Background—
Whether the myocardium in nonischemic heart failure experiences oxygen limitation remains a long-standing controversy. We addressed this question in patients with dilated cardiomyopathy (DCM) using a dual approach. First, we tested the changes in myocardial oxygenation between rest and stress states, using oxygenation-sensitive cardiovascular magnetic resonance. Second, we sought to assess the functional consequences of oxygen limitation at rest by measuring myocardial energetics before and after short-term oxygen supplementation.
Methods and Results—
Twenty-six subjects (14 DCM and 12 normal) underwent cardiac magnetic resonance imaging at 3 Tesla to assess cardiac volumes, function, oxygenation, and first-pass perfusion (0.03 mmol/kg Gd-DTPA bolus) at stress and rest (4–6 minutes IV adenosine, 140 μg/kg per minute). Signal intensity change (SIΔ) and myocardial perfusion reserve index (MPRI) were measured from oxygenation and perfusion images, respectively. Furthermore, the effect of oxygen supplementation on resting myocardial energy metabolism was tested using 31P MR spectroscopy, measuring PCr/ATP ratios in both groups at baseline and after 4 hours of oxygen via facemask in the DCM group. During stress, there were equivalent rises in rate pressure product in both groups (DCM, 76±15% and normal, 79±9%; P=0.84). MPRI was significantly reduced in DCM (1.51±0.11 versus normal 1.86±0.10; P=0.03). However, there was no difference in oxygenation between groups: SIΔ in DCM 17±3% versus normal 20±2% (P=0.38). Furthermore, at a left ventricular segmental level, there was no correlation between oxygenation-sensitive SIΔ and MPRI (R=0.06; P=0.43). Resting PCr/ATP was reduced in DCM (1.66±0.07 versus normal 2.12±0.06; P=0.002). With oxygen supplementation, there was no change in PCr/ATP (1.61±0.08; P=0.58; Δ=0.04±0.05). There was also no effect of oxygen on systolic function (ejection fraction pre oxygen, 34±1%; post oxygen, 36±2%; P=0.46; Δ 2±1%).
Conclusions—
Our results demonstrate dissociation between microvascular dysfunction and oxygenation in DCM, suggesting that the impairment of perfusion is not sufficient to cause deoxygenation during stress. Cardiac energetics are unaffected by oxygen supplementation, indicating the absence of relevant myocardial hypoxia at rest. Our study suggests that novel treatments for nonischemic heart failure should focus on efforts to directly target cardiomyocyte function and metabolism rather than oxygen delivery and microvascular function.
Keywords: adenosine, cardiac volume, dilated cardiomyopathy, heart failure, oxygen
A substantial body of evidence supports the argument that attenuated myocardial blood flow, whether at rest or during physiological/pharmacological stress, is frequently encountered in dilated cardiomyopathy (DCM).1–3 An abnormal coronary flow reserve in DCM seems to independently portend a poorer cardiac prognosis.4 In the absence of obstructive epicardial coronary disease, this reduction in flow has been inferred to represent an impaired coronary microcirculation with resultant hypoxia.
Editorial see p 1011
Clinical Perspective on p 1093
Several mechanisms have been proposed to account for this microvasculopathy. Progressive myocardial fibrosis, especially when distributed perivascularly, is a common feature of DCM, and has been proposed to reduce capillary density and increased oxygen diffusion distance.5 With progression of disease, ventricular remodeling with increased wall tension conforming to Laplace law has been proposed to compress intramyocardial vessels and contribute to microvascular disease.6 Impaired endothelial function (dependent and independent of nitric oxide) is known to contribute to microvascular dysfunction. At least in animal models of cardiomyopathy, angiotensin-converting enzyme inhibitors improve myocardial perfusion both in pattern and magnitude.7 Finally, structural and functional abnormalities of myocardial resistance vessels (ie, arterioles) have been proposed to be important determinants of myocardial perfusion in a variety of cardiac conditions (eg, ischemia) and may contribute to the perfusion patterns in DCM.6
It has been assumed that, in this scenario, impaired myocardial blood flow results in inadequate tissue oxygenation. As a corollary, hypoxia-inducible transcription factor pathway is activated in human heart failure, as adduced from the induction of ABCG2 (ATP-binding cassette sub-family G member 2), VEGF (vascular endothelial growth factor), and BNIP3 (BCL2/adenovirus E1B 19kDa interacting protein 2).8 Chronic hypoxia-inducible transcription factor-1α stabilization, putatively acting through altered metabolism, deleterious calcium fluxes and activation of proapoptotic pathways, has been proposed to accelerate DCM progression.9
Despite the intuitive logic of this pathological sequence (ie, inciting pathogenesis of DCM leading to structural and functional changes resulting in microvasculopathy with rest and stress perfusion defects possibly resulting in adverse molecular consequences of hypoxia and hence progression of disease), tissue oxygenation, especially in the context of physiological/pharmacological stress, has never been directly measured in human DCM. Likewise, at rest, abnormal cardiac energetics is well established as a predictor of mortality in DCM.10,11 However, whether tissue hypoxia contributes to the impaired cardiac high-energy phosphate metabolism and function at rest in patients with DCM is unknown.
