ABSTRACT
Objectives:
The aim of the present study was to examine the growth and tolerance of infants fed infant formulas with a caloric density closer to human milk (HM) supplemented with human milk oligosaccharides (HMOs) and to study uptake of the HMOs.
Methods:
A prospective, randomized, controlled, growth and tolerance study was conducted in healthy, singleton infants (birth weight ≥2490 g), who were enrolled by day of life (DOL) 5. Formula-fed infants were randomized to 1 of 3 formulas with a caloric density of 64.3 kcal/dL. Each formula contained galactooligosaccharides, and the 2 experimental formulas contained varying levels (0.2 and 1.0 g/L) of the HMO 2′-fucosyllactose (2′FL). The 3 formula groups were compared with an HM-fed reference group. Infants were exclusively fed either formula (n = 189) or HM (n = 65) from enrollment to 119 DOL. 2′FL was measured in the blood and urine collected from a subset of infants at DOL 42 and 119, and in HM collected from breast-feeding mothers at DOL 42.
Results:
There were no significant differences among any groups for weight, length, or head circumference growth during the 4-month study period. All of the formulas were well tolerated and comparable for average stool consistency, number of stools per day, and percent of feedings associated with spitting up or vomit. 2′FL was present in the plasma and urine of infants fed 2′FL, and there were no significant differences in 2′FL uptake relative to the concentration fed.
Conclusions:
This is the first report of infants fed 2′FL-fortified formulas with a caloric density similar to HM. Growth and 2′FL uptake were similar to those of HM-fed infants.
Keywords: galactooligosaccharides, growth, human milk oligosaccharides, infant formula, tolerance
What Is Known
Human milk oligosaccharides may provide protection and enhance the development of the gastrointestinal and immune systems of breast-fed infants.
Human milk oligosaccharides represent the third largest solid component in human milk after lactose and lipids, levels ranging from 5 to 12 g/L in mature milk to >20 g/L in preterm colostrum.
The caloric density of human milk is variable, and recent evidence suggests that its calorie content has been overestimated.
What Is New
No significant differences in weight, length, and head circumference between infants fed human milk or 64.3 kcal/dL formulas from birth to 4 months of age have been noted.
Formulas supplemented with 2′-fucosyllactose are well tolerated, and 2′-fucosyllactose absorption profiles are similar to those of breast-fed infants.
Human milk (HM) confers short- and long-term benefits to breast-fed infants, including reduced risk of otitis media, gastrointestinal (GI) and respiratory tract diseases, obesity, diabetes, atopic dermatitis, childhood leukemia, and sudden infant death syndrome (1). The precise features, however, of HM, which provide these advantages have not been clearly elucidated. Ongoing research is increasingly revealing the important role of human milk oligosaccharides (HMOs) in conferring protection and enhancing the development of breast-fed infants (2,3). HMOs represent the third largest solid component in HM after lactose and lipids, with levels ranging from ∼5 to 12 g/L in mature milk to >20 g/L in preterm colostrum (2,4,5). HMOs provide protection in a multitude of ways including enhancing the development of the immune system, binding pathogens and toxins to prevent their uptake, and enhancing the epithelial barrier function of the gut (3,6,7). These important biomolecules also play a role in shaping the intestinal microbiome. HMOs act as prebiotics, selectively promoting colonization by Bifidobacterium bifidum, which is prevalent in the intestines of HM-fed infants (2,5). Fucosylated HMOs also regulate neuronal dependent gut motility and may enhance cognition via the gut-brain axis (8).
Although the types and levels of HMOs vary considerably among women and between the stages of lactation, the major portion of HMOs include ∼20 structures, including 2′-fucosyllactose (2′FL) (2,9). The fucosylation of HMOs is determined by an individual's histo-blood group antigen status, specifically the Secretor and Lewis groups. Approximately 80% of the European and American populations are considered secretors, and women with this status secrete HMO structures containing (α1,2)-linked fucose, such as 2′FL (2,10,11). 2′FL is the most abundant HMO in this group, with levels ranging from 0.06 to 4.65 g/L (12,13).
HMOs are largely indigestible by the intestinal enzymes of infants (14). In breast-fed infants, low levels of intact HMOs are found in the stools, having passed through the gut, and in their urine and plasma, having been absorbed into the bloodstream and excreted via the kidneys (9,10,15,16). Goehring et al (10) recently reported that HMOs, including 2′FL, are found in urine and plasma of breast-fed but not formula-fed infants, and that the levels of HMOs in plasma and urine correlate with those in mothers’ breast milk; 2′FL was not present in the circulation of infants fed breast milk devoid of 2′FL.
An additional difference between HM and formula is caloric density and protein content. Currently, most commercially available infant formulas in the United States provide ∼67.6 kcal/dL (20 kcal/fl oz), which was based on initial estimates of caloric density of mature HM. One of the earliest comprehensive reviews of HM composition from the National Research Council reported that HM calorie concentration ranged from 67.0 to 71.0 kcal/dL (17). The caloric density of HM is highly variable, and more evidence suggests that its calorie content has been overestimated (18). A systematic review of 22 studies that included 1088 HM samples revealed that the reported calorie content of mature HM ranges from 50.4 ± 2.0 to 78.2 ± 3.5 kcal/dL, with a mean of 65.2 kcal/dL (19). In addition, the review reported lower protein content (1.4 g/dL) in HM than that typically found in standard infant formulas (19). Reilly et al (20), in a review of 25 studies, reported the mean calorie content of HM to be 63.9 kcal/dL. These findings are consistent with HM values from the European Commission (65.1 kcal/dL) (21) and the Institute of Medicine (65.0 kcal/dL) (22). The American Academy of Pediatrics, on the advice of the Life Sciences Research Organization (LSRO), recommends that infant formula provide 63.0 to 71.0 kcal/dL (23). Regulations in Europe are based on the opinion of the Scientific Committee on Food (21), which specifies a minimum energy content of 60 kcal/dL and a maximum energy content of 70 kcal/dL for infant formula. At the request of the European Commission, however, the European Food Safety Authority recently delivered a scientific opinion stating that it is desirable that infant formulas be designed in a way that their calorie content tends toward the lower limit of the HM range (24).
We conducted a growth and tolerance trial that evaluated 3 infant formulas with a caloric density of ∼64.3 kcal/dL (19 kcal/fl oz). This reduction in caloric density was achieved by reducing all of the macronutrients by ∼5%; two of the formulas were also fortified with 2′FL. Plasma and urine samples were collected to quantitate systemic 2′FL in formula-fed and HM-fed infants. Results from the biological samples that were collected to evaluate immune and prebiotic effects associated with feeding 2′FL-fortified formulas will be reported separately.
