Abstract
Background:
Rhoptries are unique secretory/excretory organelles that are found exclusively in the Apicomplexa, and their contents are discharged at the time of invasion and are critical in the establishment of productive infection. Several rhoptry proteins have been identified in Toxoplasma gondii, Plasmodium falciparum and Neospora caninum and have been linked not only with the parasites’ adhesion and invasion processes but also with their intracellular pathways. To date, only one Cryptosporidium parvum rhoptry protein candidate related to TgRON1 of T. gondii and PfASP of P. falciparum has been reported.
Methods:
Subcellular fractionation of sporozoites was performed to obtain highly purified organelles. One-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by liquid chromatography coupled with mass spectrometry was applied for fraction analysis, and 22 potential novel rhoptry proteins were detected by protein domain analysis using online softwares.
Results:
Twenty-two potential novel rhoptry proteins were detected. A protein with T. gondii and N. caninum rhoptry protein homologs and some proteins with domains similar to that of T. gondii rhoptry proteins were identified.
Conclusion:
These novel candidate proteins may be considered targets for researching the invasion pathway of C. parvum and the pathogenic mechanisms of rhoptry proteins. The present work provides a starting point towards the elucidation of the repertoire and function of C. parvum rhoptry proteins.
Keywords: Cryptosporidium parvum, Rhoptry, Liquid chromatography, Mass spectrometry, Protein domain analysis
Introduction
The apicomplexan parasite Cryptosporidium parvum is a waterborne enteric protozoan with significant veterinary importance (1). Cryptosporidiosis in the immunocompetent host is a self-limiting diarrheal illness, but in immunosuppressed individuals such as those with human immunodeficiency virus (HIV) infection, the disease can be serious and life threatening (1, 2). In addition to humans, C. parvum has been reported as a common serious primary cause of diarrheal outbreaks in farm animals, especially newborn ruminants such as cows that result in significant economic losses (3). To date, due to the lack of effective drugs and approved vaccines, the prevention and treatment of cryptosporidiosis remains a big problem (4).
Most apicomplexan parasites, such as Toxoplasma spp., Cryptosporidium spp., Plasmodium spp., Babesia spp., and Eimeria spp., have the capacity to invade and replicate within the cells of their vertebrate hosts (5). All of these genera possess three kinds of secretory vesicles consisting of the apical organelles (rhoptries, micronemes and dense granules), which secrete substances that enable parasites to adhere selectively to and invade host cells; once within a host, they cause further modifications and eventually escape (6). Rhoptries are perhaps the most unusual organelles found within apicomplexan parasites (7, 8). They are the largest of the apical organelles, and their name alludes to their club-like shape that is visible in the apicomplexan parasites (6).
The number of rhoptries differs among apicomplexan parasites. Each zoite has two rhoptries in Plasmodium merozoites and sporezoites, Babesia caballi has three (9), Theileria parva merozoites have six (10), and T. gondii has eight or more in every sporozoite. However, sporozoites of C. parvum reportedly have only one (11). Rhoptry proteins have been linked with both the adhesion and invasion processes of the parasites and with intracellular pathways such as the delivery of signals to the host cell nucleus that is achieved by crossing the parasitophorous vacuole membrane (PVM) (12) or through evacuoles (13). Furthermore, some rhoptry proteins were shown to be important virulence factors (14). Although C. parvum contains rhopty, few rhoptry proteins in C. parvum have been reported to date.
In the present study, subcellular fractionation was coupled with proteomic techniques to identify novel rhoptry components in C. parvum.
Materials and Methods
Oocyst preparation and sporozoite isolation
The C. parvum Iowa isolate used in this study was purchased from Waterborne™ Inc. and maintained by passage in newborn Cryptosporidium-free Holstein bull calves (15). Oocysts were isolated from calf feces using sucrose density gradient centrifugation (16) and 0.5% (wt/vol) hypochlorite treated immediately at 4°C for 10 min. Oocyts excystation was conducted in 0.75% (wt/vol) Nataurocholate (Sigma, Hercules, CA, USA) at 37°C for 2 h until it exceeded 90% (15).
