Abstract
The effect of various detergents on the stability and function of melibiose permeases of Escherichia coli (MelBEc) or Salmonella typhimurium (MelBSt) were studied. In n-dodecyl-β-d-maltoside (DDM) or n-undecyl-β-d-maltoside (UDM), WT MelBSt binds melibiose with an affinity similar to that in the membrane. However, with WT MelBEc or MelBSt mutants (Arg141→Cys, Arg295→Cys or Arg363→Cys), galactoside binding is not detected in these detergents, but binding to the phosphotransferase protein IIAGlc is maintained. In the amphiphiles lauryl maltose neopentyl glycol (MNG-3) or glyco-diosgenin (GDN), galactoside binding with all the MelB proteins is observed, with slightly reduced affinities. MelBSt is more thermostable than MelBEc, and the thermostability of either MelB is largely increased in MNG-3 or GDN. Therefore, the functional defect with DDM or UDM likely results from relative instability of the sensitive MelB proteins, and stability, as well as galactoside binding, is retained in MNG-3 or GDN. Furthermore, isothermal titration calorimetry of melibiose binding with MelBSt shows that the favorable entropic contribution to the binding free energy is decreased in MNG-3, indicating that the conformational dynamics of MelB is restricted in this detergent.
Membrane transporters, receptors, and channels play crucial roles in cellular functions by moving molecules across cell membranes. Detergents are essential tools for studying the structure and function of membrane proteins; however, selection of a detergent that retains activity and conformational stability is challenging because knowledge about detergents is still limited. The mild, nonionic detergent n-dodecyl-β-d-maltoside (DDM) is probably the most commonly used detergent for structure determination and functional analysis. The novel amphiphiles lauryl maltose neopentyl glycol (MNG-3) and glyco-diosgenin (GDN) have been shown to be superior to DDM or n-undecyl-β-d-maltoside (UDM) in maintaining solubility of several membrane proteins, including the melibiose permease of Escherichia coli (MelBEc) or Salmonella typhimurium (MelBSt), and G protein-coupled receptors.1, 2 However, their effects on substrate-binding affinity and binding thermodynamics to MelB have not been tested yet.
Both MelBEc and MelBSt catalyze symport of galactosides with H+, Na+, or Li+3-7 and are well-characterized members of the glycoside-pentoside-hexuronide/cation subfamily8, 9 of the major facilitator superfamily of membrane transport proteins.10-13 The X-ray crystal structure of MelBSt shows that MelB is composed of N- and C-terminal domains containing six irregular transmembrane helices surrounding a deep aqueous cavity open to the periplasmic side.12 This overall fold is similar to other major facilitator superfamily of transport proteins, such as the lactose permease (LacY).14, 15
MelBSt is effectively extracted from membranes with DDM or UDM, and the purified protein in UDM is monodisperse on gel filtration chromatography12 and does not precipitate when stored at 0 ºC for months. MelBSt solubilized in UDM binds melibiose,12, 16 nitrophenyl-α-galactoside (α-NPG),16 and the fluorescent sugar 2’-(N-dansyl)aminoalkyl-1-thio-β-d-galactopyranoside (D2G).12 Melibiose binding affinity with right-side-out (RSO) membrane vesicles containing WT MelBSt or with the purified proteins in UDM is comparable, with a Kd of ~1 mM.6, 12 Surprisingly with UDM-solubilized MelBEc, melibiose reversal in Trp→D2G fluorescence resonance energy transfer (D2G FRET) experiments is not detected. Thus far, all Trp→D2G FRET data with MelBEc were derived solely from studies with reconstituted proteoliposomes or membrane vesicles.17-20 In this communication, the effect of UDM, DDM, MNG-3, and GDN on ligand binding by MelBEc or MelBSt is characterized by isothermal titration calorimetry and/or D2G FRET assays. The results indicate that the functional defect with DDM or UDM likely results from relative instability of the sensitive MelB proteins, and that MNG-3 or GDN maintains the stability and galactoside binding with either MelB.
Materials and Methods
Materials
D2G was kindly provided by H. Ronald Kaback and Gérard Leblanc. Synthesis of MNG-3 and GDN was described previously.1, 2 DDM, UDM, and DM were purchased from Anatrace. All other materials were reagent grade and obtained from commercial sources.
Plasmids
The expression plasmid pK95ΔAH/WT MelBEc was from Gérard Leblanc. 21 The pK95ΔAH-based plasmids were used for overexpressing WT MelBSt6 and MelBSt mutants R141C, R295C, or R363C.13
Preparation of RSO vesicles
RSO membrane vesicles were prepared from E. coli DW2 cells by osmotic lysis,6, 22, 23 resuspended with 100 mM KPi (pH 7.5), and stored at −80 °C.
Trp→D2G FRET
RSO membrane vesicles or detergent-solubilized samples were used for Trp→D2G FRET measurements with an Amico-Bowman Series 2 (AB2) Spectrofluorometer. Trp residues were excited at 290 nm, and emission spectra were recorded between 430 and 550 nm. In time traces, emission was recorded at 465 nm for MelBEc or 490 nm for MelBSt. Successive addition of 10 μM D2G, 20 mM NaCl, and excess melibiose was done for all D2G FRET measurements.
