Supplemental Digital Content is Available in the Text.
Key Words: antiretroviral therapy, pediatric ART, loss to follow-up, patient tracing, Malawi, sub-Saharan Africa
Background:
Loss to follow-up (LTFU) challenges the success of antiretroviral therapy (ART) scale-up among pediatric patients. Little is known about children who drop out of care. We aim to analyze risk factors for LTFU among children on ART, find their true outcomes through tracing, and investigate their final outcomes after resuming ART.
Methods:
This is a descriptive, retrospective, cohort study of children on ART between April 2006 and December 2010 in 2 clinics in urban Malawi. Routine data from an electronic data system were used and matched with information obtained through routine tracing procedures.
Results:
Of 985 children (1999 child-years) on ART, 251 were LTFU: 12.6/100 child-years. At ART initiation, wasting [adjusted hazard ratio (AHR) 1.58 and 95% confidence interval (CI): 1.02 to 2.44] was independently associated with higher risk of LTFU. Of 201 LTFU children traced, 79% were found: 11% died, 25% stopped, 26% transferred-out, and 37% were still on ART. Median time between last visit and first tracing was 84 days (interquartile range: 64–101 days). Tracing reduced risk of LTFU by 38% (AHR 0.62 and 95% CI: 0.42 to 0.91) and decreased LTFU from 23.2% to 8.5%. Additional outcomes of stop, death, and transfer-out increased 4.4-fold, 1.8-fold, and 1.3-fold, respectively. Traced children with gaps in ART intake who resumed ART had higher risk of stopping (AHR 4.92 and 95% CI: 1.67 to 14.5) and transfer out (AHR 2.70 and 95% CI: 1.75 to 4.17) as final outcome.
Conclusions:
Early tracing substantially reduces LTFU; approximately one-third presumed LTFU was found to be still on ART. Children with wasting at initiation and those traced and found to have irregular ART intake require targeted interventions.
INTRODUCTION
Loss to follow-up (LTFU) among pediatric HIV-infected patients is a major challenge for the global scale-up of lifesaving antiretroviral therapy (ART). Globally, the estimated proportion of LTFU among children is 14% and 28% after one and 2 years on treatment, respectively.1 In sub-Saharan Africa, the proportion of children with unknown treatment outcomes 2 years after initiation lies between 9% in Southern Africa and 21.8% in West Africa.2 Common risk factors are advanced clinical or immunological stages, malnutrition, and young age,3–5 suggesting that many children presumed LTFU actually died.6 Only 2 studies, from Malawi and Kenya, investigate LTFU among children by reporting outcomes after telephone or home tracing.6,7
Additional information on LTFU among children, including country-specific research, is needed, so programs can tailor their efforts to retain children in care. First, LTFU rates between treatment programs can vary depending on accessibility of services, quality of record keeping, and different eligibility criteria for ART.8 For example, policies that cause a rapid expansion of eligible patients may affect service quality,9 ultimately influencing whether families maintain their appointments and avoid disengagement from care.10 Second, poor retention is a risk factor for HIV drug resistance: 20% or more LTFU 12 months after ART initiation was found to be an early warning indicator.11,12 Third, children become sexually active young adults,13 who may be able to transmit resistant virus, potentially increasing treatment costs for themselves and their future partners. Finally, a substantial number of patients considered LTFU return to HIV services and resume treatment, but little is known about their risks and subsequent outcomes.14,15 Improved understanding of how and why children drop out of care, and how many return, is vital to improve ART service delivery for HIV-infected children and their families.
Lighthouse Trust is the largest, integrated HIV/ART service provider in Malawi's public sector. Lighthouse's robust monitoring and evaluation (M&E) system and routine tracing program to return patients who miss appointments, named Back to Care (B2C), contribute to better understanding of LTFU in adults.16,17 Using this same B2C system, we conduct a similar study of children who missed an ART dispensing appointment to (1) identify risk factors for LTFU of 3 weeks or more, (2) determine these children's true outcomes through tracing, (3) examine the corrective effect of tracing and self-return on the outcomes of the ART program, and (4) investigate outcomes of successfully traced and returned patients who resume ART.
