 | 151.8 ± 6.7 | 7 (5–11) | 117.6 ± 20.5 | 5 (0–9) | 51.6 ± 28.5 | 0 (0–3) |
| 2 | H16-H16-H16 | 122.3 ± 9.1 | 5.5 (4–10) | 28.5 ± 6.8 | 0 (0–3) | 13.7 ± 3.4 | 0 (0–2) |
| 3 | Sham-H16-H16 | 0 | 0 | 102.8 ± 13.4 | 6 (0–11) | 14.4 ± 7.3 | 0 (0–2) |
| 4 | Sham-sham-H16 | 0 | 0 | 0 | 0 | 122.1 ± 21.1 | 4 (0–6) |
| 5 | H16-H13a-H13 | 54.6 ± 14.1 | 3.5 (0–5) | 0 | 0 | 72.2 ± 10.3 | 0 (0–3) |
| 6 | H13-H13-H13 | 133.7 ± 11.9 | 4.5 (1–6) | 69.6 ± 8.4 | 0 (0) | 0.8 ± 0.8 | 0 (0) |
| 7 | Sham-H13a-H13 | 0 | 0 | 1.0 ± 0.6 | 2 (0–6) | 64.0 ± 21.2 | 0 (0–7) |
| 8 | Sham-sham-H13 | 0 | 0 | 0 | 0 | 117.0 ± 12.2 | 4 (0–5) |
The second inoculation of groups 5 and 7 was unsuccessful.
The quantity of virus excretion was based on the AUC for viral RNA (i.e., CT values determined by M-RT-PCR) excreted from the cloaca from days 0 to 14 postinoculation.
Duration of virus excretion was based on the maximum duration of infectious virus excretion based on virus culture from cloaca in days.
The only known difference in the inoculation procedures between these two inoculations and all other inoculations of the eight groups was the pretreatment of the gavage tubes used for intraesophageal inoculation. Normally, one heat-sterilized gavage tube, wrapped individually in paper, was used per group. However, because there were too few heat-sterilized gavage tubes at inoculation II (3 August 2012), one or two gavage tubes (this information was not recorded) used for H13 inoculation were decontaminated with 80% ethanol, flushed with saline, and introduced loose into the glove box via air locks that had been decontaminated with 4% peracetic acid. Potentially, remnants of peracetic acid on the gavage tubes may have inactivated the virus in the inoculation fluid. Virus titrations of samples of the remaining inoculation fluid after inoculations I (6 July 2012) and III (15 July 2013) were as expected (range, 105.75 to 106.25 EID50/ml); unfortunately, samples of inoculation fluid after inoculation II were not retained for back titration.
(i) Effect of age on virus excretion.
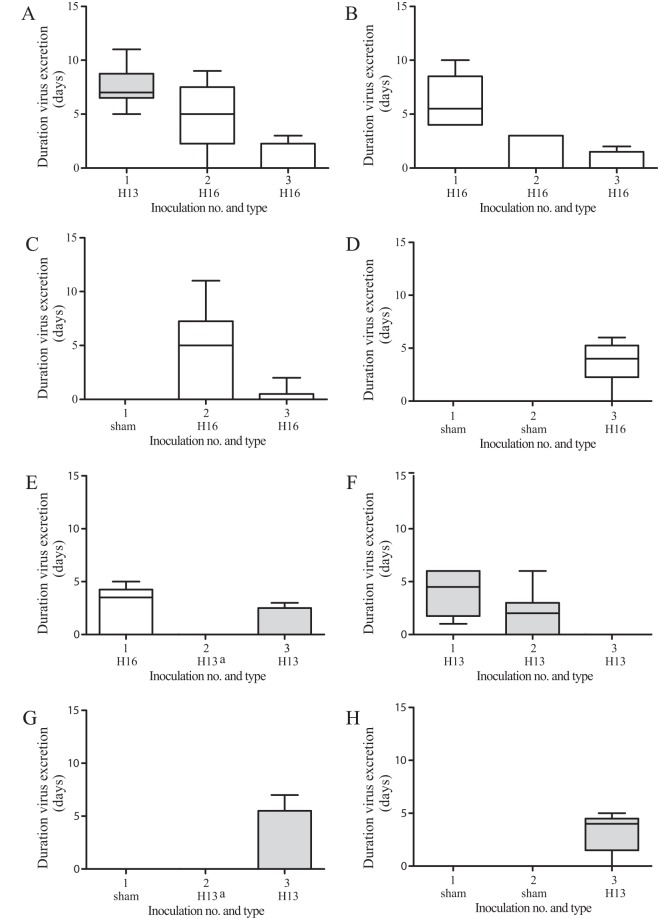
To investigate the effect of age on virus excretion, the quantity (based on AUC from days 0 to 14 postinoculation) and duration of virus excretion between previously uninfected 2-, 3-, and 14-month-old birds were compared. The mean quantity of H13 virus excreted from the cloaca after the first H13 inoculation did not differ significantly between 2-month-old birds (142.8 ± 7.1, i.e., mean for the first inoculation of groups H13-H13-H13 and H13-H16-H16) (Fig. 2A and F, black lines) and 14-month-old birds (117.0 ± 12.2) (P = 0.08) (Fig. 2H, dashed line). However, the median duration of H13 virus excretion by 2-month-old birds (6 dpi; range, 1 to 11 dpi) was significantly longer than that for 14-month-old birds (4 dpi; range, 0 to 5 dpi) (P = 0.05) (Table 2 and Fig. 3). The mean quantity of H16 virus excreted from the cloaca after the first H16 inoculation did not differ significantly between 2-month-old birds [91.5 ± 13.12, i.e., the mean for the first inoculation of groups H16-H16-H16 and H16-(H13)-H13] (Fig. 2B and E, black lines), 3-month-old birds (102.8 ± 13.4) (Fig. 2C, gray line), and 14-month-old birds (122.1 ± 21.1) (Fig. 2D, dashed line) (P = 0.23). Similarly, the median duration of H16 virus excretion did not differ significantly between 2-month-old birds (4 dpi; range, 0 to 10 dpi), 3-month-old birds (6 dpi; range, 0 to 11 dpi), and 14-month-old birds (4 dpi; range, 0 to 6 dpi) (P = 0.57) (Table 2 and Fig. 3).