Determination of IC50 of melibiose for the half-maximal displacement of bound D2G (10 μM) was carried out as described.6, 13, 24 Briefly, stepwise addition of melibiose was performed during D2G FRET until no further change in the FRET signal was observed. The IC50 was determined by hyperbolic fitting (OriginPro).
MelB overexpression and membrane preparation
Overexpression of MelB and membrane preparation were carried out according to previous protocols.12
Thermostability test
Thermostability assay of MelB in DDM or GDN was carried out as described1, 2, 25, 26 except for the use of 2% detergent concentration. Briefly, membranes containing MelBSt or MelBEc at 10 mg/mL in 20 mM Tris-HCl (pH, 7.5), 200 mM NaCl, 10% glycerol, and 20 mM melibiose were incubated with 2% (w/v) of DDM or GDN at 0 ºC for 10 min, and subsequently placed at a given temperature (0, 45, 55, and 65 ºC) for 90 min. Samples were ultracentrifuged at 355,590 g for 45 min at 4 ºC. Equal-volume solutions were analyzed by SDS-PAGE and immunoblotted with Penta-His-HRP antibody. The thermostability data of WT MelBSt27 and MelBEc1 in MNG-3 have been published.
MelB purification in different detergents
Purification of MelB has been reported.12 Briefly, E. coli DW2 cell membranes at 14 mg/ml were extracted with 1.5% UDM or 1.2% MNG-3, and purified MelB in 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 0.035% UDM or 0.01% MNG-3, and 10% glycerol was flash-frozen in liquid nitrogen, and stored at −80 °C.
Expression and purification of phosphotransferase IIAGlc
Expression and purification of IIAGlc of E. coli were performed as described.16, 28 Purified IIAGlc in 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 10% glycerol was stored at −80 °C.
Protein concentration
Protein concentration was determined with the Micro BCA Protein Assay (Pierce Biotechnology, Inc).
Isothermal titration calorimetry
ITC measurements with a Nano Isothermal Titration Calorimeter ( TA Instruments) and data process using the NanoAnalyze version 2.3.6 software29, 30 were performed as described.16, 28 MelB in 20 mM Tris-HCl buffer (pH 7.5) containing 100 mM NaCl, 10% glycerol, and a given detergent was placed into the sample cell, and melibiose and IIAGlc were prepared in the same buffer used for the permease. Data fitting using one-site independent binding model31 yields the association constant (Ka) and enthalpy change ΔH values. ΔG = - RT ln Ka. The dissociation constant (Kd) = 1/Ka. Entropy change (−TΔS) is calculated from equation ΔG = ΔH - TΔS.
Results
Trp→D2G FRET
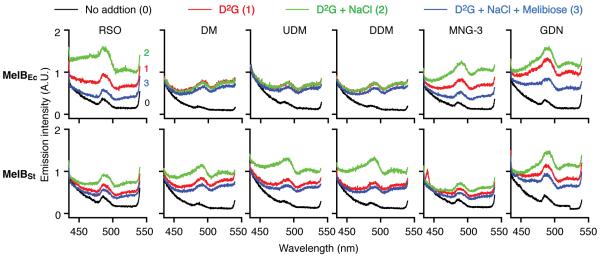
D2G is a fluorescent sugar analogue for MelB and LacY.6, 17, 18, 20, 32 As reported,6, 17 with RSO membrane vesicles containing WT MelBEc, Trp→D2G FRET is observed upon addition of D2G (Fig. 1, upper left panel, red curve), which is increased by Na+ (green curve) and decreased by consecutive addition of melibiose (blue curve), to a level slightly higher than the background (black curve). The binding affinity for Na+ or melibiose is reflected by Na+ stimulation and melibiose displacement of bound D2G, respectively. With RSO vesicles containing WT MelBSt, the D2G FRET is also obtained,6 but the FRET signal is less intense (Fig. 1, lower left panel). RSO vesicles containing MelBEc or MelBSt bind D2G in the presence of Na+ with a Kd of approximately 3 or 10 μM, respectively, and bind melibiose with a Kd of approximately 0.5 or 1 mM, respectively.6
Fig. 1. Trp→D2G FRET.
RSO vesicles prepared from DW2 cells expressing WT MelBEc (upper panel) and WT MelBSt (lower panel) at a protein concentration of 1.0 mg/ml, or solubilized with 1% of an indicated detergent, were excited at 290 nm. Emission spectra were recorded between 430 and 550 nm in the absence of (black or curve 0) or presence of 10 μM D2G (red or curve 1), with successive addition of 20 mM NaCl (green or curve 2) and 120 mM melibiose (blue or curve 3). A.U., arbitrary unit.
When RSO vesicles containing WT MelBSt were solubilized with detergent DDM, UDM or DM without purification of the protein, a specific D2G FRET signal was also detected (Fig. 1). Surprisingly, MelBEc solubilized with DDM, UDM or DM does not exhibit Na+ stimulation or melibiose reversal of fluorescent intensity, which are not improved by changing the DDM or UDM concentration from 0.5 to 2.0% (data not shown). Strikingly, with detergent MNG-3 or GDN, Na+ stimulation and melibiose reversal of D2G FRET are obtained with both MelBEc and MelBSt (Fig. 1). Notably, the intensity of D2G FRET is protein- and detergent-dependent, in addition to the fraction of bound D2G. The IC50 for melibiose displacement of bound D2G was determined to analyze galactoside-binding affinity. MelBEc in MNG-3 or GDN and MelBSt in each detergent exhibit slightly increased IC50 values than observed with RSO membrane vesicles (Table 1)
Table 1.
