Abstract
Aims
Rhabdomyosarcoma (RMS) is the most common soft tissue tumor of childhood. Patient age, size, histologic finding, and site of the tumor are primary determinants of prognosis in RMS. Chest wall RMS is a site in which the limitations of surgical excision are realized. We aim to determine the impact of surgical excision in chest wall RMS.
Methods
A retrospective chart review was conducted of all 130 pediatric patients enrolled in the Intergroup Rhabdomyosarcoma Study (IRS) with chest wall rhabdomyosarcoma from the first (I) through fourth (IV) IRS with follow-up to June 2005. Median follow-up was 12.1 years (4.6–27.2 years).
Results
There was a significant improvement in failure-free survival (FFS) and overall survival (OS) between the first IRS study, I, and IRS-IV. The estimated FFS and OS at 5 years in IRS I was 30% and 40%, respectively, compared to 68% and 78%, respectively, in IRS-IV (P = .03 and P = .05, respectively). There was no association between histologic finding or size and FFS or OS. However, all patients who presented without metastasis had an FFS and OS of 49% and 61%, respectively, compared with metastatic patients, 7% and 7%, respectively (P < .001). Five-year FFS of group I, II, and III patients was 52%, 52%, and 45%, respectively, and OS was 65%, 60%, and 59%, respectively. There was no significant difference in 5-year FFS or OS in patients who had a complete resection (group I), complete resection with positive microscopic margins (group II), or biopsy or partial resection only (group III). In groups I to III patients, the local and regional failure rate at 5 years is 25% and 6%, respectively.
Conclusions
The most significant impact on outcome in chest wall RMS patients is metastatic disease at diagnosis. The locoregional failure rate is high but does not appear to impact survival. Alternative treatment strategies are needed for chest wall RMS, but aggressive surgical excision may not be necessary.
Keywords: Rhabdomyosarcoma, Childhood, Chest wall
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and adolescents, accounting for nearly 250 cases of childhood cancer in the United States each year [1]. Despite the use of multimodal therapy, only 65% of patients with trunk and extremity RMS are expected to achieve long-term failure-free survival (FFS). Using multimodal therapy including chemotherapy, radiation therapy, and surgical excision, the prognosis for favorable sites can be up to 90% and for unfavorable sites is as low as 55% [2]. Prognosis is also dependent on histologic finding, size of the tumor, and metastatic sites [2].
Because of the rarity of this tumor, a cooperative group was established for the treatment of North American patients. The first cooperative group protocol-based treatment of RMS was in 1972 by the Intergroup Rhabdomyosarcoma Study (IRS) group. Since then, at approximately 5-year intervals, new protocols are developed that incorporate the successful regimens of the previous protocols and compare these to new chemotherapy or radiation therapy combinations in a randomized fashion. Using this system, a significant improvement in survival has been realized in most disease sites from IRS-I through the most recent 5-year follow-up from IRS-IV and 4 pilot (IVP). For unfavorable sites, such as extremity, pelvis, and trunk, improvement in overall survival (OS) has reached a plateau [3–7].
This report focuses on a very rare site, chest wall RMS. Here, we describe the outcome of chest wall RMS for the last 4 decades and critically evaluate the contribution of surgical excision.
1. Patients and methods
1.1. Patients
Records from all eligible patients enrolled on an IRS protocol (IRS-I, IRS-II, IRS-III, IRS-IVP, or IRS-IV) who had a chest wall primary site were included in this analysis. Chest wall primary site was defined as all tumors in the ribs, sternum, clavicle, and associated surround soft tissue structures of the external chest cavity. Intrathoracic or mediastinal tumors were ruled out. Patients were treated on 4 different protocols for 3 decades and included various chemotherapy and radiation therapy combinations as dictated by the protocol [3–9].
1.2. Methods
The charts for all eligible patients enrolled on an IRS protocol (IRS-I, IRS-II, IRS-III, IRS-IVP, or IRS-IV) who had a chest wall primary site (n = 134) were reviewed. There were 4 patients whose primary site was not chest wall based. Therefore, these 4 patients were deleted from the analysis set. The analysis is based on 130 eligible subjects.
