Background: Signaling by G protein-coupled melanocortin-2 receptors requires MRAP, a transmembrane accessory protein that forms antiparallel homodimers.
Results: Mutational analysis of MRAP-MRAP-receptor fusion proteins established that MRAP oriented with an extracellular amino terminus is essential.
Conclusion: MRAP acts on the outside of the cell in the ACTH-MRAP-MRAP-receptor signaling complex.
Significance: The results provide insight into molecular mechanisms of GPCR accessory proteins.
Keywords: cyclic AMP (cAMP), G protein-coupled receptor (GPCR), membrane protein, signal transduction, transmembrane domain, accessory protein, adrenocorticotropic hormone (ACTH), melanocortin receptor, melanocortin receptor accessory protein (MRAP), membrane protein topology
Abstract
The melanocortin-2 (MC2) receptor is a G protein-coupled receptor that mediates responses to ACTH. The MC2 receptor acts in concert with the MC2 receptor accessory protein (MRAP) that is absolutely required for ACTH binding and signaling. MRAP has a single transmembrane domain and forms a highly unusual antiparallel homodimer that is stably associated with MC2 receptors at the plasma membrane. Despite the physiological importance of the interaction between the MC2 receptor and MRAP, there is little understanding of how the accessory protein works. The dual topology of MRAP has made it impossible to determine whether highly conserved and necessary regions of MRAP are required on the intracellular or extracellular face of the plasma membrane. The strategy used here was to fix the orientation of two antiparallel MRAP molecules and then introduce inactivating mutations on one side of the membrane or the other. This was achieved by engineering proteins containing tandem copies of MRAP fused to the amino terminus of the MC2 receptor. The data firmly establish that only the extracellular amino terminus (Nout) copy of MRAP, oriented with critical segments on the extracellular side of the membrane, is essential. The transmembrane domain of MRAP is also required in only the Nout orientation. Finally, activity of MRAP-MRAP-MC2-receptor fusion proteins with inactivating mutations in either MRAP or the receptor was rescued by co-expression of free wild-type MRAP or free wild-type receptor. These results show that the basic MRAP-MRAP-receptor signaling unit forms higher order complexes and that these multimers signal.
Introduction
The G protein-coupled receptor (GPCR)2 superfamily is the largest family of membrane proteins. GPCRs share a basic structure with an extracellular amino terminus, seven transmembrane domains, and intracellular carboxyl terminus, and most initiate heterotrimeric G protein signaling in response to agonists. The rhodopsin-like class A family of GPCRs includes five melanocortin (MC) receptors that are activated by peptides cleaved from the pro-opiomelanocortin precursor. All five MC receptors activate Gs and stimulate cAMP formation. The MC2, or ACTH receptor, is highly expressed in the adrenal cortex and mediates normal and stress-related ACTH responses. Among natural hormones, only ACTH can activate the MC2 receptor. Inactivating mutations in the MC2 receptor cause familial glucocorticoid deficiency (type 1), a potentially fatal disease characterized by ACTH resistance (1).
Metherell et al. (2) discovered that some individuals with familial glucocorticoid deficiency harbor mutations in a small protein which is now termed MRAP for MC2 receptor accessory protein. MRAP enhances trafficking of the MC2 receptor to the plasma membrane and is absolutely required for ACTH binding and signal transduction (2–4). MRAPs are small proteins with a single predicted transmembrane domain. The amino-terminal and transmembrane domains are highly conserved and essential for MRAP function, whereas the region carboxyl-terminal to the transmembrane domain is not (5–7). Two splice variants of human MRAP differ almost completely on the carboxyl side of the transmembrane helix; however, both support strong ACTH responses (3).
Substantial evidence indicates that MRAP forms an antiparallel homodimer (4, 6, 8, 9). This structure is highly unusual and possibly unique among single pass membrane proteins. MRAP dimerization was initially demonstrated by co-immunoprecipitation of differentially tagged MRAPs. Dual topology was discovered when antibodies directed against natural or experimentally added epitopes on either the amino- or carboxyl-terminal side of the transmembrane region detected MRAP on the cell surface. Mouse MRAP contains a single potential N-glycosylation site in the amino terminus, and approximately half of mouse MRAP molecules are N-glycosylated, consistent with an antiparallel homodimer. Using bimolecular fluorescence complementation, we found that fluorescence was reconstituted when one fragment of YFP was on the amino terminus of MRAP and the other was on the carboxyl terminus (6). Analysis by bioluminescence resonance energy transfer has also supported a predominantly antiparallel orientation (10).
MRAP and MC2 receptors appear to be at least partially co-localized by conventional fluorescence microscopy (2, 3, 5, 11–13). The MRAP dimer co-precipitates with MC2 receptors from whole cell lysates or from a pool of receptors isolated from the plasma membrane (4, 7, 10, 14, 15). In addition, fluorescence complementation occurs when the YFP fragments are on the carboxyl-terminal ends of MRAP and the MC2 receptor (6, 16). Finally, MRAP and MC2 receptors undergo ACTH-dependent internalization and recycling together (12). These data imply that the accessory protein and receptor interact directly or at least very closely.
Mutational analysis has shown that MRAP has two distinct functions: to permit receptor maturation and trafficking to the plasma membrane and to allow ACTH to bind to and activate mature receptors at the surface (3, 6, 7, 9). Several regions of the conserved amino-terminal and transmembrane domains are essential for full MRAP activity. In particular, a Tyr-rich domain between residues 14 and 20 is necessary for ACTH binding and signaling but not for MC2 receptor trafficking. A stretch of positively charged residues in the juxtamembrane region of MRAP is required for dual orientation, MRAP dimer formation, and MC2 receptor trafficking (6). N-Glycosylation on the Nout MRAP partner is present but not required (4).
Despite the importance of the MRAP-MC2 receptor interaction, there is little understanding of how the accessory protein works. The dual topology of MRAP has made it impossible to determine whether highly conserved regions of the MRAP amino terminus act inside the cell, perhaps regulating G protein coupling, or on the outside where they might impact ACTH binding directly. The goals of this study were to ascertain whether the essential MRAP amino terminus is required on the extracellular or cytoplasmic surface, whether dual topology is necessary, and whether the transmembrane domain is required in both orientations. The strategy was to fix the orientation of the two antiparallel MRAP molecules and then introduce mutations on either side of the plasma membrane. This was achieved by creating fusion proteins containing tandem copies of MRAP fused to the amino terminus of the MC2 receptor. The results firmly establish that only the copy of MRAP oriented with the amino terminus on the extracellular side of the receptor is essential for signal transduction.
Experimental Procedures
Plasmids
Human MC2 receptor with an amino-terminal 3xHA epitope tag was from Missouri S&T cDNA Resource Center. MC2R-MRAP and constructs with CD8 transmembrane domains were synthesized by GeneWiz and GenScript. Mutations in MRAP and MC2 receptors were made using QuikChange (Stratagene). Mouse MRAP (mMRAP)-V5-human MRAPα (hMRAPα) tandem and fusion constructs were created using overlap extension PCR. Wild-type or mutant mMRAP was PCR-amplified using a 5′ primer with an NheI restriction site and 3′ primer containing a partial sequence for the V5 epitope. Wild-type hMRAPα was amplified using a 5′ PCR primer containing a region of overlap with the V5 primer and a 3′ primer containing an in-frame MluI site. The two PCR products were annealed and extended using no outside primers. PCR products were cloned in the pCR2.1 TA cloning vector and sequence-verified. To generate tagged tandem constructs, the tandem MRAP cDNAs were cloned in-frame between the NheI and MluI sites of a modified pCI-Neo vector containing the 3xFLAG epitope between the MluI and NotI sites. To create fusion constructs, tandem constructs were excised with NheI and MluI and cloned in-frame into a pcDNA3.1+ vector containing 3xHA-MC2 receptor between MluI and NotI sites. The same strategy was used for hMRAPα-V5-mMRAP constructs.