IC50 for melibiose displacement of bound D2Ga (mM)
| Permease | RSO membrane vesicles |
Detergent solubilization | |||
|---|---|---|---|---|---|
|
|
|||||
| DDM | UDM | MNG-3 | GDN | ||
| MelBEc | 0.66 ± 0.16b | NDc | ND | 1.20 ± 0.35 | 1.82 ± 0.18 |
| MelBSt | 2.42 ± 0.22 | 5.65 ± 1.75 | 4.79 ± 0.71 | 4.22 ± 0.82 | 3.19 ± 0.41 |
| R295C MelBSt | 4.04 ± 0.42d | /e | / | 5.49 ± 0.83 | / |
Melibiose concentration for the half-maximal displacement of bound D2G (IC50) in the presence of 20 mM NaCl.
SEM, (n = 2).
No detectable signal.
Data from reference #12;
No measurement.
Effect of detergents on MelBSt mutants
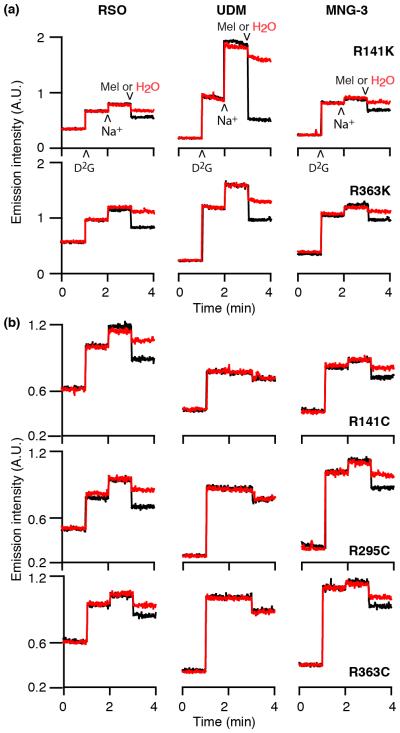
R141, R295, or R363 residue forms multiple interactions between the two domains of MelBSt13 for stabilizing the outward conformation.12 Replacement of R141, R295, or R363 of MelBSt with Cys significantly inhibits melibiose uptake with little effect on melibiose or Na+ binding as shown by melibiose transport with intact cells and the D2G FRET assay with RSO vesicles.13 When R141 or R363 are replaced with Lys, the conservative replacement mutants catalyze melibiose uptake. With RSO vesicles containing mutants R141K or R363K MelBSt, Na+-stimulation and melibiose reversal of fluorescent intensity are detected after solubilization in UDM or MNG-3 (Fig. 2a). Strikingly, there is no change upon addition of Na+ or melibiose with RSO vesicles containing MelBSt mutants (R141C, R295C, or R363C) after solubilization with UDM (Fig. 2b, middle column). When using MNG-3 (Fig. 2b, right column), Na+-stimulation and melibiose reversal of D2G FRET are observed with all three mutants, although intensity is weaker than that with RSO membrane vesicles (Fig. 2b, left column). With mutant R295C MelBSt in MNG-3, the IC50 for melibiose displacement of bound D2G is 5.49 ± 0.83 mM (Table 1).
Fig. 2. Time trace of Trp→D2G FRET.
With excitation wavelength of 290 nm, emission was recorded at 465 nm for MelBEc or 490 nm for MelBSt. 10 μM D2G, 20 mM NaCl, melibiose (Mel, black) or water (red) were successively added at a 60-sec intervals. Left column, recorded with RSO vesicles; middle and right columns, recorded with RSO vesicles after solubilization with 1% UDM or 1% MNG-3, respectively. (a) R141K and R363K MelBSt mutants; (b) R141C, R295C, and R363C MelBSt mutants.
