Background: Many soluble pyrophosphatases contain two regulatory nucleotide-binding CBS domains with or without an intercalating DRTGG domain.
Results: Linear P1,Pn-diadenosine 5′-polyphosphates (ApnAs, n = 3–6) bind with nanomolar affinity to and activate DRTGG domain-containing pyrophosphatases; Ap3A binds cooperatively.
Conclusion: Nucleotide-regulated pyrophosphatases may represent receptors for ApnAs in bacteria.
Significance: The results suggest a novel regulatory pathway in some bacteria, involving ApnAs as messengers.
Keywords: allosteric regulation, bacterial signal transduction, calorimetry, cooperativity, enzyme kinetics, stress response, CBS domain, diadenosine polyphosphate, inorganic pyrophosphatase
Abstract
Among numerous proteins containing pairs of regulatory cystathionine β-synthase (CBS) domains, family II pyrophosphatases (CBS-PPases) are unique in that they generally contain an additional DRTGG domain between the CBS domains. Adenine nucleotides bind to the CBS domains in CBS-PPases in a positively cooperative manner, resulting in enzyme inhibition (AMP or ADP) or activation (ATP). Here we show that linear P1,Pn-diadenosine 5′-polyphosphates (ApnAs, where n is the number of phosphate residues) bind with nanomolar affinity to DRTGG domain-containing CBS-PPases of Desulfitobacterium hafniense, Clostridium novyi, and Clostridium perfringens and increase their activity up to 30-, 5-, and 7-fold, respectively. Ap4A, Ap5A, and Ap6A bound noncooperatively and with similarly high affinities to CBS-PPases, whereas Ap3A bound in a positively cooperative manner and with lower affinity, like mononucleotides. All ApnAs abolished kinetic cooperativity (non-Michaelian behavior) of CBS-PPases. The enthalpy change and binding stoichiometry, as determined by isothermal calorimetry, were ∼10 kcal/mol nucleotide and 1 mol/mol enzyme dimer for Ap4A and Ap5A but 5.5 kcal/mol and 2 mol/mol for Ap3A, AMP, ADP, and ATP, suggesting different binding modes for the two nucleotide groups. In contrast, Eggerthella lenta and Moorella thermoacetica CBS-PPases, which contain no DRTGG domain, were not affected by ApnAs and showed no enthalpy change, indicating the importance of the DTRGG domain for ApnA binding. These findings suggest that ApnAs can control CBS-PPase activity and hence affect pyrophosphate level and biosynthetic activity in bacteria.
Introduction
Diadenosine polyphosphates (ApnAs)3 are ubiquitous compounds in which two adenosine moieties are linked through ribose 5′-C by a chain of three to six phosphate residues. First discovered in 1965 as by-products of chemical ATP synthesis (1), ApnAs have subsequently been identified in organisms belonging to all kingdoms of life. Many enzymatic reactions leading to ApnAs are known (2), of which the reaction catalyzed by aminoacyl-tRNA synthetase, lysyl-tRNA synthetase in particular (3), is the best known. Escherichia coli lysyl-tRNA synthetase produces ApnAs by a side reaction during lysyl-tRNA synthesis via attack of the terminal phosphate group of ATP and other monoadenosine phosphates on the enzyme-bound aminoacyl adenylate intermediate (4). Because ATP prevails in cells, the product of its reaction with aminoacyl adenylate, Ap4A, is the most prevalent ApnA. Lysyl-tRNA synthetase can additionally convert Ap4A to Ap3A (5). ApnAs are degraded in the cell by specific and nonspecific enzymes, including Ap4A hydrolase and phosphodiesterase (6, 7), which balance the intracellular concentration of ApnAs at a submicromolar level. However, their concentrations in prokaryotes can rise up to 300 μm under stress conditions (8).
Because of its association with stress, AP4A was originally classified as an intracellular “alarmone” (8–10). An alternative view is that AP4A formation represents a compensatory mechanism that helps to sustain basic physiology during stress and assist in the return to normal physiology in bacteria (11). In eukaryotes, ApnAs may have a second messenger role (12). Regardless of which theory is true, it is clear that ApnAs participate, in some as yet poorly understood ways, in a number of cellular phenomena associated with stress, such as DNA replication and repair (13) and cell division (14). In eukaryotes, ApnAs are involved in many other processes, including neurotransmission (15), apoptosis (16), and analgesia (17). Of note, Ap4A is used in hypoxia therapy in humans (18).
Understanding the roles of ApnAs requires knowledge of their target proteins. Using a radioactive photocrosslinking Ap4A analog, Johnstone and Farr (19) detected 12 Ap4A-binding proteins in E. coli extract, some of which were identified as heat shock proteins based on their electrophoretic mobilities. Guo et al. (20) and Azhar et al. (21) used pulldown assays with immobilized Ap4A analogs followed by mass-spectral analysis to identify, respectively, 6 and 13 binding proteins in E. coli. The three protein sets obtained in these studies partially overlapped. Few ApnA protein complexes have been subjected to biophysical and mechanistic studies. Apart from cases where ApnAs act as substrates or products of their metabolizing enzymes, the chaperone GroEL binds Ap4A with a dissociation constant of 10 μm; the complex exhibits increased ATPase and chaperoning activities (11). Human 5′-nucleotidase II is allosterically activated by ApnAs (n = 4–6), which bind with dissociation constants of 60–80 μm (22).
Inorganic pyrophosphatases (PPases; EC 3.6.1.1), the major PPi-metabolizing enzymes in all types of organisms, belong to three nonhomologous families (23). Family II PPases, found in bacteria and archaea, are homodimeric Mn2+- or Co2+-metalloenzymes that additionally require Mg2+ for catalysis (24). A quarter of the more than 500 putative family II PPase sequences contain a regulatory insert comprising a pair of cystathionine β-synthase (CBS) domains (Bateman module (25)) within one of the two catalytic domains. Regulatory CBS domains are found in proteins in all kingdoms of life and generally bind adenine nucleotides as regulating molecules (26–28); mutations in CBS domains of human proteins are associated with hereditary diseases (29, 30). Interestingly, only in CBS-PPases (but not all of them), are the CBS domains intercalated by another (DRTGG) domain. CBS-PPases are activated by ATP and inhibited by AMP and ADP (31, 32). Both catalysis and regulation involve marked positive cooperativity, which is Mg2+-dependent (32).
The structure of the isolated dimeric regulatory insert of Clostridium perfringens PPase (cpPPase) obtained for crystals grown in the presence of 0.25 mm Ap4A contains an AP4A molecule bound by two CBS domain pairs at the subunit interface (33), raising the possibility that Ap4A may be a physiological ligand of CBS-PPases. Preliminary activity measurements (33, 34) suggested that Ap4A activates cpPPase. Here we show that all ApnAs bind with nanomolar affinities to three DRTGG domain-containing CBS-PPases and modulate their catalytic activity and cooperative behavior. Our data thus identify a new type of ligand for CBS domains and an important target of ApnAs in the protein world.
Experimental Procedures
Enzymes and Reagents
Genes for CBS-PPases from Desulfitobacterium hafniense (dhPPase), Clostridium novyi (cnPPase), C. perfringens (cpPPase), Eggerthella lenta (elPPase), and Moorella thermoacetica (mtPPase) were expressed in E. coli, and the produced CBS-PPases were purified as described previously (32–34). Inactive aggregates were separated from soluble active proteins during size exclusion chromatography. The final products were at least 95% pure as estimated by SDS-PAGE using a Phast system with 8–25% gradient gels (GE Healthcare). Protein concentrations were determined with a Nanodrop spectrophotometer (Thermo Scientific) using A2800.1% of 0.478 for dhPPase, 0.548 for cnPPase, 0.426 for cpPPase, 0.493 for elPPase, and 0.48 for mtPPase, as calculated from their amino acid compositions with ProtParam. Molar concentrations were calculated based on subunit molecular masses of 60.4, 63.6, 60.8, 52.5, and 48.1 kDa, respectively. All enzyme concentrations are given in terms of the dimer.
P1,Pn-diadenosine 5′-polyphosphates (ApnAs) with n = 3–5 were from Sigma; Ap6A was from Jena Bioscience. All ApnAs were ≥97% pure, and Ap3A was essentially free of other ApnAs, according to the manufacturer analyses (HPLC). The concentrations of stock nucleotide solutions were calibrated by measuring absorbance in the ultraviolet region (ϵ259 = 31,800 m−1·cm−1 for dinucleotides and 15,900 m−1·cm−1 for mononucleotides).