Cell Growth and cAMP Assays
HEK293 cells were grown in DMEM with 5% fetal bovine serum and transfected using 3 μl of Lipofectamine 2000/μg of DNA. Each well of a 96-well dish was transfected with 50 ng of DNA, including plasmids encoding CRE-luciferase (15–25 ng), MC2 receptor, MRAP, fusion protein, and empty vector or GFP to balance DNA concentrations. The MC2 receptor:MRAP DNA ratio was 2:1. cAMP responses were measured in cells expressing a CRE-luciferase reporter gene with multimerized cAMP response elements from Dr. George Holz at State University of New York Upstate Medical University (Syracuse, NY). At 24–48 h after transfection, cells were stimulated with 0–1 μm human ACTH(1–24) (ACTH) or 20 μm forskolin in serum-free DMEM containing 1 mg/ml BSA and in some experiments 20 mm HEPES, pH 7.5. After 4–5 h, medium was replaced with 50 μl of Firefly Reagent from Nanolight Technology, and luciferase activity was quantified in a BioTek plate reader. Responses were expressed as a percentage of the forskolin response in the same experiment. ACTH caused no increase in cAMP unless MC2 receptor was transfected. Unless noted, values shown are mean ± range or S.E. of duplicates or triplicates. Where not visible, error bars were within symbol size. The significance of differences between groups was analyzed by analysis of variance with Tukey's post hoc analysis. EC50 values were obtained using Prism software.
For cAMP mass measurements, cells in 24-well dishes were transfected as described above and incubated for 20 min at 37 °C in serum-free medium containing 0.1% BSA and 0.5 mm isobutylmethylxanthine. cAMP was extracted in 300 μl 0.1 n HCl and quantified using cAMP Direct ELISA kits from Enzo Life Sciences.
Epitope Expression
The relative concentration of 3xHA-MC2R on the plasma membrane was measured in a fixed cell ELISA protocol. In brief, cells in a 24- or 48-well plate were washed with PBS, fixed with 3% paraformaldehyde for ∼20 min, washed, and incubated for ∼1 h with 1:5000 HA-11 monoclonal anti-HA antibody (Covance) in PBS containing 5% nonfat dry milk. Plates were then washed twice and incubated for ∼1 h with 1:5000 HRP-labeled anti-mouse IgG in PBS/milk. After two additional washes, 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich) was added. When color development was adequate (1–20 min), reactions were stopped with 10% sulfuric acid, and A450 was read. Results were expressed as percentage of HA signal obtained in cells expressing MC2 receptor/MRAP in the same experiment. Similar protocols were used to measure V5 and FLAG epitopes using 1:5000 dilutions of monoclonal anti-V5 (AbD Serotec) or M2 anti-FLAG (Sigma-Aldrich). Total concentration of epitope-tagged proteins was measured with the same protocol except that antibody incubation buffers contained 1% Triton X-100 to permeabilize cells. All experiments included control cells transfected with empty vector or GFP, and this background has been subtracted.
Western Blots
HEK293 cells in a 6-well plate were transfected with 1 μg of DNA/well. The next day cells were washed and lysed in 200 μl of buffer containing 150 mm NaCl, 50 mm Tris-Cl, 1 mm EDTA, and 1% Triton X-100, pH 8.0. Lysates were centrifuged at 10,000 × g for 20 min. Fifty microliters of 4× NuPAGE sample buffer containing 200 mm dithiothreitol was added to 150 μl of lysate supernatant, and samples were run on 10% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose and immunoblotted overnight at 4 °C in Tris-buffered saline with 0.05% Tween 20 and 5% nonfat dry milk containing 1:5000 monoclonal antibodies to HA or V5 epitopes followed by incubation with 1:5000 HRP-anti-mouse IgG at room temperature.
Microscopy
Cells were grown on glass coverslips for microscopy. To detect externally oriented epitopes, live cells were incubated with monoclonal anti-V5 or anti-HA antibodies diluted 1:1000 in PBS with 5% goat serum cells for 30 min at 18 °C to minimize internalization and then washed three times with PBS prior to fixation with 2% paraformaldehyde for 10 min. Coverslips were next washed, incubated with goat anti-rabbit Alexa Fluor 546 (Life Technologies) diluted 1:1000 in PBS/goat serum for 1 h at room temperature, and washed. To detect total epitope pools, cells were first washed with PBS and fixed and then washed and permeabilized in PBS/goat serum containing 0.5% Triton X-100. Coverslips were incubated with anti-V5 and anti-HA antibodies diluted 1:2500 in PBS/goat serum with Triton X-100 for 1 h at room temperature, washed, and incubated an additional 1 h with 1:1000 goat anti-rabbit Alexa Fluor 546 in the same buffer. Coverslips were mounted in Prolong Gold with DAPI. Images were obtained using an Olympus FV1000MP microscope in confocal mode with a 60× numerical aperture 1.35 oil objective (Olympus). Alexa Fluor 546 was excited at 559 nm with emission at 618 nm, and DAPI was excited at 405 nm with 461 nm emission.
Results
Ability of Concatenated MRAP to Support MC2 Receptor Signaling
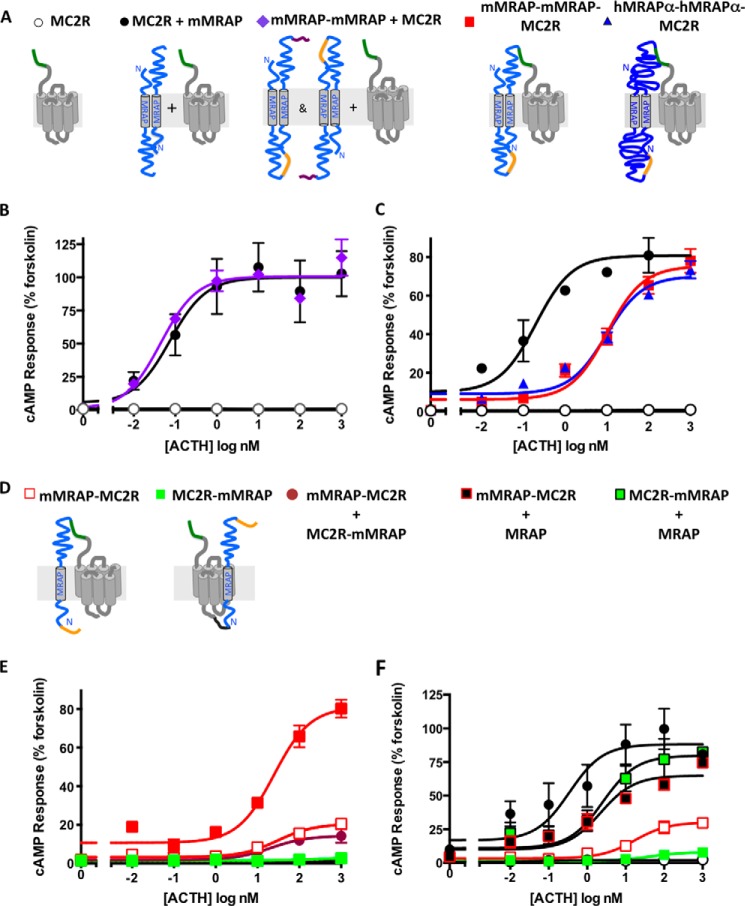
To test the feasibility of the fusion protein approach, we first determined whether a tandem MRAP protein is functional. We engineered expression cassettes containing two MRAP molecules connected with a 21-aa linker that included a V5 epitope tag and a 3xFLAG tag on the carboxyl terminus (Fig. 1A). HEK293 cells were transfected to express the MC2 receptor alone or with free MRAP or tandem MRAP-MRAP. The MC2 receptor had an amino-terminal 3xHA tag, which enabled us to estimate the relative amount of receptor on the plasma membrane of fixed but non-permeabilized cells (4). A similar assay was used for tandem MRAP-MRAP, which was found in dual orientations as depicted in Fig. 1A (data not shown).
FIGURE 1.
Tandem MRAPs and MRAP-MRAP-MC2R fusion proteins are active. A and D, presumed protein orientations are shown schematically with mMRAP (127 aa) in light blue and hMRAPα (172 aa) in dark blue. Green depicts the 3xHA tag on the amino terminus of the MC2 receptor, and orange denotes a single V5 tag. Purple shows a 3xFLAG epitope on the carboxyl terminus of tandem MRAPs, and black shows a (GGGS)3 flexible linker in MC2R-mMRAP. Note that receptor fusions did not contain a 3xFLAG epitope. B, C, E, and F, HEK293 cells were transfected with plasmids encoding CRE-luciferase, MC2 receptors, and accessory proteins as shown. The next day cells were stimulated for 5 h with either 20 μm forskolin or 0–1 μm ACTH. Responses are expressed as percentage of the forskolin response. Error bars show S.E. Surface expression of HA-tagged MC2 receptors normalized to that of MC2R/MRAP in the same experiment was 62.9% (MC2R alone), 40.0% (mMRAP-mMRAP + MC2R), 10.5% (mMRAP-MC2R), 3.8% (MC2R-mMRAP), 39.4% (mMRAP-mMRAP-MC2R), and 26.3% (hMRAPα-hMRAPα-MC2R). All values were significantly below MC2R/MRAP (p < 0.05).
