Abstract
Background:
This study investigated the in vitro and in vivo antibacterial effects of three mouthwashes on supragingival plaque microbiota. The three mouthwashes under study were 0.2% chlorhexidine (CHX), Listerine®, and Persica (PM). Water was used as negative control.
Methods:
Supragingival plaque samples were collected from 32 patients with gingivitis in the Dental School of Shahid Beheshti University of Medical Sciences in March 2014. Plaque samples were swabbed on agar plates and discs (previously immersed in the three mouthwashes) were placed on the agar. The zone of bacterial inhibition (ZOI) was measured after incubation for 24 hours. For the in vivo testing, the same plaque samples were inoculated on agar and the colony forming units (CFU) were counted. The patients were then instructed to use the mouthwashes (cases) and water (controls) for two weeks, after which plaque samples were again collected, inoculated and the CFUs were counted.
Results:
For the ZOI test, 0.2% CHX inhibited the growth of bacteria to an average diameter of 18.38 mm, while Listerine®, PM and water caused no inhibition of bacterial growth around the discs after 24 hours. The mean bacterial count after using 0.2% CHX for two weeks decreased by 23.13 CFU. This was followed by Listerine®, with a mean reduction of 19.75 CFU. PM resulted in 13.5 CFU decrease in the mean bacterial count, while water reduced the bacterial count by only 1 CFU.
Conclusion:
0.2% CHX inhibits bacterial growth considerably. All three mouthwashes can reduce total bacterial count after 2 weeks although with different mean bacterial count reduction.
Keywords: Mouthwashes, Periodontitis, Antibacterial activity, Bacterial biofilm
Introduction
Antonie van Leeuwenhoek was the first to report bacteria in the oral cavity in 1699 and described the presence of ‘living animalcules’ in dental plaques (1). Over the past hundred years, the microbial etiology of periodontal diseases was emphasized, especially after recent advances in bacterial identification and characterization (2). According to the current concept regarding the etiology of periodontal diseases, three factors determine the risk of active periodontal disease: a susceptible host, presence of pathogenic species, and absence of so-called “beneficial bacteria” (3). Bacteria colonize the oral cavity within a few hours after birth. Colonization of the gingival crevice occurs initially by bacterial interactions with the tooth and later by interbacterial interactions leading to the formation of an organized symbiotic community, called biofilm. Current evidence indicates that gingivitis and periodontitis are polymicrobial infections caused by the biofilm-associated bacteria (2).
Gram-positive cocci, especially Streptococcus spp. and Actinomyces spp. are the dominant flora of healthy gingival sulcus, while the microbial flora of a mature plaque consists of facultative anaerobic microorganisms, spirochaetes and motile rods. Strict anaerobics, Gram-negatives and motile organisms significantly increase in number as the disease progresses (3). In order to prevent periodontal disease, elimination of dental plaques is necessary by mechanical and chemical methods. The use of antimicrobial oral rinses plays an important role in maintaining oral hygiene, mainly by reducing the number of dental plaque microorganisms. Mouthwashes are very useful in reducing the number of microbial plaques.
Among the available mouthwashes, CHX (Chlorhexidine) is effective in reduction of dental plaques and pathogenic microorganisms including Streptococcus mutans (4). The action mechanism of CHX includes interactions with external cell components and the cytoplasmic membrane, inducing the leakage of intracellular components, and interactions with cytoplasmic constituents. Damage to the outer cell layers alone is insufficient to induce cell death (5). Though effective, CHX has certain side effects like brown discoloration of the teeth, oral mucosal erosion, and bitter taste (6). Therefore search for new and alternative antimicrobial substances with fewer side effects continues.
Another frequently used mouthwash, usually recommended as a part of home-care oral hygiene regimen, is Listerine®. Previous studies have found Listerine as an effective mouthwash in reduction of dental plaques and oral bacterial counts (7–10). In comparison with a CHX-based mouthwash (Peridex®) (9), Listerine had a similar one-hour antibacterial effect; however, after four hours from rinsing, Peridex showed further bacterial reduction. Listerine has no proved side effects, which is one of its advantages (11).
