Abstract
Background:
The aim of this study was to demonstrate similarities and differences between mothers and daughters regarding dietary and non-dietary risk factors for bone fractures and osteoporosis.
Methods:
The study was carried out in 2007–2010 on 712 mothers (29–59 years) and daughters (12–21 years) family pairs. In the sub-sample (170 family pairs) bone mineral density (BMD) was measured for the forearm by dual-energy x-ray absorptiometry (DXA). The consumption of dairy products was determined with a semi-quantitative food frequency questionnaire (ADOS-Ca) and calcium intake from the daily diet was calculated.
Results:
The presence of risk factors for bone fractures in mothers and daughters was significantly correlated. The Spearman rank coefficient for dietary factors of fracture risk was 0.87 (P<0.05) in whole sub-sample, 0.94 (P<0.05) in bottom tercile of BMD, 0.82 (P<0.05) in middle tercile of BMD, 0.54 (P>0.05) in upper tercile of BMD and for non-dietary factors of fracture risk was 0.83 (P<0.05) in whole sub-sample, 0.86 (P<0.05) in bottom tercile of BMD, 0.93 (P<0.05) in middle tercile of BMD, 0.65 (P<0.05) in upper tercile of BMD.
Conclusions:
Our results confirm the role of the family environment for bone health and document the stronger effect of negative factors of the family environment as compared to other positive factors on bone fracture risk.
Keywords: Fracture risk factors, BMD, Osteoporosis, Mother, Daughter
Introduction
Osteoporosis is a systemic metabolic bone disease which produces a reduction in bone density together with abnormal structure (1, 2). Currently, both the diagnosis and therapy of osteoporosis focus on determining the individual risk for bone fracture (3). This approach requires collecting information on the occurrence of dietary and non-dietary risk factors for bone fractures. Dietary risk factors for bone fractures include calcium intake (4). It is commonly thought that adequate calcium intake improves bone mineral density (5). Dietary calcium intake and dairy product consumption are the most important during childhood and adolescence (5). Non-dietary factors increasing bone fracture risk include female sex, white race, advanced age, menstruation disorders, early menopause, previous fractures, hip fractures in parents, low BMI, low bone mass, administration of corticosteroids, rheumatoid arthritis, low physical activity, poor self-evaluated health, smoking and alcohol abuse (1, 3). Bone mineral density is under the strong genetic influence (6, 7).
Epidemiological observations document the family background of osteoporosis (8, 9). Individuals with a family history of osteoporosis reveal a tendency to a more intense bone degradation process. Therefore, the probability of osteoporosis in a woman whose mother had this condition, as well as in a woman who inherited tall height or slight build bone from her mother is high (10). It is estimated that heredity determines 70–85% of the mineral density of axial skeleton bones and proximal heads of femurs and 50–60% of the appendicular skeleton (11). The rate of bone density loss and inherited tendency to develop osteoporosis can be reduced by a change of modifiable factors of bone fracture risk, including an improvement in calcium intake (12).
The family environment affects the food intake by members of the family (13, 14). Since women are more sensitive to environmental stimuli, positive attitudes of mothers towards health can have an effect on more favourable dietary patterns of the daughter and their better nutritional status (15, 16). In Polish teenagers, Jeżewska-Zychowicz (14) revealed that positive eating habits occurring in the family, hospitality and openness to knowledge were conductive to more appropriate frequency of consumption of milk, dairy products, fruit and raw and cooked vegetables. In American youth the frequency of having common family meals was positively related to the intake of fruit, vegetables, cereal products and calcium rich food, and negatively related to the consumption of soft beverages (17). Thus a strong positive correlation between family meal consumption and the quality of the diet was showed.
Mutual relations between genetic and non-genetic factors determine higher intensification of osteoporosis in some families and lower intensification in others. The improvement of public health and prevention of osteoporosis requires better understanding of family environment factors. An important element of this understanding is to determine the burden of dietary and non-dietary risk factors for bone fractures and osteoporosis in mothers and daughters.
The aim of the study was to demonstrate similarities and differences between mothers and daughters regarding risk factors for bone fractures and osteoporosis.
Materials and Methods
Sample selection
The study was carried out in 2007–2010 within the scientific project “Analysis of mother-daughter dairy products dietary patterns in relation to bone mineral status and calcium deficiency and osteoporosis risk among women. MODAF Study”.
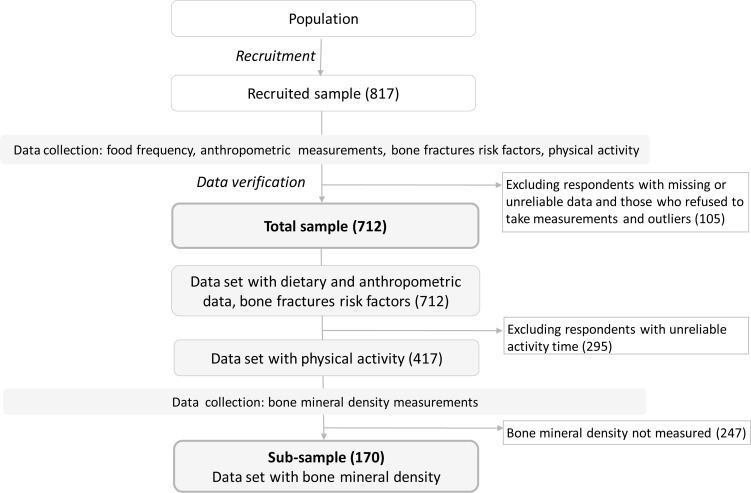
A recruitment details and inclusion/exclusion criteria can be found elsewhere (15). Briefly, the participants were a convenience sample. The study included mothers ageing under 60 years and daughters ageing above 12 years and before 21 years. Initially, 817 mother-daughter family pairs were recruited (Fig. 1). During data verification 105 mother-daughter family pairs were removed. Finally, the study included 712 mothers and daughters (Table 1). In the total sample, the dietary study was carried out and bone fractures risk factors were collected. In the subsample (170 family pairs) bone mineral density (BMD) was measured and some other osteoporosis risk factors was collected. For the subsample all mothers and daughters were chosen living in one typical area of Poland.