METHODS
Study Design
This prospective growth and tolerance study was conducted at 28 sites throughout the United States from April 2013 through January 2014. Healthy, full-term infants were enrolled by 5 days of age. A subset of parents provided consent for optional biological sampling that included the collection of urine, stool, and blood from infants, and HM samples from breast-feeding mothers. Data are presented for the HMO levels in HM, urine, and plasma samples.
Infants whose parents intended to feed their infants formula exclusively were randomized to be fed a control formula (CF) or 1 of 2 experimental formulas (EFs) that were similar to the CF, except they contained levels of galactooligosaccharides (GOS) different from those in the CF, and they contained 2′FL at 0.2 or 1.0 g/L. 2′FL is a white powdered oligosaccharide (Inalco SpA, Milan, Italy) produced through a proprietary chemical synthesis. The total amount of oligosaccharides was 2.4 g/L in all of the formula groups. A nonrandomized HM-fed group was also enrolled.
Parents were asked to feed the assigned study formula or HM as their infant's sole source of nutrition until 119 days of age. The primary outcome variable was weight gain per day from day of life (DOL) 14 to 119, whereas secondary variables included measures of tolerance and other anthropometric measures. Supportive variables included additional infant and maternal demographics, formula intake, parents’ responses to questions related to their satisfaction with the formula and their infant's behavior, the concentrations of 2′FL in HM, and infant plasma and urine and their relative absorptions.
Before enrollment, a parent or legally authorized representative of each enrolled infant signed a consent form approved by a central institutional review board for the protection of human subjects.
Subjects
Inclusion criteria were singleton birth, gestational age 37 to 42 weeks and birth weight ≥2490 g. Subjects were eligible if they were between 0 and 5 days of age at enrollment, had exclusively been fed either formula or HM since birth, were judged to be in good health, as determined from the infant's medical history and parental report, and were from smoke-free homes. Mothers of infants in the HM-fed group were instructed not to smoke during the study period, and other household members for all of the subjects were not to smoke in the home. Exclusion criteria were an adverse maternal, fetal, or infant medical history considered by investigators to have potential effects on tolerance, growth, and/or development. This included, but was not limited to, suspected maternal substance abuse. Gestational diabetes was acceptable if the infant's birth weight was equal to or less than the 2010 World Health Organization (WHO) Growth Charts 95th percentile. Infants of mothers who intended to use a combination of breast- and formula-feeding, and infants who had been treated with antibiotics other than those administered in eye drops at birth were excluded. Infants receiving medications (including over-the-counter medications such as Mylicon for gas [McNeil Consumer Pharmaceuticals, Washington, PA]), home remedies (such as juice for constipation), herbal preparations, probiotics, or rehydration fluids that might affect GI tolerance were not to be enrolled unless both the parent and the physician agreed to discontinue the use of these agents before enrollment. The use of these products was discouraged for the duration of the study, as was the provision of solid foods.
Diets and Concomitant Treatments
Infants were fed 1 of the 4 diets. The 3 formulas were targeted to contain 64.3 kcal/dL (19 kcal/fl oz) (Table 1), and their composition was similar to that of a milk-based commercially available formula. The CF contained 2.4 g/L GOS. The 2 EFs were similar to the CF but contained either 0.2 g/L 2′FL and 2.2 g/L GOS (EF1) or 1.0 g/L 2′FL and 1.4 g/L GOS (EF2). The total amount of nondigestible oligosaccharides was similar for all of the 3 formulas (ie, 2.4 g/L). Infants in the HM-fed group were fed their mothers’ own milk by breast and/or bottle. The 3 formulas were similar in appearance, consistency, and odor. The formulas were provided in ready-to-feed 32 fl oz bottles, each of which had a unique 7- character product code to ensure that parents and investigators were not aware of the formula identification. Parents were instructed to feed the assigned formulas ad libitum and to supplement infants with water ad libitum. All of the formulas met the levels of nutrients for the population as recommended by the American Academy of Pediatrics Committee on Nutrition (25) and as regulated by the Infant Formula Act of 1980 (26) and subsequent amendments (27).
TABLE 1.
Energy, macronutrient, GOS, and 2′FL concentrations in the control and EFs
| Ingredient | CF | EF 1 | EF 2 |
| Energy, kcal/dL | 64.3 | 64.3 | 64.3 |
| Protein | 13.3 | 13.3 | 13.3 |
| Fat | 34.7 | 34.7 | 34.7 |
| Total carbohydrate | 69.0 | 69.0 | 69.0 |
| GOS | 2.4 | 2.2 | 1.4 |
| 2′FL | — | 0.2 | 1.0 |
All values are expressed as g/L unless otherwise indicated. 2′FL = 2′-fucosyllactose; CF = control formula; EF = experimental formula; GOS = galactooligosaccharides.
At the time of enrollment, parents confirmed their intent to feed the study formula or HM as the sole source of nutrition for the duration of the study, unless instructed otherwise by their health care professional.
Before enrollment, infants in the formula-fed groups were not to have received any HM (mother's or donor milk), and infants in the HM-fed group were not to have received any formula or donor milk. Vitamin and mineral supplements (excluding vitamin or mineral supplements containing vitamin D for infants in the HM group or as recommended by a health care professional) were not to be given during the study period as the study formulas were nutritionally complete.
Evaluable Data
The following criteria were used to define evaluable data: from enrollment throughout the study period, formula-fed infants were not to receive alternate feedings other than assigned study product for more than a total of 5 days, or consume rehydration or receive intravenous fluids for more than a total of 3 days. Foods, juices, vitamin, and/or mineral supplements (excluding vitamin or mineral supplements containing vitamin D for infants in the HM-fed group or as recommended by a health care professional) or other sources of nutrition were not to be used for >5 consecutive days or a total of 10 days. Medications (including over-the-counter medications such as Mylicon), home remedies, herbal preparations, or probiotics that may affect GI tolerance were not to be used for more than a total of 2 days.
For the optional biological sampling conducted in a subset of study infants, from enrollment throughout the study period, formula-fed infants were not to receive >8 fl oz of an alternate feeding (HM or formula other than their assigned study formula, or >2 feedings via breast) per week. The HM-fed infants were not to receive >8 fl oz of infant formula or donor milk per week. For 48 hours before the collection of urine samples, formula-fed infants were not to consume any feedings other than the assigned study formula, and HM-fed infants were not to consume any formula or donor milk.
Randomization
Sealed envelopes containing the group assignment for formula-fed infants were prepared from computer-generated randomization schedules prepared by the sponsor. Randomization was stratified by site and sex, with each center having its own randomization schedule. Enrollment was competitive, and no goals were set for the individual sites.