In this study, we asked the question whether the nonischemic failing human myocardium is oxygen limited. We addressed this by testing whether, in DCM, the oxygenation response to stress is appropriate, and whether resting cardiac function and high-energy phosphate metabolism are improved with inhaled supplemental oxygen (increasing myocardial oxygenation as has been validated in animal models).12 If this were the case, then mechanisms to improve oxygen delivery and use would be important targets for therapeutic intervention in treating patients with chronic heart failure because of DCM. To do so, we exploited hemoglobin’s differences in magnetic susceptibility in its diamagnetic oxygenated and paramagnetic deoxygenated state (oxygenation-sensitive cardiovascular magnetic resonance [CMR] imaging).
Methods
Ethics and Study Population
The study was approved by the institutional ethics committee and informed written consent was obtained from all subjects. Fourteen patients with DCM were recruited from the Heart Failure clinic at the Oxford University Hospitals National Health Service (NHS) Trust. DCM was defined as cardiac dilatation >55 mm and left ventricular (LV) ejection fraction <40% on a clinically indicated transthoracic echocardiography, in the absence of flow limiting coronary artery disease, assessed using invasive coronary angiography. All participants were class II–III New York Heart Association and stable on optimum-tolerated heart failure treatment for at least 6 months, as assessed by their treating physician. Normal controls (12 subjects) were matched for age and sex. Control subjects had no history of cardiac disease, hypertension, family history of cardiomyopathy, or sudden death and had a normal 12-lead ECG. No subjects in either group had a history of smoking or contraindications for MR scanning. All participants were instructed to refrain from caffeine-containing drinks in the 24 hours preceding the study.
CMR Protocol
All CMR examinations were performed on a Siemens 3T Trio MR system (Siemens Healthcare Erlangen, Germany). In the oxygen supplement arm, subjects were scanned on room air, then fitted with an oxygen face mask (10 L/min using a nonrebreathing mask) outside the scan room for 4 hours while resting comfortably and supplemental oxygen was administered during the follow-up scan protocol. Figure 1 shows the scan protocol.
Figure 1.
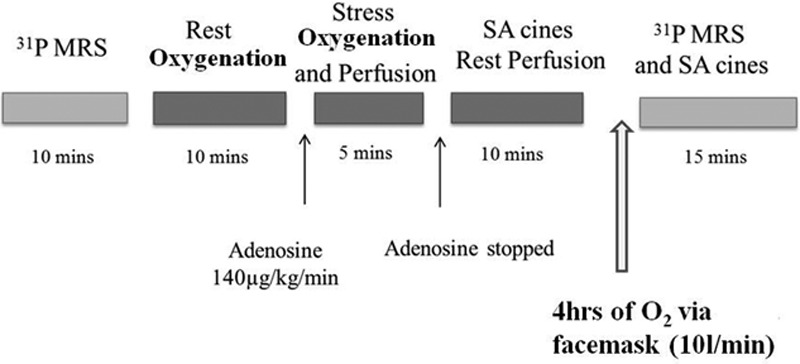
Scan protocol, gadolinium: 0.03 mmol/kg was given with stress and rest perfusion. MRS indicates magnetic resonance spectroscopy; and SA, short axis.
Cardiac Function
Cardiac volumes and ejection fraction were calculated based on a short-axis stack taken as a series of single breath-hold balanced steady-state free precession cine images as previously described.13 Each acquisition was prospectively ECG gated, with sequence parameters of flip angle, 50°; repetition time (TR), 3 ms; echo time, 1.5 ms; temporal resolution, 60.3 ms; and 8-mm thick with 3-mm gap.
Oxygenation-Sensitive CMR
Oxygenation-sensitive or blood oxygen level–dependent (BOLD) CMR was performed acquiring basal, midventricular, and apical slices at mid-diastole using a T2-prepared ECG-gated steady-state free precession sequence with the following parameters: TR/echo time, 2.86/1.43 ms; T2 preparation time, 40 ms; matrix, 168×192; field of view, 340×340 mm; slice thickness, 8 mm; and flip angle, 44°. Each BOLD image was obtained during a single breath-hold during 6 heart beats. Two images of the same slice were acquired at rest and at peak stress (3–4 minutes of adenosine infusion, 140 μg/kg per minute).14
Myocardial Perfusion Reserve Index
MPRI was measured on the same basal, midventricular, and apical slices used for oxygenation measurement. Images were acquired during peak stress after commencement of adenosine as previously described.14 Stress perfusion was measured for every heart beat during the first pass of a bolus of intravenous magnetic resonance contrast agent (0.03 mmol/kg body weight of Gadodiamide, Omniscan; GE Healthcare, Amersham, United Kingdom, injection rate, 6 mL/s) followed by a saline flush of 15 mL at the same rate, using a T1-weighted fast gradient echo sequence. Perfusion pulse sequence parameters were as follows: echo time, 0.96 ms; TR, 2 ms; saturation recovery time, 95 ms; voxel size, 2.1×2.6×8 mm3; flip angle, 17°; and slice thickness, 8 mm. Rest perfusion imaging was performed after at least 25 minutes and another dose of contrast (0.03 mmol/kg body weight) was given. Heart rate and blood pressure were measured before, at 2-minute intervals during and after adenosine stress.