METHODS
Lighthouse Trust runs 2 integrated HIV/ART facilities in Lilongwe city: the Lighthouse Clinic (LH) at Kamuzu Central Hospital and the Martin Preuss Centre (MPC) at Bwaila district hospital. By June 2011, LH and MPC had a combined 12,808 patients alive on ART under active follow-up. Eligibility criteria, treatment regimens [in 2011, either split adult or pediatric fixed dose combination of stavudine, lamivudine and nevirapine], and subsequent visit procedures adhere to national guidelines at both clinics.18,19 In brief, children on ART visit the clinic 2 weeks after initiation; then monthly for 6 months; and then every 2 months if the child's condition is stable and adherence to ART is good. Patients may come unscheduled if sick. An electronic data system (EDS) in reception, clinic rooms, and pharmacy supports real-time patient management and monitoring with touch screens and scanners, reducing errors and ensuring data completeness at each visit.20 Nurses or clinical officers record body weight, standard treatment outcomes [alive on ART, stopped ART, transferred-out (TO), died, or LTFU], and adherence assessments using pill counts. In parallel, they use paper files for specific clinical notes. The next patient visit is calculated by the EDS according to the patient's remaining medication and the new supply is determined by weight band dependant dosages and either 4- or 8-weeks between visits. In addition, the pharmacist adjusts children's pill counts and appointment dates to coincide with the carer's appointment.
Tracing Procedures
The B2C team has 3 phone and field tracers and one receptionist. The receptionist documents patients' details (residence and contact phone numbers), obtains oral consent for contacting in case of missed appointments, and updates this information during subsequent visits.21 Every week, the B2C team creates a list of patients from the EDS who missed ART dispensing appointments by 3 weeks or more. The team then checks these patients' paper files for any recent visit to verify the presumed LTFU list. To confirm that the patient ran out of pills, the tracer records the last visit date, the number of pills remaining at the last visit, the pills dispensed, and the date of the missed appointment. The estimated date when pills run out is recorded on the paper B2C follow-up form. If 3 attempts to trace the patient or caregiver by phone are unsuccessful, field tracers visit patients or carers at home with up to 3 attempts. Tracers are successful if they find the patient or carers, and information about the patient's outcome is determined. Tracing outcomes and reasons for LTFU are recorded in an off-line B2C access database and subsequently linked to the EDS through unique patient identifiers.16
Definitions and Variables
LTFU is defined by Lighthouse's B2C program as a verified, missed, and scheduled appointment for ART collection of 3 weeks or more, a definition that differs from Malawi's National ART program LTFU definition of “patients who missed an ART dispensing date by 2 or more months.”18,19 All patients who had at least one episode of LTFU (irrespective of final outcome) were included in analysis of risk factors for being LTFU. However, presumed the LTFU patients who returned spontaneously after unsuccessful tracing, but before the closure of the study in December 2010, are not counted as LTFU in the final outcomes (see definitions below) as they returned without tracing.
Successful tracing outcomes are (1) on ART (uninterrupted therapy and still taking ART), (2) on ART with gaps (taking fewer than prescribed drugs), (3) “self” TO, if transfer was arranged independently without consultation with LH or MPC, (4) “official” TO, if TO is documented in the patient's file but not in the EDS, (5) stopped ART for any reason, but still attending appointments, (6) never started ART despite having collected an initial supply of ART, and (7) death. Unsuccessful outcomes include when tracing was not attempted because a patient lives outside the catchment area for B2C, when the patient or carer refused tracing, or when patients are not found by tracers.
A patient's original standard treatment outcome is recorded in the EDS by clinical officers or nurses at each clinic visit, independently from the findings of the B2C program. Tracing outcomes replace original outcomes when they are retrospectively entered by the B2C team in the EDS. The final outcome is the last outcome registered in the EDS by December 2010, which may be the same as the original outcome if not traced, a correction made by the B2C team, an outcome that occurred after tracing for those who returned, or an outcome recorded for a patient who returned to care spontaneously. Time to original outcome is the time in months between ART initiation and the date of an original standard treatment outcome. If the child was still in care by December 2010, we took the last recorded original outcome before study closure.
Advanced clinical stages are World Health Organization (WHO) stages 3 or 4.22 Immunological stages follow WHO's age-dependant thresholds for absolute or relative CD4 counts.22 Body mass index-for-age Z-score (BAZ), height-for-age Z-score, and weight-for-age Z-score are calculated according to the WHO reference growth standards from 2007 for children 0–60 months of age, using the program WHO Anthrop Software version 3.2.223 or the SPSS macro package available online for children 5–19 years old.24 Wasted is defined as BAZ≤ −2, severely wasted as BAZ≤ −3, stunted as height-for-age Z-score≤ −2, and underweight as weight-for-age Z-score≤ −2.
The Euclidean distance is the distance in kilometers (km) between the LH or MPC and each patient's neighborhood or census area centroid (central geographic point), obtained using the ruler feature within Google Earth software.25 This approximates the distance as the crow flies but does not take transportation or geographic features into account.
Data Collection and Statistical Analysis
All children, ages 15 years and younger at ART initiation, who were on first line ART at LH or MPC between April 2006 and December 2010 are included. Transferred-in patients are excluded from the analysis as baseline information was often unknown; however, a sensitivity analysis including these patients was undertaken to rule out important differences with the selected sample (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A721). To compile the study database, visit dates, demographic and anthropometric data, WHO stage, CD4 measurements at initiation, and standard treatment outcomes from the EDS were merged with the tracing information from the off-line B2C access database using unique patient identifiers. We reviewed paper records to update missing data in the study database. Medians and interquartile ranges (IQRs) describe continuous variables, and groups are compared by the Mann–Whitney U test. Frequencies of categorical variables are described and compared with the Fisher exact or χ2 test.