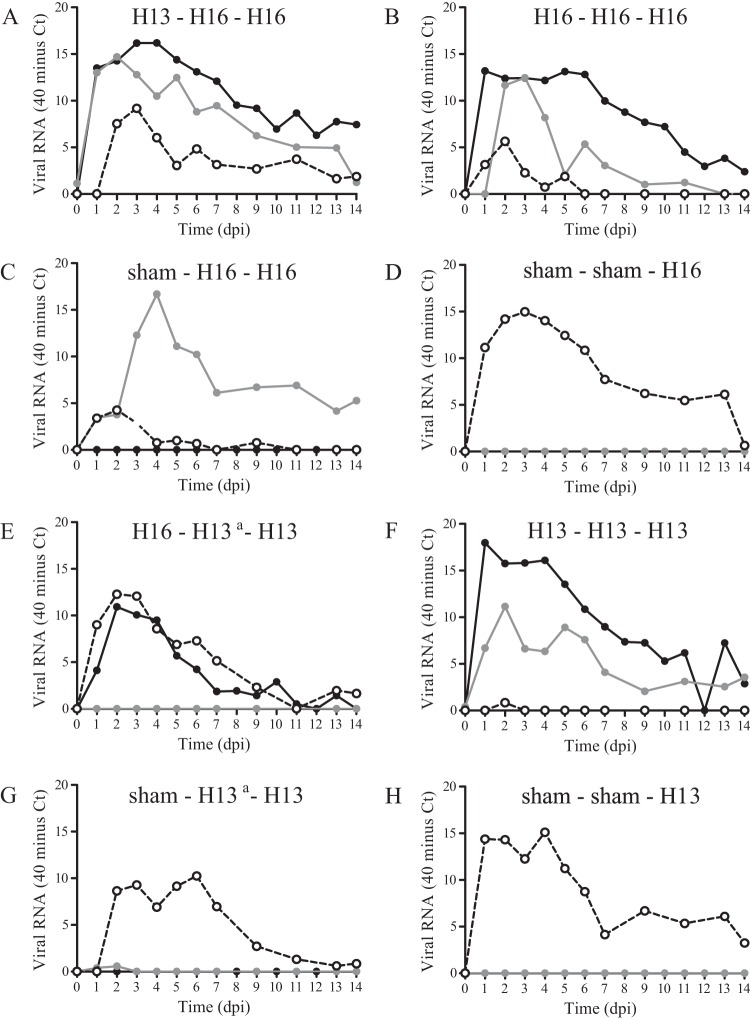
FIG 2.
Mean virus excretion from cloaca after experimental infection of black-headed gulls with one or more inoculations of LPAIV H13N2, LPAIV H16N3, or both, based on the quantity of viral RNA (i.e., CT values determined by M-RT-PCR). Each panel represents data from one group. Mean virus excretion is based on data for all birds in the group. Black lines indicate the first inoculation, gray lines indicate the second inoculation, and dashed lines indicate the third inoculation. a, the second inoculation of groups 5 and 7 was unsuccessful.
FIG 3.
Median duration of excretion of infectious virus from cloacae after experimental infection of black-headed gulls with one or more inoculations of LPAIV H13N2, LPAIV H16N3, or both, based on virus isolation. The median duration of excretion of infectious virus is based on data for all birds in the group. Gray boxes indicate H13, and white boxes indicate H16.a, the second inoculation of groups 5 and 7 was unsuccessful.
(ii) Effect of homologous LPAIV infection on virus excretion.
To investigate the effect of LPAIV infection on subsequent infection with the same virus, the quantities and durations of virus excretion of homologous inoculation groups were compared.
(a) Group H13-H13-H13.
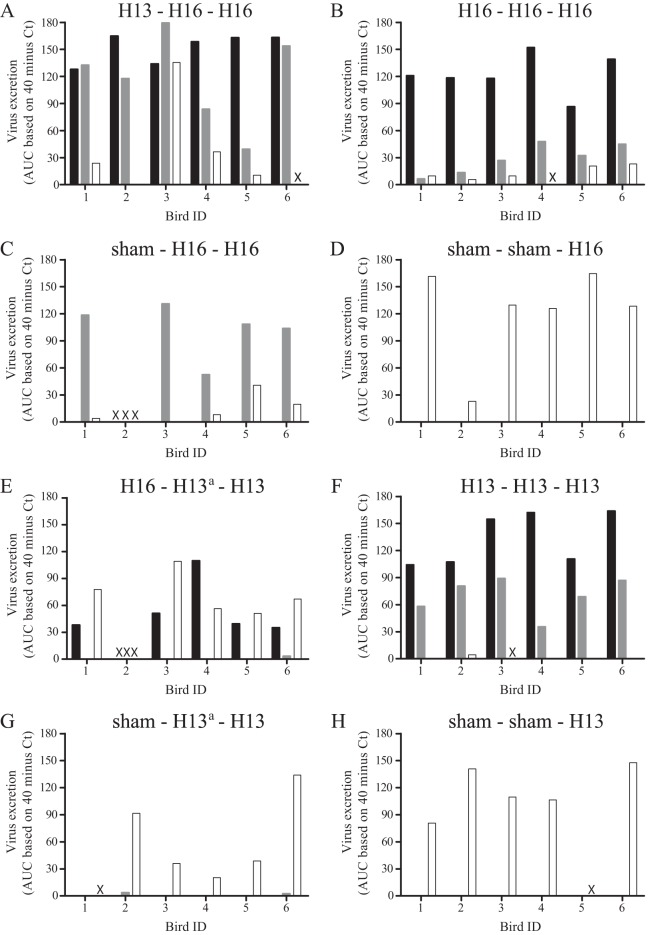
The mean quantity of H13 virus excreted from the cloaca after the second inoculation was significantly lower than that after the first inoculation (P < 0.01) (Fig. 2F, black and gray lines). Besides as a group, each individual bird excreted less virus after the second than after the first inoculation (Fig. 4). Although the median duration of virus excretion appeared to be shorter after the second inoculation (2 dpi; range, 0 to 6 dpi) than after the first inoculation (4.5 dpi; range, 1 to 6 dpi), the difference was not significant (P = 0.19) (Table 2). The mean quantity of H13 virus excreted from the cloaca after the third inoculation was significantly lower than that after the second H13 inoculation of the same group (P < 0.01) (Fig. 2F, black and dashed lines) and was significantly lower than that after H13 inoculation of immunologically naive birds at the third inoculation (P = 0.01) (Fig. 2F and H, dashed lines). In addition, after the third H13 inoculation, no infectious virus was excreted from the cloaca, and thus, the median duration of virus excretion was shorter after the third H13 inoculation than after the second H13 inoculation (2 dpi; range, 0 to 6 dpi) and than after H13 inoculation of immunologically naive birds at the third inoculation (4 dpi; range, 0 to 5 dpi) (Table 2).
FIG 4.
Excretion of LPAIV H13N2 and H16N3 by black-headed gulls, shown per individual bird. Virus excretion was based on the AUC value for viral RNA (i.e., CT value determined by M-RT-PCR). Black indicates the first inoculation, gray indicates the second inoculation, and white indicates the third inoculation. x, the bird died and was excluded from the analysis; a, the second inoculation of groups 5 and 7 was unsuccessful.
(b) Group sham-(H13)-H13.
The mean quantity of H13 virus excreted from the cloaca after the third inoculation appeared to be lower than that after H13 inoculation of immunologically naive birds at the third inoculation, but the difference was not significant (P = 0.10) (Fig. 2G and H, dashed lines). Also, the median duration of H13 virus excretion after the third inoculation (0 dpi; range, 0 to 7 dpi) appeared to be shorter than that after H13 inoculation of immunologically naive birds at the third inoculation (4 dpi; range, 0 to 5 dpi), but again, the difference was not significant (P = 0.59) (Table 2 and Fig. 3).
(c) Group H16-H16-H16.