Signaling activity was quantified by co-transfecting CRE-luciferase, a cAMP reporter (17). ACTH stimulated a robust increase in cAMP when the receptor was expressed with either MRAP or MRAP-MRAP (Fig. 1B), showing that the accessory protein is sufficiently flexible to interact with receptor in a tandem conformation. ACTH responses were indistinguishable when receptor was expressed with MRAP or tandem constructs containing mMRAP-mMRAP, mMRAP-hMRAPα, and hMRAPα-mMRAP. Surface expression of the MC2 receptor was lower in cells expressing tandem MRAP than in those expressing free MRAP (Fig. 1, legend).
Signaling by MC2 Receptors Fused to MRAP Dimers
To construct the accessory protein-receptor fusion construct, the tandem MRAPs described above were inserted in-frame to the amino terminus of the wild-type MC2 receptors with an amino-terminal 3xHA epitope (Fig. 1A). Cells were transfected to express fusion proteins containing two mMRAPs or two hMRAPαs. Both mMRAP-mMRAP-MC2R and hMRAPα-hMRAPα-MC2R responded to ACTH with maximal cAMP responses comparable with those obtained with wild-type receptor with free MRAP (Fig. 1C), although higher concentrations of ACTH were required to activate the fusion proteins (EC50 = 0.20 nm for wild-type receptor/MRAP and 10 nm for fusion proteins). Similar findings were obtained using mMRAP-hMRAPα-MC2 receptor and hMRAPα-mMRAP-MC2R as shown below. Fusion proteins displayed no constitutive activity and no response to 1 μm [Nle4,d-Phe7]-α-melanocyte-stimulating hormone (where Nle is norleucine), a potent agonist for other members of the MC receptor family (not shown). Fusion constructs were not expressed as well as free MC2 receptor on the cell surface (Fig. 1, legend).
Lack of Activity of MC2 Receptors Fused to a Single MRAP
We also tested MC2 receptors with a single MRAP fused to either the amino or carboxyl terminus of the receptor (Fig. 1, D–F). Because the 68-aa carboxyl terminus of mMRAP is not conserved and predicted to be disordered, we reasoned that fusing MRAP at the receptor amino terminus would not constrain the receptor severely. Conversely, the cytoplasmic tail of the MC2 receptor is short (∼20 aa), and the 36-aa amino terminus of MRAP is highly conserved and predicted to be partially helical. For this reason, we inserted a flexible (GGGS)3 linker between the receptor and MRAP in the MC2R-MRAP construct. MRAP-MC2R displayed weak but measurable activity, whereas MC2R-MRAP was completely inactive (Fig. 1E). Co-expressing MC2R-MRAP and MRAP-MC2R did not increase activity. Surface expression of these proteins was low, particularly for MC2R-MRAP, suggesting that these constructs are either unstable or poorly transported to the plasma membrane (Fig. 1, legend). Nonetheless, both MRAP-MC2R and MC2R-MRAP signaled strongly when co-expressed with free MRAP, proving that receptors with MRAP fused at either end reach the membrane and respond to ACTH (Fig. 1F).
Effect of Receptor Expression on Response
We explored the relationship between the amount of receptor expressed on the plasma membrane and the cAMP response by transfecting cells with various amounts of DNA encoding either free MC2 receptor and MRAP or MRAP-MRAP-MC2 receptor fusion (Fig. 2). There was no detectable cAMP response to ACTH when cells were transfected with no receptor or with MC2 receptor alone, consistent with the absence of MC2 receptor and MRAP in these cells (3, 18). With free MC2 receptor plus MRAP and with fusion protein, maximal cAMP reporter gene responses were obtained when surface receptor levels were far below maximal. Free MC2 receptor appeared to be expressed at 5-fold higher levels than MRAP-MRAP-MC2 receptor, but because the 3xHA epitope tag was on the amino terminus of the MC2 receptor and sandwiched between MRAP and the receptor in the fusion protein, the ELISA signals may not be directly comparable.
FIGURE 2.

Relationship between receptor expression and cAMP response. Top, cells in 96-well plates were transfected with a total of 50 ng of DNA/well. Each well received 12 ng of CRE-luciferase and 0–38 ng of plasmids encoding MRAP-MRAP-MC2R fusion protein or MRAP plus wild-type MC2 receptor (2:1 receptor:MRAP ratio). DNA concentrations were balanced with GFP plasmid. After 24 h, cells were incubated with vehicle, 20 μm forskolin, or 1 μm ACTH. Bottom, cells in 24-well plates were transfected with 250 ng of DNA from the same transfection mixtures, and expression of MC2 receptor on the cell surface was quantified by ELISA using anti-HA antibody on fixed, non-permeabilized cells. Dashed lines show the fusion protein. Error bars show S.E.
Orientation of MRAP-MRAP-MC2 Receptor Fusion Proteins
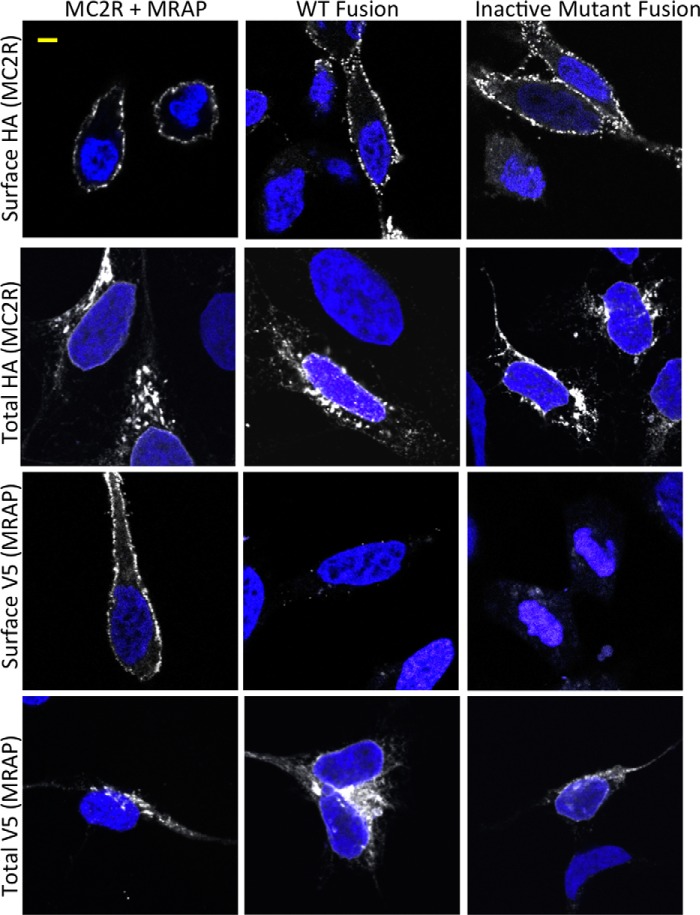
Immunofluorescence microscopy was used to determine the orientation of fusion proteins on the plasma membrane (Fig. 3). Cells were transfected to express free V5-MRAP plus free 3xHA-MC2 receptors or MRAP-V5-MRAP-3xHA-MC2 receptor fusion proteins. Both active fusion protein containing wild-type MRAP and an inactive fusion protein containing a mutant MRAP (described below) were tested. To detect epitopes on the extracellular side of the plasma membrane selectively, we incubated live cells with anti-HA and anti-V5 antibodies and then washed the cells extensively before fixation. To detect the total pool of epitope-tagged proteins, we fixed and permeabilized cells and then incubated the cells with antibody in the presence of detergent. The HA epitope of the receptor was localized at the plasma membrane in a punctate pattern in all cases. As predicted by the dual topology model, V5 was visible at the plasma membrane of cells expressing free V5-MRAP. In contrast, V5 was not detected on the surface of cells expressing fusion proteins, consistent with the orientation depicted in Fig. 1A. Not surprisingly in transient transfection studies, free MRAP, free receptor, and fusion proteins were also localized in the endoplasmic reticulum and Golgi apparatus. Cell surface ELISA approaches supported the conclusion that the predominant orientation of the MRAP-MRAP-MC2 receptor is with the receptor amino terminus facing the extracellular space (data not shown).
FIGURE 3.
