Translational application of experimental knowledge to the clinical setting is the ultimate goal of biomedical research. The reductionist strategies of the past are being rapidly replaced with a more holistic approach to the design, rationale and execution of research activities. Increasingly, the justification for basic research must be based on relevance and applicability to a clinical problem. However, as a prerequisite to clinical studies, mechanistic models to explain causality of observations and treatment outcomes are required. Although some work can be accomplished using in-vitro systems, ultimately animal systems are required for testing the safety and efficacy of data acquired before transfer to humans. Animal testing, as a prerequisite to human clinical exposure, is performed in a range of species, from laboratory mice to larger animals (such as dogs or nonhuman primates). In dental research, the animal species used is dependent largely on the research question or on the disease model. In general, smaller species are more convenient for disease-associated research, whilst larger animals are more appropriate in studies targeting tissue healing. Compared with smaller species, the anatomy of larger animals bears a closer resemblance to human dento-alveolar architecture. The recent emergence of new biologic approaches for the treatment of oral diseases, based on growth factors, active molecules and stem cells, has created a new need for animal research in dentistry in order to apply such techniques to humans. This review focuses on the animal models available for the study of regeneration in periodontal research and implantology; the advantages and disadvantages of each model; the interpretation of data acquired; and the future perspectives of animal research, with a discussion of possible nonanimal alternatives.
General overview
Animal experimentation dates back to the earliest days of research. A recent PubMed survey yielded more than 2000 peer-reviewed articles in which various animal models had been used for periodontal or peri-implant wound healing and regeneration studies. Within the context of periodontal research, animal models were first used to determine the relationship between infection and the host response in an effort to understand the disease process (53). Considerable data have been generated in animals to define the etiology and pathogenesis of periodontal and peri-implant diseases. Periodontal disease and, by analogy, peri-implant disease, are complex infections that result in a tissue-degrading inflammatory response (180). Many in-vitro systems provide strong evidence elucidating the cellular response to bacteria and bacterial products and consequently define the signaling pathways that are involved in this dynamic disease. However, it is impossible to explore the complex pathogenesis of periodontitis or peri-implantitis using only reductionist in-vitro methods. For example, multispecies biofilm models can be used for the characterization of host-parasite interactions (91). On the other hand, these techniques can never fully reproduce the complexity of in-situ biology. There will always be a need for in-vivo models (46). Adding to the disease complexity is the recognition that the inflammatory response of the host is not linear (86, 181). For instance, within the context of periodontal tissues and biofilm-induced infection of the dentition, the number of microbial species associated with disease increase as a function of inflammation whilst further stimulating the inflammatory response (92). This observation provides strong evidence of direct and complex impacts of bacteria on the host response. Homeostasis in the oral cavity does not appear to be regulated in the same way as in the rest of the digestive tract, in which the progression of disease generally results in a reduced microflora, as seen in the gut (9, 24).
In addition to the disease process, healing of the periodontal and peri-implant tissues can be studied in animals. Regeneration, after periodontal surgery, in response to various biologic materials with potential for tissue engineering is a continuous process involving various tissue compartments, including epithelia, connective tissues and the alveolar bone (14). The same principles apply to peri-implant healing. Given the complexity of the biology, animal models are necessary and serve as the standard for successful translation of regenerative materials and dental implants to the clinical setting.
Multiple animal species, including mice, rabbits, hamsters, dogs, pigs and nonhuman primates, have been used to address these questions. In some animals, periodontal disease may occur spontaneously, whereas in others, it needs to be induced experimentally. A major drawback of smaller animals is the limited similarity of their dentition to human dentition. However, small-animal models generate substantial and relevant data on the interactions between soft and hard tissue, especially during inflammation, and hence periodontal inflammatory models, as well as peri-implant pathologies, can be simulated and tested in small animals. Another major advantage of small animals is that the potential mechanisms of systemic inflammation and its impact on periodontal healing processes can be studied in vivo using genetically produced transgenic and knockout animals (53). Finally, small animals are cheaper, and the use of small animals potentially eliminates the need to use larger species before human trials. To this end, mouse, rat and rabbit represent well-defined systems, and other small animals (e.g. hamsters) are also useful.
The dental anatomy of larger species of animals resembles human dentoalveolar architecture more closely than does that of smaller animals. They allow a more direct interpretation of the data and translation of the knowledge to clinical settings. Several large-animal species, such as nonhuman primates, dogs, sheep and miniature pigs, have been traditionally used in periodontal research. Nonhuman primates have oral structures and teeth similar to those of humans and have naturally occurring dental plaque, calculus, oral microbial pathogens and periodontal disease (125, 154). Histologically, the structure of their periodontium is similar to that of humans. During inflammatory processes, connective tissues are infiltrated by plasma cells, lymphocytes and neutrophils (126). Orthodontic brackets and ligatures can be used to accelerate plaque accumulation and periodontal disease (107). The drawback of using nonhuman primates is that they are susceptible to certain infections, such as tuberculosis (154), and ethical considerations and regulations have to be adhered to, to avoid any trafficking of protected species (167). To minimize cost and the aforementioned concerns, excellent models have been generated using nonprimates such as miniature pigs. The major limitation of larger animals is that they are expensive and require specialized breeding and maintenance facilities, which limits their use to only specialized centers equipped with appropriate staff. Nonetheless, they are the in situ model systems phylogenetically closest to humans and hence highly applicable for testing materials destined for investigation in human clinical trials (125).
Rodents
Mice, rats and hamsters provide several advantages for evaluation of microbial and host responses. Rodents have only one incisor and three molars in each quadrant. Studies using rodents have shown disease development, after placement of ligatures in the gingival sulcus around the molar teeth, through an increase of biofilm accumulation (39). This model may not reproduce all aspects of human periodontitis initiation and progression; however, mice are useful animal models for using to understand host–parasite interactions. Young mice can develop periodontitis caused by their own microbiota if their ability to control their indigenous bacteria is compromised by genetic defects in their phagocytes, although the administration of antibiotics prevents the development of the disease (11). During periodontal studies, rodents are generally infected orally with select human pathogens, in an attempt to document the virulence of these species. These approaches are dependent on the use of genetically manipulated strains of rodents to focus on individual components of the host response and thereby to describe the roles of these components in the disease process (4). More recently, different investigators have used gingival tissue inoculated with chemicals, microorganisms or their products to elicit periodontal disease (117). In rats, periodontitis appears to be an infectious process. Inoculations or injections of various human periodontal pathogens can cause periodontal lesions (89). The breeding and housing costs of rodents are relatively low, making it possible to carry out studies with sufficient numbers of animals for statistical analysis. The knockout or transgenic models overexpressing various genes in mice and rats are readily available for use in studying the involvement of specific genes in periodontal and peri-implant diseases, and such approaches are being incorporated into research of regeneration. Furthermore, rodents are suitable models, not only for studies involving teeth, but also for studies investigating the dynamics of soft- and hard-tissue interactions relevant to oral inflammatory conditions. Thus, studies on rodents will continue in order to identify the pathways of regulation of inflammation, resolution and healing processes in periodontal tissues as a first step in translating the basic science into in-vivo models.
The mouse calvaria model, although not a true periodontal or peri-implant system, has potential use in a wide variety of disease applications relevant to dental research (53). Experiments utilizing the mouse calvaria model can provide valuable data on the efficacy of many compounds, including those with regenerative potential. Similar strategies have been used in other species, such as rats or rabbits. In this model, soft- and hard-tissue healing can easily be studied and the role of various molecules in the regulation of bone turnover and regeneration can be investigated. Knockout or overexpression of genes of interest during healing can be assessed. Classical inflammatory events are induced following a stimulus injection directly into the connective tissue overlying the calvarial bone, including the rapid expression of proinflammatory cytokines within a few hours and the recruitment of neutrophilic granulocytes within 24 hours (54). Bone resorption can be induced within 3–5 days, depending on the size of the stimulus (103). The mouse calvaria model is useful in studying bone turnover and the sequence of inflammation, destruction and repair relevant to large-animal models.
Mice are the smallest species used widely in dental studies. There are several advantages of the mouse model. They are extremely cost-effective compared with larger animals. Antibodies against mouse antigens are almost as widely available as human panels, which allow researchers to study a wide range of immunohistochemical end-points in mice. In recent years, mice have also become the animal of choice for knockout studies, with an extensive genetic array for different backgrounds. The mouse genome has been sequenced; therefore, the specific roles of genes in the regulation of disease processes, inflammation and regeneration, including periodontal disease, can be conveniently studied in mice. Mono-infection, virulence or mixed-disease models can be studied in mice (108, 201, 204). Periodontal disease and inflammatory processes can be induced in diabetic mice or in mice with a certain predisposition to cardiovascular diseases (6, 100, 106, 108, 124, 200, 204). The disadvantages of mice are their body size, as well as the dimensions of their teeth. Although various studies have successfully shown that ligatures can be tied around murine teeth to induce periodontal disease, this is an extremely difficult task (57, 102). The oral-gavage approach is an alternative (45, 53, 115). In this technique, an external pathogen is grown in broth and introduced into the oral cavity of the mice by gavage. Periodontal disease usually develops within 7–10 days. The mechanism of action probably involves conversion of the commensal microflora in mice to a pathogenic biofilm during this process, and tissue responses can be studied. As the dietary habits of rodents are substantially different from those of humans, oral biofilm formation in mice and rats after ligature or gavage (58) requires further and detailed investigation in order to interpret the findings appropriately.
Rats have also been extensively used in periodontal disease studies. Various approaches have been used to induce periodontal inflammation in rats. Currently, two techniques are widely employed. The first requires administration of bacterial lipopolysaccharide to the gingiva of the rat, creating an acute and severe periodontal pathology (29). This model has the advantages of generating a rapid disease process and not requiring growth of bacteria or extensive manual handling of the dental structures. Therefore, it is used especially for testing the efficacy of various agents to resolve inflammation (16, 17, 88, 122, 143, 176, 182). The disadvantages are that the model is an acute inflammatory response to the endotoxin of the bacteria, the infection is not generated by the bacteria and the host response probably represents specific pathways in certain cell types. With those limitations in mind it should be noted that valuable information on the action of various compounds, as well as testing the actions of the lipopolysaccharide and other confounding factors in periodontal destruction in vivo, has been generated using this model. Conversely, the placement of ligatures around teeth to initiate periodontal tissue loss leads to the accumulation of plaque and ulceration of the sulcular epithelium, facilitating invasion of connective tissue. Ligature-induced periodontal disease in rodents does not require human pathogens. However, alveolar bone loss in the ligature model is dependent on bacteria, as the placement of ligatures does not induce significant gingival inflammation or periodontal bone loss in gnotobiotic rats (53, 144). Thus, these models could continue to be useful for addressing specific questions, as in-vivo models.