Kinetic Assays
The activity assay medium contained 5 mm MgCl2, 140 μm PPi (yielding 50 μm MgPPi complex) and 0.1 m TES-KOH (pH 7.2), except where specified otherwise. In measurements done at higher Mg2+ concentrations, buffer concentration was decreased appropriately to maintain constant ionic strength. The reaction was initiated by adding enzyme, and Pi accumulation caused by PPi hydrolysis was continuously recorded for 2–3 min at 25 °C using an automated Pi analyzer (35). Initial velocities of PPi hydrolysis were typically estimated graphically from the slopes of the tangents to the initial portion of hydrolysis time courses recorded with the Pi analyzer.
Isothermal Calorimetry
A VP-iTC calorimeter (MicroCal Ltd.) was used. Enzyme and nucleotide solutions were made in 0.1 m MOPS/KOH (pH 7.2) buffer containing 2 mm MgCl2, 0.1 mm CoCl2, and 150 mm KCl. Titrations were performed at 25 °C by successive 10-μl injections of 0.1–10 mm mononucleotide or 33 μm dinucleotide solution into 2 ml of CBS-PPase solution (2.5–5 μm in terms of the dimer); the interval between injections was 5 min. All samples were degassed before the experiment. Binding isotherms were corrected by subtracting the ligand dilution isotherms, determined by titrating nucleotide solutions into the buffer.
Calculations and Data Analysis
The values of the apparent dissociation constants for the magnesium complexes of PPi used to maintain required free Mg2+ ion and MgPPi complex concentrations at pH 7.2 were 112 μm for MgPPi and 2.84 mm for Mg2PPi (36). Nonlinear least square fittings were performed using the program Scientist (Micromath). The dependence of hydrolysis rate on nucleotide concentration ([N]) was fit to Equation 1,
 |
where v0 and vN are activities of free and nucleotide-saturated enzyme, respectively, and KN1 and KN2 are the macroscopic dissociation constants describing successive binding of nucleotide to two regulatory sites per enzyme molecule. Cooperative kinetics of substrate (MgPPi) hydrolysis were analyzed with Equation 2,
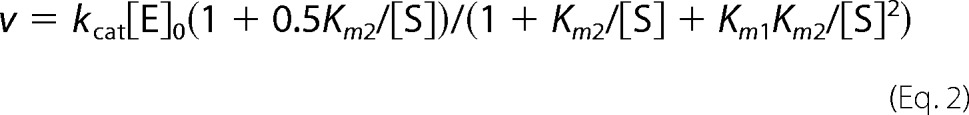 |
which assumes different Michaelis constants (Km1 and Km2) and equal kcat values for the two active sites in the dimer. [E]0 and [S] are total enzyme and substrate concentrations, respectively. The corresponding binding schemes and details of the fitting procedure were described previously (32).
The dependences of KN1, KN2, Km1, and Km2 on Mg2+ (M) concentration were fit to Equation 3,
where (KL)0 and (KL)M are the limiting values of the respective KN or Km at 0 and infinite Mg2+ concentrations, and Km is the metal binding constant.
Alternatively, rate dependences on substrate and nucleotide concentrations were fit to a Hill-type Equation 4,
where L is S or N, vL is the rate at infinite [L], and h is the Hill coefficient. The value of v0 was set to 0 when L was substrate, and the value of h was set to unity for noncooperative binding.
Isothermal titration calorimetry (ITC) data were analyzed with a MicroCal ITC subroutine in Origin 7.0 software using a single-binding site model. Thermodynamic parameters were calculated from the standard relationship, ΔG = RTlnKN = ΔH − TΔS.
Results
Effects of ApnAs on CBS-PPases at a Fixed Mg2+ Concentration
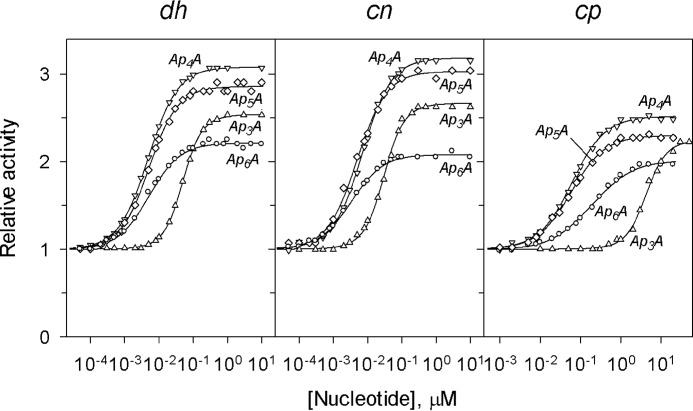
Fig. 1 shows the concentration dependences of the effects of four ApnAs with n = 3–6 on the activities of three CBS-PPases measured at fixed substrate (MgPPi) and Mg2+ concentrations (50 μm and 5 mm, respectively). Nanomolar concentrations of ApnAs caused marked activation in all cases, except that Ap3A was effective with cpPPase at micromolar concentrations.
FIGURE 1.
Concentration dependences of the effects of ApnAs on the activity of three CBS-PPases measured at fixed concentrations of substrate (50 μm MgPPi) and Mg2+ (5 mm). The lines show the best fits of Equations 1 or 4 (see text for details). Activity without nucleotides (220, 350, and 800 s−1 for dhPPase, cnPPase and cpPPase, respectively) was taken as unity. dh, dhPPase; cn, cnPPase; cp, cpPPase.
Analyses of the dependences shown in Fig. 1 and of similar dependences measured at different substrate concentrations (1 and 300 μm) were initially done using Equation 4. The value of the Hill coefficient was indistinguishable from unity (1 ± 0.05) at all substrate concentrations for ApnAs with n = 4–6. In contrast, Ap3A bound cooperatively (h = 1.4–1.7) at all substrate concentrations. Accordingly, the data for Ap3A were analyzed with Equations 1 and 4 in their general forms, whereas Equation 4 with h = 1 was used for the other ApnAs. The parameter values derived from this analysis are summarized in Tables 1 and 2.
TABLE 1.
Kinetic parameters for activation of three CBS-PPases by Ap3A in the presence of 5 mm Mg2+
| Enzyme | [MgPPi] | vN/v0a | KN1 | KN2 | 4KN1/KN2 | h | |
|---|---|---|---|---|---|---|---|
| μm | nm | ||||||
| dhPPase | 1 | 32 ± 6 | 460 ± 80 | 100 ± 20 | 213 ± 5 | 18 ± 7 | 1.60 ± 0.05 |
| 50 | 2.54 ± 0.01 | 82 ± 6 | 30 ± 2 | 50.0 ± 0.4 | 10.8 ± 1.4 | 1.51 ± 0.02 | |
| 300 | 1.78 ± 0.03 | 12 ± 4 | 4 ± 1 | 7.1 ± 0.4 | 12 ± 8 | 1.50 ± 0.10 | |
| cnPPase | 1 | 5.1 ± 0.1 | 19 ± 1 | 12 ± 1 | 14.8 ± 0.3 | 7 ± 1 | 1.42 ± 0.04 |
| 50 | 2.67 ± 0.05 | 41 ± 8 | 22 ± 4 | 30 ± 1 | 7 ± 3 | 1.43 ± 0.07 | |
| 300 | 1.77 ± 0.01 | 55 ± 9 | 22 ± 4 | 35.2 ± 0.9 | 6.3 ± 1.6 | 1.40 ± 0.04 | |
| cpPPase | 1 | 6.8 ± 0.1 | 26,000 ± 4,000 | 3,400 ± 600 | 9,000 ± 300 | 31 ± 10 | 1.66 ± 0.06 |
| 50 | 2.25 ± 0.05 | 12,000 ± 5,000 | 1,500 ± 700 | 4,200 ± 200 | 31 ± 29 | 1.69 ± 0.12 | |
| 300 | 2.24 ± 0.02 | 1,800 ± 0.2 | 700 ± 100 | 1,130 ± 30 | 10 ± 3 | 1.50 ± 0.05 |
a vN and v0 are activities extrapolated to infinite concentration of the variable nucleotide and measured in the absence of any nucleotide, respectively.
TABLE 2.
