ABSTRACT
Objectives:
This study was a comparison of growth and tolerance in premature infants fed either standard powdered human milk fortifier (HMF) or a newly formulated concentrated liquid that contained extensively hydrolyzed protein.
Methods:
This was an unblinded randomized controlled multicenter noninferiority study on preterm infants receiving human milk (HM) supplemented with 2 randomly assigned HMFs, either concentrated liquid HMF containing extensively hydrolyzed protein (LE-HMF) or a powdered intact protein HMF (PI-HMF) as the control. The study population consisted of preterm infants ≤33 weeks who were enterally fed HM. Infants were studied from the first day of HM fortification until day 29 or hospital discharge, whichever came first.
Results:
A total of 147 preterm infants were enrolled. Noninferiority was observed in weight gain reported in the intent-to-treat (ITT) analysis was 18.2 and 17.5 g · kg−1 · day−1 for the LE-HMF and PI-HMF groups, respectively. In an a priori defined subgroup of strict protocol followers (n = 75), the infants fed LE-HMF achieved greater weight over time than those fed PI-HMF (P = 0.036). The LE-HMF group achieved greater linear growth over time compared to the PI-HMF (P = 0.029). The protein intake from fortified HM was significantly higher in the LE-HMF group compared with the PI-HMF group (3.9 vs 3.3 g · kg−1 · day−1, P < 0.0001). Both fortifiers were well tolerated with no significant differences in overall morbidity.
Conclusions:
Both fortifiers showed excellent weight gain (grams per kilograms per day), tolerance, and low incidence of morbidity outcomes with the infants who were strict protocol followers fed LE-HMF having improved growth during the study. These data point to the safety and suitability of this new concentrated liquid HMF (LE-HMF) in preterm infants. Growth with this fortifier closely matches the recent recommendations for a weight gain of >18 g · kg−1 · day−1.
Keywords: breast-feeding, growth, human milk fortifier, preterm infants
What Is Known
Powdered infant milk products cannot be sterilized and is a source of bacterial infection.
Very-low-birth-weight infants often require more protein than presently can be provided with conventional human milk fortifiers.
A liquid fortifier with higher protein than conventional fortifiers is desirable to increase safety and improved growth.
What Is New
A liquid human milk fortifier that is based on extensively hydrolyzed bovine casein with higher amounts of total protein than powder human milk fortifiers confers equal to improved growth to very-low-birth-weight infants
Use of this new liquid fortifier provides sterility without any increase in feeding intolerance or short-term adverse effects.
Human milk (HM) is a source of essential nutrients and immunologic factors for the preterm infant, but alone it is not sufficient nutritionally to meet the high demands of the rapidly growing infant. Human milk fortifiers (HMFs) are nutritional supplements designed to increase total energy, protein, and micronutrient delivery to preterm infants. The primary benefits of HM fortification have been improved growth, bone mineralization, and protein status such as blood urea nitrogen (BUN) (1–5).
Increasing survival and improving growth of the preterm infant to avoid extrauterine growth restriction have resulted in demands for protein that present powdered HMF may not achieve. Although some of these infants may compensate with higher volume intake, many are unable to consume a sufficient volume because of pulmonary or other clinical issues and therefore require further concentration of protein and energy. Higher intake of protein between 3 and 4 g · kg−1 · day−1 has been associated with improved growth without complications compared with a lower consumption of protein (<3 g · kg−1 · day−1) (6). Poor weight gain has been associated with a higher risk for retinopathy of prematurity and poor neurodevelopmental outcomes (7,8). It is common practice in the neonatal intensive care units (NICUs) to add protein modular (powder or liquid) to the feeding to better meet the protein needs of the smaller preterm infant. In fact, 42% of the respondents to a recent survey on nutritional practices in the NICU reported adding protein to HM (9).
There has been a gradual transition to sterile liquid nutritionals in the neonatal environment during the last decade because of concerns about powder-based transmission of pathogens such as Cronobacteria sakasakii(10). The recent development of a liquid HM–based HMF and a partially hydrolyzed whey-acidified liquid HMF respond to these concerns (11,12). Unlike powder nutritionals, a liquid HMF may have the advantage of sterility and simpler liquid-liquid mixing with human milk (HM). One disadvantage of a liquid fortifier is volume displacement of HM.
In this study, we evaluated a novel liquid HMF containing extensively hydrolyzed protein source to determine efficacy and safety in very-low-birth-weight preterm infants.
METHODS
Study Population
A total of 14 NICUs from across the United States participated in this study, including Tampa, Florida; Wichita, Kansas; Toledo, Ohio; Salt Lake City, Utah; Birmingham, Alabama; Cleveland, Ohio; Allentown, Pennsylvania; San Diego, California; Valhalla, New York; Manhasset, New York; Portland, Oregon; Cleveland, Ohio; South Bend, India; and Brooklyn, New York. The study population consisted of preterm infants born at ≤33 weeks’ gestational age with birth weights ranging from 700 to 1500 g who were enterally fed HM in the NICU. Infants identified as eligible for randomization and for whom consent was obtained were randomly assigned to one of the 2 study regimens. Sealed envelopes containing the subject treatment group assignment were prepared from randomization schedules that were computer-generated using a pseudorandom permuted blocks algorithm. A separate computer-generated randomization schedule was produced for twins to ensure that eligible twins were both assigned to the same product. The randomization was block stratified by birth weight (700–1000 g and 1000–1500 g) and sex.