Rabbit model
Rabbits also represent a good “small animal model” for studying the impact of inflammation on periodontal wound healing and regeneration. In addition to being small enough to conduct a reproducible periodontal inflammation study around teeth, a major advantage of rabbits is that the ligature alone does not induce destruction of soft or hard tissue. Hence, it can be used to test the specific impact of periodontal pathogens of human origin, in contrast to rats and mice, in which ligature placement induces periodontal disease by conversion of the commensal flora into a pathogenic flora in the oral cavity. Another advantage of the rabbit model is its use in nondental inflammation models, such as cardiovascular diseases. Rabbit aorta is commonly used to investigate lipid deposition and vascular changes. Although rabbits do not develop spontaneous atherosclerosis, they are useful because they are highly responsive to cholesterol manipulation and develop lesions in a relatively short period of time (38). The high-cholesterol-diet rabbit model has been widely used for inducing experimental atherosclerosis. In normolipidemic rabbits, atherosclerotic lesions can develop as a result of repeated or continuous intimal injury by an aortic polyethylene catheter, balloon angioplasty or nitrogen exposure (145). Therefore, many studies have used the rabbit model with a high-cholesterol diet, arterial wall injury, or, most commonly, a combination of both. The observed lesions resemble, at least in part, those seen in human plaques, regarding the inflammatory component and the vascular smooth-muscle-cell proliferation. The rabbit model has largely been used to study the influence of lipid lowering (through diet or statins) on plaque formation and stabilization. Those studies have contributed to unveiling the mechanisms by which lipid lowering reduces macrophage accumulation and other aspects of atheroma inflammation. Rabbits with inflammation have a greater number of vascular lesions than do rabbits without inflammation. This model could represent a novel approach to the study of inflammation-associated atherosclerosis (111). A model for plaque rupture has been also developed in rabbits through the combination of aggressive vascular injury and a hyperlipidemic diet (159). This technique accurately quantifies fibrotic and lipid components of atherosclerosis in the model and may permit the serial analysis of therapeutic strategies on atherosclerotic plaque stabilization. Thus, rabbits are an excellent model for studying the inflammatory basis of periodontal diseases and healing patterns.
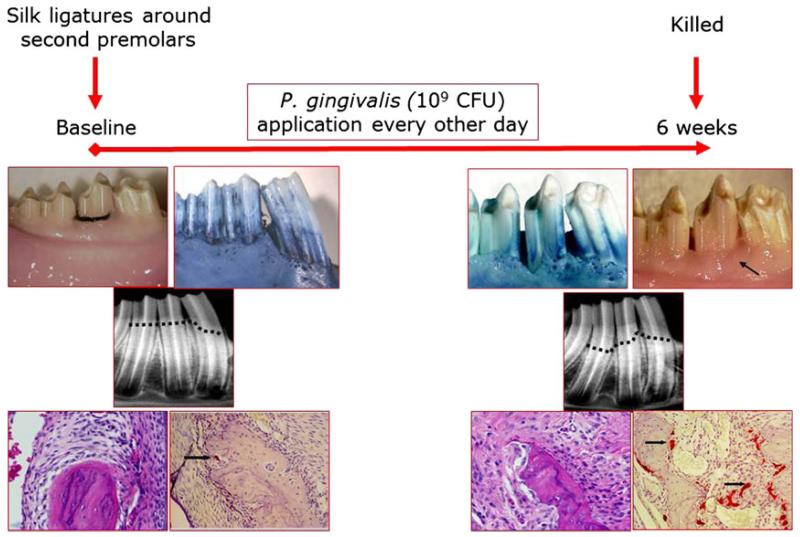
Rabbits represent a relevant model in which the physiology and the pathology of periodontal tissues resemble those of humans with respect to pro- and anti-inflammatory mechanisms. Previous work, by our group, has shown that a predictable and reproducible periodontitis can be generated in rabbits by using silk ligatures together with topical application of the periodontitis-specific microorganism, Porphyromonas gingivalis (65, 66, 77). In this model, silk ligatures are tied around the second premolars of the mandibles of rabbits (Fig. 1). Ligature alone does not result in periodontal tissue destruction. Application of P. gingivalis, every other day for 6 weeks, reproducibly generates periodontal disease in rabbits, with clinical, radiographic and histologic patterns very similar to those observed in humans during development of periodontal disease. Stopping the application of the pathogen at the end of this period does not result in spontaneous resolution of inflammation or in tissue healing. The efficacies of various compounds have been tested using this model (64-66, 68, 69). Whilst the preventive impact of therapeutics can be investigated by the simultaneous administration of bacteria and the therapeutic agent during the entire 6-week study period, treatment of the periodontal disease can also be studied by stopping the administration of bacteria and testing the ability of the compound of interest to resolve the inflammation and the impact on bone (68). Bone coupling has been shown as an outcome of resolution of periodontal inflammation and regeneration in this model. Transgenic rabbits are also available and have been used to study periodontal and atherosclerotic diseases. The major disadvantage of rabbit models is the limited availability of anti-rabbit antibodies; however, this issue is being rapidly resolved as a result of the increasing applicability of rabbit models in biomedical research.
Fig. 1.
Rabbit as a model for periodontal disease. In New Zealand White rabbit, periodontal disease can be established by placing silk ligatures around the second premolars. The rabbit oral microbiota does not result in periodontal disease development upon accumulation around the ligatures. A pathogenic bacterium (e.g. Porphyromonas gingivalis) can be applied throughout the study (6 weeks). Clinical, radiographic and histologic evidence demonstrate clear periodontal tissue destruction, similar to the disease process in humans. Established periodontal disease in rabbits is not reversible when left untreated (68). CFU, colony-forming units.
In addition to these studies, rabbits are readily used for studying the integration of dental implants into bone. Most of these techniques involve the long bones of rabbits. These studies have been designed not only to test the integration of new materials and surface characteristics introduced in implant designs (10, 18, 19, 25, 26, 30, 31, 59, 80, 90, 148, 166, 170-172, 196, 199), but also to analyze the confounding factors of bone healing, such as bisphosphonates, taking advantage of bone turnover patterns in rabbits (28, 177). The impact of various regenerative procedures around implants has been tested in rabbit models (19, 25, 48, 78, 81, 123, 147, 175, 183, 198). Some of these models have incorporated the use of the rabbit calvaria (87, 175). Very limited work has utilized the alveolar bone in rabbits for periodontal regeneration or dental implant studies (1, 23, 162).
Dog model
Dogs are among the most well-established models to study naturally occurring gingivitis and periodontitis (128). The Beagle dog is commonly used because of its size and its extremely cooperative nature; however, many studies have also used mongrel dogs (167). Periodontal tissues and the size of the teeth in dogs are, in general, similar to those in humans. However, dogs lack lateral jaw movements and occlusal contacts at the premolars. The subgingival bacterial species are predominantly anaerobic gram-negative cocci and rods (41, 61). The severity of periodontal disease in dogs increases with age and can result in natural tooth loss. Periodontitis follows gingivitis; periodontal pockets develop with a dense cellular infiltrate consisting mainly of plasma cells and lymphocytes. The osteoclastic resorption of alveolar bone results in deep, narrow lesions extending vertically around a single root, leaving the interdental space intact (60). Conversely, the extent and localization of periodontal lesions may not be always predictable in dogs (63, 167). Animal care regulations, including daily companionship, exercise, space and maintenance, make use of dogs increasingly less desirable and convenient in periodontal disease and inflammation studies (125). Nevertheless, the dog model is still extensively used in wound healing, dental implant and regeneration studies (193).
The dog as an experimental model in periodontal regeneration is not new. For decades, dog studies have been considered as the major in-vivo testing system for new regenerative devices or techniques. Some of the earliest evidence on the efficacy of guided tissue regeneration came from dog studies (120). The dog model was also used to assess the impact of biomechanical relationships in the oral cavity within the context of regenerative procedures (138). Dogs served as excellent models for studying the impact of periodontal inflammation and plaque control on regeneration and new attachment formation (73, 138, 139). These studies, and later work, demonstrated the regenerative potential of the periodontium, providing breakthrough evidence that regeneration around previously diseased root surfaces was possible provided that periodontal ligament cells could selectively repopulate onto the roots, whilst other cell types did not have the capacity to regenerate new “connective tissue” attachment, confirming the earlier observations in humans (13, 114, 120, 121, 192, 194, 195). Following these critical observations, dogs have been extensively used in various landmark studies in periodontal regeneration and eventually in implant dentistry.
During the early 1980s and 1990s, identification of the optimal materials and techniques for regeneration in periodontal therapy relied heavily on dogs as the experimental model of choice. Dogs are affordable compared with monkey models, are easy to use and their availability and size are practical. Various graft materials have been tested as bone substitutes (101, 136, 168, 169), as has the efficacy of root surface conditioning in inducing periodontal regeneration (13, 15, 118). Guided tissue regeneration and membranes have been studied in dogs, and a substantial number of publications demonstrate the efficacy and biocompatibility of expanded-polytetrafluoroethylene, synthetic and organic resorbable membranes (44, 62, 109, 110, 119, 135, 173, 174). Comparative studies of resorbable and nonresorbable membranes provided a wide selection of membrane configurations for use in guided tissue regeneration (22). The major contribution of dog studies in regenerative periodontal medicine has been the histological demonstration that guided tissue regeneration is achievable in various defect types, such as furcations and infrabony resorption sites (21). Similarly to studies in humans, dog models have helped in gaining an understanding of the limitations of regenerative approaches with regard to defect type (e.g. Class III furcations) and size, as well as of membrane-associated properties (e.g. resorption kinetics and type), providing a basis for the combined use of bone substitutes and barrier membranes (35, 110, 140). Such studies further demonstrated that defect characteristics and space are key elements for success in periodontal regeneration (188), and the combination of root-surface treatments with barrier membranes did not enhance the outcomes of guided tissue regeneration (40). Dogs were also used for other periodontal-wound healing models. In one study, no difference was observed when barrier membranes or free connective tissue grafts were used to cover the recession defects (190).
Growth factors and their regenerative potentials in periodontal defects have also been tested in dogs. Lynch and colleagues demonstrated that platelet-derived growth factor and insulin-like growth factor enhanced periodontal regeneration, resulting in significant gain in new bone formation and attachment (105). A single administration of platelet-derived growth factor plus insulin-like growth factor stimulated new-attachment formation in dogs (49). Platelet-derived growth factor was found to stimulate fibroblast proliferation during early wound healing, and barrier membranes did not significantly change the outcome (186); however, insulin-like growth factor, fibroblast growth factor and transforming growth factor-beta1 did not enhance bone formation, fibroblast production and collagen density (156). Some studies demonstrated that the response around all teeth in dogs was different and that time was critical for a significant regeneration. For example, in a study in which Class III furcations were treated with membranes alone or with platelet-derived growth factor, the second premolar teeth showed significantly better regeneration compared with the fourth premolars, and addition of platelet-derived growth factor enhanced new bone formation by up to 87% (32, 127). Such limitations, were further explored in dogs (7, 12), defect dimensions were analyzed (165) and some novel concepts, such as cell seeding, were tested (37). These investigations support the use of combined technologies in extreme defects, such as one-wall intrabony destructions (178, 206) or vertical augmentation (160), and in some new approaches, such as the application of recombinant human growth/differentiation factor-5 with poly(lactic-co-glycolic acid) constructs (96).
Specific roles of various extracellular matrix proteins, such as type I collagen, fibronectin, secreted protein acidic and rich in cysteine, vitronectin and bone sialoprotein, during regenerative healing was identified using the dog model (113). Fibronectin and vitronectin were found to be involved during early healing events (71), but their application did not enhance membrane efficacy (95). Flap designs and tensile strength were analyzed histologically in dogs (191). Collectively, these reports confirm that the regenerative potential of periodontal tissues depends on regulation by early biological events and includes an orchestrated series of events in which certain biologically active molecules (e.g. growth factors and extracellular matrix proteins) could play important roles at various stages of healing.