Kinetic parameters for activation of three CBS-PPases by diadenosine polyphosphates with n = 4–6 in the presence of 5 mm Mg2+
The value of the Hill coefficient was indistinguishable from unity in all cases.
| Enzyme/dinucleotide | [MgPPi] | vN/v0a | KNb |
|---|---|---|---|
| μm | nm | ||
| dhPPase | |||
| Ap4A | 1 | 18 ± 1 | 12.1 ± 0.3 |
| 50 | 3.0 ± 0.1 | 4.9 ± 0.2 | |
| 300 | 1.91 ± 0.02 | 4.3 ± 0.2 | |
| Ap5A | 50 | 3.32 ± 0.06 | 5.5 ± 0.2 |
| Ap6A | 50 | 2.58 ± 0.06 | 4.4 ± 0.3 |
| cnPPase | |||
| Ap4A | 1 | 6.0 ± 0.3 | 3.9 ± 0.1 |
| 50 | 3.14 ± 0.05 | 7.0 ± 0.2 | |
| 300 | 1.51 ± 0.01 | 16.5 ± 0.7 | |
| Ap5A | 50 | 3.03 ± 0.08 | 4.9 ± 0.3 |
| Ap6A | 50 | 2.08 ± 0.03 | 3.3 ± 0.2 |
| cpPPase | |||
| Ap4A | 1 | 14.7 ± 0.4 | 293 ± 5 |
| 50 | 2.52 ± 0.03 | 62 ± 2 | |
| 300 | 2.04 ± 0.02 | 33 ± 1 | |
| Ap5A | 50 | 2.30 ± 0.03 | 58 ± 2 |
| Ap6A | 50 | 2.04 ± 0.02 | 187 ± 9 |
a vN and v0 are activities extrapolated to infinite concentration of the variable nucleotide and measured in the absence of any nucleotide, respectively.
bThis parameter is equivalent to in Table 1.
The values of the activation factor (vN/v0) and their trends with changing polyphosphate length and substrate concentration were similar for the three enzymes. The value of vN/v0 was greater at low than at high substrate concentrations. In the presence of 300 μm substrate, which is in excess of the respective Michaelis constants (32), vN/v0 approached a value of ∼2 in all cases.
The apparent binding affinities of the nucleotides could be compared on the basis of the average binding constant () for Ap3A and respective KN values for the other dinucleotides. As Tables 1 and 2 make clear, the binding affinity estimated at 50 μm substrate was markedly lower for Ap3A compared with other dinucleotides for all CBS-PPases. Increasing n did not affect dhPPase affinity, but it did slightly increase cnPPase affinity and decrease cpPPase affinity. Increasing substrate concentration had opposite effects on the affinity of Ap3A and Ap4A for dhPPase and cpPPase (increased) and cnPPase (decreased). Of note, cpPPase exhibited much lower affinity for all dinucleotides compared with other CBS-PPases.
Surprisingly, neither dinucleotide at a concentration up to 10 μm affected activities of elPPase or mtPPase measured with 50 μm substrate. These CBS-PPases differ from those described above by having no DRTGG domain in their regulatory regions, which are formed by only two CBS domains. Moreover, 10 μm Ap4A did not affect the concentration dependence of ADP inhibition of elPPase or mtPPase (data not shown), indicating that the dinucleotide is unable to interact with the ADP-binding site.
Dependence of CBS-PPase Activation on Mg2+ Concentration
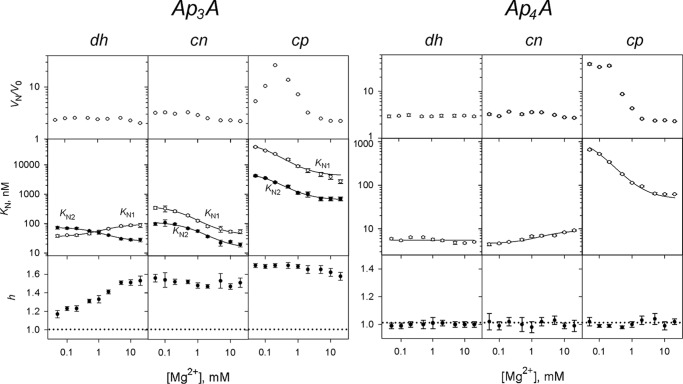
Given that cooperativity in CBS-PPases is Mg2+-dependent (32), measurements analogous to those illustrated in Fig. 1 were conducted for two representative dinucleotides, Ap3A and Ap4A, over a 0.05–20 mm Mg2+ concentration range; substrate concentration was fixed at 50 μm. The results of these experiments (Fig. 2) indicated that Ap3A bound with positive cooperativity and Ap4A bound noncooperatively to all CBS-PPases at all Mg2+ concentrations. In only one case (dhPPase with Ap3A), the degree of cooperativity, as characterized by the values of h and the ratio KN2/KN1, showed a pronounced dependence on [Mg2+] because of the opposite effects of Mg2+ on KN1 and KN2 (Fig. 2). In all other cases, KN1 and KN2 changed in the same direction to approximately the same degree and, consequently, without a marked effect on cooperativity. Of note, the ratio KN2/KN1 equals 4 in the case of noncooperative binding and is less than 4 for positively cooperative binding (37).
FIGURE 2.
Mg2+ concentration dependence of CBS-PPase activation by Ap3A (left panel) and Ap4A (right panel). The panels show (from top to bottom) the activation factor KN1 (○) and KN2 (●) values and Hill coefficients. The KN1 and KN2 lines show the best fits to Equation 3. The horizontal dotted lines (h = 1) mark the boundary between positive and negative cooperativity. dh, dhPPase; cn, cnPPase; cp, cpPPase.
In most cases (except for dhPPase with Ap4A), Mg2+ modulated dinucleotide binding, with the direction of the effect depending on both the nature of the nucleotide and the CBS-PPase origin (Figs. 2 and 3). Mg2+ generally stimulated Ap3A binding, except for dhPPase, where it exerted the opposite effect on KN1 (Fig. 2). Mg2+ exhibited a full range of effects on Ap4A binding (Fig. 2): stimulation (cpPPase), suppression (cnPPase), and no effect (dhPPase). The effect of Mg2+ on dinucleotide binding could be described by Equation 3, yielding the parameter values summarized in Table 3. The values of Km governing the Mg2+ effects were in the millimolar range and were similar for both steps of Ap3A binding and Ap4A binding for a given CBS-PPase.
FIGURE 3.
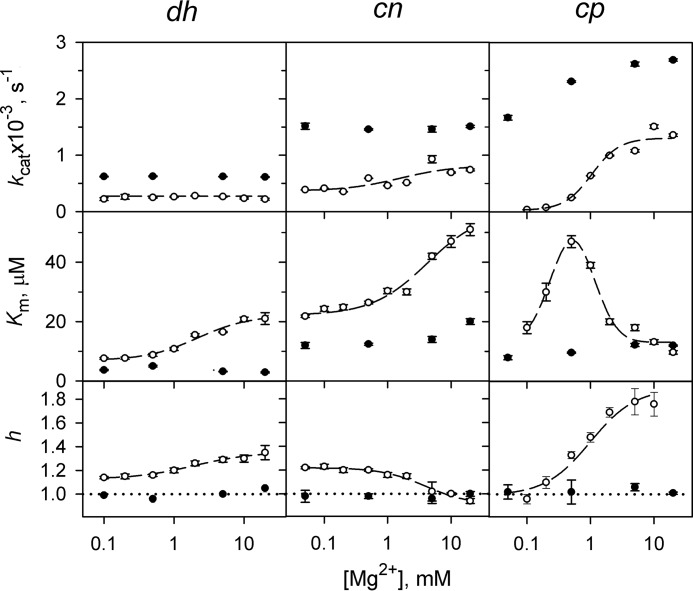
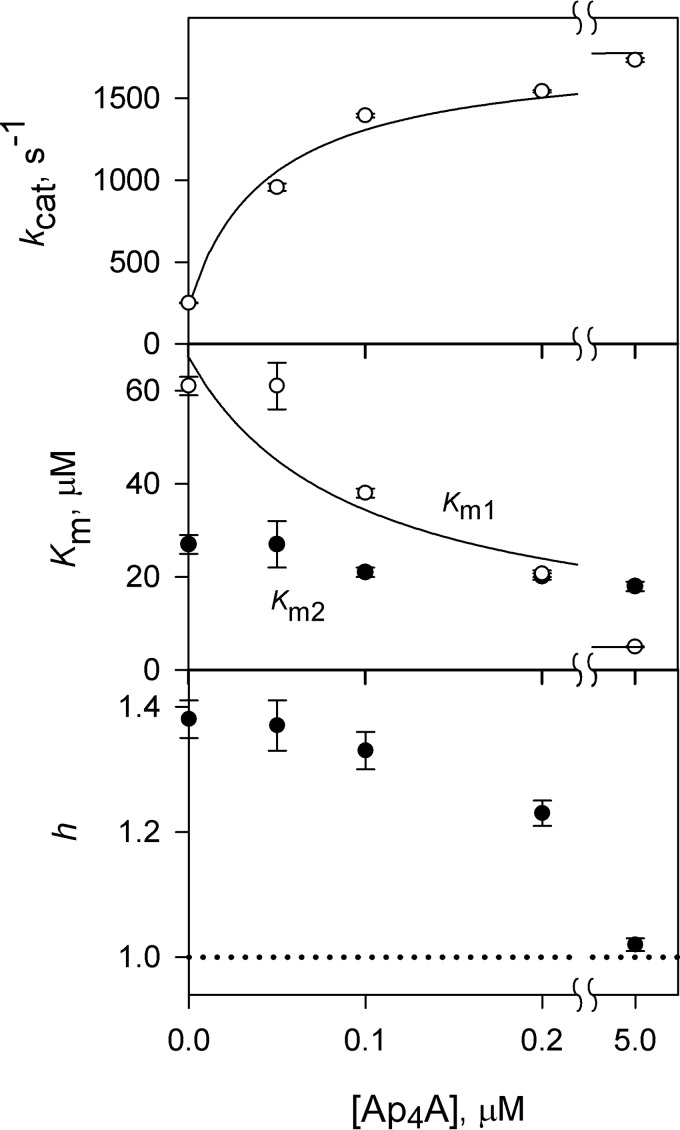
Lack of kinetic cooperativity in CBS-PPases in the presence of 10 μm Ap4A. The panels show (from top to bottom) the catalytic constant kcat, the Michaelis constant Km, and the Hill coefficient h. The dashed lines and corresponding points refer to the early estimated parameter values in the absence of Ap4A (32); Km values refer to the average Michaelis constants () in this case. The horizontal dotted lines (h = 1) mark the boundary between positive and negative cooperativity. Km values are measured in terms of the MgPPi complex. dh, dhPPase; cn, cnPPase; cp, cpPPase.
