Abstract
Exposure to intense sound can damage or kill cochlear hair cells. This loss of input typically manifests as noise induced hearing loss, but it can also be involved in the initiation of other auditory disorders such as tinnitus or hyperacusis. In this study we quantify changes in HC number following exposure to one of four sound damage paradigms. We exposed adult, anesthetized Long-Evans rats to a unilateral 16 kHz pure tone that varied in intensity (114 dB or 118 dB) and duration (1, 2, or 4 hours) and sacrificed animals 2–4 weeks later. We compared two different methods of tissue preparation, plastic embedding/sectioning and whole mount dissection, for quantifying hair cell loss as a function of frequency We found that the two methods of tissue preparation produced largely comparable cochleograms, with whole mount dissections allowing a more rapid evaluation of hair cell number. Both inner and outer hair cell loss was observed throughout the length of the cochlea irrespective of sound damage paradigm. IHC loss was either equal to or greater than OHC loss. Increasing the duration of sound exposures resulted in more severe HC loss which included all HC lesions observed in an analogous shorter duration exposure.
Keywords: Hearing Loss, Hair Cell, Cochleogram, Sound Exposure
1. Introduction
The primary sites of damage in the peripheral auditory system following acoustic overexposure are the sensory hair cells and supporting structures of the cochlea [1, 2], while secondary damage occurs in the central auditory system [3]. Damage to the sensory hair cells in the auditory periphery manifests functionally as noise-induced hearing loss (NIHL) [4, 5]. Hyperacusis and tinnitus can also occur as the result of peripheral or central damage and remodeling [6, 7]. The relationship between hair cell (HC) damage and subsequent manifestations of auditory pathology is complex[8], and HC lesions alone do not always provide a sufficient explanation for any resulting functional pathology post sound-exposure. In combination with other experimental methods, however, the cochleogram is a useful tool for studying auditory disorders since it quantifies an aspect of the initial peripheral insult that initiates central remodeling.
Hair cell lesions cannot always be predicted accurately on the basis of parameters of a given sound damage paradigm, and HC loss induced by acoustic trauma is highly variable across animals of the same strain[9, 10]. Therefore, it is necessary to directly quantify HC lesions. A variety of tissue preparation methods exist for HC quantification, which can be separated into two major categories: embedding and sectioning[11] or surface preparations [12, 13]. Embedding and sectioning the cochlea is perhaps the first method utilized for examining hair cells, with early examples using celloidin embedding[14]. This preparation method involves the cochlea being embedded in a media (e.g., celloidin, paraffin, plastic, etc.) and then serially sectioned to expose the hair cells either parallel or perpendicular to the modiolar axis. This approach allows for examination of virtually all cochlear structures, including HCs, spiral ganglion neurons [11], and other supporting structures [15]. When the plane of section is parallel to the modiolar axis, the reconstruction of the numerous, discontinuous segments of the organ of Corti necessary to assess frequency-specific hair cell loss is tedious.. Alternatively, the surface preparations approach involves microdissecting the cochlea into cochlear turns. This method allows for a stable view of the HCs across the entire length of the organ of Corti, but at the expense of trimming away the majority of other cochlear structures. Reconstruction of the few large, continuous fragments is straight-forward. Tissues prepared using either method can be labeled with a wide array of staining protocols and visualized using a variety of microscopic techniques. While both methods of preparation are in widespread use, few studies have directly compared cochleograms generated using both approaches. Ostensibly both methods are believed to produce comparable results, yet this has not been thoroughly documented in the literature.
In the present study we sought to identify the HC loss profiles that result from exposure to four different, though related, sound damage paradigms in a rat model of noise-induced hearing loss. We utilized two tissue preparation methods, one a surface preparation and the other an embedding and sectioning method, in an effort to examine the comparability of the resulting cochleograms. We also simultaneously sought to assess how the method of tissue preparation and reconstruction might influence the resulting cochleograms and areas of significant HC loss.
2. Methods
2.1. Animals
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA) weighing between 250 to 300 grams were used in these experiments. The Institutional Animal Care and Use Committee of the University of Kansas Medical Center approved all experimental protocols.
2.1.1. Sound Exposure
Rats were anesthetized for either 4 hours using isoflurane, or 1–2 hours using a mixture of ketamine (50 mg/kg), atropine (0.05 mg/kg), and xylazine (10 mg/kg), and then placed inside a sound attenuated chamber (Industrial Acoustics Company, Bronx, NY) for the noise-exposure procedure. Rats were unilaterally exposed to one of four 16 kHz pure tones (114 dB SPL for 1 hour; 114 dB SPL for 2 hours; 118 dB SPL for 1 hour; and 118 dB SPL for 4 hours) from a loudspeaker (model 40–1310-B, Radio Shack, Fort Worth, TX) inside a plastic case. The loudspeaker was coupled to the pinna using ½ inch flexible plastic tubing and sealed, using Audalin ear mold compound (All American Mold Lab, Oklahoma City, OK), to minimize any bilateral damage. The intensity level of the stimulus measured outside the tubing was 45 dB less than the intensity of the stimulus within the tubing sealed to the head of the animal [16]. A Macintosh computer with a MaLab synthesizer, event processor, and software (Kaiser Instruments, Irvine, CA) was used to control noise waveform synthesis.
2.2. Plastic embedded cochlea: tissue processing, HC counting, and cochlear reconstruction
2.2.1. Tissue Harvest and Processing
For evaluation of hair cell numbers from tissue sections, we employed a protocol for plastic embedding developed by Kujawa et al. [11]. Rats were euthanized by injection of Beuthenasia (5 cc/kg) and then either decapitated or perfused with 4% paraformaldehyde and decapitated. Harvested temporal bones were placed in 4% paraformaldehyde.
At the time of embedding the stapes was removed and the oval window, round window, and apex of the cochlea were punctured. The cochlea was then submerged for one hour in a 1% osmium tetroxide solution, which was flushed through the cochlea at 15-minute intervals. Following washes with distilled water, the cochlea was decalcified with RDO (Apex Engineering Products, Aurora, IL) for one hour. The decalcified cochlea was then rinsed with phosphate buffered saline (PBS), dehydrated using a graded series of ethanol, and embedded in Araldite plastic (Electron Microscopy Sciences, Hatfield, PA). The cochlea was cut into 40 um sections parallel to the modiolar axis, using a tungsten-carbide knife (Fig. 1A). Sections were mounted on charged glass slides, counterstained with toluidine blue, and coverslipped with Permount (Fisher Scientific, Waltham, MA).
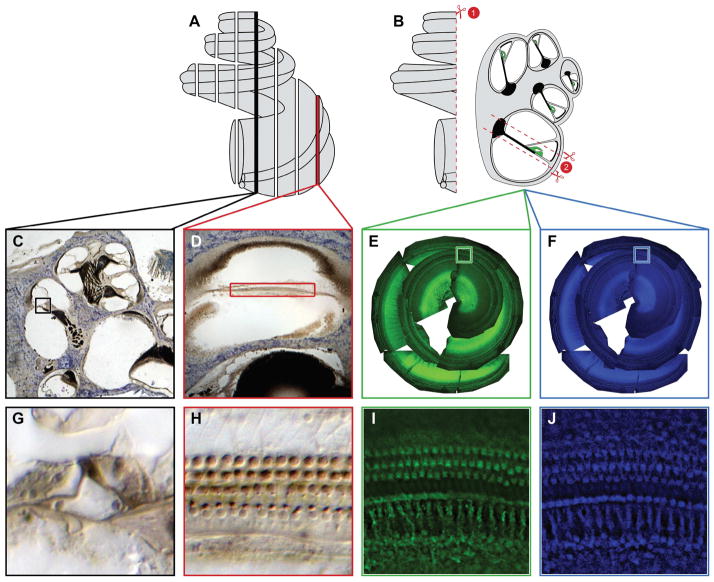
Figure 1. Tissue Preparation and Staining Methods.
