Dear Editor
In 2000, there was an outbreak of sequence type (ST)-11, serogroup W meningococcal disease in Mecca, Saudi Arabia, with subsequent global spread. Since then, serogroup W meningococcal infection has become an increasing problem in many parts of the world, including Latin America. In this Journal, the presence of the Hajj 2000-associated clone, which is characterized as 2-145:P1.5,2:F1-1:ST-11 (cc11) and 16S type 31, was recently reported in Brazil,1 as were other highly-related ST-11 serogroup W isolates in Brazil1,2 and Argentina.3
In Rio de Janeiro State, Brazil, serogroup W meningococcal disease was uncommon until 2003, when a sudden increase of W:2a Neisseria meningitidis sporadic cases and outbreaks occurred.4 From 1988 to 2009, 12,917 (1988–1999 = 8906 [3728 culture-proven] and 2000–2009 = 4011 [1060 culture-proven]) cases of meningococcal disease were reported to the Rio de Janeiro State Department of Health. Although serogroup B N. meningitidis was recorded as the leading cause of invasive meningococcal disease before 2000, it has gradually been replaced by isolates of se-rogroup C, which have been responsible for over 80% of culture-confirmed cases since 2008. In this context, there were 34 culture-positive serogroup W cases, 14 from 1988 to 1999 and 20 from 2003 to 2009, representing an average of 1.2 and 2.9 cases per year, respectively. From 2003 to 2009, an additional 44 PCR-positive serogroup W cases were reported, 30 (68%) of which occurred during 2004–2005.
We analysed the genetic and antigenic diversity of serogroup W N. meningitidis and serotype 2a isolates of other serogroups from patients during 1988–2009 in Rio de Janeiro State with the goal of understanding the sudden increase in serogroup W disease and its role in meningococcal disease outbreaks. N. meningitidis serogroup was determined by slide agglutination with specific rabbit antisera (BD Difco, Maryland, USA). Serotyping was performed at the National Meningitis Reference Centre. MLST and antigen sequence typing of the genes encoding PorB, PorA, and FetA were performed on 31 serogroup W and 21 serotype 2a (B = 2; C = 19) invasive isolates and directly from 37 CSF samples available from which serogroup W N. meningitidis had been detected by a two-step PCR assay; 16S rRNA gene typing was also performed on selected isolates of the ST-11 clonal complex.1,5
Of the 78 cases (4 missing data) of serogroup W meningococcal disease, the average age was 15 years (range 24 days–63 years); 64% were ≤10 years. The disease affected more males (59%) than females (41%) (p = 0.01). The proportion diagnosed with septicaemia increased from 7% in 1988–1999 to 22% in 2000–2009 (p = 0.1). The case fatality rate also increased from 14% in the early period to 21% since 2000 (p = 0.4).
All of 21 serotype 2a isolates and 98% of 57 W isolates from 1999 to 2009 belonged to the ST-11 clonal complex (Table 1). In contrast, 2 (18%) of the W isolates before 1999 belonged to the ST-11 clonal complex. Beginning in 1999, two predominant ST-11 or single locus variant [SLV] of ST-11 serogroup W clones emerged (2–145:P1.5,2:F1-1 [79% of 56 cases] and 2–69:P1.5,2:F1-1 [16%]). The 2–145:P1.5,2:F1-1:ST-11 (cc11) clone was first identified in 1997 and was found to exhibit multiple capsules, an indication of possible capsular switching, and to be 16S type 13 (Table 1). For example, two ST-11 clonal complex isolates from 1997 (one ST-11 and one ST-475, an SLV of ST-11) were found to bear serogroup B capsule. Subsequently, serogroup W and C variants of the clone were identified in 1999 and 2000, respectively.
Table 1.