Enamel matrix proteins have been tested in periodontal regeneration in dogs, and the efficacy of such preparations in this respect has been demonstrated (3, 5, 47, 55, 70, 74, 76, 134, 146, 189). These studies are significant in the light of the wide use of enamel matrix proteins in several periodontal and peri-implant applications and have shown that new alveolar bone, as well as new cementum formation, can be facilitated through their use whilst preventing the apical migration of the epithelium (5, 146). An interesting study also demonstrated that while nicotine impaired membrane-induced regeneration, the efficacy of enamel matrix proteins was not affected (134). Another valuable contribution was the observation that regenerative techniques using enamel matrix derivatives not only resulted in enhanced hard-tissue formation, but also increased gingival tissue thickness (3).
Regeneration of bone around dental implants has also been tested in the dog model since the early 1990s. Various surface preparations, as well as designs of implants, were tested with the concomitant use of barrier membranes, and the results suggested that the use of these materials facilitated peri-implant regeneration (202), even when the implants were placed in fresh extraction sockets (27), providing some of the early evidence for the feasibility of immediate implant placement. However, not all implant designs and membranes tested in dogs were found to be successful in regenerating bone around implants. For example, a hydrolyzable polyester material (polyhydroxybutyrate-hydroxyvalerate reinforced with polyglactin 910 fibers) not only did not enhance new bone formation, it actually resulted in an increased inflammatory reaction with significantly less bone formation compared with the control side, which was treated without any barrier membrane (52). Likewise, the prototypes of some membrane designs, such as one with two collagen layers and an internal polylactide layer for lateral ridge augmentation, did not show any benefit in dogs (185). Nevertheless, the results of many dog studies on regeneration around implants have proven to be beneficial in providing data applicable for use in humans (8, 203), whilst demonstrating the efficacy of such methods (184). These studies further justified the need for the use of carefully designed studies in animal models before clinical application in humans.
In addition to clinical efficacy, factors complicating guided bone regeneration outcomes following the experimental induction of peri-implantitis in dogs have also been studied. For example, the lack of oral hygiene and membrane exposure during healing were found to impair bone regeneration and integration (51, 56, 158). Likewise, inflammation has been shown to be a complicating factor during implant loading (93). Implant stability was tested in peri-implantitis models in dogs (50, 157), and novel technologies, such as laser treatment, were studied (36), with significant findings suggesting that the inflammatory process and the resulting alveolar bone destruction in peri-implantitis in dogs are similar to those in humans and can be successfully treated. These studies also initiated a debate on defect types and material composition, as well as on the surface characteristics of implants (130, 132). For example, dehiscence-type defects have been proposed to have a higher chance for predictable restoration of tissues. In addition, infection and inflammation were shown to result in a challenge for resolution and periodontal/peri-implant regeneration. In several studies, resolution of inflammation was shown to lead to healing and “re-osseointegration” on the surfaces of previously contaminated implants after removal of accumulated plaque (82, 112, 131), whilst connective tissue, to some extent, was also found between the implants and the bone (129). Titanium-reinforced membranes were found to be predictable in protecting the space for blood-clot stabilization around implants and resulted in supracrestal bone formation, even without the addition of bone grafts (83). This approach was tested around implants in which the threads were left exposed, and vertical bone growth was observed when nonresorbable membranes were used as space maintainers (142).
Taken together, the dog is still being used as an experimental model, although its use has substantially decreased for several ethical reasons as well as because of the introduction of other small-animal models or, in many cases, replacement with alternative models such as the miniature pig. Nevertheless, it continues to serve as an excellent model for identification of the mechanism underlying the tissue response to regenerative approaches in periodontology and implantology.
Miniature pigs
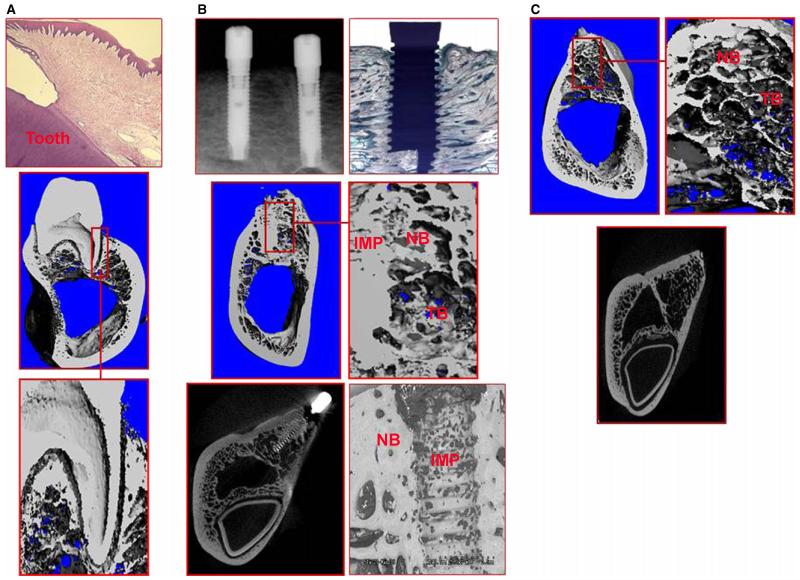
The miniature pig model has emerged as a good alternative to the dog model (Fig. 2). Varieties of miniature pig have been extensively used in biomedical research, studies of artificial organs and dermal wound-healing models since the 1950s (42, 137, 155, 179, 197). These animals are similar to humans in gross anatomy and physiology and have economic advantages over other large species. Some of the miniature pigs develop human diseases, such as diabetes, through similar processes and therefore represent an excellent model for systemic diseases (98). A recent and excellent review has described the dental characteristics of miniature pigs in detail (187). The periodontium of miniature pigs shows many similarities to that of humans. The depth of the gingival sulcus is 2–3 mm with a long junctional epithelium. Natural gingivitis can be observed at 6 months of age. The pattern of disease progression follows the same stages as that in humans: gingival swelling, plaque accumulation, calculus formation and bleeding on probing. Histologically, these clinical features are accompanied by inflammatory cell infiltration and vasodilatation. As of 16 months of age, miniature pigs may develop advanced periodontitis, with pocket depths up to 5 mm and alveolar bone resorption. In an experimental periodontitis model in miniature pigs, in which ligatures were placed and human periodontopathogens were administered, periodontitis developed in 4 weeks, with increased periodontal destruction by 8 weeks and no further progression until 20 weeks (187). Removal of the ligatures did not result in complete restoration of the periodontal destruction, with remaining substantial tissue loss supporting the suitability of the model. Almost the earliest nondisease use of miniature pigs in periodontal research was for the creation of mucogingival defects (84, 85). In this model, facial gingiva was excised from primary incisors and resulted in recession by 3 months. This model was not further explored in follow-up studies but certainly would be of use. Miniature pigs have also been tested for suitability in experimental peri-implantitis studies. Silk ligatures were placed around dental implants for 45 days and disease was assessed by measuring increases in pocket depth, loss of attachment and gingival inflammation (72). During peri-implantitis in miniature pigs, the microbiota changed from primarily gram-positive facultative organisms to gram-negative obligate anaerobes, and, similarly to human disease, bone loss was evident and implant threads became exposed.
Fig. 2.
The miniature pig as a model for periodontal and peri-implant studies. (A) Miniature pigs can be used in periodontal disease studies as a large animal model as a result of the resemblance of their dentition with human dentition. Clinical measurements can be accompanied by radiographic measurements and by three-dimensional analysis using micro-computed tomography. (B) Miniature pigs can be used as a model for implantology research. Histologic, clinical and radiographic, as well as electron microscopic, analyses can be made. IMP, implant; NB, new bone; TB, trabecular bone. (C) Miniature pigs can also be a good model for socket preservation studies in which trabecular bone and new bone formation can be studied using radiographic and qualitative methods.
Over the last two decades, miniature pigs have been heavily used for testing the regenerative potential of various compounds and technologies. In a series of studies, the osseointegration of dental implants was tested in grafted bone, demonstrating that iliac crest grafts were significantly better compared with hydroxyapatite (150-153). Lack of proper bone formation in response to alloplastic materials was further confirmed in miniature pigs (116). In addition to testing bone substitutes, miniature pigs were used for guided bone regeneration procedures around implants (161). Combined use of autogenous grafts and membranes demonstrated that guided bone regeneration was predictable and that osseointegration, bone regeneration and disease processes could be accurately assessed in the miniature pig (20, 149). Histologic examinations demonstrated that bone repair involved a programmed maturation and that bone grafts did not enhance membrane-induced regeneration. Histomorphometric analysis showed that autologous bone grafts had the best osteoconductive properties during the initial healing period, with 39% of newly formed bone inside the membrane-covered defects at 4 weeks of healing, and that the regenerative process was similar to that in humans (20). We have developed a miniature pig model for immediate implant placement and loading and tested a novel biodegradable polymeric material combined with a light/chemical hardening technology for bone grafts. Implant stability and function; microcomputed tomography, histologic and histomorphometric analyses of bone–implant contact; and the characteristics of newly formed bone around implants and extraction sockets have been tested. The results contribute to our knowledge on the importance of primary stability in the success of immediate implants and immediate loading (67).
As novel technologies developed, many of the new devices were tested in miniature pigs. Enamel matrix proteins have been tested in miniature pigs; the results suggest that application of enamel matrix proteins alters the type of periodontal connective tissue interface (33). In another study, autogenous periodontal cell grafts, with or without the application of enamel matrix derivative, were tested on the implant–connective tissue interface. The study demonstrated that the implants, treated with periodontal cells but not with enamel matrix derivatives, demonstrated good bone contact with some epithelium; however, the best results were obtained for the group in which the implants were treated with periodontal cells and enamel matrix derivatives – significant bone contact was observed without any evidence of the epithelium (34). The miniature pig model was also used to test the efficacy of platelet concentrate in bone regeneration; no supportive role was found for platelet concentrate in the regenerative actions of autografts or bone substitutes (79). In one study, the impact of platelet-rich plasma was tested on flap strength at various postsurgical time points in a miniature pig model. Platelet-rich plasma did not seem to contribute to greater flap strength and did not enhance wound healing (141). One of the recent applications of miniature pigs in periodontal regeneration has been the testing of growth factors and their regenerative capacity. The regenerative capacity of recombinant human bone morphogenetic protein 7 was tested following the extraction of immature primary incisors and the total removal of periodontal ligament and cementum. Recombinant human bone morphogenetic protein 7 improved both the survival and the eruption of replanted teeth (164). A recent bone-augmentation study compared recombinant human bone morphogenetic protein 7 with autogenous bone particles using maxillary sinus augmentation techniques in minipigs (99). The results of the study clearly indicated that minipigs are useful models in various bone-regeneration procedures and produce relevant data that can be easily translated into standard clinical applications in humans. Overall, whilst a series of studies on miniature pigs supported human data, it also opened new avenues of potential therapeutic applications.