Fig. 1:
Sample selection and data collection. Notes: () in the brackets indicated the number of mothers and daughters
Table 1:
Description of the total sample and sub-sample of mothers and daughters tested with bone densitometry
| Variables | Total sample | Sub-sample | ||
|---|---|---|---|---|
| Mothers | Daughters | Mothers | Daughters | |
| Sample size | 712 | 712 | 170 | 170 |
| Age § (years) | 43.8±5.9 (29–59) | 16.9±2.6 (12–21) | 45.5±5.8 (32–59) | 18.1±2.9 (12–21) |
| BMI § (kg/m2) | 25.6±4.2 | 20.7±2.6 | 26.2±4.6 | 20.5±2.8 |
| Mothers’ age groups † (% of the sample) | ||||
| 29–39 years | 28 | 16 | ||
| 40–49 years | 56 | 61 | ||
| 50–59 years | 16 | 22 | ||
| Daughters’ age groups # (% of the sample) | ||||
| <15 years | 30 | 18 | ||
| 15–18 years | 29 | 17 | ||
| >18 years | 41 | 65 | ||
| Education ‡ (% of the sample) | ||||
| elementary | 1 | 41 | 1 | 25 |
| secondary | 67 | 38 | 71 | 20 |
| higher | 28 | 21 | 28 | 55 |
| Place of living ¥ (% of the sample) | ||||
| village | 48 | 63 | ||
| town <50 000 residents | 16 | 6 | ||
| town 50–100 000 residents | 14 | 11 | ||
| city >100 000 residents | 22 | 20 | ||
| Self-declared economic situation ¥ (% of the sample) | ||||
| bad | 1 | 1 | ||
| satisfactorily | 23 | 29 | ||
| good | 66 | 63 | ||
| very good | 10 | 7 | ||
| Description of household ¥ (% of the sample) | ||||
| we live very poorly | 0 | 1 | ||
| we live poorly | 1 | 2 | ||
| we live modestly | 7 | 8 | ||
| we live very thriftily | 14 | 13 | ||
| we live relatively thriftily | 54 | 51 | ||
| we live very good | 23 | 25 | ||
| Physical activity § (MET-minutes/week) | 1609±1105 | 1111±820 | 1996±1359 | 1309±1026 |
mean ± standard deviation; significant differences between total sample and sub-sample at P<0.05 as follows:
in mothers,
in daughters,
for daughters was given present educational level
in family; () in the brackets indicated minimum-maximum range;
for 417 mother-daughter family pairs with complete physical activity data
The research received permission from: (i) the Bioethics Committee of the Regional Medical Chamber in Olsztyn in 2001, on June 27, 2001, Resolution No. 49/2001 and (ii) the Bioethics Committee of the Faculty of Medical Sciences, University of Warmia and Mazury in Olsztyn on June 17, 2010, Resolution No. 20/2010.
General information
All information was collected by personal interview and measured by well-trained researchers. The respondents were asked about their date of birth, place of residence and education level (“elementary”, “secondary” or higher”). Two closed-questions were used for description of the family economic situation:
– self-declared economic situation with four answers: “bad”, “satisfactory”, “good” and “very good”,
– self-declared situation of the household with six answers (based on the Polish Central Office of Statistics): (i) “we live very poorly – we do not have enough resources even for the cheapest food and clothing”, (ii) “we live poorly – we do not have enough resources for housing fees”, (iii) “we live modestly – we have enough resources only for food and clothing”, (iv) “we live very thriftily”, (v) “we live relatively thriftily”, (vi) “we live very well – we can afford everything without limitations”
Assessment of consumption of dairy products and dietary calcium
The consumption of dairy products was determined using a validated semi-quantitative food frequency questionnaire (acronym ADOS-Ca) (16). Calcium intake from each dairy product and daily diet was calculated. More details for this calculation can be found elsewhere (15, 16). Briefly, the data was collected on the frequency of consumption and portion size usually consumed (during the last six months) of 11 groups of dairy products. Respondents were questioned about typically-eaten servings. The calcium intake (mg/day) from dairy products was calculated by summing up the calcium intakes from all groups of dairy products. Finally, the calcium intake from the daily diet was calculated on the basis of calcium intake from dairy products according to the formula worked out in the validation study (16).
The number of mothers and daughters (in %) who met the Polish calcium intake recommendations and did not meet calcium intake recommendations was calculated (18) using the probability method. The established cut-off points (i.e., z-values of individual calcium intake <−1SD or >1SD) produced conclusions with a probability of 0.85.
Collection of risk factors for bone fractures and osteoporosis
Information on risk factors influencing bone mass and presence of osteoporosis was collected. A questionnaire with closed-questions was used. A comprehensive list of dietary and non-dietary risk factors was prepared. In total, information regarding 21 risk factors was collected, including dietary (6 items) and non-dietary risk factors (15 items) (2, 3, 5). Low BMI was not included in any subgroup because of the difficulty of classifying it as a non-dietary risk factor, although weight and BMI are strongly correlated with diet.
Information about the customary consumption of ready-made calcium enriched cereal products and calcium enriched juices was collected as well as everyday consumption of dairy products at pre-school and school age (yes, no, I don’t know).
During the interview information about non-dietary risk factors affecting bone weight and occurrence was collected, such as:
occurrence of bone pains (yes, no, I don’t know),
current smoking (no, occasionally ≤5 pcs/day, >pcs/day),
past fractures (yes, no, I don’t know),
calcium supplement use (yes, no, I don’t know),
incidence of other chronic diseases than tthyroid diseases and rheumatoid arthritis, e.g. lung diseases, cardiac diseases, vascular diseases, cancer, diabetes mellitus, stroke (19) (yes, no),
menstruation (yes – regularly, yes – irregularly, I haven’t started menstruating, I have stopped menstruating),
incidence of osteoporosis in the family (yes, no, I don’t know),
physical activity (low, moderate, high),
incidence of thyroid diseases (yes, no),
hormonal contraceptive use (yes, no),
glucocorticosteroid use (yes, no),
incidence of rheumatoid arthritis (yes, no),
sun exposure frequency (I avoid sun exposure, rarely, often, very often),
health status in own assessment (very good, good, quite good, poor),
consumption of significant amounts of alcohol (yes, no, I don’t know).