Distribution of the outer membrane protein (OMP) gene sequence profiles (porB, porA, and fetA) and sequence types (STs) of Neisseria meningitidis, by serogroup (serotype) and MLST clonal complex, 1988–2009.
| Year | Genotype | No. of isolates |
|---|---|---|
| Serogroup B (2a) | ST-11 clonal complex | |
| 1997 | 2-145:P1.5,2:F1-1:ST-11a | 1b |
| 2-145:P1.5-2:F1-1:ST-475a | 1 | |
| Serogroup C (2a) | ST-11 clonal complex | |
| 1990 | 2-184:P1.5-1,2-2:F1-1:ST-5121 | 1 |
| 1995–1997 | 2-2:P1.5,2:F3-6:ST-11 | 5 |
| 1997 | 2-60:P1.5,2:F3-6:ST-7849 | 1 |
| 2000 | 2-145:P1.5,2:F1-1:ST-11a | 1b |
| 2001 | 2-184:P1.5-1,2-2:F1-1:ST-5121 | 1 |
| 2007–2009 | 2-2:P1.5-1,10-8:F3-6: ST-11 | 10 |
| Serogroup W (2a) | ST-11 clonal complex | |
| 1988 | 2-2:P1.5,2:F1-46:ST-11 | 1 |
| 1993 | 2-2:P1.5,2:F1-1:ST-11 | 1 |
| 1999 | 2-145:P1.5,2:F1-1:ST-11a | 2b |
| 2003–2005 | 2-69:P1.5,2:F1-1:ST-11 | 8 |
| 2-69:P1.5,2:F1-1:ST-4091 | 1 | |
| 2003–2006 | 2-145:P1.5,2:F1-1:ST-11a | 41b |
| 2-145:P1.5,2:F1-1:ST-7815a | 1 | |
| 2009 | 2-2:P1.5-1,10-8:F3-6:ST-7816 | 1 |
| Serogroup W (2b, NT) | ST-11 clonal complex | |
| 2005 | 2-3:P1.5,2:F1-1:ST-11 | 1 |
| 2009 | 2-148:P1.5,2:F1-1:ST-11 | 1 |
| Serogroup W (19) | ST-174 clonal complex | |
| 1988 | 3-35:P1.21,16:F5-13:ST-6302 | 1 |
| 1990 | 3-35:P1.21,16:F5-13:ST-2914 | 1 |
| 3-35:P1.21,16:F5-13:ST-6307 | 1 | |
| 3-35:P1.21,16:F5-13:ST-7715 | 1 | |
| 1991–1993 | 3-35:P1.21,16:F5-13:ST-174 | 1 |
| 3-35:P1.21,16:F5-13:ST-6307 | 2 | |
| 2003 | 3-292:P1.7-2,13:F5-13:ST-7707 | 1 |
| Serogroup W (NT) | ST-22 clonal complex | |
| 1991 | 2-23:P1.18-1,3:F4-1:ST-22 | 1 |
| Serogroup W (7) | No clonal complex | |
| 1990 | 3-296:P1.5-1,10-4:F1-7:ST-7706 | 1 |
16S rRNA type 13.
Successful clone exhibiting multiple capsules associated with outbreak and field clusters (see text).