An interesting model involved the replantation of cultured cells from the periodontium of miniature pigs, demonstrating the formation of new cementum and new bone (97). This study was significant in showing that regeneration in the periodontium was determined by the availability of precursor cells capable of forming calcified tissues. Recent studies further suggest that not only is the miniature pig an excellent large-animal model for regenerative studies, it also harbors a good source for pluripotent cells, which may be harvested for use in regeneration (75). Thus, tissue-engineering studies extensively utilize miniature pigs. For example, a new population of stem cells isolated from the root apical papilla of human teeth was transplanted into miniature pigs to generate a root–periodontal complex capable of supporting a porcelain crown and normal tooth function (43, 163), demonstrating that complete periodontal regeneration was possible (104). These studies support the feasibility of using stem-cell-mediated tissue engineering to treat periodontal defects. The most recent research has tested how the regeneration of multiple periodontal tissues is coordinated in miniature pigs. The hybrid tooth–bone constructs combined tooth-bud-cell-seeded scaffolds with autologous iliac crest bone-marrow-derived stem-cell-seeded scaffolds that were then transplanted back into surgically created mandibular defects in the same miniature pigs. The constructs were harvested after 12 and 20 weeks of growth; the tooth-like structures consisted of dentin, enamel, pulp, cementum and periodontal ligament and were surrounded by regenerated alveolar bone (205). This strategy was also tested for coordinated autologous reconstruction of tooth and mandible (2). Stem cells were shown to promote tooth regeneration in autogenic cell transplantation on various scaffolds (94). Combining the mesenchymal stem cells and platelet-rich plasma incorporated into a fluorohydroxyapatite scaffold in cylindrical defects in the edentulous mandibular ridge of miniature pigs demonstrated the formation of new vital bone (133).
Conclusion
Animal experimentation in dental research is necessary and will stay relevant. Animal models are often superior to in-vitro or clinical studies in addressing mechanistic questions and serve as an essential link between hypotheses and human patients. There is no single animal model that represents all aspects of human disease, tissue architecture and healing and aging processes. However, human studies cannot always be coupled with harvesting the tissues and body parts necessary for microscopic analyses, which are necessary for defining the biologic impact of the regenerative methods and materials. Therefore, animal studies are critical for establishing cause-and-effect relationships and, just as importantly, for initial tests of principles in the development of new regenerative devices and advanced therapeutics. In selecting the animal model, the goal should not be to define the model which is most similar to humans but rather to describe how various models can be used to provide insights into the mechanisms of periodontal diseases, wound healing around implants, tissue architecture and responses, and whether a given model is suitable for studying a specific hypothesis. As animals are vulnerable, models should be carefully planned and justified. Power calculations in such studies are crucial to target a sample size large enough to generate statistically useful data, whilst at the same time small enough to prevent the unnecessary use of animals.
References
- 1.Abrahamsson P. Intra-oral soft tissue expansion and volume stability of onlay bone grafts. Swed Dent J Suppl. 2011;211:11–66. [PubMed] [Google Scholar]
- 2.Abukawa H, Zhang W, Young CS, Asrican R, Vacanti JP, Kaban LB, Troulis MJ, Yelick PC. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J Oral Maxillofac Surg. 2009;67:335–347. doi: 10.1016/j.joms.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hezaimi K, Al-Fahad H, O’Neill R, Shuman L, Griffin T. The effect of enamel matrix protein on gingival tissue thickness in vivo. Odontology. 2012;100:61–66. doi: 10.1007/s10266-011-0022-5. [DOI] [PubMed] [Google Scholar]
- 4.Alayan J, Ivanovski S, Farah CS. Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient mice. J Periodontal Res. 2007;42:97–103. doi: 10.1111/j.1600-0765.2006.00920.x. [DOI] [PubMed] [Google Scholar]
- 5.Alhezaimi K, Al-Shalan T, O’Neill R, Shapurian T, Naghshbandi J, Levi P, Jr, Griffin T. Connective tissue-cementum regeneration: a new histologic regeneration following the use of enamel matrix derivative in dehiscence-type defects. A dog model. Int J Periodontics Restorative Dent. 2009;29:425–433. [PubMed] [Google Scholar]
- 6.Amar S, Wu SC, Madan M. Is Porphyromonas gingivalis cell invasion required for atherogenesis? Pharmacotherapeutic implications. J Immunol. 2009;182:1584–1592. doi: 10.4049/jimmunol.182.3.1584. [DOI] [PubMed] [Google Scholar]
- 7.Araujo MG, Berglundh T, Albrekstsson T, Lindhe J. Bone formation in furcation defects. An experimental study in the dog. J Clin Periodontol. 1999;26:643–652. doi: 10.1034/j.1600-051x.1999.261003.x. [DOI] [PubMed] [Google Scholar]
- 8.Artzi Z, Givol N, Rohrer MD, Nemcovsky CE, Prasad HS, Tal H. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 1: description of a dog model and histological observations. J Periodontol. 2003;74:1143–1152. doi: 10.1902/jop.2003.74.8.1143. [DOI] [PubMed] [Google Scholar]
- 9.Arulampalam V, Greicius G, Pettersson S. The long and winding road to gut homeostasis. Curr Opin Gastroenterol. 2006;22:349–353. doi: 10.1097/01.mog.0000231806.65030.ed. [DOI] [PubMed] [Google Scholar]
- 10.Badr NA, El Hadary AA. Hydroxyapatite-electroplated cptitanium implant and its bone integration potentiality: an in vivo study. Implant Dent. 2007;16:297–308. doi: 10.1097/ID.0b013e31805d7dc4. [DOI] [PubMed] [Google Scholar]
- 11.Beertsen W, Willenborg M, Everts V, Zirogianni A, Podschun R, Schroder B, Eskelinen EL, Saftig P. Impaired phagosomal maturation in neutrophils leads to periodontitis in lysosomal-associated membrane protein-2 knockout mice. J Immunol. 2008;180:475–482. doi: 10.4049/jimmunol.180.1.475. [DOI] [PubMed] [Google Scholar]
- 12.Bianucci HC, Smith MM, Saunders GK, Reddy MS, Cox CF, Till LG, Feldman BF. Periodontal healing of canine experimental grade-III furcation defects treated with autologous fibrinogen and absorbable barrier membrane. Am J Vet Res. 1998;59:1329–1338. [PubMed] [Google Scholar]
- 13.Bogle G, Claffey N, Egelberg J. Healing of horizontal circumferential periodontal defects following regenerative surgery in beagle dogs. J Clin Periodontol. 1985;12:837–849. doi: 10.1111/j.1600-051x.1985.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 14.Bosshardt DD, Sculean A. Does periodontal tissue regeneration really work? Periodontol 2000. 2009;51:208–219. doi: 10.1111/j.1600-0757.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 15.Bostanci HS, Arpak MN, Gunhan O. New attachment formation following periodontal surgery in a dog. J Nihon Univ Sch Dent. 1990;32:159–166. doi: 10.2334/josnusd1959.32.159. [DOI] [PubMed] [Google Scholar]
- 16.Breivik T, Gundersen Y, Osmundsen H, Fonnum F, Opstad PK. Neonatal dexamethasone and chronic tianeptine treatment inhibit ligature-induced periodontitis in adult rats. J Periodontal Res. 2006;41:23–32. doi: 10.1111/j.1600-0765.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 17.Breivik T, Opstad PK, Engstad R, Gundersen G, Gjermo P, Preus H. Soluble beta-1,3/1,6-glucan from yeast inhibits experimental periodontal disease in Wistar rats. J Clin Periodontol. 2005;32:347–352. doi: 10.1111/j.1600-051X.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 18.Bumgardner JD, Chesnutt BM, Yuan Y, Yang Y, Appleford M, Oh S, McLaughlin R, Elder SH, Ong JL. The integration of chitosan-coated titanium in bone: an in vivo study in rabbits. Implant Dent. 2007;16:66–79. doi: 10.1097/ID.0b013e3180312011. [DOI] [PubMed] [Google Scholar]
- 19.Burgos PM, Rasmusson L, Meirelles L, Sennerby L. Early bone tissue responses to turned and oxidized implants in the rabbit tibia. Clin Implant Dent Relat Res. 2008;10:181–190. doi: 10.1111/j.1708-8208.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- 20.Buser D, Hoffmann B, Bernard JP, Lussi A, Mettler D, Schenk RK. Evaluation of filling materials in membrane-protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin Oral Implants Res. 1998;9:137–150. doi: 10.1034/j.1600-0501.1998.090301.x. [DOI] [PubMed] [Google Scholar]
- 21.Caffesse RG, Dominguez LE, Nasjleti CE, Castelli WA, Morrison EC, Smith BA. Furcation defects in dogs treated by guided tissue regeneration (GTR) J Periodontol. 1990;61:45–50. doi: 10.1902/jop.1990.61.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Caffesse RG, Nasjleti CE, Morrison EC, Sanchez R. Guided tissue regeneration: comparison of bioabsorbable and non-bioabsorbable membranes. Histologic and histometric study in dogs. J Periodontol. 1994;65:583–591. doi: 10.1902/jop.1994.65.6.583. [DOI] [PubMed] [Google Scholar]
- 23.Caiazza S, Taruscio D, Ciaralli F, Crateri P, Chistolini P, Bedini R, Colangelo P, Pintucci S. Evaluation of an experimental periodontal ligament for dental implants. Biomaterials. 1991;12:474–478. doi: 10.1016/0142-9612(91)90145-z. [DOI] [PubMed] [Google Scholar]
- 24.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 2008;56:305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Carmagnola D, Abati S, Celestino S, Chiapasco M, Bosshardt D, Lang NP. Oral implants placed in bone defects treated with Bio-Oss, Ostim-Paste or PerioGlas: an experimental study in the rabbit tibiae. Clin Oral Implants Res. 2008;19:1246–1253. doi: 10.1111/j.1600-0501.2008.01584.x. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho CM, Carvalho LF, Costa LJ, Sa MJ, Figueiredo CR, Azevedo AS. Titanium implants: a removal torque study in osteopenic rabbits. Indian J Dent Res. 2010;21:349–352. doi: 10.4103/0970-9290.70798. [DOI] [PubMed] [Google Scholar]
- 27.Caudill R, Lancaster D. Histologic analysis of the osseointegration of endosseous implants in simulated extraction sockets with and without e-PTFE barriers. Part II: histomorphometric findings. J Oral Implantol. 1993;19:209–215. [PubMed] [Google Scholar]
- 28.Chacon GE, Stine EA, Larsen PE, Beck FM, McGlumphy EA. Effect of alendronate on endosseous implant integration: an in vivo study in rabbits. J Oral Maxillofac Surg. 2006;64:1005–1009. doi: 10.1016/j.joms.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Chang KM, Ryan ME, Golub LM, Ramamurthy NS, McNamara TF. Local systemic factors in periodontal disease increase matrix-degrading enzyme activities in rat gingiva: effect of micocycline therapy. Res Commun Mol Pathol Pharmacol. 1996;91:303–318. [PubMed] [Google Scholar]