Sugar binding by ITC
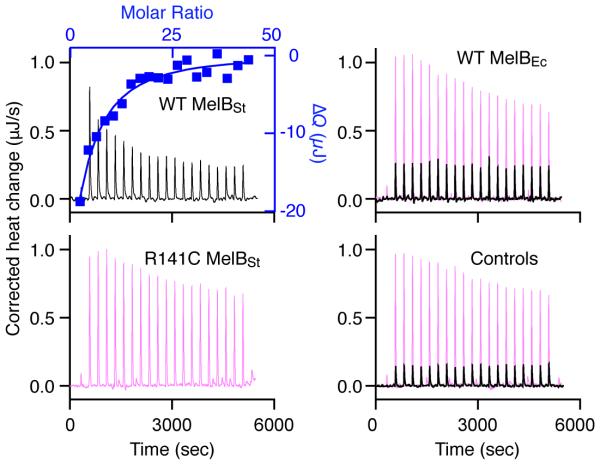
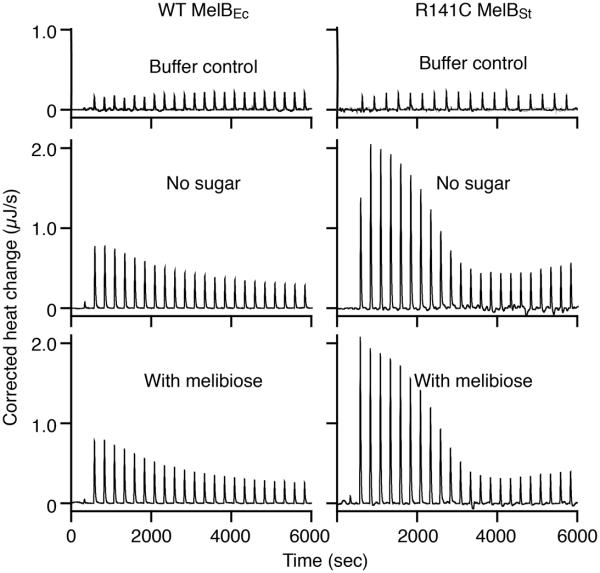
To determine the Kd value for melibiose binding with MelB purified after solubilization with UDM or MNG-3, ITC measurements were carried out. As shown previously, titration of WT MelBSt in UDM with melibiose yields a Kd of 0.97 ± 0.02 mM and ΔG of −17.20 ± 0.07 kJ/mol in the presence of Na+ (Fig. 3, Table 2).16 Energetically, melibiose binding is driven by both favorable enthalpy (ΔH of −10.33 ± 0.36 kJ/mol) and entropy (−TΔS of −6.87 ± 0.28 kJ/mol) (Fig. 4b). Remarkably, titration of the WT MelBEc in UDM with melibiose (10 mM), even at an increased concentration (100 mM), yields a similar heat change to that observed by injection of melibiose into buffer in the absence of protein (Fig. 3, right column). Similarly, titration of mutant R141C MelBSt with melibiose at 100 mM also exhibits no binding isotherm (Fig. 3). These data clearly indicate that WT MelBEc and mutant R141C MelBSt in UDM do not bind melibiose, which correlate well with the D2G FRET results.
Fig. 3. ITC measurement of melibiose binding with MelB in UDM.
MelB was extracted with UDM and purified in buffer containing 0.035% UDM. Thermograms (with y-axis on the left side) were recorded at 25 °C during the titration of WT MelBSt, mutant R141C MelBSt, or WT MelBEc (80 μM) with melibiose at 10 mM (black curves) or 100 mM (pink curves). Injection of melibiose to the buffer in the absence of a protein is used for the control. Melibiose binding to the WT MelBSt has been reported.12, 16 Accumulated heat change (ΔQ, with y-axis on the right side) of each injection was plotted against the melibiose/MelB molar ratio (on the top), and fitted to a one-site independent binding model.
Table 2.
Detergent effect on energetics of melibiose binding to MelBa
| Permease |
Ka
(/mol) |
Kd
(mM) |
ΔG
(kJ/mol) |
ΔH (kJ/mol) |
-TΔS
(kJ/mol) |
|---|---|---|---|---|---|
| MelBSt in UDM | 1030 (27.50) | 0.97 (0.02) | −17.20 (0.07) | −10.33 (0.36) | −6.87 (0.28) |
| MelBSt in MNG-3 | 402.18 (20.60) | 2.51 (0.13) | −14.68 (0.13) | −13.65 (0.36) | −1.21 (0.40) |
| MelBEc in UDM | NDc | ND | ND | ND | ND |
| MelBEc in MNG-3 | 782.05 (38.65) | 1.28 (0.06) | −16.51 (0.12) | −9.33 (0.04) | −7.18 (0.16) |
All measurements were carried out at 25 °C. The data are presented in Figs. 3 and 4.
b SEM, number of tests = 2 - 4.
No detectable signal.
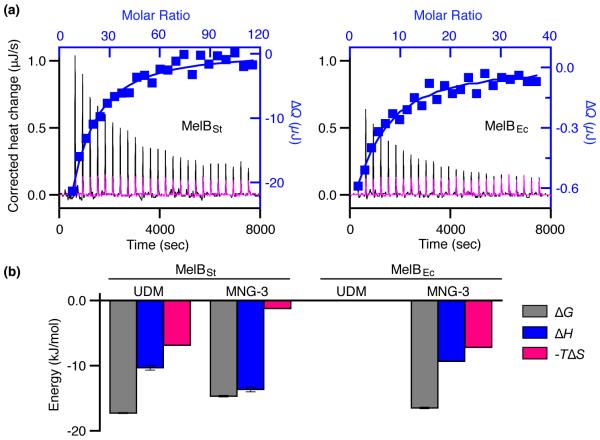
Fig. 4. Thermodynamics of melibiose binding with MelB in MNG-3.
WT MelBSt or MelBEc was solubilized with MNG-3 and purified in buffers containing 0.01% MNG-3. (a) Thermograms (with y-axis on the left side) were recorded at 25 °C during the titration of MelB (95 μM) with melibiose at 30 mM (MelBSt, black curve) or 10 mM (MelBEc, black curve). Injection of melibiose at 30 mM or 10 mM to the buffer in the absence of a protein is used for the control (magenta curves). ΔQ (with y-axis on the right side) was plotted against the melibiose/MelB molar ratio (on the top), and fitted to a one-site independent binding model. (b) Energetics of melibiose binding with MelB in UDM or MNG-3. Free energy change (ΔG), enthalpy change (ΔH), and entropy change (−TΔS) are obtained from curve fitting in Figs. 3 and 4 as described in the materials and methods. Errors, SEM, number of tests = 2 - 4.