Cochleae in (A) were embedded in araldite plastic and then cut into 40 um sections parallel to the modiolus. This method of sectioning resulted in two different views of the HCs. A midmodiolar section (C) allows the counter to view the HCs in cross section while a non-modiolar section (D) allows the counter to view the HCs from above over a much longer distance. Cochleae in (B) were decalcified and then dissected under PBS. The first cut (B1) bisects the cochlea, allowing the half-turns of the Organ of Corti to be dissected. Each half-turn is then removed and trimmed down as indicated in (B2). Once the dissection is complete the tissue is labeled with phalloidin and DAPI (E and F). Following staining the tissue is mounted and photographed; the photographs are then collaged together, which is what is seen in (E and F). High magnification views of the phalloidin and DAPI staining (I and J respectively) provide views of the hair cells from above, similar to the non-modiolar sections seen in (H).
2.2.2. Hair Cell Counts of Nuclei in Plastic Sections
One person with no knowledge of the treatment group to which the cochlea belonged performed hair cell counts. Inner and outer hair cells were counted at 20x using Nomarski illumination on a Nikon Optiphot-2 microscope. The presence of a hair cell was defined as an intact, spherical nucleus located in the basal half of the cell. The same person who performed the initial counts recounted a select group of cochleae to determine a measure of intra-rater reliability.
2.2.3 Reconstruction
To begin reconstruction of the basilar membrane, low magnification images of serial cochlear sections were acquired using a QImaging EXi Aqua camera in combination with QImaging software (QImaging, Surry, British Columbia, Canada). These images were aligned using Adobe Photoshop and the (x, y) coordinates of each basilar membrane point containing hair cells were identified and exported as a Microsoft Excel file. Coordinates of the basilar membrane were synced with the corresponding HC count data in Excel and ordered from the basal-most point to the apex of the cochlea. The equation developed by Greenwood [17] was used to convert relative cochlear location, expressed as percent distance from the apex (100% is the base, 0% is the apex) to characteristic frequency for evaluation of the data. The equation is:
Where F is frequency in kHz; x is the relative location (% distance) along the basilar membrane; A, a are species-specific coefficients; and k is the constant of integration.
2.3 Whole Mount Dissection: Tissue Processing, cochlear reconstruction, and HC counting
2.3.1 Tissue Processing
For whole-mount dissections we employed the same animal sacrifice and tissue harvesting procedures as previously described for plastic sections. Post-fixation, the cochlea was decalcified using RDO for 1 hour. The excess temporal bone around the cochlea was trimmed away, and the cochlea was bisected in a midmodiolar plane. The bisected cochlea was then submerged in PBS. A series of cuts through the modiolus separated the half-turns from one another (Fig. 1B). Each half-turn was then removed from the surrounding temporal bone and the tectorial membrane was removed.
Dissected half-turns were permeabilized using 0.2% Triton X-100 (Sigma Aldrich, St. Louis, MO) for 30 minutes. Excess Triton X-100 was washed off using PBS. Tissue was blocked for 30 minutes using a 2% bovine serum albumin solution, which was removed using PBS. Half-turns were incubated with alexafluor 488 conjugated phalloidin (Life Technologies, Carlsbad, CA) diluted in PBS (1:400) for 40 minutes. Tissue was then washed with PBS to remove residual, unbound phalloidin. The stained tissue was mounted on charged slides, using Vectashield containing DAPI (Vector Laboratories, Burlingame, CA).
2.3.2 Reconstruction
Photographs of each half-turn were acquired using a Nikon Eclipse E800 microscope and a QImaging EXi Aqua camera in combination with QImaging software. Two sets of images were taken for each half-turn to visualize DAPI and phalloidin staining. In Adobe Photoshop, images from both channels were superimposed and linked together. The linked images of the half-turns were then aligned to reconstruct the cochlear spiral, using the heads of the pillar cells as a reference point. The total length of the cochlea was then subdivided into 20 equal sections in preparation for counting.
2.3.3 Hair Cell Counts
Using the images created in Adobe Photoshop, separate hair cell counts were generated using the phalloidin and DAPI staining. Intact, phalloidin-stained stereocilia bundles were counted directly when visible. In some instances the phalloidin staining was robust and actin in other, non-stereocilia structures was labeled. Fluorescence from the staining of cytoskeletal actin and the cuticular plate rendered stereocilia difficult to distinguish, in which case the cuticular plate was used as the criteria for the presence of a hair cell. In DAPI stained tissue, intact spherical nuclei were counted to estimate HC number. Care was taken when photographing tissue, so that the nuclei in focus were HC nuclei and not the nuclei of nearby supporting cells. Two different individuals completed HC counts for the entire set of whole-mount dissected tissue. These two sets of HC counts were used to calculate inter-rater reliability measures. As with the plastic section based HC counts, one individual also recounted a subset of the whole-mount dissected tissue. This set of HC counts was used to calculate a measure of intra-rater reliability.
2.4 Data Analysis & Transformation
We used intra-class correlations (ICC) to determine the reliability of our data sets. Reliability statistics were calculated in SPSS v22.0 (IBM, New York, NY). We used an intra-class correlation coefficient of ≥ 0.7 as a measure of strong absolute agreement between the two sets of HC counts being compared. All statistical tests for examining differences in HC number between treatment groups were calculated using Prism v6.0 (GraphPad, La Jolla, CA) with the level of significance set at p ≤0.05. T-tests were used to assess differences in the total numbers of HCs, inner hair cells (IHC) and outer hair cells (OHC) across treatment groups for both methods of tissue preparation. Two-way ANOVAs for repeated measures were used to determine the frequency-specific regions of HC loss relative to control animals. The Dunnett post hoc method compared data sets to a control (Figs. 4–5), while comparisons of data sets among methods (Figs. 2–3) utilized Tukey’s multiple comparisons.
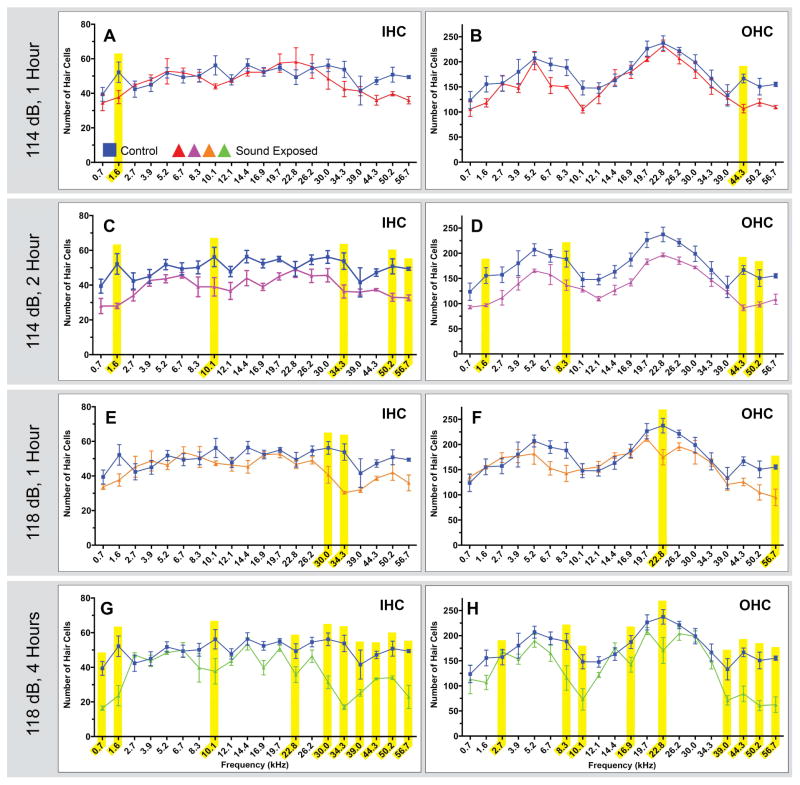
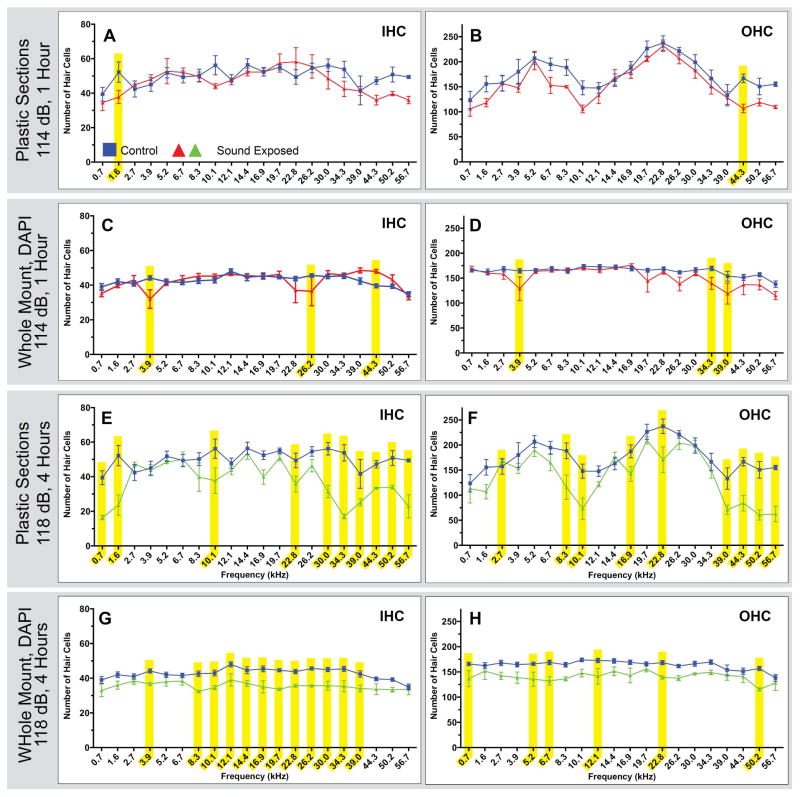
Figure 4. Comparisons of noise exposed animals to unexposed controls in plastic embedded and sectioned tissues.