- 30.Chen F, Ouyang H, Feng X, Gao Z, Yang Y, Zou X, Liu T, Zhao G, Mao T. Anchoring dental implant in tissue-engineered bone using composite scaffold: a preliminary study in nude mouse model. J Oral Maxillofac Surg. 2005;63:586–591. doi: 10.1016/j.joms.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Z, Zhang F, He F, Zhang L, Guo C, Zhao S, Yang G. Osseointegration of titanium implants with a roughened surface containing hydride ion in a rabbit model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:e5–e12. doi: 10.1016/j.tripleo.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Cho MI, Lin WL, Genco RJ. Platelet-derived growth factor-modulated guided tissue regenerative therapy. J Periodontol. 1995;66:522–530. doi: 10.1902/jop.1995.66.6.522. [DOI] [PubMed] [Google Scholar]
- 33.Craig RG, Kallur SP, Inoue M, Rosenberg PA, LeGeros RZ. Effect of enamel matrix proteins on the periodontal connective tissue-material interface after wound healing. J Biomed Mater Res A. 2004;69:180–187. doi: 10.1002/jbm.a.20140. [DOI] [PubMed] [Google Scholar]
- 34.Craig RG, Kamer AR, Kallur SP, Inoue M, Tarnow DP. Effects of periodontal cell grafts and enamel matrix proteins on the implant-connective tissue interface: a pilot study in the minipig. J Oral Implantol. 2006;32:228–236. doi: 10.1563/820.1. [DOI] [PubMed] [Google Scholar]
- 35.Crigger M, Bogle GC, Garrett S, Gantes BG. Repair following treatment of circumferential periodontal defects in dogs with collagen expanded polytetrafluoroethylene barrier membranes. J Periodontol. 1996;67:403–413. doi: 10.1902/jop.1996.67.4.403. [DOI] [PubMed] [Google Scholar]
- 36.Deppe H, Horch HH, Neff A. Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants. 2007;22:79–86. [PubMed] [Google Scholar]
- 37.Dogan A, Ozdemir A, Kubar A, Oygur T. Assessment of periodontal healing by seeding of fibroblast-like cells derived from regenerated periodontal ligament in artificial furcation defects in a dog: a pilot study. Tissue Eng. 2002;8:273–282. doi: 10.1089/107632702753725030. [DOI] [PubMed] [Google Scholar]
- 38.Drobnik J, Dabrowski R, Szczepanowska A, Giernat L, Lorenc J. Response of aorta connective tissue matrix to injury caused by vassopressin-induced hypertension or hypercholesterolemia. J Physiol Pharmacol. 2000;51:521–533. [PubMed] [Google Scholar]
- 39.Duarte PM, Tezolin KR, Figueiredo LC, Feres M, Bastos MF. Microbial profile of ligature-induced periodontitis in rats. Arch Oral Biol. 2010;55:142–147. doi: 10.1016/j.archoralbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Dyer BL, Caffesse RG, Nasjleti CE, Morrison EC. Guided tissue regeneration with dentin biomodification. J Periodontol. 1993;64:1052–1060. doi: 10.1902/jop.1993.64.11.1052. [DOI] [PubMed] [Google Scholar]
- 41.Egelberg J. Local effect of diet on plaque formation and development of gingivitis in dogs. 3. Effect of frequency of meals and tube feeding. Odontol Revy. 1965;16:50–60. [PubMed] [Google Scholar]
- 42.England DC, Winters LM, Carpenter LE. The development of a breed of miniature swine; a preliminary report. Growth. 1954;18:207–214. [PubMed] [Google Scholar]
- 43.Fang D, Seo BM, Liu Y, Sonoyama W, Yamaza T, Zhang C, Wang S, Shi S. Transplantation of mesenchymal stem cells is an optimal approach for plastic surgery. Stem Cells. 2007;25:1021–1028. doi: 10.1634/stemcells.2006-0576. [DOI] [PubMed] [Google Scholar]
- 44.Fleisher N, de Waal H, Bloom A. Regeneration of lost attachment apparatus in the dog using Vicryl absorbable mesh (Polyglactin 910) Int J Periodontics Restorative Dent. 1988;8:44–55. [PubMed] [Google Scholar]
- 45.Franco LH, Paula MO, Wowk PF, Fonseca DM, Sergio CA, Fedatto PF, Gembre AF, Ramos SG, Silva CL, Medeiros AI, Faccioli LH, Bonato VL. Leukotrienes are not essential for the efficacy of a heterologous vaccine against Mycobacterium tuberculosis infection. Braz J Med Biol Res. 2010;43:645–650. doi: 10.1590/s0100-879x2010007500053. [DOI] [PubMed] [Google Scholar]
- 46.Freire MO, Sedghizadeh PP, Schaudinn C, Gorur A, Downey JS, Choi JH, Chen W, Kook JK, Chen C, Goodman SD, Zadeh HH. Development of an animal model for Aggregatibacter actinomycetemcomitans biofilm-mediated oral osteolytic infection: a preliminary study. J Periodontol. 2011;82:778–789. doi: 10.1902/jop.2010.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita T, Yamamoto S, Ota M, Shibukawa Y, Yamada S. Coverage of gingival recession defects using guided tissue regeneration with and without adjunctive enamel matrix derivative in a dog model. Int J Periodontics Restorative Dent. 2011;31:247–253. [PubMed] [Google Scholar]
- 48.Garetto LP, Chen J, Parr JA, Roberts WE. Remodeling dynamics of bone supporting rigidly fixed titanium implants: a histomorphometric comparison in four species including humans. Implant Dent. 1995;4:235–243. doi: 10.1097/00008505-199500440-00002. [DOI] [PubMed] [Google Scholar]
- 49.Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol. 1994;65:1158–1168. doi: 10.1902/jop.1994.65.12.1158. [DOI] [PubMed] [Google Scholar]
- 50.Gotfredsen K, Berglundh T, Lindhe J. Bone reactions at implants subjected to experimental peri-implantitis and static load. A study in the dog. J Clin Periodontol. 2002;29:144–151. doi: 10.1034/j.1600-051x.2002.290209.x. [DOI] [PubMed] [Google Scholar]
- 51.Gotfredsen K, Nimb L, Buser D, Hjorting-Hansen E. Evaluation of guided bone generation around implants placed into fresh extraction sockets: an experimental study in dogs. J Oral Maxillofac Surg. 1993;51:879–884. doi: 10.1016/s0278-2391(10)80108-9. [DOI] [PubMed] [Google Scholar]
- 52.Gotfredsen K, Nimb L, Hjorting-Hansen E. Immediate implant placement using a biodegradable barrier, polyhydroxybutyrate-hydroxyvalerate reinforced with polyglactin 910. An experimental study in dogs. Clin Oral Implants Res. 1994;5:83–91. doi: 10.1034/j.1600-0501.1994.050204.x. [DOI] [PubMed] [Google Scholar]
- 53.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graves DT, Naguib G, Lu H, Desta T, Amar S. Porphyromonas gingivalis fimbriae are pro-inflammatory but do not play a prominent role in the innate immune response to P. gingivalis. J Endotoxin Res. 2005;11:13–18. doi: 10.1179/096805105225006722. [DOI] [PubMed] [Google Scholar]
- 55.Gruenbaum-Cohen Y, Tucker AS, Haze A, Shilo D, Taylor AL, Shay B, Sharpe PT, Mitsiadis TA, Ornoy A, Blumenfeld A, Deutsch D. Amelogenin in cranio-facial development: the tooth as a model to study the role of amelogenin during embryogenesis. J Exp Zool B Mol Dev Evol. 2009;312B:445–457. doi: 10.1002/jez.b.21255. [DOI] [PubMed] [Google Scholar]
- 56.Grunder U, Hurzeler MB, Schupbach P, Strub JR. Treatment of ligature-induced peri-implantitis using guided tissue regeneration: a clinical and histologic study in the beagle dog. Int J Oral Maxillofac Implants. 1993;8:282–293. [PubMed] [Google Scholar]
- 57.Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall J, Miranda-Burgos P, Sennerby L. Stimulation of directed bone growth at oxidized titanium implants by macroscopic grooves: an in vivo study. Clin Implant Dent Relat Res. 2005;7(Suppl. 1):S76–S82. doi: 10.1111/j.1708-8208.2005.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 60.Hamp SE, Lindberg R. Histopathology of spontaneous periodontitis in dogs. J Periodontal Res. 1977;12:46–54. doi: 10.1111/j.1600-0765.1977.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 61.Hamp SE, Lindhe J, Loe H. Experimental periodontitis in the beagle dog. J Periodontal Res. 1972;7:13–14. [PubMed] [Google Scholar]
- 62.Haney JM, Nilveus RE, McMillan PJ, Wikesjö UM. Periodontal repair in dogs: expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J Periodontol. 1993;64:883–890. doi: 10.1902/jop.1993.64.9.883. [DOI] [PubMed] [Google Scholar]
- 63.Haney JM, Zimmerman GJ, Wikesjö UM. Periodontal repair in dogs: evaluation of the natural disease model. J Clin Periodontol. 1995;22:208–213. doi: 10.1111/j.1600-051x.1995.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 64.Hasturk H, Goguet-Surmenian E, Blackwood A, Andry C, Kantarci A. 1-Tetradecanol complex: therapeutic actions in experimental periodontitis. J Periodontol. 2009;80:1103–1113. doi: 10.1902/jop.2009.090002. [DOI] [PubMed] [Google Scholar]
- 65.Hasturk H, Jones VL, Andry C, Kantarci A. 1-Tetradecanol complex reduces progression of Porphyromonas gingivalis-induced experimental periodontitis in rabbits. J Periodontol. 2007;78:924–932. doi: 10.1902/jop.2007.060293. [DOI] [PubMed] [Google Scholar]
- 66.Hasturk H, Kantarci A, Ebrahimi N, Andry C, Holick M, Jones VL, Van Dyke TE. Topical H2 antagonist prevents periodontitis in a rabbit model. Infect Immun. 2006;74:2402–2414. doi: 10.1128/IAI.74.4.2402-2414.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasturk H, Kantarci A, Ghattas M, Schmidt M, Giordano RA, Ashman A, Diekwisch TG, Van Dyke T. The use of light/chemically hardened polymethylmethacrylate, polyhydroxyethylmethacrylate, and calcium hydroxide graft material in combination with polyanhydride around implants in minipigs: part I: immediate stability and function. J Periodontol. 2011;82:1339–1352. doi: 10.1902/jop.2011.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 69.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 70.Haze A, Taylor AL, Haegewald S, Leiser Y, Shay B, Rosenfeld E, Gruenbaum-Cohen Y, Dafni L, Zimmermann B, Heikinheimo K, Gibson CW, Fisher LW, Young MF, Blumenfeld A, Bernimoulin JP, Deutsch D. Regeneration of bone and periodontal ligament induced by recombinant amelogenin after periodontitis. J Cell Mol Med. 2009;13:1110–1124. doi: 10.1111/j.1582-4934.2009.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herr Y, Matsuura M, Lin WL, Genco RJ, Cho MI. The origin of fibroblasts and their role in the early stages of horizontal furcation defect healing in the beagle dog. J Periodontol. 1995;66:716–730. doi: 10.1902/jop.1995.66.8.716. [DOI] [PubMed] [Google Scholar]
- 72.Hickey JS, O’Neal RB, Scheidt MJ, Strong SL, Turgeon D, Van Dyke TE. Microbiologic characterization of ligature-induced peri-implantitis in the microswine model. J Periodontol. 1991;62:548–553. doi: 10.1902/jop.1991.62.9.548. [DOI] [PubMed] [Google Scholar]
- 73.Hugoson A, Schmidt G. Influence of plaque control on the healing of experimentally-induced bone defects in the dog. J Periodontol. 1978;49:135–141. doi: 10.1902/jop.1978.49.3.135. [DOI] [PubMed] [Google Scholar]
- 74.Hurzeler MB, Zuhr O, Schupbach P, Rebele SF, Emmanouilidis N, Fickl S. The socket-shield technique: a proof-of-principle report. J Clin Periodontol. 2010;37:855–862. doi: 10.1111/j.1600-051X.2010.01595.x. [DOI] [PubMed] [Google Scholar]
