Abstract
Background
Desmoid fibromatosis (desmoid tumor, DT) is a soft tissue neoplasm prone to recurrence despite complete surgical resection. Numerous small retrospective reports suggest that non-cytotoxic chemotherapy using tamoxifen and sulindac may be effective for DT. We evaluated the safety and efficacy of tamoxifen and sulindac in a prospective phase II study within the Children’s Oncology Group.
Procedures
Eligible patients were <19 years of age who had measurable DT that was recurrent or not amenable to surgery or radiation. The primary objective was to estimate progression-free survival (PFS). Patients received tamoxifen and sulindac daily for 12 months or until disease progression or intolerable toxicity occurred. Response was assessed by magnetic resonance imaging.
Results
Fifty-nine eligible patients were enrolled from 2004 to 2009; 78% were 10–18 years old. Twenty-two (38%) were previously untreated; 15 (41%) of the remaining 37 enrolling with recurrent DT had prior systemic chemotherapy and six (16%) had prior radiation. No life-threatening toxicity was reported. Twelve (40%) of 30 females developed ovarian cysts, which were asymptomatic in 11 cases. Ten patients completed therapy without disease progression or discontinuing treatment. Responses included four partial and one complete (5/59, 8%). The estimated 2-year PFS and survival rates were 36% (95% confidence interval: 0.23–0.48) and 96%, respectively. All three deaths were due to progressive DT.
Conclusions
Tamoxifen and sulindac caused few serious side effects in children with DT, although ovarian cysts were common. However, the combination showed relatively little activity as measured by response and PFS rates.
Keywords: chemotherapy, desmoid-type fibromatosis, sulindac, tamoxifen
INTRODUCTION
Desmoid tumor (DT), also known as desmoid-type and aggressive fibromatosis, is a clonally derived, locally aggressive neoplasm that lacks the capacity to metastasize [1]. Historically, therapy has focused on achieving disease control by surgery with or without radiation therapy (RT) [2,3]. In some patients, especially children, complete excision is not feasible without compromising form and/or function, and RT has both acute and late effects that can cause significant morbidity [4–6]. DT has also been treated with chemotherapeutic agents to decrease tumor size and either facilitate delayed operative removal or provide disease control without other therapy. A variety of combinations of cytotoxic and non-cytotoxic agents have been shown to have some activity in DT, but most reports represent small, retrospective series. One relatively large prospective trial in pediatric DT, carried out by the Pediatric Oncology Group (POG 9650), showed that vinblastine and methotrexate could control DT in a substantial proportion of children (estimated progression-free survival [PFS] was 46% at 2 years) [7].
While the POG 9650 data were maturing, the Children’s Oncology Group (COG) investigated whether non-cytotoxic therapy could provide at least as good disease control with less toxicity. The approach was based on numerous reports of small groups of adults with DT treated using sulindac and tamoxifen [8–11]. The rationale for sulindac, a non-steroidal anti-inflammatory drug, included the fact that DT is associated with germ-line mutations in the Adenomatous Polyposis Coli (APC) gene leading to familial adenomatous polyposis (FAP) and the related Gardner syndrome (FAP plus DT, osteoma, and other anomalies) [12,13]. Further, DT occurring outside of the setting of Gardner syndrome/FAP often demonstrates somatic mutations in APC or CTNNB1 encoding β-Catenin, a downstream effector of APC [14]; the net effect of either mutation is increased β-Catenin protein activity. APC gene mutation is known to enhance the activity of the peroxisome proliferator-activated receptor δ (PPARδ), and this effect can be blocked by the NSAID sulindac [15]. Further, pharmacological or genetic cyclooxygenase-2 inhibition suppresses intestinal polyp formation in FAP patients [16] and in mice with mutations in the orthologous mouse APC gene [17], respectively.
