Abstract
Background:
Twin pregnancies are commonly associated with low birth weight (LBW) infants. Most studies focus on growth of LBW compared with normal infants in singleton. However, there has not been any study on twins to compare LBW with normal birth weight (NBW) infants as a match control of the same twin.
Methods:
This cohort study was conducted at Healthcare centers of Kashan, in 2013. Twins differing in birth weight (one with LBW and the other with NBW) were assessed using the care charts in Well Care program in regard to weight, height and head circumference measurements at birth and after 6, 12, 24 months of age and were compared separately between all matched pairs and in four sex-twin subgroups which was defined according to the sex of each twin in pairs; SF (Same-sex: Female), SM (Same-sex: Male), LF (LBW: Female) and LM (LBW: Male).
Results:
Incidence of dissimilar twins in birth weight was 28.4%. The weight of LBW was significantly lower than the NBW cohort in SF and LF subgroups up to 24 months. The height of LBW was not significantly different from NBW cohort in SM and LM subgroups up to 24 month. Head circumference was significantly higher in LBW compared to NBW cohort in LM subgroup up to 24 month. However, these indices were significantly lower in LBW compared to NBW cohort in all pairs.
Conclusion:
Although LBW cohort was significantly lower than the NBW in growth indices at birth to 2 years old in all twins, however, highly variable results was observed in four subgroups of sex-twin.
Keywords: Growth indicator, Low birth weight, Twins
Introduction
The prevalence of twin pregnancy is 1 out of 208 deliveries in Iran (1) and 1 out of every 90 worldwide and it is commonly related to race and family factors (2). However, this rate changes from 6 to 9 twins per every 1000 live births in the United States, South and South-east Asia (the lowest rate), to 9–16 in North America and Europe, and to 18–30 in Central Africa (3). However, there are reports that show a 76% increase of twin births during 1980–2009 in the United States (from 18.9 to 33.3 per 1000 births) (4). This raising multiple pregnancy rate is due mainly to increasing use of fertility treatments such as in vitro fertilization (IVF) (5).
The health problems of multiple pregnancies in mothers and newborns are more pronounced than the single type. Twin pregnancies are commonly associated with preterm delivery, lower birth weight, and weight discordance defined by birth weight difference of 15% or more (the difference of the larger twin weight from the small one in twin pairs) (6–9). The incidence of weight discordance has been reported between 9.6 to 28.8% in a study in Nigeria (10).
There have been studies on twins in different areas worldwide. For instance, a study introduces height, weight and BMI growth chart for LBW children in Germany (11). The majority of studies on twins have focused on intrauterine growth or evaluating after birth growth status and developmental aspects of discordant twins. However, there is no standardized cut-off point for discordancy regarding the birth weight of twins, therefore, the incidence and status of subsequent neonatal outcomes in this regard varies depending on the cut-off used for discordancy (12).
The birth weight of less than 2500gr per live born infant is considered as low birth weight (LBW), based on the WHO (13). Results of a study by the Agency for Healthcare Research and Quality showed that approximately 6.1% of the births in the US in 2011 were LBW (14) whereas 15.5% of live births in the world are LBW of which 95% are born in developing countries like Iran (15). In some references, 6–7% of all births are reported as LBW neonates. However, these cases constitute 75% of neonate mortality. In addition, this problem is an important cause of some diseases in the childhood (16). In a study reported by Onyiriuka in Nigeria LBW incidence in twins was 51.7% (7).
The risk factors that may lead to LBW in mothers consist of multiple pregnancies, insufficient nutrition, young ages, precedence of LBW infants, hypertension or heart disease, alcohol abuse and obesity, insufficient weight gaining, poor prenatal care, low level of educations and environmental factors such as air pollutions and smoking (6, 17, 18). Presently, the complications of LBW are well known and include the increased probability of infant’s mortality as the most serious one (19). There are also other complications such as the increased chance of chronic diseases later in life, morbidity, neuropathologic disease and secondary physical and mental disabilities. Moreover, LBW is a health index at the population level. Recently, several studies have showed the effect of LBW on the cognitive development of infants (20–21).