Study Visits
At the enrollment visit, prestudy feeding regimens, present infant medication/supplement use, birth anthropometric measurements, and gestational age were recorded, and present length, weight, and head circumference were measured. Demographic data were collected, including race, number, and ages of siblings in the home, and mode of delivery. Data regarding maternal medication/supplement use, prepregnancy height and weight, and maternal weight gain during pregnancy were recorded for the HM group. Eligible subjects were randomized to one of the formula groups or enrolled into the HM group. The parents were instructed to exclusively feed HM or begin feeding the assigned study formula as the first feeding following enrollment.
After enrollment, infants were seen at 5 additional clinic visits at DOL 14, 28, 42, 84, and 119. The DOL 14, 28, 42, and 84 visits had a window of ±3 days, and the DOL 119 visit had a window of ±5 days. At each visit, growth was measured, and detailed interval diet and clinical histories were taken that included any adverse events, changes in mother's intake of medications/supplements (HM group), smoking status in the home, and whether the infant had received any medications, home remedies, or nonstudy feedings.
Anthropometric Measures
Research staff was trained to weigh and measure infants. A video explaining procedures for obtaining accurate anthropometric measures was provided to each site, and completion of staff training on measuring anthropometrics was documented. All of the measurements were made twice, with a third being made if the difference between the 2 measurements exceeded defined limits. Infant weights were measured to the nearest 10 g using a digital, electronic scale. The scale was calibrated annually by a qualified technician, accuracy testing was performed before infants were weighed, and a log of scale weight checks was maintained. Individual infant's growth was plotted on WHO growth charts. Infant length and head circumference were measured to the nearest 0.1 cm.
Tolerance Measures
At enrollment and the DOL 42, 84, and 119 visits, the parents were given intake and stool records, and thorough instructions regarding their proper completion. The parents recorded detailed, 24-hour information about the volume of formula consumed at each feeding or the number of HM feedings, incidence of spitting up and vomiting associated with feedings, and each infant's stool characteristics (frequency, consistency, and color). Records were maintained by parents starting with the first feeding after enrollment, continuing until DOL 28, and for 3 consecutive days before the DOL 42, 84, and 119 visits. The study staff reviewed the completed forms with the parents at each visit to ensure they were completed correctly and thoroughly. Parents also completed infant feeding and stool patterns questionnaires and formula satisfaction questionnaires (formula-fed group only) at the DOL 28 and 119 visits. Parents completed an infant behavior questionnaire at the DOL 119 visit.
Breast Milk Samples
At the DOL 28 visit, breast-feeding mothers who consented to the optional biological sampling were given kits for the collection of breast milk at home within 24 hours of the DOL 42 visit. Alternatively, samples were collected at the DOL 42 visit. Breast milk samples were not collected at DOL 119. For each collection, a 20-mL mid-milk sample was collected from 1 breast starting ∼5 minute after the infant had begun sucking or the breast had begun to be pumped. The goal was to collect the sample at the regular feeding time of the infant, with a 2-hour gap since the previous feeding. The time of HM collection, the time that the breast being used for collection was last used for feeding, and the time the mother ate her last meal were recorded. Samples collected at home were collected within 2 hours of the study visit. They were immediately placed into the provided insulated cooler bag and refrigerated. Frozen ice packs were added to the cooler bag before transport to the study visit. Samples were stored frozen at the clinic sites (−20°C) before being transported to the central laboratory on dry ice.
Urine Samples
At the DOL 28 and 84 visits, parents who consented to the optional biological sampling were given urine sample collection kits, and samples were collected during the DOL 42 and 119 visits. Parents were instructed to place a urine collection pad in front of a clean diaper within 1 hour of their scheduled study visit. During the visit, study staff extracted 2 individual samples of urine, each a minimum of 1 mL, by placing a syringe tip into a wet area of the pad and withdrawing the plunger; samples were then transferred to a vial. Urine samples contaminated by feces were not collected. Samples were immediately frozen and stored at −20°C before being shipped to the central laboratory on dry ice. The parents were asked if their infant had received any alternate feedings within the previous 48 hours. If they had, parents were told to continue the assigned study feeding, and the sample collection was rescheduled within the study window or within 3 days, whichever was greater. At the time of the rescheduled sample collection, parents were again asked whether the infant had received any alternate feedings; if they had, the sample was not collected.
Blood Samples
At the DOL 42 and 119 visits, parents who consented to the optional biological sampling were asked whether their infant had received any alternate feedings or oral nonsteroidal anti-inflammatory medications within the previous 48 hours or had a present respiratory tract infection. If they had, parents were told to continue the assigned study feeding, and the sample collection was rescheduled within the study window or within 3 days, whichever was greater. In addition, mothers of HM-fed infants were not to have used nonsteroidal anti-inflammatory drugs within 48 hours of the blood collection. During the visit, 2 to 3 mL of nonfasting venous blood was drawn by a trained nurse into sodium heparin vacutainer tubes. Blood samples were shipped at ambient temperature to the laboratory and received within 24 hours of collection. Plasma was obtained by standard centrifugation procedure, dispensed into small plastic vials, and stored at −80°C until analysis.
Stool Samples
At the DOL 42 and 119 visits, parents who consented to the optional biological sampling also provided stool samples from their infants. This data will be used to examine the concentration of IgA, characterization of microbiota, and characterization of biological factors influential to GI health. This data will be presented in a subsequent publication.
2′FL Analyses
The plasma, urine, and HM samples were stored at less than −20°C and shipped frozen for analysis to Metabolon, Inc (Durham, NC). No more than 1 sample per subject per time point was analyzed for plasma, urine, and HM. HM samples were first diluted 1:500 in water. All of the samples were spiked with an internal standard and subjected to protein precipitation with methanol. Following centrifugation, supernatant was removed. Plasma supernatant was further evaporated to dryness and reconstituted in methanol:water (75:25, vol/vol). Aliquots of urine and HM supernatant, reconstituted plasma extract, and freshly prepared calibration standards were injected onto an Agilent 1290/AB Sciex QTrap 5500 liquid chromatography with tandem mass spectrometry system (AB Sciex, Framingham, MA) equipped with a BEH Amide UHPLC column (Waters Corporation, Milford, MA). Data were acquired using electrospray ionization in negative ionization mode. 2′FL concentrations were calculated based on the area ratios of 2′FL and internal standard peaks using a weighted (1/×) least squares regression analysis generated from external calibration standards included in each run.
2′FL Uptake
The relative absorption of 2′FL from the diet was estimated by dividing the concentration of 2′FL in the plasma by the concentration of 2′FL in the formula or HM. Relative excretion was estimated by dividing the concentration of 2′FL in the urine by the concentration of 2′FL in the feed (10).