Eligibility criteria included appropriate intrauterine growth and maternal intent to provide breast milk during the study. The use of donor HM was not permitted during the study period unless indicated by the clinical staff or PI but could have been used in the first week of life before study initiation. Infants were excluded for enteral feeds not started within 21 days of life, severe congenital anomalies, expectant transfer to another facility, 5-minute Apgar <5, severe intraventricular hemorrhage (grade 3 or 4), mechanical ventilation, major abdominal surgery, severe asphyxia, and necrotizing enterocolitis (NEC). Use of probiotics or postnatal corticosteroids was not permitted.
Study Design
This was an unblinded randomized controlled multicenter study conducted on preterm infants receiving HM supplemented with 2 randomly assigned HMFs, either a newly formulated concentrated liquid HMF containing extensively hydrolyzed protein (Abbott Nutrition, Columbus, OH; LE-HMF) or a conventional powdered intact protein HMF (Similac Human Milk Fortifier, PI-HMF, Abbott Nutrition) as control. For every 25 mL of HM, HMF was added as a 5-mL dose of LE-HMF or 1 single packet of PI-HMF. Study Day (SDAY) 1 was defined as the first day of HM fortification and occurred within 72 hours after the subject had reached an intake of at least 100 mL · kg−1 · day−1 of HM. The primary study period was from SDAY 1 until SDAY 29 or hospital discharge, whichever came first. This study was approved by institutional research ethics board as appropriate at each study sites. Table 1 shows the key study fortifier differences.
TABLE 1.
Approximate nutrient composition of PI-HMF or LE-HMF added to HM
| Nutrient PI-HMF | LE-HMF | |
| Energy, cal | 100 | 100 |
| Fat, g | 5.2 | 5.1 |
| CHO, g | 10.4 | 10.1 |
| Protein, g | 3 | 3.6 |
| Source/type of protein | Intact whey protein concentrate | Extensively hydrolyzed casein |
| DHA, mg | 12 | 24 |
| Vitamin D, IU | 150 | 150 |
| Calcium, mg | 175 | 153 |
| Phosphorus, mg | 98 | 86 |
| Osmolality, mOsm/kg water | 385 | 450 |
| Lutein, μg | * | 23 |
Values per 100 calories mixed at a ratio of 1 pkt or 5 mL:25 mL HM (as fed). CHO = carbohydrate; DHA = docosahexaenoic acid; HM = human milk; LE-HMF = liquid HMF containing extensively hydrolyzed protein; PI-HMF = powdered intact protein HMF.
*Lutein not added to product but available in varying amounts from HM.
Anthropometric indices (weight, length, and head circumference [HC]), tolerance, serum biochemistries, intake, and morbidity data were assessed. Anthropometric variables and tolerance outcomes were collected after SDAY 29 if the infant remained on study HMF.
Weight, length, and HC of infants were measured according to standardized procedures from SDAY 1 to SDAY 29 or hospital discharge, whichever came first. Weight measures were taken daily using the hospital scales (incubator or bedside). Documentation of scale calibration was reviewed during routine visits. The other anthropometric measurements were performed weekly. Recumbent length was obtained with a fixed headboard and moveable footboard and HC using a nonstretchable tape.
Feeding tolerance was assessed by variables such as stool characteristics (bloody, hard, black, and/or watery) and the incidence of feedings withheld because of abdominal distention, gastric residuals, and vomiting. Any nil per os periods were also collected.
Enteral intake was collected from enrollment to SDAY 29. Intake of HM (including donor/banked HM) or other enteral feeding (including supplements such as protein modulars) were recorded. Although the LE-HMF contained the same amount of energy as the PI-HMF, it contained higher protein and a different source of protein. It also contained added lutein, docosahexaenoic acid, and arachidonic acid.
Blood samples were drawn from each infant by venipuncture or, if necessary, by heelstick on SDAYs 1, 15, and 29. Serum electrolytes, bicarbonate, calcium, phosphorus, magnesium, alkaline phosphatase, BUN, and prealbumin were analyzed at the hospital site. Confirmed NEC (determined by using modified Bell staging criteria) and sepsis were recorded. The occurrence of these and other serious adverse events was documented throughout the study.
Statistical Analysis
Study data were analyzed on an intent-to-treat (ITT) basis including all enrolled infants who received study fortifier. Based on anticipated protocol deviations in this high-risk population, a subgroup analysis was prospectively planned to analyze data from infants who strictly adhered to the assigned HMF. The strict protocol followers (SPFs) were defined a priori as those infants who received <20% of total energy from sources other than the assigned study HMF; and <3 consecutive days on modular supplements (eg, protein supplements, another study HMF, nonstudy formula, or donor milk) for at least 2 weeks from SDAY 1 to SDAY 29.
Sample size was calculated to test the hypothesis that LE-HMF was noninferior to PI-HMF using an equivalence limit of 1.6 g · kg−1 · day−1 in weight gain per day. With a noninferiority hypothesis and assuming that the expected difference in means is zero and the common standard deviation is 2.56 g · kg−1 · day−1, the total sample size required to have 80% power was 66 subjects who are SPF (33 per group). The power for this unbalanced sample size distribution is 83%. Assuming an attrition rate of approximately 46%, the target number for enrollment was 124 subjects (62 per group). A study designed for noninferiority does not preclude testing for superiority (13). Weight gain (grams per kilogram per day) for each subject was calculated by an exponential model that involved a regression line fit on loge (wt), where wt is weight (in grams) on each day (13). Weight gain (grams per kilogram per day) was analyzed using analysis of variance with factors for center and feeding (primary). Analyses were also made adjusting for sex, birth weight, and average fortified HM intake (milliliters per kilogram per day) diluted full strength during the study period. A 95% 1-sided confidence interval for the difference in means between groups was used for noninferiority evaluation.