The mean quantity of H16 virus excreted from the cloaca after the second inoculation was significantly lower than those after the first H16 inoculation (P < 0.01) (Fig. 2B, black and gray lines) and after H16 inoculation of immunologically naive birds at the second inoculation (P < 0.01) (Fig. 2B and C, gray lines). The decrease in virus excretion was consistent for each individual bird of group H16-H16-H16 (Fig. 4). Also, the median duration of excretion of infectious virus was significantly shorter after the second inoculation (0 dpi; range, 0 to 3 dpi) than after the first inoculation (5.5 dpi; range, 4 to 10 dpi) (P < 0.01) and than after H16 inoculation of immunologically naive birds at the second inoculation (6 dpi; range, 0 to 11 dpi) (P = 0.04) (Table 2 and Fig. 3). The mean quantity of H16 virus excreted from the cloaca after the third inoculation did not differ significantly from that after the second inoculation of the same group (P = 0.22) (Fig. 2B, black and dashed lines) but was significantly lower than that after H16 inoculation of immunologically naive birds at the third inoculation (P < 0.01) (Fig. 2B and D, dashed lines). Similarly, the median duration of virus excretion after the third inoculation (0 dpi; range, 0 to 2 dpi) did not differ significantly from that after the second inoculation of the same group (0 dpi; range, 0 to 3 dpi) (P = 0.80) but was significantly shorter than that after H16 inoculation of immunologically naive birds at the third inoculation (4 dpi; range, 0 to 6 dpi) (P = 0.04) (Table 2 and Fig. 3).
(d) Group sham-H16-H16.
The mean quantity of H16 virus excreted from the cloaca after the third inoculation was significantly lower than that after the second inoculation (P < 0.01) (Fig. 2C, gray and dashed lines) and was significantly lower than that after H16 inoculation of immunologically naive birds at the third inoculation (P < 0.01) (Fig. 2C and D, dashed lines). Also, the median duration of excretion of infectious H16 virus was significantly shorter after the third inoculation (0 dpi; range, 0 to 2 dpi) than after the second inoculation (6 dpi; range, 0 to 11 dpi) (P = 0.04) and than after H16 inoculation of immunologically naive birds at the third inoculation (4 dpi; range, 0 to 6 dpi) (P = 0.03) (Table 2 and Fig. 3).
(iii) Effect of heterologous LPAIV infection on virus excretion.
To investigate the effect of H13 virus infection on subsequent infection with H16 virus, the quantity and duration of virus excretion after second inoculation of group H13-H16-H16 were compared with those in immunologically naive birds inoculated with H16 at the second inoculation. The effect of H16 virus on subsequent infection with H13 virus could not be investigated due to the unsuccessful inoculation of immunologically naive birds with H13 at the second inoculation and, thus, the lack of a control group [i.e., group sham-(H13)-H13].
(a) Group H13-H16-H16.
The mean quantity of H16 virus excreted from the cloaca after the second inoculation did not differ significantly between birds preexposed to H13 and immunologically naive birds (P = 0.54) (Fig. 2A and C, gray lines). In line with this, the median duration of H16 virus excretion did not differ significantly between birds preexposed to H13 virus (5 dpi; range, 0 to 9 dpi) and immunologically naive birds (6 dpi; range, 0 to 11 dpi) (P = 0.85) (Table 2 and Fig. 3).
(b) Group H16-(H13)-H13.
The mean quantity of H13 virus excreted from the cloaca after the second inoculation was low, as H13 virus was detected in the cloaca by M-RT-PCR in only one of six birds on day 7 after the second inoculation, and no virus was isolated from the cloaca. In the same bird, H13-specific antibodies were detected after the second inoculation. Despite the fact that no virus was detected in other birds until 14 days after the second inoculation, the H16-specific antibody titer was boosted in three of five birds after the second inoculation (Fig. 5). The mean quantity of H13 virus excreted from the cloaca after the third inoculation was significantly lower than that for immunologically naive birds inoculated with H13 at the third inoculation (P = 0.03) (Fig. 2E and H, dashed lines). The median duration of excretion of infectious virus after the third inoculation (0 dpi; range, 0 to 3 dpi) did not differ significantly from that for immunologically naive birds inoculated with H13 at the third inoculation (4 dpi; range, 0 to 5 dpi) (P = 0.08) (Table 2 and Fig. 3).
FIG 5.
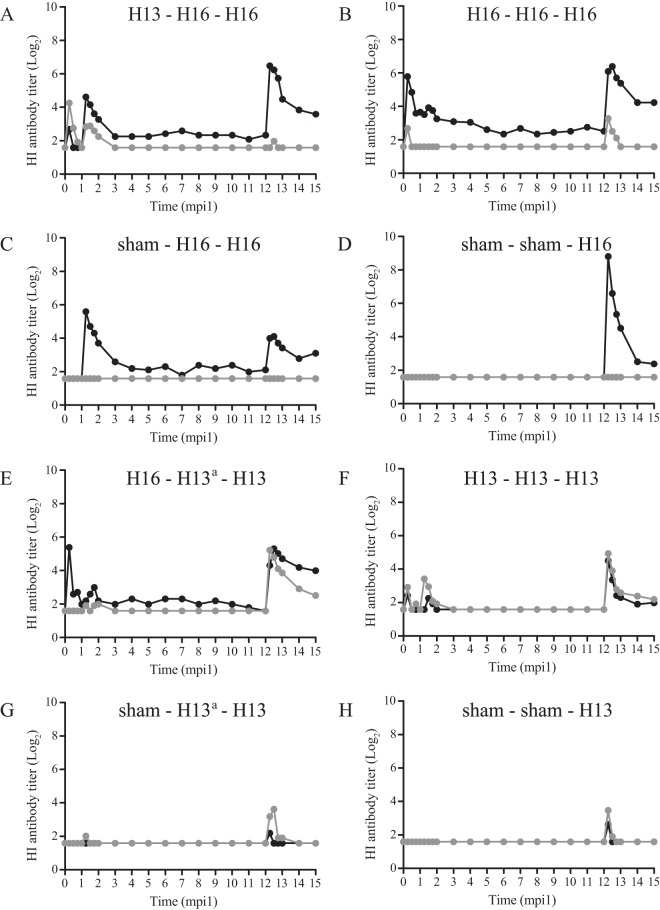
Mean HI antibody titers after one or more inoculations of black-headed gulls with LPAIV H13N2, LPAIV H16N3, or both. Mean antibody titers are based on data for all birds in the group. Gray indicates H13-specific antibodies, and black indicates H16-specific antibodies. a, the second inoculation of groups 5 and 7 was unsuccessful.
Humoral immune response. (i) Effect of age on AIV-specific antibody production.
To investigate the effect of age on the immune response, the proportions of birds that raised AIV-specific antibodies within 1 month after inoculation and the concentrations of these antibodies in serum were compared in 2-, 3- and 14-month-old birds. The proportions of birds that produced H13-specific antibodies after the first inoculation with H13 did not differ significantly between 2-month-old birds (6 of 12 [50%], i.e., the total for the first inoculation of group H13-H16-H16 and group H13-H13-H13) and 14-month-old birds (3 of 5 [60%]) (P = 0.38). The proportions of birds with NP-specific antibodies after the first inoculation with H13 also did not differ significantly between 2-month-old birds (7 of 12 [58%]) and 14-month-old birds (4 of 5 [80%]) (P = 0.32). The mean quantities of H13-specific antibodies generated after the first inoculation with H13 virus did not differ significantly between 2-month-old birds (2.92 ± 0.94; n = 12) and 14-month-old birds (2.20 ± 1.02) (P = 0.74) (Table 3).
TABLE 3.