Statistical Analysis
The sample size was calculated by using the software package nQuery Advisor 5.0 (Statistical Solutions Ltd, Cork, Ireland). The study hypothesis was that growth would be similar between the control and the 2 experimental feeding groups. The trial was designed to show noninferiority instead of superiority. Therefore, in calculating sample size and power, no adjustment was made for the number of groups (multiple comparisons) that were studied. A sample size of 64 subjects in each formula feeding group has 80% power to detect a difference in means of ≥3 g/day assuming that the common standard deviation is 6 g/day, using a 2-group t test with a 0.05 2-sided significance level. With an assumed attrition rate of 30%, the target enrollment was ∼92 subjects per formula feeding group. In addition, a HM reference group was enrolled with approximately the same number of subjects as each of the formula feeding groups. Therefore, the targeted number of subjects in the study was 368. Subjects were added to the study to obtain the targeted number of laboratory samples.
Three sets of models were fitted for most of the variables (model 1 CF vs EF1, model 2 CF vs EF2, and model 3 all 4 study groups). Analysis of variance (ANOVA) was used in baseline comparisons of continuous variables, whereas Cochran-Mantel-Haenszel test statistics were used for baseline comparisons of categorical variables. Anthropometric and intake data were compared among treatment groups using ANOVA techniques, including analysis of covariance and repeated measures analyses. If there was an overall significant treatment group effect (or significant treatment group interaction), then least squares means were compared between each pair of treatment groups and adjusted for multiple comparisons by using the step-down Bonferroni adjustment. Questionnaire and adverse event data were compared among groups using categorical analyses such as Cochran-Mantel-Haenszel and Fisher exact test.
2′FL concentrations and relative absorption for infant plasma were compared among treatment groups using ANOVA. 2′FL concentrations for infant urine and relative excretion for infant urine were compared among treatment groups using nonparametric methods (Kruskal-Wallis test and Wilcoxon rank-sum test). Change in 2′FL concentrations from 42 to 119 days in infant plasma and urine were calculated using paired t tests separately for each treatment group. SAS version 9.2 (SAS Institute, Cary, NC) was used to perform the statistical analyses.
RESULTS
Of the 424 infants enrolled, 420 were included in the intent-to-treat analysis (101 CF, 104 EF1, 109 EF2, and 106 HM); 4 subjects were excluded from the intent-to-treat group because they never received any study product. A total of 338 infants completed the study (84 CF, 81 EF1, 83 EF2, and 90 HM), 304 of whom completed the study on the assigned feeding or HM (79 CF, 70 EF1, 72 EF2, and 83 HM). The number of premature discontinuations of the study formulas was not different among the formula-fed groups. There were no significant differences among feeding groups for age at enrollment, sex, weight, length, or head circumference at birth, and mode of delivery except a significant difference between CF and HM for age at enrollment (P = 0.020). There was a significant difference between the EF2 and HM groups with respect to race, with HM having more infants that were white and EF2 having more infants that were black or of other races (Table 2). The remaining study results (Tables 3 and 4 and Figs. 1 and 2) are based on infants who completed the study per protocol and whose data were included in the evaluable analyses.
TABLE 2.
Demographic and clinical characteristics of infants fed CF, EF with different levels of GOS and 2′-fucosyllactose (EF1 or EF2), or HM
| Characteristic | CF | EF1 | EF2 | HM |
| n = 101 | n = 104 | n = 109 | n = 106 | |
| Age at enrollment, days* | 3.8 ± 0.1 | 3.5 ± 0.1 | 3.7 ± 0.1 | 3.4 ± 0.1 |
| Males, n (%) | 51 (50) | 51 (49) | 54 (50) | 57 (54) |
| Gestational age, wk | 39.3 ± 0.1 | 39.2 ± 0.1 | 39.2 ± 0.1 | 39.3 ± 0.1 |
| Birth weight, g | ||||
| Males | 3453 ± 60 | 3306 ± 61 | 3344 ± 53 | 3480 ± 62 |
| Females | 3327 ± 66 | 3311 ± 66 | 3240 ± 55 | 3397 ± 59 |
| Birth length, cm | ||||
| Males | 50.9 ± 0.3 | 50.8 ± 0.3 | 51.2 ± 0.3 | 51.5 ± 0.3 |
| Females | 50.7 ± 0.3 | 50.1 ± 0.3 | 50.1 ± 0.3 | 50.8 ± 0.4 |
| Birth head circumference, cm | ||||
| Males | 34.9 ± 0.3 | 34.3 ± 0.3 | 34.4 ± 0.2 | 34.8 ± 0.3 |
| Females | 34.0 ± 0.4 | 33.7 ± 0.3 | 33.6 ± 0.3 | 34.0 ± 0.3 |
| Race, n (%)† | ||||
| White | 71 (70) | 71 (68) | 62 (57) | 83 (78) |
| Black | 23 (23) | 21 (20) | 31 (28) | 15 (14) |
| Other | 7 (7) | 12 (12) | 16 (15) | 8 (8) |
| Mode of delivery, n (%) | ||||
| Vaginal | 65 (64) | 74 (71) | 80 (73) | 80 (75) |
| Cesarean section | 36 (36) | 30 (29) | 29 (27) | 26 (25) |
Data represent the mean ± SEM unless otherwise indicated. CF = control formula; EF = experimental formula; GOS = galactooligosaccharides; HM = human milk.
*Significant difference between CF and HM, P = 0.020.
†Significant difference between EF2 and HM, P = 0.029.
TABLE 3.
Gains in weight, length, and head circumference from DOL 14 to 119 in infants fed CF, EF (EF1 or EF2), or HM
| Characteristic | CF | EF1 | EF2 | HM |
| Weight gain, g/day* | ||||
| Males (n) | 30.4 ± 1.2 (34) | 30.2 ± 1.3 (29) | 31.4 ± 1.0 (30) | 30.9 ± 1.1 (33) |
| Females (n) | 26.8 ± 0.8 (34) | 25.8 ± 1.1 (33) | 26.5 ± 1.2 (29) | 25.0 ± 1.0 (32) |
| Length gain, cm/day | ||||
| Males (n) | 0.106 ± 0.003 (32) | 0.107 ± 0.003 (28) | 0.109 ± 0.002 (30) | 0.102 ± 0.004 (32) |
| Females (n) | 0.097 ± 0.003 (34) | 0.102 ± 0.003 (32) | 0.101 ± 0.004 (29) | 0.095 ± 0.002 (32) |
| Head circumference gain, cm/day | ||||
| Males (n) | 0.061 ± 0.001 (31) | 0.059 ± 0.002 (28) | 0.061 ± 0.002 (30) | 0.057 ± 0.001 (32) |
| Females (n) | 0.055 ± 0.001 (33) | 0.056 ± 0.002 (31) | 0.053 ± 0.003 (29) | 0.049 ± 0.001 (31) |
Data represent the mean ± SEM. Sex-combined differences in gains during different time intervals are shown in footnotes. CF = control formula; DOL = day of life; EF = experimental formula; HM = human milk; LSM = least squared means.