Growth evaluation is one of the most basic elements of child health, specifically, at birth and infancy period. Physical growth is the most distinctive development in a child. Therefore, physical growth parameters such as weight, height and head circumference may be considered as one of the best mean for assessing the health status. Different studies have indicated that LBW children face poor growth trends compared to the normal children (22). As a result, specific methods have been designed to evaluate of the LBW growth (23). Since the light weighing is more likely in twins, trend assessment of growth needs more studies in twin pairs. Studies on twins are of specific interest, since twins are naturally matched and many probable confounding factors such as length of gestation, maternal nutrition, or socioeconomic status, that both infant in a pair share them, are eliminated (24). A careful review of literature revealed that the majority of the studies have focused on examining the growth trend of discordant twins (exclusive LBW as a cutoff for discordancy) or have compared the growth of LBW with normal infants in singleton. However, there is a lack of information regarding twins when only one is born with normal birth weight (birth weight of 2500gr to 4000gr) whereas the other one suffers from low birth weight.
Therefore, this study was designed to compare the growth indices of twins with dissimilar weight at birth (LBW vs. normal twin) for two consecutive years.
Methods and Materials
This historical cohort study was conducted at the primary health care centers of Kashan (located in the central region of Iran) in 2013.
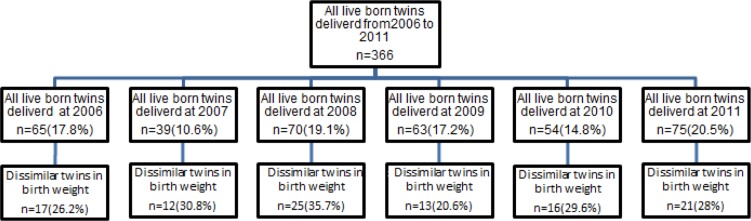
The twins who only one was born with normal birth weight (birth weight of 2500 gr to 4000 gr) and the other one with low birth weight were selected from the total twins born over a six years period from April 2006 to March 2011. This twins followed for two years and the weight, height and head circumference of these neonates were measured at the time intervals of birth, after 6, 12, 24 months of chronological age using care charts of children in ‘Well Care Children’ (WCC) (25). In addition, their background characteristics and breastfeeding duration recorded for each pair. Figure 1 presents the flowchart of the study population according to the years of study.
Fig. 1:
Study population according to the years of study
Statistical analysis
Following the completion of data collection, in order to clarify the sex role in the growth of infant, twins were stratified into four subgroups, named sex-twin subgroups, according to the sex of each twin in pairs ; SF subgroup (Same-sex: Female), SM subgroup (Same-sex: Male), LF subgroup (LBW: Female, NBW: male) and LM subgroup (LBW: Male, NBW: female). The relationship between different subgroups and the background characteristics and subsequent neonatal outcomes were assessed.
Percent of difference between both twins birth weight in each pair (difference of Birth weight of smaller twin from Birth weight of larger twin/Birth weight of larger twin and multiplied by 100) was calculated to show the discordance status in the twins.
For the background characteristics, differences in means among the categories of sex-twin subgroups were determined by analysis of variance and reported as mean±sd and ‘Chi-square’ test used for comparing the categorical data.
The growth outcomes were compared separately between all matched pairs and in sex-twin subgroups using paired t-test or Wilcoxon. Repeated measurement analysis was also used to assess the growth trend of twins in four subgroups over time. The analyses were performed by the SPSS 18.0 software (Chicago, IL, USA). All of the tests were performed with P-values set to 0.05 or less by two-sided.
Results
The prevalence of live born twins delivered from the April 2006 to March of 2011 in Kashan was 11.6 per 1000 birth. From the 366live born twins in this period, 104 (28.4%) twins (with minimum of 20.6% in 2009 and maximum 35.7% in 2008) were dissimilar in weight at birth. Less than half (47%) of these neonates were same-sex and the rest were from unlike-sex. Table 1 presents the birth weight in the LBW and NBW cohort for all twins and according to the subgroups as well. There was a significant difference between the weight of LBW and NBW twins (P<0.001).