Length (centimeters per week) and HC gains (centimeters per week) were analyzed using the same models. Weight, length, and HC collected at 1-week intervals were analyzed with repeated measures analysis of covariance (ANCOVA) testing effects of center, feeding, sex, study day, interaction of feeding with sex, feeding with study day, and covariate birth weight. By time point analyses of weight, length, and HC using ANCOVA were made post-hoc using 1-sided tests consistent with a noninferiority design.
Average daily volume enteral intake (milliliters per kilogram per day) was analyzed using analysis of variance. Complete blood cell counts with differential and serum blood biochemistries were analyzed using repeated measures ANCOVA with covariate SDAY 1 measure.
Outcomes expressed as percent of infants (tolerance, morbidity, and respiratory variables) were analyzed using the Cochran-Mantel-Haenzsel test stratified by center. The frequencies of occurrence of adverse events by system organ class and preferred terms using MedDRA codes were tabulated and analyzed using Fisher exact test. Hypothesis testing for this study was done using 2-sided, 0.05 level tests. All analyses were made using SAS version 9.2 (SAS Institute, Cary, NC) on a computer.
RESULTS
Study Population
A total of 147 subjects were randomized into the study. Of the 147 subjects, 129 were included in the ITT group, that is, all randomized subjects who received study HMF. Of those subjects in the ITT group, 75% completed the study duration (45 PI-HMF, 52 LE-HMF). More than half the infants in the ITT group met the definition for the SPFs (Fig. 1). The number of days on the assigned study fortifier was 25 and 29 for the PI-HMF (n = 63) and LE-HMF (n = 66) groups, respectively. The median number of days on the assigned study fortifier for SPF was 29 days for both the PI-HMF and LE-HMF groups as some extended their use beyond the study period. Of note, some SPF subjects did not complete the study duration because they were discharged from the hospital.
FIGURE 1.
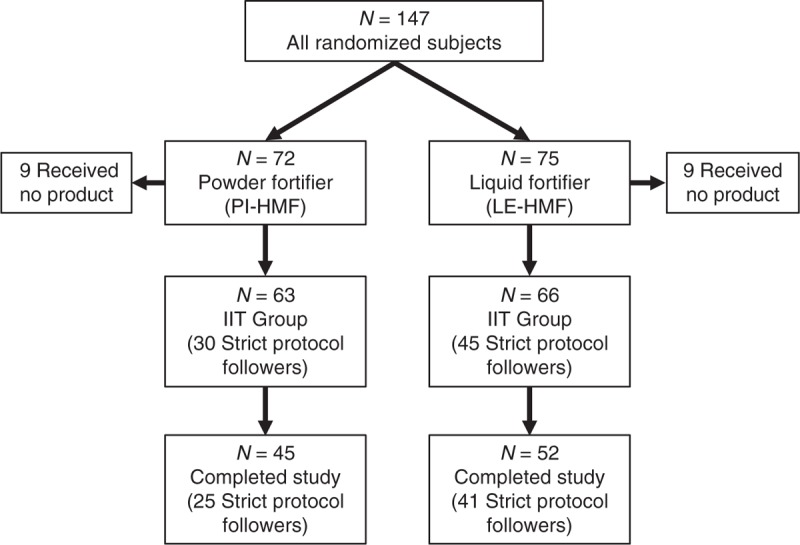
Disposition of subjects.
Demographic and Other Baseline Characteristics
Characteristics of the study patients are summarized in Table 2. There were no statistically significant differences among study subjects randomized to the PI-HMF or the LE-HMF group in gestational age, sex, race, mode of delivery and multiple birth status. There were, however, more Hispanic infants in the PI-HMF as compared to the LE-HMF group (28% vs 13%, P = 0.041). In addition, there were no statistical differences between groups at birth or SDAY 1 for weight, length, and HC. Furthermore, there were no differences in clinical history and progression of enteral feeds. Infants in the 2 feeding groups who were SPF reflect comparable demographic and baseline characteristics patterns.
TABLE 2.
Neonatal and perinatal characteristics of preterm infants
| Treatment group* | ||
| PI-HMF (n = 63) | LE-HMF (n = 66) | |
| Gestational age, wk | 28.7 ± 0.2 | 28.8 ± 0.2 |
| Birth weight, g | 1156 ± 24 | 1193 ± 26 |
| Birth length, cm | 37.4 ± 0.3 | 37.7 ± 0.3 |
| Birth HC, cm | 26.1 ± 0.2 | 26.5 ± 0.2 |
| Male sex, n (%) | 35 (56) | 36 (55) |
| Ethnicity: Hispanic, n (%) | 17 (28) | 8 (13)† |
| Race, n (%) | ||
| White | 42 (67) | 43 (65) |
| Black | 13 (21) | 17 (26) |
| Asian | 1 (2) | 1 (2) |
| Other | 7 (11) | 3 (5) |
| White/other | 0 (0) | 2 (3) |
| C-section, n (%) | 38 (60) | 42 (64) |
| Twin, n (%) | 16 (25) | 12 (18) |
| Age at study day 1, d | 12.3 ± 0.7 | 12.8 ± 0.6 |
| Birth class, n (%) | ||
| ≤1000 g | 16 (24) | 12 (19) |
| >1000 g | 66 (76) | 63 (81) |
LE-HMF = liquid HMF containing extensively hydrolyzed protein; PI-HMF = powdered intact protein HMF.