Immunolocalization of free MRAP, free receptor, and fusion proteins. Cells were transfected with DNA encoding wild-type receptor and MRAP, MRAP-MRAP-MC2R (WT Fusion), or (18–21A)MRAP-MRAP-MC2R (Inactive Mutant Fusion) as shown. After 24 h, live cells were incubated with monoclonal anti-HA antibody to detect surface-localized 3xHA-tagged MC2 receptors or monoclonal anti-V5 antibody to detect V5-tagged MRAP. Cells were then washed and fixed, and epitopes were visualized with Alexa Fluor 546-anti-mouse IgG (shown in white). Total HA and V5 epitopes were detected in cells that were fixed and permeabilized with detergent before incubation with anti-HA or anti-V5 antibodies followed by Alexa Fluor 546-anti-mouse IgG, all in the continued presence of detergent. Blue shows nuclei stained with DAPI. Images are 0.5-μm slices through the middle of cells; a 5-μm scale bar is shown in the upper left. Intensity and contrast were adjusted identically for all images, which were typical in terms of epitope localization and intensity. Surface V5 staining was not detected in any cells expressing fusion proteins. Control cells transfected with empty vector showed no surface or total staining for either epitope.
Activity of Mutant MRAP-MRAP-MC2 Receptor Fusion Proteins
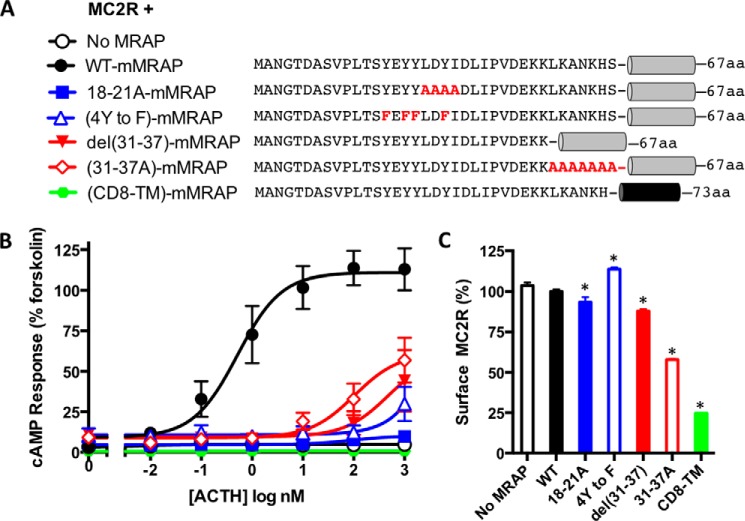
The sequence of the amino terminus of mMRAP is shown in Fig. 4A. To identify regions critical for activity, we tested a series of mutants for their ability to support signaling by the MC2 receptor (Fig. 4B). The receptor again showed no response to ACTH in the absence of MRAP and a robust cAMP response in the presence of wild-type MRAP. MRAP activity was strongly inhibited by substituting Ala for the LDYI sequence at residues 18–21 (18–21A), and by replacing Tyr-14, -16, -17, and -20 with Phe (4Y to F). Alanine substitution or deletion of most of the basic residues immediately preceding the transmembrane domain (31–37A and del31–37) also inhibited activity. The EC50 for ACTH (0.52 nm with wild-type MRAP) was increased over 100-fold with all of these amino-terminal mutants of MRAP, and the maximal responses were reduced. We also replaced most of the transmembrane domain of MRAP with the transmembrane domain from human CD8; this caused a complete loss of activity. The MC2 receptor was expressed at levels expected to be sufficient for maximal activity in all cases (Fig. 4C). MRAP mutants 18–21A and del31–37 were previously studied in CHO cells where they also had greatly reduced activity (6).
FIGURE 4.
Activity of mutant MRAPs. A, MC2R was expressed with mMRAPs containing the mutations shown in the conserved amino-terminal domain. In one construct, residues 37–59 of the MRAP transmembrane domain (SIVIALWLSLATFVVLLFLILLY) were replaced with the transmembrane domain of human CD8α (YIWAPLAGTCGVLLLSLVITLYC) depicted in black. B, cAMP responses. C, surface expression of receptors. *, p < 0.05 versus WT. Error bars show S.E.
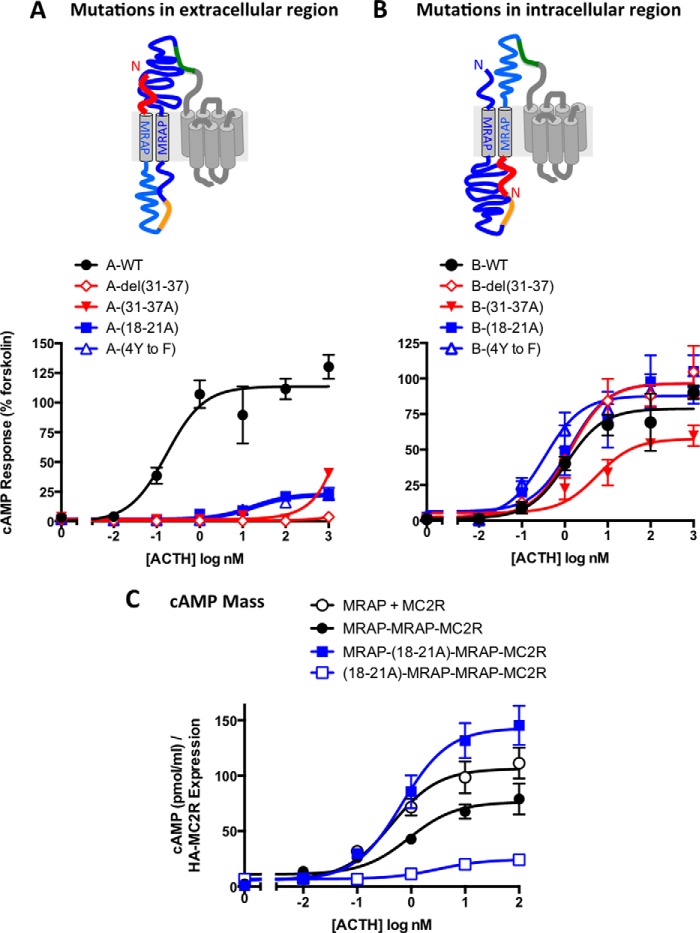
A critical objective of this study was to ascertain whether the important amino-terminal domains of MRAP are required on the extracellular or intracellular face of the receptor. For this purpose, mutations that inactivate MRAP were introduced in the first copy of MRAP (termed A) or the second copy (termed B), placing potentially inactivating mutations (Fig. 5, shown in red) on either the extracellular or intracellular side of the plasma membrane. MRAP-MRAP-MC2R responded strongly to ACTH (Fig. 5A). Substitution of residues 18–21 or the four amino-terminal Tyr residues of the extracellular (A) MRAP reduced maximal cAMP responses by over 75% and increased EC50 values for ACTH by over 100-fold. Deletion or alanine substitution of 7 aa in the positively charged stretch just before the transmembrane domain of the A MRAP likewise reduced or abolished activity. Results were much different when the same mutations were introduced into the second, intracellular (B) MRAP (Fig. 5B). In this case, the mutations had little or no effect on the ACTH response.
FIGURE 5.
Essential amino-terminal residues are required on the Nout copy of MRAP. Schematic representations of the presumed orientations of MRAP-MRAP-MC2R fusion proteins containing mutations in either the first copy of MRAP (mutations on the extracellular face) (A) or the second copy of MRAP (mutations on the intracellular face) (B) are shown. Mutated segments are shown in red. In A and B, cells were incubated for 4–5 h with ACTH, and the response was measured with the CRE-luciferase assay and normalized to the forskolin response. In C, cells were incubated with ACTH for 20 min in buffer containing 0.5 mm isobutylmethylxanthine, and cAMP mass was measured by ELISA. The data were normalized to surface HA-receptor measured in parallel cultures. Relative to the value in cells transfected with MRAP/MC2R, surface receptor levels were 58.3% (MRAP-MRAP-MC2R), 144% (MRAP-(18–21A)MRAP-MC2R), and 35.5% ((18–21A)MRAP-MRAP-MC2R). Color schemes and sequences are given in Figs. 1 and 4. Error bars show S.E.