Beyond the aforementioned therapeutic reports, the use of tamoxifen was supported by clinical and pathological observations. Estrogen signaling has long been implicated in DT biology based on the fact that DT has a faster growth rate and higher incidence in pregnant or fertile women [18]. Histological studies at the time the study was conceived and more recent pathological findings confirm that a substantial number of DT cases express estrogen-binding proteins, especially estrogen receptor β [19]. Hence, both sulindac and tamoxifen could be considered molecularly targeted therapy for DT. This prospective Phase II study, COG ARST0321, was undertaken to evaluate their safety and efficacy in children and adolescents with DT.
METHODS
Patients
All patients had a diagnosis of DT verified by review of central pathology specimens (by DAH) at study enrollment. Patients fell into three categories: (1) those with newly diagnosed, previously untreated disease that was not amenable to complete surgical removal or RT; (2) those who had undergone tumor excision, provided that gross residual DT remained; and (3) those with recurrent DT documented by magnetic resonance imaging (MRI). Other eligibility criteria included (a) age less than 19 years at initial diagnosis; (b) adequate performance status (Lansky score of 50 or more); (c) normal blood count and renal, liver, and cardiac function; (d) measurable disease present on gadolinium-enhanced MRI; and (e) signed informed consent from the patient, parent, or guardian, according to institutional review board guidelines.
Patients were excluded if they (a) had received prior antitumor therapy with tamoxifen, sulindac, or other non-steroidal anti-inflammatory agents (NSAIDs) or estrogen antagonists; (b) had received other cytotoxic chemotherapy or radiation for the current disease recurrence; (c) were post-pubertal females who would not agree to use non-hormonal methods of contraception; or (d) had a history of deep venous thrombosis, a clinically significant bleeding disorder, aspirin allergy, or current pregnancy. Of note, prior occasional use of NSAIDs for pain was allowed. In patients with progressive DT who had received therapy prior to study enrollment, 1–4 weeks must have elapsed, depending on the specific prior therapy. Corticosteroid use was not allowed while on study.
Therapy
Sulindac and tamoxifen, administered twice daily with food, were dosed based on body weight: 3 mg/kg, with the maximum daily dose of 300 mg for each agent. Four, 3-month courses were to be delivered for (a) up to 1 year or (b) up to 1 month following a complete response (CR), whichever came first. Other formal criteria for ceasing protocol therapy included: (a) progressive disease (PD); (b) intolerable toxicity, including any toxicity interrupting therapy for more than 4 weeks; (c) refusal of further protocol therapy by patient, parent, or guardian, or (d) as determined by the treating physician.
If the tumor became suitable for surgical removal during protocol therapy, surgery was allowed; if the microscopic surgical margins were uninvolved by DT, sulindac and tamoxifen were to be continued for 1 month or through the completion of the 12-month period, whichever came first. Otherwise, protocol therapy was to be continued as originally planned. Adjuvant RT was not allowed during protocol therapy or in the follow-up period.
Appropriate dose modification or interruption of sulindac/tamoxifen was allowed for serious or life-threatening toxicity—NCI CTCAE v.3.0 Grade 3 or 4, respectively—or for Grade 2 toxicity lasting for more than 8 weeks. With resolution of toxicity, gradual escalation to original dose was attempted.
Response Assessment
Initial assessment and follow-up examinations included history, physical examination, MRI scans of the tumor, serum chemistry tests and complete blood counts, electrocardiography, and ophthalmologic examination before courses 1 and 3. Periodic endocrine evaluations included assessment of growth, bone age/bone density, hormone levels (LH, FSH, estradiol or testosterone, insulin-like growth factor [IGF]-1, IGF binding protein, thyroid tests), and leuprolide stimulation tests, plus pelvic ultrasound examinations in females (details of endocrine findings will be reported in a separate manuscript).
Response was assessed by institutional report of MRI obtained at the end of each 3-month course. Degree of response, based on comparison to initial MRI, was prospectively defined based on the maximal product of the two greatest perpendicular dimensions, to be consistent with the prior POG 9650 study [7]. Responses were coded as CR (no evidence of residual tumor), partial response (PR; decrease by >50%, with no new lesions), minor response (MR; decrease by >25% but ≤50%), stable disease (SD; decrease or increase by ≤25%), or PD (increase by >25%, or the appearance of one or more new sites of disease). Of note, all patients, including those electively discontinuing therapies, were followed for recurrence and survival.