*Mean ± SEM.
†P = 0.0407.
Growth
There were no statistical differences in the primary outcome of weight gain (grams per kilogram per day) during the study period regardless of whether the statistical analysis was performed on the ITT group or SPFs. Hence, noninferiority was achieved. Respective weight gains were 17.5 and 18.2 g · kg−1 · day−1 for PI-HMF and LE-HMF (Table 3). Likewise in the subgroup (SPF) analysis weight gains were 18.2 and 18.4 g · kg−1 · day−1 for PI-HMF and LE-HMF. There was, however, a main feeding effect that was the infants fed LE-HMF compared with infants fed PI-HMF had increased weight during the study among SPFs as depicted in Fig. 2A (P = 0.036). When analyzing the data at separate time points the weight at SDAY 29 was significantly higher in LE-HMF group versus the PI-HMF group (P = 0.024). Likewise, infants in the ITT group fed LE-HMF had higher weights at SDAYs 15, 22, and 29 than infants fed PI-HMF whether or not adjusted for differences in ethnicity. The SPF infants receiving LE-HMF reached 1800 g 7 days sooner than the infants fed PI-HMF (19 vs 26 days, respectively, P = 0.049).
TABLE 3.
Anthropometric gains
| Treatment group* | Targeted growth†,‡ | ||
| PI-HMF (n = 63) | LE-HMF (n = 66) | ||
| Weight gain, g kg−1 day−1 | 17.5 ± 0.6 | 18.2 ± 0.3 | >18 |
| Length gain, cm/wk | 1.2 ± 0.07 | 1.2 ± 0.06 | >0.9 |
| HC gain, cm/wk | 1.0 ± 0.04 | 1.0 ± 0.05 | >0.9 |
FIGURE 2.
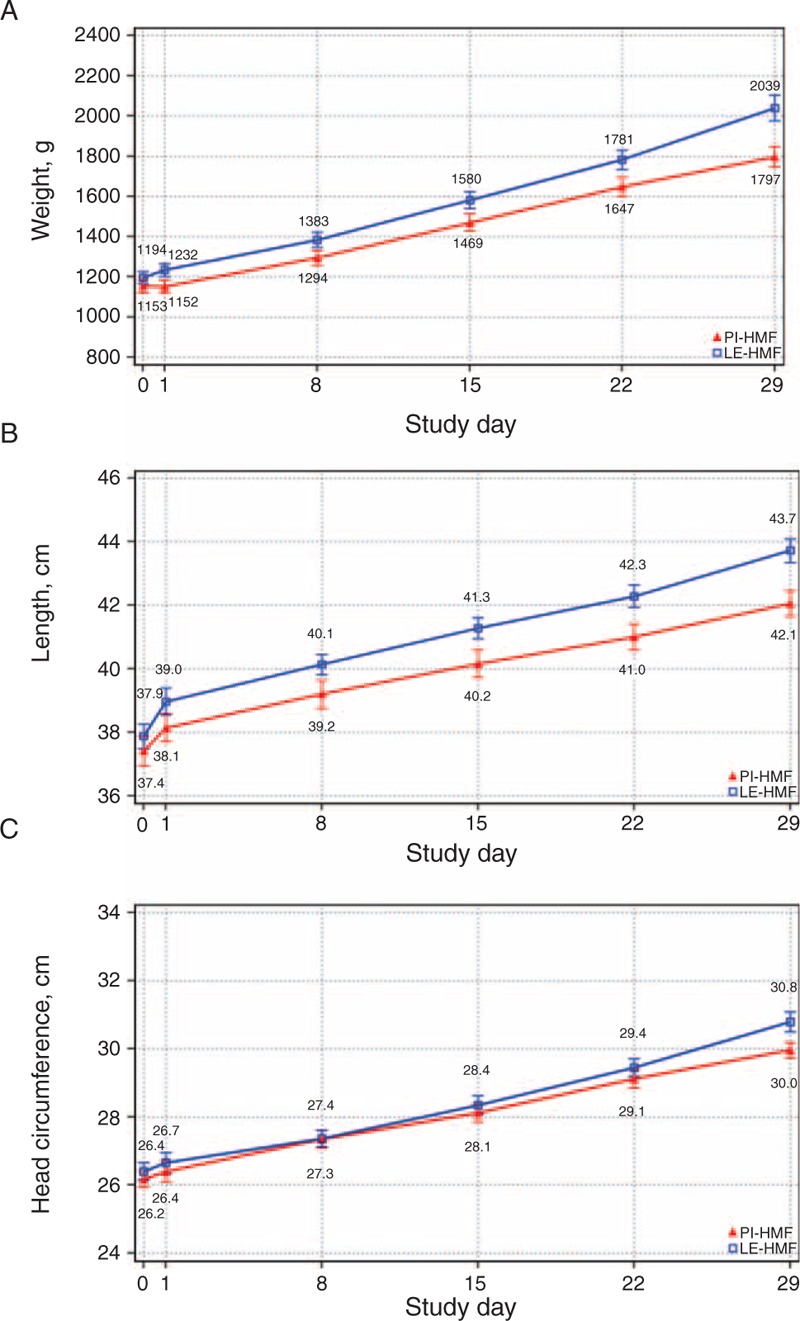
Evaluable analysis: A, weight (in grams); B, length (in centimeters); C, head circumference (in centimeters). A, Weight (in grams). Repeated measures analysis main effect, P = 0.036; post-hoc per time point analysis: SDAY 29, P = 0.024. B, Length (in centimeters). Repeated measures analysis main effect, P = 0.029; post-hoc per time point analysis: SDAY 22, P = 0.006, SDAY 29, P = 0.037. C, Head circumference (in centimeters).