Because the CRE-luciferase reporter is downstream and requires several hours for activation, desensitization could have a substantial effect on the readout of the assay. For this reason, we measured a more proximal step in receptor activation, generation of the second messenger cAMP (Fig. 5C). cAMP mass measurements were normalized to the relative concentration of receptors on the surface, which is reported in the legend. ACTH stimulated an 18-fold increase in cAMP mass in cells expressing free receptor plus MRAP and 7–25-fold increases in cells expressing fusion proteins. When cAMP mass was quantified, EC50 values were fairly similar for free receptors and fusion proteins containing wild-type MRAPs (1.08 ± 0.48 and 1.81 ± 0.9 nm, respectively). The fusion proteins displayed better activity, relative to free receptor, when cAMP mass rather than cAMP reporter activity was measured, suggesting possible differences in desensitization or other downstream processes.
In agreement with measurements of the CRE-luciferase responses, fusion proteins containing the inactive (18–21A)MRAP in the A copy were much less active than those with the same mutation in the B position when cAMP was quantified. These results indicate that the Nout copy of MRAP is needed for the initial signaling event.
Mutant Fusion Protein Expression
Fig. 6A presents data compiled from multiple studies in which ACTH responses and surface expression of MRAP-MRAP-receptor fusion proteins and wild-type MC2 receptor were compared in the same experiments. Fusion proteins containing wild-type MRAP displayed 70–80% of the activity of free MC2 receptor/MRAP in response to 1 μm ACTH, a concentration sufficient to generate maximal responses. All fusion proteins containing mutations in the A subunit were significantly less active than the corresponding wild-type version, whereas none of the fusions with mutations in the B copy of MRAP differed from control.
FIGURE 6.
Responses and expression of MRAP-MRAP-MC2R fusion proteins. A, responses to 1 μm ACTH and surface expression of MRAP-MRAP-MC2R fusion proteins were measured in 4–11 experiments, each in duplicate or triplicate. Means ± S.E. are plotted. *, p < 0.05 versus wild type. Error bars show S.E. B, Western blot of lysates prepared from cells expressing MC2R alone, V5-MRAP-3xFLAG alone, or fusion proteins. Each lysate was run on two separate gels, which were blotted with either anti-HA or anti-V5 antibody to detect receptor or MRAP, respectively. Wild-type free MRAP was transfected at a lower level than fusion proteins.
Surface expression of all of the fusion proteins was compared with MC2 receptor alone, again measured at the same time. There was more variability in surface expression than in activity. Western blots provided an estimate of total receptor concentration (Fig. 6B). All of the fusion proteins ran at high molecular weight in broad bands on SDS-PAGE as expected because both MRAP and MC2 receptors have consensus sites for N-glycosylation and are known to be glycosylated (4, 19). In general, mutations in the A MRAP reduced expression, suggesting less efficient folding or trafficking.
We also performed titration experiments using DNA encoding both the inactive (A copy) and active (B copy) versions of fusion proteins containing (18–21A)- and del(31–37)MRAP (Fig. 7A). MRAP-(18–21A)MRAP-MC2R and free MC2R were expressed at high levels, but the highly active MRAP-del(31–37)MRAP-MC2R was present on the surface at lower density as were the two inactive fusions.
FIGURE 7.
Effect of receptor density on concentration-response curves of fusion proteins. A, free MC2R and MRAP and mutant fusion proteins were tested as described for Fig. 2. B and C, cells were transfected using either 3 or 30 ng of receptor DNA/well in a 96-well plate and equivalent amounts in 24-well plates. ACTH responses (B) and surface expression (C) were measured 24 h later. Dashed lines show the lower concentrations of DNA. In all cases, receptor expression was significantly lower with the lower amount of DNA (p < 0.05). Error bars show S.E.
To determine whether the concentration-response relationship depended on receptor expression, we transfected cells with 10-fold different amounts of receptor DNA and measured CRE-luciferase responses and surface expression (Fig. 7B). None of the constructs with mutations in the A copy of MRAP displayed much activity, whereas fusion proteins with wild-type or mutant MRAPs in the B position responded well at both low and high expression levels. Effects of receptor concentration on the EC50 values for ACTH varied in different directions, and the differences were less than 10-fold in all cases. For comparison, using the same assay, we found that overexpression of the β2-adrenergic receptor shifted the EC50 for isoproterenol to the left by more than 100-fold.
Importance of the MRAP Transmembrane Domain
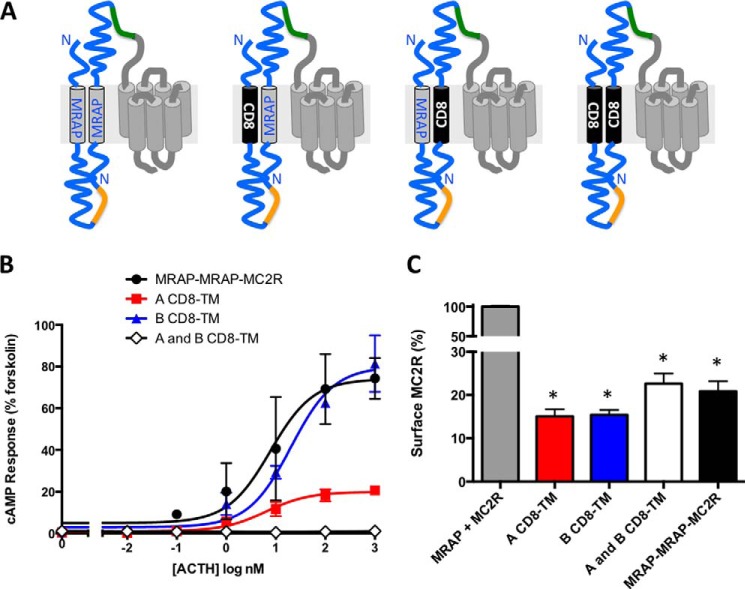
If the primary function of the highly conserved transmembrane domain is simply to bring the antiparallel MRAP dimer to the receptor, it seemed conceivable that this segment could be replaced in MRAP-MRAP-receptor fusions where the orientation of the two MRAP molecules was fixed. We therefore replaced 23 aa of the MRAP transmembrane region with the 23-aa transmembrane sequences from human CD8 in fusion proteins. CD8 normally has an Nout orientation. When the CD8 transmembrane domain was placed in the A copy of MRAP, activity of the (CD8-TM)MRAP-MRAP-MC2R was reduced but not entirely absent (Fig. 8). When the CD8 transmembrane domain was inserted into the B copy of MRAP, the MRAP-(CD8-TM)MRAP-MC2R was fully active. This result is in striking contrast to the finding that free (CD8-TM)MRAP was completely non-functional (compare Figs. 4 and 8). When both copies of MRAP in the fusion protein contained CD8 transmembrane helices, the MC2 receptor was inactive. Surface expression of all CD8-substituted fusions appeared to be adequate (Fig. 8C).
FIGURE 8.
The transmembrane domain is required on the Nout copy of MRAP. A, presumed orientations of MRAP-MRAP-MC2R fusions in which the transmembrane domains of MRAP in either the A, B, or A and B copies of MRAP were replaced with the transmembrane domain of human CD8α (shown in black). B, ACTH responses. C, surface expression. Color schemes and sequences are described in Figs. 1 and 4. Error bars show S.E. *, p < 0.05 versus MC2R/MRAP.
Evidence for Formation of Multimeric MRAP-Receptor Complexes
We tested responses of active and inactive MRAP-MRAP-receptor fusions in the presence of co-transfected MRAP. When MRAP-MRAP-MC2R was expressed with free wild-type MRAP, the concentration-response curve shifted to the left such that the free receptor and the fusion protein gave equivalent ACTH responses (Fig. 9A). Furthermore, co-expressed free MRAP completely restored the activity of the fusion protein inactivated by the 18–21A substitution in the A copy of MRAP (Fig. 9B).
FIGURE 9.
Free MRAP restores activity of MC2R fused to wild-type or mutant MRAP dimers, and MRAP-MRAP fused to a signaling-incompetent receptor activates free MC2R. MC2 receptor alone or fusion proteins containing wild-type (A) or mutant (B) MRAPs were expressed with or without wild-type MRAP. C and D, an E80K mutation was introduced into MC2 receptor and MRAP-MRAP-MC2R. Mutant receptors were expressed with or without MRAP and wild-type MC2 receptor as shown. C, ACTH responses. E80K-substituted receptors gave no ACTH response with or without co-expressed wild-type MRAP (not shown for fusion protein). D, surface expression of E80K-receptors. Error bars show S.E. *, p < 0.05 versus MC2R/MRAP. E, models of MRAP/MRAP/MC2 receptor multimers.