1.3. Statistical analysis
Failure-free survival was defined as the time from enrollment on study to disease progression or death. Overall survival was defined as the time from enrollment to death from any cause. Failure-free survival and OS for patients who had not experienced an event were censored at the patient’s last date of contact. The Kaplan-Meier method was used to estimate the FFS and OS distributions [10]. Differences between survival curves were analyzed by the log-rank test [11]. A Cox proportional hazards regression model was used to adjust comparisons between patient groups for possible confounding factors [12]. The distributions of categorical patient characteristics were compared between the groups using a χ2 test or Fisher’s Exact test. Cumulative incidence curves were used to estimate the local, regional, and distant failure rates. Analyses were based on data available by June 2005. P values of less than .05 were considered statistically significant.
2. Results
There were 130 patients with chest wall RMS treated on IRS-I through IRS-IV. Age ranged from 0 to 20 years (median, 9 years), and 48% were female. Most patients had alveolar histologic finding (37%), and 39% of patients presented with stage 4 disease. There are no stage 1 patients in this study because only patients with favorable sites have stage 1 disease (Table 1).
Table 1.
Baseline demographic, disease, and treatment characteristic summary: percentages are calculated based on the number of nonmissing values
| Variable | Nonmetastatic patients (n = 100) | Metastatic patients (n = 30) | All chest wall patients (n = 130) |
|---|---|---|---|
| Study | |||
| IRS-I | 20 (20%) | 4 (13%) | 24 (18%) |
| IRS-II | 28 (28%) | 14 (47%) | 42 (32%) |
| IRS-III | 27 (27%) | 5 (17%) | 32 (25%) |
| IRS-IV | 19 (19%) | 2 (7%) | 21 (16%) |
| IRS-IVP | 6 (6%) | 5 (17%) | 11 (8%) |
| XRT given | |||
| Yes | 67 (74%) | 23 (77%) | 90 (74%) |
| Sex | |||
| Female | 41 (41%) | 21 (70%) | 62 (48%) |
| Histologic finding | |||
| Alveolar | 34 (34%) | 14 (47%) | 48 (37%) |
| Embryonal/spindle cell | 27 (27%) | 8 (27%) | 35 (27%) |
| Rhabdo NOS | 1 (1%) | 1 (1%) | |
| Undifferentiated | 20 (20%) | 4 (13%) | 24 (18%) |
| Sarcoma, not classifiable | 8 (8%) | 2 (7%) | 10 (8%) |
| Other | 10 (10%) | 2 (7%) | 12 (9%) |
| Age (y) | |||
| <1 | 6 (6%) | 2 (7%) | 8 (6%) |
| 1–9 | 53 (53%) | 11 (37%) | 64 (49%) |
| 10+ | 41 (41%) | 17 (57%) | 58 (45%) |
| Stage | |||
| 1 | |||
| 2 | 14 (31%) | 14 (19%) | |
| 3 | 30 (67%) | 30 (42%) | |
| 4 | 1 (2%) | 27 (100%) | 28 (39%) |
| Group | |||
| I A | 19 (19%) | 19 (15%) | |
| I B | 4 (4%) | 4 (3%) | |
| II A | 30 (30%) | 30 (23%) | |
| II B | 3 (3%) | 3 (2%) | |
| III | 44 (44%) | 44 (34%) | |
| IV | 30 (100%) | 30 (23%) | |
| Size | |||
| ≤5 cm | 15 (35%) | 1 (13%) | 16 (31%) |
| T-Stage | |||
| T-1 | 23 (52%) | 23 (43%) | |
| T-2 | 21 (48%) | 9 (100%) | 30 (57%) |
| Nodal status | |||
| N-0 | 33 (72%) | 6 (67%) | 39 (71%) |
| N-1 | 6 (13%) | 3 (33%) | 9 (16%) |
| N-X | 7 (15%) | 7 (13%) | |
XRT, radiation therapy.
The most significant risk factor was the presence of metastatic disease. The 5-year FFS and OS for patients with nonmetastatic disease were 49% and 61%, respectively, compared to 7% and 7%, respectively, for patients with metastatic disease (Fig. 1). After adjustment for age and histologic finding, subjects with metastatic disease are at increased risk of failure based on FFS (hazard ratio [HR], 3.64; 95% confidence interval [CI], 2.23–5.95; P < .001) and OS (HR, 4.93; 95% CI, 2.96–8.20; P < .001). Subsequent data summaries are presented separately according to metastatic disease status.