- 75.Ibi M, Ishisaki A, Yamamoto M, Wada S, Kozakai T, Nakashima A, Iida J, Takao S, Izumi Y, Yokoyama A, Tamura M. Establishment of cell lines that exhibit pluripotency from miniature swine periodontal ligaments. Arch Oral Biol. 2007;52:1002–1008. doi: 10.1016/j.archoralbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Iqbal MK, Bamaas N. Effect of enamel matrix derivative (EMDOGAIN) upon periodontal healing after replantation of permanent incisors in beagle dogs. Dent Traumatol. 2001;17:36–45. doi: 10.1034/j.1600-9657.2001.170107.x. [DOI] [PubMed] [Google Scholar]
- 77.Jain A, Batista EL, Jr, Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infect Immun. 2003;71:6012–6018. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jemt T, Lekholm U, Johansson CB. Bone response to implant-supported frameworks with differing degrees of misfit preload: in vivo study in rabbits. Clin Implant Dent Relat Res. 2000;2:129–137. doi: 10.1111/j.1708-8208.2000.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 79.Jensen SS, Broggini N, Weibrich G, Hjorting-Hansen E, Schenk R, Buser D. Bone regeneration in standardized bone defects with autografts or bone substitutes in combination with platelet concentrate: a histologic and histomorphometric study in the mandibles of minipigs. Int J Oral Maxillofac Implants. 2005;20:703–712. [PubMed] [Google Scholar]
- 80.Johansson CB, Gretzer C, Jimbo R, Mattisson I, Ahlberg E. Enhanced implant integration with hierarchically structured implants: a pilot study in rabbits. Clin Oral Implants Res. 2012;23:943–953. doi: 10.1111/j.1600-0501.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 81.Johnsson AA, Sawaii T, Jacobsson M, Granstrom G, Turesson I. A histomorphometric and biomechanical study of the effect of delayed titanium implant placement in irradiated rabbit bone. Clin Implant Dent Relat Res. 2000;2:42–49. doi: 10.1111/j.1708-8208.2000.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 82.Jovanovic SA, Kenney EB, Carranza FA, Jr, Donath K. The regenerative potential of plaque-induced peri-implant bone defects treated by a submerged membrane technique: an experimental study. Int J Oral Maxillofac Implants. 1993;8:13–18. [PubMed] [Google Scholar]
- 83.Jovanovic SA, Schenk RK, Orsini M, Kenney EB. Supracrestal bone formation around dental implants: an experimental dog study. Int J Oral Maxillofac Implants. 1995;10:23–31. [PubMed] [Google Scholar]
- 84.Kalkwarf KL, Krejci RF. Effect of inflammation on periodontal attachment levels in miniature swine with mucogingival defects. J Periodontol. 1983;54:361–364. doi: 10.1902/jop.1983.54.6.361. [DOI] [PubMed] [Google Scholar]
- 85.Kalkwarf KL, Krejci RF, Berry WC., Jr Chronic mucogingival defects in miniature swine. J Periodontol. 1983;54:81–85. doi: 10.1902/jop.1983.54.2.81. [DOI] [PubMed] [Google Scholar]
- 86.Kantarci A, Van Dyke TE. Resolution of inflammation in periodontitis. J Periodontol. 2005;76:2168–2174. doi: 10.1902/jop.2005.76.11-S.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SJ, Shin HS, Shin SW. Effect of bone block graft with rhBMP-2 on vertical bone augmentation. Int J Oral Maxillofac Surg. 2010;39:883–888. doi: 10.1016/j.ijom.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Kirkwood KL, Li F, Rogers JE, Otremba J, Coatney DD, Kreider JM, D’Silva NJ, Chakravarty S, Dugar S, Higgins LS, Protter AA, Medicherla S. A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther. 2007;320:56–63. doi: 10.1124/jpet.106.112466. [DOI] [PubMed] [Google Scholar]
- 89.Klausen B. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. J Periodontol. 1991;62:59–73. doi: 10.1902/jop.1991.62.1.59. [DOI] [PubMed] [Google Scholar]
- 90.Klokkevold PR, Johnson P, Dadgostari S, Caputo A, Davies JE, Nishimura RD. Early endosseous integration enhanced by dual acid etching of titanium: a torque removal study in the rabbit. Clin Oral Implants Res. 2001;12:350–357. doi: 10.1034/j.1600-0501.2001.012004350.x. [DOI] [PubMed] [Google Scholar]
- 91.Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 92.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 93.Kozlovsky A, Tal H, Laufer BZ, Leshem R, Rohrer MD, Weinreb M, Artzi Z. Impact of implant overloading on the peri-implant bone in inflamed and non-inflamed peri-implant mucosa. Clin Oral Implants Res. 2007;18:601–610. doi: 10.1111/j.1600-0501.2007.01374.x. [DOI] [PubMed] [Google Scholar]
- 94.Kuo TF, Lin HC, Yang KC, Lin FH, Chen MH, Wu CC, Chang HH. Bone marrow combined with dental bud cells promotes tooth regeneration in miniature pig model. Artif Organs. 2001;35:113–121. doi: 10.1111/j.1525-1594.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 95.Kurtis B, Balos K, Oygur T. Effect of a collagen membrane enriched with fibronectin on guided tissue regeneration in dogs. Periodontal Clin Investig. 2002;24:11–19. [PubMed] [Google Scholar]
- 96.Kwon DH, Bennett W, Herberg S, Bastone P, Pippig S, Rodriguez NA, Susin C, Wikesjo UM. Evaluation of an injectable rhGDF-5/PLGA construct for minimally invasive periodontal regenerative procedures: a histological study in the dog. J Clin Periodontol. 2010;37:390–397. doi: 10.1111/j.1600-051X.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 97.Lang H, Schuler N, Arnhold S, Nolden R, Mertens T. Formation of differentiated tissues in vivo by periodontal cell populations cultured in vitro. J Dent Res. 1995;74:1219–1225. doi: 10.1177/00220345950740051201. [DOI] [PubMed] [Google Scholar]
- 98.Larsen MO, Rolin B. Use of the Gottingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J. 2004;45:303–313. doi: 10.1093/ilar.45.3.303. [DOI] [PubMed] [Google Scholar]
- 99.Lee J, Susin C, Rodriguez NA, de Stefano J, Prasad HS, Buxton AN, Wikesjö UM. Sinus augmentation using rhBMP-2/ACS in a mini-pig model: relative efficacy of autogenous fresh particulate iliac bone grafts. Clinical Oral Implants Res. 2013;24:497–504. doi: 10.1111/j.1600-0501.2011.02419.x. [DOI] [PubMed] [Google Scholar]
- 100.Leone CW, Bokhadhoor H, Kuo D, Desta T, Yang J, Siqueira MF, Amar S, Graves DT. Immunization enhances inflammation and tissue destruction in response to Porphyromonas gingivalis. Infect Immun. 2006;74:2286–2292. doi: 10.1128/IAI.74.4.2286-2292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levy P, Nevins A, LaPorta R. Healing potential of surgically-induced periodontal osseous defects in animals using mineralized collagen gel xenografts. J Periodontol. 1981;52:303–306. doi: 10.1902/jop.1981.52.6.303. [DOI] [PubMed] [Google Scholar]
- 102.Li CH, Amar S. Morphometric, histomorphometric, and microcomputed tomographic analysis of periodontal inflammatory lesions in a murine model. J Periodontol. 2007;78:1120–1128. doi: 10.1902/jop.2007.060320. [DOI] [PubMed] [Google Scholar]
- 103.Li L, Khansari A, Shapira L, Graves DT, Amar S. Contribution of interleukin-11 and prostaglandin(s) in lipopolysaccharide-induced bone resorption in vivo. Infect Immun. 2002;70:3915–3922. doi: 10.1128/IAI.70.7.3915-3922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lynch SE, de Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, Antoniades HN. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991;62:458–467. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 106.Madan M, Bishayi B, Hoge M, Amar S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. 2008;197:504–514. doi: 10.1016/j.atherosclerosis.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madden TE, Caton JG. Animal models for periodontal disease. Methods Enzymol. 1994;235:106–119. doi: 10.1016/0076-6879(94)35135-x. [DOI] [PubMed] [Google Scholar]
- 108.Maekawa T, Takahashi N, Tabeta K, Aoki Y, Miyashita H, Miyauchi S, Miyazawa H, Nakajima T, Yamazaki K. Chronic oral infection with Porphyromonas gingivalis accelerates atheroma formation by shifting the lipid profile. PLoS ONE. 2011;6:e20240. doi: 10.1371/journal.pone.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Magnusson I, Batich C, Collins BR. New attachment formation following controlled tissue regeneration using biodegradable membranes. J Periodontol. 1988;59:1–6. doi: 10.1902/jop.1988.59.1.1. [DOI] [PubMed] [Google Scholar]
- 110.Magnusson I, Stenberg WV, Batich C, Egelberg J. Connective tissue repair in circumferential periodontal defects in dogs following use of a biodegradable membrane. J Clin Periodontol. 1990;17:243–248. doi: 10.1111/j.1600-051x.1990.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 111.Marcus AJ, Hajjar DP. Vascular transcellular signaling. J Lipid Res. 1993;34:2017–2031. [PubMed] [Google Scholar]
- 112.Marinello CP, Berglundh T, Ericsson I, Klinge B, Glantz PO, Lindhe J. Resolution of ligature-induced peri-implantitis lesions in the dog. J Clin Periodontol. 1995;22:475–479. doi: 10.1111/j.1600-051x.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 113.Matsuura M, Herr Y, Han KY, Lin WL, Genco RJ, Cho MI. Immunohistochemical expression of extracellular matrix components of normal and healing periodontal tissues in the beagle dog. J Periodontol. 1995;66:579–593. doi: 10.1902/jop.1995.66.7.579. [DOI] [PubMed] [Google Scholar]
- 114.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 115.Mkonyi LE, Bakken V, Sovik JB, Mauland EK, Fristad I, Barczyk MM, Bletsa A, Berggreen E. Lymphangiogenesis is induced during development of periodontal disease. J Dent Res. 2012;91:71–77. doi: 10.1177/0022034511424747. [DOI] [PubMed] [Google Scholar]
- 116.Naaman B-AN, Patat JL, Guillemin G, Issahakian S, Forest N, Ouhayoun JP. Evaluation of the osteogenic potential of biomaterials implanted in the palatal connective tissue of miniature pigs using undecalcified sections. Biomaterials. 1994;15:201–207. doi: 10.1016/0142-9612(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 117.Nakajima K, Hamada N, Takahashi Y, Sasaguri K, Tsukinoki K, Umemoto T, Sato S. Restraint stress enhances alveolar bone loss in an experimental rat model. J Periodontal Res. 2006;41:527–534. doi: 10.1111/j.1600-0765.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 118.Nalbandian J, Cote N. Direct histological comparison of periodontal wound healing in the beagle dog with and without citric acid conditioning. J Periodontal Res. 1982;17:552–562. doi: 10.1111/j.1600-0765.1982.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 119.Nalbandian J, Helldén L. Response of the periodontal tissues of the beagle dog to polytetrafluorethylene implants. Oral Surg Oral Med Oral Pathol. 1982;54:452–460. doi: 10.1016/0030-4220(82)90395-4. [DOI] [PubMed] [Google Scholar]