Isolation of rhoptry organelles
A total of 1 × 109 sporozoites were collected by centrifugation at 1,300 × g for 10 min at 4°C. The subsequent purification steps were performed as previously described (17). The sporozoites were washed once in phosphate-buffered saline (PBS) and in R buffer (250 mM sucrose, 10 mM MOPS, pH 7.2, 2 mM dithiothretol, 1 mM ethylenediaminetetraacetic acid [EDTA], complete protease inhibitor mixture), respectively. The pellet was resuspended in R buffer (5 × 108 sporozoites/ml) and then disrupted by passage through a French® Type Pressure Cell Disrupter (STANSTED FLUID POWER Ltd.) as previously described (18). After being centrifuged at 1,300 × g for 20 min, the supernatant was transferred to a new micro-centrifuge tube.
The organellar pellet was created by centrifugation at 25,000 × g for 25 min and resuspended in R buffer containing 30% (wt/vol) Percoll. The mixture was centrifuged at 61,500 × g for 25 min and a brownish band was collected. The band was diluted in R buffer and pelleted again at 100,000 × g for 90 min to remove the Percoll. The mixture (after Percoll removal and R buffer dilution) was overlaid with steps of sucrose (36%, 39%, 42%, 45%, 48%, and 60%) in S buffer (10 mM MOPS, 2 mM dithiothreitol, 1 mM EDTA, and complete protease inhibitor mixture) and centrifuged at 150,000 × g for 18 h. Fractions in each gradient were collected, diluted in R buffer, and centrifuged at 100,000 × g for 90 min. These fractions were stored in R buffer at −80°C.
Electron microscopy detection
Pooled gradient fractions were respectively fixed in 2.5% glutaraldehyde, 4% sucrose, and 0.05 M phosphate buffer (pH 7.4) for 2 h, washed three times with ice-cold PBS, and centrifuged at 14,000 rpm for 10 min. Each sample was processed for electron microscopy using standard methods (19).
Protein extraction
Total protein was extracted using a ReadyPrep™ Protein Extraction Kit (Total Protein) (BIO-RAD, USA) according to the manufacturer’s instructions. The extraction process could solubilize many types of cell proteins, including both soluble and membrane fractions. Briefly, the purified rhoptry fraction was added to 1 ml of sample buffer (7 M urea, 2 M thiourea, 1% [wt/vol] ASB-14 detergent, 40 mM Tris base, and 0.001% bromophenol blue) in a 2-ml microcentrifuge tube and sonicated on ice for 30-sec bursts (typically 3–4 times). The mixture was centrifuged at 16,000 × g for 30 min at 20°C. We removed and transferred the supernatant to a clean tube. Unused extract was stored in aliquots at −80 °C.
One-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis (1-D SDS-PAGE)
Rhoptry proteins were separated in 15% SDS-PAGE and visualized using Coomassie Blue staining. The entire protein gel profile was excised from the gel and maintained in the new tube with MilliQ water until the protein was digested.
Liquid chromatography coupled with mass spectrometry (LC/MS-MS) and bioinformatics analysis
The entire 1-D SDS-PAGE gel profile was hydrolyzed to cleave the peptides and then analyzed using LC/MS-MS as described below.
First, the sample was minced and washed twice with MilliQ water, decolorized twice with 50% MeOH for 30 min and subsequently washed with MilliQ water, 50% acetonitrile and 100% acetonitrile. After the sample was reduced with 10 mM DTT for 1 h at 55 °C, alkylated 55 mM iodoacetamide was added for 45 min in the dark and the sample was washed again with MilliQ water, 50% acetonitrile and 100% acetonitrile. Finally, the sample was dried in vacuo for 30 min.
Second, the dried sample was digested with 3 μl of 12.5 ng/μl trypsin (Promega V5280) overnight at 37°C and centrifuged, and the supernatant was acidified using 1% formic acid. Digested peptides were dissolved using 25 mM NH4HCO3 (0.1% formic acid and 2% acetonitrile) were analyzed using LC/MS-MS on an LTQ-Orbitrap mass spectrometer (Thermo Electron, Bremen, Germany). The peptides were separated on a BioBasic C18 PicoFit column (100 μm, 10 cm long, 3 μm resin; Michrom Bioresources, Auburn, CA, USA) at a flow rate of 300 nl/min. Water and acetonitrile added with 0.1% formic acid were used as solvents A and B, respectively. The gradient was started at 5% solvent B and increased to 35% solvent B for 120 min. Peptide ions were analyzed in data-dependent MS experiments and detected in a survey scan from 400 to 1700 amu (3 íscans) followed by five data-dependent MS/MS scans (5 íscans each; isolation width, 3 amu; 35% normalized collision energy; dynamic exclusion for 3 min). Bioworks 3.2 software was used to process the data and convert it to an MGF file.