Four related sound damaged paradigms were used to generate the tissue for the above counts. Two paradigms were a 114 dB, 16 kHz pure tone for 1 or 2 hours, and two paradigms were a 118 dB, 16 kHz pure tone for 1 or 4 hours. Each point represents the mean number of HCs ± SEM for a given frequency range (control n=5 animals ;114 dB, 1 hour n=4 animals; 114 dB, 2 hours n=3 animals; 118 dB, 1 hour n=3 animals; 118 dB, 4 hours n=5 animals). Frequency ranges with statistically significant HC loss are highlighted in yellow (p<0.05). Short duration exposures (A & B and E & F) resulted in few areas of significant HC loss Longer duration exposures (C & D and G & H) showed more areas of significant HC loss across a greater range of frequencies, with high frequencies showing the most damage.
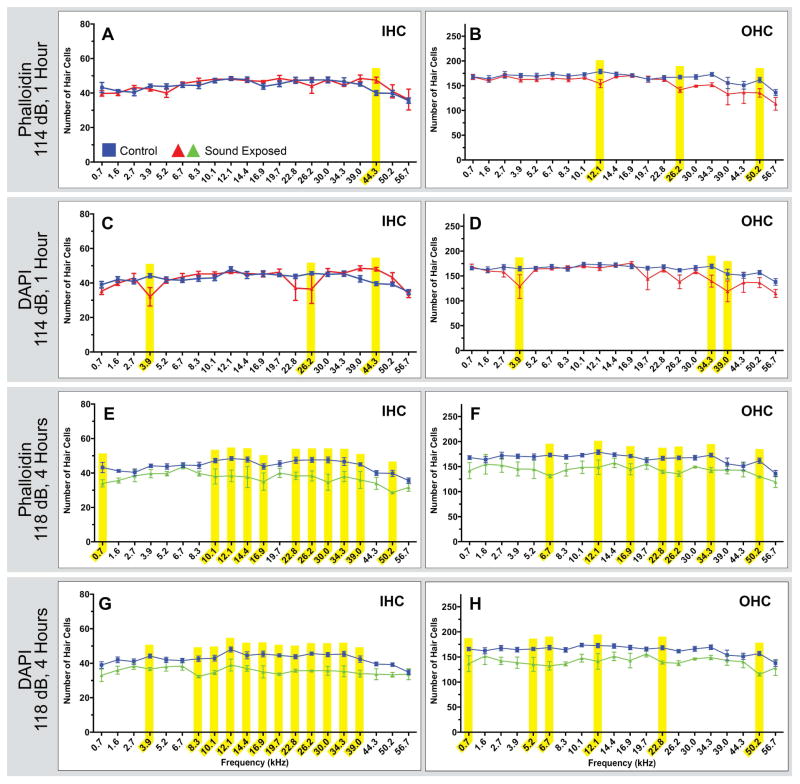
Figure 5. Comparisons of noise exposed animals to unexposed controls in whole mount dissected tissue.
Two sound damage paradigms were used to generate tissue for this analysis. Tissue was stained with both phalloidin and DAPI, and cells were counted separately using each stain. Each point represents the mean number of HCs ± SEM for a given frequency range (control n=5 animals; 114 dB, 1 hour n=4 animals; 118 dB, 4 hours n=3 animals). Frequency ranges with statistically significant HC loss are highlighted in yellow (p<0.05 ). We observe similar overall patterns of loss using these two stains, even though some specific ranges of HC loss are not seen in both the phalloidin and DAPI based counts.
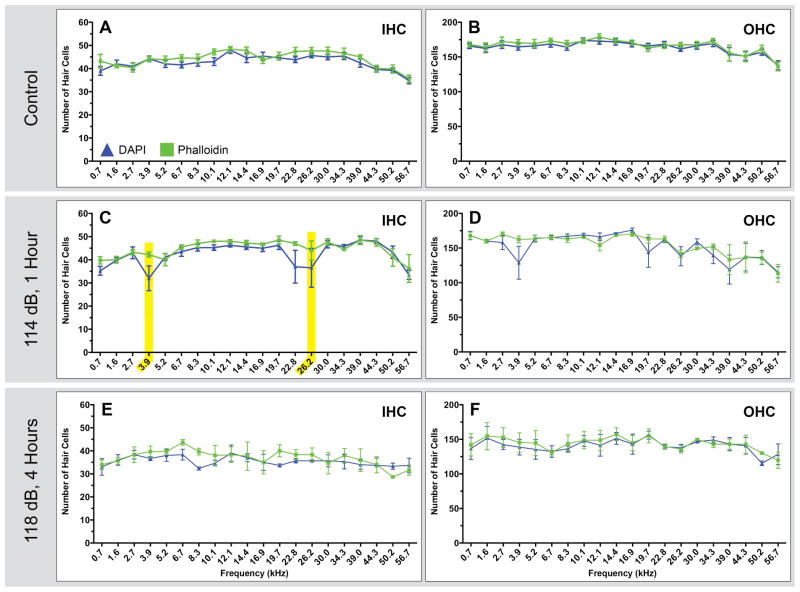
Figure 2. Comparisons of phalloidin and nuclei based counts in dissected tissue.
Each pair of HC counts (A & B, C & D, E & F) shows the IHC and OHC counts for a given treatment group. Tissue used to generate these counts was co-labeled using DAPI (blue triangles) and phalloidin (green squares). Both stains were counted separately in an effort to determine whether the feature used to identify HCs had a significant impact on the resulting counts. Each point represents the mean number of HCs ± SEM for a given frequency range (control n=5 animals; 114 dB, 1 hour n=4 animals; 118 dB, 4 hours n=3 animals). Only two areas of statistical significance are observed, highlighted in yellow, in the IHCs counts for animals exposed to a 114 dB, 16 kHz pure tone for 1 hour.