31P-Magnetic Resonance Spectroscopy
All 31P -magnetic resonance spectroscopy (MRS) were acquired at 3 Tesla using acquisition-weighted chemical shift imaging,15 with 10 averages in the center of k-space. Subjects lay prone, with the left ventricle at magnet iso-center. Pilot images of vertical-long-axis, horizontal-long-axis, and short-axis (three-dimensional stack) were acquired. A 16×8×8 chemical shift imaging grid with a 240×240×200 mm field of view was centered in the midventricular septum on the first short-axis image showing the papillary muscles. To minimize contamination from surrounding tissue, 3 saturation bands, each 25-mm thick, were placed over chest wall muscle and liver. Five 2.5 ms long nuclear overhauser enhancement pulses were played out at an interpulse delay of 80.5 ms, 222.2 V, and average flip angle of 150° in between the ECG wave and the 31 P excitation to increase the signal-to-noise ratio in the acquired spectra.
Nonlocalized inversion-recovery spectra were acquired and used in conjunction with a field-map to calculate the flip angle (nominally 35°) achieved in each voxel for each acquisition. MRS data were acquired with 720-ms TR, using an optimized radiofrequency pulse16 centered between γ- and α-ATP peaks for uniform excitation of the spectrum, and ultrashort echo time17 to minimize T2 effects and first order phase artifacts. Each MRS acquisition took 8 minutes to acquire, as previously described.18
Image Analysis
Evaluation of LV Systolic Function
LV short-axis epicardial and endocardial borders were manually contoured at end-diastole and end-systole for determining end-diastolic volume, end-systolic volume, and stroke volume (SV). Commercially available software (Argus version VA60C, Siemens Healthcare, Erlangen, Germany) was used. LV mass was calculated based on previous knowledge of myocardial-specific density (1.05 g/cm3).18
Oxygenation Analysis
For oxygenation analysis, QMass software (version 6.2.3, Medis, Leiden, The Netherlands) was used. Myocardial SI was measured after manually tracing the endocardial and epicardial contours. Mean signal intensities were calculated for resting and stress conditions by averaging signal measurements from images during rest and adenosine stress, respectively. BOLD SI measurements were corrected for variations in heart rate between resting and stress as previously described.19
Perfusion Analysis
QMass software (version 6.2.3) was used for perfusion analysis. SI over time curves were generated by tracing endocardial and epicardial contours after manual correction for displacement during breathing. A region of interest was drawn in the LV blood pool, avoiding any papillary muscles therein, to permit the derivation of an arterial input function. Rest and stress myocardial perfusion upslopes were calculated using 5-point linear fit model of SI versus time and normalized to the LV blood pool upslope. MPRI was then calculated by dividing the results at vasodilation through the results at rest. MPRI is a semiquantitative method and has been widely adapted for perfusion analysis.14
31P-MRS Analysis
Spectra were direct current corrected and baseline corrected based on the last half of acquired data points. Spectral peaks were imported into Java Magnetic Resonance User Interface20 and fitted using the Advanced Method of Accurate, Robust, and Efficient Spectroscopic fitting21 algorithm making use of previous knowledge relating to the relative peak frequencies, amplitudes, phases, and j-coupling patterns.
Spectral peak areas were corrected for nuclear overhauser effect, using correction factors determined by previous experiment16: nuclear overhauser effect correction factors used were PCr 0.80, β-ATP 0.88, α-ATP 0.88, γ-ATP 0.79, 2, 3-DPG 0.70. Correction for radiofrequency saturation was calculated for each subject using the TR and experimentally determined excitation flip angle at the chosen voxel and T1 values from the literature22: PCr 3.8 s, γ-ATP 2.4 s, α-ATP 2.5 s, β-ATP 2.7 s, 2,3-DPG 1.39 s, and PDE 1.1 s. The resulting peak areas of the 3 ATP signals were averaged and corrected for blood contamination by subtracting 11% of the 2, 3-DPG peak area.23
Statistical Analysis
All data are expressed as mean±SE and checked for normality using Kolmogorov–Smirnov test. In addition, all data were plotted to confirm that they are normally distributed. Comparisons between the groups were performed using independent t tests, whereas comparisons within groups were performed using paired t tests. Fisher exact test was used to compare discrete data.