TABLE 3.
Kinetic parameters for nucleotide activation derived from the Mg2+ dependencies of KN1 and KN2 for Ap3A or KN for Ap4A (Fig. 2)
| Enzyme | Parameter value |
Km | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ap3A |
Ap4A |
|||||||||
|
KN1 dependence |
KN2 dependence |
KN,0 | KN,M | |||||||
| KN1,0 | KN1,M | Km | KN2,0 | KN2,M | Km | |||||
| nm | nm | mm | nm | nm | mm | nm | nm | mm | ||
| dhPPase | 35 ± 2 | 96 ± 3 | 0.7 ± 0.3 | 75 ± 2 | 24 ± 1 | 1.5 ± 0.4 | 5.5 ± 0.5 | 5.5 ± 0.5 | NAa | |
| cnPPase | 390 ± 10 | 41 ± 6 | 3.2 ± 0.7 | 110 ± 10 | 14 ± 4 | 5 ± 3 | 4.3 ± 0.3 | 8.6 ± 0.5 | 0.8 ± 0.3 | |
| cpPPase | 59,000 ± 5,000 | 4,200 ± 700 | 1.4 ± 0.4 | 5,600 ± 200 | 640 ± 50 | 1.1 ± 0.2 | 1,200 ± 200 | 55 ± 3 | 1.4 ± 0.2 | |
aNA, not attendant.
The degree of activation (vN/v0) of dhPPase and cnPPase by Ap3A and Ap4A demonstrated no or only small variations with Mg2+ concentration (Fig. 2). In contrast, activation of cpPPase showed a bell-shaped dependence (Ap3A) or markedly decreased (Ap4A) with increasing Mg2+ concentration.
Analysis of CBS-PPase Activation in Terms of Michaelis-Menten Parameters
As previously reported, the rate of MgPPi hydrolysis by CBS-PPases does not obey Michaelis-Menten kinetics, requiring the use of a more complex equation with two Michaelis constants (32). Their ratio, Km2/Km1, was less than 4, and the Hill coefficient was greater than 1, indicating positive kinetic cooperativity.
Surprisingly, Ap3A and Ap4A completely abolished or markedly suppressed the kinetic cooperativity in dhPPase, cnPPase and cpPPase, as indicated by a Hill coefficient with a value close to 1 (Table 4 and Fig. 3). That the h value is greater than 1 for cpPPase in the presence of Ap3A may reflect incomplete saturation of this enzyme by the dinucleotide, which binds much more weakly to cpPPase compared with the other CBS-PPases, especially at low substrate concentrations (Table 1).
TABLE 4.
Kinetic parameters for PPi hydrolysis in the presence of 50 μm Ap3A and 5 mm Mg2+ estimated with Equation 2
The values in parentheses refer to parameter values previously measured in the absence of Ap3A (32).
| Enzyme | kcat | Km1 | Km2 | 4Km1/Km2 | h | |
|---|---|---|---|---|---|---|
| s−1 | μm | |||||
| dhPPase | 565 ± 4 (350) | 1.47 ± 0.03 (26) | 5.8 ± 0.3 (10) | 2.9 ± 0.1 (20) | 1.01 ± 0.07 | 1.00 ± 0.02 (1.29) |
| cnPPase | 1,120 ± 20 (540) | 5.0 ± 0.4 (23) | 18 ± 3 (80) | 9.5 ± 0.6 (44) | 1.1 ± 0.2 | 1.02 ± 0.04 (1.02) |
| cpPPase | 3,090 ± 20 (1,080) | 13 ± 1 (80) | 16 ± 1 (4) | 14.5 ± 0.4 (18) | 3.2 ± 0.3 | 1.23 ± 0.02 (1.8) |
The kinetics of activation by Ap4A was investigated over a range of Mg2+ concentrations (Fig. 3). The results showed that 10 μm activator increased kcat, decreased the Michaelis constant, and abolished kinetic cooperativity. Again the largest effects were observed with cpPPase, which was therefore explored in greater detail.
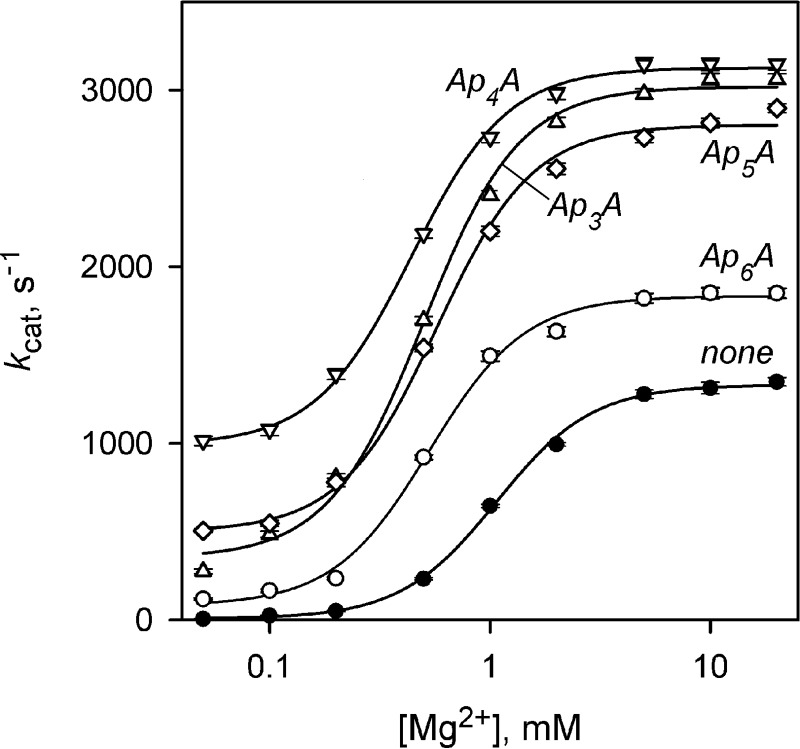
The effects of four ApnAs on the Mg2+ concentration dependence of kcat for cpPPase were qualitatively similar (Fig. 4). Mg2+ induced a transition from low to high activity over a narrow range of concentrations, requiring a term with [Mg2+]2 in the corresponding equation (see Fig. 3 legend) (32). All four activators increased the limiting value of kcat at infinite [Mg2+] (kcat,M) and decreased the Mg2+ binding constant (Km) ∼2-fold (Table 5). Most surprisingly, ApnA binding conferred catalytic activity to the otherwise inactive cpPPase at low [Mg2+] (see kcat,0 values in Table 5). The activity of Ap4A-activated cpPPase in these conditions approached its maximum activity observed at high [Mg2+] in the absence of Ap4A (Fig. 4).