The search was performed using the MASCOT in the NCBI, CryptoDB v5.0, and EupathDB v2.16 databases. Protein functions were predicted using SMART, TMpred and SignalP 4.0 software.
Results
Isolation of rhoptry organelles
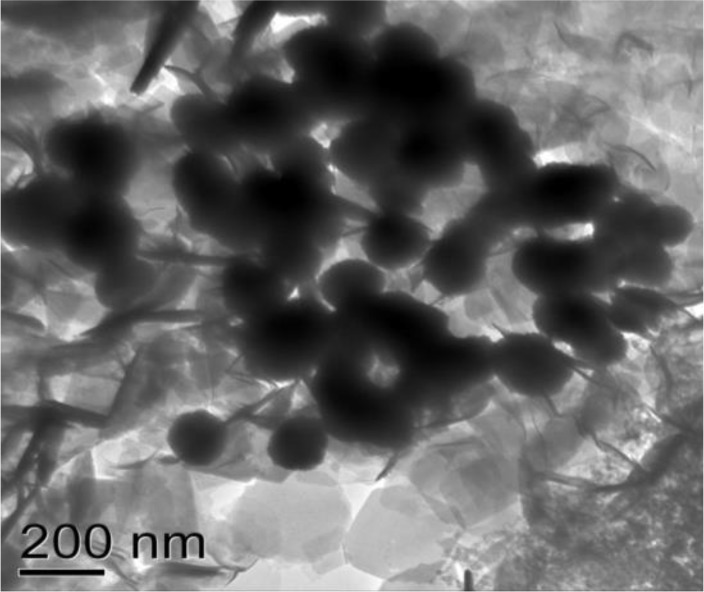
The Cryptosporidium oocysts with >90% viability were used to excystate and disrupte. The disrupeted sporozoite composition was subjected to sucrose gradient floatation using a gradient of 36– 60% sucrose. Fractions of each gradient (36%, 39%, 42%, 45%, 48%, and 60% sucrose) were processed for electron microscopy, and those that were enriched with rhoptry proteins were identified. The only fraction between 36% and 39% sucrose was enriched with rhoptries as indicated by its black tadpole-like appearance (Fig. 1).
Fig. 1:
Electron microscopy of sucrose graction of rhoptry fraction
Other sucrose concentrations did not find any similar structures. The crystalline objects in the background may be the leaving sugars. Based on these results, pooled gradient fractions of C. parvum were used for the subsequent studies.
Proteomic identification of rhoptry organelles using gel LC/MS-MS
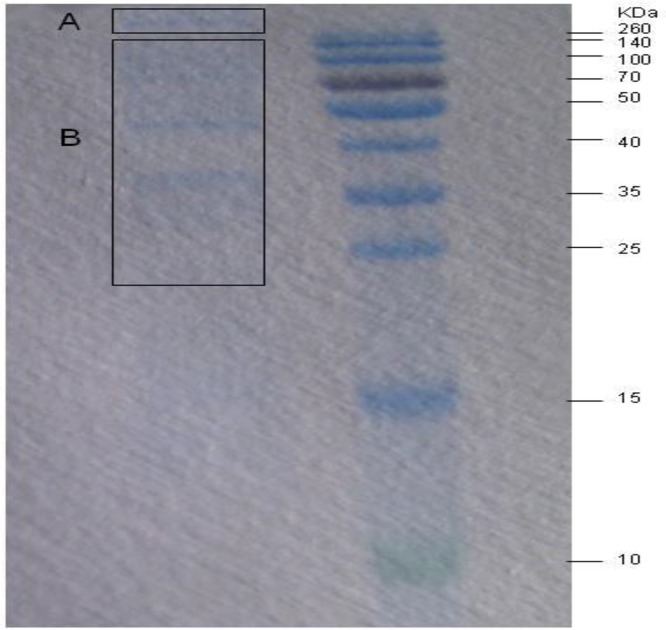
Different protein compositions were observed among the subcellular fractions in 1-D SDS-PAGE (Fig. 2). The molecular weights of some of the proteins were >260 kDa (A), while the others were 25–260 kDa (B). These two sections were excised and identified using LC/MS-MS. A total of 22 proteins (four from part A and 18 from part B) were identified, including kinases, secret proteins, large proteins, membrane protein, ATPase, peptidase, dikinase, transmembrane protein, and hypothetical proteins (Table 1). Among these proteins, a serine threonine (Ser/Thr) protein kinase of C. parvum (cgd7_590) possessed 40% amino acid sequence identity with T. gondii ME49 ROP17 (TgROP17) and 34% identity with N. caninum ROP20 (NcROP20).