Statistical tests were 2 tailed, and P<0.05 was considered to indicate a statistically significant difference. All statistical analysis was performed with commercially available software packages (IBM SPSS Statistics, version 19.0 and MedCalc version 12). Power calculations using G* Power 3.2.1. software based on the use of independent t test and pilot data (DCM PCr/ATP, 1.65±0.4 versus normal, 2.1±0.2) suggested 9 subjects would be needed in each group to detect a difference in PCr/ATP ratio (α=0.05; power=80%).
Results
Study Groups and Functional Measurements
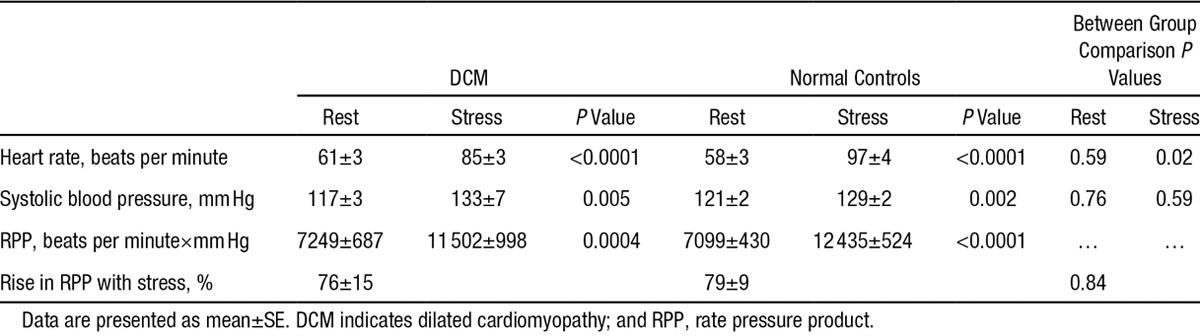
Subject characteristics are described in Table 1. There were no differences in age and sex between the DCM and normal groups. As expected, the DCM group had significantly lower ejection fraction than the normal group (33±1% versus 69±1%; P<0.0001) as well as significantly higher end-diastolic volumes (200±20 mL versus 142±12 mL; P=0.02). During adenosine stress, there was an equivalent rise in rate pressure product in both the groups (DCM, 76±15%; normal, 79±9%; P=0.84), Table 2.
Table 1.
Baseline Characteristics of Study Groups
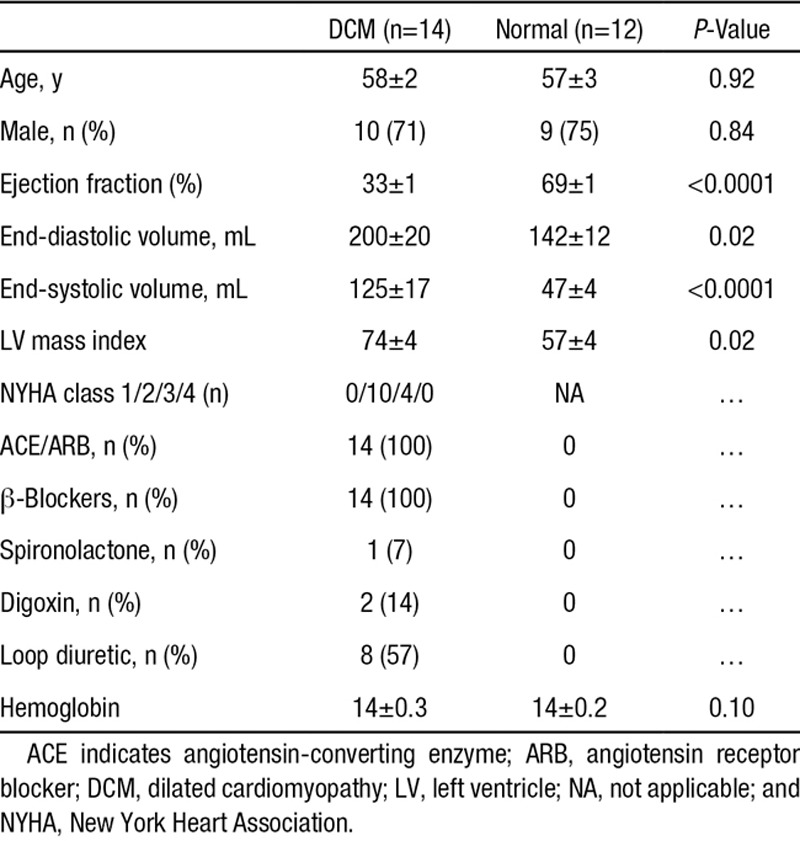
Table 2.