The physical activity level was determined by using a validated International Physical Activity Questionnaire (IPAQ), a long version last 7 days, expressed as a standard Metabolic Energy Turnover in MET-minutes/week (20, 21). According to the procedure made by the IPAQ Research Committee, 295 family pairs were excluded (activity time >960 minutes/24h). Three levels of physical activity were classified: low (<600 MET-minutes/week), moderate (600–2999), high (3000 or more).
Assessment of Bone Mineral Density and body mass
Bone mineral density (BMD, g/cm2) was measured with a pDEXA densitometer with a dual-energy X-ray absorptiometry (DXA) in the distal part of the radius and the ulna (2). Three subgroups were created according to BMD terciles (bottom, middle, upper) for mothers (mT1BMD, mT2BMD, mT3BMD) and separately for daughters (cT1BMD, cT2BMD, cT3BMD).
Weight and height of the respondents were measured and body mass index was calculated (BMI, kg/m2).
Statistical analysis
The average intake of calcium of dairy products, age, BMI, BMD and T-score BMD were expressed with the mean (x) and the standard deviation (SD).
The Spearman’s rank correlation for calcium in-take from dairy products and dietary calcium in-take of mothers and daughters were calculated (22). Lists of dairy products according to their diminishing share in calcium supply in the daily diet were created separately for mothers and daughters. Similarity of the calcium source ranking in diets of mothers and daughters as well as similarity of dietary and non-dietary bone risk factor ranking was verified using a Spearman rank order test.
Spearman’s rank correlation for BMD and three fracture risk factors with numerical data (age, dietary calcium intake, physical activity) were calculated separately for mothers and daughters. Before the analysis, BMD (in g/cm2), dietary calcium in-take (in mg/day) and physical activity (in MET-minutes/week) were converted to SD scores by using values obtained for the participants.
The relationship between BMD mothers and BMD daughters in a complex system was evaluated with a correspondence analysis using Burt’s panels. We include 6 features into the correspondence analysis. We analyzed the relations between BMD mothers and BMD daughters divided into terciles (mT1BMD, mT2BMD, mT3BMD, dT1BMD, dT2BMD, dT3BMD) and obtained a high level of explained inertia in this model (56%).
The statistical analysis was performed with Statistica 10.0 PL software (StatSoft, Poland).
Results
The women from the total sample and in the sub-sample did not significant differ in social and demographic parameters as well as BMI in either mothers or daughters (Table 1).
Calcium intake from all dairy products by mothers and daughters was correlated (P<0.05) (Table 2). The correlation coefficients ranged from 0.29 for cheese for spreading to 0.17 for cream. The correlation coefficient for the dietary calcium intake of mothers and daughters was 0.25 in the total sample and 0.23 in sub-sample (both P<0.05) (Table 2).
Table 2:
Comparison of calcium intake from dairy products and dietary calcium intake by mothers and daughters in the total sample (mg/day; mean±standard deviation)
| Variables | Mothers | Daughters | Mother-daughter differences | Mother-daughter correlation coefficient |
|---|---|---|---|---|
| Sample size | 712 | 712 | ||
| Milk | 156±205 | 160±195 | −4 | 0.19* |
| Rennet cheese | 90±123 | 135±172 | −45*** | 0.25* |
| Fruit yoghurt | 58±79 | 101±131 | −43*** | 0.22* |
| Natural yoghurt | 54±105 | 32±70 | 22*** | 0.19* |
| Kefir, buttermilk | 22±46 | 19±44 | 3* | 0.22* |
| Processed cheese | 20±48 | 29±65 | −9** | 0.27* |
| Fresh cheese | 15±24 | 10±18 | 5*** | 0.25* |
| Homogenized cheese | 9±16 | 17±24 | −8*** | 0.22* |
| Ice-creams | 8±13 | 28±33 | −20*** | 0.23* |
| Cream | 5±12 | 2±6 | 3*** | 0.17* |
| Cheese for spreading | 2±16 | 2±6 | 0 | 0.29* |
| Calcium from dairy products in total | 441±314 | 535±354 | −94*** | 0.25* |
| Dietary calcium | 596±424 | 723±479 | −127*** | 0.25* |
significance of differences at:
P<0.05;
P<0.01;
P<0.001
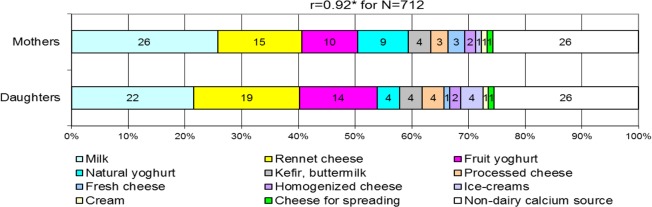
A high correlation (r=0.92) between mothers and daughters was found for calcium sources and their share in diet (Fig. 2). The main sources of calcium in the daily diet were in mothers: milk, rennet cheese, fruit yoghurt and natural yoghurt and in daughters: milk, rennet cheese and fruit yoghurt. Mothers supplied in their daily diet significantly less calcium than daughters (596 vs. 723 mg/day) (Table 2), both intakes were extremely below the Polish national recommendation (RDA for calcium: 1000–1300 mg/day) (18). Daughters, in comparison to mothers, consumed significantly more calcium from rennet cheese, fruit yoghurt, ice-creams, processed cheese and homogenized cheese.
Fig. 2:
Comparison of the calcium source ranking similarity according to calcium sources share in daily diet of mothers and daughters in the total sample (mean value in % calcium). Notes: r – correlation coefficient for dietary calcium source ranking; significance of differences at: * P <0.05
The presence of risk factors for bone fractures in mothers and daughters was significantly correlated (Table 3). The Spearman rank coefficients were significant for dietary factors of fracture risk: 0.87 in whole sub-sample, 0.94 in bottom and 0.82 in middle tercile of BMD, and for non-dietary factors of fracture risk: 0.83 in whole sub-sample, 0.86 in bottom, 0.93 in middle, 0.65 in upper tercile of BMD.