Table 1:
Birth weight (gr) in twin pairs
| Sex-twin subgroups | n(%) | Minimum | Maximum | Mean | Std. Deviation | P-value | |
|---|---|---|---|---|---|---|---|
| SF | Birth weight of LBW twin | 28(26.9) | 1500.00 | 2410.00 | 2125.0 | 312.0 | <0.001 |
| Birth weight of NBW twin | 2550.00 | 3150.00 | 2786.4 | 240.0 | |||
| Difference* | 50.00 | 1250.00 | 461.43 | 290.6 | |||
| SM | Birth weight of LBW twin | 21(20.2) | 1600.00 | 2400.00 | 2105.2 | 258.9 | <0.001 |
| Weight of NBW twin | 2500.00 | 3200.00 | 2686.7 | 200.4 | |||
| Difference* | 200.00 | 800.00 | 481.43 | 193.6 | |||
| LF | Birth weight of LBW twin | 33(31.7) | 1550.00 | 2450.00 | 2235.8 | 310.3 | <0.001 |
| Birth weight of NBW twin | 2600.00 | 3400.00 | 2679.5 | 354.3 | |||
| Difference* | 50.00 | 1000.00 | 443.79 | 225.2 | |||
| LM | Birth weight of LBW twin | 22(21.2) | 1800.00 | 2400.00 | 2264.1 | 224.8 | <0.001 |
| Birth weight of NBW twin | 2500.00 | 3200.00 | 2612.3 | 262.7 | |||
| Difference* | 120.00 | 800.00 | 348.2 | 188.3 | |||
| Total | Birth weight of LBW twin | 104(100) | 1500.00 | 2450.00 | 2205.8 | 285.5 | <0.001 |
| Birth weight of NBW twin | 2500.00 | 3400.00 | 2641.7 | 278.9 | |||
| Difference* | 50.00 | 1250.00 | 435.9 | 233.8 | |||
Difference of birth weight of LBW twin from Birth weight of NBW twin
The result of analysis indicated that 82 twins (79%) examined in this research had discordance of 10% or more. The majority of the twins had discordance of 10% to 20%. However, the result of Chi square test showed that there was no significant difference in the frequency of discordance among the four subgroups of sex-twin (P=0.68). This result is presented in Table 2. Background characteristics in four sex-twin subgroups are presented in Table 3. There were no significant imbalances among the four subgroups. Cesarean deliveries was more frequent than the vaginal deliveries (98(94.2%) vs. 6 (5.8%)) and mean gestational age was more than 36 weeks (near to full term (26)) in all subgroups.
Table 2:
Birth weight (gr) discordance in twin pairs
| Discordance cut-off | SF n(%) | SM n(%) | LF n(%) | LM n(%) | Total n(%) | P-value |
|---|---|---|---|---|---|---|
| <10 | 6(21.4) | 5(23.8) | 6(18.2) | 5(23) | 22(21.2) | 0.68 |
| 10<=....<20 | 13(46.4) | 7(33.3) | 16(48.5) | 11(50) | 47(45.2) | |
| 20<=...<30 | 4(14.3) | 7(33.3) | 9(27.3) | 5(23) | 25(24) | |
| >=30 | 5(17.9) | 2(9.5) | 2(6.1) | 1(4) | 10(9.6) | |
| total | 28(100) | 21(100) | 33(100) | 22(100) | 104(100) |
Table 3:
Background characteristics in four sex-twin subgroups
| Sex-twin subgroups | |||||||
|---|---|---|---|---|---|---|---|
| SF n(%) | SM n(%) | LF n(%) | LM n(%) | Total n(%) | P-value | ||
| Maternal education | under diploma | 11(25.6) | 10(23.2) | 15(34.9) | 7(16.3) | 43(100) | 0.7 |
| Diploma + | 17(27.9) | 11(18.03) | 18(29.5) | 15(24.6) | 61(100) | ||
| Maternal job | Housewife | 26(28.6) | 20(22) | 28(30.8) | 17(18.7) | 91(100) | 0.24 |
| working | 2(15.4) | 1(7.6) | 5(38.5) | 5(38.5) | 13(100) | ||
| Type of delivery | Vaginal | 2(33.3) | 2(33.3) | 1(16.7) | 1(16.7) | 6(100) | 0.76 |
| c/s | 26(26.5) | 19(19.4) | 32(32.6) | 21(21.4) | 98(100) | ||
| Maternal age in years | 26.5±4.3 | 26.9±4.8 | 28.1±5.2 | 29.7 ±5.9 | 27.8±5.1 | 0.22 | |
| Mother’s BMI in kg/m2 | 28.4±4.4 | 27.1±5.1 | 26.7±4.1 | 26.5 ±3.1 | 27.3±4.3 | 0.4 | |
| Gestational age in weeks | 36.9±0.4 | 36.8±0.7 | 36.6±0.9 | 36.6 ±0.8 | 36.7±0.8 | 0.37 | |
The mean duration of breastfeeding in both LBW and NBW cohorts was approximately 10 months in all twins. In addition, there was no significant difference in duration of breastfeeding of LBW and NBW infants or the four sex-twin subgroups either (Table 4). The growth indicators of LBW and NBW cohorts are summarized in Table 5.