Antibody detection in black-headed gulls after one or more inoculations with LPAIV H13N2, H16N3, or both
| Group | Inoculation schedule | Assay | Antibody detection at inoculation: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I |
II |
III |
|||||||||
| Antibody titer at 0 dpi | Mean quantity of antibody production (log2 AUC) ± SEb | No. of seropositive birds/total no. of birdsc | Antibody titer at 0 dpi | Mean quantity of antibody production (log2 AUC) ± SEb | No. of seropositive birds/total no. of birdsc | Antibody titer at 0 dpi | Mean quantity of antibody production (log2 AUC) ± SEb | No. of seropositive birds/total no. of birdsc | |||
| 1 | H13-H16-H16 | H13 HI | 0 | 4.17 ± 1.38 | 4/6 | 0 | 3.90 ± 2.03 | 4/6 | 0 | 0.40 ± 0.40 | 1/4 |
| H16 HI | 0 | 1.10 ± 0.50 | 3/6 | 0 | 8.47 ± 2.69 | 5/6 | 2.33 ± 1.50 | 15.51 ± 6.03 | 4/4 | ||
| NP bELISA | 3/6 | 4/6 | 2/4 | ||||||||
| 2 | H16-H16-H16 | H13 HI | 0 | 1.10 ± 0.82 | 2/6 | 0 | 0.00 ± 0.00 | 0/6 | 0 | 3.47 ± 2.49 | 2/5 |
| H16 HI | 0 | 10.49 ± 2.54 | 6/6 | 3.65 ± 1.73 | 8.30 ± 2.38 | 5/6 | 2.18 ± 0.89 | 16.52 ± 6.78 | 4/5 | ||
| NP bELISA | 4/6 | 1/6 | 3/5 | ||||||||
| 3 | Sham-H16-H16 | H13 HI | 0 | 0.00 ± 0.00 | 0/5 | 0 | 0.00 ± 0.00 | 0/5 | 0 | 0.00 ± 0.00 | 0/5 |
| H16 HI | 0 | 0.00 ± 0.00 | 0/5 | 0 | 10.89 ± 3.24 | 4/5 | 2.10 ± 1.16 | 8.21 ± 4.60 | 3/5 | ||
| NP bELISA | 0/5 | 4/5 | 1/5 | ||||||||
| 4 | Sham-sham-H16 | H13 HI | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.00 ± 0.00 | 0/6 |
| H16 HI | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.00 ± 0.00 | 0/6 | 0 | 17.45 ± 1.97 | 6/6 | ||
| NP bELISA | 0/6 | 0/6 | 5/6 | ||||||||
| 5 | H16-H13a-H13 | H13 HI | 0 | 0.00 ± 0.00 | 0/5 | 0 | 0.83 ± 0.83 | 1/5 | 0 | 10.48 ± 3.61 | 4/5 |
| H16 HI | 0 | 6.12 ± 2.65 | 3/5 | 1.98 ± 0.89 | 3.50 ± 1.33 | 4/5 | 0 | 11.43 ± 5.03 | 3/5 | ||
| NP bELISA | 3/5 | 0/5 | 4/5 | ||||||||
| 6 | H13-H13-H13 | H13 HI | 0 | 1.67 ± 1.17 | 2/6 | 0 | 3.86 ± 2.19 | 4/6 | 0 | 7.37 ± 4.31 | 4/5 |
| H16 HI | 0 | 0.83 ± 0.54 | 2/6 | 0 | 1.00 ± 1.00 | 1/6 | 0 | 5.88 ± 2.74 | 3/5 | ||
| NP bELISA | 4/6 | 2/6 | 3/5 | ||||||||
| 7 | Sham-H13a-H13 | H13 HI | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.43 ± 0.43 | 1/6 | 0 | 4.11 ± 1.53 | 4/5 |
| H16 HI | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.60 ± 0.60 | 1/5 | ||
| NP bELISA | 0/6 | 0/6 | 2/5 | ||||||||
| 8 | Sham-sham-H13 | H13 HI | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.00 ± 0.00 | 0/6 | 0 | 2.20 ± 1.02 | 3/5 |
| H16 HI | 0 | 0.00 ± 0.00 | 0/6 | 0 | 0.00 ± 0.00 | 0/6 | 0 | 1.03 ± 0.43 | 3/5 | ||
| NP bELISA | 1/6 | 0/6 | 4/5 | ||||||||
The second inoculation of groups 5 and 7 was unsuccessful.
Antibody production is based on the AUC on a log2 scale between days 0 and 28 postinoculation.
Total number of birds that seroconverted between days 0 and 28 postinoculation.
The proportion of birds that produced H16-specific antibodies after the first inoculation with H16 virus did not differ significantly between 2-month-old birds (9 of 11 [82%]) and 3-month-old birds (4 of 5 [80%]) (P = 0.49), between 3-month-old birds and 14-month-old birds (6 of 6 [100%]) (P = 0.45), and between 2-month-old birds and 14-month-old birds (P = 0.40). Also, the proportion of birds with NP-specific antibodies after the first inoculation with H16 virus did not differ significantly between 2-month-old birds (7 of 11 [64%]) and 3-month-old birds (4 of 5 [80%]) (P = 0.38), between 3-month-old birds and 14-month-old birds (5 of 6 [83%]) (P = 0.55), and between 2-month-old birds and 14-month-old birds (P = 0.32). The mean quantities of H16-specific antibodies generated after the first H16 inoculation of 2-month-old birds (8.5 ± 1.9), 3-month-old birds (10.89 ± 3.24), and 14-month-old birds (17.45 ± 1.97) differed significantly (P = 0.05), with a significantly larger quantity of H16-specific antibodies detected in 14-month-old birds than in 2-month-old birds (P = 0.01) (Table 3).
(ii) Detection of AIV-specific cross-reactive antibodies.
The detection of cross-reactive antibodies differed between H13 and H16 viruses and by age. After the first H13 inoculation, H16-cross-reactive antibodies were detected on day 7 in 5 of 12 (42%) 2-month-old birds and in 3 of 5 (60%) 14-month-old birds. After the first H16 inoculation, H13-cross-reactive antibodies were detected on day 7 in 2 of 11 (18%) 2-month-old birds and were not detected in 5 3-month-old birds and 6 14-month-old birds (Table 3 and Fig. 5).
(iii) AIV-specific antibody production after multiple LPAIV inoculations.
Of birds that had been exposed to the same virus more than once (i.e., H13-H13-H13 and H16-H16-H16), no significant differences in the quantities of specific antibody titers after the first, second, and third H13 infections (P = 0.33) or after the first, second, and third H16 infections (P = 0.62) were detected (Table 3).
(iv) Persistence of AIV-specific antibodies between breeding seasons.
To investigate the persistence of AIV-specific antibodies in the different inoculation groups, the periods of detection of H13-, H16-, and NP-specific antibodies for the different groups were compared. During the months between the second and third inoculations, AIV-specific antibodies were detected in a limited number of birds for a limited period of time, except for H16-specific antibodies, which stayed detectable until 11 months after the second inoculation. Within this period, H16-specific antibodies were most frequently detected in birds of group H16-H16-H16. In contrast to H16-specific antibodies, H13-specific antibodies were detected only until 1 month after the second inoculation [in groups H13-H16-H16, H16-(H13)-H13, and H13-H13-H13 only]. NP-specific antibodies were detected until 3 months after the second inoculation (i.e., H13-H13-H13) (Table 4). On the day of the third inoculation, H16-specific antibodies were detected (in groups H13-H16-H16, H16-H16-H16, and sham-H16-H16 only), while no H13-specific antibodies were detected on that day (Fig. 5).