A competing risks model is used to estimate the LTFU proportions at different follow-up times: competing risks include death, TO, and stopped ART. To identify independent risk factors for LTFU, we use Cox proportional hazards models. All statistically significant variables (P < 0.05) in univariate analysis are included in multivariate regression together with other a priori variables and possible confounders such as sex, advanced clinical stage at ART initiation, and distance to clinic. The proportionality hazards assumption is tested by constructing time-dependent variables for each covariate (adding an interaction term of time to the Cox model) and testing for significance. The proportionality assumption holds true for each variable included in the final multivariate model. The effect of tracing on the final outcome of LTFU is measured using a Cox regression model with one frailty. Among those children LTFU who were successfully traced, found alive not TO, and returned to care, we estimate adjusted hazard ratios (AHRs) of the different final outcomes stratified by the tracing outcomes alive on ART, on ART with gaps, and stopped ART. The effect of active tracing of LTFU patients on the occurrence of LTFU as the final outcome is analyzed using a Cox regression model with one frailty. Results are adjusted for sex, HIV facility, distance to clinic, and advanced WHO stage, wasting, age, and severe immunosuppression at ART initiation.
For all variables with missing values, we perform multiple imputation using the ice26 procedure in Stata13 to create 20 imputed data sets; estimates of results are averaged across the 20 imputed data sets according to Rubin's rules27 using the mim procedure in Stata.28 The imputation models include all variables in Table 2, the outcome of interest, and the Nelson Aalen estimator of the cumulative baseline hazard.29,30 We include results for complete cases and for imputed missing data. Data are analyzed in SPSS 18.0 and Stata 13.0, and significance was set at P < 0.05.
TABLE 2.
Effect of Baseline Characteristics on the Original Outcome, Lost to Follow Up, Among Children on ART: Results From Cox Proportional Hazards Model (N = 985)