- 120.Nyman S, Karring T, Lindhe J, Planten S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol. 1980;7:394–401. doi: 10.1111/j.1600-051x.1980.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 121.Nyman S, Sarhed G, Ericsson I, Gottlow J, Karring T. Role of “diseased” root cementum in healing following treatment of periodontal disease. An experimental study in the dog. J Periodontal Res. 1986;21:496–503. doi: 10.1111/j.1600-0765.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 122.Oka H, Miyauchi M, Furusho H, Nishihara T, Takata T. Oral administration of EP4 antagonist inhibits LPS-induced osteoclastogenesis in rat periodontal tissue. J Periodontol. 2012;83:506–513. doi: 10.1902/jop.2011.110301. [DOI] [PubMed] [Google Scholar]
- 123.Oliveira RV, de Souza Nunes LS, Filho HN, de Andrade Holgado L, Ribeiro DA, Matsumoto MA. Fibrovascularization and osteogenesis in high-density porous polyethylene implants. J Craniofac Surg. 2009;20:1120–1124. doi: 10.1097/SCS.0b013e3181abb4ab. [DOI] [PubMed] [Google Scholar]
- 124.Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-kappaB signaling cascade. Neurobiol Dis. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 125.Oz HS, Puleo DA. Animal models for periodontal disease. J Biomed Biotechnol. 2011;2011:754857. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Page R, Schroeder H. Periodontitis in man and other animals. a comparative review. Krager; Basel: 1982. [Google Scholar]
- 127.Park JB, Matsuura M, Han KY, Norderyd O, Lin WL, Genco RJ, Cho MI. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J Periodontol. 1995;66:462–477. doi: 10.1902/jop.1995.66.6.462. [DOI] [PubMed] [Google Scholar]
- 128.Pavlica Z, Petelin M, Nemec A, Erzen D, Skaleric U. Measurement of total antioxidant capacity in gingival crevicular fluid and serum in dogs with periodontal disease. Am J Vet Res. 2004;65:1584–1588. doi: 10.2460/ajvr.2004.65.1584. [DOI] [PubMed] [Google Scholar]
- 129.Persson LG, Araujo MG, Berglundh T, Grondahl K, Lindhe J. Resolution of peri-implantitis following treatment. An experimental study in the dog. Clin Oral Implants Res. 1999;10:195–203. doi: 10.1034/j.1600-0501.1999.100302.x. [DOI] [PubMed] [Google Scholar]
- 130.Persson LG, Berglundh T, Lindhe J, Sennerby L. Re-osseointegration after treatment of peri-implantitis at different implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2001;12:595–603. doi: 10.1034/j.1600-0501.2001.120607.x. [DOI] [PubMed] [Google Scholar]
- 131.Persson LG, Ericsson I, Berglundh T, Lindhe J. Guided bone regeneration in the treatment of periimplantitis. Clin Oral Implants Res. 1996;7:366–372. doi: 10.1034/j.1600-0501.1996.070410.x. [DOI] [PubMed] [Google Scholar]
- 132.Persson LG, Ericsson I, Berglundh T, Lindhe J. Osseintegration following treatment of peri-implantitis and replacement of implant components. An experimental study in the dog. J Clin Periodontol. 2001;28:258–263. doi: 10.1034/j.1600-051x.2001.028003258.x. [DOI] [PubMed] [Google Scholar]
- 133.Pieri F, Lucarelli E, Corinaldesi G, Fini M, Aldini NN, Giardino R, Donati D, Marchetti C. Effect of mesenchymal stem cells and platelet-rich plasma on the healing of standardized bone defects in the alveolar ridge: a comparative histomorphometric study in minipigs. J Oral Maxillofac Surg. 2009;67:265–272. doi: 10.1016/j.joms.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 134.Pimentel SP, Sallum AW, Saldanha JB, Casati MZ, Nociti FH, Jr, Sallum EA. Enamel matrix derivative versus guided tissue regeneration in the presence of nicotine: a histomorphometric study in dogs. J Clin Periodontol. 2006;33:900–907. doi: 10.1111/j.1600-051X.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 135.Pitaru S, Tal H, Soldinger M, Grosskopf A, Noff M. Partial regeneration of periodontal tissues using collagen barriers. Initial observations in the canine. J Periodontol. 1988;59:380–386. doi: 10.1902/jop.1988.59.6.380. [DOI] [PubMed] [Google Scholar]
- 136.Plotzke AE, Barbosa S, Nasjleti CE, Morrison EC, Caffesse RG. Histologic and histometric responses to polymeric composite grafts. J Periodontol. 1993;64:343–348. doi: 10.1902/jop.1993.64.5.343. [DOI] [PubMed] [Google Scholar]
- 137.Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- 138.Polson AM. Interrelationship of inflammation and tooth mobility (trauma) in pathogenesis of periodontal disease. J Clin Periodontol. 1980;7:351–360. doi: 10.1111/j.1600-051x.1980.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 139.Polson AM. The relative importance of plaque and occlusion in periodontal disease. J Clin Periodontol. 1986;13:923–927. doi: 10.1111/j.1600-051x.1986.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 140.Pontoriero R, Nyman S, Ericsson I, Lindhe J. Guided tissue regeneration in surgically-produced furcation defects. An experimental study in the beagle dog. J Clin Periodontol. 1992;19:159–163. doi: 10.1111/j.1600-051x.1992.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 141.Powell CA, Bannister SR, Mackey SA, Maller SC, McDonnell HT, Deas DE. Periodontal wound healing with and without platelet-rich plasma: histologic observations and assessment of flap tensile strength. J Periodontol. 2009;80:985–992. doi: 10.1902/jop.2009.080626. [DOI] [PubMed] [Google Scholar]
- 142.Renvert S, Claffey N, Orafi H, Albrektsson T. Supracrestal bone growth around partially inserted titanium implants in dogs. A pilot study. Clin Oral Implants Res. 1996;7:360–365. doi: 10.1034/j.1600-0501.1996.070409.x. [DOI] [PubMed] [Google Scholar]
- 143.Rogers JE, Li F, Coatney DD, Otremba J, Kriegl JM, Protter TA, Higgins LS, Medicherla S, Kirkwood KL. A p38 mitogen-activated protein kinase inhibitor arrests active alveolar bone loss in a rat periodontitis model. J Periodontol. 2007;78:1992–1998. doi: 10.1902/jop.2007.070101. [DOI] [PubMed] [Google Scholar]
- 144.Rovin S, Costich ER, Gordon HA. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J Periodontal Res. 1966;1:193–204. doi: 10.1111/j.1600-0765.1966.tb01860.x. [DOI] [PubMed] [Google Scholar]
- 145.Ruberg FL, Viereck J, Phinikaridou A, Qiao Y, Loscalzo J, Hamilton JA. Identification of cholesteryl esters in human carotid atherosclerosis by ex vivo image-guided proton MRS. J Lipid Res. 2006;47:310–317. doi: 10.1194/jlr.M500431-JLR200. [DOI] [PubMed] [Google Scholar]
- 146.Sakallioglu U, Acikgoz G, Ayas B, Kirtiloglu T, Sakallioglu E. Healing of periodontal defects treated with enamel matrix proteins and root surface conditioning – an experimental study in dogs. Biomaterials. 2004;25:1831–1840. doi: 10.1016/s0142-9612(03)00468-x. [DOI] [PubMed] [Google Scholar]
- 147.Salata LZ, Rasmusson L, Kahnberg KE. Effects of a mechanical barrier on the integration of cortical onlay bone grafts placed simultaneously with endosseous implant. Clin Implant Dent Relat Res. 2002;4:60–68. doi: 10.1111/j.1708-8208.2002.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 148.Sanz A, Oyarzun A, Farias D, Diaz I. Experimental study of bone response to a new surface treatment of endosseous titanium implants. Implant Dent. 2001;10:126–131. doi: 10.1097/00008505-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 149.Schliephake H, Aleyt J. Mandibular onlay grafting using prefabricated bone grafts with primary implant placement: an experimental study in minipigs. Int J Oral Maxillofac Implants. 1998;13:384–393. [PubMed] [Google Scholar]
- 150.Schliephake H, Klosa D, Neukam FW. Automated micro-densitometric quantification of bone ingrowth into porous implants. Int J Oral Maxillofac Implants. 1991;6:168–176. [PubMed] [Google Scholar]
- 151.Schliephake H, Neukam FW. Bone replacement with porous hydroxyapatite blocks and titanium screw implants: an experimental study. J Oral Maxillofac Surg. 1991;49:151–156. doi: 10.1016/0278-2391(91)90103-s. [DOI] [PubMed] [Google Scholar]
- 152.Schliephake H, Neukam FW, Klosa D. Influence of pore dimensions on bone ingrowth into porous hydroxylapatite blocks used as bone graft substitutes. A histometric study. Int J Oral Maxillofac Surg. 1991;20:53–58. doi: 10.1016/s0901-5027(05)80698-8. [DOI] [PubMed] [Google Scholar]
- 153.Schliephake H, van den Berghe P, Neukam FW. Osseointegration of titanium fixtures in onlay grafting procedures with autogenous bone and hydroxylapatite. An experimental histometric study. Clin Oral Implants Res. 1991;2:56–61. doi: 10.1034/j.1600-0501.1991.020202.x. [DOI] [PubMed] [Google Scholar]
- 154.Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993;64:497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- 155.Screaton NJ, Coxson HO, Kalloger SE, Baile EM, Nakano Y, Hiorns M, Mayo JR. Detection of lung perfusion abnormalities using computed tomography in a porcine model of pulmonary embolism. J Thorac Imaging. 2003;18:14–20. doi: 10.1097/00005382-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 156.Selvig KA, Wikesjo UM, Bogle GC, Finkelman RD. Impaired early bone formation in periodontal fenestration defects in dogs following application of insulin-like growth factor (II). Basic fibroblast growth factor and transforming growth factor beta 1. J Clin Periodontol. 1994;21:380–385. doi: 10.1111/j.1600-051x.1994.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 157.Sennerby L, Persson LG, Berglundh T, Wennerberg A, Lindhe J. Implant stability during initiation and resolution of experimental periimplantitis: an experimental study in the dog. Clin Implant Dent Relat Res. 2005;7:136–140. doi: 10.1111/j.1708-8208.2005.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 158.Sevor JJ, Meffert RM, Cassingham RJ. Regeneration of dehisced alveolar bone adjacent to endosseous dental implants utilizing a resorbable collagen membrane: clinical and histologic results. Int J Periodontics Restorative Dent. 1993;13:71–83. [PubMed] [Google Scholar]
- 159.Shimizu T, Nakai K, Morimoto Y, Ishihara M, Oishi H, Kikuchi M, Arai H. Simple rabbit model of vulnerable atherosclerotic plaque. Neurol Med Chir (Tokyo) 2009;49:327–332. doi: 10.2176/nmc.49.327. [DOI] [PubMed] [Google Scholar]
- 160.Simion M, Rocchietta I, Kim D, Nevins M, Fiorellini J. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26:415–423. [PubMed] [Google Scholar]
- 161.Singh G, O’Neal RB, Brennan WA, Strong SL, Horner JA, Van Dyke TE. Surgical treatment of induced peri-implantitis in the micro pig: clinical and histological analysis. J Periodontol. 1993;64:984–989. doi: 10.1902/jop.1993.64.10.984. [DOI] [PubMed] [Google Scholar]
- 162.Slotte C, Lundgren D, Sennerby L, Lundgren AK. Influence of preimplant surgical intervention and implant placement on bone wound healing. Clin Oral Implants Res. 2003;14:528–534. doi: 10.1034/j.1600-0501.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- 163.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Springer IN, Acil Y, Spies C, Jepsen S, Warnke PH, Bolte H, Kuchenbecker S, Russo PA, Wiltfang J, Terheyden H. RhBMP-7 improves survival and eruption in a growing tooth avulsion trauma model. Bone. 2005;37:570–577. doi: 10.1016/j.bone.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 165.Stavropoulos A, Wikesjö UM. Influence of defect dimensions on periodontal wound healing/regeneration in intrabony defects following implantation of a bovine bone biomaterial and provisions for guided tissue regeneration: an experimental study in the dog. J Clin Periodontol. 2010;37:534–543. doi: 10.1111/j.1600-051X.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- 166.Stenport VF, Johansson CB. Evaluations of bone tissue integration to pure and alloyed titanium implants. Clin Implant Dent Relat Res. 2008;10:191–199. doi: 10.1111/j.1708-8208.2007.00077.x. [DOI] [PubMed] [Google Scholar]