Fig. 2:
1-D SDS-PAGE resolution of the rhoptry fraction
A: The proteins with molecular weight more than 260 kDa; B: The proteins with molecular weight between 25 kDa to 260 kDa
Table. 1:
Identification of rhoptry proteins by LC/MS-MS
| Protein name | Genome localization | Predicted Molecular Weight (kDa) | Score * | No. matched peptides ** | Amino acid sequence of peptides *** | Acession No. | Blast homology (score/Identities/Positives) |
|---|---|---|---|---|---|---|---|
| Ser/thr protein kinase | Chr VII | 26.66 | 106 | 8 | ENAIKYDLYCIGMI-MI-FIEMWTSALK/NLSGQR/LAADPAWSAGK | cgd7_590 | TGME49_058580 Toxoplasma_gondii_ME49 Rhoptry kinase family protein ROP17 (83/40%/66%); NCLIV_028170 Neospora_caninumRhoptry kinase family protein ROP20, putative,(83/34%/48%) |
| Large protein with a SPRY domain and HECT domain | Chr I | 538.22 | 806 | 22 | DSVIDTKSSFLDYNEL-EK/IIYESR/KSPELIDDLFVK/NVNQLIELI NAITK/NVLYNIPDTEIYSLK/TPNNDSFS NNVNHNLNILHDTYSNHDIIK | cgd1_1920 | |
| Secreted protein with signal peptide, fringe-like glycosyltransferase domain and a WcaK like glycosyltransferase domain | Chr VI | 95.52 | 538 | 16 | FDEHLNNTNIPLQFKYNSLEI-EHPCK/SLFFFLELSLIITNSSTYIPNDNK/A IIFHGGG-NFGDLYSHHHELR/TLDGMAFLSR | cgd6_1450 | |
| Large protein with signal peptide. cysteine-rich, threonine-rich, possible mucin | Chr III | 184.65 | 551 | 19 | ELSIIPFEDVEDHIK/CTNSNDK/GWPIG-MEAL-NGNWDLNR/ALSSTYIYTK/EMPIPFFTAS LPLIGQLAANAFPMIK | cgd3_1540 | |
| Ser/thr protein kinase | Chr III | 178.11 | 279 | 10 | NLIIDEKTQNEINCNER/NPQIEYRLTQI-INIIK/SGNPLVLCNNILK/LLNFLQK | cgd3_3230 | |
| Signal peptide plus GPI anchored membrane protein with N-terminal multiple cysteines | Chr VII | 45.22 | 235 | 10 | TKETMTMISR/LFGLTPEEQCK/EDEDK-DEAEK | cgd7_4820 | |
| Vacuolar proton translocatingATpase with 7 transmembrane regions near C-terminus | Chr IV | 104.81 | 356 | 6 | GDGILFAPLNHMR/NINLIK/VVFVI-YFQGATTSAVYDKISR/FTSFTNSSK | cgd4_1470 | |
| WD repeat protein | Chr V | 127.45 | 306 | 9 | IHIQESQQDISDSK/EENCLELSK/LIHSSA WMNNNIVIFGGEDHIVR | cgd5_2540 | |
| WD repeat protein | Chr IV | 63.01 | 260 | 11 | YLISSAHDGTIKLWNSLNGK/YENLEIP-ENIYSLFGRK | cgd4_950 | |
| Secreted insulinase like peptidase, signal peptide | Chr III | 146.83 | 127 | 12 | LSDILQEIS-SPT(2)/VFMVYDK/SIPTQNITQNSLK | cgd3_4280 | |
| R1 like alpha-glucan water dikinase | Chr II | 192.83 | 148 | 8 | HSNSMKLI-WEK/INFLYR/LLAQSAETASLTLISK | cgd2_2340 | |
| 3 transmembrane region protein | Chr VIII | 43.85 | 168 | 3 | MAK-NLWLFSQEIMESNAAK/RFLGFCVNGIK | cgd8_4880 | |
| Very large secreted protein, signal peptide | Chr VII | 374.84 | 484 | 15 | LYLNSSQIEGLEIPK/LYLIPMQK/VNELF DK/GDLFDFLGCIR/INLTVTTPGK | cgd7_4340 | |
| Hypothetical protein with signal peptide and one or more transmembrane domain regions | Chr II | 135.07 | 406 | 12 | LNY-NILLNSILENSR/LVQSSK/NYSINMANEEF K/INFFANNFIFELNDLK | cgd2_1710 | |
| Hypothetical protein with signal peptide and possible transmembrane region at C-terminus | Chr V | 56.88 | 245 | 8 | VEIIDREISILD-GQMSLR/PIISELFDMLLENI-EAKR/PIQFSSFNIVTSK | cgd5_4280 | |
| Extracellular protein with a signal peptide, FN3 domain and a predicted transmembrane region | Chr IV | 284.24 | 731 | 18 | MKLSNLIIPGR/FFDYEYDLSR/LSD-FLYVTFDNNIR/LDWKFYNFGNFPLDLEI FEK/IINTFSISYLESDTIEQVEEEEWQVK/ELNGNNEEVSISR | cgd4_640 | |
| Multiprotein bridging factor type 1 like transcriptional co-activator | Chr III | 177.24 | 111 | 6 | LAMPNAARLDQDTGDYR/QNIPQNAAK | cgd3_3750 | |
| Nuclear VCP like protein with 2 AAA ATpase domains | Chr V | 77.24 | 206 | 10 | VVNQLLTELD-GVGER/LTALYSK/IIYVPLPNEMGR | cgd5_2010 | |