*From DOL 14 to 28, HM > EF1 (43.6 ± 1.8 vs 36.7 ± 1.9 g/day [LSM ± SE]; P = 0.016). From DOL 84 to 119, EF2 > HM (25.0 ± 1.2 vs 20.5 ± 1.2 g/day [LSM ± SE]; P = 0.022).
TABLE 4.
2′FL concentrations in feeds, plasma and urine, and relative absorption and excretion of 2′FL
| DOL | Characteristic | Units | CF | EF1 | EF2 | HM |
| 42 | Feed (n) | g/L | 0* | 0.20* | 1.00* | 1.98 ± 0.17 (76) |
| Plasma (n) | mg/L | <0.03 ± 0.00 (36) | 0.13a ± 0.02 (32) | 0.52b ± 0.07 (33) | 1.00c ± 0.17 (36) | |
| Relative absorption† | % | NC | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | |
| Urine (n) | mg/L | 0.08a ± 0.01 (59) | 3.00b ± 0.33 (54) | 12.60c ± 1.92 (61) | 35.55c ± 6.89 (58) | |
| Relative excretion† | % | NC | 1.50 ± 0.17 | 1.26 ± 0.19 | 1.35 ± 0.23 | |
| 119 | Feed (n) | g/L | 0* | 0.20* | 1.00* | NT |
| Plasma (n) | mg/L | <0.03 ± 0.00 (12) | 0.05 ± 0.01 (12) | 0.29 ± 0.09 (14) | 0.43 ± 0.17 (11) | |
| Relative absorption† | % | NC | 0.02 ± 0.01 | 0.03 ± 0.01 | NC | |
| Urine (n) | mg/L | 0.09a ± 0.01 (53) | 2.88b ± 0.71 (45) | 11.18c ± 1.95 (45) | 19.52c ± 4.51 (54) | |
| Relative excretion† | % | NC | 1.44 ± 0.35 | 1.12 ± 0.19 | NC |
Groups were fed CF, EF with different levels of GOS and 2′FL (EF1 or EF2), or HM. Data represent the mean ± SEM unless otherwise indicated. DOL 119 breast milk sample not collected. Means with different superscripts are significantly different from each other, (P < 0.05). 2′FL = 2′-fucosyllactose; CF = control formula; DOL = day of life; EF = experimental formula; GOS = galactooligosaccharides; HM = human milk; n = number of samples tested (1 sample per subject per time point for each variable); NC = not calculable; NT = not tested.
*Concentration of 2′FL in formula, g/L.
†Concentration of 2′FL in plasma or urine relative to concentration in feed.
FIGURE 1.
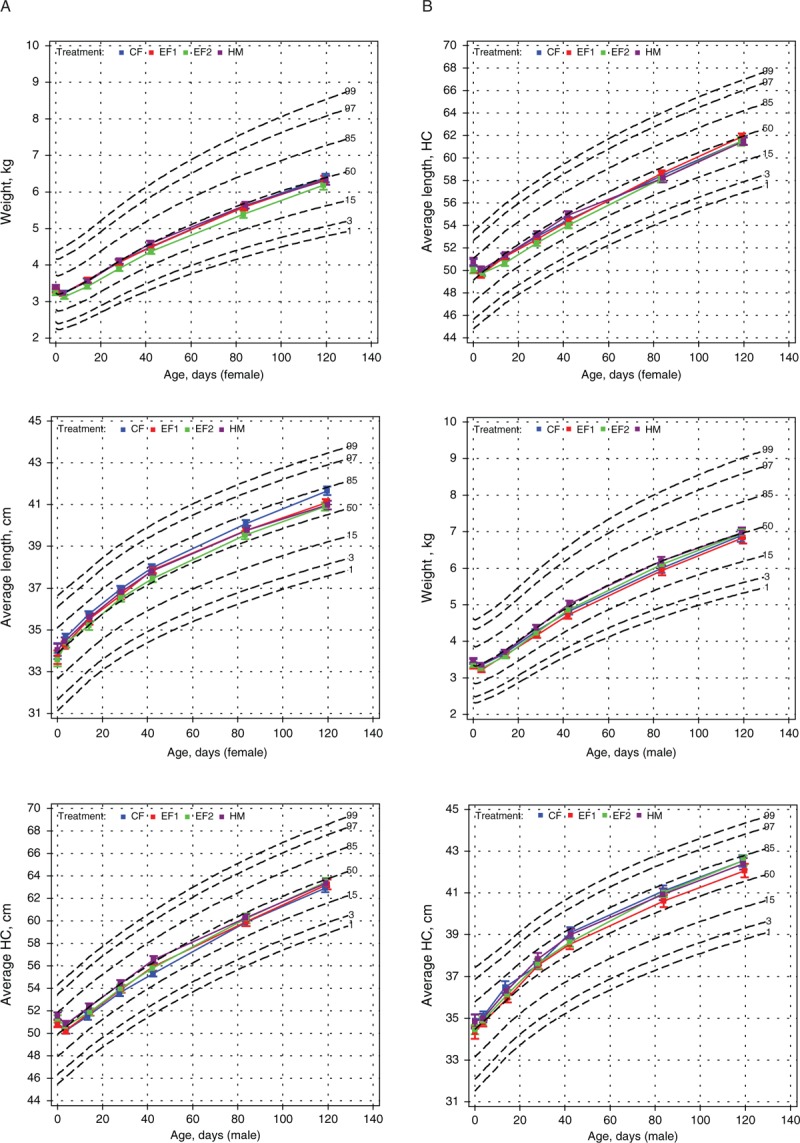
A and B, Weight, length, and head circumference growth of female (A) and male (B) infants plotted on WHO growth charts. CF = control formula; EF = experimental formula; HC = head circumference; HM = human milk; WHO = World Health Organization.
FIGURE 2.