Statistical Evaluation
The primary goal of the study was to estimate the PFS rate for patients treated with sulindac/tamoxifen. Survival estimates in POG 9650 showed no difference between patients enrolled with primary or recurrent DT, and a similar analysis was planned for the sulindac/tamoxifen patients. The estimated PFS was compared to a fixed outcome based on the POG 9650 results using Woolson’s 1-sample log-rank test [20], with interim results monitored using O’Brien and Fleming boundaries and four interim analyses [21]. Data were current as of October 1, 2011.
RESULTS
Accrual and Demographics
Seventy patients were enrolled on ARST0321 from February 2004 to May 2009. Eleven were declared ineligible due to no pre-study MRI (7) or other reasons (4), leaving 59 eligible patients. Tables I and II show the characteristics of the patients, sites of primary disease, and types of prior non-surgical therapy, where available, for those with recurrent disease. Seventy-eight percent of the patients were 10–18 years old at diagnosis. Twenty-two patients had newly diagnosed DT and 37 (63%) were enrolled with recurrent disease. Thirteen (24%) of 55 patients reporting had multifocal DT, and 10 (20%) of 50 reporting had a diagnosis or family history of FAP.
TABLE I.
Characteristics of Patients Enrolled on Two Prospective Phase II Clinical Trials for Children With Desmoid Tumor
| COG ARST0321 | POG 9650 | |
|---|---|---|
| Years of accrual | 2004–2009 | 1997–2001 |
| Period of accrual | 63 months | 35 months |
| Eligible subjects | 59 | 27 |
| Age at Dx [median (range)] | 13 years (<1–18 years) | 10 years (<1–18 years) |
| Males/females | 30/29 | 20/7 |
| Newly diagnosed | 22 (37%) | 11 (41%) |
| Recurrent disease | 37 (63%) | 16 (59%) |
| Prior chemotherapy | 15/37 (41%) | NA |
| Prior radiation therapy | 6/37 (16%) | NA |
| Multifocal disease | 13/55 (24%); 4 Unk | NA |
| FHx of FAP | 10/50 (20%); 9 Unk | NA |
COG, Children’s Oncology Group; POG, Pediatric Oncology Group; FAP, familial adenomatous polyposis; Dx, diagnosis; NA, not available; Unk, unknown.
TABLE II.
Anatomic Sites of Disease
| Site of disease | Patients (#)a |
|---|---|
| Extremity | 7 |
| Trunk | 12 |
| Face/neck | 4 |
| Other | 3 |
Data are from 26 patients reporting anatomic site of primary tumor.
Also shown in Table I are results of the prior Phase II study from the Pediatric Oncology Group, POG 9650, for the same categories. Accrual rate was lower for POG 9650 (9.6/year) than for ARST0321 (11.2/year) because the former study was open at fewer institutions. Ages at diagnosis were similar in the two studies, as was the proportion of patients with newly diagnosed disease. Note that only two of 27 eligible patients enrolling on POG 9650 were noted to have FAP [7]–as opposed to 20% of patients on the current study; this information was not systematically collected as part of POG 9650.
Twenty-four of the 59 patients (41%) discontinued the study therapy for reasons other than completing it or developing PD: 10 patients/parents refused further treatment; physicians determined that it was four patients’ best interest to stop; two patients had intolerable toxicity; and eight stopped for other reasons, including non-compliance with treatment (n = 3), family/patient decision (n = 2), declined any treatment (n = 1), ovarian cysts (n = 1), and consent withdrawal (n = 1).