Table 3:
Comparison of the occurrence of fracture risk factors in mothers and daughters by BMD terciles in the sub-sample
| Variables | Mothers | Daughters | Mothers and daughters T1BMD | Mothers and daughters T2BMD | Mothers and daughters T3BMD | |||
|---|---|---|---|---|---|---|---|---|
| mT1BMD | dT1BMD | mT2BMD | dT2BMD | mT3BMD | dT3BMD | |||
| Sample size | 170 | 170 | 57 | 57 | 57 | 56 | 56 | 57 |
| Age (years) | 45.5±5.8 | 18.1±2.9 | 47.1±6.8A,B | 16.5±3.3a,b | 44.3±5.0A | 19.2±2.0a | 45.3±5.3B | 18.7±2.4b |
| BMD (mg/cm2)*** | 379±59 | 337±56 | 322±29α | 276±34α | 374±15β | 340±13β | 443±45γ | 396±28γ |
| Dietary calcium intake §(mg/day) | 507±363 | 577±395 | 536±363 | 613±417 | 461±279 | 552±407 | 522±433 | 567±364 |
| Physical activity § (MET-minutes/week)*** | 1996±1359 | 1309±1026 | 2256±1469δ,C | 1188±996δ | 1571±1317C,D | 1227±1079 | 2165±1193ɛ,D | 1508±990ɛ |
| Dietary risk factors (% of the sample) | ||||||||
| Consumption of calcium-enriched juices | 72 | 76 | 70 | 79 | 75 | 75 | 71 | 75 |
| Daily consumption of dairy products during pre-school periods** | 64 | 54 | 60 | 54 | 86 | 48 | 75 | 58 |
| Consumption of ready-made calcium enriched cereal products* | 63 | 74 | 61 | 79 | 70 | 73 | 57 | 68 |
| Daily consumption of dairy products during school periods*** | 61 | 31 | 61 | 37 | 47 | 25 | 73 | 30 |
| Respondents who did not meet# calcium intake recommendations | 11 | 3 | 12 | 7 | 9 | 0 | 11 | 2 |
| Respondents who met# calcium intake recommendations | 2 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Correlation coefficient for dietary risk factor ranking | 0.87* | 0.94* | 0.82* | 0.54 | ||||
| Non-dietary risk factors (% of the sample) | ||||||||
| Bone pains*** | 75 | 56 | 74 | 53 | 75 | 64 | 77 | 51 |
| Current smoking*** | 25 | 7 | 18 | 7 | 32 | 5 | 25 | 9 |
| Past fractures | 24 | 25 | 25 | 26 | 32 | 23 | 16 | 25 |
| Calcium supplementation | 22 | 16 | 25 | 14 | 21 | 16 | 21 | 18 |
| Other chronic diseases †*** | 22 | 10 | 25 | 12 | 14 | 7 | 27 | 11 |
| Menstruation disorders** | 18 | 9 | 28 | 23 | 7 | 4 | 20 | 2 |
| Osteoporosis in the family | 16 | 16 | 16 | 20 | 11 | 16 | 21 | 17 |
| Low physical activity ¥** | 15 | 29 | 7 | 26 | 26 | 38 | 11 | 23 |
| Thyroid diseases** | 10 | 3 | 11 | 2 | 5 | 4 | 14 | 4 |
| Hormonal contraceptive use | 7 | 9 | 9 | 4 | 9 | 7 | 2 | 16 |
| Glucocorticosteroid use* | 6 | 2 | 5 | 2 | 9 | 2 | 4 | 2 |
| Rheumatoid arthritis** | 5 | 0 | 5 | 0 | 5 | 0 | 4 | 0 |
| Avoiding sun exposure*** | 5 | 0 | 7 | 0 | 5 | 0 | 4 | 0 |
| Poor health condition** | 4 | 0 | 5 | 0 | 2 | 0 | 4 | 0 |
| Consumption of significant amounts of alcohol | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Correlation coefficient for non-dietary risk factor ranking | 0.83* | 0.86* | 0.93* | 0.65* | ||||
mean ± standard deviation;
used cut-off points produced conclusions with a probability of 0.85 and, because of this, the number respondents who met or did not meet calcium intake recommendations do not sum up to 100%;
other chronic diseases than thyroid diseases and rheumatoid arthritis, e.g. lung diseases, cardiac diseases, vascular diseases, cancers, diabetes mellitus, stroke;
low physical activity: <600 MET-minutes/week; mT1BMD – bottom tercile BMD by mothers, mT2BMD – middle tercile BMD by mothers, mT3BMD – upper tercile BMD by mothers; dT1BMD – bottom tercile BMD by daughters, dT2BMD – middle tercile BMD by daughters, dT3BMD – upper tercile BMD by daughters; significance of differences between mothers and daughters in whole sub-sample at:
P <0.05;
P<0.01;
P<0.001; significance of differences at P<0.05:
between mothers’ BMD terciles
between daughters’ BMD terciles,
between mothers and daughters’ BMD terciles;
Mothers from the bottom tercile of BMD were significantly older than mothers from the middle or upper tercile of BMD (Table 3). Daughters from the bottom tercile of BMD were significantly younger than daughters from the middle or upper tercile of BMD.
BMD of mothers was significantly higher than daughters in the whole sub-sample (379 vs. 337 mg/cm2, respectively) as well as in each BMD tercile (Table 3). More mothers than daughters consumed dairy products daily during pre-school and school periods. In comparison to daughters, fewer mothers consumed ready-made cereal products enriched with calcium. Significantly more mothers than daughters suffered from bone pains, smoked, suffered from chronic diseases, menstruation disorders, thyroid diseases, rheumatoid arthritis, poor health condition, used glucocorticosteroids avoided sun exposure. The total physical activity was significantly higher in mothers than daughters in the whole sub-sample as well as in the bottom and upper BMD terciles.