The Malawi National Health Science Research Committee and the Liverpool School of Tropical Medicine Research Ethics Committee approved the study.
RESULTS
Baseline Characteristics and Original Outcomes
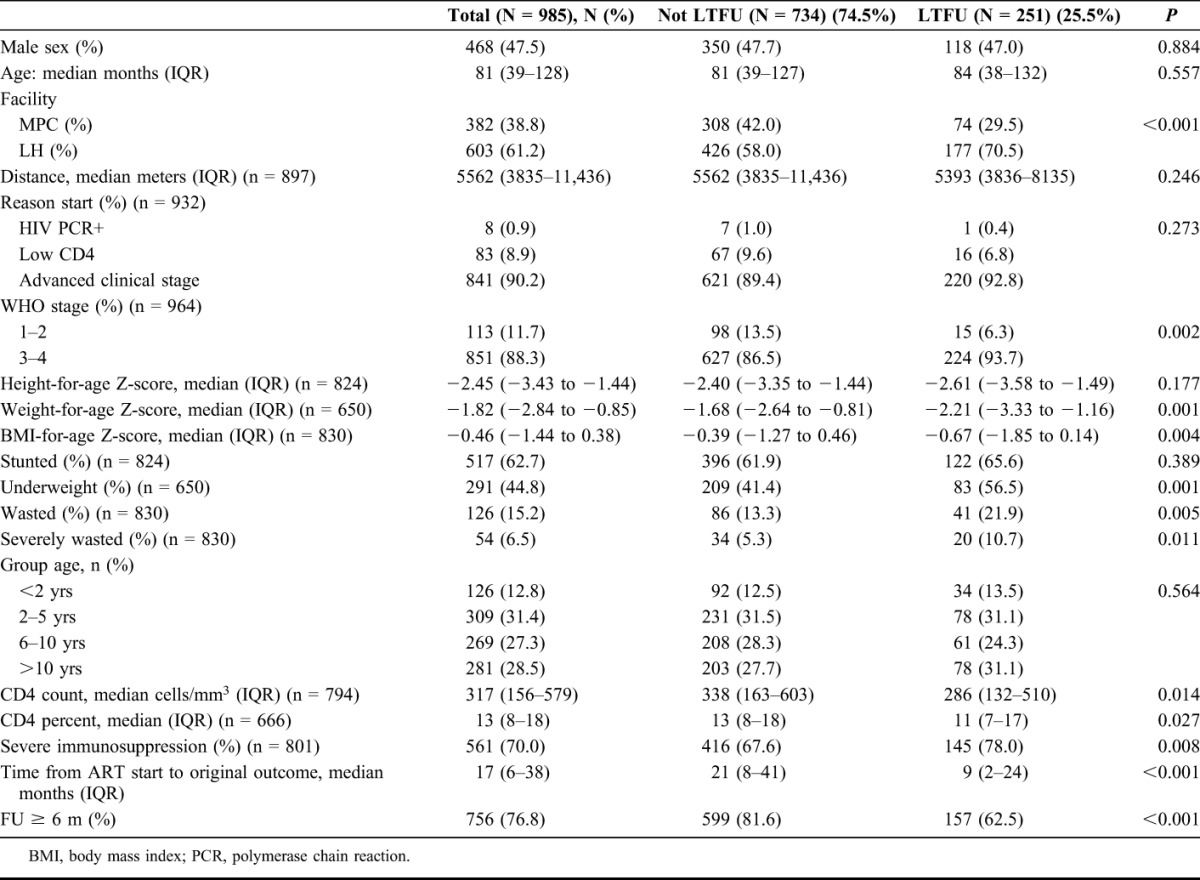
A total of 1182 children were on ART between April 2006 and December 2010 at LH and MPC; 197 were excluded because there were older than 15 years or were transfer-in (Fig. 1). Of the 985 children included in analysis, 47.5% were male; median age at ART initiation was 81 months (IQR: 39–128); and they contributed 1999 years of ART follow-up. Most children had advanced clinical stage (88.3%) and were severely immunosuppressed (70.0%) (Table 1). At this stage in the flowchart (Fig 1), we present the baseline characteristics of the 985 includede children and then return to Figure 1. Of the 985 children, 251 missed at least 1 appointment by 3 weeks and were LTFU, 25 died, and 187 were TO by December 2010. The B2C team attempted tracing for 201/251 patients; 50 were not traced as they lived outside the B2C catchment area.
FIGURE 1.
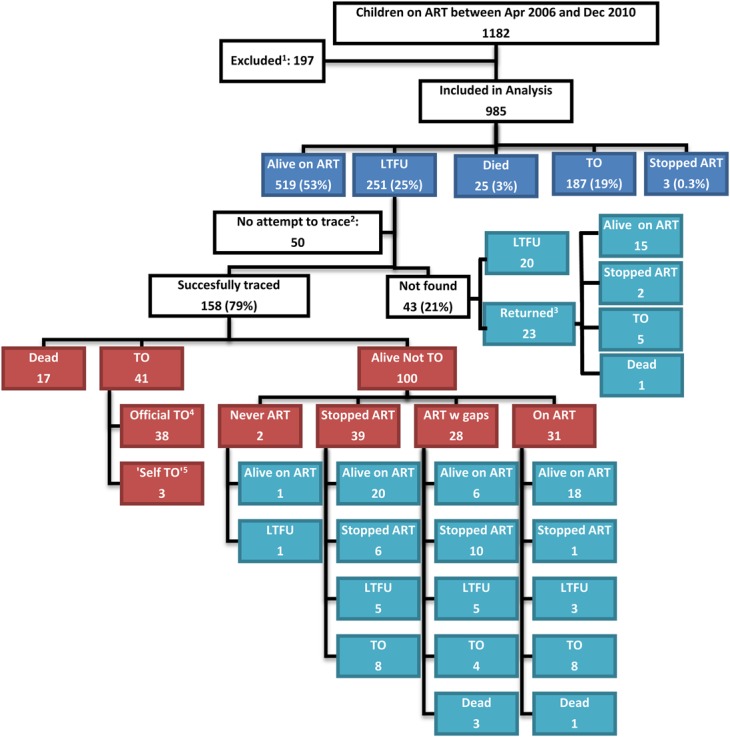
Study profile and outcomes. Dark blue—original standard treatment outcomes as recorded in the EDS before active tracing; red—successful tracing outcomes as recorded by the tracing (Back2care) team; light blue—final outcomes as recorded in the EDS by study closure in December 2010. 1—above 15 years of age or transfer-in from other ART clinic; 2—outside the catchment area of the tracing team; 3—returned on their own after unsuccessful tracing; 4—TO by health care workers at the HIV clinic but not correctly recorded in the EDS; 5—patient decided to be treated at another HIV clinic without notifying the health care worker. Apr—April; Dec—December; LTFU—lost to follow-up; w gaps—with gaps.
TABLE 1.
Baseline Characteristics Stratified by Original Outcome, LTFU
The LTFU rate of the 251 children (Fig 1) was of 12.6 per 100 child years. Competing risks model estimates of proportion of LTFU were of 8.10% (95% CI: 6.4% to 10.0%) at 6 months, 12.96% (95% CI: 10.8% to 15.3%) at 12 months, and 19.35% (95% CI: 16.6% to 22.2%) at 24 months (see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/A721).