We next asked whether an MRAP-MRAP-receptor fusion protein containing an inactive receptor would be able to activate a wild-type receptor in the absence of free MRAP. Ala substitution for Glu-80 near the top of the second transmembrane helix of the MC2 receptor has previously been shown to cause a 10-fold reduction in ACTH binding (20). This residue is conserved and believed to interact directly with the melanocortin pharmacophore His-Phe-Arg-Trp. We replaced Glu-80 with Lys and found a complete loss of activity (Fig. 9C). The MRAP-MRAP-(E80K)MC2R was likewise completely inactive, and addition of free MRAP did not unveil any signaling. When MRAP-MRAP-(E80K)MC2 receptor was expressed with free wild-type MC2 receptor, however, a significant ACTH response was observed (Fig. 9C). E80K substitution did not inhibit surface receptor expression (Fig. 9D). These findings are most easily explained if multiple MRAP molecules and multiple MC2 receptors interact (Fig. 9E).
Activity of MRAP-MRAP-MC2 Receptors in CHO Cells
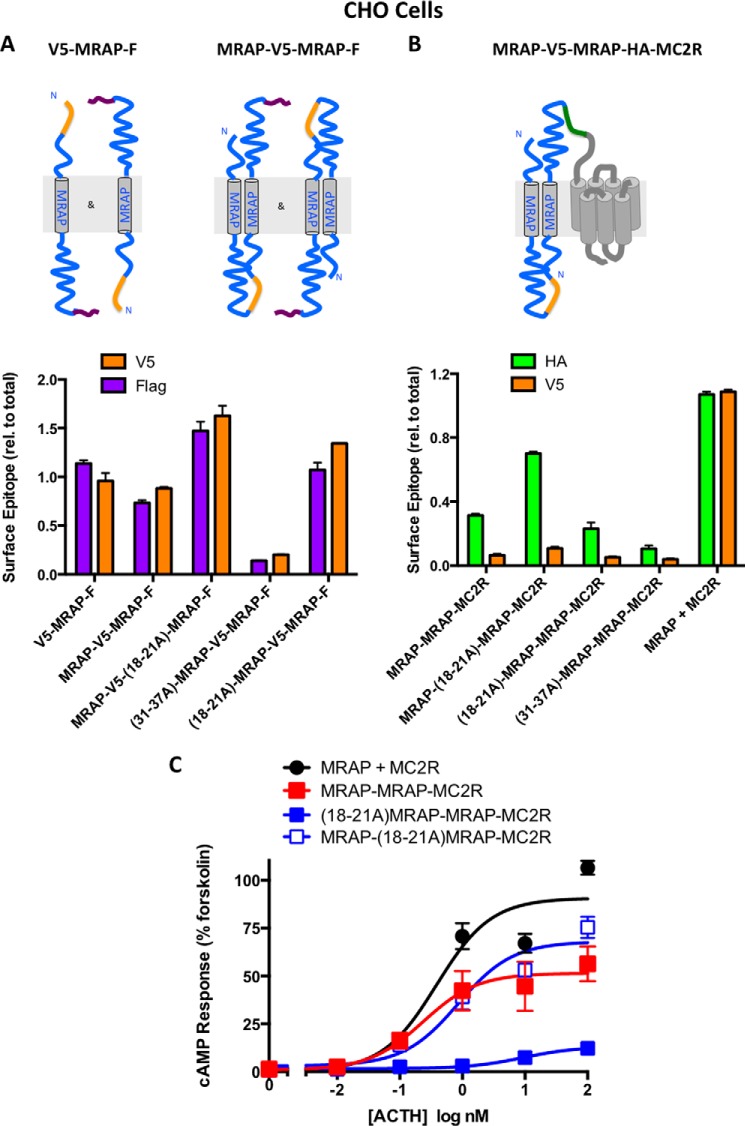
We elected to work with HEK293 cells because their strong cAMP responses were helpful for analysis of weakly active mutant fusion proteins. HEK293 cells have no detectable MRAP, but they do contain mRNA encoding MRAP2 (19), which can promote MC2 receptor trafficking and dimerize with MRAP (6, 8). When cells are transfected with MRAP2 and MC2 receptors, micromolar concentrations of ACTH are required to stimulate cAMP formation as opposed to subnanomolar concentrations with MRAP (6). As shown by the open circles in Figs. 1 and 4 of this study, HEK293 cells transfected with MC2 receptor but without an accessory protein failed to respond to ACTH at any concentration; i.e. endogenous MRAP2 could not support signaling. Surface expression of MC2 receptor is more dependent on transfected MRAP in CHO cells than in HEK293 cells (2–4) likely because endogenous MRAP2 is absent or less abundant in CHO cells. For this reason, we repeated key experiments using CHO cells and confirmed that free MRAP and tandem versions of both wild-type and mutant MRAP assumed dual topology (Fig. 10A, left). The predominant orientation of both active (wild-type and (18–21A)MRAP in the B copy) and inactive fusion proteins ((18–21A)- and (31–37A)MRAPs in the A copy) was the same as that determined in HEK293 cells with the HA-tagged receptor amino terminus on the extracellular face of the membrane (Fig. 10B). Most importantly, the MRAP copy with an Nout topology was again required for activity in CHO cells (Fig. 10C), establishing that the results reported here were not confounded by the possible presence of MRAP2 in HEK293 cells.
FIGURE 10.
Orientation and responses of MRAPs and MRAP-MRAP-receptor fusion constructs in CHO cells. A, CHO cells were transfected with either V5-MRAP-3xFLAG or tandem MRAP-V5-MRAP-3xFLAG proteins with mutations as shown. Relative expression of V5 and FLAG epitopes on the cell surface and in permeabilized cells was determined as described under “Experimental Procedures” and expressed relative to total for each epitope. B, CHO cells were transfected with wild-type or mutant MRAP-MRAP-MC2R fusion proteins. Total and surface HA and V5 epitopes were measured, and results were expressed relative (rel.) to total for each epitope. Although relative expression of an epitope can be compared for different mutants, surface expression of proteins cannot be compared directly with total because of differences in assay conditions. C, CHO cells were transfected to express MRAP plus MC2 receptor or the MRAP-MRAP-MC2 receptor constructs shown, and responses to ACTH were determined. Error bars show S.E.
Discussion
The fusion protein approach has provided conclusive evidence that signaling by the MC2 receptor requires MRAP actions on the outside of the cell where it is poised to affect ACTH binding and receptor activation. Two regions of ACTH are particularly important for activating the MC2 receptor: the His-Phe-Arg-Trp pharmacophore (residues 6–9 of ACTH) and Lys-Lys-Arg-Arg-Pro (residues16–20) (20, 21). Although no MC receptor structure has been solved, analyses of evolutionary patterns, responses to ACTH analogs, and effects of receptor mutations have led to structural models in which the ACTH peptide interacts with amino acids at the extracellular face of multiple transmembrane helices (20, 22). Liang et al. (21) have suggested that binding is a two-step process in which the KKRRP segment of ACTH engages the receptor first and causes conformational changes that enable HFRW binding and G protein coupling. The MRAP partner in the Nout orientation could contribute at either step.
Roy et al. (5) have reported that hMRAPα is present in a single Nin orientation on the plasma membrane. It is difficult to reconcile this finding with our contention that Nout MRAP is essential for activity. Experiments utilizing bioluminescence resonance energy transfer led Cooray et al. (10) to conclude that an antiparallel dimer was the active form of hMRAPα, and we have found that surface-localized V5-hMRAPα-3xFLAG assumes a dual topology (data not shown). These contradictory results may reflect differences in expression levels or the use of different epitope tags, although algorithms such as TM-PRED and TMHMM predict that the epitope-tagged MRAPs used will in all cases strongly prefer an Nout orientation. Our finding that Nout MRAP is required does not mean that Nin MRAP has no function. In fact, the MRAP-MC2 receptor, where the single MRAP was presumably orientated with Nin, had low but real activity; without MRAP, there was no detectable ACTH response.
A key question is whether mutant fusion proteins failed to respond to ACTH because they were not expressed well on the plasma membrane or because they were intrinsically less active. Some of the inactive mutants were in fact expressed weakly. Nonetheless, titration experiments showed that increasing the levels of surface-localized receptors failed to increase maximal responses or lower EC50 values substantially for either wild-type or mutant fusion proteins. These results support the conclusion that the Tyr-rich and juxtamembrane Lys-rich regions of the Nout copy of MRAP, which faces the outside of cells, are required for either ACTH binding or ACTH-dependent conformational changes initiating the signal transduction cascade. The results are in accord with our previous finding that free MRAPs with mutations in these regions support MC2 receptor trafficking but not the binding of radiolabeled ACTH or signaling (6).