TABLE 4.
Year-round antibody detection after one or more inoculations of black-headed gulls with LPAIV H13N2, H16N3, or both
| Group | Inoculation schedule | Assay | No. of seropositive birds/total no. of birds after inoculationb: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I (0 mo) | II at mo: |
III at mo: |
||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||||
| 1 | H13-H16-H16 | H13 HI | 0/6 | 0/6 | 2/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| H16 HI | 0/6 | 0/6 | 4/6 | 1/6 | 3/6 | 2/6 | 2/6 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 3/4 | 2/4 | 2/4 | ||
| NP bELISA | 0/6 | 0/6 | 1/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 1/4 | 1/4 | ||
| 2 | H16-H16-H16 | H13 HI | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 |
| H16 HI | 0/6 | 4/6 | 4/6 | 3/6 | 4/6 | 3/6 | 3/6 | 3/6 | 2/6 | 3/6 | 3/6 | 3/6 | 2/5 | 3/5 | 2/5 | 2/5 | ||
| NP bELISA | 0/6 | 1/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | ||
| 3 | Sham-H16-H16 | H13 HI | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| H16 HI | 0/5 | 0/5 | 3/5 | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 | 2/5 | 1/5 | 3/5 | 3/5 | 3/5 | ||
| NP bELISA | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 1/5 | ||
| 4 | Sham-sham-H16 | H13 HI | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/4 |
| H16 HI | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 6/6 | 2/5 | 2/4 | ||
| NP bELISA | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/4 | ||
| 5 | H16-H13a-H13 | H13 HI | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 4/5 | 3/5 | 2/5 |
| H16 HI | 0/5 | 1/5 | 1/5 | 1/5 | 2/5 | 1/5 | 2/5 | 2/5 | 1/5 | 2/5 | 2/5 | 1/5 | 0/5 | 3/5 | 3/5 | 3/5 | ||
| NP bELISA | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 3/5 | 1/5 | 1/5 | ||
| 6 | H13-H13-H13 | H13 HI | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | 1/5 | 1/5 | 1/5 |
| H16 HI | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 1/5 | 1/5 | ||
| NP bELISA | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 1/5 | 0/5 | ||
| 7 | Sham-H13a-H13 | H13 HI | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 1/5 | 0/5 | 0/5 |
| H16 HI | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | ||
| NP bELISA | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | ||
| 8 | Sham-sham-H13 | H13 HI | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| H16 HI | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | ||
| NP bELISA | 0/6 | 0/6 | 0/6 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | ||
The second inoculation of groups 5 and 7 was unsuccessful.
Boldface type indicates detection of seropositivity.
(v) Link between AIV-specific antibodies and H13 and H16 virus excretion.
To investigate if AIV-specific antibodies had a protective effect against subsequent infection, the presence of H13- and H16-specific antibodies on the day of subsequent inoculation was compared with the subsequent excretion of homologous virus. On the day of the second or third inoculation, only H16-specific antibodies were detected (Table 4 and Fig. 5). There were no significant differences in the quantity of virus excretion (P = 0.54), peak virus excretion (P = 0.84), timing of peak virus excretion (P = 0.14), and duration of virus excretion (P = 0.37) between birds with (n = 7) and those without (n = 9) H16-specific antibodies belonging to group H16-H16-H16 and sham-H16-H16.
Clinical signs of infection.
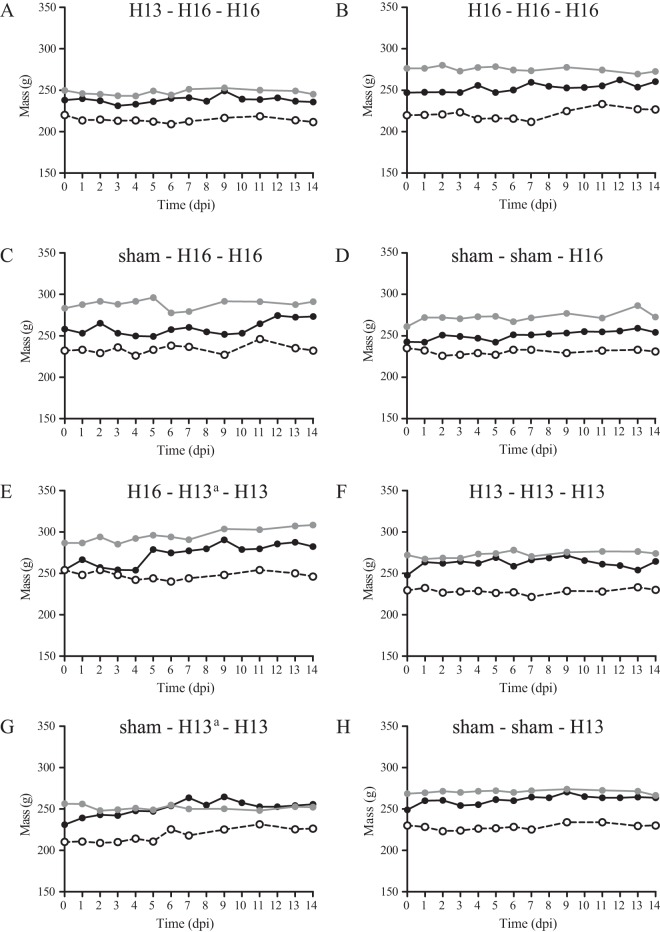
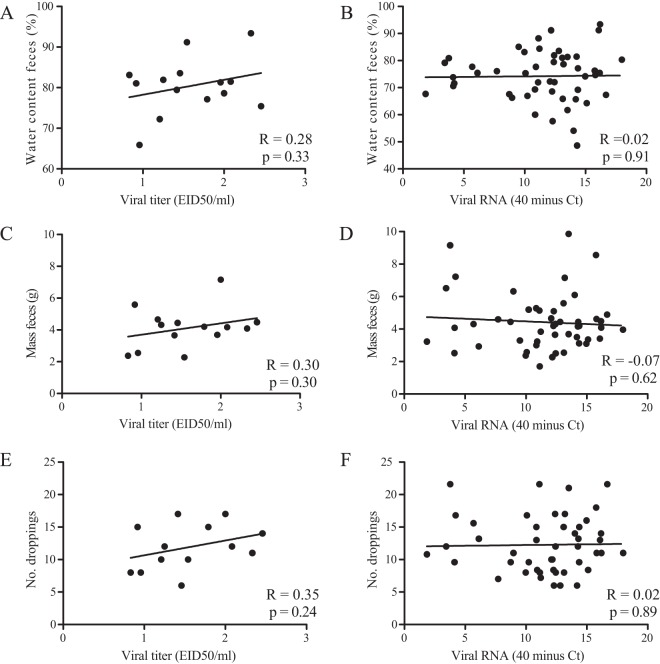
To investigate clinical signs of infection, body mass, bird behavior, and fecal water content were monitored. Body mass was constant in time from days 0 to 14 postinoculation independent of LPAIV or sham inoculation (Fig. 6). After each inoculation, bird behavior, as observed for 5 min per group each morning, varied inconsistently between days (data not shown). After the first inoculations with H13 or H16 virus, the water content of feces, as a proxy for diarrhea, varied inconsistently in time from days 0 to 7 postinoculation and did not correlate with the quantity of virus excretion (R = 0.02 and P = 0.91). The mass of feces and number of droppings were not associated with the quantity of virus excretion (R = −0.07 and P = 0.62, and R = 0.02 and P = 0.89, respectively) (Fig. 7).