Haemodynamic Data at Rest and During Adenosine Infusion for the Study Population
Perfusion and Oxygenation Response to Stress
During adenosine infusion, stress perfusion was blunted in DCM compared with normal (DCM, 1.51±0.11; normal, 1.86±0.10; P=0.03; Figure 2), in keeping with previous reports of abnormal perfusion in DCM.1–3 However, in spite of this, in DCM, the oxygenation response to vasodilator stress was not significantly different from normal (BOLD SIΔ DCM, 17±3%; normal, 20±2%; P=0.56; Figure 2). To put these numbers in context, using similar methods, we have previously published significantly lower BOLD SIΔ in patients with coronary artery disease (3±1%),19 hypertrophic cardiomyopathy (7±1%),14 and aortic stenosis (5±9%).24 Furthermore, in DCM on a segmental level, there was no significant correlation between BOLD SIΔ and MPRI (R=0.06; P=0.43).
Figure 2.
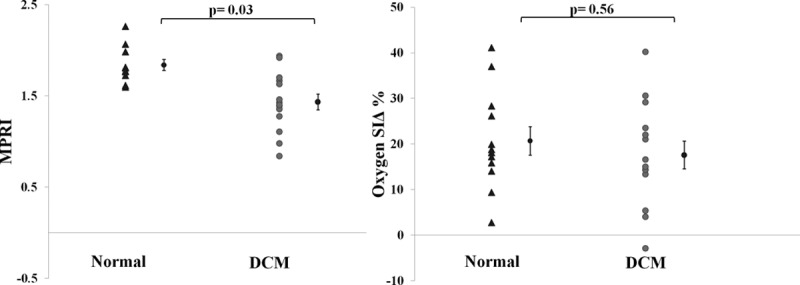
Oxygenation and stress perfusion measurements. Error bars are ±SE. Blood oxygen level–dependent (BOLD) signal intensity change (SIΔ) indicates BOLD SIΔ. DCM indicates dilated cardiomyopathy; and MPRI, myocardial perfusion reserve index.
Cardiac Energetics During Short-Term Oxygen Supplementation
BOLD imaging can detect oxygenation difference between rest and stress states, but does not measure absolute oxygenation levels. The myocardial oxygenation findings, therefore, did not rule out the possibility of an oxygen deficit during resting conditions. To test whether oxygen was limiting in the nonischemic failing myocardium at rest, we measured cardiac energetics before and after 4 hours of oxygen supplementation. At baseline, the PCr/ATP ratio was significantly reduced in DCM (1.66±0.07; normal, 2.12±0.06; P=0.002; Figure 3). In DCM, 4 hours of oxygen supplementation did not result in any change in the cardiac PCr/ATP ratio (1.61±0.08; P=0.58; Δ=0.04±0.05; Figure 3). Moreover, there was no effect of oxygen supplementation on systolic function (EF pre oxygen, 34±1%; post oxygen, 36±2%; P=0.46; Δ 2±1%; Figure 3). These findings suggest that in DCM neither cardiac function nor cardiac energetics were acutely limited by oxygen availability. Figure 4 shows an example of spectra obtained pre and post oxygen supplementation.
Figure 3.
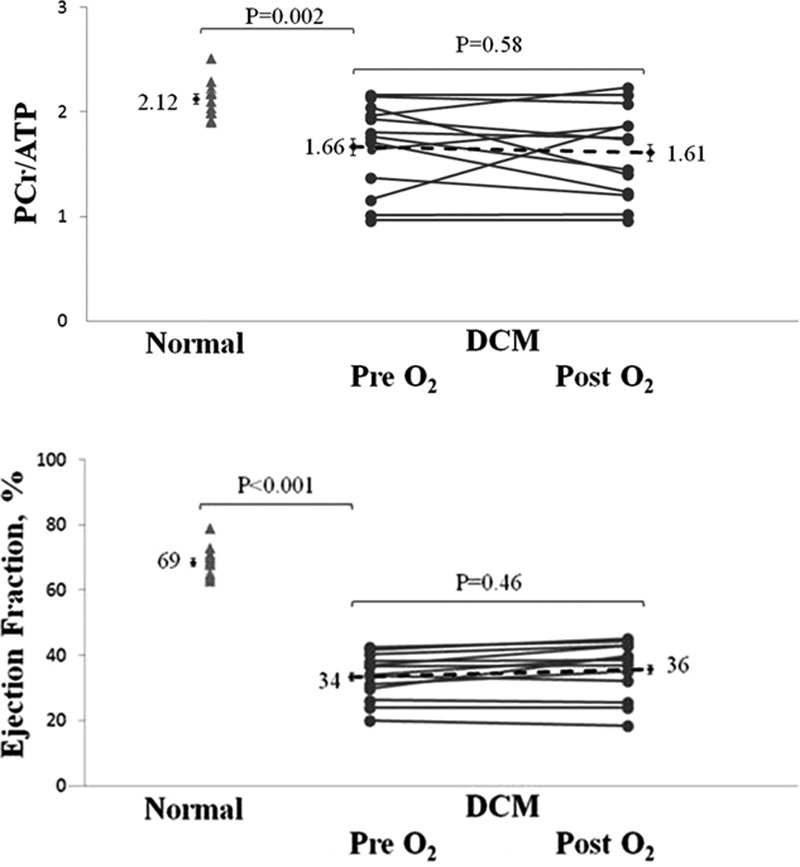
The effect of oxygen supplementation on PCr/ATP and ejection fraction in dilated cardiomyopathy (DCM). Error bars are ±SE.