The length and HC gains (centimeters per week) during the study period revealed no statistical differences between the groups and met growth targets (Table 3). The infants fed LE-HMF compared with infants fed PI-HMF had increased linear growth during the study among SPFs as depicted in Fig. 2B (P = 0.029). When analyzing the data at separate time points adjusted for birth length, the length at SDAY 22 and SDAY 29 were significantly higher in LE-HMF group versus the PI-HMF group (P < 0.05). HC was not different between the fortifier groups (Fig. 2C).
Feeding Tolerance and Stool Characteristics
In both the ITT and SPF groups, both fortifiers were well tolerated with similar number and percentage of infants having feedings withheld because of abdominal distention, gastric residuals and/or vomiting. There was no difference in the percentage of infants who were nil per os between the groups (22.7 LE-HMF, 19 PI-HMF). The stool characteristics in both groups were similar with no differences in bloody stools, hard stools or black stools. Loose stools were commonly reported—56% in the PI-HMF group and 53% in the LE-HMF group—and were considered normal for infants who are receiving HM as their primary feeding.
Enteral Nutrition
The mean caloric and protein intakes are reported for both HMF groups. For the SPFs, the average percentage of calories from fortified HM was ∼96% in both the PI-HMF and LE-HMF groups. The mean intake of fortified HM was 116 and 114 kcal · kg−1 · day−1 in the PI-HMF and LE-HMF groups, respectively. The calculated protein intake from fortified HM was significantly higher in the LE-HMF group as compared to the PI-HMF group (3.9 vs 3.3 g · kg−1 · day−1, P < 0.0001). This difference was expected as LE-HMF contains more protein than PI-HMF. Energy intakes were not different between the groups.
Blood Chemistries
The blood chemistries reported in Table 4 include bicarbonate, BUN, prealbumin, calcium, phosphorus, magnesium, alkaline phosphatase, and electrolytes. In general, the blood biochemistries at SDAYs 1, 15, and 29 were within the normal reference ranges for preterm infants for both the ITT and SPF groups fed milk fortified with either fortifier (14,15). There were significant differences between groups in both the ITT and SPF analyses for BUN (P < 0.001) and prealbumin (P < 0.01), with both being higher in the LE-HMF group. Both groups were well within reference ranges for these parameters. Bicarbonate was significantly higher in the LE-HMF group only at SDAY1 in the ITT analysis.
TABLE 4.
Blood chemistry data
| Characteristics | Reference ranges | Study day | Treatment group* | |
| PI-HMF | LE-HMF | |||
| Bicarbonate, mEq/L† | 17–24 | 1 | 23.27 ± 0.45 (59) | 25.05 ± 0.45 (62) |
| 15 | 24.32 ± 0.50 (49) | 25.40 ± 0.39 (58) | ||
| 29 | 25.04 ± 0.43 (40) | 25.54 ± 0.44 (50) | ||
| BUN, mg/dL‡ | 2.5–31.4 | 1 | 11.47 ± 0.78 (56) | 11.89 ± 1.03 (61) |
| 15 | 8.30 ± 1.15 (50) | 11.72 ± 0.68 (58) | ||
| 29 | 5.81 ± 0.38 (40) | 9.31 ± 0.53 (49) | ||
| Prealbumin, mg/dL§ | 7.0–39.0 | 1 | 10.05 ± 0.37 (58) | 9.69 ± 0.33 (54) |
| 15 | 10.11 ± 0.37 (47) | 11.40 ± 0.41 (46) | ||
| 29 | 9.08 ± 0.35 (36) | 10.01 ± 0.35 (37) | ||
| Calcium, mg/dL | 8.0–11.0 | 1 | 10.10 ± 0.08 (56) | 9.93 ± 0.08 (60) |
| 15 | 9.93 ± 0.10 (50) | 9.95 ± 0.07 (57) | ||
| 29 | 9.89 ± 0.09 (40) | 9.82 ± 0.06 (49) | ||
| Phosphorus, mg/dL | 4.2–8.7 | 1 | 6.41 ± 0.17 (54) | 6.20 ± 0.13 (58) |
| 15 | 6.71 ± 0.13 (46) | 6.50 ± 0.12 (56) | ||
| 29 | 6.66 ± 0.10 (40) | 6.46 ± 0.12 (47) | ||
| Magnesium, mg/dL | 1.5–2.1 | 1 | 1.90 ± 0.03 (54) | 1.88 ± 0.02 (59) |
| 15 | 1.80 ± 0.03 (47) | 1.86 ± 0.03 (55) | ||
| 29 | 1.81 ± 0.02 (40) | 1.82 ± 0.03 (46) | ||
| Alkaline phosphatase, U/L | 150–400 | 1 | 443.89 ± 24.50 (55) | 415.40 ± 15.78 (60) |
| 15 | 366.13 ± 21.80 (48) | 332.68 ± 10.87 (57) | ||
| 29 | 335.28 ± 21.84 (40) | 342.36 ± 13.10 (47) | ||
| Sodium, mEq/L | 129–143 | 1 | 137.49 ± 0.49 (61) | 138.42 ± 0.34 (65) |
| 15 | 137.46 ± 0.55 (52) | 137.56 ± 0.29 (59) | ||
| 29 | 139.07 ± 0.41 (41) | 138.70 ± 0.40 (50) | ||
| Potassium, mEq/L | 4.5–7.1 | 1 | 5.39 ± 0.11 (61) | 5.20 ± 0.09 (65) |
| 15 | 5.25 ± 0.09 (52) | 5.23 ± 0.09 (59) | ||
| 29 | 5.25 ± 0.10 (41) | 5.06 ± 0.07 (50) | ||
| Chloride, mEq/L | 100–117 | 1 | 104.16 ± 0.60 (58) | 104.03 ± 0.55 (63) |
| 15 | 104.10 ± 0.72 (49) | 103.88 ± 0.43 (57) | ||
| 29 | 106.00 ± 0.57 (40) | 106.14 ± 0.37 (49) | ||
BUN = blood urea nitrogen; LE-HMF = liquid HMF containing extensively hydrolyzed protein; PI-HMF = powdered intact protein HMF; HC = head circumference.