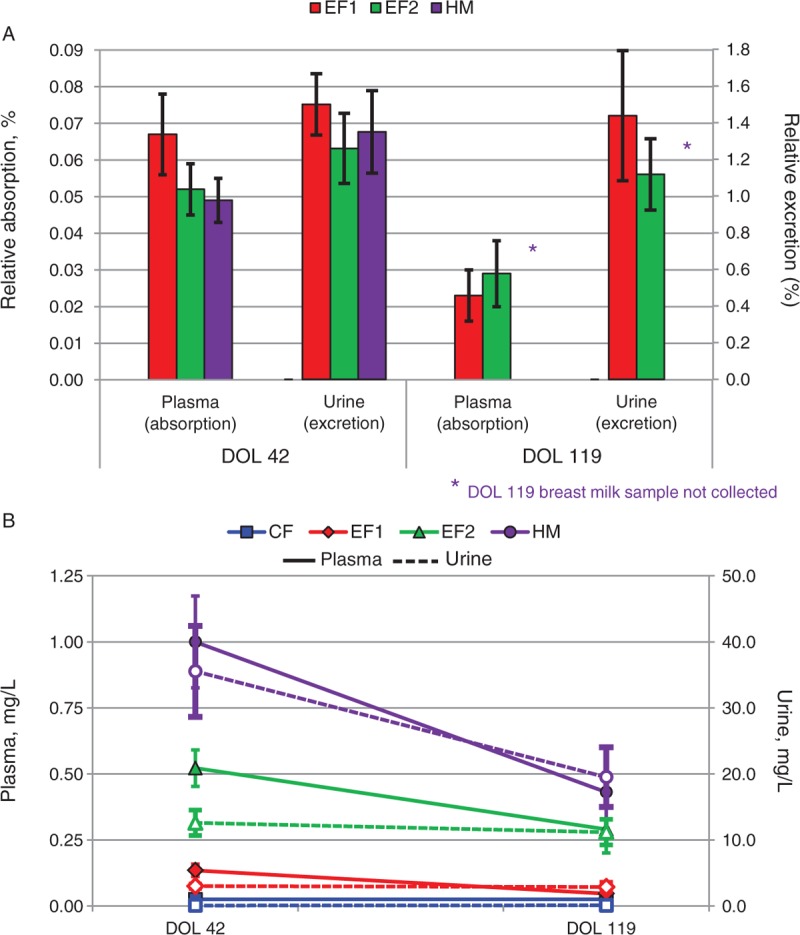
A and B, The relative absorption and excretion of 2′FL at DOL 42 and 119 (A) and the levels of 2′FL in plasma (solid line) and urine (dotted line) at DOL 42 and 119 (B). 2′FL = 2′-fucosyllactose; CF = control formula; DOL = day of life; EF = experimental formula; HM = human milk.
Growth
There were no significant differences (sex-specific or sex-combined) in mean weight, length, or head circumference among feeding groups during the study, and no significant differences among feeding groups in mean gains in these measures from DOL 14 to 119 (Table 3). Secondary analyses of the sex-combined data for several shorter time periods revealed that from DOL 14 to 28, the HM group gained significantly more weight than the EF1 group (P = 0.016), and from DOL 84 to 119 the EF2 group gained significantly more weight than the HM group (P = 0.022) (Table 3). Sex-specific weight-, length-, and head circumference-for-age percentiles are shown in Figure 1.
Intake
The mean daily volume of study formula consumed from enrollment to DOL 28 and for the 3-day periods before the DOL 42, 84, and 119 visits was similar between the CF, EF1, and EF2 groups (data not shown), with the exception of the period from enrollment to DOL 28, during which the CF group consumed significantly more formula than the EF1 group (661 ± 17 vs 614 ± 18 mL/day [least squares means ± standard error of the mean], respectively, P = 0.024).
Tolerance
The mean number of stools per day was significantly greater for the HM group versus the CF, EF1, and EF2 groups from enrollment to DOL 28 (4.9 ± 0.2, 2.0 ± 0.1, 2.2 ± 0.2, and 2.5 ± 0.2, respectively, P < 0.0001) and for the 3-day periods before DOL 42 (3.8 ± 0.2, 1.4 ± 0.1, 1.4 ± 0.1, and 1.5 ± 0.1, respectively, P < 0.0001) and DOL 84 (2.6 ± 0.2, 1.4 ± 0.1, 1.4 ± 0.1, and 1.4 ± 0.1, respectively, P = 0.004), and it was higher for the HM group versus the CF group for the 3-day period before DOL 119 (2.0 ± 0.2 and 1.2 ± 0.1, respectively, P = 0.008). The percent of feedings with spitting up or vomit within 1 hour of feeding was quite variable among groups, but from the enrollment to DOL 28 it was significantly higher in the CF, EF1, and EF2 groups versus the HM group (17.5 ± 2.6, 21.5 ± 2.9, 18.0 ± 2.5, and 10.5 ± 1.6, respectively, P ≤ 0.05). There were no differences among all of the groups after DOL 28. The mean rank stool consistency (MRSC) (1 = watery, 5 = hard) was significantly greater for the EF2 (2.26 ± 0.05) versus HM group (2.04 ± 0.05) from enrollment to DOL 28 (P = 0.021). Repeated measure analysis during the DOL 42, 84, and 119 visits revealed that the MRSC was significantly greater for the formula groups versus the HM group (CF > HM, P = 0.004; EF1 > HM, P = 0.001; EF2 > HM, P = 0.009). MRSC was not significantly different among the 3 formulas groups. The range of MRSC from enrollment to DOL 28 to 119 for the formula groups was as follows: CF 2.2 ± 0.06 to 2.4 ± 0.09, EF1 2.21 ± 0.06 to 2.38 ± 0.09, and EF2 2.26 ± 0.05 to 2.404 ± 0.11.
Safety
There were no significant differences in the overall percentage of subjects with adverse events or serious adverse events in the CF versus the EF1 or EF2 groups. The CF and EF2 groups had significantly more subjects with reported adverse events in the “infections and infestations” category compared with the EF1 group (P < 0.05) with 28 and 38, respectively, compared with 11 in the EF1 group. The types of adverse events were similar among groups with a high proportion of upper respiratory tract symptoms, otitis media, viral infections, and oral candidiasis. Within this category, however, there were no significant differences among study groups for any specific preferred term. The CF group had a significantly higher percentage of subjects (n = 5) with reported eczema compared with the EF1 and EF2 groups who had zero (P < 0.05). Overall, there were no safety concerns noted with either of the EFs (EF1 and EF2).
2′FL Uptake
Levels of 2′FL in the DOL 42 and 119 plasma samples of infants fed CF were below the limit of detection; therefore, relative absorption and excretion data were not calculable. The mean plasma concentrations of 2′FL at DOL 42 were significantly different for each pair of treatment groups (HM > EF2 > EF1) and reflected the amounts in the feeds; however, relative absorption of 2′FL was similar (0.07%, 0.05%, and 0.05% for EF1, EF2, and HM, respectively; not significant) (Table 4; Fig. 2A). Mean urine concentrations were significantly different for each pair of treatment groups (HM, EF2 > EF1 > CF), with the exception of the EF2 and HM groups. Relative excretion was similar among the groups fed 2′FL (1.50%, 1.26%, and 1.35% for the EF1, EF2, and HM groups, respectively; not significant).
HM was not collected at DOL 119; therefore, relative absorption and excretion values for 2′FL were not calculated for the HM group. In contrast to DOL 42, mean plasma concentrations at DOL 119 were not significantly different for each pair of treatment groups. Relative absorption was similar between the EF1 and EF2 groups (0.02% and 0.03%, respectively; not significant). Mean urine concentrations were significantly different for each pair of treatment groups (HM, EF2 > EF1 > CF), with the exception of the EF2 and HM groups. Relative excretion was similar between the EF1 and EF2 groups, 1.44% for EF1 and 1.12% for EF2.