Table 4:
Breast-feeding duration in twin pairs in months
| Cohorts | SF | SM | LF | LM | Total |
|---|---|---|---|---|---|
| LBW twin | 11.0±7.5 | 10.3±6.8 | 11.4±7.5 | 9.0±6.02 | 10.3±7.3 |
| NBW twin | 10.8±7.8 | 10.8±7.5 | 11.2±8 | 9.2±5.8 | 10.2±7.0 |
| P-value | 0.46 | 0.6 | 0.8 | 0.31 | 0.81 |
Table 5:
Difference of weight, height and head circumference between LBW and NBW cohorts from birth to 2 years old
| outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Sex-twin subgroups | time | Weight (in grams) | Height (in centimeters) | head circumference (in centimeters) | |||
| Difference | P-value | Difference | P-value | Difference | P-value | ||
| SF (mean±sd) | At birth | 461.43±290 | <0.001 | 1.4±2.2 | 0.013 | 1.2±0.92 | <0.001 |
| 6 month | 508.3±659.8 | 0.001 | 1.2±2 | 0.007 | 0.8±1.1 | 0.008 | |
| 12 month | 503.9 ±845 | 0.009 | 1.1 ±2.2 | 0.03 | 0.65±1.1 | 0.012 | |
| 24 month | 576±1111.6 | 0.016 | 0.98 ±2.6 | 0.073 | 0.22±1.04 | 0.3 | |
| SM (mean±sd) | At birth | 481.43±193.6 | <0.001 | 0.82±2.4 | 0.15 | 0.46±1.4 | 0.17 |
| 6 month | 419.5±743.3 | 0.024 | 0.87±2 | 0.07 | 1.1±0.97 | <0.001 | |
| 12 month | 547.4±1134.5 | 0.05 | 0.92 ±2.8 | 0.18 | 1.03±1.25 | 0.002 | |
| 24 month | 521.4±1454.5 | 0.12 | 0.86 ±2.6 | 0.15 | 1.2±1.5 | 0.003 | |
| LF (mean±sd) | At birth | 443.79±225.2 | <0.001 | 1.7±2.4 | 0.001 | 1.5±1.2 | <0.001 |
| 6 month | 1017.1 ±893.3 | <0.001 | 2.3 ±2.4 | <0.001 | 1.6±1.2 | <0.001 | |
| 12 month | 980 ±1214 | <0.001 | 1.3 ±3.2 | 0.03 | 1.65±1.6 | <0.001 | |
| 24 month | 989.4±2087.1 | 0.01 | 1.2 ±3.7 | 0.085 | 1.7±1.7 | <0.001 | |
| LM (mean±sd) | At birth | 348.2±188.3 | <0.001 | 0.88±1.6 | 0.025 | 0.5±1.1 | 0.07 |
| 6 month | −335 ±759 | 0.051 | −0.1 ±2.3 | 0.84 | −0.74±0.96 | 0.002 | |
| 12 month | −136.6 ±995.8 | 0.54 | −0.28 ±2.2 | 0.56 | −0.82±1.05 | 0.002 | |
| 24 month | 15.4±1418.7 | 0.91 | −0.23±2.9 | 0.72 | −0.68±0.94 | 0.004 | |
| Total (mean±sd) | At birth | 435.9±233.8 | <0.001 | 1.2±2.2 | <0.001 | 0.99±1.2 | <0.001 |
| 6 month | 467.5±916.9 | <0.001 | 1.2±2.4 | <0.001 | 0.66±1.4 | <0.001 | |
| 12 month | 531.4 ±1131.2 | <0.001 | 0.81±2.7 | 0.005 | 0.74±1.6 | <0.001 | |
| 24 month | 581.9±1630.2 | 0.001 | 1.03±4.4 | 0.021 | 0.68±1.6 | <0.001 | |
Despite the fact that the LBW cohort were significantly less than the NBW in terms of weight, height and head circumference tested separately from birth to 2 years of age in the total of twins (sex excluded), however, highly variable results was observed in the four sex-twin subgroups.