| Hypothetical integral membrane protein with 12 transmembrane domains | Chr VI | 63.49 | 411 | 11 | LCWVFIFCLSLFGMLDIITSLM-NNYR/VNCLTDGHLK/FALTLHGIIGFFLF VTPCIFTYDMDNLVAK | cgd6_3870 | |
| Signal peptide, serine proline rich, possible low mw mucin glycoprotein locus of 6 genes | Chr II | 19.34 | 72 | 3 | DFVSVVGLNTENRNSVELGNK/GSIEIGN PTQPK | cgd2_450 | |
| Hypothetical protein with signal peptide and one or more transmembrane domain regions | Chr II | 135.07 | 480 | 13 | LNYNILLNSILENSR/VFSIIIILISLVFTA-TIFIPLILIESEK/ISQHSLK/KQLMGIFR | cgd2_1710 | |
| Hypothetical protein with 4 transmembrane domains, possible unusual phyletic distribution | Chr III | 134.07 | 299 | 7 | MESNELNSLSIDIP-SGNEQNLK/TSSEGMKGGYSSDTK/GFPL CHIYPAPSIGMNR | cgd3_590 |
MASCOT MS protein score, the probability score was <0.05.
Number of peptide masses values matched.
Amino acid sequence identified by MS-MS.
Protein domain analysis by software online
Most hypothetical C. parvum rhoptry proteins have N-terminal signal peptides, C- or N-terminal transmembrane domains, and several low complexity regions. The cgd7_4820 protein has a predicted GPI anchor signal. The cgd7_590 and cgd3_3230 proteins contain Ser/Thr kinase domain, which usually results in a functional change of the target protein by changing enzyme activity, cellular location, or the association with other proteins. The cgd7_590 protein is located on chromosome VII and contains the signature Ser/Thr kinase domains such as TgROP16, TgROP17, and TgROP18. The putative RON protein (cgd3_1540) identified in C. parvum, which islocated on chromosome III, owned two signal peptides in the N-terminal, two internal repeats, and several low complexities such as TgRON 4. The cgd6_3870 protein was located on chromosome VI and had 12 transmembrane domains, similar to TgROP14. The cgd3_590 protein had DUF900, a hydrolase-like domain. Despite the presence of N-terminal signal peptides and C-terminal trans-membrane domains, the cdg2_450 protein also had a serine proline-rich domain.
Discussion
Rhoptries are unique secretory/excretory organelles that are found exclusively in Apicomplexa, and their contents are discharged at the time of invasion and are critical to the establishment of a productive infection. Rhoptries were first isolated from Eimeria nieschilzi (20) and have since been isolated from several Apicomplexa including P. falciparum, T. gondii, Babesia spp., Eimeria spp., and N. caninum. Bradley et al. reported 38 novel proteins identified by MS of a purified rhoptry fraction from T. gondii (17). Marugán-Hernández et al. identified an extensive number of potential rhoptry proteins in N. caninum using proteomic approaches (21). Sanderson et al. assessed the potential array of rhoptry-related proteins detected in the C. parvum sporozoite proteome by performing homologous BLAST searches using protein sequences and detected 12 putative sporozoite rhoptry proteins (22).However, the functions of these proteins were not studied here. To date, only one C. parvum putative rhoptry protein, CpPRP1, has been reported, and it is the only C. parvumprotein that shows significant homology to the known rhoptry neck proteins (23). The reason for the low primary sequence conservation between C. parvum and other apicomplexan parasites may be that the parasites are able to adapt to distinct biological niches, i.e., nucleated cells for T. gondii and N. caninum, erythrocytes/hepatocytes for P. falciparum, and enterocytes for C. parvum(23). Therefore, it is difficult to use homology comparisons to obtain novel C. parvum rhoptry proteins. In this study, the subcellular fractionation technique was used to gain enriched rhoptry organelle fractions prior to the use of 1-D SDS-PAGE and LC/MS-MS.