- 167.Struillou X, Boutigny H, Soueidan A, Layrolle P. Experimental animal models in periodontology: a review. Open Dent J. 2010;4:37–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sugaya A, Minabe M, Hori T, Tatsumi J, Watanabe Y, Ikeda K, Numabe Y, Hayashi H, Kamoi K. Effects on wound healing of tricalcium phosphate-collagen complex implants in periodontal osseous defects in the dog. J Periodontal Res. 1990;25:60–63. doi: 10.1111/j.1600-0765.1990.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 169.Sugaya A, Minabe M, Tamura T, Hori T, Watanabe Y. Effects on wound healing of hydroxyapatite-collagen complex implants in periodontal osseous defects in the dog. J Periodontal Res. 1989;24:284–288. doi: 10.1111/j.1600-0765.1989.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 170.Sul YT, Byon ES, Jeong Y. Biomechanical measurements of calcium-incorporated oxidized implants in rabbit bone: effect of calcium surface chemistry of a novel implant. Clin Implant Dent Relat Res. 2004;6:101–110. doi: 10.1111/j.1708-8208.2004.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 171.Sul YT, Jeong Y, Johansson C, Albrektsson T. Oxidized, bioactive implants are rapidly and strongly integrated in bone. Part 1 – experimental implants. Clin Oral Implants Res. 2006;17:521–526. doi: 10.1111/j.1600-0501.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 172.Sul YT, Johansson C, Wennerberg A, Cho LR, Chang BS, Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005;20:349–359. [PubMed] [Google Scholar]
- 173.Tal H, Kozlovsky A, Pitaru S. Healing of sites within the dog periodontal ligament after application of cold to the periodontal attachment apparatus. J Clin Periodontol. 1991;18:543–547. doi: 10.1111/j.1600-051x.1991.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 174.Tal H, Pitaru S. Formation of new periodontal attachment apparatus after experimental root isolation with collagen membranes in the dog. Int J Periodontics Restorative Dent. 1992;12:231–242. [PubMed] [Google Scholar]
- 175.Tamimi F, Torres J, Gbureck U, Lopez-Cabarcos E, Bassett DC, Alkhraisat MH, Barralet JE. Craniofacial vertical bone augmentation: a comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials. 2009;30:6318–6326. doi: 10.1016/j.biomaterials.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 176.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 177.Tsetsenekou E, Papadopoulos T, Kalyvas D, Papaioannou N, Tangl S, Watzek G. The influence of alendronate on osseointegration of nanotreated dental implants in New Zealand rabbits. Clin Oral Implants Res. 2012;23:659–666. doi: 10.1111/j.1600-0501.2011.02189.x. [DOI] [PubMed] [Google Scholar]
- 178.Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, Ohno T, Lin K, Yamato M, Ishikawa I, Okano T, Izumi Y. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32:5819–5825. doi: 10.1016/j.biomaterials.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 179.Van Dorp AG, Verhoeven MC, Koerten HK, Van Der Nat-Van Der Meij TH, Van Blitterswijk CA, Ponec M. Dermal regeneration in full-thickness wounds in Yucatan miniature pigs using a biodegradable copolymer. Wound Repair Regen. 1998;6:556–568. doi: 10.1046/j.1524-475x.1998.60608.x. [DOI] [PubMed] [Google Scholar]
- 180.Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79:1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Van Dyke TE. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. J Clin Periodontol. 2011;38(Suppl. 11):119–125. doi: 10.1111/j.1600-051X.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- 182.Vardar-Sengul S, Buduneli E, Turkoglu O, Buduneli N, Atilla G, Wahlgren J, Sorsa T, Baylas H. The effects of selective COX-2 inhibitor/celecoxib and omega-3 fatty acid on matrix metalloproteinases, TIMP-1, and laminin-5gamma2-chain immunolocalization in experimental periodontitis. J Periodontol. 2008;79:1934–1941. doi: 10.1902/jop.2008.080001. [DOI] [PubMed] [Google Scholar]
- 183.Veis AA, Dabarakis NN, Parisis NA, Tsirlis AT, Karanikola TG, Printza DV. Bone regeneration around implants using spherical and granular forms of bioactive glass particles. Implant Dent. 2006;15:386–394. doi: 10.1097/01.id.0000243317.57261.86. [DOI] [PubMed] [Google Scholar]
- 184.von Arx T, Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Lateral ridge augmentation and implant placement: an experimental study evaluating implant osseointegration in different augmentation materials in the canine mandible. Int J Oral Maxillofac Implants. 2001;16:343–354. [PubMed] [Google Scholar]
- 185.von Arx T, Cochran DL, Schenk RK, Buser D. Evaluation of a prototype trilayer membrane (PTLM) for lateral ridge augmentation: an experimental study in the canine mandible. Int J Oral Maxillofac Surg. 2002;31:190–199. doi: 10.1054/ijom.2001.0205. [DOI] [PubMed] [Google Scholar]
- 186.Wang HL, Pappert TD, Castelli WA, Chiego DJ, Jr, Shyr Y, Smith BA. The effect of platelet-derived growth factor on the cellular response of the periodontium: an autoradiographic study on dogs. J Periodontol. 1994;65:429–436. doi: 10.1902/jop.1994.65.5.429. [DOI] [PubMed] [Google Scholar]
- 187.Wang S, Liu Y, Fang D, Shi S. The miniature pig: a useful large animal model for dental and orofacial research. Oral Dis. 2007;13:530–537. doi: 10.1111/j.1601-0825.2006.01337.x. [DOI] [PubMed] [Google Scholar]
- 188.Warrer K, Karring T. Guided tissue regeneration combined with osseous grafting in suprabony periodontal lesions. An experimental study in the dog. J Clin Periodontol. 1992;19:373–380. doi: 10.1111/j.1600-051x.1992.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 189.Watanabe K, Kikuchi M, Okumura M, Kadosawa T, Fujinaga T. Efficacy of enamel matrix protein applied to spontaneous periodontal disease in two dogs. J Vet Med Sci. 2003;65:1007–1010. doi: 10.1292/jvms.65.1007. [DOI] [PubMed] [Google Scholar]
- 190.Weng D, Hurzeler MB, Quinones CR, Pechstadt B, Mota L, Caffesse RG. Healing patterns in recession defects treated with ePTFE membranes and with free connective tissue grafts. A histologic and histometric study in the beagle dog. J Clin Periodontol. 1998;25:238–245. doi: 10.1111/j.1600-051x.1998.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 191.Werfully S, Areibi G, Toner M, Bergquist J, Walker J, Renvert S, Claffey N. Tensile strength, histological and immunohistochemical observations of periodontal wound healing in the dog. J Periodontal Res. 2002;37:366–374. doi: 10.1034/j.1600-0765.2002.01375.x. [DOI] [PubMed] [Google Scholar]
- 192.Wikesjö UM, Bogle GC, Nilveus RE. Periodontal repair in dogs: effect of a composite graft protocol on healing in supraalveolar periodontal defects. J Periodontol. 1992;63:107–113. doi: 10.1902/jop.1992.63.2.107. [DOI] [PubMed] [Google Scholar]
- 193.Wikesjö UM, Kean CJ, Zimmerman GJ. Periodontal repair in dogs: supraalveolar defect models for evaluation of safety and efficacy of periodontal reconstructive therapy. J Periodontol. 1994;65:1151–1157. doi: 10.1902/jop.1994.65.12.1151. [DOI] [PubMed] [Google Scholar]
- 194.Wikesjö UM, Nilveus R. Periodontal repair in dogs. Healing patterns in large circumferential periodontal defects. J Clin Periodontol. 1991;18:49–59. doi: 10.1111/j.1600-051x.1991.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 195.Wikesjö UM, Selvig KA, Zimmerman G, Nilveus R. Periodontal repair in dogs: healing in experimentally created chronic periodontal defects. J Periodontol. 1991;62:258–263. doi: 10.1902/jop.1991.62.4.258. [DOI] [PubMed] [Google Scholar]
- 196.Wu J, Bai YX, Wang BK. Biomechanical and histomorphometric characterizations of osseointegration during mini-screw healing in rabbit tibiae. Angle Orthod. 2009;79:558–563. doi: 10.2319/031108-138.1. [DOI] [PubMed] [Google Scholar]
- 197.Xu Q, Yu D, Qiu Y, Zhang H, Ding Y. Function of a new internal bioartificial liver: an in vitro study. Ann Clin Lab Sci. 2003;33:306–312. [PubMed] [Google Scholar]
- 198.Yang GL, He FM, Hu JA, Wang XX, Zhao SF. Effects of biomimetically and electrochemically deposited nanohydroxyapatite coatings on osseointegration of porous titanium implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:782–789. doi: 10.1016/j.tripleo.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 199.Yang GL, He FM, Yang XF, Wang XX, Zhao SF. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:516–524. doi: 10.1016/j.tripleo.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 200.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, Levine SJ. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am J Respir Crit Care Med. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yoneda M, Hirofuji T, Anan H, Matsumoto A, Hamachi T, Nakayama K, Maeda K. Mixed infection of Porphyromonas gingivalis and Bacteroides forsythus in a murine abscess model: involvement of gingipains in a synergistic effect. J Periodontal Res. 2001;36:237–243. doi: 10.1034/j.1600-0765.2001.036004237.x. [DOI] [PubMed] [Google Scholar]
- 202.Zablotsky M, Meffert R, Caudill R, Evans G. Histological and clinical comparisons of guided tissue regeneration on dehisced hydroxylapatite-coated and titanium endosseous implant surfaces: a pilot study. Int J Oral Maxillofac Implants. 1991;6:294–303. [PubMed] [Google Scholar]
- 203.Zhang Q, Yao K, Liu L, Sun Y, Xu L, Shen X, Lai Q. Evaluation of porous collagen membrane in guided tissue regeneration. Artif Cells Blood Substit Immobil Biotechnol. 1999;27:245–253. doi: 10.3109/10731199909117697. [DOI] [PubMed] [Google Scholar]
- 204.Zhang T, Kurita-Ochiai T, Hashizume T, Du Y, Oguchi S, Yamamoto M. Aggregatibacter actinomycetemcomitans accelerates atherosclerosis with an increase in atherogenic factors in spontaneously hyperlipidemic mice. FEMS Immunol Med Microbiol. 2010;59:143–151. doi: 10.1111/j.1574-695X.2010.00674.x. [DOI] [PubMed] [Google Scholar]
- 205.Zhang W, Abukawa H, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue engineered hybrid tooth-bone constructs. Methods. 2009;47:122–128. doi: 10.1016/j.ymeth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 206.Zhou Y, Li Y, Mao L, Peng H. Periodontal healing by periodontal ligament cell sheets in a teeth replantation model. Arch Oral Biol. 2012;57:169–176. doi: 10.1016/j.archoralbio.2011.08.008. [DOI] [PubMed] [Google Scholar]