Toxicity
Toxicity data were collected from 57 patients; one withdrew consent before starting therapy and a second had not submitted toxicity data at the time the study report was generated. All 57 submitted toxicity data through the first 3 months (Study Period 1); 36 patients submitted data from months 4 through 6 (Study Period 2); 27 from months 7 through 9 (Study Period 3); and 17 from months 10 through 12 months (Study Period 4). Targeted toxicities of concern at the outset of the study, such as gastritis or emesis secondary to NSAID use, or ocular problems or thrombo-embolism related to tamoxifen, were very rare (Table III). Similarly, serious (Grade 3) or life-threatening (Grade 4) non-targeted toxicities were also rare. For example, tinnitus, prolonged QTc interval, fever, fatigue, skin breakdown/decubitus, nausea, elevated ALT/AST, hypomagnesemia, and dizziness were each reported only once in Study Period 1. The most frequent Grade 3 toxicity in study period 1 was development of ovarian cysts (see details below). Grade 3 or more toxicity did not appear to increase in subsequent Study Periods.
TABLE III.
Targeted Toxicities in Each of Four Treatment Periods
| Period | Toxicity | Gradea | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| 1 | Gastritis | 55 | 0 | 2 | 0 |
| Vomiting | 42 | 10 | 4 | 1 | |
| Hemorrhage, GI—abdomen NOS | 56 | 1 | 0 | 0 | |
| Hemorrhage, other | 56 | 1 | 0 | 0 | |
| Ocular, other | 54 | 3 | 0 | 0 | |
| Pain—abdomen NOS | 43 | 10 | 2 | 2 | |
| Pain—head/headache | 49 | 3 | 4 | 1 | |
| Thrombosis/thrombus/embolism | 57 | 0 | 0 | 0 | |
| 2 | Gastritis | 35 | 0 | 1 | 0 |
| Vomiting | 35 | 1 | 0 | 0 | |
| Hemorrhage, GI—abdomen NOS | 36 | 0 | 0 | 0 | |
| Hemorrhage, other | 36 | 0 | 0 | 0 | |
| Ocular, other | 34 | 2 | 0 | 0 | |
| Pain—abdomen NOS | 27 | 7 | 2 | 0 | |
| Pain—head/headache | 34 | 0 | 2 | 0 | |
| Thrombosis/thrombus/embolism | 36 | 0 | 0 | 0 | |
| 3 | Gastritis | 27 | 0 | 0 | 0 |
| Vomiting | 25 | 0 | 2 | 0 | |
| Hemorrhage, GI—abdomen NOS | 27 | 0 | 0 | 0 | |
| Hemorrhage, other | 27 | 0 | 0 | 0 | |
| Ocular, other | 26 | 1 | 0 | 0 | |
| Pain—abdomen NOS | 23 | 2 | 1 | 1 | |
| Pain—head/headache | 24 | 1 | 2 | 0 | |
| Thrombosis/thrombus/embolism | 27 | 0 | 0 | 0 | |
| 4 | Gastritis | 16 | 0 | 1 | 0 |
| Vomiting | 15 | 1 | 1 | 0 | |
| Hemorrhage, GI—abdomen NOS | 17 | 0 | 0 | 0 | |
| Hemorrhage, other | 16 | 1 | 0 | 0 | |
| Ocular, other | 17 | 0 | 0 | 0 | |
| Pain—abdomen NOS | 13 | 0 | 2 | 2 | |
| Pain—head/headache | 13 | 2 | 1 | 1 | |
| Thrombosis/thrombus/embolism | 17 | 0 | 0 | 0 | |
GI, gastrointestinal; NOS, not otherwise specified.
Note that no Grade 4 or 5 targeted toxicities were observed.
Two AdEERS reports were filed for (a) Grade 4 depression during the third course of treatment, possibly due to sulindac and (b) Grade 5 acute kidney toxicity. This latter event had probable attribution to disease progression with a 29 × 38 cm mass arising from the root of the mesentery and only possibly related to study drugs. A single MedWatch report was filed for Grade 4 hepatopathy in a patient subsequently deemed ineligible due to inadequate pre-study staging.
Ovarian Cysts
Eleven (37%) of 30 girls with data from Study Period 1 developed one or more ovarian cysts in the first 3 months of therapy. Although the cysts were greater than 3 cm in 10 of them, only one patient was symptomatic. All 11 patients were diagnosed by routine, required pelvic ultrasonography. No adverse consequences of the cysts were reported, and no patient underwent surgical treatment of the cysts. Age was associated with the frequency of ovarian cysts, which were found in 11 (50%) of 22 girls 10 years of age and older and in none of eight girls less than age 10 (P = 0.014; Fisher’s exact test).