FIGURE 4.
Mg2+ concentration dependence of kcat for cpPPase measured in the presence of 50 μm Ap3A or 10 μm Ap4A or Ap5A. The values of kcat were fit to the equation kcat = kcat,0 + (kcat,M − kcat,0)/{1 + (Km/[M])2}, where kcat,0 and kcat,M are the limiting values of kcat at 0 and infinite Mg2+ concentrations, respectively, and Km is the metal binding constant.
TABLE 5.
Kinetic parameters describing effects of Mg2+ on kcat for cpPPase in the presence of diadenosine polyphosphates
| ApnA | kcat,0 | kcat,m | Km |
|---|---|---|---|
| s−1 | s−1 | mm | |
| None | 6 ± 13 | 1,330 ± 20 | 1.07 ± 0.03 |
| Ap3A (50 μm) | 350 ± 50 | 3,020 ± 40 | 0.50 ± 0.03 |
| Ap4A (10 μm) | 990 ± 30 | 3,130 ± 20 | 0.44 ± 0.02 |
| Ap5A (10 μm) | 500 ± 40 | 2,800 ± 30 | 0.57 ± 0.03 |
| AP6A (10 μm) | 80 ± 30 | 1,830 ± 30 | 0.53 ± 0.03 |
Fig. 5 illustrates the concentration dependence of cpPPase activation by Ap4A in the presence of 0.5 mm Mg2+, analyzed in terms of kcat and Km values. The value of kcat increased ∼7.5-fold (from 240 ± 100 to 1800 ± 100 s−1), Km1 decreased ∼18-fold (from 70 ± 10 to 4 ± 1 μm), and Km2 changed insignificantly with increasing Ap4A concentration from 0 to 5 μm. The Ap4A binding constant estimated from kcat and Km1 dependences was 0.04 ± 0.01 and 1.7 ± 1.0 μm, respectively. Because kcat and Km dependences report on Ap4A binding to substrate-free enzyme and enzyme-substrate complex, respectively, a likely implication is that Ap4A and the first bound substrate molecule mutually stabilize binding of each other to cpPPase 20–40-fold.
FIGURE 5.
Ap4A concentration dependence of kinetic cooperativity in cpPPase in the presence of 0.5 mm Mg2+. Notations are as in Fig. 4. The values of kcat were fit to the equation kcat = kcat,0 + (kcat,N − kcat,0)/(1 + KN/[M]), where kcat,0 and kcat,M are the limiting values of kcat at 0 and infinite Ap4A concentrations, respectively, and KN is the nucleotide binding constant. The line for Km1 shows the best fit to Equation 3.
Thermodynamics and Stoichiometry of Nucleotide Binding CBS-PPases
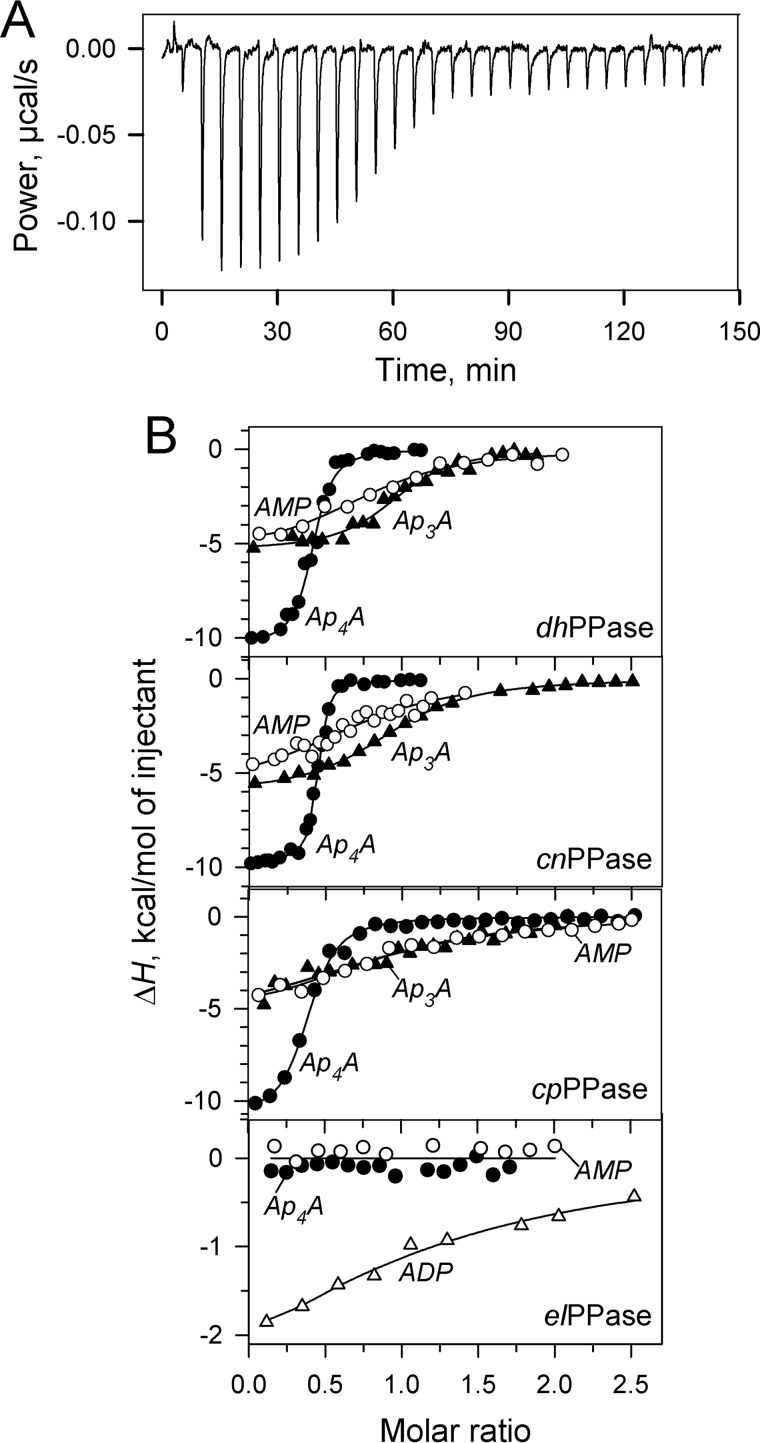
Using ITC allowed the direct measurement of changes in free energy (ΔG), enthalpy (ΔH), and entropic free energy (TΔS) components of nucleotide binding to CBS-PPases. A typical titration profile is shown in Fig. 6A. The results of similar titrations performed with different CBS-PPases and nucleotides are summarized in Fig. 6B and Table 6.
FIGURE 6.
ITC measurements of nucleotide binding to CBS-PPases. A, typical raw data for successive injections of Ap4A into dhPPase solution. B, integrated heats for titration of four CBS-PPases by selected mono- and dinucleotides after correction for dilution. Enzyme dimer concentration was 4 μm (dhPPase), 5 μm (cnPPase), 3.5 μm (cpPPase), or 2.5 μm (elPPase). The lines show the best fits for a single-binding site model.
TABLE 6.