In the SF subgroup, even though the weight of LBW twin was significantly lower than the NBW twin from birth up to 2 years of age (P-values were <0.001, 0.001, 0.009 and 0.016 respectively for birth, 6, 12 and 24 month), but in spite of the smaller height and head circumference of LBW compared to the NBW cohort up to 12 months, these difference in parameters leveled off between the two cohorts at 24 months.
In the SM subgroup, there was no significant difference between the weight of LBW and NBW cohorts after six month (P>0.05). Moreover, there were no significant differences between the heights of the two cohorts from birth to 2 years of age. However, there was a significant difference between the head circumference in the LBW and NBW infants after birth up to 24 months later (P-values were <0.001, 0.002 and 0.003 respectively for 6, 12 and 24 month).
In the LF subgroup, except of height at 24 months (P=0.085), the LBW cohort were significantly lighter and shorter and had a smaller head circumference compared to the NBW cohort.
In the LM subgroup, although weight and height was higher in NBW compared to LBW cohort, but these differences were not statistically significant (P>0.05) and the lower weight (P<0.001) and height (P=0.025) of the LBW cohort was observed only at the birth time. From 6 months to 2 years old, the differences in head circumference were even more considerable since LBW cohort was significantly larger than the NBW cohort (P<0.01).
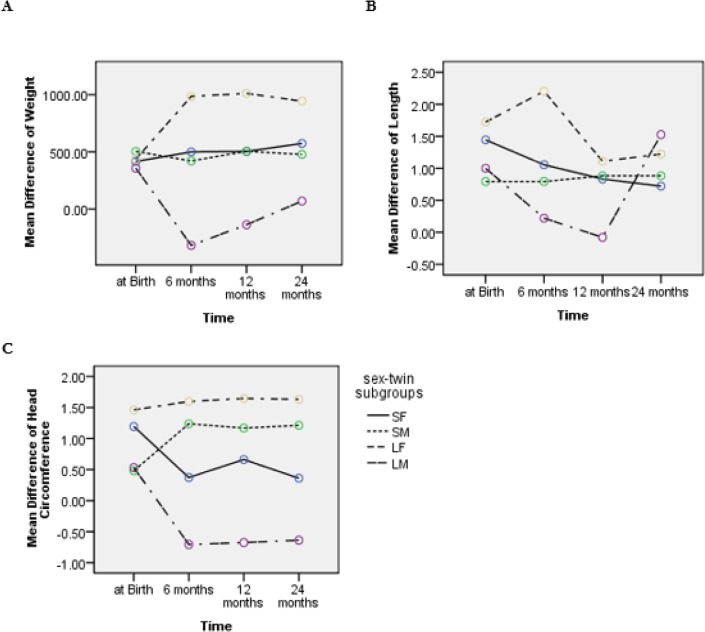
Figure 2 demonstrates the mean difference of twin pairs (NBW from LBW twin) in weight, height and head circumference at birth and 6, 12 and 24 months after the birth in four subgroups. Repeated measures analysis showed significant trends difference in weight (P=0.006) and head circumference (P<0.001) in the four sex-twin subgroups, however, no such result was found for the height difference (P=0.56).
Fig. 2:
Trends of mean difference of weight (A), height (B) and head circumference (C) between NBW and LBW twin in four sex-twin subgroups from birth to 2 years of age
A constant trend for SM and SF was found in the mean difference in weight of the subgroups from birth to 24 months. However, in LF subgroup, the difference of weight increased at the age of six months and then leveled off afterward. Conversely, in LM subgroup, following a decreasing trend in weight difference up to the 6 month, started to show an increase in trend from 6 to 24 months.
Similarly, despite the negligible discrepancy for SM and SF at birth, the trend of differences in height nearly remained constant after 6 months. In LF subgroup, the difference in height after an increase at 6 month decreased at 12 month; it had an invariable trend up to 24 months. Beside in LM subgroup, the difference in height showed a decreasing trend up to 12 months and then it increased.
In SM, unlike SF subgroup, the difference in head circumference increased at 6 month, and then it showed a steady trend. In LF subgroup, a constant trend with larger difference compared with the other subgroups was present from birth to 2 years of age. However, in LM subgroup, after decreasing at 6 months, it did not change noticeably and remained less than the other subgroups.