Response and PFS Rates
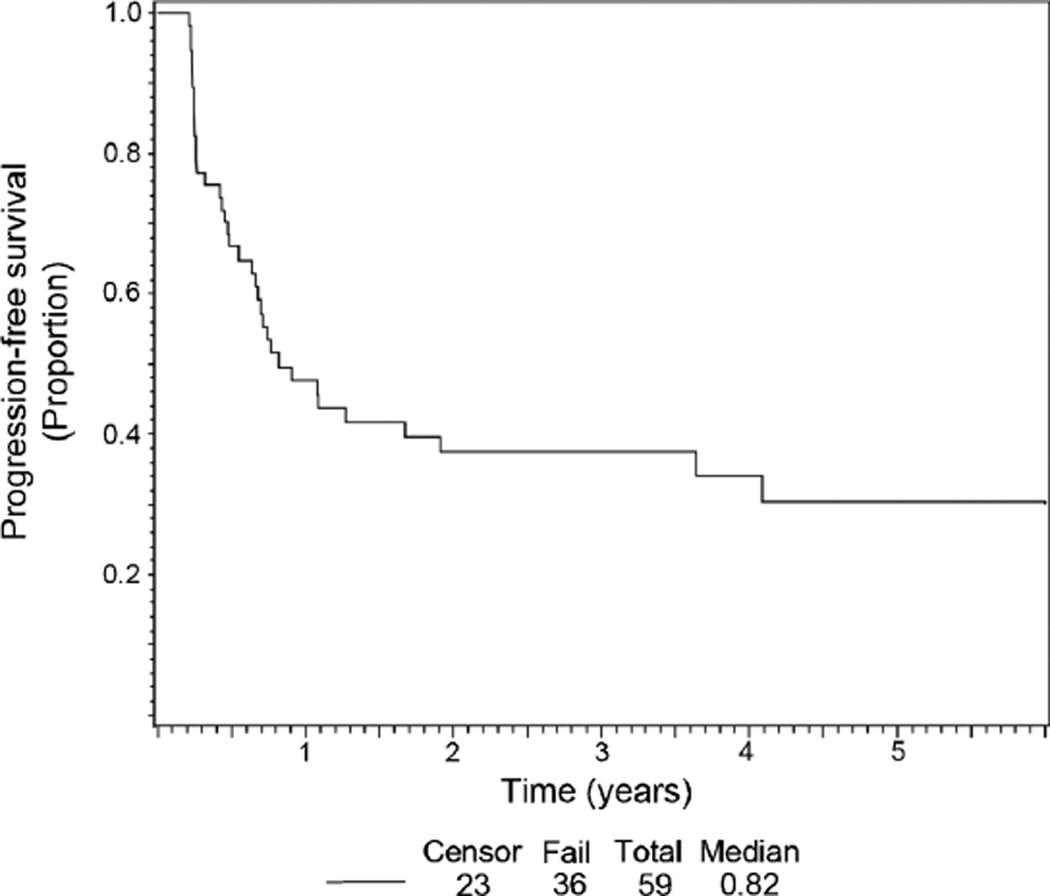
Table IV shows the results of treatment and outcome, with data from the previous POG Study 9650 displayed for comparison. Among the 59 eligible ARST0321 patients, four achieved a PR and one achieved a complete response (8% overall response rate). The estimated PFS rate at 2 years was 36% (Fig. 1), somewhat lower than the 2-year PFS in the POG 9650 study [22]. Three participants died due to disease progression. Of note, response rate and survival were not influenced by whether the patient had previously received chemotherapy.
TABLE IV.