Both WT MelBEc and MelBSt were purified after solubilization with MNG-3. Titration of MelBSt in MNG-3 with melibiose exhibits slightly reduced binding affinity with a Kd of 2.51 ± 0.13 mM (Fig. 4a, Table 2), which is 2.5-fold higher than that in UDM (Table 2). Interestingly, the favorable entropic contribution to the total free energy (ΔG) is largely reduced from 40% in UDM to less than 10% in MNG-3 (Fig. 4b, red bars), yielding a −TΔΔSMNG-3 - UDM of 5.66 kJ/mol. As a partial compensation, the favorable enthalpic change (ΔH) is increased and contributes to ΔG at greater than 90%. Thus, the decrease in melibiose binding affinity with MelBSt in MNG-3 is due solely to a loss of entropy.
While no sugar binding is observed with MelBEc in UDM, titration of MelBEc in MNG-3 with melibiose reveals a typical binding isotherm with a Kd of 1.28 ± 0.06 mM (Fig. 4a), which is about 2.5-fold higher than that measured with RSO by D2G FRET6 but similar to the Kd obtained with proteoliposomes by flow-dialysis assay using [3H]nitrophenyl-α-d-galactopyranoside.33 Energetically, both enthalpy and entropy contribute favorably to the free energy, which is similar to melibiose binding with MelBSt in UDM (Fig. 4b).
IIAGlc binding by ITC
Previous ITC measurements show that the phosphotransferase IIAGlc, a regulatory protein, binds to MelBSt,16 MelBEc,16 or LacY28 in UDM, yielding Kd of ca. 3, 25, or 5 μM, respectively. When melibiose is pre-incubated with MelBSt, IIAGlc affinity is 3-fold decreased, and the binding rate is faster.16 However, melibiose has no effect on IIAGlc binding to MelBEc (Fig. 5, also see ref. 16), which is now recognized as lack of melibiose binding. When titration of mutant R141C MelBSt in UDM with IIAGlc, a binding curve similar to WT MelBSt is obtained at a Kd of 2 μM (Fig. 5). Again, melibiose shows no effect, which is consistent with a lack of melibiose binding.
Fig. 5. Thermogram of IIAGlc binding.
Titration of WT MelBEc (30 μM, at 25 °C) or R141C MelBSt (50 μM, at 20 °C) with IIAGlc (400 or 455 μM) in the absence or presence of 10 mM melibiose was recorded, respectively. Both MelB were purified in buffer containing 0.035% UDM after extracted with UDM. Injection of IIAGlc to the buffer in the absence of a protein is used as the control. The Kd for IIAGlc binding to WT MelBEc has been reported.16
Comparison of thermostability between MelBSt and MelBEc
Thermostability studies with MelBSt and MelBEc in DDM, MNG-3, or GDN have been reported,1, 2, 27 but the previous focus was not on direct comparison between the two MelB proteins. Data on DDM or GDN (Fig. 6) were obtained from similar studies but at higher concentration; the MNG-3 data are from two publications.1, 27 All the tests were done in the presence of melibiose and NaCl.
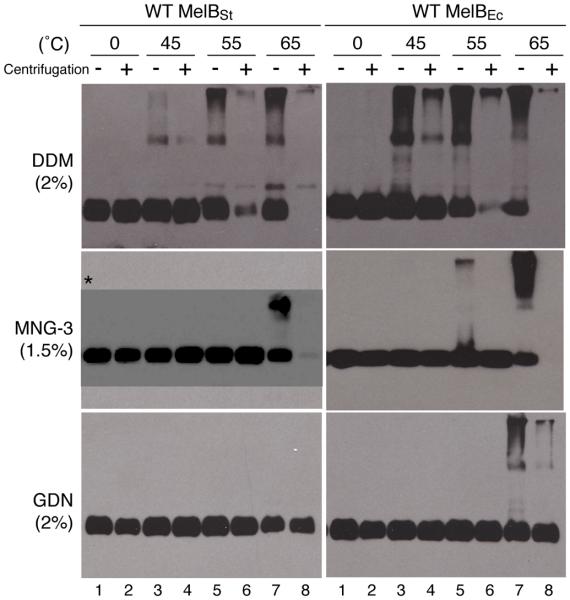
Fig. 6. Thermostability.
Equal volume of MelB samples after solubilization with 2% DDM or GDN, as well as 1.5% MNG-3 in the presence of 20 mM melibiose and 200 mM NaCl without (−) or with (+) ultracentrifugation was loaded onto SDS-12% PAGE. MelB proteins were detected by Western blotting using anti–His tag antibody. 10 μg membrane proteins were used for test under each condition. *, SDS-16% PAGE. The data of MNG-3 have been reported1, 27.