From DOL 42 to 119, plasma concentrations of 2′FL decreased significantly for the EF1, EF2, and HM groups (P = 0.017, 0.008, and 0.015, respectively). Urine concentrations decreased significantly for the HM group (P = 0.018) but did not change significantly for the EF1 and EF2 groups (Fig. 2B).
DISCUSSION
To our knowledge, this is the first growth and tolerance study of infant formulas with a caloric density similar to that of HM. There were no significant differences in weight, length, and head circumference between infants fed HM or the 64.3 kcal/dL formulas during the 4-month study period. Each of the 3 formulas contained GOS and 2 contained 2′FL. All of the formulas were well tolerated, and the relative absorption and excretion of 2′FL were similar to those of HM-fed infants.
There are few published studies that report the feeding of infant formulas with caloric densities lower than standard formulas. Foman et al (28,29) published 2 studies nearly 40 years ago in which infants were fed diets with caloric densities much lower than that of HM; thus, their relevance to the present study is limited. More recently, Timby et al (30) randomly assigned infants <2 months of age to be fed a standard 66 kcal/dL formula or an experimental 60 kcal/dL formula until 6 months of age; the EF also contained bovine milk fat globule and had a lower protein content. Infants fed the EF ingested larger volumes of formula; however, there were no significant differences between groups in linear growth, weight gain, body mass index, percentage of body fat, or head circumference. In our study, the formula with lower caloric density was not associated with higher volumes of intake, compared with previous study data, which may be because of the more modest, 5% decrease in caloric density.
In a recent systematic review, we showed that in the first 2 weeks of life, infants fed standard formulas have a 1.2- to 9.5-fold greater energy intake and a 1.2- to 4.8-fold greater protein intake than breast-fed infants (24). Numerous studies have shown that formula-fed infants grow at a faster rate than HM-fed infants during early life (31–36). The slower growth rate of HM-fed infants may explain, in part, the long-term advantages of breast-feeding (37,38). Greater weight gain during early life has been associated with adverse outcomes, including higher risk of obesity (34,39–41), hypertension (42), diabetes (43,44), and cardiovascular disease (38,45). The risk for these outcomes is reportedly higher in formula versus breast-fed infants (34,43,46–48). Despite accumulating data supporting a connection between higher weight gain early in life and later adverse outcomes, there is a paucity of data showing that formulas with a caloric density more similar to HM are safe and support adequate growth. In the present study, the growth patterns of infants fed the 64.3 kcal/dL formula were similar to those of HM-fed infants, indicating that formula with a caloric density similar to HM is safe and supports growth patterns similar to those of HM-fed infants.
2′FL has previously been found in the plasma and urine of breast-fed but not exclusively formula-fed infants (10,16). Here, we show for the first time that infants fed a formula supplemented with 2′FL exhibit uptake similar to that of HM-fed infants and at levels relative to the concentration fed. Although there were no significant differences in relative absorption between treatment groups, somewhat unexpectedly, both plasma concentrations and relative absorptions decreased from DOL 42 to 119. The urine concentrations for the HM group also decreased; however, the urine concentrations for EF1 and EF2 were consistent between time points.
It is known that both the structure and function of the GI tract mucosa are immature at birth (49). Additionally, the composition of intestinal microbiota transforms throughout infancy (5). These developmental changes may account for the decline in plasma concentrations as the gut becomes less permeable and the microbiota populations evolve to better use 2′FL.
Renal excretion mechanisms, such as glomerular filtration rate and tubular secretory pathways, are also underdeveloped at birth and steadily rise until adult values are reached by 8 to 12 months (49). This increase in renal function from DOL 42 to 119 may be sufficient to counter the decline in absorption of 2′FL and account for the lack of decrease in the 2′FL concentration in urine of formula-fed infants. The decrease in the concentration of 2′FL in the urine of the HM group may be because of the decrease in the concentration of 2′FL in the breast milk fed because levels of 2′FL in HM are known to diminish during the course of lactation (13).
Our study has a limitation. We did not include infants fed formula with the standard caloric density, therefore cannot compare growth and intake between infants fed formula with the standard versus lower caloric density. In a previous study comparing the lower energy formula and a CF (20 kcal/fl oz), there were no significant differences in the average volume of study formula intake per day or average weight gains through 28 days of life. Infants, however, fed the lower calorie formula grew at a rate similar to that of HM-fed infants—the criterion standard (Abbott Nutrition, unpublished results). A strength of our study was the statistical power and study design which assured careful monitoring of growth and tolerance.
In conclusion, the feeding of infant formula with a caloric density similar to that of HM results in growth similar to that of breast-fed infants. In addition, formulas supplemented with 2′FL are well tolerated, and 2′FL absorption profiles are similar to those of breast-fed infants.
Acknowledgment
The authors thank Jane Carver, PhD, for assistance in the preparation of this manuscript.
Footnotes
www.clinicaltrials.gov registration number: NCT01808105.
The study was funded by Abbott Nutrition, Abbott Laboratories, and all of the authors are employees of Abbott Nutrition.
The authors report no conflicts of interest.