Wood sticks are traditionally and widely used for cleaning the teeth in several countries of the Middle East and Africa. The plant most commonly used as cleaner is Salvadora persica, a small tree growing wildly in a geographical distribution. It has been used in many centuries and by different communities as an oral hygiene aid. The therapeutic effect of persica could be due to certain chemical constituents such as fluoride, silicones, essential alkaloids, tannins, resins and anthraquinones. Using this herb or its extract could support periodontal health, and reduces the accumulation of microbial plaques as well as bleeding during brushing (12).
The objective of this study was to compare the antimicrobial effects of 0.2% CHX, persica mouthwash (PM) and Listerine® on aerobic and facultative bacteria gathered from supragingival plaques of patients with gingivitis.
Materials and Methods
This experimental study was performed in the Dental School of Shahid Beheshti University of Medical Sciences under the supervision of one of the faculty members (MR T) in March 2014. Sample processing and all other laboratory procedures were done at the Department of Clinical Microbiology Laboratory of Medical School.
Sources and samples
The participants in this study were recruited from patients seeking periodontal treatment at the Dental School of Shahid Beheshti University of Medical Sciences, Department of Periodontics. They signed an informed consent form provided by the university. The samples were gathered from supragingival plaques of patients with gingivitis. The eligible subjects were selected based on the following clinical parameters:
Erythema
Bleeding on probing
Age between 25–35 years old
Moderate plaque index (40–70%)
No bone loss
Exclusion from the study was based on the following criteria:
Smoking
Systemic disease
Pregnancy and lactation
Orthodontic or prosthodontic appliances
Antibiotic therapy within the past three months
Adverse reaction to the three mouthwashes
Instruments for gathering data and validation
Supragingival samples were collected with a sterile curette and the aid of a mouth mirror and cotton rolls. A sterile tube was used for transferring the samples to the laboratory.
Procedure for gathering data
For standardization, first, the patients were given oral prophylaxis. Oral B toothbrush and toothpaste were given to all patients and they were instructed to brush their teeth by the modified Bass technique three times in a day and use mouthwashes twice a day according to the manufacturer’s instructions. Plaque index was measured every 5 days. The samples were gathered before and two weeks after using the mouthwashes.
The 32 patients were randomly divided into four groups (eight patients in each group) using CHX, PM, Listerine® or water. Each group of patients used the mouthwashes every 12 hours as follows:
CHX group: 30 seconds mouth rinsing with 30 ml of CHX mouthwash.
PM group: 20 seconds mouth rinsing with 15 drops of PM in 15 ml of water.
Listerine® group: 30 seconds mouth rinsing with 20 ml of Listerine mouthwash.
Control group: 30 seconds mouth rinsing with 30 ml of water.
Sample Collection
A supragingival plaque sample equal to 1 mg was collected with a sterile curette and directly immersed in a sterile vial containing 1 ml of phosphate buffered saline (PBS).
To disperse the bacterial cells, the solution was homogenized by an agitator for five minutes. Tenfold serial dilutions were made in PBS with a repeat homogenization on the agitator for 60 s at the start and between successive dilutions. Dilutions of 1 × 10−5 were then used for culturing.
Preparation of Mueller Hinton agar plates and inoculation of bacteria
For bacterial colony counting, 0.1 ml of diluted samples was transferred into empty plates. Mueller Hinton agar was cooled to 50 °C, and poured into each plate. After the agar had solidified, the plates were incubated for 24 hours and the colony forming units (CFU) were counted. The same method of sample collection and inoculation was repeated after two weeks when the subjects completed the two-week regimen of mouthwash or water use.
The zone of growth inhibition (ZOI) test was done in vitro on the diluted samples gathered before the patients used the mouthwashes or water. Bacteria were streaked on the agar surface with a swab. The plates were then divided into four equal sections, labeled as C, L, P and W for CHX, Listerine®, PM, and water, respectively. Filter paper discs impregnated with each of the mouthwashes and water were then placed at the center of each section and pressed lightly on to the agar.
Then the plates were incubated in the inverted position at 37 °C for 24 hours. After 24 hours, ZOI around the disc was measured. The measurement was between disc edge and bacterial growth border.
Data analysis
Collected data were studied by coding the variables in Excel format and using the pHstate2 software to obtain the descriptive statistical analysis. The difference between the four groups was determined using the analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant.