DDM quantitatively solubilizes either MelBSt or MelBEc at 0 °C (Fig. 6, lanes 1-2). After incubation at elevated temperatures (45, 55, or 65 °C) for 90 min, MelBEc exhibits strong aggregations on the western blot (Fig. 6, lanes 3-7). With MelBSt, only slight aggregation is observed at 45 °C; at 55 °C, the soluble fraction of MelBSt is greater than that of MelBEc, as shown with the samples after ultracentrifugation (lane 6); at 65 °C, both MelB proteins disappear from the solutions (lane 8).
MNG-3 or GDN maintains either MelB in soluble fraction completely after incubation at 55 °C; GDN also keeps all MelB in solution even after incubation at 65 °C for 90 min (Fig. 6).2 The data indicate that either MelB exhibits an increased thermostability in MNG-3 or GDN, by approximately 10 °C or 20 °C, respectively. In addition, as observed in DDM, MelBSt in both MNG-3 and GDN shows less aggregation, clearly indicating that MelBSt is more thermostable than MelBEc (Fig. 6).
Discussion
MelBSt and MelBEc share more than 85% sequence identity, and the residues necessary for cation and sugar binding are highly conserved.10 The common detergents DDM and UDM completely extract either permease from the membrane,1, 2 and the purified proteins exhibit no aggregation on ice for weeks to months, but the thermostability of WT MelBSt is clearly better than WT MelBEc (Fig. 6). Interestingly, DDM and UDM, which work well for MelBSt, do not support galactoside binding by MelBEc, or by some MelBSt mutants (Figs. 1-3). Thus, DDM or UDM causes abnormal conformations of these sensitive proteins, but not MelBSt. It is likely that these effects are due to relatively poor stability in these detergents. The DDM and UDM effects are subtle and reversible without denaturation/aggregation (Fig. 6) because the same protein samples bind the regulatory protein IIAGlc (Fig. 5). In addition, WT MelBEc after reconstitution into proteoliposomes binds Na+ or Li+ and galactosides.17, 18, 20, 34 When using MNG-3 or GDN, galactosides binding with WT MelBEc and the MelBSt mutants are obtained (Figs. 1, 2, 4; Tables 1 and 2). ITC measurement with WT MelBEc in MNG-3 yields a Kd value of 1.2 mM for melibiose in the presence of NaCl.
The effect of DDM and MNG-3 on a LacY mutant with Cys154→Gly has also been studied,35 The C154G LacY, which likely favors an intermediate periplasmic-open conformation in the membrane, collapses to a lower-energy, periplasmic-closed conformation in DDM. Notably, MNG-3 stabilizes C514G LacY in the membrane-embedded form.35 It has been also reported that the muscarinic acetylcholine receptor requires cholesterol or its derivatives for protein stability and that MNG-3 stabilizes the receptor without cholesterol derivatives.36 In addition, the off-rate of MNG-3 from the G protein-coupled beta(2)-adrenoreceptor has been shown to be four orders of magnitude lower than that of DDM,37 clearly demonstrating that MNG-3 binds the membrane protein tightly.
Structurally, both MNG-3 and GDN have a branched dimaltoside hydrophilic headgroup,1, 2 which plays an important role in membrane protein stabilization. The two alkyl-chains in MNG-3 could have a multivalent effect, binding to membrane proteins tighter than DDM or UDM. GDN has a flat panel-like structure with lipophilic groups, and thus likely forms self-assemblies with strong intermolecular interactions around membrane proteins. Both MNG-3 and GDN also have critical micelle concentration (CMC) values (0.001%1 and 0.002%2, respectively) lower than DDM (0.008%). While the CMC is only one of many factors determining membrane protein stability, a detergent with a low CMC value has high tendency to form stable micelles, which could contribute to formation of stable protein-detergent complexes. Thus, the unique structural and biophysical features of the novel amphiphiles and resulting tight interactions with membrane proteins could be responsible for the increased stability and for retaining the galactoside binding in MelB. The tight binding of MNG-3 is supported by the thermodynamic characterization using ITC. The results clearly show that the favorable entropy change contributing to melibiose binding with MelBSt in MNG-3 is reduced with a −TΔΔSMNG-3 - UDM of 5.66 kJ/mol. Unfortunately, this parameter for MelBEc is not available because sugar binding is not observed with MelBEc in UDM. Loss of entropy implies that the conformational dynamics of MelBSt is restricted, in all probability because MNG-3 interacts tightly with the protein.
Interestingly, melibiose binding with MelBSt in MNG-3 exhibits a 2.5-fold increase in Kd value. The decrease in galactoside binding affinity likely results from the restricted conformational dynamics. The galactoside binding with MelB is proposed to use an induced-fit mechanism,16, 28 which is similar to the sugar binding in LacY.38, 39 Based on this notion, a galactoside induces conformational change of MelB from an open state to an occluded state to form a completely liganded binding site. It has been proposed that IIAGlc inhibits such a process by restricting conformational entropy of MelB, resulting a largely reduced melibiose binding.16 It seems that MNG-3 also hinders the induced-fit process in MelBSt. Thus, for an optimal galactoside binding of each MelB, a balance between structural stability and conformational dynamics is needed, which is largely affected by detergent micelles.
Acknowledgements
The authors appreciate the technique support of Shalika Nurva. The authors also appreciate H. Ronald Kaback and Luis Reuss for critically reading this manuscript.