The molecular basis of MC2 receptor-MRAP association in unknown. Interestingly, missense mutations in human MC2 receptors responsible for clinical ACTH resistance do not prevent receptors from interacting with hMRAPα but do prevent them from reaching the plasma membrane (23). All teleost and tetrapod MC2 receptors require MRAP, but MC2 receptors from a cartilaginous fish do not (11, 24). Unfortunately, the mammalian and elephant shark receptors are sufficiently different that a basis for MRAP dependence cannot be deduced readily from their sequences (11). Several groups have generated MC2/MC4 receptor chimeras in an effort to pinpoint the differences that account for MRAP dependence (25–28). This approach has not provided any simple answers either, but the data are consistent with involvement of the receptor amino terminus, outer regions of transmembrane helices 2 and 3, and the second extracellular loop, which could interact with the Nout MRAP partner, as well as receptor transmembrane domains.
The existence of MC2 receptor dimers has been reported previously based on co-immunoprecipitation, bimolecular fluorescence complementation, and bioluminescence resonance energy transfer approaches (10, 16). Here we found that free MC2 receptor, which is incapable of signaling on its own, becomes functional in the presence of a fusion protein containing wild-type MRAPs fused to a dead receptor. This implies that multiple receptors can interact with a single MRAP dimer; it was not previously known whether receptors in oligomers actually signal. We also found that free MRAP rescues responses of fusion proteins containing inactivating MRAP mutations; this is most easily explained if a single receptor is in a complex with multiple MRAP dimers. In accord with the present data, Cooray et al. (10) observed bioluminescence resonance energy transfer signals consistent with antiparallel MRAP dimers and a weak signal suggesting additional interactions between MRAPs in a parallel orientation but only when MC2 receptors were included. They concluded that at least two antiparallel MRAP dimers and one MC2 receptor are present in a signaling complex. Fridmanis et al. (27) also proposed that MRAPs interact with a receptor at different positions. We failed to detect fluorescence when YFP fragments were placed on the MRAP carboxyl terminus, perhaps due to the particular geometry of the constructs or lack of sensitivity (6).
There is a large and ongoing debate in the literature concerning the role of monomers versus oligomers in signaling by rhodopsin family GPCRs (29, 30), and the data described here should be interpreted cautiously. Because relatively few receptors are needed for maximal signaling, multimers of MRAP and MC2 receptors may have been abundant enough to generate a robust cAMP response but a minor species overall. Overexpression is expected to drive the formation of multimeric complexes. Despite these caveats, it is clear that at least for the fusion proteins characterized here 1) multiple MRAP dimers can interact with a single receptor, 2) multiple receptors can interact with a single MRAP dimer, and 3) higher order structures can generate ACTH-mediated cAMP responses.
The MC receptor family has a number of unusual features. Multiple endogenous agonists are derived from a single peptide precursor, and endogenous inverse agonists (agouti signaling protein and agouti-related protein) powerfully suppress MC1, MC3, and MC4 receptor signaling. Although only the MC2 receptor requires an accessory protein, MRAP and MRAP2 both co-precipitate with all five MC receptors, and MRAP2 regulates responses of several of them, most notably the MC4 receptor involved in food intake and energy expenditure (6, 13–15, 31, 32). MRAP and MRAP2 heterodimerize readily, and MRAP2 can act in a dominant negative fashion to antagonize the effects of MRAP on the MC2 receptor (8). The results shown here raise the possibility that multiple dimers of MRAP and MRAP2 can interact with a given receptor, adding a new dimension to an already complex signaling system.
Many GPCRs can be expressed easily and signal well in widely used model cell systems; MC1 and MC3–5 receptors fall into this category. Others, including most in the large odorant and taste receptor families, are notoriously difficult to express. A smaller set of GPCRs, including MC2 receptors, are inactive unless bound to specific accessory proteins. One group of accessory proteins, the receptor transport proteins (RTP1–4), act selectively at certain GPCRs to promote folding and trafficking to the plasma membrane. RTP1S acts at multiple steps to enable odorant receptors to reach the plasma membrane (33, 34), whereas RTP4 promotes trafficking of μ-δ-opioid receptor heterodimers (35). RTPs are single transmembrane domain proteins with an Nin-Cout orientation, but they are thought to act intracellularly because a cytoplasmic version is active (36). Receptor activity-modifying proteins (RAMPs) (37) interact with secretin-like (B family) GPCRs characterized by a long, structured amino terminus. RAMPs are small proteins with large extracellular amino-terminal domains, single transmembrane helices, and very short intracellular tails. RAMPs form stable complexes with receptors and exert dramatic effects on ligand specificity. In the presence of RAMPs 1–3, the calcitonin receptor becomes an amylin receptor, whereas the calcitonin receptor-like receptor binds calcitonin gene-related peptide with RAMP1 but is converted to an adrenomedullin-binding form in the presence of RAMP2 or -3. The crystal structure of the complex between the extracellular domains of RAMP1 and calcitonin receptor-like receptor has been solved and shows a large interaction surface between RAMP1 and the receptor (38).
Like RAMP1 and calcitonin receptor-like receptor, MRAP accompanies its receptor from the endoplasmic reticulum to the plasma membrane where the accessory protein remains stably associated and necessary for agonist binding. However, a minimum of one MRAP dimer associates with each MC2 receptor. Both the Nout MRAP and the MC2 receptor have small extracellular domains. It is not clear whether the MRAP dimer interacts with the MC2 receptor exclusively through the extracellular regions or, as seems more likely, through interactions that also involve transmembrane domains. Novel approaches will be needed to determine whether MRAP or multiple MRAPs participate directly in ACTH binding or act indirectly to drive the receptor into a conformation with high ACTH affinity.
Given the evidence that the Nout MRAP is the business end of the antiparallel MRAP dimer, it is reasonable to ask what the Nin copy of MRAP is doing. The dual topology MRAP2 protein arose more than 500 million years ago at about the same time as MC receptors, and gene duplication events gave rise to MRAP and additional MC receptors (24, 39). We can speculate that the antiparallel MRAP dimer is needed for binding to MC receptors or for MRAP stability, requirements that were bypassed in the fusion proteins, but additional work will be required to test these ideas. The findings described here may serve as a stepping stone to a deeper insight into the important ACTH signaling pathway.
Author Contributions
S. M. and Z. J. M. designed and generated constructs. P. M. H. designed experiments. P. M. H., S. M., and T. M. D. performed experiments. P. M. H. wrote the manuscript.
This work was supported by National Institutes of Health Grants DK19974 and DK67214. The authors declare that they have no conflicts of interest with the contents of this article.
- GPCR
- G protein-coupled receptor
- MC
- melanocortin
- MC2R
- MC2 receptor
- MRAP
- MC2 receptor accessory protein
- Nin
- intracellular amino terminus
- Nout
- extracellular amino terminus
- mMRAP
- mouse MRAP
- hMRAP
- human MRAP
- CRE
- cAMP response element
- aa
- amino acid(s)
- TM
- transmembrane domain
- RTP
- receptor transport protein
- RAMP
- receptor activity-modifying protein.