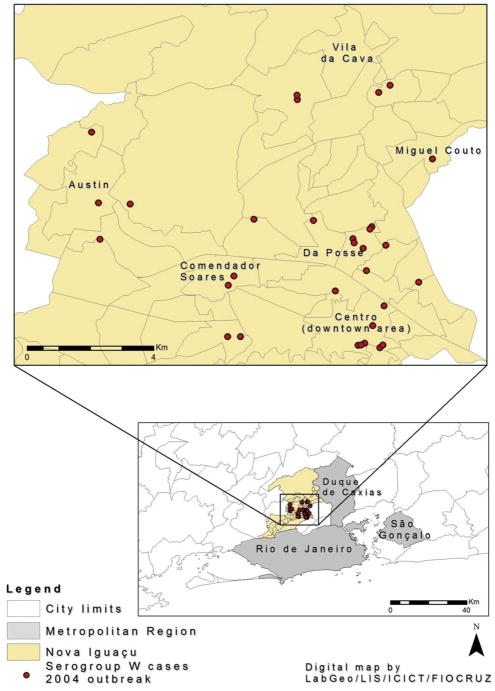
During the period of 2003–2006, there were 42 serogroup W cases caused by this clone (41 ST-11 and 1 ST-7815, another SLV of ST-11). This was due in part to an outbreak caused by the 2–145:P1.5,2:F1-1:ST-11 (cc11) clone in 16 of 68 neighbourhoods in the city of Nova Iguaçu (Fig. 1), where 31 individuals (74% ≤ 6 years) were diagnosed with meningococcal disease from April through mid-June 2004; 22 (71%) patients had a haemorrhagic rash at admission and 4 (13%) patients died. The affected neighbourhoods are contiguous areas belonging to 6 districts (260,133 inhabitants), for which the overall incidence rate was 12/100,000 (range, 5.4 in Miguel Couto to 17.9/100,000 in Centro [downtown area]). Also, there were 2 additional clusters caused by this clone, one in 2004 involving 2 military recruits during a 4 day outdoor training exercise in the city of São Gonçalo (attack rate, 2/94 = 2.1%) and one in 2006 affecting 2 brothers (1 year and 3 years old) living in the city of Duque de Caxias, which is adjacent to Nova Iguaçu (Fig. 1).
Figure 1.
Geographic location of meningococcal disease cases in the city of Nova Iguaçu during the 2004 serogroup W outbreak.
In this study, we found ST-11 clonal complex meningococcal isolates of multiple capsular groups, namely C, with which this clonal complex is most commonly associated, as well as B and W. This finding is within the context that Brazil has recently instituted a national program of routine childhood meningococcal vaccination with serogroup C conjugate vaccine.6 Capsular switching and subsequent substantial disease from serogroup C to serogroups W and B has important public health implications. Although ST-11 serogroup W disease has been reported from southern Brazil2 and Argentina,3 to our knowledge neither has been associated with outbreaks in region. These capsular switching events were associated with a substantial number of clinical cases in Rio de Janeiro State, outbreak and field clusters, a preponderance of disease in children, an increase in septicaemia, and a high case fatality.
Recent studies have demonstrated that serogroup W ST-11 isolates in several countries were distinguishable from the Hajj-associated clone by means of fine molecular typing.1,7,8 These results suggest the expansion of local isolates and the emergence of new genetic lineages of serogroup W that differ from the Hajj-associated clone, which is believed to have arisen from serogroup C to W capsular switching. Whether these differences reflect the evolution of the Hajj clone over time or multiple serogroup C to W capsular switches is not known but might be discernible by DNA sequencing of the capsular operon of well-selected isolates. The re-emergence of N. meningitidis belonging to serogroup W and to the ST-11 clonal complex has been observed globally since 2010 in regions such as the African meningitis belt, France, and Chile, giving rise to new questions about the origin and expansion of these isolates.9,10 Our study underscores the need to conduct molecular surveillance of meningococcal disease to monitor the impact of meningococcal immunization programs and determine whether use of multivalent meningococcal vaccines is warranted.
Acknowledgments
Funding
This work was supported in part by a Fogarty International Center Global Infectious Diseases Research Training Program grant, National Institutes of Health, to the University of Pittsburgh (D43TW006592).
We thank the staff of the National Meningitis Reference Centre (Adolfo Lutz Institute, São Paulo, Brazil) for serological typing; the staff of the microbiology laboratories for providing meningococcal isolates; Mônica de A. F. M. Magalhães and Fabiane Bertoni of the Geoprocessing Laboratory/LIS/ICICT/FIOCRUZ for the digital map; Julia Bennett for curation of the MLST database for Neisseria meningitidis.