Response Rates and Survival in Two Prospective Phase II Protocols for Children With Desmoid Tumor
| COG ARST0321 | POG 9650 | |
|---|---|---|
| Evaluable (#) | 59 | 26 |
| Complete response (CR; #) | 1 | 1 |
| Partial response (PR; #) | 4 | 4 |
| CR + PR [#(%)] | 5/59 (8%) | 5/26 (19%) |
| 2 year PFS (95% CI) | 36% (23–48) | 46% (25–65) |
| Follow-up [median (range)] | 3.3 years (0–6.1 years) | 3.6 years (1–5.9 years) |
| 2 year survival (%) | 56 (96) | 26 (100) |
COG, Children’s Oncology Group; POG, Pediatric Oncology Group; PFS, progression-free survival; #, number; %, percentage.
Fig. 1.
Kaplan–Meier progression-free survival curve for eligible participants enrolled on the ARST 0321 study.
This study was designed so that a total of 33 failures would constitute full information, based on the previous POG 9650 experience using vinblastine/methotrexate [7]. At the time of a scheduled interim analysis in November 2009, 32 patients had experienced disease progression. Using a fixed outcome based on POG 9650 (with a PFS at two years of 46%), only 24 patients would have been expected to have disease progression if the true outcome with sulindac and tamoxifen was the same as that seen with vinblastine/methotrexate. The P-value associated with the comparison of observed to expected failures is 0.045 (one-sided); thus there is sufficient evidence to conclude that failure-free survival in the first 2 years for ARST0321 is not as good as that observed with vinblastine and methotrexate on POG 9650.
DISCUSSION
This report represents the largest, prospective phase II clinical trial of chemotherapy for children with DT, and the first prospective trial of non-cytotoxic, “molecularly-targeted” therapy for children with DT. Based on our findings, we can first conclude that these agents were not often associated with severe, acute side effects. This point stands in contrast to the previous prospective trial of vinblastine and methotrexate in which, for example, just over 50% of the eligible patients experienced Grade 3 or 4 neutropenia [7]. Although the neutropenia was not associated with adverse consequences in that study, the NCI defines this degree of neutropenia as severe or life threatening. Given the relative paucity of severe side effects from sulindac and tamoxifen, the fact that close to 40% of subjects electively discontinued therapy was even more surprising; only 2 of these 24 stopped due to intolerable toxicity. Other than knowing whether the study therapy was discontinued for patient/parent wishes or physician’s judgment, further details regarding the decisions were not systematically collected; in anecdotal experiences from one of us (SXS), patient/parent decision to discontinue therapy can be driven by not viewing SD as a satisfying outcome.
We can also conclude that tamoxifen and sulindac administration was associated with disease control in only a small minority of patients. Our study does not have the power to detect whether outcome is influenced by FAP status, anatomic site of disease, or prior therapy. Obviously, including the relatively large number of patients electively discontinuing therapy in our analysis could have contributed to the poor disease control. However, we feel this is important because inability or unwillingness to take chronic oral therapy represents a potential limitation for this therapeutic approach.
Tumor-specific factors could also contribute, although we could not prospectively discern obvious tumor subsets based on routine histology findings on study entry. It may be interesting to determine whether hormone receptor status correlates with response; however, if the most recent immunostaining showing estrogen receptor β expression in over 80% of cases in adults [19] is recapitulated in pediatric cases, receptor status will not likely discriminate the small subset with apparent disease control. CTNNB1 status represents another potential molecular determinant of outcome. In one recent report, 83% of sporadic, extra-abdominal DT specimens were noted to have mutation, mostly involving exon 3, in this gene and the subset of patients with wild type CTNNB1 seemed to fare better [23]. Presence of specific CTNNB1 mutation has been correlated with recurrence following surgery [24], but this association was not found in an independent study [23]. Whether the CTNNB1 or hormone receptor status correlates with response to tamoxifen/sulindac or other chemotherapy has yet to be investigated.