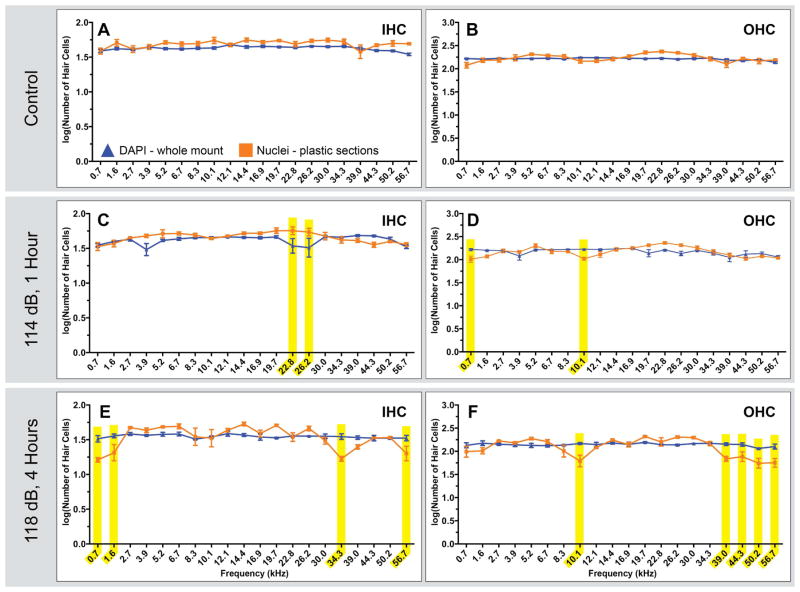
Figure 3. Comparison of nuclei based counts in plastic sections and dissected tissue.
Two methods of tissue preparation were used to generate the above HC counts - plastic sections (orange squares) and whole mount cochlear dissection (blue triangles). Counts are based on the nucleus, a feature visible in both preparations. Comparisons of IHC and OHCs in control animals (A and B) showed no significant differences. Each point represents the mean number of HCs ± SEM for a given frequency range (Whole Mount Dissections: control n=5; 114 dB, 1 hour n=4; 118 dB, 4 hours n=3. Plastic Sections: control n=5 animals;114 dB, 1 hour n=4 animals; 118 dB, 4 hour n=5 animals). Comparisons of IHCs and OHCs in sound exposed animals (C–F) resulted in multiple areas of statistically significant differences, highlighted in yellow, in low, mid and high frequency ranges.
3. Results
Utilizing the data collected during these experiments we sought to address two questions. First, what patterns of HC loss result from exposure to each of the 4 sound damage paradigms? Second, how comparable are the cytocochleograms generated from each method of tissue preparation?
3.1 Reliability
Prior to evaluating hair cell number as a function of sound exposure or tissue preparation, we performed a series of intra-class correlation analyses to determine the reliability of HC count data. We used an “absolute agreement” analysis, as opposed to “consistency,” because we were interested in degree of absolute agreement between our data sets. Blinded HC counts from two independent raters, generated using whole-mount dissected tissue stained with DAPI and phalloidin, were compared using ICC analysis set to a two-way mixed model with absolute agreement. Individual ICCs were performed utilizing count data for each stain and HC type (e.g. one analysis for DAPI stained IHCs and a subsequent analysis for DAPI stained OHCs). Similarly, intra-class correlation analyses were performed on blinded recounts of tissue from both tissue preparation methods to provide a measure of intra-rater reliability. We performed three distinct sets of correlation analyses: rater 1 v. rater 2 counts of phalloidin and DAPI stained whole-mount dissections; rater 1 initial count v. rater 1 recount of phalloidin and DAPI stained whole mount dissections; and rater 1 initial count v. rater 1 recounts of plastic embedded tissue.
When we examined the ICC coefficients from the rater 1 v. rater 2 counts of whole-mount dissection, we determined that regardless of stain, HC type, or treatment condition, the two counts were in good agreement, with all ICC coefficients exceeding 0.7 (Fig. S1). We observed an even higher degree of agreement in the rater 1 recount comparisons (Table S1), with all ICC coefficients exceeding 0.9 irrespective of stain, HC type, or treatment group. Based on these data, both raters consistently, and accurately, assessed HC number.
3.2 Tissue Preparation & Staining
We compared two methods of tissue preparation for examining HC loss: sectioning an embedded cochlea (Fig. 1A) and dissecting and staining a whole-mount preparation (Fig. 1B). Plastic embedding and sectioning allowed HC nuclei to be stained and viewed with light microscopy for assessment of HC number. This preparation also allowed for evaluation of other structural features, such as supporting cell integrity, spiral ganglion neurons, and the stria vascularis (Fig. 1C and G). The primary disadvantage of this method was that the basilar membrane and Organ of Corti were divided into multiple discontinuous segments of variable lengths and appearance (modiolar [Fig. 1C and G] v. non-modiolar [Fig. 1D and H]), as a result of sectioning. The need to reconstruct the basilar membrane made assessment of HC number as a function of relative location challenging.
Whole-mount dissections (Fig. 1B) allowed for a consistent view of the HCs for the entire length of the Organ of Corti, and this tissue preparation allowed us to label various features using immunohistochemical markers. The stable and consistent perspective facilitated the counting process, and although dissection disrupted the basilar membrane, the segments were continuous, which simplified reconstruction. The fixed perspective, however, limited what cochlear features could be examined, since the cells were not seen in cross-section.
3.3 Analysis of Total HC Number
The total numbers of IHCs, OHCs, and combined HC types, as determined using both methods of tissue preparation, are reported in Table 1. First, we compared the results of toluidine blue stained nuclei counts in plastic sections to DAPI stained nuclei counts in whole-mount dissections for IHCs, OHCs and total number of HCs. Second, we compared the results of HC counts based on different HC features in whole mount dissected tissue (DAPI nuclear stain and phalloidin actin stain) for IHCs, OHCs, and total number of HCs. In control animals, comparisons of nuclei based counts (DAPI and toluidine blue) were found to be significantly different for IHCs, OHCs, and total number of HCs (p=0.0005 for IHC, p=0.0108 for OHC, p=0.0028 for total number). However, we observed no significant differences when DAPI and phalloidin based HC counts were compared in control animals. In sound exposed animals, we observed no significant differences for any of the analogous comparisons.
Table 1.
Total HC numbers
| Number of Hair Cells* | |||||
|---|---|---|---|---|---|
| Exposure | Tissue Prep | Stain | IHC | OHC | Total |
| Control | Plastic Sections | Toluidine Blue | 1001 ± 17 | 3509 ± 19 | 4511 ± 25 |
| Control | Whole-mount | DAPI | 853 ± 20 | 3278 ± 68 | 4132 ± 86 |
| Phalloidin | 916 ± 21 | 3325 ± 64 | 4241 ± 82 | ||
| 114 dB, 16 kHz for 1 hour | Plastic Sections | Toluidine Blue | 931 ± 41 | 3063 ± 43 | 3994 ± 76 |
| 114 dB, 16 kHz for 1 hour | Whole-mount | DAPI | 848 ± 23 | 3044 ± 158 | 3892 ± 180 |
| Phalloidin | 893 ± 17 | 3099 ± 99 | 3992 ± 113 | ||
| 114 dB, 16 kHz for 2 hours | Plastic Sections | Toluidine Blue | 780 ± 29 | 2713 ± 15 | 3493 ± 32 |
| 118 dB, 16 kHz for 1 hour | Plastic Sections | Toluidine Blue | 876 ± 35 | 3161 ± 111 | 4037 ± 144 |
| 118 dB, 16 kHz for 4 hours | Plastic Sections | Toluidine Blue | 741 ± 30 | 2735 ± 144 | 3476 ± 174 |
| 118 dB, 16 kHz for 4 hours | Whole-mount | DAPI | 709 ± 32 | 2812 ± 145 | 3521 ± 174 |
| Phalloidin | 739 ± 52 | 2872 ± 163 | 3611 ± 210 | ||
3.4 Comparing HC Counts based on different features of HCs
Using tissue co-labeled with both DAPI (Fig. 1F and J) and phalloidin (Fig. 1E and I) we performed HC counts as a function of location along the length of the basilar membrane (Fig. 2). Comparing these two sets of data allowed us to compare counts based on different HC features in the same tissue. In control animals, we observed no significant differences between DAPI and phalloidin based counts for either IHC or OHC (Fig. 2A and B) as a function of frequency. In rats exposed to a 118 dB, 16 kHz pure-tone for 4 hours, which resulted in significant HC loss, we also observed no difference between DAPI and phalloidin based counts (Fig. 2E and F). However, statistically significant differences in two regions were found in IHC counts (Fig. 2C) for animals exposed to a 114 dB, 16 kHz pure-tone for 1 hour, although no corresponding differences were observed in the OHCs (Fig. 2D). These IHC differences are directly attributable to one rat with atypically severe HC loss for this sound damage paradigm. The minor differences that exist for the 114 dB, 1 hour exposure, and the lack of differences for the 118 dB, 4 hour exposure, suggest either feature can be used to determine HC loss for these sound damage paradigms.