FIG 6.
Body mass of black-headed gulls after the first, second, and third LPAIV H13N2 or H16N3 inoculations from days 0 to 14 postinoculation. Black lines indicate the first inoculation, gray lines indicate the second inoculation, and dashed lines indicate the third inoculation. a, the second inoculation of groups 5 and 7 was unsuccessful.
FIG 7.
Monitoring of feces and virus excretion from cloacae based on viral titer (A, C, and E) and viral RNA (B, D, and F) from day 0 until day 7 after the first LPAIV H13N2 or H16N3 inoculation of black-headed gulls during the first and second breeding seasons.
Head movements as a measure of activity after the second inoculation varied inconsistently among groups and in time. Bird activity, as measured during daily 15-min observations after the third inoculation, varied inconsistently among groups and in time (data not shown).
DISCUSSION
The results of this study on H13 and H16 LPAIV infections in BHGU provided answers to the main questions posed above. LPAIV infection induced long-lasting, partial protection against infection with a homologous virus, which was boosted at each subsequent exposure, but no protection against infection with a heterologous virus. In general, first-year birds and second-year birds were equally susceptible to LPAIV infection. Finally, LPAIV infection did not cause detectable disease in BHGU.
LPAIV infection induced partial protection against subsequent infection with the homologous virus in birds in this study. Furthermore, this protection was boosted upon a second exposure to the homologous virus, with no excretion of infectious virus in H13-inoculated birds (0 of 5 birds) and only limited excretion in H16-inoculated birds (2 of 5 birds, until 2 dpi). This implies that after two or, at most, three serial infections with a homologous LPAIV, BHGU are no longer productively infected with LPAIV. Although the age at which BHGU will have had two LPAIV infections is unknown, it is likely that BHGU are exposed to LPAIV every year at the end of the breeding season (late summer) at large-colony breeding sites (6). Even if first-year BHGU have not been infected at their breeding colony site, they are most likely to become infected shortly afterwards, when nonbreeding BHGU and BHGU from multiple breeding sites mix abundantly. Thus, BHGU typically will probably have had at least two LPAIV infections after their second summer. Given the long-term protective effect of prior infections with homologous LPAIVs, BHGU older than 1.5 years of age may not be important for the persistence of LPAIV in the population.
LPAIV infection did not induce protection against subsequent infection with the heterologous virus in birds in this study. The level and duration of H16 virus excretion in group H13-H16-H16, 1 month after H13 virus infection, were similar to those of H16 virus excretion by immunologically naive birds (group sham-H16-H16). The effect of H16 virus infection on subsequent H13 virus infection [group H16-(H13)-H13] was ambiguous due to unsuccessful H13 virus inoculation 1 month after H16 inoculation: no H13 virus excretion was detected, although AIV-specific immunity was boosted. However, H13 virus inoculation 1 year after H16 virus inoculation in this group resulted in H13 virus excretion that was significantly smaller in quantity than but similar in duration to those in immunologically naive birds. The absence of a protective effect of H13 virus infection on subsequent H16 virus infection suggests that the epidemiological dynamics of H13 and H16 in BHGU are largely independent of each other; however, a partially protective effect of H16 virus infection on subsequent H13 virus infection cannot be excluded.
Previously, in other aquatic bird species, LPAIV infection induced partial protection against subsequent infection with a homologous virus and partial to nearly complete protection against subsequent infection with a heterologous virus. Similar to our findings in BHGU, LPAIV infection also induced partial protection against reinfection with the homologous virus in mallards (H7N7) (8) and Pekin ducks (H5N3 and H7N2, respectively) (7, 18). In those studies, the time interval between the first and second inoculations was relatively short, ranging from 21 to 84 days. In contrast to our findings in BHGU, LPAIV infection induced partial (H5N3 followed by H7N2 and H3N8 followed by H5N2 and vice versa, respectively) (7, 9) to nearly complete (8) protection against subsequent infection with a heterologous virus in Pekin ducks (7) and mallards (8, 9). The following differences in study design may play a role in this discrepancy. Compared to our study, the LPAIV subtypes used in those studies were different (H5N3 and H7N2 [7], H7N7 and H5N2 [8], and H3N8 and H5N2 [9]); the time interval between the first and second inoculations, 14 (8) or 21 days (7, 9), was shorter; the inoculum dose, 4 × 106 PFU (7) or 108.7 EID50 (8), was higher; and there were two prior infections with a heterologous virus (8) rather than one. In free-living mallards, heterosubtypic LPAIV immunity has been described for different HA subtypes belonging to the same phylogenetic clade (19). For the above-described mallard and Pekin duck studies with homologous as well as heterologous inoculations, the time interval between subsequent infections was relatively short; consequently, it is unknown if protection would have lasted for 1 year, which is the typical interval between epizootics in mallards (2) and BHGU (6).
Overall, the results of this study showed no effect of age on susceptibility to LPAIV infection: there were no differences between immunologically naive 2- and 14-month-old BHGU in the proportion of birds infected, quantity of LPAIV excreted, or duration of LPAIV excretion. An exception was the duration of H13 virus excretion, which was significantly longer in 2-month-old than in 14-month-old birds. The latter result needs to be interpreted with caution, because there was already quite a high degree of variability in the virus excretion results between groups of 2-month-old birds inoculated with the same virus (Table 2). These results correspond with those reported previously by Costa et al. (11), who inoculated LPAIV H5N2 or LPAIV H3N8 into mallards ranging from 2 weeks to 4 months of age and found no significant effect of age on the proportion of birds infected or the level of LPAIV excretion. However, these results are in contrast with the results of VanDalen et al. (12), who inoculated LPAIV H4N6 into 3- or 6-month-old mallards and found a significantly larger quantity of excreted viral RNA in 6-month-old birds. Together, these results indicate that the more frequent detection of LPAIV in juvenile than in adult free-living water birds (e.g., see references 2, 20, and 21) cannot be explained by age-dependent susceptibility.
There was no evidence of clinical disease from LPAIV infection in the birds in this study. In order to be able to detect possible clinical signs as sensitively as possible, we measured several parameters (fecal water content, fecal mass, and number of droppings) related to diarrhea, which is often associated with intestinal infections (1). However, none of these parameters were correlated with LPAIV excretion. In addition, there was no loss of body weight, decreased activity level, or any other clinical sign. These results indicate that LPAIV H13 and H16 infections do not cause clinical disease in BHGU. Hypothetically, selection for such a lack of virulence of LPAIV may be driven by the mobility of wild water birds, because any virulence would render the infected bird less mobile, as well as inducing it to separate from the rest of its group and thus reducing the contact rate and therefore the transmission rate (22). However, a caveat of this study, as for any laboratory infection of wild animals, is that the circumstances were very different from those in the field (1). For example, birds were not exposed to a harsh climate or food scarcity and instead were kept at a constant temperature and fed ad libitum. Therefore, a failure to observe clinical signs under laboratory circumstances does not mean that LPAIV is not virulent for BHGU under field circumstances.