Footnotes
Competing interests
Dr. Harrison receives funding from the Centers for Disease Control and Prevention and the National Institute of Allergy and Infectious Diseases. He has received research support and lecture fees from Sanofi Pasteur; lecture fees from Novartis Vaccines; and has served as a consultant to GlaxoSmithKline, Merck, Novartis Vaccines, Sanofi Pasteur, and Pfizer. Dr. Harrison’s financial ties with industry were terminated before he became a voting member of the Advisory Committee on Immunization Practices in July 2012.
Contributor Information
David E. Barroso, Laboratory of Biochemical Systematics, Oswaldo Cruz Institute, FIOCRUZ, Av. Brasil 4365, 21040-900 Rio de Janeiro, RJ, Brazil.
Terezinha M.P.P. Castiñeiras, Department of Preventive Medicine, Federal University, School of Medicine, Rio de Janeiro, Brazil
Ana C.P. Vicente, Laboratory of Molecular Genetics of Microorganisms, Oswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, Brazil
Elaine O. Cerqueira, Central Laboratory Noel Nutels and Meningitis Advisory Committee, State Department of Health, Rio de Janeiro, Brazil
Lee H. Harrison, Infectious Diseases Epidemiology Research Unit, University of Pittsburgh School of Medicine and Graduate, School of Public Health, Pittsburgh, United States
References
- 1.Lemos APS, Harrison LH, Lenser M, Sacchi CT. Phenotypic and molecular characterization of invasive serogroup W135 Neisseria meningitidis strains from 1990 to 2005 in Brazil. J Infect. 2010;60:209–17. doi: 10.1016/j.jinf.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weidlich L, Baethgen LF, Mayer LW, Moraes C, Klein CC, Nunes LS, et al. High prevalence of Neisseria meningitidis hypervirulent lineages and emergence of W135:P1. 5,2:ST-11 clone in Southern Brazil. J Infect. 2008;57:324–31. doi: 10.1016/j.jinf.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Efron AM, Sorhouet C, Salcedo C, Abad R, Regueira M, Vázquez JA. W135 invasive meningococcal strains spreading in South America: significant increase in incidence rate in Argentina. J Clin Microbiol. 2009;47:1979–80. doi: 10.1128/JCM.02390-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barroso DE, Rebelo MC. Recognition of the epidemiological significance of Neisseria meningitidis capsular serogroup W135 in the Rio de Janeiro region. Brazil Mem Inst Oswaldo Cruz. 2007;102:773–5. doi: 10.1590/s0074-02762007005000104. [DOI] [PubMed] [Google Scholar]
- 5.Pedro LGF, Boente RF, Madureira DJ, Matos JA, Rebelo MC, Igreja RP, et al. Diagnosis of meningococcal meningitis in Brazil by use of PCR. Scand J Infect Dis. 2007;39:28–32. doi: 10.1080/00365540600904761. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso CW, Pinto LL, Reis MG, Flannery B, Reis JN. Impact of vaccination during an epidemic of serogroup C meningococcal disease in Salvador, Brazil. Vaccine. 2012;30:5541–6. doi: 10.1016/j.vaccine.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Yazdankhah SP, Lindstedt BA, Caugant DA. Use of variable-number tandem repeats to examine genetic diversity of Neisseria meningitidis. J Clin Microbiol. 2005;43:1699–705. doi: 10.1128/JCM.43.4.1699-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taha M-K, Giorgini D, Ducos-Galand M, Alonso J-M. Continuing diversification of Neisseria meningitidis W135 as a primary cause of meningococcal disease after emergence of the serogroup in 2000. J Clin Microbiol. 2004;42:4158–63. doi: 10.1128/JCM.42.9.4158-4163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parent du Chatelet I, Barboza P, Taha MK. W135 invasive meningococcal infections imported from Sub-Saharan Africa to France, January to April 2012. Euro Surveill. 2012;17 pii: 20181. [PubMed] [Google Scholar]
- 10.Situación enfermedad meningocócica por serogrupo W-135. Chile: 2012. Available from. http://epi.minsal.cl/vigilancia-epidemiologica/enfermedades-de-noticacion-obligatoria/enfermedad-meningococica. [Google Scholar]