3.5 Comparing HC Counts using different cochlear preparations
HC counts based on the presence of nuclei, using the two previously described methods of tissue preparation, were performed to examine how tissue preparation and the corresponding reconstruction methods influenced HC counts. Prior to analysis, the data sets from each method (plastic sections and whole-mount dissections) were logarithmically transformed (Fig. S2). Data transformation was necessary due to artifacts in the reconstruction process for plastic embedded cochleae, which caused the resulting numbers of HCs to have unique distributions as a function of frequency. The source of this artifact was the changing orientation of the Organ of Corti and the corresponding need to subdivide regions of hair cell in non-modiolar sections (Fig. 1D and H). Without such a transformation, any statistical evaluation might have reflected differences resulting from artifacts and not sound exposure.
Although the total number of IHCs and OHCs in control animals were found to be significantly different for tissue preparation methods (Table 1), we observed no significant differences for either IHC or OHC number as a function of frequency (Fig. 3A and B). In animals exposed to a 114 dB, 16 kHz pure-tone for 1 hour (Fig. 3 C and D) two regions with significant differences for both IHC and OHC were observed. The more severe exposure of 118 dB, 16 kHz pure-tone for 4 hours resulted in four regions with significant differences in IHC counts and five regions with differences in OHC counts (Fig. 3 E and F). The source of these differences in HC counts could have been a result of methods used to quantify them and/or variability in sound damage in each group of animals. The substantially different reconstruction processes for plastic embedded and whole-mount dissected tissue could have resulted in distributions of HCs into different frequency ranges. Alternatively, individual animal responses to sound damage may have been the source of the variability of the HC counts, as counts from different animals are compared in these analyses.
3.6 Identifying Areas of Damage
Following comparisons of tissue preparation and staining methods for HC counts, we next applied these methods to determine patterns of HC loss in each of 4 sound damage paradigms by comparing them to undamaged control animals. For these analyses, sound damaged cochleae prepared with a given method were compared to corresponding tissue from control animals prepared using the same method. No statistical comparisons were made across methods unless explicitly stated.
3.6.1 Plastic Sections
Figure 4 shows HC counts from plastic embedded tissue sections. Animals exposed to a 114 dB, 16 kHz pure-tone for 1 hour had statistically significant HC loss in one low frequency region for IHC and one high frequency region for OHC (Fig. 4A and B). A longer duration (2 hour) exposure to the 114 dB, 16 kHz pure-tone resulted in a more extensive pattern of HC loss in comparison to the shorter duration exposure. With this longer exposure duration, 5 regions of statistically significant IHC loss across low, mid and high frequencies were observed, along with 4 areas of OHC loss in similar frequency regions (Fig. 4C and D). All regions of statistically significant HC loss seen following 1 hour exposure were also observed in the longer duration exposure. As expected, we observed more regions of HC loss as sound exposure duration increased. However, although we presented a 16 kHz pure-tone to create cochlear damage, we did not observe a discrete region of damage centered on the 16 kHz region of the cochlea. Rather, regions of damage were patchy, both above and below the 16 kHz location, particularly for longer duration exposures.
Animals exposed to the more intense 118 dB, 16 kHz pure-tone for 1 hour had significant HC loss in two high frequency regions for IHC and two regions (mid and high frequency) for OHC (Fig. 4E and F). Exposure to a 118 dB, 16 kHz pure-tone for 4 hours resulted in many areas of significant HC loss. Once again, all regions of statistically significant HC loss seen in the 1 hour exposure were also observed in the longer duration exposure. As seen with the 114 dB sound damage paradigms, longer exposure duration resulted in more regions of HC loss. Ten areas of significant IHC loss were observed in low, mid, and high frequency regions, with the majority of the damage seen in the high frequencies (Fig. 4G); nine areas of significant OHC loss were also observed across all frequency regions, but the damage was most prevalent in the high frequency regions (Fig. 4H).
3.6.2 Whole-Mount Dissections
Hair cell loss was also evaluated using whole-mount dissection in animals exposed either to a 114 dB, 16 kHz pure-tone for 1 hour or a 118 dB, 16 kHz pure-tone for 4 hours. Each of the cochleae used for these HC counts was co-labeled with DAPI and phalloidin, and each stain was used to count HCs separately in the co-labeled tissue.
When examining phalloidin based counts, animals exposed to a 114 dB, 16 kHz pure-tone for 1 hour had only one region of significant IHC loss in the high frequencies (Fig. 5A) and three regions of OHC loss in the mid and high frequencies (Fig. 5B). Corresponding DAPI counts were similar, however, three regions of IHC loss were seen in low and high frequencies (Fig. 5C) and three regions of OHC loss were seen in the low and high frequencies (Fig. 5D). For animals exposed to a 118 dB, 16 kHz pure-tone for 4 hours, the phalloidin based counts revealed 11 areas of significant IHC loss, seen predominantly in the mid to high frequency ranges, although minor low frequency damage was observed (Fig 5. E). Only seven total areas of significant OHC loss were observed in the low, mid and high frequency regions (Fig 5F). Corresponding DAPI based counts showed 12 regions of significant IHC loss (Fig. 5G) throughout the cochlea, and 6 regions of significant OHC loss, also throughout the cochlea (Fig. 5H). In all cases, damage was observed both above and below the 16 kHz frequency region.
3.6.3 Cross Method HC Loss Comparisons
The last comparison we made is shown in figure 6, which depicts the regions of statistically significant HC loss for the 114 dB, 16 kHz pure-tone 1 hour exposure and the 118 dB, 16 kHz pure-tone 4 hour exposure for both methods of tissue preparation. When the cochleograms for the same sound exposure were compared, the regions of significant HC loss were found to be similar, but not identical (Fig. 6A–D and E–H). The milder, 114 dB, 1 hour exposure resulted in regions of statistically significant HC loss that were similar for each method of tissue preparation (Fig. 6A and C, B and D). The regions of significant HC loss were minimal, patchy, and affected an equal number of IHC and OHC regions. However, HC counts in plastic sections revealed slightly fewer affected regions than whole mount dissections.
Figure 6. Comparisons of HC loss profiles as determined by both methods of tissue preparation.
Each point represents the mean number of HCs ± SEM for a given frequency range, and frequency ranges with statistically significant HC loss are highlighted in yellow (p<0.05 ). Significant hair cell loss resulting from exposure to a 114 dB, 16 kHz pure-tone for 1 hour is minimal as determined by either method of tissue preparation (panels A–B and C–D). Hair cell loss resulting from exposure to a 118 dB, 16 kHz pure-tone for 1 hour is more severe as determined by either method of tissue preparation (panels E–F and G–H). The HC counts derived from plastic sections (E–F) show more high frequency regions of HC loss and fewer mid frequency regions of HC loss than HC counts derived from whole mount dissected tissue (G–H).
A more dramatic difference between the HC counts generated by the two methods of tissue preparation was seen for the more severe, 118 dB, 4 hour exposure. The whole-mount dissection method of tissue preparation revealed more regions of significant HC loss in the low to mid frequency regions, but less damage in the highest frequency regions compared to counts in plastic embedded tissue. Whole-mount dissection does not show the more extensive high-frequency HC loss seen in plastic sections. Surprisingly, both methods revealed more IHC loss than OHC.
4. Discussion
The data presented address several aspects of hair cell quantification. We will first discuss the reliability of the data set as determined through internal and external measures as well as comparability and suggested applications of the two methods of tissue preparation. Next, we will compare the patterns of HC loss we observed in sound exposed animals to that observed by other investigators.