No significant correlation was found between BMD and dietary calcium intake or BMD and physical activity in the total sub-sample and each BMD tercile in mothers as well as daughters (Table 4). In daughters whole sub-sample, the correlation between age and BMD was significant (r=0.30).
Table 4:
Correlation coefficients for bone mineral density§ and fracture risk factors in mothers and daughters by BMD terciles within the sub-sample with Spearman’s rank test
| Variables | Mothers | Daughters | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | mT1BMD | mT2BMD | mT3BMD | Total | dT1BMD | dT2BMD | dT3BMD | |
| Sample size | 170 | 57 | 57 | 56 | 170 | 57 | 56 | 57 |
| Age | −0.11 | −0.25 | 0.16 | 0.08 | 0.30* | 0.41* | 0.04 | 0.03 |
| Dietary calcium intake§ | −0.06 | 0.05 | 0.03 | −0.04 | −0.08 | −0.25 | −0.09 | −0.02 |
| Physical activity§ | −0.02 | 0.11 | −0.09 | −0.16 | 0.13 | −0.10 | −0.03 | −0.01 |
values were converted to SD scores; mT1BMD – bottom tercile BMD by mothers, mT2BMD – middle tercile BMD by mothers, mT3BMD – upper tercile BMD by mothers; dT1BMD – bottom tercile BMD by daughters, dT2BMD – middle tercile BMD by daughters, dT3BMD – upper tercile BMD by daughters; significance of differences at:
P <0.05;
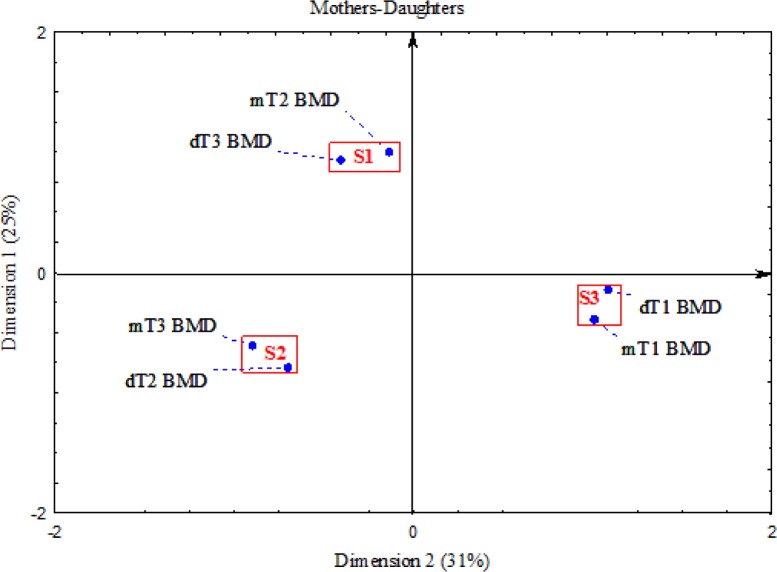
The same terciles of BMD were stated in 39% of mother-daughter pairs, in the bottom tercile of BMD in 16% of pairs, in the middle tercile of BMD in 11% pairs and in the upper tercile of BMD in 12% pairs (Table 5). The correspondence analysis demonstrated the existence of the strong relation between BMD of mothers and BMD of daughters. Three clusters were distinguished (Fig. 3). The S1 cluster was made up by the middle BMD tercile of mothers and the upper BMD tercile of daughters. The S2 cluster was made up of the upper BMD tercile of mothers and the middle BMD tercile of daughters. The S3 cluster was made up of the both bottom BMD terciles of mothers and daughters.
Table 5:
Distribution of mothers and daughters by BMD terciles
| Daughters’ BMD terciles n (%) | ||||
|---|---|---|---|---|
| dT1BMD 57 (34) | dT2BMD 56 (33) | dT3BMD 57 (34) | ||
| Mothers’ BMD terciles | mT1BMD 57 (34%) | 27 (16) | 14 (8) | 16 (9) |
| mT2BMD 57 (34%) | 18 (11) | 19 (11) | 20 (12) | |
| mT3BMD 56 (33%) | 12 (7) | 23 (14) | 21 (12) | |
| Sum of mother-daughter pairs with compatible BMD terciles | 67 (39) | |||
| Sum of mother-daughter pairs with non-compatible BMD terciles | 103 (61) | |||
() in the brackets percentage of mother-daughter pairs
Fig. 3:
Graphic presentation of the relationship between bone mineral density in mothers and daughters in the sub-sample; () in brackets are the given explained inertia in two dimensions; areas marked by rectangles and signed S1, S2 and S3 point three clusters grouping features correlated with each other. Notes: mT1BMD – bottom tercile BMD by mothers, mT2BMD – middle tercile BMD by mothers, mT3BMD – upper tercile BMD by mothers; dT1BMD – bottom tercile BMD by daughters, dT2BMD – middle tercile BMD by daughters, dT3BMD – upper tercile BMD by daughters
Discussion
The study showed the existence of a similarity between mothers and daughters, both in the occurrence of dietary and non-dietary factors of fracture risk. Differences between mothers and daughters concerned the level of threat with risk factors. In comparison to daughters, mothers were more burdened with adverse factors increasing bone fracture risk, but they revealed more favourable dietary habits reducing the risk of bone fractures.
A strong similarity in BMD concerned mothers and daughters with low BMD. The similarity between mothers and daughters regarding medium and higher BMD was weaker. The same BMD terciles were found in nearly 40% mother-daughter family pairs. Our results are difficult to compare with previous findings. We used a unique approach by grouping subjects before the statistical analysis, but agreement with previous studies may be found. Authors who used correlation analysis showed a significant association between mothers and daughters in BMD (23–25), which partially confirms our results.
There are three possible explanations for the similarity of low BMD in mothers and daughters. The first is the effect of age. Mothers with the lowest BMD were older (by 2–3 years) and daughters with the low BMD were younger (by 2–3 years) than those with moderate and high BMD. Thus, our findings in older women group might result from the intensifying of bone resorption and in younger girls might result from the slowing of bone mineralisation within 2–3 years after menarche (26).