Unexpected results of this study were that AIV-specific serum antibodies had little value as a correlate of protection or as evidence of prior infection. First, although LPAIV infection (either H13 or H16) induced partial protection against reinoculation with the homologous virus, this protective effect, at the between-group level, was independent of the presence of AIV-specific antibodies on the day of reinoculation. Moreover, at the within-group level, the presence or titer of H16-specific antibodies on the day of reinoculation was not associated with decreased or shortened H16 virus excretion. Also, at the between-group level (sham-H16-H16 versus H13-H16-H16), the detection of H16-specific antibodies at 1 week post-H13 inoculation was not associated with a protective effect against subsequent H16 infection at 4 weeks post-H13 inoculation. These results show that the presence or titer of AIV-specific serum antibodies in BHGU is not a correlate of protection against LPAIV infection. These results in BHGU correspond to those in mallards (8), where virus excretion after challenge with homologous LPAIV was independent of AIV-specific ELISA antibodies on the day of challenge (8).
Mucosal rather than serum antibodies may be a better correlate of protection, as LPAIV in BHGU (15) and mallards (23) infects the digestive tract. Although already suggested in 1980 by Kida and colleagues (18), mucosal antibodies against virus infections in birds have received little attention, perhaps because of technical difficulties in measurements. AIV-specific antibodies have been detected in bile of ducks infected with AIV (24). Also, mucosal antibodies have been detected in tears of chickens after infection with Newcastle disease virus and infectious bronchitis virus and were associated with partial protection against virus challenge (25, 26). In humans, rotavirus-specific IgA in fecal specimens was directly correlated with protection against rotavirus illness (27, 28). Therefore, the use of mucosal antibodies in feces as a potential correlate of protection of water birds against LPAIV infection in the digestive tract deserves further research.
Second, this study shows that the use of AIV-specific serum antibodies to provide evidence of prior AIV infection is limited. Experimental infections using wild-caught or farm-raised birds often rely on the absence of AIV-specific antibodies to indicate the absence of past infection (8, 29–31). However, in our study, the vast majority of BHGU did not have NP- or HA-specific antibodies 1 month after primary H13 or H16 infection, with the exception of H16-specific antibodies after H16 infection. Furthermore, even in the birds that seroconverted, AIV-specific antibodies remained detectable for a maximal period of only 2 to 3 months (for NP- and H13-specific antibodies) or 11.5 months (for H16-specific antibodies) after primary H13 or H16 infection. Thus, our results indicate that a lack of AIV-specific serum antibodies does not exclude past LPAIV infection in BHGU.
The differences in patterns of virus excretion and immunogenicity between the two LPAIV isolates used in this study are most likely due to genetic differences in three gene segments, HA, NA, and NS; the other gene segments were genetically highly similar (sequences are available online [see Materials and Methods]). There were obvious differences in the HA genes (H13 versus H16) and NA genes (N2 versus N3). Therefore, exposure to a LPAIV with the same HA and NA genes may have strengthened the protective effect against reinoculation with the homologous virus. The NS gene segment of H13 belonged to allele B, and that of H16 belonged to allele A (the most common NS allele) (32, 33). The NS1 protein, one of the two proteins encoded by the NS gene segment, is able to inhibit the host innate immune response by antagonizing interferon. Viruses with allele A of the NS1 protein replicated more than did those with allele B in chicken and turkey cells; in contrast, viruses with allele B of the NS1 protein replicated more and to higher titers than did those with allele A in duck cells (34).
In conclusion, we demonstrate that experimental LPAIV infection of BHGU has a protective effect, lasting up to 1 year, on reinfection with a homologous virus but no protective effect on subsequent infection with a heterologous virus. The information generated in this study (e.g., quantity of virus excreted and duration of excretion of infectious virus after the first, second, and third infections with homologous or heterologous viruses) should be useful information to help design surveillance programs of AIV in wild birds and to interpret data generated by these programs. It should also help to build mathematical models to study the epidemiology of LPAIV in BHGU and other free-living aquatic birds. Nevertheless, additional research is needed to show if the same AIV dynamics apply to other bird species and other AIV subtypes and strains. Given the lack of correlation between AIV-specific serum antibodies and protection against LPAIV infection, further research is required to elucidate the mechanism of protection of LPAIV infection and which parameters (e.g., mucosal antibody levels) can be used as correlates of protection. In addition, this study points out that the lack of detectable AIV-specific serum antibodies in birds does not exclude the possibility of a past LPAIV infection. Knowledge on long-term protection against homologous and heterologous LPAIV infections in an aquatic bird species like BHGU, which are annually exposed at its breeding colony sites in West Europe, is essential to understand LPAIV epidemiology and persistence in wild birds.
ACKNOWLEDGMENTS
We thank Angéla Gommersbach and Tanja Schouten (RIVM animal caretakers), Ellis Mulder and Cor Dijkstra (University of Groningen, for sexing of BHGU), Monique de Vrijer (Vogelklas Karel Schot, for advice on hand-raising and nutrition of birds), and Francisca Velkers (Utrecht University, for advice on hand-raising and nutrition of birds).
U.H. was partly funded by a Netherlands Genomics Institute/Virgo Consortium visiting scientist stipend. This work was sponsored by grants from the Dutch Ministry of Economic Affairs, European Research Council project FLUPLAN (250136), and NIH NIAID contracts HHSN266200700010C (2007-2014) and HHSN272201400008C (2014-2021).