References
- 1.Meimaridou E., Hughes C. R., Kowalczyk J., Chan L. F., Clark A. J., and Metherell L. A. (2013) ACTH resistance: genes and mechanisms. Endocr. Dev. 24, 57–66 [DOI] [PubMed] [Google Scholar]
- 2.Metherell L. A., Chapple J. P., Cooray S., David A., Becker C., Rüschendorf F., Naville D., Begeot M., Khoo B., Nürnberg P., Huebner A., Cheetham M. E., and Clark A. J. (2005) Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 37, 166–170 [DOI] [PubMed] [Google Scholar]
- 3.Roy S., Rached M., and Gallo-Payet N. (2007) Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol. Endocrinol. 21, 1656–1669 [DOI] [PubMed] [Google Scholar]
- 4.Sebag J. A., and Hinkle P. M. (2007) Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc. Natl. Acad. Sci. U.S.A. 104, 20244–20249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy S., Roy S. J., Pinard S., Agulleiro M. J., Cerdá-Reverter J. M., Parent J. L., and Gallo-Payet N. (2012) The C-terminal domains of melanocortin-2 receptor (MC2R) accessory proteins (MRAP1) influence their localization and ACTH-induced cAMP production. Gen. Comp. Endocrinol. 176, 265–274 [DOI] [PubMed] [Google Scholar]
- 6.Sebag J. A., and Hinkle P. M. (2009) Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J. Biol. Chem. 284, 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webb T. R., Chan L., Cooray S. N., Cheetham M. E., Chapple J. P., and Clark A. J. (2009) Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 150, 720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebag J. A., and Hinkle P. M. (2010) Regulation of G protein-coupled receptor signaling: specific dominant-negative effects of melanocortin 2 receptor accessory protein 2. Sci. Signal. 3, ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinkle P. M., and Sebag J. A. (2009) Structure and function of the melanocortin2 receptor accessory protein (MRAP). Mol. Cell. Endocrinol. 300, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooray S. N., Chung T. T., Mazhar K., Szidonya L., and Clark A. J. (2011) Bioluminescence resonance energy transfer reveals the adrenocorticotropin (ACTH)-induced conformational change of the activated ACTH receptor complex in living cells. Endocrinology 152, 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinick C. L., Liang L., Angleson J. K., and Dores R. M. (2012) Identification of an MRAP-independent melanocortin-2 receptor: functional expression of the cartilaginous fish, Callorhinchus milii, melanocortin-2 receptor in CHO cells. Endocrinology 153, 4757–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy S., Roy S. J., Pinard S., Taillefer L. D., Rached M., Parent J. L., and Gallo-Payet N. (2011) Mechanisms of melanocortin-2 receptor (MC2R) internalization and recycling in human embryonic kidney (HEK) cells: identification of Key Ser/Thr (S/T) amino acids. Mol. Endocrinol. 25, 1961–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kay E. I., Botha R., Montgomery J. M., and Mountjoy K. G. (2013) hMRAPa increases αMSH-induced hMC1R and hMC3R functional coupling and hMC4R constitutive activity. J. Mol. Endocrinol. 50, 203–215 [DOI] [PubMed] [Google Scholar]
- 14.Chan L. F., Webb T. R., Chung T. T., Meimaridou E., Cooray S. N., Guasti L., Chapple J. P., Egertová M., Elphick M. R., Cheetham M. E., Metherell L. A., and Clark A. J. (2009) MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc. Natl. Acad. Sci. U.S.A. 106, 6146–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay E. I., Botha R., Montgomery J. M., and Mountjoy K. G. (2013) hMRAPa specifically alters hMC4R molecular mass and N-linked complex glycosylation in HEK293 cells. J. Mol. Endocrinol. 50, 217–227 [DOI] [PubMed] [Google Scholar]
- 16.Sebag J. A., and Hinkle P. M. (2009) Opposite effects of the melanocortin-2 (MC2) receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J. Biol. Chem. 284, 22641–22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chepurny O. G., and Holz G. G. (2007) A novel cyclic adenosine monophosphate responsive luciferase reporter incorporating a nonpalindromic cyclic adenosine monophosphate response element provides optimal performance for use in G protein coupled receptor drug discovery efforts. J. Biomol. Screen. 12, 740–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rached M., El Mourabit H., Buronfosse A., Blondet A., Naville D., Begeot M., and Penhoat A. (2005) Expression of the human melanocortin-2 receptor in different eukaryotic cells. Peptides 26, 1842–1847 [DOI] [PubMed] [Google Scholar]
- 19.Roy S., Perron B., and Gallo-Payet N. (2010) Role of asparagine-linked glycosylation in cell surface expression and function of the human adrenocorticotropin receptor (melanocortin 2 receptor) in 293/FRT cells. Endocrinology 151, 660–670 [DOI] [PubMed] [Google Scholar]
- 20.Chen M., Aprahamian C. J., Kesterson R. A., Harmon C. M., and Yang Y. (2007) Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry 46, 11389–11397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang L., Angleson J. K., and Dores R. M. (2013) Using the human melanocortin-2 receptor as a model for analyzing hormone/receptor interactions between a mammalian MC2 receptor and ACTH(1–24). Gen. Comp. Endocrinol. 181, 203–210 [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Chen M., Kesterson R. A. Jr., and Harmon C. M. (2007) Structural insights into the role of the ACTH receptor cysteine residues on receptor function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1120–R1126 [DOI] [PubMed] [Google Scholar]
- 23.Chung T. T., Webb T. R., Chan L. F., Cooray S. N., Metherell L. A., King P. J., Chapple J. P., and Clark A. J. (2008) The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. J. Clin. Endocrinol. Metab. 93, 4948–4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valsalan R., Krishnan A., Almén M. S., Fredriksson R., and Schiöth H. B. (2013) Early vertebrate origin of melanocortin 2 receptor accessory proteins (MRAPs). Gen. Comp. Endocrinol. 188, 123–132 [DOI] [PubMed] [Google Scholar]
- 25.Hinkle P. M., Serasinghe M. N., Jakabowski A., Sebag J. A., Wilson K. R., and Haskell-Luevano C. (2011) Use of chimeric melanocortin-2 and -4 receptors to identify regions responsible for ligand specificity and dependence on melanocortin 2 receptor accessory protein. Eur. J. Pharmacol. 660, 94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Chen M., Dimmitt R., and Harmon C. M. (2014) Structural insight into the MC4R conformational changes via different agonist-mediated receptor signaling. Biochemistry 53, 7086–7092 [DOI] [PubMed] [Google Scholar]
- 27.Fridmanis D., Petrovska R., Kalnina I., Slaidina M., Peculis R., Schiöth H. B., and Klovins J. (2010) Identification of domains responsible for specific membrane transport and ligand specificity of the ACTH receptor (MC2R). Mol. Cell. Endocrinol. 321, 175–183 [DOI] [PubMed] [Google Scholar]
- 28.Fridmanis D., Petrovska R., Pjanova D., Schiöth H. B., and Klovins J. (2014) Replacement of short segments within transmembrane domains of MC2R disrupts retention signal. J. Mol. Endocrinol. 53, 201–215 [DOI] [PubMed] [Google Scholar]
- 29.Bouvier M., and Hébert T. E. (2014) Crosstalk proposal: weighing the evidence for class A GPCR dimers, the evidence favours dimers. J. Physiol. 592, 2439–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert N. A., and Javitch J. A. (2014) Crosstalk opposing view: weighing the evidence for class A GPCR dimers, the jury is still out. J. Physiol. 592, 2443–2445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asai M., Ramachandrappa S., Joachim M., Shen Y., Zhang R., Nuthalapati N., Ramanathan V., Strochlic D. E., Ferket P., Linhart K., Ho C., Novoselova T. V., Garg S., Ridderstråle M., Marcus C., Hirschhorn J. N., Keogh J. M., O'Rahilly S., Chan L. F., Clark A. J., Farooqi I. S., and Majzoub J. A. (2013) Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 341, 275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebag J. A., Zhang C., Hinkle P. M., Bradshaw A. M., and Cone R. D. (2013) Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science 341, 278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Björk S., Hurt C. M., Ho V. K., and Angelotti T. (2013) REEPs are membrane shaping adapter proteins that modulate specific G protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS One 8, e76366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito H., Kubota M., Roberts R. W., Chi Q., and Matsunami H. (2004) RTP family members induce functional expression of mammalian odorant receptors. Cell 119, 679–691 [DOI] [PubMed] [Google Scholar]
- 35.Décaillot F. M., Rozenfeld R., Gupta A., and Devi L. A. (2008) Cell surface targeting of μ-delta opioid receptor heterodimers by RTP4. Proc. Natl. Acad. Sci. U.S.A. 105, 16045–16050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu L., Pan Y., Chen G. Q., Matsunami H., and Zhuang H. (2012) Receptor-transporting protein 1 short (RTP1S) mediates translocation and activation of odorant receptors by acting through multiple steps. J. Biol. Chem. 287, 22287–22294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sexton P. M., Poyner D. R., Simms J., Christopoulos A., and Hay D. L. (2012) RAMPs as drug targets. Adv. Exp. Med. Biol. 744, 61–74 [DOI] [PubMed] [Google Scholar]
- 38.ter Haar E., Koth C. M., Abdul-Manan N., Swenson L., Coll J. T., Lippke J. A., Lepre C. A., Garcia-Guzman M., and Moore J. M. (2010) Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure 18, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 39.Dores R. M., and Garcia Y. (2015) Views on the co-evolution of the melanocortin-2 receptor, MRAPs, and the hypothalamus/pituitary/adrenal-interrenal axis. Mol. Cell. Endocrinol. 408, 12–22 [DOI] [PubMed] [Google Scholar]