Fig. 1.
A, Failure-free survival and B, OS distributions by metastatic disease.
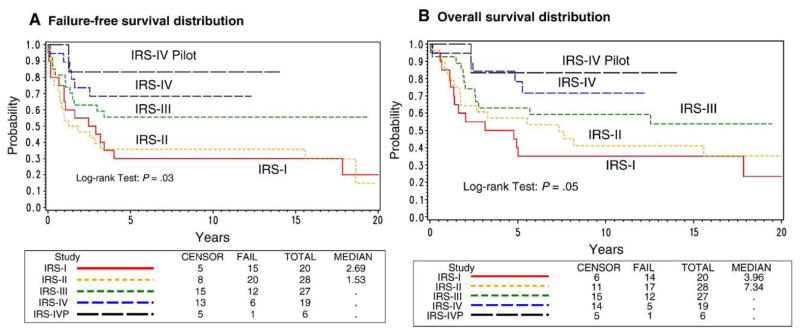
A significant improvement in FFS and OS distributions was seen from IRS-I to IRS-IV for patients without metastatic disease (overall, P = .03 and P = .05, respectively) (Fig. 2), whereas no significant change was seen among the patients with metastatic disease (overall, P value = .9 and P = .7, respectively). For patients with nonmetastatic disease, the 5-year FFS rates ranged from 30% to 68% over the 4 studies and OS ranged from 40% to 78%.
Fig. 2.
A, Failure-free survival and B, OS distributions for nonmetastatic disease patients by study.
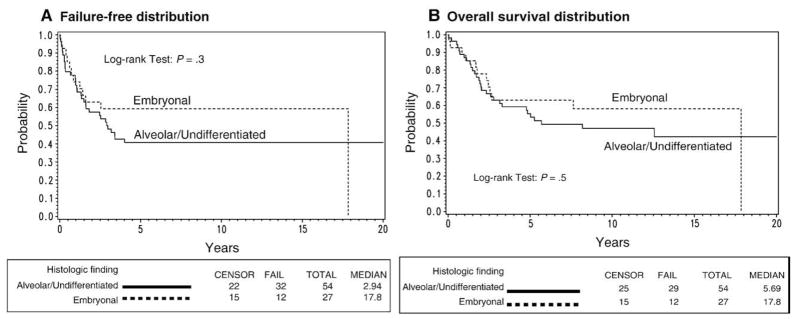
Complete data were available on histologic finding and group status. There was no significant difference in FFS (P = .3) or OS (P = .3) for patients with nonmetastatic alveolar or undifferentiated histologic finding compared to patients with nonmetastatic embryonal or spindle cell disease (Fig. 3). Results were similar based on a model adjusted for age. Among patients with nonmetastatic disease, the 5-year FFS rates were 41% (95% CI, 28%–53%) for patients with alveolar disease and 59% (95% CI, 39%–75%) in patients with embryonal disease. The 5-year OS rates for patients with alveolar or undifferentiated histologic finding were 55% (95% CI, 41%–67%) compared to 63% (95% CI, 42%–78%) for embryonal or spindle cell patients with nonmetastatic disease.
Fig. 3.
A, Failure-free survival and B, OS distributions for nonmetastatic disease patients by histologic finding.
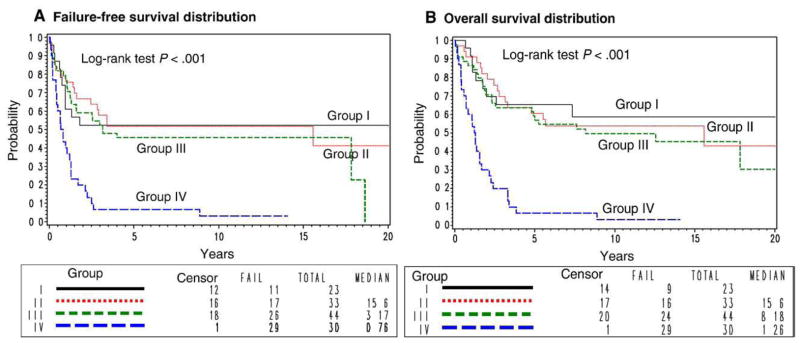
Group Ia patients had complete removal of tumor 5 cm or less, and group II patients had positive microscopic margins at the time of resection, but all gross tumor was removed. Group III patients had a biopsy only or incomplete resection. The 5-year FFS rates for patients with group I, II, III, and IV disease were 52%, 52%, 45%, and 7%, respectively, and the 5-year OS rates were 65%, 60%, 59%, and 7%, respectively. This difference was statistically significant for FFS (P < .001, overall) and OS (P < .001, overall). This is driven by the comparison with group IV patients.