Figure 4.
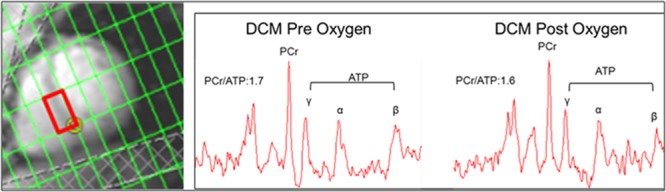
Example of voxel location for measurement of PCr/ATP (red rectangle) and examples of typical 31P-spectra obtained. DCM indicates dilated cardiomyopathy.
Discussion
Whether myocardial oxygenation is a limiting factor in human nonischemic heart failure is an important question. On the one hand, experimental studies using 31P- and 1H-MRS in large animals led by Zhang group have not found evidence for such deoxygenation.25 On the other hand, this question has, to date, not been examined in human myocardium, and a body of literature is accumulating describing both microvascular dysfunction in DCM and maladaptive ensuing sequelae of hypoxia, including hypoxia-inducible transcription factor-1α stabilization.8,9 Consequently, the noninvasive detection of hypoxia in cardiac tissue has the potential to be an important prognostic biomarker. The presence of hypoxia has been inferred as a consequence of microvascular dysfunction. The observation that even healthy subjects experience deterioration in cardiac function and energy metabolism in the context of sustained hypoxia represents a rationale for this pathological sequence.13
Set against this background, we now measured the myocardial oxygen response to stress in DCM with a direct comparison with perfusion reserve on a per segment basis. The principal finding of our study is that, although we confirm that patients with DCM have impaired myocardial perfusion reserve, our oxygenation-sensitive CMR studies, reflecting tissue hemoglobin saturation, suggest that there is no disturbance of the oxygen supply/demand balance in these patients during stress. Additionally in DCM, although there are alterations in cardiac volumes, function and energy metabolism, short-term oxygen supplementation does not improve these changes. In aggregate, these data suggest that hypoxia does not contribute significantly to the pathogenesis of DCM.
Oxygenation Response to Vasodilator Stress
The lack of a correlation between BOLD SIΔ and MPRI (R=0.06; P=0.43) suggests that despite the confirmation of microvascular dysfunction as indicated by reduced myocardial perfusion reserve in DCM, this seems not to be severe enough to affect the oxygen supply/demand balance. This contrasts with observations in coronary artery disease, reporting that BOLD SIΔ was significantly lower in segments supplied by coronary arteries with fractional flow reserve <0.8.26 Nevertheless, Karamitsos et al19 validated BOLD against myocardial perfusion assessed by positron emission tomography in patients with coronary artery disease and concluded that in this population, reduced perfusion does not always lead to deoxygenation. Moreover, Arnold et al,27 in comparing BOLD with first-pass perfusion in patients with CAD on a segment-based analysis, provided further evidence that a dissociation between oxygenation and perfusion can exist even in coronary artery disease.
This study confirms the finding of reduced resting PCr/ATP ratio in nonischemic heart failure.28 However, our data now provide further mechanistic insight and suggest that abnormal resting PCr/ATP ratio in DCM is not explained by the acute consequences of tissue hypoxia. Therefore, the more likely mechanisms responsible are the chronic consequences of molecular remodeling, including changes in substrate use,29,30 mitochondrial structure and function,31 and high-energy transfer and use.32
Limitations
Although this study was relatively small with 14 patients, it was powered adequately for the novel end point of oxygenation-sensitive CMR in DCM. Nevertheless, it remains an important caveat that the lack of myocardial hypoxia may not be germane to all categories of DCM. Furthermore, in this study, oxygen supplementation was given for 4 hours. Whether the adaptive consequences of chronic hypoxia are sustained (eg, hibernation) and require prolonged tissue oxygenation to ameliorate gene expression and lead to an improvement in cardiac function and energy metabolism remains unclear, and further studies would be needed to investigate this possibility.
Conclusions and Clinical Implications
Our results demonstrate dissociation between microvascular dysfunction and oxygenation in DCM, suggesting that the impairment of perfusion is inadequate to cause myocardial deoxygenation during stress. We also demonstrate that cardiac function and energetics are unaffected by short-term oxygen supplementation, suggesting that there is no significant disturbance of the oxygen supply/demand balance at rest. Our findings therefore suggest that novel treatments for nonischemic heart failure should focus on efforts to directly target cardiomyocyte function and metabolism, rather than oxygen delivery and microvascular function.
Sources of Funding
This study was supported by research fellowship grant FS/07/030 from the British Heart Foundation and by the Oxford National Institute for Health Research Biomedical Research Centre. Drs Neubauer and Watkins acknowledge support from the Oxford British Heart Foundation Centre of Research Excellence.