Funding Source Statement: This work was supported in part by National Science Foundation-Directorate for Biological Sciences (grant MCB-1158085 to L.G.), by the U.S. Department of Health and Human Services-National Institutes of Health (grant R01 GM095538 to L.G.), and by Korean government (MSIP)-the National Research Foundation of Korea (grant 2013R1A2A2A03067623 to P.S.C.).
Abbreviations and Textual Footnotes
- MelBSt
melibiose permease of Salmonella typhimurium
- MelBEc
melibiose permease of Escherichia coli
- E. coli
Escherichia coli
- MNG-3
lauryl maltose neopentyl glycol
- GDN
glyco-diosgenin
- DDM
n-dodecyl-β-d-maltoside
- UDM
n-undecyl-β-d-maltoside
- DM
n-decyl-β-d-maltoside
- D2G
2’-(N-dansyl)aminoalkyl-1-thio-β-d-galactopyranoside
- CMC
critical micelle concentration
- D2G FRET
Trp→dansyl-galactoside fluorescence resonance energy transfer
- ITC
isothermal titration calorimetry
- Kd
dissociation constant
- RSO
right-side-out membrane vesicles
- ΔQ
accumulated heat change
- ΔG
free energy change
- ΔH
enthalpy change
- −TΔS
entropy change
References
- [1].Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka B, Gellman SH. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chae PS, Rasmussen SG, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK, Gellman SH. A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chemistry. 2012;18:9485–9490. doi: 10.1002/chem.201200069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wilson DM, Wilson TH. Cation specificity for sugar substrates of the melibiose carrier in Escherichia coli. Biochim Biophys Acta. 1987;904:191–200. doi: 10.1016/0005-2736(87)90368-3. [DOI] [PubMed] [Google Scholar]
- [4].Tsuchiya T, Raven J, Wilson TH. Co-transport of Na(+) and methul-beta-d-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977;76:26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- [5].Bassilana M, Pourcher T, Leblanc G. Facilitated diffusion properties of melibiose permease in Escherichia coli membrane vesicles. Release of co-substrates is rate limiting for permease cycling. J Biol Chem. 1987;262:16865–16870. [PubMed] [Google Scholar]
- [6].Guan L, Nurva S, Ankeshwarapu SP. Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium. J Biol Chem. 2011;286:6367–6374. doi: 10.1074/jbc.M110.206227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guan L, Jakkula SV, Hodkoff AA, Su Y. Role of Gly117 in the cation/melibiose symport of MelB of Salmonella typhimurium. Biochemistry. 2012;51:2950–2957. doi: 10.1021/bi300230h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Poolman B, Knol J, van der Does C, Henderson PJ, Liang WJ, Leblanc G, Pourcher T, Mus-Veteau I. Cation and sugar selectivity determinants in a novel family of transport proteins. Mol Microbiol. 1996;19:911–922. doi: 10.1046/j.1365-2958.1996.397949.x. [DOI] [PubMed] [Google Scholar]
- [9].Saier MH., Jr. Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- [10].Yousef MS, Guan L. A 3D structure model of the melibiose permease of Escherichia coli represents a distinctive fold for Na(+) symporters. Proc Natl Acad Sci U S A. 2009;106:15291–15296. doi: 10.1073/pnas.0905516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Granell M, Leon X, Leblanc G, Padros E, Lorenz-Fonfria VA. Structural insights into the activation mechanism of melibiose permease by sodium binding. Proc Natl Acad Sci U S A. 2010;107:22078–22083. doi: 10.1073/pnas.1008649107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ethayathulla AS, Yousef MS, Amin A, Leblanc G, Kaback HR, Guan L. Structure-based mechanism for Na(+)/melibiose symport by MelB. Nat Commun. 2014;5:3009. doi: 10.1038/ncomms4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Amin A, Ethayathulla AS, Guan L. Suppression of conformation-compromised mutants of Salmonella enterica serovar Typhimurium MelB. J Bacteriol. 2014;196:3134–3139. doi: 10.1128/JB.01868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci U S A. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hariharan P, Guan L. Insights into the inhibitory mechanisms of the regulatory protein IIA(Glc) on melibiose permease activity. J Biol Chem. 2014;289:33012–33019. doi: 10.1074/jbc.M114.609255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cordat E, Mus-Veteau I, Leblanc G. Structural studies of the melibiose permease of Escherichia coli by fluorescence resonance energy transfer. II. Identification of the tryptophan residues acting as energy donors. J Biol Chem. 1998;273:33198–33202. doi: 10.1074/jbc.273.50.33198. [DOI] [PubMed] [Google Scholar]
- [18].Maehrel C, Cordat E, Mus-Veteau I, Leblanc G. Structural studies of the melibiose permease of Escherichia coli by fluorescence resonance energy transfer. I. Evidence for ion-induced conformational change. J Biol Chem. 1998;273:33192–33197. doi: 10.1074/jbc.273.50.33192. [DOI] [PubMed] [Google Scholar]