Most proteins examined here from C. parvum rhoptry-enriched fractions possessed an N-terminal signal peptide that was recognized by SignaIP. However, the internal transmenbrane domains were not recognized by TMpred in all proteins. Additionally, many proteins contained several stretches of low complexity regions, which were predicted to form α-helical regions within the T. gondii rhoptry proteins. These characteristics of the 22 novel predicted proteins we gained here were similar in overall structure to those of the T. gondii rhoptry proteins.
These novel proteins identified by LC/MS-MS could be categorized into two classes. In the first class, only one novel protein (cgd7_590) identified in C. parvum had T. gondii and N. caninum homologs. The sequences of the C. parvum cdg7_590 protein shared 40% and 34% identity with TgROP17 and NcROP20, respectively. The cgd7_590 protein was located on chromosome VII and contained the signature Ser/Thr kinase domain just like TgROP16, TgROP17, and TgROP18. There are few studies on TgROP17, but it shared high structural similarity with TgROP16 and TgROP18 of the TgROP2 super-family (24, 25). With regard to domain structures, TgROP17 and TgROP18 contained a conserved catalytic triad and showed excellent conservation of other key residues (26). Moreover, TgROP17 showed stronger conservation within the ATP-binding clefts like TgROP16 and TgROP18, which were related to kinase activity (27). As we all know, TgROP16 and TgROP18 are the main virulence factors with kinase activity (14, 28). TgROP18 remains at the PVM (29); however, TgROP16 is translocated into the host cell nucleus (14). It is highly possible that TgROP17 will work as a functional kinase for regulating the virulence of T. gondii from this point. On the other hand, the cgd3_3230 protein contained an Ser/Thr kinase domain and seemed to be a functional kinase as well.
In the other class, some proteins with domains similar to that of T. gondii rhoptry proteins were identified. One putative RON protein (cgd3_1540) identified in C. parvum located on chromosome III contained two signal peptides in the N-terminal, two internal repeats, and several low complexities like TgRON 4, a secreted rhoptry neck protein of T. gondii that migrates at the moving junction in association with TgAMA1 during invasion (17). The TgRON4 protein also contains two amino acid repeats that may have particular importance to the function of their trafficking to the rhoptries. The cgd6_3870 protein was located on chromosome VI and had 12 transmembrane domains, similar to TgROP14. TgROP14 lacks an apparent signal peptide but contains multiple predicted transmembrane domains, a feature common to other Toxoplasma proteins that have been demonstrated to enter the secretory pathway in the absence of a signal peptide (30). At the same time, the cgd3_590 protein contained DUF900, a hydrolase-like domain. In addition to the signal peptide and transmembrane region, the cgd2_450 protein was the owner of a serine- and proline-rich domain and a possible low molecular weight mucin glycoprotein locus of six genes. These functional structures above were similar to those of other known rhoptry proteins, but their detailed functions remain unknown.
Prior to this research, only one C. parvum rhoptry protein candidate (cgd8_2530) related to TgRON1 of T. gondii and PfASP of P. falciparum had been reported. However, this protein was not detected in the present study. Naturally, several peptides from C. parvum rhoptry were not identified due to fewer total proteins or their low abundance. The identification of several non-rhoptry proteins proved that rhoptry-enriched fractions possibly contain other proteins. In T. gondii (31), E. tenella (32) and N. caninum(21), different degrees of contamination (such as dense granules) (6) were also observed in the secretory organelle-enriched fractions.
In this study, 22 novel proteins were identified by LC/MS-MS from purified rhoptry fractions of C. parvum. The molecular weights of the 18 proteins from part B were between 25–260 kDa, while those from part A were >260 kDa. Due to protein abundance and other reasons, some proteins were not detected. However, the present work provided a starting point for the study of the repertoire and function of C. parvum rhoptry proteins. Nonetheless, further studies must be conducted to confirm their characteristics, localization within rhoptries, synergistic effect with other proteins, and functional role in the C. parvumlife cycle.