Pair-wise comparisons for FFS distributions, after adjustment for histologic finding and age, are as follows: group I vs group II (P = .8; HR [I vs II], 1.12; 95% CI, 0.52–2.42) and group II vs group III (P = .3; HR [II vs III], 0.73; 95% CI, 0.39–1.37), and comparing OS distributions, after adjustment for histologic finding and age, are as follows: group I vs group II (P = .8; HR [I vs II], 0.92; 95% CI, 0.40–2.11) and group II vs group III (P = .4; HR [II vs III], 0.78; 95% CI, 0.41–1.47) (Fig. 4). The proportion of patients who received radiation therapy across the groups was 26%, 79%, 80%, and 77%, respectively, with significantly fewer group I patients receiving radiation therapy (P < .001).
Fig. 4.
A, Failure-free survival and B, OS distributions by group.
For nonmetastatic disease patients, the local failure (any failure involving a local site) rate at 5 years was 25%, the regional failure (any failure involving a regional site) rate was 6%, and the distant failure (any failure involving a distant site) rate was 18%. For metastatic disease patients, the local failure rate at 5 years was 37%, the regional failure rate was 20%, and the distant failure rate was 30%.
3. Discussion
This is the largest published retrospective series for chest wall RMS. Other studies include chest wall tumors of all histologic origins [13–16]. Across these studies, OS averages 50%. The improvement in survival in chest wall RMS for the last 3 decades of treatment in IRS as documented in this article can be attributed to the improvement in chemotherapy and radiotherapy regimens as refined over the 4 protocols. Under this cooperative group effort, FFS and OS in children with nonmetastatic chest wall RMS has nearly doubled for the last 3 decades.
Surgical techniques have not significantly changed over the same period, and surgical resection has not appeared to contribute to the improvement in survival. Complete surgical resection of the tumor, either before or after induction chemotherapy, does not significantly change the 5-year OS of groups I to III patients, ranging from 65% to 59%. In accordance with IRS protocol, most patients with positive microscopic margins after surgical excision received radiation therapy. Failure-free survival was identical in group I patients who underwent complete surgical excision with negative margins compared to group II patients in which microscopic tumor was found at the edge of the specimen. Even after adjustment for histologic finding and age, there was no statistical significance FFS or OS in group III patients who had a biopsy followed by neoadjuvant chemotherapy and resection, compared to patients who had “up-front” surgical resection. These data imply that surgical excision does not significantly contribute to OS or FFS, although the results are based on observational data, and inference should not extend to causality because there may be factors related to outcome that differ between subjects who did and did not undergo surgical excision. If a surgeon cannot assure excision with negative margins, initial biopsy only, followed by chemotherapy, should be considered.
Factors contributing to poor prognosis include alveolar histologic finding and metastatic disease. In chest wall and other sites of RMS, alveolar histologic finding has been found to characterize disease with poor prognosis compared to embryonal or botryoid histologic finding [2]. Here, although there is a trend toward poorer survival in patients with alveolar disease, in nonmetastatic patients, the histologic difference is not statistically significant.
Patients with metastatic disease have realized no improvement in survival. From previous IRS studies, we know that patients with metastatic RMS have an average 3-year OS of 25%. The 7% survival in metastatic patients in this study of chest wall RMS is much less than that of IRS-III and IRS-IV [3,4,8]. Williams and colleagues [17] in Toronto identified a subset of patients with embryonal histologic finding, younger than 10 years, and metastasis confined to the lung with a 3-year survival of 100% when treated with surgical excision and high-dose chemotherapy and stem cell rescue. In our article, none of the patients in this cohort of chest wall RMS was treated with high-dose chemotherapy and stem cell rescue because it was not part of the protocol treatment plan. In addition, approximately 40% of patients in this study had alveolar histologic finding.