Secondly, the daughters’ low BMD might be inherited from mothers. Osteoporosis is determined by multiple genes (27). There is evidence of the occurrence of similar “skeleton features” among family members (28). A high correlation of BMD was observed among the three female Mexican generations (23). In Japanese daughter-mother pairs, BMD was significantly correlated, but no significant correlation was found in mother-grandmother or daughter-grandmother pairs (24). A family similarity concerning bone strength in girls and women probably is established in the first year of life, but the familial correlation of BMD might disappear due to a menopause-associated estrogen deficiency (24, 25).
The next explanation of low BMD similarity in mothers and daughters might involve environmental factors (1–7, 27). Some of these factors may be “inherited” such as lifestyle, habits and behaviors. An “osteoporotic environment”, defined as “the sum of influences which depend on the surroundings, opportunities, or conditions of life”, may promote osteoporosis in certain families. In our study, a significant correlation between mothers and daughters in the occurrence of both dietary and non-dietary fracture risk factors was observed. It should be underlined that correlation coefficients were higher in mothers and daughters with low or moderate mineral density (from 0.82 to 0.94) than those with high bone mineral density (below 0.66). Non-dietary risk factors for bone fractures which might be under the influence of family behaviours and create an “osteoporotic environment” in the family include: past fractures, calcium supplementation, chronic diseases, osteoporosis in the family and hormonal contraceptive use.
Surprising, we found significant differences (lack of similarity) between mothers and daughters in the occurrence of low physical activity. Swee and Win (29) also found no correlation in physical activity in pairs of premenopausal mothers and their daughters aged 10–19 years. Physical activity level is age- and gender-specific. Teenage girls are more likely to have a sedentary lifestyle than boys at the same age (30, 31). It may be supposed that a lack of similarity in physical activity between mothers and daughters resulted from girls’ specific lifestyle, so the impact of “family environment” was weaker.
Family similarity between mothers and daughters was reflected by the occurrence of similar dietary factors of fracture risk. Mothers and daughters had similar sources of dietary calcium in their diets. These were mainly milk, cheese and fruit yoghurt, which supplied over 50% of dietary calcium. A study by Harris (32) of 902 families found a strong association between the number of servings of dairy foods consumed by the adolescents and their parents. A similar relation was observed in American (33) and Malay mother-daughter pairs (29) with conclusion that mothers play an important role in influencing the dietary habits of their daughters which, in turn, may affect their bone health status.
The similarity between mothers and daughters was also reflected by lack of significant correlation between BMD and dietary calcium intake or physical activity. Our results are compatible with some previous findings (34, 35). However, there are some contradictory findings (36–39). In contrast to our findings, physical activity is clearly recognized as factor positively associated with an increase in bone mass during a developmental period (6). This was confirmed in subjects reporting recreational exercise (40, 41). In our study, mothers with low or high BMD were significantly more physically active than mothers with moderate BMD, and all of them were under the influence of many different fracture risk factors. The impact of environmental factors on BMD is complex, since it is difficult to explain the association between bone mineral density and a single risk factor (1, 2, 5, 42).
We showed that mothers were more burdened with factors increasing bone fracture risk than daughters. It is an expected result because of age differences (31). The main five non-dietary risk factors occurred (in decreasing order) in 75% to 22% of the mothers, and 56% to 16% of the daughters. We cannot identify which factors (from 21 which were assessed) were more effective than others in low BMD. Based on previous findings, it may be assumed that past fractures, osteoporosis in the family, current smoking and low physical activity in mothers or daughters were the most important factors increasing the risk of bone fractures (42–45).
Our study revealed an interesting finding. We found more protective dietary habits in mothers reduced the risk of bone fractures. In the past, during pre-school and school periods, more mothers than daughters consumed dairy products (and calcium) daily. This might have resulted from generation transformation and changes in food supply as well as food consumption in Poland over the past fifty years. Mothers were in pre-school and school age when dairy consumption in Poland was relatively high, and peaked in the 1980s (on average, 260–280 litres/person/year after recalculation to milk) (46). Daughters were in pre-school and school age when dairy consumption decreased significantly by 30%, but at the same time increased the consumption of fast-food and highly-prepared food and the traditional Polish diet became westernized (31). Therefore, we documented that the occurrence of protective factors of bone fracture may be irrespective of age. Secondly, we showed the limitation of “inheritance” dietary habits shared with family members. Our findings demonstrate that family habits in food consumption are also influenced by the external impact such as food supply on domestic market.
Study weakness
Bone densitometric measurements were carried out in a medium-sized subsample, which was non-randomly selected from the general population. However, in mothers/daughters from a large-sized basic sample and sub-sample, a similarity in dietary calcium intake was revealed – the correlation coefficient for dietary calcium intake of mothers and daughters was nearly equal. This allows generalization of results obtained in the sub-sample and stronger conclusions to be drawn.
Study strengths
The study strength is the assessment of the occurrence of dietary and non-dietary factors of fracture risk (1–3). Such a broad scope of research is realized in family pairs. An advantage of this research is the use of validated tools to assess dietary calcium intake (16) and physical activity (20).
Conclusion
In mothers and daughters, similarity was found in the occurrence of dietary and non-dietary factors of fracture risk. Differences between mothers and daughters concerned the level of threat with risk factors. In comparison to daughters, mothers were more burdened with factors increasing bone fracture risk, but they revealed more favourable dietary habits reducing the risk of bone fractures.
Our results demonstrate the stronger effect of adverse factors of family environment than protective factors on bone fracture risk and osteoporosis. Improvement of public health in Poland requires the elimination of adverse dietary and non-dietary habits in women and the development and strengthening of health-promoting habits in girls.
Ethical considerations
Ethical issues (including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
The work was completed on behalf of the MODAF project (No. N N312 2862 33) entitled “Analysis of mother-daughter dairy products food patterns in relation to bone mineral status and calcium deficiency and osteoporosis risk among women. MODAF Study” and funded under the Polish Ministry of Science and Higher Education. We thank the women who participated in the study. The authors declare that there is no conflict of interests.