Results
Table 1 summarizes the bacterial counts before and after two-week use of 0.2% CHX, Listerine® and Persica mouthwashes in patients with gingivitis. The summary statistics on the bacterial counts before and after two-week use of water (negative control) in patients with gingivitis are also presented in Table 1. Analysis of Variance (ANOVA) was used to analyze the differences in the reduction of bacterial counts after using 0.2% CHX, PM, Listerine and water rinses. The results showed that the mean bacterial count after using 0.2% CHX for two weeks decreased by 23.13 CFU. This was followed by Listerine®, with a mean CFU reduction of 19.75. PM resulted in 13.5 CFU decrease of the mean bacterial count, while water reduced the bacterial count only by 1 CFU. The above observations are illustrated in Fig. 1.
Table 1:
Bacterial colony counts (CFU) pre and post treatment with different mouthwashes and water
| Before | After | ||
|---|---|---|---|
| CHX | Mean± SD | 26.88±7.59 | 3.75±2.25 |
| Min/Max | 21/43 | 1/8 | |
| Count | 8 | 8 | |
| Listerine | Mean± SD | 27.5±6.25 | 7.75±2.66 |
| Min/Max | 19/36 | 4/12 | |
| Count | 8 | 8 | |
| PM | Mean± SD | 22.13±7.88 | 8.63±3.11 |
| Min/Max | 12/32 | 4/13 | |
| Count | 8 | 8 | |
| Water | Mean± SD | 26.88±7.51 | 25.88±9.01 |
| Min/Max | 18/37 | 17/40 | |
| Count | 8 | 8 |
Fig. 1:
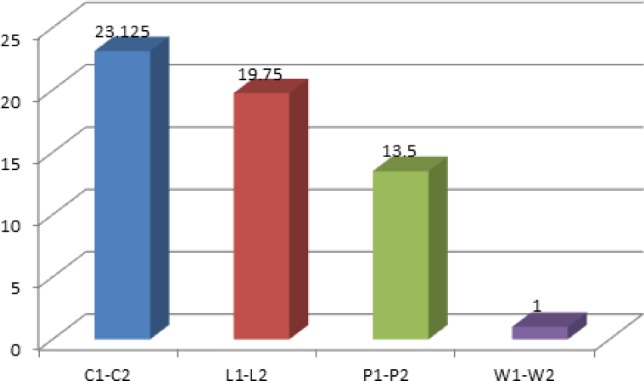
- C1–C2 Amount of the mean bacterial reduction after use of 0.2% CHX
- L1–L2 Amount of the mean bacterial reduction after use of Listerine®
- P1–P2 Amount of the mean bacterial reduction after use of PM
- W1–W2 Amount of the mean bacterial reduction after use of water
It can be observed in Fig. 1 that 0.2% CHX caused the highest reduction in bacterial counts, followed by Listerine® and PM. Water was ineffective in reducing bacterial counts.
Subsequent ANOVA revealed that highly significant differences existed regarding the reduction capability on bacterial counts between the three mouthwashes and water. This is shown by the P-value of 6.74 × 10−9, which is lower than 0.05. To determine significantly different mouthwashes, Tukey-Kramer post hoc test was employed and the results are summarized in Table 2. Figure 1 shows that 0.2% CHX was slightly more effective than Listerine® in reducing bacterial counts after two weeks of use as mouthwash; however, the difference turned out to be insignificant (Table 2) by Tukey-Kramer post hoc test.
Table 2:
Tukey-Kramer test on the mean bacterial count reduction between different methods
| Comparison | Absolute difference of colony forming units | std. Error of Difference | Critical Range | Results |
|---|---|---|---|---|
| 0.2% CHX vs. Listerine | 3.375 | 1.77831054 | 8.5359 | P-value>0.05 |
| 0.2% CHX vs. PM | 9.625 | 1.77831054 | 8.5359 | P-value<0.05 |
| 0.2% CHX vs. water | 22.125 | 1.77831054 | 8.5359 | P-value<0.05 |
| Listerine vs. PM | 6.25 | 1.77831054 | 8.5359 | P-value>0.05 |
| Listerine vs. Water | 18.75 | 1.77831054 | 8.5359 | P-value<0.05 |
| PM vs. Water | 12.5 | 1.77831054 | 8.5359 | P-value<0.05 |
The 0.2% CHX mouthwash was significantly more effective than PM and water. The bacterial reduction was not statistically significant between Listerine® and PM (P>0.05). The mean bacterial count after two weeks of using Listerine® was reduced by 19.75 CFU compared to 13.5 CFU for PM.