Surgical excision is the mainstay of local disease control in chest wall RMS, but with large tumors, there are clear limitations to surgical excision. Although obtaining negative margins is ideal, we did not show any survival advantage to chest wall tumor excision with positive compared to negative margins. However, almost all patients with positive margins were required to undergo radiotherapy. The addition of radiotherapy can expose patients to serious risks such as decreased lung capacity, pulmonary fibrosis, decreased diffusion capacity, restrictive defects secondary to altered development of the thoracic cavity, and scoliosis [18]. For this reason, only if the surgeon is sure, negative margins can be obtained do we recommend “up-front” surgical resection of chest wall RMS. If negative margins cannot be obtained, we recommend biopsy only, followed by chemotherapy, and then operative excision. Certainly, resectable and unresectable tumors are biologically different and more studies of chest wall RMS are needed to determine the biological differences in this rare disease.
Footnotes
Presented at the 39th Annual Meeting of the Canadian Association of Pediatric Surgeons, August 23–26, 2007, St John’s Newfoundland, Canada.
References
- 1.Ries LAG, et al. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. Bethesda (Md): National Cancer Institute, SEER Program; 1999. [Google Scholar]
- 2.Breitfeld PP, Meyer WH. Rhabdomyosarcoma: new windows of opportunity. Oncologist. 2005;10(7):518–27. doi: 10.1634/theoncologist.10-7-518. [DOI] [PubMed] [Google Scholar]
- 3.Crist W, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13(3):610–30. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 4.Crist WM, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence W, Jr, et al. Prognostic significance of staging factors of the UICC staging system in childhood rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS-II) J Clin Oncol. 1987;5(1):46–54. doi: 10.1200/JCO.1987.5.1.46. [DOI] [PubMed] [Google Scholar]
- 6.Maurer HM, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71(5):1904–22. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Maurer HM, et al. The intergroup rhabdomyosarcoma study: a preliminary report. Cancer. 1977;40(5):2015–26. doi: 10.1002/1097-0142(197711)40:5<2015::aid-cncr2820400505>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson SS, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma—a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51(3):718–28. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 9.Raney RB, Jr, et al. Primary chemotherapy with or without radiation therapy and/or surgery for children with localized sarcoma of the bladder, prostate, vagina, uterus, and cervix. A comparison of the results in Intergroup Rhabdomyosarcoma Studies I and II. Cancer. 1990;66(10):2072–81. doi: 10.1002/1097-0142(19901115)66:10<2072::aid-cncr2820661006>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan GL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 11.Peto R, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient II. Analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 13.Andrassy RJ, et al. Thoracic sarcomas in children. Ann Surg. 1998;227(2):170–3. doi: 10.1097/00000658-199802000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang NC, Siegel SE, Phillips JD. Malignant chest wall tumors in children and young adults. J Pediatr Surg. 1999;34(12):1773–8. doi: 10.1016/s0022-3468(99)90310-x. [DOI] [PubMed] [Google Scholar]
- 15.Saenz NC, et al. Chest wall rhabdomyosarcoma. Cancer. 1997;80(8):1513–7. doi: 10.1002/(sici)1097-0142(19971015)80:8<1513::aid-cncr20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Shamberger RC, Grier HE. Chest wall tumors in infants and children. Semin Pediatr Surg. 1994;3(4):267–76. [PubMed] [Google Scholar]
- 17.Williams BA, Williams KM, Doyle J, et al. Metastatic rhabdomyosarcoma: a retrospective review of patients treated at the hospital for sick children between 1989 and 1999. J Pediatr Hematol Oncol. 2004;26(4):243–7. doi: 10.1097/00043426-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Young MM, et al. Treatment of sarcomas of the chest wall using intensive combined modality therapy. Int J Radiat Oncol Biol Phys. 1989;16(1):49–57. doi: 10.1016/0360-3016(89)90009-6. [DOI] [PubMed] [Google Scholar]