References
- 1. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton JM, III, Khaltaev A. (2008). A reference standard for the description of osteoporosis. Bone, 42: 467–75. [DOI] [PubMed] [Google Scholar]
- 2. WHO (1994). Assessment of facture risk and its application to screening for postmenopausal osteoporosis, Technical Report, WHO Study Group: Geneva, Switzerland, 843, pp.: 1–129. [PubMed] [Google Scholar]
- 3. Badurski JE, Czerwiński E, Marcinkowska-Suchowierska E. (2007). Zalecenia Polskiej Fundacji Osteoporozy i Polskiego Towarzystwa Osteoartrologii wobec osteoporozy w oparciu o stanowisko Światowej Organizacji Zdrowia (WHO) i Międzynarodowej Fundacji Osteoporozy (IOF). Ortop Traumatol Rehabil, 9: 45–53. [Google Scholar]
- 4. McCloskey E, Johansson H, Oden A, Kanis JA. (2012). Fracture risk assessment. Clin Biochem, 45: 887–93. [DOI] [PubMed] [Google Scholar]
- 5. Nieves JW, Barrett-Connor E, Siris ES, Zion M, Barlas S, Chen YT. (2008). Calcium and vitamin D intake influence bone mass, but not short-term fracture risk, in Caucasian postmenopausal women from the National Osteoporosis Risk Assessment (NORA) study. Osteoporos Int, 19: 673–79. [DOI] [PubMed] [Google Scholar]
- 6. Rizzoli R, Bianch ML, Garabédian M, McKay HA, Moreno LA. (2010). Maximizing bone mineral mass gain during growth for the prevention of fractures in adolescents and the elderly. Bone, 46( 2): 294–305. [DOI] [PubMed] [Google Scholar]
- 7. Urano T, Shiraki M, Ezura Y, Fujita M, Sekine E, Hoshino S, Hosoi T, Orimo H, Emi M, Ouchi Y, Inoue S. (2004). Association of a single-nucleotide polymorphism in low-density lipoprotein receptor-related protein 5 gene with bone mineral density. J Bone Miner Metab, 22( 4): 341–45. [DOI] [PubMed] [Google Scholar]
- 8. Richards J, Kavvoura F, Rivadeneira F, Styrkársdóttir U, Estrada K, Halldórsson B, et al. (2009). For the GEFOS (Genetic Factors for Osteoporosis) consortium, collaborative meta-analysis: Associations of 150 Candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med, 151: 528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrew T, Antioniades L, Scurrah K, Macgregor A, Spector T. (2005). Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res, 20: 67–74. [DOI] [PubMed] [Google Scholar]
- 10. Lata P, Elliott ME. (2007). Patient assessment in the diagnosis, prevention, and treatment of osteoporosis. Nutr Clin Pract, 22( 3): 261–75. [DOI] [PubMed] [Google Scholar]
- 11. Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, et al. (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet, 3( 371): 1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebeling P, Daly RM, Kerr DA, Kimlin MG. (2013). Building healthy bones throughout life: an evidence-informed strategy to prevent osteoporosis in Australia. MJA Open, 2( 1): 1–46. [DOI] [PubMed] [Google Scholar]
- 13. Larson N, Story M, Wall M, Neumark-Sztainer D. (2006). Calcium and dairy intakes of adolescents are associated with their home environment, taste preferences, personal health beliefs, and meal patterns. J Am Diet Assoc, 106: 1816–24. [DOI] [PubMed] [Google Scholar]
- 14. Jeżewska-Zychowicz M. (2004). Family environment as a predictor of selected food habits among adolescents from Warsaw. Pol J Food Nutr Sci, 13: 307–12. [Google Scholar]
- 15. Wądołowska L, Sobaś K, Szczepanska JW, Slowinska MA, Czlapka-Matyasik M, Niedzwiedzka E. (2013). Dairy products, dietary calcium and bone health: possibility of prevention of osteoporosis in women: The Polish experience. Nutrients, 5( 7): 2684–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szymelfejnik EJ, Wądołowska L, Cichon R, Przysławski J, Bolesławska I. (2006). Dairy products frequency questionnaire (ADOS-Ca) calibration for calcium intake evaluation. Pol J Food Nutr Sci, 15: 229–36. [Google Scholar]
- 17. Neumark-Sztainer D, Hannan P, Story M, Croll J, Perry C. (2003). Family meal patterns: Associations with sociodemographic characteristics and improved dietary intake among adolescents. J Am Diet Assoc, 103: 317–22. [DOI] [PubMed] [Google Scholar]
- 18. Jarosz M. (2012). Normy żywienia dla populacji polskiej - nowelizacja (Dietary reference intakes for Polish population - amendment). IŻŻ, Warszawa, Poland. Available from: http://mail.izz.waw.pl/~it/NORMY/NormyZywieniaNowelizacjaIZZ2012.
- 19. Oksanen T, Kivimaki M, Pentti J, Virtanen M, Klaukka T, Vahtera J. (2010). Self-report as an indicator of incident disease. Ann Epidemiol, 20: 547–54. [DOI] [PubMed] [Google Scholar]
- 20. International Physical Activity Questionnaire (IPAQ) (2005). Guidelines for Data Processing and Analysis of the IPAQ. www.ipaq.ki.se (accessed on 30 July 2013).