Risk Factors for LTFU
In univariate analysis, LH registered a higher proportion of children LTFU (177/603) compared with MPC (74/382); LH contributed 70.5% of LTFU children in the study. LTFU children were more frequently in advanced clinical and immunological stage at ART initiation and had a poorer nutritional status at baseline (Table 1). In an additional sensitivity analysis including children transferred-in, significance of associations did not change, so transfer-in children were excluded from the main analysis (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A721).
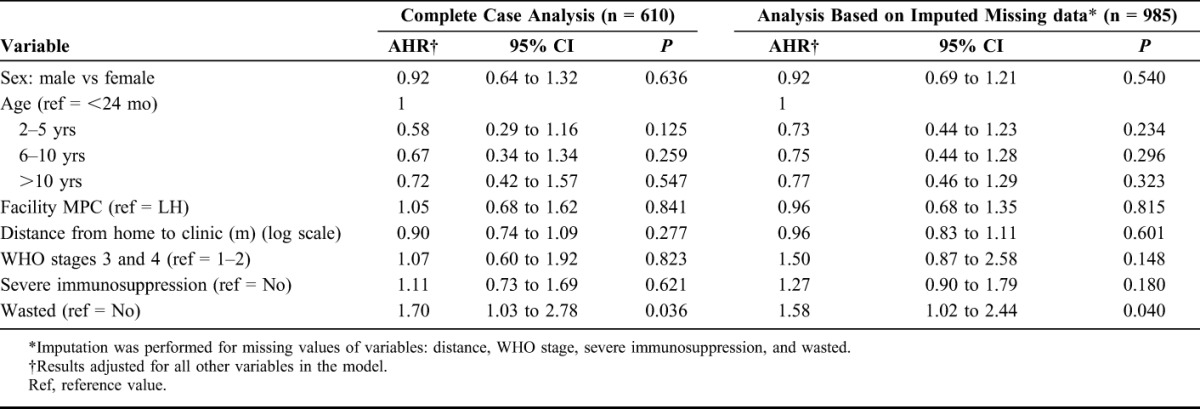
In multivariate Cox regression analysis with imputed missing data, wasting (AHR 1.58 and 95% CI: 1.02 to 2.44) at ART initiation was independently associated with a higher risk of being LTFU. Results were adjusted for sex, age, HIV facility, advanced WHO clinical stage, severe immunosuppression, and distance to the HIV facility (Table 2).
Tracing Outcomes
Among the 201 children with tracing attempts, the median time on ART before presumed LTFU was 8 months (IQR: 2–22 months). Median time between last visit and first tracing attempt was 84 days (IQR: 64–101 days).
Of the 201 children, 158 (79%) were interviewed and 43 (21%) were not found by tracers: 42 had incorrect addresses and 1 had moved outside the tracing catchment area. Of the 158 children found, 17 (11%) died; 41 (26%) TO (3 were self-transfer); and 100 (63%) were alive and not TO, and 59 of these 100 (37.3% of the total 158) were still taking ART from LH or MPC (Fig. 1).
Final Outcomes
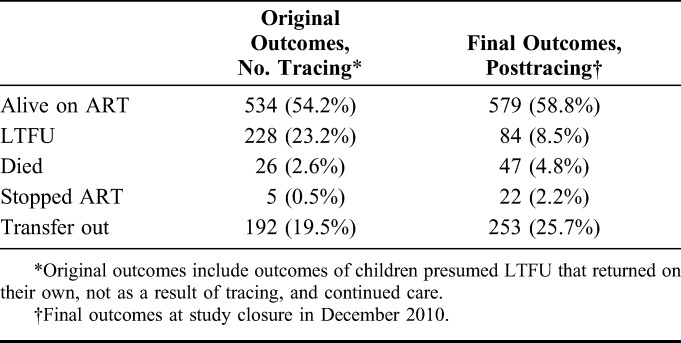
Although tracing reduced the proportion of children classified as LTFU by nearly 2/3 (63.2%, 228 vs 84), it increased the number of children who stopped, died, or TO by 4.4-fold, 1.8-fold, and 1.3-fold, respectively, compared with the original outcomes (Table 3). The LTFU rate decreased from 11.2 to 4.2 per 100 child years, and the mortality rate increased from 1.3 to 2.4 per 100 child years after correction for mortality among children LTFU.
TABLE 3.
Effect of Tracing on Original Outcomes in Children LTFU From ART at Lighthouse Trust (N = 985)
We also analyzed the effect of active tracing of LTFU patients on the occurrence of LTFU as the final outcome, using a Cox regression model with one frailty adjusted for sex, HIV facility, distance to clinic, advanced WHO stage, wasting, age, and severe immunosuppression at ART initiation. Active tracing reduced the risk of being LTFU at the end of the study period by 38% (AHR 0.62 and 95% CI: 0.42 to 0.91, and P = 0.014) (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A721).