Discussion
The prevalence of live born twins was 11.6 per 1000 birth similar to 9 to 16 per 1000 birth in north America and Europe reported by Martin and associates in year 2012 (4). In addition, the rate of discordance determined by LBW as a cutoff point was 28.4%. This rate was in agreement with the 28.8% reported by Onyiriuka who determined discordance by a 15% difference in twin birth weight as the cut-off point (10).
Nearly half of the twins (47%) were from the same sex confirming the anticipated rate in twin pregnancy cases. Onyiriuka also have indicated that based on 15% cut-off point, 60 percent of the newborn are from the same sex (10).
The birth weight difference of 30% or more was observed in 9.6% of the twins examined in this study. Such condition has been present in 66.7% of the twins who both infants in a pair suffered from LBW reported by Onvirjuka (7).
Juneja et al. examined the growth of full term neonates up to the 18th month versus the LBW newborns weighing less than 2000gr and reported that all the growth parameters in these LBW children regardless of their gender was lower than the NBW (22). In addition the results of a research comparing the growth parameters of LBW in China have indicated that LBW infant have less growth in weight, height and head circumference measures compared to the normal birth weight neonate up to three years of age (27). As was observed in the present study, all the twins showed similar patterns but when they were stratified based on four sex subgroups, the different results were revealed.
However, Reuner et al. in a research including low risk LBW neonates (birth weight of 1500 to 2500 gr) showed that weight growth of LBW neonates compared to the matched control group (Normal Birth Weigh) was not different during the adulthood (28). There was a similarity between design of the present study and the design employed by Reuner et al. (28). Both researches compared the LBW and NBW neonates in a match design, however, in the present research the two cohorts were naturally matched. In addition, the minimum birth weight for LBW included in this study was 1500 gr similar to the former study.
In the both female groups (SF and LF), the mean weight of LBW and NBW in the present research was significantly different until two years of age, however, in the male groups (SM and LM) this significant difference was present only at six months and disappeared in the second year of birth. Such discrepancy of weight gaining may be expected based on the fact that higher rate of low weight compensation in male infant compared to the female.
Height growth of LBW neonates compared to the matched control group (Normal Birth Weight) was significantly less during the adulthood (28). However, in the present research no significant difference was found among the four subgroups of LBW and NBW infants at the age of 2 years, despite the fact that the height of female twins of LBW was significantly lower than the NBW prior to the second year birth. This difference was not present in boys. Such condition is expected since there is a higher rate of height growth in male compared to female.
Mackay et al. also examined 139 very low birth weight neonates (birth weight of less than 1500gr) in South Africa and claimed that these infants had poor growth at the early months after birth and showed gradual catch up but the growth deficit was still present20 months after birth (29).
Concerning the head circumference, there was a significant difference between the LBW twins and NBW neonates at all times except in the SF group at 24 month of age. However, in LM subgroup, the head circumference of LBW was significantly higher than the NBW cohort. Such condition may be attributed to the fact that there is a faster growth rate in male gender.
The time of compensation of growth indices was nearly similar to the finding of the present study. For instance, Eslami and associates in 2012 showed that the growth indices of LBW children partially improved at the age of one year (30).
The gestational age in this study was more than 36 weeks. That was close to the full term (26). Perhaps this may be because one of the neonates in a pair was normal. Therefore, it was suggested that further research on estimating the GA includes twins in which at least one of them has normal birth weight.
The recommendation for vaginal delivery in twins is usually based on the cases when the age of pregnancy is between 37 to 38 weeks and healthy twin pregnancy is detected (31). In the present study despite of the fact that the gestational age was near the full term, but method of delivery for 94 percent of the cases was caesarian section. In most countries, the rate of caesarian deliveries is lower than this study. For instance, this rate in the United States was 75 percent (32).
The mean duration of breastfeeding in the present study was 10 months. This time is considerably less than that the recommended time of 2 years set by WHO (13). Therefore, because of the superiority of breastfeeding to any alternative infant feeding method and provides immunity to many viral and bacterial diseases and shorted period of breastfeeding for the twins studied in this research, further studies for the purpose of examining the role of breastfeeding duration in preventing the diseases specially the infectious diseases and other complications associated with reduced breastfeeding duration is recommended.
Considering the findings of different studies concerning LBW as a risk factor causing developmental delay (33), further research about the growth and development in discordant twins based on LBW as a cut-off point is recommended.