REFERENCES
- 1.Kuiken T. 2013. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc Biol Sci 280:20130990. doi: 10.1098/rspb.2013.0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Baas C, Lexmond P, Waldenstrom J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WE, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog 3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol Rev 56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graves IL. 1992. Influenza viruses in birds of the Atlantic flyway. Avian Dis 36:1–10. doi: 10.2307/1591706. [DOI] [PubMed] [Google Scholar]
- 5.Velarde R, Calvin SE, Ojkic D, Barker IK, Nagy E. 2010. Avian influenza virus H13 circulating in ring-billed gulls (Larus delawarensis) in southern Ontario, Canada. Avian Dis 54:411–419. doi: 10.1637/8808-040109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 6.Verhagen JH, Majoor F, Lexmond P, Vuong O, Kasemir G, Lutterop D, Osterhaus AD, Fouchier RA, Kuiken T. 2014. Epidemiology of influenza A virus among black-headed gulls, the Netherlands, 2006-2010. Emerg Infect Dis 20:138–141. doi: 10.3201/eid2001.130984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaise C, Lalmanach AC, Marty H, Soubies SM, Croville G, Loupias J, Marc D, Quere P, Guerin JL. 2014. Protection patterns in duck and chicken after homo- or hetero-subtypic reinfections with H5 and H7 low pathogenicity avian influenza viruses: a comparative study. PLoS One 9:e105189. doi: 10.1371/journal.pone.0105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jourdain E, Gunnarsson G, Wahlgren J, Latorre-Margalef N, Brojer C, Sahlin S, Svensson L, Waldenstrom J, Lundkvist A, Olsen B. 2010. Influenza virus in a natural host, the mallard: experimental infection data. PLoS One 5:e8935. doi: 10.1371/journal.pone.0008935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa TP, Brown JD, Howerth EW, Stallknecht DE. 2010. Effect of a prior exposure to a low pathogenic avian influenza virus in the outcome of a heterosubtypic low pathogenic avian influenza infection in mallards (Anas platyrhynchos). Avian Dis 54:1286–1291. doi: 10.1637/9480-072210-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepin KM, VanDalen KK, Mooers NL, Ellis JW, Sullivan HJ, Root JJ, Webb CT, Franklin AB, Shriner SA. 2012. Quantification of heterosubtypic immunity between avian influenza subtypes H3N8 and H4N6 in multiple avian host species. J Gen Virol 93:2575–2583. doi: 10.1099/vir.0.045427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa TP, Brown JD, Howerth EW, Stallknecht DE. 2010. The effect of age on avian influenza viral shedding in mallards (Anas platyrhynchos). Avian Dis 54:581–585. doi: 10.1637/8692-031309-ResNote.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanDalen KK, Franklin AB, Mooers NL, Sullivan HJ, Shriner SA. 2010. Shedding light on avian influenza H4N6 infection in mallards: modes of transmission and implications for surveillance. PLoS One 5:e12851. doi: 10.1371/journal.pone.0012851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fereidouni SR, Grund C, Hauslaigner R, Lange E, Wilking H, Harder TC, Beer M, Starick E. 2010. Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenicity avian influenza viruses. Avian Dis 54:79–85. doi: 10.1637/9005-073109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 14.Tolf C, Latorre-Margalef N, Wille M, Bengtsson D, Gunnarsson G, Grosbois V, Hasselquist D, Olsen B, Elmberg J, Waldenstrom J. 2013. Individual variation in influenza A virus infection histories and long-term immune responses in mallards. PLoS One 8:e61201. doi: 10.1371/journal.pone.0061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofle U, Van de Bildt MW, Leijten LM, Van Amerongen G, Verhagen JH, Fouchier RA, Osterhaus AD, Kuiken T. 2012. Tissue tropism and pathology of natural influenza virus infection in black-headed gulls (Chroicocephalus ridibundus). Avian Pathol 41:547–553. doi: 10.1080/03079457.2012.744447. [DOI] [PubMed] [Google Scholar]
- 16.Munster VJ, Baas C, Lexmond P, Bestebroer TM, Guldemeester J, Beyer WE, de Wit E, Schutten M, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2009. Practical considerations for high-throughput influenza A virus surveillance studies of wild birds by use of molecular diagnostic tests. J Clin Microbiol 47:666–673. doi: 10.1128/JCM.01625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirst GK. 1943. Studies of antigenic differences among strains of influenza A by means of red cell agglutination. J Exp Med 78:407–423. doi: 10.1084/jem.78.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kida H, Yanagawa R, Matsuoka Y. 1980. Duck influenza lacking evidence of disease signs and immune response. Infect Immun 30:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latorre-Margalef N, Grosbois V, Wahlgren J, Munster VJ, Tolf C, Fouchier RA, Osterhaus AD, Olsen B, Waldenstrom J. 2013. Heterosubtypic immunity to influenza A virus infections in mallards may explain existence of multiple virus subtypes. PLoS Pathog 9:e1003443. doi: 10.1371/journal.ppat.1003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmley EJ, Bastien N, Booth TF, Bowes V, Buck PA, Breault A, Caswell D, Daoust PY, Davies JC, Elahi SM, Fortin M, Kibenge F, King R, Li Y, North N, Ojkic D, Pasick J, Pryor SP, Robinson J, Rodrigue J, Whitney H, Zimmer P, Leighton FA. 2008. Wild bird influenza survey, Canada, 2005. Emerg Infect Dis 14:84–87. doi: 10.3201/eid1401.061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stallknecht DE, Shane SM. 1988. Host range of avian influenza virus in free-living birds. Vet Res Commun 12:125–141. doi: 10.1007/BF00362792. [DOI] [PubMed] [Google Scholar]
- 22.Kuiken T, Harder T. 2012. H5N1 highly pathogenic avian influenza: breaking the rules in disease emergence, p 228–238. In Aguirre AA, Ostfeld RS, Daszak P (ed), New directions in conservation medicine: applied cases of ecological health. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 23.Daoust PY, Kibenge FS, Fouchier RA, van de Bildt MW, van Riel D, Kuiken T. 2011. Replication of low pathogenic avian influenza virus in naturally infected mallard ducks (Anas platyrhynchos) causes no morphologic lesions. J Wildl Dis 47:401–409. doi: 10.7589/0090-3558-47.2.401. [DOI] [PubMed] [Google Scholar]
- 24.Higgins DA, Shortridge KF, Ng PL. 1987. Bile immunoglobulin of the duck (Anas platyrhynchos). II. Antibody response in influenza A virus infections. Immunology 62:499–504. [PMC free article] [PubMed] [Google Scholar]
- 25.Gelb J Jr, Nix WA, Gellman SD. 1998. Infectious bronchitis virus antibodies in tears and their relationship to immunity. Avian Dis 42:364–374. doi: 10.2307/1592487. [DOI] [PubMed] [Google Scholar]
- 26.Takada A, Kida H. 1996. Protective immune response of chickens against Newcastle disease, induced by the intranasal vaccination with inactivated virus. Vet Microbiol 50:17–25. doi: 10.1016/0378-1135(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 27.Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. 1992. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol 30:1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matson DO, O'Ryan ML, Herrera I, Pickering LK, Estes MK. 1993. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis 167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 29.Reperant LA, van de Bildt MW, van Amerongen G, Buehler DM, Osterhaus AD, Jenni-Eiermann S, Piersma T, Kuiken T. 2011. Highly pathogenic avian influenza virus H5N1 infection in a long-distance migrant shorebird under migratory and non-migratory states. PLoS One 6:e27814. doi: 10.1371/journal.pone.0027814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keawcharoen J, van Riel D, van Amerongen G, Bestebroer T, Beyer WE, van Lavieren R, Osterhaus AD, Fouchier RA, Kuiken T. 2008. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown J, Poulson R, Carter D, Lebarbenchon C, Pantin-Jackwood M, Spackman E, Shepherd E, Killian M, Stallknecht D. 2012. Susceptibility of avian species to North American H13 low pathogenic avian influenza viruses. Avian Dis 56:969–975. doi: 10.1637/10158-040912-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wille M, Robertson GJ, Whitney H, Bishop MA, Runstadler JA, Lang AS. 2011. Extensive geographic mosaicism in avian influenza viruses from gulls in the northern hemisphere. PLoS One 6:e20664. doi: 10.1371/journal.pone.0020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zohari S, Gyarmati P, Ejdersund A, Berglof U, Thoren P, Ehrenberg M, Czifra G, Belak S, Waldenstrom J, Olsen B, Berg M. 2008. Phylogenetic analysis of the non-structural (NS) gene of influenza A viruses isolated from mallards in Northern Europe in 2005. Virol J 5:147. doi: 10.1186/1743-422X-5-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams S, Xing Z, Li J, Mendoza K, Perez D, Reed K, Cardona C. 2013. The effect of avian influenza virus NS1 allele on virus replication and innate gene expression in avian cells. Mol Immunol 56:358–368. doi: 10.1016/j.molimm.2013.05.236. [DOI] [PubMed] [Google Scholar]