The antibacterial effect of 0.2% CHX was significantly higher than that of PM (P-value<0.05) and water but the differences between the antimicrobial effects of 0.2% CHX and Listerine® and also PM and Listerine® were not significant (P-value>0.05).
The ZOI test demonstrated no bacterial growth inhibition for Listerine®, PM and water after 24-hour incubation and complete bacterial growth was seen on the concordant part of plates despite of the presence of mouthwash discs. However, CHX prevented the growth of bacteria in all 32 plates.
The 0.2% CHX inhibited the growth of bacteria to an average diameter of 18.38 mm with a standard deviation of 6.08. The smallest zone without bacterial growth had a diameter of 12 mm while the largest zone had a diameter of 27 mm.
Discussion
The culture medium of this study was Mueller Hinton Agar, which is used in procedures commonly performed on aerobic and facultative anaerobic bacteria (neogen.com) as it is growth-specific to those bacteria. It would seem logical to assume, therefore, that the bacteria cultivated on the agar plates were indeed both aerobic and facultative.
The results of this study indicated a discrepancy between the demonstrated antibacterial effects of Listerine® and PM because while both mouthwashes showed bacterial count reduction after two weeks of in vivo use, they were not able to produce ZOIs after 24 hours of in vitro incubation. This can be explained by the fact that for the ZOI tests, the culture medium was treated with the mouthwashes only once and then the results were read after 24 hours; whereas, for the in vivo tests, bacteria of the oral cavity were repeatedly exposed to the effects of mouthwashes for two weeks. This may mean that continuous exposure to the mouthwashes is necessary to reduce bacterial counts, especially for the Listerine® and PM.
Due to limitations of the laboratory, the ZOI could only be checked after 24 hours of incubation. It seems very probable that Listerine® and PM had lost their antibacterial effect within that time, allowing bacterial growth in the plates during incubation. The antibacterial effect of Listerine® mostly comes from its alcohol content, which may have evaporated by the time the ZOI was measured. Further studies are need to be conducted for PM to determine whether or not, like Listerine®, its main antibacterial component loses its effectiveness within a relatively shorter time than CHX. Therefore, in this study, no short-term antibacterial effect was shown for Listerine® and PM. Only CHX had antibacterial effect even after 24 hours of incubation. It may be reasonable to assume that Listerine® and PM could have produced a measurable ZOI during the first few hours of incubation. This assumption is further based on a study by Almas et al. (13) which showed that PM had antibacterial activity although much lower compared to CHX. What is apparent from the results of this study is that CHX antimicrobial effect has more longevity than the effects of Listerine® or PM.
It is difficult to remove all bacteria by mechanical plaque control; thus, antibacterial mouthwashes can be useful adjuncts for this purpose. In the current study, the antibacterial effects of three mouthwashes were compared in vitro and in vivo. ZOI test (in vitro) was used for supragingival plaque samples of 32 patients with gingivitis before using mouthwashes while colony counting test (in vivo) was used for these patients before and two weeks after using the mouthwashes. All three commercial mouthwashes (0.2% CHX, Listerine® and PM) turned out to be more effective than water in reducing bacterial counts after two weeks of mouth washing. Although the 0.2% CHX was slightly more effective than Listerine®, the difference turned out to be insignificant. PM was also effective in reducing bacterial counts but to a significantly less degree than 0.2% CHX. These findings were similar to those of Tomas et al. (14) who found that the bacterial count in the oral cavity decreased after using CHX mouthwash. Their results showed a significant reduction in the total bacterial population at 30 seconds and one hour after mouth rinsing with both CHX concentrations of 0.12% and 0.2%; also, CHX had the highest antimicrobial effects in orthodontic patients.
Kasuga et al. (8) also demonstrated a reduction in bacterial counts after using Listerine® mouthwash. They reported that mouth washing with Listerine® for 30 seconds resulted in decrease of viable bacterial counts in the saliva. Al-Bayati and Sumaiman’s (15) study also showed total bacterial reduction after using PM. They reported that the strongest antibacterial activity was observed using the aqueous extract of Salvadora persica against S. faecalis.
Both mouthwashes (CHX and Listerine®) significantly reduced bacterial counts one and 4 hours after treatment in their volunteers, although the Peridex® (CHX) oral rinse showed a further reduction in the bacterial colony count (9).