It is difficult to judge how our results compare to other reports of chemotherapy for DT, especially in children. Earlier reports favoring the use of non-cytotoxic therapy with tamoxifen and sulindac largely represented small, retrospective analyses [8–11]. However, our PFS rate was similar to that reported in a subset of patients within a large retrospective series of 183 adult DT patients requiring systemic therapy [25]; the median PFS (measured by RECIST) for a subset of 26 getting hormonal therapy was only 12 months. Whether non-cytotoxic therapy is better than cytotoxic chemotherapy is also unanswered. For example, a retrospective series in adult patients indicated that 10 of 13 had PFS [26], but the follow-up period was not clearly indicated. When we consider even larger pediatric series, a recent report of the Italian experience described a 5-year PFS rate of 35% in children with gross disease [27]. The 29 patients in this subset were treated during a 35-year period with a variety of chemotherapy regimens, most of which included alkylating agents or vinca alkaloids. Finally, making an effort to pool institutional results with those of five other pediatric series, Buitendijk et al. [28] reported a response rate of 59%. As in the Italian experience, most of the patients were treated with cytotoxic chemotherapy regimens. Although it may be easier to compare our current findings to the former POG 9650 study of vinblastine and methotrexate because both were accomplished in multi-center prospective trials with similar eligibility and disease response criteria, we still can only cautiously conclude that vinblastine/methotrexate provides better disease control than tamoxifen/sulindac in children.
The results of ARST0321 again illustrate the feasibility of conducting prospective, cooperative group trials in children with DT, which provide more information than limited institution retrospective studies. But, as a single-arm, Phase II study, it is difficult to unequivocally attribute a cause-effect relationship between the tamoxifen and sulindac and the few cases with disease control, especially because DT is reported to remain stable or sometimes undergo spontaneous regression [29]. Alternative study designs for DT may include randomized studies or trials in which a window of expectant management is provided for children with asymptomatic and non-life-threatening disease. Such a “wait and see” approach has been advocated in recent reports of adults with DT [30,31], and might be incorporated into a prospective trial in children.
ACKNOWLEDGMENT
The authors gratefully acknowledge helpful suggestions and advice from members of the Soft Tissue Sarcoma Committee of the Children’s Oncology Group (COG); statistical analyses from the COG Statistics and Data Center; and administrative support from the COG Publications Office.
Grant sponsor: National Cancer Institute, Bethesda, Maryland; Grant numbers: CA-24507, CA-29511, CA-72989, CA-98543.
Footnotes
Conflict of Interest: Nothing to report.
REFERENCES
- 1.Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of soft tissue arnd bone. Lyon: IARC Press; 2002. World Health Organization classification of tumours. [Google Scholar]
- 2.Pritchard DJ, Nascimento AG, Petersen IA. Local control of extra-abdominal desmoid tumors. J Bone Joint Surg Am. 1996;78:848–854. doi: 10.2106/00004623-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Anthony T, Rodriguez-Bigas MA, Weber TK, et al. Desmoid tumors. J Am Coll Surg. 1996;182:369–377. [PubMed] [Google Scholar]
- 4.Jabbari S, Andolino D, Weinberg V, et al. Successful treatment of high risk and recurrent pediatric desmoids using radiation as a component of multimodality therapy. Int J Radiat Oncol Biol Phys. 2009;75:177–182. doi: 10.1016/j.ijrobp.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 5.Merchant TE, Nguyen D, Walter AW, et al. Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys. 2000;47:1267–1271. doi: 10.1016/s0360-3016(00)00566-6. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Lewis JJ, Merchant NB, et al. Surgical management of intra-abdominal desmoid tumours. Br J Surg. 2000;87:608–613. doi: 10.1046/j.1365-2168.2000.01400.x. [DOI] [PubMed] [Google Scholar]