Thermodynamic parameters for nucleotide complexes of CBS-PPases obtained by isothermal calorimetry
| Enzyme/nucleotide | KN | n | ΔH | −TΔS |
|---|---|---|---|---|
| μm | kcal/mol | kcal/mol | ||
| dhPPase | ||||
| AMP | 0.8 ± 0.3 | 0.79 ± 0.05 | −5.6 ± 0.5 | −2.7 ± 0.6 |
| ADP | 1.0 ± 0.2 | 0.85 ± 0.04 | −5.9 ± 0.4 | −2.4 ± 0.5 |
| ATP | 1.2 ± 0.1 | 0.80 ± 0.02 | −5.8 ± 0.2 | −2.4 ± 0.2 |
| Ap3A | 0.12 ± 0.05 | 0.97 ± 0.02 | −5.3 ± 0.2 | −4.0 ± 0.3 |
| Ap4A | 0.41 ± 0.01 | −10.4 ± 0.3 | ||
| Ap5A | 0.41 ± 0.01 | −10.3 ± 0.3 | ||
| cnPPase | ||||
| AMP | 0.9 ± 0.4 | 0.80 ± 0.08 | −5.9 ± 0.9 | −2.4 ± 1.0 |
| ADP | 5 ± 1 | 0.91 ± 0.08 | −3.0 ± 0.6 | −4.1 ± 1.0 |
| ATP | 0.6 ± 0.2 | 0.89 ± 0.07 | −5.8 ± 0.7 | −2.8 ± 1.1 |
| Ap3A | 0.94 ± 0.01 | −6.1 ± 0.1 | ||
| Ap4A | 0.44 ± 0.01 | −9.7 ± 0.3 | ||
| cpPPase | ||||
| AMP | 0.97 ± 0.03 | 0.87 ± 0.06 | −6.0 ± 0.6 | −2.3 ± 0.7 |
| ADP | 2.6 ± 0.5 | 0.94 ± 0.09 | −6.0 ± 0.8 | −1.6 ± 0.3 |
| ATP | 0.23 ± 0.06 | 1.00 ± 0.03 | −5.6 ± 0.3 | −3.6 ± 0.9 |
| Ap3A | 0.7 ± 0.3 | 0.97 ± 0.11 | −5.7 ± 1.1 | −2.7 ± 1.2 |
| Ap4A | 0.42 ± 0.01 | −10.4 ± 0.3 | ||
| elPPase | ||||
| AMP | ≤0.1 | |||
| ADP | 9 ± 4 | 0.95 ± 0.1 | −4.5 ± 1.6 | −2.4 ± 2.0 |
| ATP | 5 ± 1 | 1.0 ± 0.4 | −8 ± 4 | −0.5 ± 0.3 |
| Ap3A | ≤0.1 | |||
| Ap4A | ≤0.1 | |||
One important result was that titrations of the DRTGG domain-lacking elPPase or mtPPase with up to 10 μm Ap4A or Ap3A produced no ITC signal, consistent with the inability of the dinucleotides to activate these CBS-PPases and modulate their inhibition by ADP. Because the lack of effect on activity did not rule out the possibility of a “silent” binding, the ITC data, which report on a different aspect of the binding reaction, provided an important support for the lack of complex formation between the DRTGG domain-lacking CBS-PPases and ApnAs. This interpretation was supported by parallel measurements employing AMP, ADP, and ATP (Table 6), which produced similar enthalpy changes in the cases, where previous measurements (32) revealed effects on activity, but no or reduced enthalpy change (elPPase with AMP and cnPPase with ADP, respectively), where no effect on activity was observed (32). Together, these findings suggest that modulation of activity and heat production are coupled phenomena and that the DRTGG domain is required for tight binding of diadenosine polyphosphates, but not monoadenosine phosphates, to CBS-PPases. The inability of elPPase to bind AMP is not associated with the absence of the DRTGG domain because another DRTGG domain-lacking CBS-PPase, mtPPase, is inhibited by AMP and hence binds it (31).
Another important finding was that ΔH, as calculated per mole of nucleotide, was nearly two times greater for Ap4A and Ap5A than for Ap3A and the mononucleotides in the titrations with the DRTGG domain-containing CBS-PPases. This effect correlated with a 2-fold lower binding stoichiometry for Ap4A and Ap5A compared with that for mononucleotides and Ap3A.
Because of the very tight binding, KN and, accordingly, TΔS values could not be estimated with adequate precision in most ApnA titrations. Where KN (and hence ΔG) values were available, the free energy change of nucleotide binding was dominated by ΔH, with a significant contribution from TΔS, likely because of a hydrophobic effect. The KN values derived from ITC measurements are in a fair agreement with those obtained from nucleotide effects on activity (see Ref. 32 for mononucleotides and Table 1 for Ap3A). It should be noted that ITC measurements can hardly distinguish positive binding cooperativity and yield an average ΔH value for all binding sites.
Discussion
CBS domains, found in many proteins, are known for their ability to bind adenine nucleotides and in this way regulate activities of their carrier proteins. The list of regulating adenine nucleotides includes AMP, ADP, ATP, S-adenosyl methionine, NADH, and analogs of AMP and ATP (27). Examples of less common CBS domain ligands include Mg2+ (38), DNA, and RNA (39, 40). We earlier reported that crystals of the isolated dimeric regulatory region of cpPPase grown in the presence of Ap4A contains one Ap4A molecule per dimer bridging two pairs of CBS domains, whereas each CBS domain pair binds an AMP molecule (33). We also found that Ap4A induces a significant opening of the interface compared with the AMP-bound form. The results reported above extend these earlier findings by showing that (a) ApnAs with n = 3–6 bind three CBS-PPases with nanomolar affinity and activate them in vitro; (b) ApnA binding is only observed in CBS-PPases that have an intercalating DRTGG domain in the regulatory region; and (c) unlike common adenine nucleotides, long chain ApnAs (n > 3) abolish or markedly reduce kinetic cooperativity (non-Michaelian behavior) in CBS-PPases. The unique features of ApnA complexes of CBS-PPases compared with those of their complexes with mononucleotides and complexes of other CBS proteins with their regulating ligands are described below. Notably, ApnAs have not been reported as ligands for any other CBS protein.
Based on their binding properties, ApnAs can be divided into two groups. Ap3A bound to CBS-PPases cooperatively and with lower affinity, as characterized by either KN1 and KN2 or their average value ) (Table 1). The other dinucleotides (n = 4–6) bound noncooperatively and with a higher affinity that did not depend significantly on the n value (Table 2). The affinities of ApnAs with n = 4–6 for CBS-PPases surpassed that of adenine mononucleotides (32) by 2–3 orders of magnitude. Such high affinities are unprecedented among other CBS proteins, which generally bind their nucleotide ligands in the millimolar range. The difference in the binding affinities of the two ApnA groups was most pronounced with cpPPase, amounting to 3 orders of magnitude. As previously demonstrated (33), Ap4A interacts through both of its adenine moieties with two CBS domain pairs of different subunits in cpPPase. Such an arrangement is also likely with Ap5A and Ap6A, consistent with their similar ΔH values and binding stoichiometries, determined from ITC measurements (Table 6). In contrast, ΔH for Ap3A was half that of Ap5A and Ap6A, and the binding stoichiometry was 2-fold higher, similar to values for mononucleotides (Table 6). These observations likely indicate that Ap3A predominantly binds CBS-PPases through only one adenine moiety.
The binding affinities of ApnAs showed a complex dependence on substrate and metal cofactor concentrations. At a constant Mg2+ concentration, substrate increased the binding affinities of dhPPase and cpPPase for all ApnAs but exerted an opposite effect on cnPPase (Tables 1 and 2). Accordingly, Ap3A (Table 4) and Ap4A (Fig. 3) decreased the average Michaelis constant ( and Km). The effect of Ap3A on for cpPPase measured in the presence of 5 mm Mg2+ was quite modest, but keeping in mind the bell-shaped dependence of on [Mg2+] for this enzyme in the absence of adenine nucleotides (Fig. 3), one would expect, by analogy, greater effects of Ap3A at low [Mg2+].
However, the most striking effect of ApnAs on substrate binding was abolition of kinetic cooperativity. This effect was observed with both Ap3A and Ap4A, representing the two dinucleotide groups and might be explained by two different mechanisms. First, the effectors may disrupt the communication between active sites, allowing them to function independently. Alternatively, the dinucleotides may induce asymmetry in the enzyme dimer such that only one active site operates in the dimer (ultimate negative cooperativity). Determining the three-dimensional structure of the enzyme with bound dinucleotide would make it possible to discriminate between these alternative explanations.
Mg2+ effects on nucleotide binding also varied depending on the enzyme (Fig. 2) and differed from those observed with adenine mononucleotides (32). With Ap3A, values of KN1 and KN2 for dhPPase changed in different directions, decreasing the degree of cooperativity at low [Mg2+] (Fig. 2A). No bound Mg2+ ion was observed in the structure of the regulatory region of cpPPase (33), suggesting that the modulatory Mg2+ resides in the active site. Notable in this regard, three Mg2+ ions per active site participate in catalysis among homologous nonregulated family II PPases (24, 41). The effects of Mg2+ on nucleotide binding may, in part, be a consequence of its effects on substrate binding, because these measurements were carried out at a nonsaturating substrate concentration (50 μm).