*Values are mean ± SEM (n).
†Bicarbonate (mEq/L): (SDAY 1) LE-HMF > PI-HMF, P = 0.0419, LSM ± SE: LE-HMF = 24.71 ± 0.56, PI-HMF = 23.33 ± 0.62.
‡BUN (mg/dL): Feeding main effect: LE-HMF > PI-HMF, P = 0.0013, LSM ± SE: LE-HMF = 11.99 ± 0.73, PI-HMF = 8.99 ± 0.83.
§Prealbumin (mg/dL): Feeding main effect: LE-HMF > PI-HMF, P = 0.0049, LSM ± SE: LE-HMF = 10.61 ± 0.35, PI-HMF = 9.32 ± 0.38.
Safety and Morbidity Data
In the ITT group, fewer infants discontinued fortifier because of feeding intolerance in the LE-HMF group as compared to the PI-HMF group (2% vs 10%, P = 0.048). There was a low incidence of confirmed NEC (1.5% in the LE-HMF group and 3.2% in the PI-HMF group) and confirmed sepsis (4.5% vs 3.2%, respectively)
DISCUSSION
The purpose of developing LE-HMF was to provide a concentrated liquid fortifier that would be superior to conventional powder HMF by virtue of sterility, higher protein concentration, and absence of intact cow's-milk protein. An extensively hydrolyzed protein source is included to promote feeding tolerance in preterm infants. The extensively hydrolyzed protein may be tolerated better for infants who are sensitive to the intact cow's-milk protein.
The primary purpose of the present clinical trial was to assess whether the new HMF would promote targeted weight gain, with good tolerance and without association with specific comorbidities in a noninferiority comparison with a commercially available powder HMF that has demonstrated safety and efficacy in preterm infants (13).
Weight gain and linear growth approaching intrauterine rates are important goals in the management of premature infants. The mean weight gain for both groups (PI-HMF and LE-HMF) exceeded the intrauterine growth rate of 15 g · kg−1 · day−1 and closely matched recent recommendations for a weight gain of >18 g · kg−1 · day−1(7). The mean HC gain for both groups also closely matched recent recommendations for a HC gain of >0.9 cm/wk (7). This result was not surprising given the excellent weight, length, and HC gains previously reported in infants fed PI-HMF powder (13).
Ehrenkranz et al (7) have reported that as the rate of weight gain increased in hospitalized preterm infants, the incidence of cerebral palsy, neurodevelopmental impairment, and need for re-hospitalization decreased significantly. A weight gain rate of >18 g · kg−1 · day−1 and a HC growth rate of >0.9 cm/wk were associated with better neurodevelopmental and growth outcomes. Lower quartile growth was associated with the poorest neurodevelopmental outcomes.
Weight and length differed between the groups. Although there were no significant differences in mean weight at birth or SDAY 1, infants receiving LE-HMF had ∼½ lb greater mean weight than the infants in the PI-HMF group at the end of the study period. Although the rate of linear growth was not statistically different, infants in the LE-HMF group had greater achieved linear growth during the study period. It is possible that the greater weight and length in the LE-HMF infants was because of the higher number of infants in this group that adhered to the assigned study feeding.
New expert recommendations suggest that extremely-low-birth-weight infants (<1000 g birth weight) have higher protein requirements (3.5–4.5 g/100 kcal) (16). HMFs provide an important strategy to overcoming nutrient deficits for preterm and low-birth-weight infants. Differences in the level and ingredient sources of the macronutrients, especially the protein quantity, in PI-HMF versus LE-HMF may have contributed to the overall performance of the LE-HMF group. The higher protein intake in infants receiving LE-HMF (∼3.6 g/100 kcal) as compared to PI-HMF (∼3.0 g/100 kcal) was likely one of the reasons for the improved growth observed in these infants. Although infants in the LE-HMF group had higher protein intakes, energy intakes were not different between the groups.
Preterm infants fed fortified HM have variable rates of growth at least partly because of differences in intake of calories, carbohydrates, electrolytes, calcium, phosphate, and protein. The acid-base status of the preterm infant also, however, affects growth. In preterm infants the kidney may not tolerate an acid load, leading to the development of metabolic acidosis. In a recent study, a liquid acidified HMF caused metabolic acidosis and poor growth in preterm infants in the NICU (17,18). In another study, Rochow et al (19) described a commercially available fortifier in Europe that had to be reformulated because of the development of metabolic acidosis from an imbalance of electrolytes. The authors recorded a mean weight gain of only 9.7 g · kg−1 · day−1 and decreased bone mineralization with metabolic acidosis. No infants in our study developed metabolic acidosis.