Conclusion
The results simply revealed that mouthwashes are effective in maintaining low bacterial counts in the mouth. However, since the beneficial role of the presence of commensal species in the oral cavity has been established, the need to maintain constantly low bacterial counts in the mouth is still under debate. The results obtained from this study revealed that the three mouthwashes might decrease the number of bacteria in the oral cavity.
Ethical considerations
Ethical issues (including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgment
We would like to thank warmly the staff of Microbiology Laboratory of Shahid Beheshti Medical School for their kind collaboration. The authors of this paper had no conflicts of interests and no funding was paid for this study.
References
- 1. Tatakis DN, Kumar PS. (2005). Etiology and pathogenesis of periodontal diseases. Dent Clin North Am, 49( 6): 491–516. [DOI] [PubMed] [Google Scholar]
- 2. Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. (2005). Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol, 43( 8): 3944–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ljiljana K, Jelena M, Marija I, Radmila O. (2008). Microbial etiology of periodontal disease. FU Med Biol, 15( 12): 616–621. [Google Scholar]
- 4. Saffari F, Danesh Ardakani M, Zandi H, Heidarzadeh H, Moshafi MH. (2015). The Effects of Chlorhexidine and Persica Mouthwashes on Colonization of Streptococcus mutans on Fixed Orthodontics O-rings. J Dent Shiraz Univ Med Sci, 16( 1): 54–57. [PMC free article] [PubMed] [Google Scholar]
- 5. Pietruska M, Paniezko A, Waszkiel D, Pietruska J, Bernaez A. (2006). Efficacy of local treatment with chlorhexidine gluconate drugs on the clinical status of periodontium in chronic periodontitis patients. Adv Med Sci, 51( 7): 162–165. [PubMed] [Google Scholar]
- 6. Lakade LS, Shah P, Shirol D. (2014). Comparison of antimicrobial efficacy of chlorhexidine and combination mouth rinse in reducing the Mutans streptococcus count in plaque. J Indian Soc Pedod Prev Dent, 32: 91–6. [DOI] [PubMed] [Google Scholar]
- 7. Fornell J, Sundin Y, Lindhe J. (2007). Effect of listerine on dental plaque and gingivitis. Scand J Dent Res, 83( 1): 18–25. [DOI] [PubMed] [Google Scholar]
- 8. Kasuga Y, Ikenoya H, Okuda K. (1997). Bactericidal effects of mouth rinses on oral bacteria. The Bulletin of Tokyo Dental College, 38( 4): 297–302. [PubMed] [Google Scholar]
- 9. Balbuena L, Stambaugh KI, Ramirez SG, Yeager C. (1998). Effects of topical oral antiseptic rinses on bacterial counts of saliva in healthy human subjects. Otolaryngol Head Neck Surg, 118( 5): 625–629. [DOI] [PubMed] [Google Scholar]
- 10. Aneja KR, Joshi R, Sharma C. (2010). The antimicrobial potential of ten often used mouthwashes against four dental caries pathogens. Jundishapur J Microbiol, 3( 1): 15–27. [Google Scholar]
- 11. Al Habashneh R, Qubain TG, Alsalman W, Khader Y. (2014). The Effect of Listerine Mouthwash on Dental Plaque, Gingival Inflammation and C - reactive protein (CRP). Dentistry, 4( 1): 191–195. [Google Scholar]
- 12. Darout IA, Albandar JM, Skaug N. (2000). Periodontal status of adult Sudanese habitual users of miswak chewing sticks or tooth brushes. Acta Odontol Scand, 58( 1): 25–30. [DOI] [PubMed] [Google Scholar]
- 13. Almas K, Skaug N, Ahmad I. (2005). An in vitro antimicrobial comparison of miswak extract with commercially available non-alcohol mouthrinses. Int J Dent Hyg, 3( 1): 18–24. [DOI] [PubMed] [Google Scholar]
- 14. Tomas I, Cousido MC, Tomas MJL, Caballero L, Diz P. (2008). In vivo bactericidal effect of 0.2% chlorhexidine but not 0.12% on salivary obligate anaerobes. Arch Oral Biol, 53( 3): 1186–1191. [DOI] [PubMed] [Google Scholar]
- 15. Al-Bayati FA, Sulaiman KD. (2008). In vitro antimicrobial activity of salvadora persica l. extracts against sole isolated oral pathogens in Iraq. Turk J Biol, 32( 1): 57–62. [Google Scholar]