Returned patients
Of the 100 successfully traced patients found alive and not TO (Fig. 1), all returned to LH or MPC to resume care. The median time to return to care from B2C team contact was 10 days (IQR: 6–22 days), and the median time to the final outcome after returning to care was 13 months (IQR: 0–33 months).
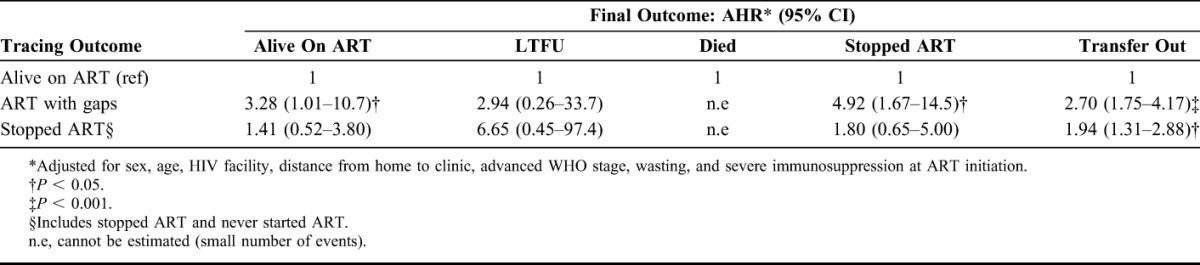
Adjusted Cox proportional hazard analyses showed that successfully traced children found taking ART with gaps or stopped ART had a greater risk of being TO (AHR 2.70 and 95% CI: 1.75 to 4.17, and AHR 1.94 and 95% CI: 1.31 to 2.88 respectively) when returned to care than those who were found taking their ART regularly (Table 4). Although children found to be on ART with gaps had an increased risk of stopping ART (AHR 4.92 and 95% CI: 1.67 to 14.5), they also had a 3-fold increased risk of being alive on ART at the study end vs those children found to be on uninterrupted ART when traced (Table 4). The association with the final outcome of death was not estimated because of the small number of children who died (4 of 100).
TABLE 4.
AHR of Final Outcomes Stratified by Tracing Outcome Among Children on ART Who Were Presumed LTFU and Found Alive Not TO After Successful Tracing (n = 100)
DISCUSSION
In our study, 25% (251/985) of children on ART were presumed LTFU; wasting was the main risk factor. When attempted, tracing was successful in 78.6% (158/201) of children, of whom 37.3% (59/158) were found still taking ART (either uninterrupted or with gaps), 25.9% (41/158) were receiving treatment elsewhere, 24.6% (39/158) had stopped ART, and 10.7% (17/158) had died. Tracing resulted in a reduction of children classified as LTFU by 2/3 in final outcomes and a 4.4-fold, 1.8-fold, and 1.3-fold increase in children classified as stop, death, and transfer-out, respectively. Active tracing of LTFU children on ART reduced the risk of being LTFU by 38% at the end of the follow-up period. Traced children found taking ART with gaps who resumed ART were nearly 5 times more likely to stop and 2.7 times more likely to transfer out as compared with traced children found on ART without gaps who also resumed care.
Many sites in southern Africa trace children LTFU. Among those collaborating in the International Epidemiologic Databases to Evaluate AIDS network, 83% use telephone calls and 71% complete outreach worker home visits.30 However, this study is among the few quantitative studies that systematically investigate tracing success and outcomes. We show that tracing of pediatric patients is acceptable and feasible, reducing LTFU and misclassification in this group of patients.
Our study highlights some operational challenges. Tracers frequently found children still taking ART dispensed by LH or MPC [31/158 (20%)], despite estimating the child's lack of medication before a tracing attempt. Adults on treatment within the household may share their tablets (most children were on split adult tablets) until both, adult and child, visit the clinic together to avoid an additional clinic visit, a possibility that may occur in the short time between missing an appointment and active tracing for some children. Patients may also provide incorrect information about remaining tablets at home, or health workers may not count remaining tablets correctly during visits, resulting in incorrect dates for next appointments. In addition, 41/158 (24%) of LTFU who were successfully traced were actually officially TO, indicating weak record keeping using the EDS and adding unnecessary work for the B2C team. Furthermore, the time between last contact and tracing was a median of 84 days-well beyond the target of 21 days, although this period is still shorter than in the national recommendations18,19 and other studies.6,7 Documentation in the EDS, communication between health workers, and communication between health workers and patients need improvement.