Conclusion
Although LBW cohort was significantly lower than the NBW in weight, height and head circumference at the assessment time at birth to 2 years of age in all twins, however, highly variable results was observed in four subgroups of sex-twin. Therefore, further research in different countries and races is recommended in order to compare the growth pattern of different anthropometric indices including height, weight and head circumference in discordant twins based on gender and LBW as a cut-off point.
Ethical considerations
Ethical issues (including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was funded by a grant from the Deputy of Research of Tehran University of Medical Sciences, Tehran, Iran. The authors declare that there is no conflict of interests.
References
- 1. Rezavand N, Veisi F, Malek-Khosravi Sh, Zangeneh M, Kohzadi M. ( 2014). Assessment of Frequency of Twin Pregnancy and Neonatal Outcome in Deliveries of Mo’tazedi Hospital, Kermanshah in 2004–2007. J Obstet Gynaecol India, 64( 1): 19– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matte U, Le Roux MG, Bénichou B, Moisan JP, Giugliani R. ( 1996). Study on possible increase in twinning rate at a small village in south Brazil. Acta Genet Med Gemellol (Roma), 45( 4): 431– 437. [DOI] [PubMed] [Google Scholar]
- 3. Smits J, Monden C. ( 2011). Twinning across the Developing World. PLoS One, 6( 9): e25239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin JA, Hamilton BE, Osterman MJK. ( 2012). Three Decades of Twin Births in the United States, 1980–2009. NCHS data brief, no 80. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 5. Anonymous ( 2011). Multiple pregnancy. The management of twin and triplet pregnancies in the antenatal period. NICE clinical guideline 129. Available from: www.nice.org.uk/guidance/cg129/resources/guidance-multiple-pregnancy-pdf [PubMed]
- 6. da Fonseca CR, Strufaldi MW, de Carvalho LR, et al. ( 2012). Risk factors for low birth weight in Botucatu city, SP state, Brazil: a study conducted in the public health system from 2004 to 2008. BMC Res Notes, 5: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Onyiriuka AN. ( 2010). Incidence of delivery of low birth weight infants in twin gestations. Niger J Clin Pract, 13( 4): 365– 70. [PubMed] [Google Scholar]
- 8. Elliott JP. ( 2008). Preterm labor in twins and high-order multiples. Clinics in Perinatology, 34( 4): 599– 609. [DOI] [PubMed] [Google Scholar]
- 9. Hamedi A, Poyurjavad M, Dadgar S. ( 2009). The Prevalence and Complications of Multifetal Deliveries in Omalbanin Women Hospital in Mashhad. The Iranian Journal of Obstetrics, Gynecology and Infertility, 12( 1): 55– 60. [Google Scholar]
- 10. Onyiriuka AN. ( 2009). Intra pair birth weight discordance in twins. Ann Afr Med, 8( 2): 110– 114. [DOI] [PubMed] [Google Scholar]
- 11. Van Dommelen P, De Gunst MC, Van Der Vaart AW, Van Buuren S, Boomsma DI. ( 2004). Growth charts for height, weight and body-mass index of twins during infancy. Ned Tijdschr Geneeskd, 148( 27): 1345– 50. [PubMed] [Google Scholar]
- 12. Yalçin HR, Zorlu CG, Lembet A, Özden S, Gökmen O. ( 1998). The significance of birth weight difference in discordant twins: a level to standardize? Acta Obstet Gynecol Scand, 77: 28– 31. [PubMed] [Google Scholar]
- 13. Anonymous ( 2013). World Health Organization. ICD-10: International statistical classification of diseases and related health problems, 10th revision. Volume 2 2nd ed Geneva: WHO; 2004. Available from: www.who.int/classifications/icd/ICD-10_2nd_ed_volume2.pdf. [Google Scholar]
- 14. Kowlessar NM, Jiang HJ, Steiner C. ( 2013). Hospital Stays for Newborns, 2011. HCUP Statistical Brief #163. Agency for Healthcare Research and Quality, Rockville, MD: Available: www.hcupus.ahrq.gov/reports/statbriefs/sb163.pdf. [PubMed] [Google Scholar]