Disclosures
None.
Supplementary Material
Footnotes
Drs Karamitsos and Neubauer contributed equally to this work.
The data in this manuscript was awarded The Early Careers Award SCMR 2013 and was also featured in the AHA Scientific Sessions newsletter 2013.
CLINICAL PERSPECTIVE
It remains controversial whether the myocardium in nonischemic heart failure is oxygen deprived. It is well recognized that in spite of normal epicardial coronary anatomy, a decreased coronary flow reserve does exist, and this is an independent predictor of mortality in nonischemic heart failure. It has been assumed that this abnormal flow reflects inadequate tissue oxygenation. However, tissue oxygenation in human hearts with nonischemic heart failure has never been directly measured. We sought to answer these unknowns by investigating the changes in myocardial oxygenation in these hearts at rest and during stress. We also investigated the effect of oxygen supplementation on myocardial energetics at rest, hypothesizing that, if oxygen availability limits energy metabolism and function at rest, oxygen supplementation would improve both. We employed sophisticated cardiac magnetic resonance imaging techniques to measure perfusion, oxygenation, and energetics. We show that in nonischemic heart failure, as expected, there is significantly reduced myocardial perfusion reserve with stress, and now demonstrate that, despite this, there is no impairment of oxygenation. Furthermore, at rest cardiac energetics are significantly reduced and administration of supplemental oxygen does not improve this abnormal metabolic profile and left ventricular systolic function. This observed dissociation between microvascular dysfunction and preserved oxygenation during stress and the lack of improvement in cardiac function and energetics with oxygen supplementation at rest suggest the absence of relevant hypoxia in these hearts at both rest and stress. We suggest development of treatments for patients with nonischemic heart failure should target cardiomyocyte function and metabolism rather than optimization of oxygen delivery.
References
- 1.Rigo F, Ciampi Q, Ossena G, Grolla E, Picano E, Sicari R. Prognostic value of left and right coronary flow reserve assessment in nonischemic dilated cardiomyopathy by transthoracic Doppler echocardiography. J Card Fail. 2011;17:39–46. doi: 10.1016/j.cardfail.2010.08.003. doi: 10.1016/j.cardfail.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Rigo F, Gherardi S, Galderisi M. The prognostic impact of coronary flow-reserve assessed by Doppler echocardiography in non-ischaemic dilated cardiomyopathy. Eur Heart J. 2006;27:1319–1323. doi: 10.1093/eurheartj/ehi795. [DOI] [PubMed] [Google Scholar]
- 3.Neglia D, Michelassi C, Trivieri MG. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–193. doi: 10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 4.Sobajima M, Nozawa T, Suzuki T. Impact of myocardial perfusion abnormality on prognosis in patients with non-ischemic dilated cardiomyopathy. J Cardiol. 2010;56:280–286. doi: 10.1016/j.jjcc.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Sabbah HN, Sharov VG, Lesch M, Goldstein S. Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem. 1995;147:29–34. doi: 10.1007/BF00944780. [DOI] [PubMed] [Google Scholar]
- 6.Opherk D, Schwarz F, Mall G, Manthey J, Baller D, Kübler W. Coronary dilatory capacity in idiopathic dilated cardiomyopathy: analysis of 16 patients. Am J Cardiol. 1983;51:1657–1662. doi: 10.1016/0002-9149(83)90205-9. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaidis LA, Doverspike A, Huerbin R, Hentosz T, Shannon RP. Angiotensin-converting enzyme inhibitors improve coronary flow reserve in dilated cardiomyopathy by a bradykinin-mediated, nitric oxide-dependent mechanism. Circulation. 2002;105:2785–2790. doi: 10.1161/01.cir.0000017433.90061.2e. [DOI] [PubMed] [Google Scholar]
- 8.Zolk O, Solbach TF, Eschenhagen T, Weidemann A, Fromm MF. Activation of negative regulators of the hypoxia-inducible factor (HIF) pathway in human end-stage heart failure. Biochem Biophys Res Commun. 2008;376:315–320. doi: 10.1016/j.bbrc.2008.08.152. doi: 10.1016/j.bbrc.2008.08.152. [DOI] [PubMed] [Google Scholar]