The LE-HMF protein source may be beneficial for this population because it was extensively hydrolyzed casein formulation without any intact cow's-milk protein. It has been suggested that a combination of free amino acids and short chain peptides (di- and tri peptides) may allow more optimal nitrogen absorption (20,21). Intact bovine protein powder HMF has an excellent safety record; however, a recent study by Sullivan et al (11) suggested the possibility that even in the presence of a HM base diet, the addition of intact bovine protein powder HMF is associated with higher rates of total and surgical NEC. The mechanism for the higher NEC risk is not known yet. Although this study was not powdered for NEC there was no difference in the NEC or sepsis rates between the infants fed an intact bovine protein and the extensively hydrolyzed protein. Both groups had rates lower than previously reported (22–24).
Intact bovine protein has higher associated long-term risk for allergy and atopy compared with HM-fed infants. Protein intolerance is seen in premature infants and in term infants (25). Because preterm infants have a similar risk for allergy and atopy compared with term infants and in the NICU have presented with symptoms suggestive of allergic colitis, avoiding intact bovine protein may be a desirable objective. For preterm infants fed HM the use of an extensively hydrolyzed protein-based HMF is an appropriate option.
In general, blood chemistries were within normal reference ranges for preterm infants. The higher BUN and prealbumin seen in the LE-HMF group can be attributed to the higher protein content of LE-HMF. These higher values may be indicative of improved protein nutriture. It should be noted that although BUN is influenced by renal function and hydration state, all other influences being equal, it is proportional to protein intake and responds rapidly to changes in protein intake (4,5,26,27).
Postnatal growth failure remains common in premature infants. Nearly 25 years ago Kashyap et al showed that even a small deficit in protein intake impairs both growth in lean body mass and linear growth (28). In recent years, Arslanoglu et al reported that addition of protein to preterm feedings of recovering VLBW infants resulted in significantly improved linear growth (4,5). This was accomplished by monitoring the BUN level so that when it was less than 9 mg/dL, increased protein was added to their feedings. It was observed in the present study that the mean BUN level fell <9 mg/dL by week 2 in infants receiving PI-HMF; however, in infants receiving LE-HMF it never fell <9 mg/dL during the entire study period. Our results, in part, agree with other investigators that an increased protein-to-calorie ratio in the feeds of preterm infants will improve linear growth (4,5,9,28). It is becoming increasingly evident that promoting catch-up growth in the NICU may have implications for long-term development and health (7,29).
Our study did have several limitations. The study examined the combined effects of changing both protein content and type (hydrolyzed vs intact). Future studies may want to capture effects of changing one of these variables. A number of subjects in this study did not complete the protocol to SDAY 29. This partially diluted the effects seen in the ITT groups but still permitted demonstration of differential effects seen in the SPF subgroup. A larger study design may improve this in the future. Infants <700 g birth weight were excluded from this study and therefore the study findings cannot be readily extrapolated to this vulnerable group. It is expected however that this group would have higher protein demands than infants in this study and therefore would be as likely or more to have a favorable response to higher protein. Although no differences were seen between both groups for NEC and sepsis the study size was too small to discern true differences for these outcomes.
CONCLUSIONS
Both fortifiers showed excellent tolerance and a low rate of morbidity outcomes, with the infants who were SPFs fed LE-HMF having improved growth. These data confirm the safety and suitability of this new concentrated liquid HMF for preterm infants.
Acknowledgments
The authors thank the following individuals for their hard work and dedication: Coryn Commare, MS, RD; Christy Saulters, BS; Debra Lee-Butcher, BSN, RN; Holy Boyko, BSN, RN; Angela Worley; Carolyn Richardson; Sue Zhang, MS, MAS; Mustafa Vurma, PhD; Maggie Hroncich, BS; Aimee Diley; Kristen Fithian; Sue Nicholson, MS, RD; and Jennifer Teran, BS, RD. The authors also thank study investigators and their staff for their cooperation: Terri Ashmeade, MD; Anthony Killian, MD; Lance Parton, MD; Robert Schelonka, MD; Robert White, MD; Ivan Hand, MD, FAAP; Michelle Walsh, MD; Jeffrey Blumer, PhD, MD; Paula Delmore, RN; Carrie Rau, RN; Renee Bridge, RN; Lisa Lepis, RN; Judy Zaritt, RN; Claire Roane, RN, MSN; Julie Gualtier, RN; Diane Fierst, RN; Christina Gogal; Natalie Dweck; Debra Potak, RN; Barbara Wilkens, RN; Nakia Clay, BS; Mashelle Monhaut, NNP-BC; Rickey Taing, NPL; Susan Bergant, RN, CCRP; and Bonnie Rosolowski, RPT.
Footnotes
www.clinicaltrials.gov registration number: NCT01373073.
This study was funded by Abbott Nutrition.
J.H.K., B.B., G.C., R.S. and S.G.-W. received research funds from the study sponsor, Abbott Nutrition, to conduct the study. J.H.K. is on the speakers’ bureaus for Abbott Nutrition, Mead Johnson Nutrition, Nestle Nutrition, Nutricia, and Medela. J.H.K. and R.S. are on the medical advisory board for Medela. J.H.K. owns shares in PediaSolutions and has provided medical expert testimony. B.B. received a grant from the Wichita Medical Research and Education Foundation. G.C. received a research grant from the University of Utah and has provided medical expert testimony. S.G.-W. is on the speakers’ bureau of Abbott Nutrition. B.B.-R., L.W., and G.B. are employees of Abbott Nutrition.