4.1 Reliability
Although hair cell loss following acoustic trauma is an objective phenomenon that results in an absolute number of lost HCs, its quantification is subjective and dependent, in part, on the rater evaluating it. Our internal comparisons consisted of intra-class correlations, which compared two sets of HC counts to one another to determine the extent to which the two data sets were in absolute agreement. The ICC coefficients for all inter-rater comparisons returned values ≥ 0.7, irrespective of the method of tissue preparation and HC stain, which suggests strong agreement between the two raters. Additionally, our intra-rater comparisons yielded ICC coefficients ≥ 0.9 indicating that the rater had a high degree of internal consistency when assessing hair cell loss. To lend further support to the reliability of our data we compared our HC counts to those of Keithley and Feldman (1982), who reported an average of 4700 HCs, occurring in a ratio of 3470 OHC: 960 IHC in age-matched, unexposed Sprague-Dawley rats. These values are highly similar to our data for control animals (Table 1). Thus internal analysis and external reporting support our conclusion that our full data set is both reliable and consistent, irrespective of tissue preparation, stain, and HC type.
4.2 Methods comparisons
Multiple tissue preparation methods exist for examining the cochlear sensory epithelium. The pros, cons, and limitations of each of these methods occur with respect to both identification of hair cells as well as the reconstruction methods used for creating a cytocochleogram [19]. In the present study, we applied both methods of tissue preparation to animals exposed to the same sound damage paradigms, enabling us to make direct comparisons of the resulting cytocochleograms. When examining tissue preparation methods we routinely compared two sets of HC count data within a given treatment group: (1) phalloidin staining and DAPI staining in whole mount dissection, and (2) toluidine blue stained nuclei from plastic sections and DAPI stained nuclei in whole-mount dissections. These within-group comparisons seek to identify differences in cytocochleograms that are attributable to the method of tissue preparation, in addition to our ultimate goal of determining regions of hair cell loss due to sound damage.
The absolute number of hair cells in control animals, as determined by either method of tissue preparation we employed, approximated the findings of Keithley and Feldman’s series of age-graded HC counts [18], and the ratio of OHC:IHC is between ~3.5–3.8, as expected. An initial comparison of the total number of nuclei-based HCs from control animals (Table 1) returned statistically significant differences between IHC, OHC, and total number of HCs, with,. counts from plastic sections consistently higher then analogous counts in whole mount dissections. We believe this discrepancy is due to the bias inherent in profile counts, particularly here where nuclei were counted in adjacent serial sections. Since we were not concerned as much with absolute HC numbers as we were with relative changes in HC number, we did not employ correction factors[20]. We did not employ stereology-based counts[21] due to our desire to subdivide the cochlea into small regions for determining frequency-specific hair cell loss.
In noise-exposed animals, no statistically significant differences were found when comparing DAPI and phalloidin based total HC counts in co-labeled whole mount dissections for any treatment group. This result was not unexpected, as the two sets of counts were taken from the same animal. No statistically significant differences in total HC counts were observed in noise exposed animals, most likely due to the higher inter-animal variability introduced by noise damage.
The more detailed between-method comparison of nuclei based HC counts seen in Figure 3, allows us to view the distribution of the HCs across frequency regions. Both data sets were transformed for this comparison due to residual artifacts in the reconstruction process used for plastic sections (S. Fig 2). The need to transform data prior to comparison further suggests the cytocochleograms generated using each method of tissue preparation might not be entirely comparable. In this detailed analysis, no statistically significant differences were observed when the control groups for both methods of tissue-preparation were compared (Fig. 3 A and B). The regions of significant differences were few for animals exposed to a 114 dB, 16 kHz pure-tone for 1 hour (Fig. 3C and D), while the number of differences further increased when examining the results of the 118 dB, 16 kHz pure-tone exposures for 4 hours (Fig. 3E and F). The increasing number of regions of difference could be attributable to either the variable response of rats to sound trauma, or the differences in the reconstruction methods used for the two methods of tissue preparation. The lack of differences when control groups were compared may suggest the reconstruction methods were similar and did not skew the distributions of HCs across frequency regions.
In Figure 2 we compare our two HC markers (DAPI and phalloidin) in whole-mount dissected tissue. These comparisons revealed only two areas of statistical difference (Fig. 2C) in the IHC counts of animals exposed to a 114 dB, 16 kHz pure-tone for 1 hour. Examination of individual animals’ patterns of HC loss in this group (data not shown) suggest these differences are directly attributable to an atypical pattern of HC loss seen in a few frequencies regions in one single animal. Thus, a severe response from a single animal in our small sample directly affected the statistical analysis. Following exposure to the same sound, this degree of inter-animal variability is not unexpected [9, 10], so we chose not to exclude the animal from our analysis. We might not expect to observe these significant differences in a larger sample. Given the possible variability in an individual animal’s response to sound damage, we expected the differences based on tissue preparation to be the most evident when comparing plastic sections and whole-mount dissections from sound exposed animals.
Given the results of our between-method comparisons, we recommend utilizing the whole-mount dissection method of tissue preparation for assessment of HC survival or quantification of some HC features (e.g., ribbon synapses) visible with confocal microscopy[11], This dissection is more technically demanding, but the relative simplicity and speed of cell counting and reconstruction are appreciated. Additionally, immunohistochemistry can be more easily performed on dissected tissue than on plastic embedded sections. However, if a study seeks to quantify the effects of sound exposure on non-HC structures within the cochlea, it may be more reasonable to pursue an embedding and sectioning method of tissue preparation.
4.3 Hair Cell Loss
The sound damage paradigms used to induce hearing loss and other auditory disorders in animal models vary greatly, as do the patterns of HC loss that result from exposure to different sound damage paradigms. The intensity, frequency, and duration of the damaging stimulus all contribute to the extent and pattern of cochlear damage. This array of possible parameter combinations, along with animal species, complicates comparisons of results across studies. Figures 4–6 identify frequency regions of sound exposed cochleae with statistically significant HC loss. Each figure contains comparisons within a given method of tissue-preparation, and no direct comparisons are made across tissue preparation methods.
4.3.1. Frequency regions containing significant HC loss
We observed that exposure to a shorter duration (1 hour) lower intensity (114 dB) stimulus resulted in markedly less severe HC loss (Fig. 4A–B and Fig. 5A–D) than a longer duration (4 hour) higher intensity (118 dB) stimulus (Fig. 4 G–H and Fig. 5E–H). This finding held true when comparing HC loss evaluated with either tissue preparation method (Fig. 6). Somewhat surprisingly, we observed in both cases, that HC loss was seen throughout the cochlea and was not restricted to a specific frequency region at or near the 16 kHz frequency of the damaging tone. Given the use of sound damage to elucidate the tonotopic map in some species (e.g. Ryals and Rubel, 1982 in chick)[22], it is commonly believed that hair cell loss that occurs as result of exposure to a pure-tone or narrowband stimulus will result in a discrete band of HC loss. Despite this common expectation, one of our most consistent observations is that HC loss occurs in low, mid, and high frequency regions of cochlea, irrespective of the sound damage paradigm and method of tissue preparation utilized (Figs. 4–6).
Under specific conditions, sound-induced cochlear damage can occur in frequency specific regions of the cochlea. The dendritic terminals that make contact with IHC have been shown to swell in regions with CFs greater than or equal to the frequency of the damaging stimulus[2, 23]. Stereocilia can also be immediately damaged in specific frequency regions, although studies on this topic are in conflict showing exclusively IHC[24], exclusively OHC[25], or a mixture of IHCs and OHCs[6] being damaged in the area of maximal basilar membrane displacement. Lateral wall pathology has also been observed in discrete regions as a result of acoustic trauma[15]. Much of this damage is limited to the temporary threshold shift window before secondary damage and repair processes have begun[1]. These processes contribute to additional neuronal and HC damage and loss. Ultimately, the damage observed during temporary threshold shift is not correlated with PTS and HC loss[26]. Therefore these examples of frequency specific damage would not lead us to expect frequency specific damage from our sound damage paradigms.