The proportion of LTFU in our study before tracing (25%) is higher than expected.2 Wasting is a common risk factor for LTFU.2,31,32 Previous studies suggest that many children LTFU from ART died,33 and wasting also predicts death in untreated children.33 However, death is the least common cause of LTFU in our study (11%). Rather, in our study, the majority, 100/158 (65%) LTFU patients successfully traced, were found alive, not TO, at the time of tracing. Carers of malnourished children might be particularly vulnerable to irregular visits and missed appointments although many of them still ensure the children continue ART. Of those successfully traced patients, 59/158 (37%) were still on ART either uninterrupted or with gaps. Possible explanations for missing children's appointments might also include poor patient's or carer's health and receiving treatment elsewhere [41/158 (26%) successfully traced], potentially closer to home with fewer transport costs and less travel time. New universal treatment policies under consideration in Malawi should counteract poor health status because of late ART initiation, and further decentralization of HIV services should improve accessibility. However, a massive influx of patients may also have negative repercussions on the proportion of LTFU.9 Therefore, interventions to prevent LTFU must be deployed to complement tracing efforts, such as promotion and better documentation of transfer-outs and text messaging appointment reminders.34
Although baseline characteristics, settings, and sample sizes differ between studies, the short time between the LTFU classification and tracing (median 84 days) may be the most relevant difference between our and previous studies. In our study, the relatively short time between LTFU and tracing likely increased the chances to find lost children before death,7 as has been shown in adults.35 The difference in time to tracing may also account for the lower mortality rate in LTFU children in our study compared with adult studies.36 Therefore, our findings do not support the use of adult tracing data to correct mortality for LTFU in children.37
Finally, we observed an association between outcomes at the time of tracing and final outcomes at the end of the study. In our case, children with previous missed visits and incomplete tablet intake at the time of tracing (“on ART with gaps”) were more likely to have stopped ART as the final outcome. It is plausible that patients who missed appointments in the past may have future problems with adherence.15 However, these same children were also more likely to be alive on ART as a final outcome as compared with those found alive on ART when traced. Although these findings merit further investigation, they suggest that children with missed visits or incomplete tablet taking should be targets for immediate adherence counseling to prevent falling completely out of care or stopping treatment. As adult returners after default showed substantial HIV drug resistance and virological failure once they resumed ART,14 children might be at similar risk.
Our study has several limitations. First, data are missing. To mitigate bias through missing values, we used multiple imputation methods. Second, a number of factors potentially associated with LTFU were not available for analysis (eg, tuberculosis status, adherence to ART, parent education, or parent HIV status). Third, we did not consider change of variables over the 4-year period of the study such as for CD4 count, weight, height, or operational changes affecting quality of service delivery and retention such as health worker-patient ratio and time per patient visit. Fourth, the type of ART regimen may influence LTFU.2,4 All patients were on a fixed dose combination of stavudine, lamivudine and nevirapine but used different formulations; this was not considered in this analysis. Fifth, our LTFU definition (missed appointment for more than 3 weeks) is different from what is used in other studies (missed an appointment for 3–6 months), which limits the generalizability of our findings. Sixth, the catchment area of our clinic is urban. The situation in rural settings may differ.
Overall, tracing was accepted by families and health workers and was a feasible intervention. Tracing reduced the LTFU rate below WHO's former early warning sign threshold and increased retention. However, communication and documentation within the clinic should be strengthened to improve identification of LTFU children running out of ART and reduce the time between identification and tracing. Increased attention to synchronizing ART appointments between children and their carers on ART may be also needed. Among those children traced, fewer children died than expected, whereas the number of children identified with ART with gaps, stopped ART, or TO increased. Wasted children are most vulnerable for LTFU. Among those who return after tracing, children who had treatment gaps might be at risk for HIV drug resistance and may require viral load testing. Interventions should prioritize children most at risk for LTFU and possibly treatment failure.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and staff of the Lighthouse Trust in Lilongwe, Malawi.
Footnotes
C.A.-G. is currently funded by Wellcome Trust Grant Number 099938/B/12/Z. C.F. was supported with funding from Cooperative Agreement U91HA06801 from the US Department of Health and Human Services, Health Resources and Services Administration (HRSA).
Findings of the study were partly presented at the 19th International AIDS Conference, July 22-27, 2012, Washington, DC. Abstract No. WEAE0203.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed on March 3, 2014.
- 2.Leroy V, Malateste K, Rabie H, et al. Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. J Acquir Immune Defic Syndr. 2013;62:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braitstein P, Katshcke A, ChangYu S, et al. Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Trop Med Int Health. 2010;15:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatti G, Bock P, Grimwood A, et al. Increased vulnerability of rural children on antiretroviral therapy attending public health facilities in South Africa: a retrospective cohort study. J Int AIDS Soc. 2010;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengayi M, Dwane N, Marinda E, et al. Predictors of loss to follow-up among children in the first and second years of antiretroviral treatment in Johannesburg, South Africa. Glob Health Action. 2013;6:19248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braitstein P, Songok J, Vreeman RC, et al. Wamepotea (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in Western Kenya. J Acquir Immune Defic Syndr. 2011;57:e40–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire M, Munyenyembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15:55–62. [DOI] [PubMed] [Google Scholar]
- 8.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;28:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware NC, Wyatt MA, Geng EH, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10:e1001369; discussion e1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. IV drug resistance early warning indicators. World Health Organization indicators to monitor HIV drug resistance prevention at antiretroviral treatment sites. 2010. Available at: http://www.unaids.org.br/biblioteca/links/OPAS-OPS/OPAS%2014.pdf. Accessed on March 3, 2014.