- [19].Abdel-Dayem M, Basquin C, Pourcher T, Cordat E, Leblanc G. Cytoplasmic loop connecting helices IV and V of the melibiose permease from Escherichia coli is involved in the process of Na(+)-coupled sugar translocation. J Biol Chem. 2003;278:1518–1524. doi: 10.1074/jbc.M210053200. [DOI] [PubMed] [Google Scholar]
- [20].Ganea C, Meyer-Lipp K, Lemonnier R, Krah A, Leblanc G, Fendler K. G117C MelB, a mutant melibiose permease with a changed conformational equilibrium. Biochimica et biophysica acta. 2011;1808:2508–2516. doi: 10.1016/j.bbamem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- [21].Pourcher T, Leclercq S, Brandolin G, Leblanc G. Melibiose permease of Escherichia coli: large scale purification and evidence that H(+), Na(+), and Li(+) sugar symport is catalyzed by a single polypeptide. Biochemistry. 1995;34:4412–4420. doi: 10.1021/bi00013a033. [DOI] [PubMed] [Google Scholar]
- [22].Kaback HR. Bacterial Membranes. Methods in Enzymol XXII. 1971:99–120. [Google Scholar]
- [23].Short SA, Kaback HR, Kohn LD. D-lactate dehydrogenase binding in Escherichia coli dld- membrane vesicles reconstituted for active transport. Proc Natl Acad Sci U S A. 1974;71:1461–1465. doi: 10.1073/pnas.71.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jakkula SV, Guan L. Reduced Na(+) affinity increases turnover of Salmonella enterica serovar Typhimurium MelB. J Bacteriol. 2012;194:5538–5544. doi: 10.1128/JB.01206-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cho KH, Du Y, Scull NJ, Hariharan P, Gotfryd K, Loland CJ, Guan L, Byrne B, Kobilka BK, Chae PS. Novel Xylene-Linked Maltoside Amphiphiles (XMAs) for Membrane Protein Stabilisation. Chemistry. 2015;21:10008–10013. doi: 10.1002/chem.201501083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chae PS, Kruse AC, Gotfryd K, Rana RR, Cho KH, Rasmussen SG, Bae HE, Chandra R, Gether U, Guan L, Kobilka BK, Loland CJ, Byrne B, Gellman SH. Novel tripod amphiphiles for membrane protein analysis. Chemistry. 2013;19:15645–15651. doi: 10.1002/chem.201301423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cho KH, Husri M, Amin A, Gotfryd K, Lee HJ, Go J, Kim JW, Loland CJ, Guan L, Byrne B, Chae PS. Maltose neopentyl glycol-3 (MNG-3) analogues for membrane protein study. Analyst. 2015;140:3157–3163. doi: 10.1039/c5an00240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hariharan P, Balasubramaniam D, Peterkofsky A, Kaback HR, Guan L. Thermodynamic mechanism for inhibition of lactose permease by the phosphotransferase protein IIAGlc. Proc Natl Acad Sci U S A. 2015;112:2407–2412. doi: 10.1073/pnas.1500891112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Velazquez Campoy A, Freire E. ITC in the post-genomic era…? Priceless. Biophys Chem. 2005;115:115–124. doi: 10.1016/j.bpc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- [30].Zakariassen H, Sorlie M. Heat capacity changes in heme protein-ligand interactions. Thermochimica Acta. 2007;464:24–28. [Google Scholar]
- [31].Freire E, Mayorga OL, Straume M. Isothermal titration calorimetry. Analytical Chemistry (Washington) 1990;62:950A–959A. [Google Scholar]
- [32].Schuldiner S, Kerwar GK, Kaback HR, Weil R. Energy-dependent binding of dansylgalactosides to the beta-galactoside carrier protein. J Biol Chem. 1975;250:1361–1370. [PubMed] [Google Scholar]
- [33].Mus-Veteau I, Pourcher T, Leblanc G. Melibiose permease of Escherichia coli: substrate-induced conformational changes monitored by tryptophan fluorescence spectroscopy. Biochemistry. 1995;34:6775–6783. doi: 10.1021/bi00020a024. [DOI] [PubMed] [Google Scholar]
- [34].Garcia-Celma JJ, Dueck B, Stein M, Schlueter M, Meyer-Lipp K, Leblanc G, Fendler K. Rapid activation of the melibiose permease MelB immobilized on a solid-supported membrane. Langmuir. 2008;24:8119–8126. doi: 10.1021/la800428h. [DOI] [PubMed] [Google Scholar]
- [35].Jiang X, Guan L, Zhou Y, Hong WX, Zhang Q, Kaback HR. Evidence for an intermediate conformational state of LacY. Proc Natl Acad Sci U S A. 2012;109:E698–704. doi: 10.1073/pnas.1201107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, Shaw DE, Weis WI, Wess J, Kobilka BK. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chung KY, Kim TH, Manglik A, Alvares R, Kobilka BK, Prosser RS. Role of detergents in conformational exchange of a G protein-coupled receptor. J Biol Chem. 2012;287:36305–36311. doi: 10.1074/jbc.M112.406371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kumar H, Kasho V, Smirnova I, Finer-Moore JS, Kaback HR, Stroud RM. Structure of sugar-bound LacY. Proc Natl Acad Sci U S A. 2014;111:1784–1788. doi: 10.1073/pnas.1324141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kaback HR. A chemiosmotic mechanism of symport. Proc Natl Acad Sci U S A. 2015;112:1259–1264. doi: 10.1073/pnas.1419325112. [DOI] [PMC free article] [PubMed] [Google Scholar]