Bohne and Harding have shown that if the parameters of an acoustic overexposure fall within a certain range, HC loss can occur in discrete region of the cochlea[1]. This observation was made in a chinchilla model of sound exposure that utilized a 4 kHz octave band, 86 dB, 2-day sound damage paradigm. Inner HC lesions were more narrowly restricted to the ~8 kHz region, and this region was flanked by more severe OHC loss. These initial findings are supported by a 2009 meta-analysis that showed a similar pattern of cochlear lesions can occur if both the sound exposure intensity and duration are low to moderate. The parameters of the four sound damage paradigms presented in this paper far exceed these criteria for causing discrete cochlear HC lesions[27], so we would not expect to observe a discrete band of HC loss.
4.3.2 Duration of Sound Exposure
All four of the 16 kHz pure-tone sound exposures (114 dB, 1 hour; 114 dB, 2 hours; 118 dB, 1 hour; and 118 dB, 4 hours) were analyzed for HC loss using plastic sections (Fig. 4). We observed that increases in exposure duration, while holding stimulus intensity constant, resulted in more severe IHC and OHC loss (fig 4 A–D and EH) than the analogous short duration exposures. More specifically, all regions of HC loss observed in the one-hour exposures were also observed in the longer duration sound exposures in addition to new regions of HC loss (e.g. Fig. 4A and 4C).
Mulders et. al used a guinea pig model of noise-induced tinnitus and generated HC counts from animals exposed to 10 kHz, 124 dB sound for 1 or 2 hours [8]. Their findings show HC loss exclusively at or above the frequency of the damaging stimulus with IHC and OHC similarly affected. Increases in exposure duration did not result in increased HC loss. We suspect a difference in species might underlie these contrasting findings. Bauer and Brozoski exposed Long-Evans rats to narrowband noise centered at 16 kHz, 105 dB, for either 1 or 2 hours[28]. The animal species, as well as the the frequency and duration of this sound exposure closely match our study, although the intensity of our exposures is more than double what Bauer and Brozoski utilized. Additionally, they allowed for a 5-month recovery period prior to sacrifice, while we allowed for a 2-4 week post-exposure recovery period. Their HC counts revealed nearly complete OHC loss throughout the cochlea. Inner HC loss either sloped from ~20% – 50% (1 hour) or ~20% – 25% (2 hour) distance from the apex with the remainder of the basal portion of the cochlear having complete IHC loss. These unexpectedly severe patterns of IHC and OHC are substantially different from our findings, where we show scattered loss of IHC and OHC throughout the cochlea with neither HC type approaching complete loss in any given frequency region. The difference in severity may be, in part, due to the difference in recovery periods, which would allow for more secondary HC death and damage to occur in their animal model. Lastly, Harding and Bohne conducted two meta-analyses using a large sample of noise-exposed chinchilla (n >400) [27, 29]. These two studies suggest acoustic trauma above and below a critical threshold produce distinct patterns of HC loss, and that basal and apical regions of the cochlea are affected differently. Hair cell loss in above critical level exposures is likely to begin as distinct focal lesions, which then expand, over time, and grow in size before merging with neighboring lesions. Bohne and Harding’s analyses suggest specific values for these thresholds in chinchilla, but caution that critical levels will vary as a function of species. Conceptually their findings support our observations, but the species difference (chinchilla vs. rat) makes us cautious in regard to extrapolating their findings to our rat model. Had we included additional sound damage paradigms of intermediate durations a more precise picture of the expansion and merging of HC lesions could be observed. Available data and studies regarding the effects of exposure duration on HC loss in rats are varied, with no clear consensus. The data we present add a new contribution to this discussion, and lend support to the concepts put forth by Bohne and Harding in their chinchilla model of noise exposure.
4.3.3. IHC v OHC susceptibility to sound damage
Outer hair cells are typically regarded as being more susceptible to sound damage than inner hair cells, particularly in regions with higher characteristic frequencies than the damaging stimulus[6, 30]. Effects of sound trauma on IHC and OHC stereocilia are conflicting concerning IHC and OHC stereocilia susceptibility to acoustic trauma, although more studies seem to suggest OHCs may be more easily damaged. Excitotoxic damage caused by overstimulation during acoustic trauma more severely affects IHC than OHC[31], which may result in either HC damage and loss or loss of afferent synapses. Comparing cytocochleograms from various studies is also a useful, practical approach to comparing the relative susceptibility of IHC and OHC to noise. In the noise-exposed cochlea examined in this study we observed roughly equivalent regions of statistically significant OHC and IHC loss (Fig. 6), and in our most severe sound damage paradigm, IHC loss exceeded OHC loss (Fig. 6 E–H). Our findings suggest IHCs are no more susceptible or resistant to noise-induced damage than OHCs. We believe our findings follow a trend observed in other studies[28] with roughly comparable sound damage paradigms. When sound exposures become more severe and HC damage and loss becomes more extensive it is less likely to occur in a clearly ordered fashion preferentially damages OHC prior to IHC.
4.3.4 Hair Cell loss as determined by both methods of tissue preparation
Figure 6 enables direct comparison of the cytocochleograms generated using both methods of tissue preparation. For both sound exposures we observed that the two methods of tissue preparation identified slightly different areas of statistically significant HC loss, but they did show similar degrees of HC loss through throughout the cochlea. In the 114 dB, 1 hour exposure we see minimal hair cell loss scattered throughout the cochlea, while in the 118 dB, 4 hour exposure we observe more extensive hair cell loss throughout the length of the cochlea. Even though the specific statistically significant regions differ somewhat across methods, both still suggested IHC and OHC were lost in roughly equal proportions. Ultimately the two methods show functionally similar regions of hair cell loss.
5. Conclusion
In this study we have utilized 4 sound damage paradigms and two methods of tissue preparation to determine what patterns of HC loss occur and whether the method of tissue preparation used biases the resulting cytocochleograms. We found that both methods of tissue preparation produce comparable, but not identical, cytocochleograms. With short duration exposure to a 114dB pure tone, very few regions exhibit hair cell loss and the exact location of damage varies between methods. However, with a 118dB 4 hour exposure, hair cell loss is observed along most of the length of the cochlea with either method of tissue preparation. Any differences in the regions identified as having significant hair cell loss are not substantial enough to alter any conclusions we may draw regarding how peripheral damage will influence the central nervous system. The differences we do observe may be partially attributable to smaller sample sizes combined with variable animal responses to acoustic trauma. To assess damage to hair cells themselves we strongly recommend a whole-mount cochlear preparation. Assessment can be done more rapidly and reconstruction is simpler. In instances where non hair cell features are under examination, an embedding and sectioning approach may be warranted.
Using either method, hair cell loss was seen throughout the length of the cochlea and was not limited to specific frequency ranges related to the damaging stimulus. Inner HCs and OHCs were lost in nearly equal proportions for all sound exposures, suggesting equal susceptibility to damage. Lastly, we also observed that HC lesions observed in short duration exposures were seen in the analogous longer duration exposures, suggesting that areas of damage expanded and merged with a longer duration exposure.
Supplementary Material
The table, left, shows the descriptive information for each of the 9 animals used for whole mount dissections that were counted by both raters. The two right-most columns display the intra-class correlation coefficients. Values ≥ 0.7 indicate a strong degree of absolute agreement between the counts of the two raters. The graphs in A and B show the HC counts as a function of frequency from rater 1 and rater 2 for phalloidin stained tissue in a single animal exposed to a 118 dB, 16 kHz pure-tone for 4 hours..
Panels A–E and F–J illustrate the reconstruction process required for plastic sections for both IHC and OHC, respectively. The raw counts, A and F, contain periodic spikes in HC number. These spikes correspond to the non-modiolar regions of the cochlea as seen in Fig. 1 D and H. As part of the reconstruction process (B and G) these long regions of HCs were subdivided into multiple shorter lengths prior to dividing the cochlea into frequency regions. The results of the reconstruction process are shown in C and H, and the dramatic spiking pattern is noticeably lessened. The reconstruction process (G) was not able to fully remove the spike-like artifacts for OHCs. We sought to directly compare the distribution of HCs across frequency regions as determined by both tissue preparation methods; however, the data set required an additional transformation due to the remaining artifacts seen the cochleogram for OHCs (H). A log transformation (D and I) was performed to further reduce the effects of the artifacts in the raw HC count data. The resulting log transformation of HC number is seen in E and J.