Conclusion
Twenty-two potential novel rhoptry proteins were detected from Cryptosporidium parvum rhoptry-enriched fractions by one-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by liquid chromatography coupled with mass spectrometry analysis. These novel candidate proteins may be considered targets for researching the invasion pathway of C. parvum and the pathogenic mechanisms of rhoptry proteins.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was supported partly by Shanghai Municipal Agriculture Commission (Grant No. 2015-1-10 and 2005-3-4), Funds of Branch Projects and Quality and Safety Risk Assessment for Agricultural Products of Ministry of Agriculture (Grant No. GJFP201500803), National S & T Major Program of the People’s Republic of China (Grant No2012ZX10004220), Basic Foundation for Scientific Research of State-level Public Welfare Institutes of China (Grant No. 2013JB13), Sciences and Technology Innovation Project Fund of Chinese Academy of Agricultural Sciences and Minhang District Human Resources and Social Security Administration. The authors declare that there is no conflict of interests.
References
- 1. O'Donoghue PJ. (1995). Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol, 25( 2): 139–95. [DOI] [PubMed] [Google Scholar]
- 2. Chen XM, Keithly JS, Paya CV, LaRusso NF. (2002). Cryptosporidiosis. N Engl J Med, 346( 22): 1723–31. [DOI] [PubMed] [Google Scholar]
- 3. Harp JA, Woodmansee DB, Moon HW. (1990). Resistance of calves to Cryptosporidium parvum: Effects of age and previous exposure. Infect Immun, 58( 7): 2237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark DP. (1999). New insights into human cryptosporidiosis. Clin Microbiol Rev, 12( 4): 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubremetz JF, Garcia-Reguet N, Conseil V, Fourmaux MN. (1998). Apical organelles and host-cell invasion by Apicomplexa. Int J Parasitol, 28( 7): 1007–13. [DOI] [PubMed] [Google Scholar]
- 6. Blackman MJ, Bannister LH. (2001). Apical organelles of Apicomplexa: Biology and isolation by subcellular fractionation. Mol Biochem Parasitol, 117( 1): 11–25. [DOI] [PubMed] [Google Scholar]
- 7. Nichols BA, Chiappino ML, O'Connor GR. (1983). Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J Ultrastruct Res, 83( 1): 85–98. [DOI] [PubMed] [Google Scholar]
- 8. Sam-Yellowe TY. (1996). Rhoptry organelles of the apicomplexa: Their role in host cell invasion and intracellular survival . Parasitol Today , 12 ( 8 ): 308 – 16 . [DOI] [PubMed] [Google Scholar]
- 9. Kawai S, Igarashi I, Abgaandorjiin A, Miyazawa K, Ikadai H, Nagasawa H, Fujisaki K, Mikami T, Suzuki N, Matsuda H. (1999). Ultrastructural characteristics of Babesia caballi in equine erythrocytes in vitro. Parasitol Res, 85( 10): 794–9. [DOI] [PubMed] [Google Scholar]
- 10. Shaw MK, Tilney LG. (1992). How individual cells develop from a syncytium: Merogony in Theileria parva (Apicomplexa). J Cell Sci, 101( pt 1): 109–23. [DOI] [PubMed] [Google Scholar]
- 11. Tetley L, Brown SM, McDonald V, Coombs GH. (1998). Ultrastructural analysis of the sporozoite of Cryptosporidium parvum. Microbiology, 144( pt 12): 3249–55. [DOI] [PubMed] [Google Scholar]
- 12. Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ. (2007). Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot Cell, 6( 1): 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hakansson S, Charron AJ, Sibley LD. (2001). Toxoplasma evacuoles: A two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J, 20( 12): 3132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. (2007). Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature, 445( 7125): 324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riggs MW, McGuire TC, Mason PH, Perryman LE. (1989). Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J Immunol, 143( 4): 1340–5. [PubMed] [Google Scholar]
- 16. Arrowood MJ, Sterling CR. (1987). Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol, 73( 2): 314–9. [PubMed] [Google Scholar]