- 15. Ali Abadi F, Nazy S, Maghfori B. ( 2011). Gross Motor Development of low birth weight infants with the history of being in Aliasghar hospital Corrected aged 8 to 12 months. Journal of Modern Rehabilitation, 5( 2): 36– 39. [Google Scholar]
- 16. Hosseinzadeh K, Azima S, Keshavarz T, Karamizadeh Z, Zare N. ( 2012). The Effects of Massage on the Process of Physical Growth among Low-Weight Neonates. Journal of Isfahan Medical School, 29( 165): 11– 18. [Google Scholar]
- 17. Veloso HJF, da Silva AAM, Bettiol H, et al. ( 2014). Low birth weight in São Luís, northeastern Brazil: trends and associated factors. BMC Pregnancy Childbirth, 14( 1): 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kabiri M, Parsinia M, Goodarzi M, Babaei GhR. ( 2003). Relation between physical growth of 0–2 year-old children and socioeconomic and educational situation of their parents in Karaj/Iran. Iran J Pediatr, 13( 1): 47– 52. [Google Scholar]
- 19. Norton M. ( 2005). New evidence on birth spacing: promising findings for improving newborn, infant, child, and maternal health. Int J GynObs, 89: 51– 56. [DOI] [PubMed] [Google Scholar]
- 20. Stevens-Simon C, Orleans M. ( 1999). Low-birth weight prevention programs: the enigma of failure. Birth, 26( 3): 184– 91. [DOI] [PubMed] [Google Scholar]
- 21. Dorling JS , Field DJ. ( 2006). Follow up of infants following discharge from the neonatal unit : structure and process. Early Hum Dev, 82: 151– 156. [DOI] [PubMed] [Google Scholar]
- 22. Juneja M, Shanker A, Ramji S. ( 2005). Neurodevelopmental, functional and growth status of low birth weight infants at eighteen months. Indian Pediatr, 42: 1134– 1140. [PubMed] [Google Scholar]
- 23. Furmaga-Jabłońska W. ( 2003). Methods of growth assessment for low-birth-weight children. Med Wieku Rozwoj, 7( 2): 109– 20. [PubMed] [Google Scholar]
- 24. Carlin JB, Gurrin LC, Sterne AC, Morley R, Dwyer T. ( 2005). Regression models for twin studies: a critical review. Int J Epidemiol, 34( 5): 1089– 1099. [DOI] [PubMed] [Google Scholar]
- 25. Schor EL. ( 2004). Rethinking well-child care. Pediatrics, 114( 1): 210– 216. [DOI] [PubMed] [Google Scholar]
- 26. American College of Obstetricians and Gynecologists ( 2013). ACOG Committee Opinion no 579: Definition of term pregnancy. Obstet Gynecol, 122( 5): 1139– 1140. [DOI] [PubMed] [Google Scholar]
- 27. Li HR, Feng LY, Zheng MS. ( 1996). A longitudinal study of growth and development of low birth weight infants. Zhonghua Hu Li ZaZhi, 31( 2): 63– 67. [PubMed] [Google Scholar]
- 28. Reuner G, Hassenpflug A, Pietz J, Philippi H. ( 2009). Long-term development of low-risk low birth weight preterm born infants: neurodevelopmental aspects from childhood to late adolescence. Early Hum Dev, 85( 7): 409– 13. [DOI] [PubMed] [Google Scholar]
- 29. Mackay CA, Ballot DE, Cooper PA. ( 2011). Growth of a cohort of very low birth weight infants in Johannesburg, South Africa. BMC Pediatr, 11: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Islami Z, Fallah R, Mosavian T, Pahlavanzadeh MR. ( 2012). Growth parameters of NICU admitted low birth weight preterm neonates at corrected ages of 6 and 12 month. Iran J Reprod Med, 10( 5): 459– 464. [PMC free article] [PubMed] [Google Scholar]
- 31. Biswas A, Su LL, Mattar C. ( 2013). Caesarean section for preterm birth and, breech presentation and twin pregnancies. Best Pract Res Clin Obstet Gynaecol, 27( 2): 209– 19. [DOI] [PubMed] [Google Scholar]
- 32. Lee HC, Gould JB, Boscardin WJ, El-Sayed YY, Blumenfeld YJ. ( 2011). Trends in cesarean delivery for twin births in the United States: 1995–2008. Obstet Gynecol, 118( 5): 1095– 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karimi M, Fallah R, Dehghanpoor A, Mirzaei M. ( 2011). Developmental status of 5-year-old moderate low birth weight children. Brain Dev, 33( 8): 651– 5. [DOI] [PubMed] [Google Scholar]