- 12.WHO. Meeting Report on Assessment of WHO HIV Drug Resistance Early Warning Indicators. Report of the Early Warning Indicator Advisory Panel Meeting. 2012. Available at: http://apps.who.int/iris/bitstream/10665/75186/1/9789241503945_eng.pdf?ua=1. Accessed on March 3, 2014. [Google Scholar]
- 13.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luebbert J, Tweya H, Phiri S, et al. Virological failure and drug resistance in patients on antiretroviral therapy after treatment interruption in Lilongwe, Malawi. Clin Infect Dis. 2012;55:441–448. [DOI] [PubMed] [Google Scholar]
- 15.Nakiwogga-Muwanga A, Musaazi J, Katabira E, et al. Patients who return to care after tracking remain at high risk of attrition: experience from a large HIV clinic, Uganda. Int J STD AIDS. 2015;26:42–47. [DOI] [PubMed] [Google Scholar]
- 16.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the “Back-to-Care” project in Lilongwe, Malawi. Trop Med Int Health. 2010;15:82–89. [DOI] [PubMed] [Google Scholar]
- 17.Tweya H, Feldacker C, Estill J, et al. Are they really lost? “true” status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PLoS One. 2013;8:e75761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health Malawi. Treatment of AIDS. Guidelines for the Use of Antiretroviral Therapy in Malawi. 2nd ed Lilongwe, Malawi: Ministry of Health of Malawi; 2006. [Google Scholar]
- 19.Ministry of Health of Malawi. Treatment of AIDS. Guidelines for the Use of Antiretroviral Therapy in Malawi. 3rd ed Lilongwe, Malawi: Ministry of Health of Malawi; 2008. [Google Scholar]
- 20.Douglas GP, Gadabu OJ, Joukes S, et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLoS Med. 2010;7:e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Health of Malawi. Clinical Management of HIV in Children and Adults. Malawi Integrated Guidelines for Providing HIV Services. 1st ed Lilongwe, Malawi: Ministry of Health of Malawi; 2011. [Google Scholar]
- 22.WHO. WHO Case Definitions of HIV for Surveillance and Revised Clinical <br/>staging and Immunological Classification of HIV-Related Disease in Adults and Children. Geneva, Switzerland: World Health Organization; 2007. Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. Accessed on March 3, 2014. [Google Scholar]
- 23.WHO. WHO AnthroII PC. Version 3.2.2. 2007. Available at: http://www.who.int/childgrowth/software/en/. Accessed on March 3, 2014. [Google Scholar]
- 24.WHO. Reference 2007 SPSS Macro Package. 2007. Available at: http://www.who.int/growthref/tools/en/. Accessed on March 3, 2014. [Google Scholar]
- 25.Google Inc. Google Earth Software. Version 5.1.3533.1731. Mountain View, CA: 2009. Available at: http://www.google.com/earth/download/ge/agree.html. Accessed on March 3, 2014. [Google Scholar]
- 26.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, New Jersey; John Wiley & Sons; 2004. [Google Scholar]
- 28.Royston P, Carlin JB, White IR. Multiple imputation of missing values: new features for mim. Stata J. 2009;9:252. [Google Scholar]
- 29.White IR, Royston P. Imputing missing covariate values for the cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IeDEA Pediatric Working Group. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa–the international epidemiologic databases to evaluate AIDS (IeDEA). J Int AIDS Soc. 2013;16:17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. J Acquir Immune Defic Syndr. 2013;62:e70–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weigel R, Estill J, Egger M, et al. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS. 2012;26:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross Continents Collaboration for Kids (3Cs4kids) Analysis and Writing Committee. Markers for predicting mortality in untreated HIV-infected children in resource-limited settings: a meta-analysis. AIDS. 2008;22:97–105. [DOI] [PubMed] [Google Scholar]
- 34.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9:e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakiwogga-Muwanga A, Musaazi J, Katabira E, et al. Early tracking after a missed return visit reduces the proportion of untraceable patients at a large HIV clinic in Kampala, Uganda. J Int Assoc Provid AIDS Care. 2014. [Epub ahead of print, April 9, 2014]. [DOI] [PubMed] [Google Scholar]
- 36.Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fenner L, Brinkhof MWG, Keiser O, et al. Early mortality and loss to follow-up in HIV-infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010;54:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