- 17. Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, Dunn JD, Ferguson DJ, Sanderson SJ, Wastling JM, Boothroyd JC. (2005). Proteomic analysis of rhoptry organelles reveals many novel comsitituents for host-parasite interactions in Toxoplasma gondii. J Biol Chem, 280( 40): 34245–58. [DOI] [PubMed] [Google Scholar]
- 18. Leriche MA, Dubremetz JF. (1991). Characterization of the protein contents of rhoptries and dense granules of Toxoplasma gondii tachyzoites by subcellular fractionation and monoclonal antibodies. Mol Biocheml Parasitol, 45( 2): 249–59. [DOI] [PubMed] [Google Scholar]
- 19. Sam-Yellowe TY, Del Rio RA, Fujioka H, Aikawa M, Yang JC, Yakubu Z. (1998). Isolation of merozoite rhoptries, identification of novel rhoptry-associated proteins from Plasmodium yoelii, P. chabaudi, P. berghei, and conserved inter-species reactivity of organelles and proteins with P. falciparum rhoptry-specific antibodies. Exp Parasitol, 89( 3): 271–84. [DOI] [PubMed] [Google Scholar]
- 20. Dubremetz JF, Ferreira E, Dissous C. (1989). Isolation and partial characterization of rhoptries and micronemes from Eimeria nieschulzi zoites (Sporozoa, Coccidia). Parasitol Res, 75( 6): 449–54. [DOI] [PubMed] [Google Scholar]
- 21. Marugán-Hernández V, Alvarez-García G, Tomley F, Hemphill A, Regidor-Cerrillo J, Ortega-Mora LM. (2011). Identification of novel rhoptry proteins in Neospora caninum by LC/MS-MS analysis of subcellular fractions. J Proteomics, 74( 5): 629–42. [DOI] [PubMed] [Google Scholar]
- 22. Sanderson SJ, Xia D, Prieto H, Yates J, Heiges M, Kissinger JC, Bromley E, Lal K, Sinden RE, Tomley F, Wastling JM. (2008). Determining the protein repertoire of Cryptosporidium parvum sporozoites. Proteomics, 8( 7): 1398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Valentini E, Cherchi S, Possenti A, Dubremetz JF, Pozio E, Spano F. (2012). Molecular characterisation of a Cryptosporidium parvum rhoptry protein candidate related to the rhoptry neck proteins TgRON1 of Toxoplasma gondii and PfASP of Plasmodium falciparum. Mol Biochem Parasitol, 183( 1): 94–9. [DOI] [PubMed] [Google Scholar]
- 24. Laliberté J, Carruthers VB. (2008). Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci, 65( 12): 1900–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sibley LD, Qiu W, Fentress S, Taylor SJ, Khan A, Hui R. (2009). Forward genetics in Toxoplasma gondii reveals a family of rhoptry kinases that mediates pathogenesis. Eukaryot Cell, 8( 8): 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu W, Wernimont A, Tang K, Taylor S, Lunin V, Schapira M, Fentress S, Hui R, Sibley LD. (2009). Novel structural and regulatory features of rhoptry secretory kinases in Toxoplasma gondii. EMBO J, 28( 7): 969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Labesse G, Gelin M, Bessin Y, Lebrun M, Papoin J, Cerdan R, Arold ST, Dubremetz JF. (2009). ROP2 from Toxoplasma gondii: A virulence factor with a protein-kinase fold and no enzymatic activity. Structure, 17( 1): 139–46. [DOI] [PubMed] [Google Scholar]
- 28. El Hajj H, Lebrun M, Arold ST, Vial H, Labesse G, Dubremetz JF. (2007). ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog, 3 (2): e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Hajj H, Demey E, Poncet J, Lebrun M, Wu B, Galéotti N, Fourmaux MN, Mercereau-Puijalon O, Vial H, Labesse G, Dubremetz JF. (2006). The ROP2 family of Toxoplasma gondii rhoptry proteins: Proteomic and genomic characterization and molecular modeling. Proteomics, 6( 21): 5773–84. [DOI] [PubMed] [Google Scholar]
- 30. Brossier F, Jewett TJ, Sibley LD, Urban S. (2005). A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc Natl Acad Sci U S A, 102( 11): 4146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia JL, Gennari SM, Navarro IT, Machado RZ, Sinhorini IL. (2004). Toxoplasma gondii: Isolation of tachyzoites rhoptries and incorporation into Iscom. Exp Parasitol, 108( 1–2): 40–6. [DOI] [PubMed] [Google Scholar]
- 32. Bromley E, Leeds N, Clark J, McGregor E, Ward M, Dunn MJ, Tomley F. (2003). Defining the protein repertoire of microneme secretory organelles in the apicomplexan parasite Eimeria tenella Proteomics, 3( 8): 1553–61. [DOI] [PubMed] [Google Scholar]