Both Ap3A and Ap4A activated CBS-PPases under the conditions tested because of favorable changes in both kcat and the average Michaelis constant () (Table 4 and Fig. 3). Accordingly, the degree of activation was greater at low substrate concentrations (Table 1) and varied from severalfold to several ten-fold. The largest effects were observed with cpPPase. Based on its kcat and Km values (Fig. 3), this enzyme is predicted to be activated by Ap4A in the presence of 1 mm Mg2+ by a factor of ∼51 and ∼19 at substrate concentrations of 1 and 10 μm, respectively. At low [Mg2+], the activating effect of ApnA is dominated by kcat, especially with cpPPase (Fig. 4 and Table 5). In this enzyme, kcat is strongly Mg2+-dependent and ApnA markedly released this dependence by allowing catalysis in the enzyme with a vacant Mg2+ site and by somewhat increasing its affinity for Mg2+ (Table 5). In this respect, ApnAs partially substitute for Mg2+ as an enzyme activator.
Qualitatively similar activating effects on CBS-PPases were previously observed with ATP (31, 32), although ATP effects were smaller in size and required much higher effector concentrations. A further difference is that ATP bound cooperatively, like Ap3A. The effects of ATP and Ap3A are thus similar in many aspects. As noted above, activator binding induces significant opening of the CBS domain interface (33). Such opening can be achieved upon binding of a single molecule of Ap4A or a longer dinucleotide that binds to both subunits of CBS-PPase through two adenine moieties. Structure modeling of the cpPPase regulatory region indicated that the polyphosphate chain of Ap3A is too short for this binding mode. In this case, and with ATP, interface opening apparently results from repulsion between two molecules of the effector bound to different subunits.
The requirement for an intercalating DRTGG domain for ApnA binding to CBS domains provides another interpretive challenge. In the structure of the regulatory region and the modeled structure of the whole cpPPase, both the DRTGG domain and CBS domain pairs participate in forming the subunit interface (33). DRTGG domain-containing CBS-PPases apparently have a larger binding cavity for the regulating ligands or increased flexibility of the CBS domains at the expense of their smaller contribution to the subunit contact area, allowing them to accommodate more bulky ApnA molecules. This interpretation is supported by data showing that the DRTGG domain-deficient elPPase (32) and mtPPase (31) bind ATP with an affinity 1–2 orders of magnitude lower than that of the less bulky AMP and ADP. In contrast, no such discrimination is observed in DRTGG domain-containing CBS-PPases (32). Notably, the primary structures of the CBS domains in DRTGG domain-deficient CBS-PPases (Fig. 7) do not contain specific mutations that would disallow their binding of ApnAs. Despite a generally low degree of residue conservation in CBS domains, all residues involved in nucleotide binding are found in at least one of the DRTGG domain-deficient CBS-PPases. Based on these considerations, ApnAs are not expected to bind with comparable affinity to the numerous other CBS proteins that lack a DRTGG or other intercalating domain.
FIGURE 7.
Aligned amino acid sequences of the two CBS domains of the characterized CBS-PPases. Amino acid residues making contacts with Ap4A or AMP in the crystal structures of cpPPase (33) are shown in boxes. Consensus residues based on 180 CBS-PPase sequences are indicated in the two bottom lines. Residue numbering is for full-length cpPPase. Consensus residues for different levels of identity are indicated below the set of sequences.
ApnA binding is expected to significantly change CBS-PPase activity in vivo, particularly under low energy conditions, when the concentration of the alternative activator, ATP, is low. Although basal intracellular levels of ApnAs are 4 orders of magnitude lower than those of adenine mononucleotides, ApnA concentrations can rise by 2 orders of magnitude under stress conditions (42, 43). Also taking into consideration their extraordinarily high affinity, ApnAs could be expected to efficiently compete with mononucleotides for CBS-PPase binding in these circumstances. An increase in CBS-PPase activity is expected to decrease the concentration of PPi and thus release PPi-mediated inhibition of numerous biosynthetic reactions in which PPi is produced as a by-product (44). That the affinity of CBS-PPases for ApnAs markedly surpasses that of all known ApnA-binding proteins suggests that this enzyme is a dominant target through which ApnAs fulfill their stress response-related functions in bacteria.
Author Contributions
V. A. A. designed, performed and analyzed the experiments, and contributed to writing the manuscript. A. S. designed and constructed vectors, expressed and purified proteins, and performed bioinformatics analyses. H. K. T. performed ITC experiments with dhPPase. V. N. O. supervised ITC experiments and data analysis. R. L. designed and supervised the experiments. A. A. B. designed and analyzed experiments and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Dr. P. Semenyuk for help with ITC measurements.
This work was supported by Academy of Finland Grant 139031 and Russian Foundation for Basic Research Grants 12-04-01002 and 15-04-04828. The authors declare that they have no conflicts of interest with the contents of this article.
- ApnA
- 5′,5-P1,Pn-diadenosine polyphosphate with n phosphate residues
- CBS
- cystathionine β-synthase
- CBS-PPase
- CBS domain-containing pyrophosphatase
- cnPPase
- C. novyi pyrophosphatase
- cpPPase
- C. perfringens pyrophosphatase
- dhPPase
- D. hafniense pyrophosphatase
- elPPase
- E. lenta pyrophosphatase
- mtPPase
- M. thermoacetica pyrophosphatase
- PPase
- pyrophosphatase
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid
- ITC
- isothermal titration calorimetry.
References
- 1.Reiss J. R., and Moffatt J. G. (1965) Dismutation reactions of nucleoside polyphosphates: III. the synthesis of α,ω-dinucleoside 5′-polyphosphates. J. Org. Chem. 30, 3381–3387 [DOI] [PubMed] [Google Scholar]
- 2.Fraga H., and Fontes R. (2011) Enzymatic synthesis of mono and dinucleoside polyphosphates. Biochim. Biophys. Acta 1810, 1195–1204 [DOI] [PubMed] [Google Scholar]
- 3.Zamecnik P. C., Stephenson M. L., Janeway C. M., and Randerath K. (1966) Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem. Biophys. Res. Commun. 24, 91–97 [DOI] [PubMed] [Google Scholar]
- 4.Goerlich O., Foeckler R., and Holler E. (1982) Mechanism of synthesis of adenosine(5′)tetraphospho(5′)adenosine (AppppA) by aminoacyl-tRNA synthetases. Eur. J. Biochem. 126, 135–142 [DOI] [PubMed] [Google Scholar]
- 5.Wright M., Boonyalai N., Tanner J. A., Hindley A. D., and Miller A. D. (2006) The duality of LysU, a catalyst for both Ap4A and Ap3A formation. FEBS J. 273, 3534–3544 [DOI] [PubMed] [Google Scholar]
- 6.Guranowski A. (2000) Specific and nonspecific enzymes involved in the catabolism of mononucleoside and dinucleoside polyphosphates. Pharmacol. Ther. 87, 117–139 [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y.-L., Zhang J.-W., Yu W.-L., Cheng W., Zhang C.-C., Frolet C., Di Guilmi A.-M., Vernet T., Zhou C.-Z., and Chen Y. (2011) Structural and enzymatic characterization of the streptococcal ATP/diadenosine polyphosphate and phosphodiester hydrolase Spr1479/SapH. J. Biol. Chem. 286, 35906–35914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee P. C., Bochner B. R., and Ames B. N. (1983) AppppA, heat shock stress, and cell oxidation. Proc. Natl. Acad. Sci. U.S.A. 80, 7496–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bochner B. R., Zylicz M., and Georgopoulos C. (1986) Escherichia coli DnaK protein possesses a 5′-nucleotidase activity that is inhibited by AppppA. J. Bacteriol. 168, 931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varshavsky A. (1983) Diadenosine 5′,5‴-P1,P4-tetraphosphate: a pleiotypically acting alarmone. Cell 34, 711–712 [DOI] [PubMed] [Google Scholar]