The authors report no conflicts of interest.
REFERENCES
- 1.Schanler RJ. Suitability of human milk for the low-birthweight infant. Clin Perinatol 1995; 22:207–222. [PubMed] [Google Scholar]
- 2.Schanler RJ, Abrams SA. Postnatal attainment of intrauterine macromineral accretion rates in low birth weight infants fed fortified human milk. J Pediatr 1995; 126:441–447. [DOI] [PubMed] [Google Scholar]
- 3.Kuschel CA, Harding JE. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Database Syst Rev 2004; 1:CD000343. [DOI] [PubMed] [Google Scholar]
- 4.Arslanoglu S, Bertino E, Coscia A, et al. Update of adjustable fortification regimen for preterm infants: a new protocol. J Biol Regul Homeost Agents 2012; 26 (3 suppl):65–67. [PubMed] [Google Scholar]
- 5.Arslanoglu S, Moro GE, Ziegler EE. Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol 2006; 26:614–621. [DOI] [PubMed] [Google Scholar]
- 6.Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev 2006; 1:CD003959. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006; 117:1253–1261. [DOI] [PubMed] [Google Scholar]
- 8.Vanderveen DK, Martin CR, Mehendale R, et al. Early nutrition and weight gain in preterm newborns and the risk of retinopathy of prematurity. PLoS One 2013; 8: e64325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitfield J, Punjabi-Gupta S, Hendrikson H, et al. Improved linear growth in VLBW infants at discharge: impact of increasing the protein/kcal ratio (PCR) of feeds. E-PAS Abstract 2012; 4510:122. [Google Scholar]
- 10.Taylor C. Health Professionals Letter on Enterobacter sakazakii Infections Associated With the Use of Powdered (Dry) Infant Formulas in Neonatal Intensive Care Units. Bethesda, MD: US Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling and Dietary Supplements; 2002. [Google Scholar]
- 11.Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010; 156:562–567. [DOI] [PubMed] [Google Scholar]
- 12.Moya F, Sisk PM, Walsh KR, et al. A new liquid human milk fortifier and linear growth in preterm infants. Pediatrics 2012; 130:e928–e935. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Reis B, Hall RT, Schanler RJ, et al. Enhanced growth of preterm infants fed a new powdered human milk fortifier: a randomized, controlled trial. Pediatrics 2000; 106:581–588. [DOI] [PubMed] [Google Scholar]
- 14.The Harriet Lane Handbook (The Johns Hopkins Hospital). 19th ed. New York: Elsevier Health Sciences; 2011: chap 27. [Google Scholar]
- 15.Ramel SE, Georgieff MK. Nutrition. In: Avery's Neonatology—Pathophysiology and Management of the Newborn. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2015. [Google Scholar]
- 16.Koletzko B, Poindexter B, Uauy R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Rev Nutr Diet 2014; 110:297–299. [DOI] [PubMed] [Google Scholar]
- 17.Thoene M, Hanson C, Lyden E, et al. Comparison of the effect of two human milk fortifiers on clinical outcomes in premature infants. Nutrients 2014; 6:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cibulskis CC, Armbrecht ES. Association of metabolic acidosis with bovine milk-based human milk fortifiers. J Perinatol 2015; 35:115–119. [DOI] [PubMed] [Google Scholar]
- 19.Rochow N, Jochum F, Redlich A, et al. Fortification of breast milk in VLBW infants: metabolic acidosis is linked to the composition of fortifiers and alters weight gain and bone mineralization. Clin Nutr 2011; 30:99–105. [DOI] [PubMed] [Google Scholar]
- 20.Grimble GK, Keohane PP, Higgins BE, et al. Effect of peptide chain length on amino acid and nitrogen absorption from two lactalbumin hydrolysates in the normal human jejunum. Clin Sci (Lond) 1986; 71:65–69. [DOI] [PubMed] [Google Scholar]
- 21.Boza JJ, Martinez-Augustin O, Baro L, et al. Protein v. enzymic protein hydrolysates. Nitrogen utilization in starved rats. Br J Nutr 1995; 73:65–71. [PubMed] [Google Scholar]
- 22.Patole S. Prevention and treatment of necrotising enterocolitis in preterm neonates. Early Hum Dev 2007; 83:635–642. [DOI] [PubMed] [Google Scholar]
- 23.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007; 196:147.e1-8. [DOI] [PubMed] [Google Scholar]
- 24.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Netto MA, Herson VC, Hussain N, et al. Allergic gastroenteropathy in preterm infants. J Pediatr 2000; 137:480–486. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler EE. Breast-milk fortification. Acta Paediatr 2001; 90:720–723. [PubMed] [Google Scholar]
- 27.Polberger SK, Axelsson IE, Raiha NC. Urinary and serum urea as indicators of protein metabolism in very low birthweight infants fed varying human milk protein intakes. Acta Paediatr Scand 1990; 79:737–742. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap S, Schulze KF, Forsyth M, et al. Growth, nutrient retention, and metabolic response in low birth weight infants fed varying intakes of protein and energy. J Pediatr 1988; 113:713–721. [DOI] [PubMed] [Google Scholar]
- 29.Hanson C, Sundermeier J, Dugick L, et al. Implementation, process, and outcomes of nutrition best practices for infants <1500 g. Nutr Clin Pract 2011; 26:614–624. [DOI] [PubMed] [Google Scholar]
- 30.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999; 104 (2 Pt 1):280–289. [DOI] [PubMed] [Google Scholar]