- 7.Skapek SX, Ferguson WS, Granowetter L, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: Results of a pediatric oncology group phase II trial. J Clin Oncol. 2007;25:501–506. doi: 10.1200/JCO.2006.08.2966. [DOI] [PubMed] [Google Scholar]
- 8.Tsukada K, Church JM, Jagelman DG, et al. Noncytotoxic drug therapy for intra-abdominal desmoid tumor in patients with familial adenomatous polyposis. Dis Colon Rectum. 1992;35:29–33. doi: 10.1007/BF02053335. [DOI] [PubMed] [Google Scholar]
- 9.Izes JK, Zinman LN, Larsen CR. Regression of large pelvic desmoid tumor by tamoxifen and sulindac. Urology. 1996;47:756–759. doi: 10.1016/s0090-4295(96)00026-x. [DOI] [PubMed] [Google Scholar]
- 10.Lackner H, Urban C, Kerbl R, et al. Noncytotoxic drug therapy in children with unresectable desmoid tumors. Cancer. 1997;80:334–340. doi: 10.1002/(sici)1097-0142(19970715)80:2<334::aid-cncr22>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Bauernhofer T, Stoger H, Schmid M, et al. Sequential treatment of recurrent mesenteric desmoid tumor. Cancer. 1996;77:1061–1065. [PubMed] [Google Scholar]
- 12.Soravia C, Berk T, McLeod RS, et al. Desmoid disease in patients with familial adenomatous polyposis. Dis Colon Rectum. 2000;43:363–369. doi: 10.1007/BF02258303. [DOI] [PubMed] [Google Scholar]
- 13.Miyaki M, Konishi M, Kikuchi-Yanoshita R, et al. Coexistence of somatic and germ-line mutations of APC gene in desmoid tumors from patients with familial adenomatous polyposis. Cancer Res. 1993;53:5079–5082. [PubMed] [Google Scholar]
- 14.Alman BA, Li C, Pajerski ME, et al. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors) Am J Pathol. 1997;151:329–334. [PMC free article] [PubMed] [Google Scholar]
- 15.He TC, Chan TA, Vogelstein B, et al. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 17.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 18.Reitamo JJ, Scheinin TM, Hayry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg. 1986;151:230–237. doi: 10.1016/0002-9610(86)90076-0. [DOI] [PubMed] [Google Scholar]
- 19.Deyrup AT, Tretiakova M, Montag AG. Estrogen receptor-beta expression in extraabdominal fibromatoses: An analysis of 40 cases. Cancer. 2006;106:208–213. doi: 10.1002/cncr.21553. [DOI] [PubMed] [Google Scholar]
- 20.Woolson RF. Rank-tests and a one-sample logrank test for comparing observed survival-data to a standard population. Biometrics. 1981;37:687–696. [Google Scholar]
- 21.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 22.Skapek SX, Hawk BJ, Hoffer FA, et al. Combination chemotherapy using vinblastine and methotrexate for the treatment of progressive desmoid tumor in children. J Clin Oncol. 1998;16:3021–3027. doi: 10.1200/JCO.1998.16.9.3021. [DOI] [PubMed] [Google Scholar]
- 23.Domont J, Salas S, Lacroix L, et al. High frequency of beta-catenin heterozygous mutations in extra-abdominal fibromatosis: a potential molecular tool for disease management. Br J Cancer. 2010;102:1032–1036. doi: 10.1038/sj.bjc.6605557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol. 2008;173:1518–1527. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Camargo VP, Keohan ML, D’Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor) Cancer. 2010;116:2258–2265. doi: 10.1002/cncr.25089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansmann A, Adolph C, Vogel T, et al. High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer. 2003;100:612–620. doi: 10.1002/cncr.11937. [DOI] [PubMed] [Google Scholar]
- 27.Meazza C, Bisogno G, Gronchi A, et al. Aggressive fibromatosis in children and adolescents: The Italian experience. Cancer. 2010;116:233–240. doi: 10.1002/cncr.24679. [DOI] [PubMed] [Google Scholar]
- 28.Buitendijk S, van de Ven CP, Dumans TG, et al. Pediatric aggressive fibromatosis: A retrospective analysis of 13 patients and review of literature. Cancer. 2005;104:1090–1099. doi: 10.1002/cncr.21275. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JJ, Boland PJ, Leung DH, et al. The enigma of desmoid tumors. Ann Surg. 1999;229:866–872. doi: 10.1097/00000658-199906000-00014. discussion 872–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: A front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16:2587–2593. doi: 10.1245/s10434-009-0586-2. [DOI] [PubMed] [Google Scholar]
- 31.Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: A wait-and-see policy according to tumor presentation. J Clin Oncol. 2011;29:3553–3558. doi: 10.1200/JCO.2010.33.5489. [DOI] [PubMed] [Google Scholar]