- 11.Tanner J. A., Wright M., Christie E. M., Preuss M. K., and Miller A. D. (2006) Investigation into the interactions between diadenosine 5′,5‴-P1,P4-tetraphosphate and two proteins: molecular chaperone GroEL and cAMP receptor protein. Biochemistry 45, 3095–3106 [DOI] [PubMed] [Google Scholar]
- 12.Tshori S., Razin E., and Nechushtan H. (2013) Amino-acyl tRNA synthetases generate dinucleotide polyphosphates as second messengers: functional implications. Top. Curr. Chem, 344, 10–12 [DOI] [PubMed] [Google Scholar]
- 13.Sillero M. A., De Diego A., Osorio H., and Sillero A. (2002) Dinucleoside polyphosphates stimulate the primer independent synthesis of poly(A) catalyzed by yeast poly(A) polymerase. Eur. J. Biochem. 269, 5323–5329 [DOI] [PubMed] [Google Scholar]
- 14.Nishimura A., Moriya S., Ukai H., Nagai K., Wachi M., and Yamada Y. (1997) Diadenosine 5′,5‴-P1,P4-tetraphosphate (Ap4A) controls the timing of cell division in Escherichia coli. Genes Cells 2, 401–413 [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Villafuertes R., Pintor J., Gualix J., and Miras-Portugal M. T. (2004) GABA modulates presynaptic signalling mediated by dinucleotides on rat synaptic terminals. J. Pharmacol. Exp. Ther. 308, 1148–1157 [DOI] [PubMed] [Google Scholar]
- 16.Vartanian A. A., Suzuki H., and Poletaev A. I. (2003) The involvement of diadenosine 5′,5′′′-P1,P4-tetraphosphate in cell cycle arrest and regulation of apoptosis. Biochem. Pharmacol. 65, 227–235 [DOI] [PubMed] [Google Scholar]
- 17.Giraldez L., Díaz-Hernández M., Gómez-Villafuertes R., Pintor J., Castro E., and Miras-Portugal M. T. (2001) Adenosine triphosphate and diadenosine pentaphosphate induce [Ca2+]i increase in rat basal ganglia aminergic terminals. J. Neurosci. Res. 64, 174–182 [DOI] [PubMed] [Google Scholar]
- 18.Conant A. R., Theologou T., Dihmis W. C., and Simpson A. W. (2008) Diadenosine polyphosphates are selective vasoconstrictors in human coronary artery bypass grafts cells. Vascul. Pharmacol. 48, 157–164 [DOI] [PubMed] [Google Scholar]
- 19.Johnstone D. B., and Farr S. B. (1991) AppppA binds to several proteins in Escherichia coli, including the heat shock and oxidative stress proteins DnaK, GroEL, E89, C45 and C40. EMBO J. 10, 3897–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W., Azhar M. A., Xu Y., Wright M., Kamal A., and Miller A. D. (2011) Isolation and identification of diadenosine 5′,5‴-P1,P4-tetraphosphate binding proteins using magnetic bio-panning. Bioorg. Med. Chem. Lett. 21, 7175–7179 [DOI] [PubMed] [Google Scholar]
- 21.Azhar M. A., Wright M., Kamal A., Nagy J., and Miller A. D. (2014) Biotin-c10-AppCH2ppA is an effective new chemical proteomics probe for diadenosine polyphosphate binding proteins. Bioorg. Med. Chem. Lett. 24, 2928–2933 [DOI] [PubMed] [Google Scholar]
- 22.Marques A. F., Teixeira N. A., Gambaretto C., Sillero A., and Sillero M. A. (1998) IMP-GMP 5′-nucleotidase from rat brain: activation by polyphosphates. J. Neurochem. 71, 1241–1250 [DOI] [PubMed] [Google Scholar]
- 23.Kajander T., Kellosalo J., and Goldman A. (2013) Inorganic pyrophosphatases: one substrate, three mechanisms. FEBS Lett. 587, 1863–1869 [DOI] [PubMed] [Google Scholar]
- 24.Parfenyev A. N., Salminen A., Halonen P., Hachimori A., Baykov A. A., and Lahti R. (2001) Quaternary structure and metal-ion requirement of family II pyrophosphatases from Bacillus subtilis, Streptococcus gordonii and Streptococcus mutans. J. Biol. Chem. 276, 24511–24518 [DOI] [PubMed] [Google Scholar]
- 25.Bateman A. (1997) The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem. Sci. 22, 12–13 [DOI] [PubMed] [Google Scholar]
- 26.Ereño-Orbea J., Oyenarte I., and Martínez-Cruz L. A. (2013) CBS domains: ligand binding sites and conformational variability. Arch. Biochem. Biophys. 540, 70–81 [DOI] [PubMed] [Google Scholar]
- 27.Baykov A. A., Tuominen H. K., and Lahti R. (2011) The CBS domain: a protein module with an emerging prominent role in regulation. ACS Chem. Biol. 6, 1156–1163 [DOI] [PubMed] [Google Scholar]
- 28.Kemp B. E. (2004) Bateman domains and adenosine derivatives form a binding contract. J. Clin. Invest. 113, 182–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ignoul S., and Eggermont J. (2005) CBS domains: structure, function, and pathology in human proteins. Am. J. Physiol. Cell Physiol. 289, C1369–C1378 [DOI] [PubMed] [Google Scholar]
- 30.Scott J. W., Hawley S. A., Green K. A., Anis M., Stewart G., Scullion G. A., Norman D. G., and Hardie D. G. (2004) CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 113, 274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jämsen J., Tuominen H., Salminen A., Belogurov G. A., Magretova N. N., Baykov A. A., and Lahti R. (2007) A CBS domain-containing pyrophosphatase of Moorella thermoacetica is regulated by adenine nucleotides. Biochem. J. 408, 327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salminen A., Anashkin V. A., Lahti M., Tuominen H. K., Lahti R., Baykov A. A. (2014) Cystathionine β-synthase (CBS) domains confer multiple forms of Mg2+-dependent cooperativity to family II pyrophosphatases. J. Biol. Chem. 289, 22865–22876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuominen H., Salminen A., Oksanen E., Jämsen J., Heikkilä O., Lehtiö L., Magretova N. N., Goldman A., Baykov A. A., and Lahti R. (2010) Crystal structures of the CBS and DRTGG domains of the regulatory region of Clostridium perfringens pyrophosphatase complexed with the inhibitor, AMP, and activator, diadenosine tetraphosphate. J. Mol. Biol. 398, 400–413 [DOI] [PubMed] [Google Scholar]
- 34.Jämsen J., Baykov A. A., and Lahti R. (2012) Fast kinetics of nucleotide binding to Clostridium perfringens family II pyrophosphatase containing CBS and DRTGG domains Biochemistry 77, 165–170 [DOI] [PubMed] [Google Scholar]
- 35.Baykov A. A., and Avaeva S. M. (1981) A simple and sensitive apparatus for continuous monitoring of orthophosphate in the presence of acid-labile compounds. Anal. Biochem. 116, 1–4 [DOI] [PubMed] [Google Scholar]
- 36.Baykov A. A., Bakuleva N. P., and Rea P. A. (1993) Steady-state kinetics of substrate hydrolysis by vacuolar H+-pyrophosphatase. A simple three-state model. Eur. J. Biochem. 217, 755–762 [DOI] [PubMed] [Google Scholar]
- 37.Bisswanger H. (2008) Enzyme Kinetics: Principles and Methods, 2nd Ed., pp. 14–17, Wiley-VCH Verlag, Weinheim, Germany [Google Scholar]
- 38.Hattori M., Tanaka Y., Fukai S., Ishitani R., and Nureki O. (2007) Crystal structure of the MgtE Mg2+ transporter. Nature 448, 1072–1075 [DOI] [PubMed] [Google Scholar]
- 39.McLean J. E., Hamaguchi N., Belenky P., Mortimer S. E., Stanton M., and Hedstrom L. (2004) Inosine 5′-monophosphate dehydrogenase binds nucleic acids in vitro and in vivo. Biochem. J. 379, 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguado-Llera D., Oyenarte I., Martínez-Cruz L. A., and Neira J. L. (2010) The CBS domain protein MJ0729 of M. jannaschii binds DNA. FEBS Lett. 584, 4485–4489 [DOI] [PubMed] [Google Scholar]
- 41.Fabrichniy I. P., Lehtiö L., Tammenkoski M., Zyryanov A. B., Oksanen E., Baykov A. A., Lahti R., and Goldman A. (2007) A trimetal site and ground-state substrate distortion mark the active site of family II inorganic pyrophosphatase. J. Biol. Chem. 282, 1422–1431 [DOI] [PubMed] [Google Scholar]
- 42.Garrison P. N., and Barnes L. D. (1992) Determination of dinucleoside polyphosphates, in Ap4A and Other Dinucleoside Polyphosphates (McLennan A.G., ed) pp. 29–61, CRC Press, Boca Raton, FL [Google Scholar]
- 43.Plateau P., and Blanquet S. (1994) Dinucleoside oligophosphates in micro-organisms. Adv. Microb. Physiol. 36, 81–109 [DOI] [PubMed] [Google Scholar]
- 44.Heinonen J. K. (2001) Biological Role of Inorganic Pyrophosphate, pp. 123–188, Kluwer Academic Publishers, London [Google Scholar]