REFERENCES
- 1.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evidence Report/Technology Assessment No. 153. AHRQ Publication No. 07-E007. Rockville, MD: Agency for Healthcare Research and Quality; 2007:1–186. [PMC free article] [PubMed] [Google Scholar]
- 2.Ninonuevo MR, Lebrilla CB. Mass spectrometric methods for analysis of oligosaccharides in human milk. Nutr Rev 2009; 67 suppl 2:S216–S226. [DOI] [PubMed] [Google Scholar]
- 3.Tao N, Wu S, Kim J, et al. Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res 2011; 10:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrielli O, Zampini L, Galeazzi T, et al. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011; 128:e1520–e1531. [DOI] [PubMed] [Google Scholar]
- 5.Zivkovic AM, German JB, Lebrilla CB, et al. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A 2011; 108 suppl 1:4653–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smilowitz JT, O'Sullivan A, Barile D, et al. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr 2013; 143:1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Liu S, Leone S, et al. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol 2014; 7:1326–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienenstock J, Buck RH, Linke H, et al. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One 2013; 8:e76236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Leoz ML, Wu S, Strum JS, et al. A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal Bioanal Chem 2013; 405:4089–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goehring KC, Kennedy AD, Prieto PA, et al. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS One 2014; 9:e101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Leoz ML, Gaerlan SC, Strum JS, et al. Lacto-N-tetraose, fucosylation, and secretor status are highly variable in human milk oligosaccharides from women delivering preterm. J Proteome Res 2012; 11:4662–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asakuma S, Urashima T, Akahori M, et al. Variation of major neutral oligosaccharides levels in human colostrum. Eur J Clin Nutr 2008; 62:488–494. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi P, Warren CD, Altaye M, et al. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001; 11:365–372. [DOI] [PubMed] [Google Scholar]
- 14.Gnoth MJ, Kunz C, Kinne-Saffran E, et al. Human milk oligosaccharides are minimally digested in vitro. J Nutr 2000; 130:3014–3020. [DOI] [PubMed] [Google Scholar]
- 15.Rudloff S, Pohlentz G, Borsch C, et al. Urinary excretion of in vivo (1)(3)C-labelled milk oligosaccharides in breastfed infants. Br J Nutr 2012; 107:957–963. [DOI] [PubMed] [Google Scholar]
- 16.Ruhaak LR, Stroble C, Underwood MA, et al. Detection of milk oligosaccharides in plasma of infants. Anal Bioanal Chem 2014; 406:5775–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macy IG, Kelley H, Sloan R. The composition of milks: a compilation of the comparative composition and properties of human, cow and goat milk, colostrum, and transition milk. Bulletin of the National Research Council, no. 119. Washington, DC: National Research Council; 1950. [Google Scholar]
- 18.Hosoi S, Honma K, Daimatsu T, et al. Lower energy content of human milk than calculated using conversion factors. Pediatr Int 2005; 47:7–9. [DOI] [PubMed] [Google Scholar]
- 19.Hester SN, Hustead DS, Mackey AD, et al. Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? J Nutr Metab 2012; 2012:891201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly JJ, Ashworth S, Wells JC. Metabolisable energy consumption in the exclusively breast-fed infant aged 3–6 months from the developed world: a systematic review. Br J Nutr 2005; 94:56–63. [DOI] [PubMed] [Google Scholar]
- 21.European Scientific Committee on Food. Report on the revision of essential requirements of infant formulae and follow-on formulae. SCF/CS/NUT/IF/65/Final.2003; p17 ec.europa.eu/food/fs/sc/scf/out199_en.pdf Accessed August 28, 2014. [Google Scholar]
- 22.National Research Council. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). Washington, DC: The National Academies Press; 2005:171. [Google Scholar]
- 23.Life Sciences Research Office. Executive Summary for the Report: Assessment of Nutrient Requirements for Infant Formulas. Washington DC: Center for Food Safety and Applied Nutrition, Food and Drug Administration, Department of Health and Human Services; 1998. http://jn.nutrition.org/content/128/11/suppl/DC1 Accessed August 28, 2014. [Google Scholar]
- 24.European Food Safety Authority. Scientific opinion on the essential composition of infant and follow-on formulae. EFSA J 2014; 12:3760. [Google Scholar]
- 25.American Academy of Pediatrics Committee on Nutrition. Commentary on breast-feeding and infant formulas, including proposed standards for formulas. Pediatrics 1976; 57:278–285. [PubMed] [Google Scholar]
- 26.Infant Formula Act of 1980. Public Law No. 96–359, 94 Stat. 1190 [codified at 21 U.S.C. §350(a), 301, 321 (aa), 331, 374 (a)]. Sept 26, 1980. 80 IFA 1980. [Google Scholar]
- 27.Infant Formula I. 21 Code of Federal Regulations (as amended) [412] Sect. 350a, Infant Formulas, October 27, 198633. [Google Scholar]
- 28.Fomon SJ, Ziegler EE, Bergmann KE, et al. Skim milk in infant feeding. Acta Paediatr Scand 1977; 66:17–30. [DOI] [PubMed] [Google Scholar]
- 29.Fomon SJ, Filmer LJ, Jr, Thomas LN, et al. Influence of formula concentration on caloric intake and growth of normal infants. Acta Paediatr 1975; 64:172–181. [DOI] [PubMed] [Google Scholar]
- 30.Timby N, Domellof E, Hernell O, et al. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr 2014; 99:860–868. [DOI] [PubMed] [Google Scholar]
- 31.Baird J, Poole J, Robinson S, et al. Milk feeding and dietary patterns predict weight and fat gains in infancy. Paediatr Perinat Epidemiol 2008; 22:575–586. [DOI] [PubMed] [Google Scholar]
- 32.Dewey KG. Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate 1998; 74:94–105. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Fein SB, Grummer-Strawn LM. Do infants fed from bottles lack self-regulation of milk intake compared with directly breastfed infants? Pediatrics 2010; 125:e1386–e1393. [DOI] [PubMed] [Google Scholar]
- 34.Ong KK, Preece MA, Emmett PM, et al. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr Res 2002; 52:863–867. [DOI] [PubMed] [Google Scholar]
- 35.Sievers E, Oldigs HD, Santer R, et al. Feeding patterns in breast-fed and formula-fed infants. Ann Nutr Metab 2002; 46:243–248. [DOI] [PubMed] [Google Scholar]
- 36.Taveras EM, Scanlon KS, Birch L, et al. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics 2004; 114:e577–e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agostoni C, Fattore G. Growth outcome: nutritionist perspective. World Rev Nutr Diet 2013; 106:12–18. [DOI] [PubMed] [Google Scholar]
- 38.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 2004; 363:1642–1645. [DOI] [PubMed] [Google Scholar]
- 39.Baird J, Fisher D, Lucas P, et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005; 331:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harder T, Bergmann R, Kallischnigg G, et al. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005; 162:397–403. [DOI] [PubMed] [Google Scholar]
- 41.Stettler N, Stallings VA, Troxel AB, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation 2005; 111:1897–1903. [DOI] [PubMed] [Google Scholar]
- 42.Law CM, Shiell AW, Newsome CA, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation 2002; 105:1088–1092. [DOI] [PubMed] [Google Scholar]
- 43.Knip M, Virtanen SM, Akerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr 2010; 91:1506S–1513S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singhal A, Fewtrell M, Cole TJ, et al. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 2003; 361:1089–1097. [DOI] [PubMed] [Google Scholar]
- 45.Singhal A, Cole TJ, Fewtrell M, et al. Is slower early growth beneficial for long-term cardiovascular health? Circulation 2004; 109:1108–1113. [DOI] [PubMed] [Google Scholar]
- 46.Frederiksen B, Kroehl M, Lamb MM, et al. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr 2013; 167:808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen CG, Martin RM, Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 2005; 115:1367–1377. [DOI] [PubMed] [Google Scholar]
- 48.Owen CG, Martin RM, Whincup PH, et al. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 2006; 84:1043–1054. [DOI] [PubMed] [Google Scholar]
- 49.van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handb Exp Pharmacol 2011; 205:51–75. [DOI] [PubMed] [Google Scholar]