- 21. Craig C, Marshall A, Sjostrom M, Bauman A, Booth M, Ainsworth B, Pratt M, Ekelund U, Yngve A, Sallis J, Oja P. (2003). International Physical Activity Questionnaire: 12 country reliability and validity. Med Sci Sports, 35( 8): 1381–95. [DOI] [PubMed] [Google Scholar]
- 22. Stanisz A. (2007). Przystępny kurs statystyki z zastosowaniem STATISTICA PL na przykładach z medycyny. Tom 1. Statystyki Podstawowe; StatSoft Polska: Kraków, Poland, pp.: 292, 296. [Google Scholar]
- 23. Lazcano-Ponce E, Tamayo J, Díaz R, Burguete AI, Salmerón J. (2009). Correlation trends for bone mineral density in Mexican women: Evidence of familiar predisposition. Salud Publica Mex, 51( 1): 93–9. [DOI] [PubMed] [Google Scholar]
- 24. Ohta H, Kuroda T, Onoe Y, Nakano Ch, Yoshikata R, Ishitani K, Hashimoto K, Kume M. (2010). Familial correlation of bone mineral density, birth data and lifestyle factors among adolescent daughters, mothers and grandmothers. J Bone Miner Metab, 28( 6): 690–5. [DOI] [PubMed] [Google Scholar]
- 25. Wang Q, Alén M, Lyytikäinen A, Xu L, Tylavsky FA, Kujala UM, Kröger H, Seeman E, Cheng S. (2010). Familial resemblance and diversity in bone mass and strength in the population are established during the first year of postnatal life. J Bone Miner Res, 25( 7): 1512–20. [DOI] [PubMed] [Google Scholar]
- 26. Schönau E. (2004). The peak bone mass concept: Is it still relevant? Pediatr Nephrol, 9( 8): 825–31. [DOI] [PubMed] [Google Scholar]
- 27. Kung AW, Huang Q-Y. (2007). Genetic and environmental determinants of osteoporosis. JMNI, 7( 1): 26–2. [PubMed] [Google Scholar]
- 28. Urano T, Shiraki M, Yagi H, Ito M, Sasaki N, Sato M, et al. (2012). GPR98/Gpr98 gene is involved in the regulation of human and mouse bone mineral density. JCEM, 97( 4): 565–74. [DOI] [PubMed] [Google Scholar]
- 29. Swee ChS, Win LS. (2003). Resemblance in dietary habits and calcaneal ultrasound attenuation in Malay mother-daughter pairs. Mal J Nutr, 9( 2): 85–93. [PubMed] [Google Scholar]
- 30. Gubbels JS, van Assema P, Kremers SPJ. (2013). Physical Activity, Sedentary Behavior, and Dietary Patterns among Children. Curr Nutr Rep, 2: 105–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wądołowska L. (2010). Żywieniowe podłoże zagrożeń zdrowia w Polsce. (Nutrition as an Underlying Cause of Health Hazards in Poland). Olsztyn, Poland: UWM; (in Polish), pp.: 70–72. [Google Scholar]
- 32. Harris Interactive (2009). Influencing milk consumption. Conducted for MilkPEP and Deutsch. 1, 505 mothers (with children ages 1–17), 878 children (ages 8–17), Available from: www.harrisinteractive.com.
- 33. Birch LL, Lee Y. (2002). Family influences: mothers’ and daughters’ use of multivitaminmineral supplements. Nutrition Today, 37: 173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Townley M, Burt LA, Boyd S. (2013). Determinants of bone quality: heredity versus lifestyle factors. JURA, 3 (1): 1. [Google Scholar]
- 35. Skowrońska-Jóźwiak E, Jaworski M, Grzywa A, Lorenc R, Lewiński A. (2014). Influence of calcium intake on bone mineral density and incidence of fractures in treatmentnaive women from Lodz urban area – a part of EPOLOS study. AAEM, 21( 1): 201–4. [PubMed] [Google Scholar]
- 36. Wlodarek D, Glabska D, Kolota A, Adamczyk P, Czekajlo A, Grzeszczak W, Drozdzowska B, Pluskiewicz W. (2014). Calcium intake and osteoporosis: the influence of calcium intake from dairy products on hip bone mineral density and fracture incidence - a population-based study in women over 55 years of age. Public Health Nutr, 17( 2): 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ito S, Ishida H, Uenishi K, Murakami K, Sasaki S. (2011). The relationship between habitual dietary phosphorus and calcium intake, and bone mineral density in young Japanese women: a cross-sectional study. Asia Pac J Clin Nutr, 20( 3): 411–17. [PubMed] [Google Scholar]
- 38. Lanou AJ, Berkow SE, Barnard ND. (2005). Calcium, Dairy Products, and Bone Health in Children and Young Adults: A Reevaluation of the Evidence. Pediatrics, 115( 3): 736–43. [DOI] [PubMed] [Google Scholar]
- 39. Matkovic V, Landoll JD, Badenhop-Stevens NE, Ha EY, Crncevic-Orlic Z, Li B, Goel P. (2004). Nutrition influences skeletal development from childhood to adulthood: a study of hip, spine, and forearm in adolescent females. J Nutr, 134( 3): 701–5. [DOI] [PubMed] [Google Scholar]
- 40. Callréus M, McGuigan F, Ringsberg K, Akesson K. (2012). Self-reported recreational exercise combining regularity and impact is necessary to maximize bone mineral density in young adult women. A population-based study of 1, 061 women 25 years of age. Osteoporos Int, 23( 10): 2517–26. [DOI] [PubMed] [Google Scholar]
- 41. Helge EW, Aagaard P, Jakobsen MD. (2010). Recreational football training decreases risk factors for bone fractures in untrained premenopausal women. Scand J Med Sci Sports, 20(suppl. 1): 31–9. [DOI] [PubMed] [Google Scholar]
- 42. Kanis JA. (2008). World Health Organisation Scientific Group: Assessment of osteoporosis at primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK, pp.: 126. [Google Scholar]
- 43. Khosla S, Riggs BL. (2005). Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin N Am, 34: 1015–30. [DOI] [PubMed] [Google Scholar]
- 44. Frax®: WHO Fracture Risk Assessment Tool http://www.sheffield.ac.uk/FRAX/tool.jsp?lang=en (accessed on 27 December 2014).
- 45. Cauley JA, Lui L-Y, Barnes D, Ensrud KE, Zmuda JM, Hillier TA, Hochberg MC, Schwartz AF, Yaffe K, Cummings SR, Newman AB. (2009). Successful skeletal aging: A marker of low fracture risk and longevity. The Study of Osteoporotic Fractures (SOF). J Bone Miner Res, 24: 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Statistical Yearbook of the Republic of Poland (2010). Central Statistical Office, Statistical Publishing Establishment, Warsaw, pp.: 322. [Google Scholar]