- 9.Holscher M, Schafer K, Krull S. Unfavourable consequences of chronic cardiac HIF-1alpha stabilization. Cardiovasc Res. 2012;94:77–86. doi: 10.1093/cvr/cvs014. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S, Horn M, Cramer M. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 11.Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic rates of ATP transfer through creatine kinase (CK Flux) predict clinical heart failure events and death. Sci Transl Med. 2013;5:215re3. doi: 10.1126/scitranslmed.3007328. doi: 10.1126/scitranslmed.3007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gogna R, Madan E, Khan M, Pati U, Kuppusamy P. p53’s choice of myocardial death or survival: oxygen protects infarct myocardium by recruiting p53 on NOS3 promoter through regulation of p53-Lys(118) acetylation. EMBO Mol Med. 2013;5:1662–1683. doi: 10.1002/emmm.201202055. doi: 10.1002/emmm.201202055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway CJ, Montgomery HE, Murray AJ. Cardiac response to hypobaric hypoxia: persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J. 2011;25:792–796. doi: 10.1096/fj.10-172999. [DOI] [PubMed] [Google Scholar]
- 14.Karamitsos TD, Dass S, Suttie J, Sever E, Birks J, Holloway CJ, Robson MD, Jerosch-Herold M, Watkins H, Neubauer S. Blunted myocardial oxygenation response during vasodilator stress in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61:1169–1176. doi: 10.1016/j.jacc.2012.12.024. doi: 10.1016/j.jacc.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pohmann R, von Kienlin M. Accurate phosphorus metabolite images of the human heart by 3D acquisition-weighted CSI. Magn Reson Med. 2001;45:817–826. doi: 10.1002/mrm.1110. [DOI] [PubMed] [Google Scholar]
- 16.Tyler DJ, Emmanuel Y, Cochlin LE, Hudsmith LE, Holloway CJ, Neubauer S, Clarke K, Robson MD. Reproducibility of 31P cardiac magnetic resonance spectroscopy at 3 T. NMR Biomed. 2009;22:405–413. doi: 10.1002/nbm.1350. doi: 10.1002/nbm.1350. [DOI] [PubMed] [Google Scholar]
- 17.Robson MD, Tyler DJ, Neubauer S. Ultrashort TE chemical shift imaging (UTE-CSI). Magn Reson Med. 2005;53:267–274. doi: 10.1002/mrm.20344. doi: 10.1002/mrm.20344. [DOI] [PubMed] [Google Scholar]
- 18.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–822. doi: 10.1161/CIRCULATIONAHA.113.001719. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 19.Karamitsos TD, Leccisotti L, Arnold JR, Recio-Mayoral A, Bhamra-Ariza P, Howells RK, Searle N, Robson MD, Rimoldi OE, Camici PG, Neubauer S, Selvanayagam JB. Relationship between regional myocardial oxygenation and perfusion in patients with coronary artery disease: insights from cardiovascular magnetic resonance and positron emission tomography. Circ Cardiovasc Imaging. 2010;3:32–40. doi: 10.1161/CIRCIMAGING.109.860148. doi: 10.1161/CIRCIMAGING.109.860148. [DOI] [PubMed] [Google Scholar]
- 20.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 21.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 22.Bottomley PA, Ouwerkerk R. Optimum flip-angles for exciting NMR with uncertain T1 values. Magn Reson Med. 1994;32:137–141. doi: 10.1002/mrm.1910320120. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer S, Krahe T, Schindler R. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 24.Mahmod M, Francis JM, Pal N, Lewis A, Dass S, De Silva R, Petrou M, Sayeed R, Westaby S, Robson MD, Ashrafian H, Neubauer S, Karamitsos TD. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J Cardiovasc Magn Reson. 2014;16:29. doi: 10.1186/1532-429X-16-29. doi: 10.1186/1532-429X-16-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jameel MN, Hu Q, Zhang J. Myocytes oxygenation and high energy phosphate levels during hypoxia. PLoS One. 2014;9:e101317. doi: 10.1371/journal.pone.0101317. doi: 10.1371/journal.pone.0101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walcher T, Manzke R, Hombach V, Rottbauer W, Wöhrle J, Bernhardt P. Myocardial perfusion reserve assessed by T2-prepared steady-state free precession blood oxygen level-dependent magnetic resonance imaging in comparison to fractional flow reserve. Circ Cardiovasc Imaging. 2012;5:580–586. doi: 10.1161/CIRCIMAGING.111.971507. doi: 10.1161/CIRCIMAGING.111.971507. [DOI] [PubMed] [Google Scholar]
- 27.Arnold JR, Karamitsos TD, Bhamra-Ariza P, Francis JM, Searle N, Robson MD, Howells RK, Choudhury RP, Rimoldi OE, Camici PG, Banning AP, Neubauer S, Jerosch-Herold M, Selvanayagam JB. Myocardial oxygenation in coronary artery disease: insights from blood oxygen level-dependent magnetic resonance imaging at 3 tesla. J Am Coll Cardiol. 2012;59:1954–1964. doi: 10.1016/j.jacc.2012.01.055. doi: 10.1016/j.jacc.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 28.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 29.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 30.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, Hoppel CL, Imai M, Rastogi S, Sabbah HN, Stanley WC. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2004;287:H1538–H1543. doi: 10.1152/ajpheart.00281.2004. doi: 10.1152/ajpheart.00281.2004. [DOI] [PubMed] [Google Scholar]
- 31.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 32.Nascimben L, Ingwall JS, Pauletto P. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901. doi: 10.1161/01.cir.94.8.1894. [DOI] [PubMed] [Google Scholar]