The table, left, shows the descriptive information for each of the 3 animals used for blinded recounts from whole mount dissections. The two right-most columns display the intra-class correlation coefficients. The table on the right shows the analogous information for HC counts derived from plastic sections. Values ≥ 0.9 indicate a very strong degree of absolute agreement between the initial counts and recounts by this rater.
Four 16 kHz sound exposures are utilized of varying intensities and durations
Cochlear hair cells are counted using plastic sections or whole mount dissections
Hair cell loss was observed throughout the cochlea
Increases in exposure duration led to more severe hair cell loss
HC loss in short duration exposures is observed in the related long duration exposure
Acknowledgments
We would like to thank the Kansas Intellectual and Development Disabilities Research Center (NICHD HD02528), Department of Defense Grant # CDMRP PR08124, and the Madison and Lila Self Graduate Fellowship at the University of Kansas for their funding and support.
Abbreviations Used
- NIHL
Noise induced hearing loss
- HC
Hair Cell
- PBS
Phosphate buffered saline
- ICC
Intra-class correlation
- IHC
Inner hair cell
- OHC
Outer hair cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21(4):505–9. [PubMed] [Google Scholar]
- 2.Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1971;71(2):166–76. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- 3.Gold JR, Bajo VM. Insult-induced adaptive plasticity of the auditory system. Front Neurosci. 2014;8:110. doi: 10.3389/fnins.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen GD, Fechter LD. The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res. 2003;177(1–2):81–90. doi: 10.1016/s0378-5955(02)00802-x. [DOI] [PubMed] [Google Scholar]
- 5.Hamernik RP, et al. The quantitative relation between sensory cell loss and hearing thresholds. Hear Res. 1989;38(3):199–211. doi: 10.1016/0378-5955(89)90065-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen YS, et al. Changes of hair cell stereocilia and threshold shift after acoustic trauma in guinea pigs: comparison between inner and outer hair cells. ORL J Otorhinolaryngol Relat Spec. 2003;65(5):266–74. doi: 10.1159/000075224. [DOI] [PubMed] [Google Scholar]
- 7.Knipper M, et al. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Mulders WH, et al. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J Comp Neurol. 2011;519(13):2637–47. doi: 10.1002/cne.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence M, Yantis PA. Individual differences in functional recovery and structural repair following overstimulation of the guinea pig ear. Ann Otol Rhinol Laryngol. 1957;66(3):595–621. doi: 10.1177/000348945706600301. [DOI] [PubMed] [Google Scholar]
- 10.Cody AR, Robertson D. Variability of noise-induced damage in the guinea pig cochlea: electrophysiological and morphological correlates after strictly controlled exposures. Hear Res. 1983;9(1):55–70. doi: 10.1016/0378-5955(83)90134-x. [DOI] [PubMed] [Google Scholar]
- 11.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–85. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohne BA. Location of small cochlear lesions by phase contrast microscopy prior to thin sectioning. Laryngoscope. 1972;82(1):1–16. doi: 10.1002/lary.5540820101. [DOI] [PubMed] [Google Scholar]
- 13.Akil O, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75(2):283–93. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guild SR. Correlations of Histologic Observations and the Acuity of Hearing. Acta Otolaryngol. 1932;1(7):207–249. [Google Scholar]
- 15.Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4(3):339–52. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490(4):391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood DD. Comparing octaves, frequency ranges, and cochlear-map curvature across species. Hear Res. 1996;94(1–2):157–62. doi: 10.1016/0378-5955(95)00229-4. [DOI] [PubMed] [Google Scholar]
- 18.Keithley EM, Feldman ML. Hair cell counts in an age-graded series of rat cochleas. Hear Res. 1982;8(3):249–62. doi: 10.1016/0378-5955(82)90017-x. [DOI] [PubMed] [Google Scholar]
- 19.Saunders JC, Dear SP, Schneider ME. The anatomical consequences of acoustic injury: A review and tutorial. J Acoust Soc Am. 1985;78(3):833–60. doi: 10.1121/1.392915. [DOI] [PubMed] [Google Scholar]
- 20.Abercrombie M, Johnson ML. Quantitative histology of Wallerian degeneration: I. Nuclear population in rabbit sciatic nerve. J Anat. 1946;80(Pt 1):37–50. [PubMed] [Google Scholar]
- 21.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryals BM, Rubel EW. Patterns of hair cell loss in chick basilar papilla after intense auditory stimulation. Frequency organization. Acta Otolaryngol. 1982;93(3–4):205–10. doi: 10.3109/00016488209130873. [DOI] [PubMed] [Google Scholar]
- 23.Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear Res. 1983;9(3):263–78. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- 24.Canlon B, et al. Pure tone overstimulation changes the micromechanical properties of the inner hair cell stereocilia. Hear Res. 1987;30(1):65–72. doi: 10.1016/0378-5955(87)90184-5. [DOI] [PubMed] [Google Scholar]
- 25.Gao WY, et al. A comparison of changes in the stereocilia between temporary and permanent hearing losses in acoustic trauma. Hear Res. 1992;62(1):27–41. doi: 10.1016/0378-5955(92)90200-7. [DOI] [PubMed] [Google Scholar]
- 26.Ward WD. Proceedings of the International Congress on Noise as Public Health Problem. 1973. Susceptibility to TTS and PTS; pp. 281–292. Publication No. 550/9-73-008. [Google Scholar]
- 27.Harding GW, Bohne BA. Relation of focal hair-cell lesions to noise-exposure parameters from a 4- or a 0.5-kHz octave band of noise. Hear Res. 2009;254(1–2):54–63. doi: 10.1016/j.heares.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J Assoc Res Otolaryngol. 2001;2(1):54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harding GW, Bohne BA. Noise-induced hair-cell loss and total exposure energy: analysis of a large data set. J Acoust Soc Am. 2004;115(5 Pt 1):2207–20. doi: 10.1121/1.1689961. [DOI] [PubMed] [Google Scholar]
- 30.Sha SH, et al. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155(1–2):1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 31.Amarjargal N, et al. Differential vulnerability of outer and inner hair cells during and after oxygen-glucose deprivation in organotypic cultures of newborn rats. Physiol Res. 2009;58(6):895–902. doi: 10.33549/physiolres.931466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The table, left, shows the descriptive information for each of the 9 animals used for whole mount dissections that were counted by both raters. The two right-most columns display the intra-class correlation coefficients. Values ≥ 0.7 indicate a strong degree of absolute agreement between the counts of the two raters. The graphs in A and B show the HC counts as a function of frequency from rater 1 and rater 2 for phalloidin stained tissue in a single animal exposed to a 118 dB, 16 kHz pure-tone for 4 hours..
Panels A–E and F–J illustrate the reconstruction process required for plastic sections for both IHC and OHC, respectively. The raw counts, A and F, contain periodic spikes in HC number. These spikes correspond to the non-modiolar regions of the cochlea as seen in Fig. 1 D and H. As part of the reconstruction process (B and G) these long regions of HCs were subdivided into multiple shorter lengths prior to dividing the cochlea into frequency regions. The results of the reconstruction process are shown in C and H, and the dramatic spiking pattern is noticeably lessened. The reconstruction process (G) was not able to fully remove the spike-like artifacts for OHCs. We sought to directly compare the distribution of HCs across frequency regions as determined by both tissue preparation methods; however, the data set required an additional transformation due to the remaining artifacts seen the cochleogram for OHCs (H). A log transformation (D and I) was performed to further reduce the effects of the artifacts in the raw HC count data. The resulting log transformation of HC number is seen in E and J.
The table, left, shows the descriptive information for each of the 3 animals used for blinded recounts from whole mount dissections. The two right-most columns display the intra-class correlation coefficients. The table on the right shows the analogous information for HC counts derived from plastic sections. Values ≥ 0.9 indicate a very strong degree of absolute agreement between the initial counts and recounts by this rater.