Abstract
Background
The relationship between arterial stiffness and kidney function has not been clearly demonstrated although observations of higher arterial stiffness in patients with advanced stages of chronic kidney disease (CKD) were reported. In longitudinal analyses, there was no close association between basal arterial stiffness and progression of kidney function in the general population. In the present study, we assessed the relationship between arterial stiffness and progression of renal dysfunction in patients with CKD stages 3-5 using two types of measures of arterial stiffness, i.e., carotid-femoral pulse wave velocity (cfPWV) and brachial-ankle pulse wave velocity (baPWV), over a 10-year period.
Methods
110 patients with CKD stages 3-5 (aged 57.8 ± 10.6 years; female/male: 72/38) were followed for 10 years. Before and at the end of the 10-year period, cfPWV and baPWV were measured using form PWV/ABI (Omron Colin Co. Ltd.).
Results
Throughout the study, systolic blood pressure was well-controlled in all patients. Twenty-nine patients (26%) received renal replacement therapy, 12 patients (11%) developed cardiovascular diseases (CVDs), 5 patients were found to have neoplasm, and 9 patients dropped out of the study during the 10-year observation period. In patients who developed end-stage renal disease, the baseline estimated glomerular filtration rate (eGFR) was significantly lower, and in patients who developed CVD, the basal value of baPWV was significantly higher (p < 0.05). Throughout the study, blood pressures were controlled (136.1/77.0 ± 15.6/7.1 to 137.5/77.6 ± 14.9/11.2), kidney function worsened (eGFR, 30.8 ± 16.5 to 22.9 ± 17.6 ml/min/1.73 m2; p < 0.01), and baPWV but not cfPWV showed a significant change [1, 672.2 ± 209.6 vs. 1,753.1 ± 333.2 cm/s (p = 0.04) and 918.9 ± 153.2 vs. 939.4 ± 133.2 cm/s]. Moreover, the difference in PWV between the start and the end of the 10-year observation period was positively correlated with the difference in eGFR.
Conclusion
With moderate progression of renal dysfunction and under well-controlled blood pressure, peripheral but not central arterial stiffness is possibly one of the strongest predictors of CVD in patients with CKD stages 3-5.
Key Words: Estimated glomerular filtration rate, Blood pressure, Pulse wave velocity
Introduction
Risk factors for the progression of renal dysfunction over the long term have been discussed. However, it remains uncertain what factors play important roles.
Previously, blood pressure (BP) control was considered to be most important [1] and antihypertensive drugs have been aggressively used for the control of BP. Among the antihypertensive drugs, renin-angiotensin inhibitors are recommended as the first-line therapy for hypertension in patients with chronic kidney disease (CKD) [2].
In addition to a decline in renal function, the risk of cardiovascular events increased as the glomerular filtration rate (GFR) decreased in a large, community-based population [3]. Considerable data are available on the association between arterial stiffness and decline in renal function [4,5]. Furthermore, arterial stiffness increases progressively with decline in renal function [6,7]. Carotid-femoral pulse wave velocity (cfPWV), a measure of aortic stiffness, and brachial-ankle pulse wave velocity (baPWV), a measure of both central and peripheral arterial stiffness, are associated with increased cardiovascular mortality and have been identified as potential nontraditional risk factors for CKD [6,8,9,10]. At present, the association between arterial stiffness and decline in renal function still remains controversial, probably because the data were obtained from a cross-sectional cohort study. Several studies have reported that in patients with early CKD, increased arterial stiffness was strongly associated with decline in GFR [11,12,13]. On the other hand, other studies have reported that PWV is not associated with decline in GFR in patients with early CKD [14,15,16,17]. In the present study, we examined the longitudinal relationship between the change in renal function and the change in cfPWV or baPWV in patients with nondiabetic CKD stages 3, 4 and 5 excluding dialysis.
Subjects and Methods
This was a prospective, observational, single-center cohort study, and was conducted in accordance with the Declaration of Helsinki. Approval for the study was obtained from the Saitama Medical University Ethics Committee, and written informed consent was obtained from each participant.
Patients were recruited from specialist renal clinics at the Renal Disease Center, Saitama Medical University, from October 2002 to September 2003. All participating patients were followed for 10 years or until death, the commencement of dialysis therapy or renal transplantation, the detection of neoplasm or the occurrence of a cardiovascular event (fatal or nonfatal myocardial infarction, cerebrovascular disease or aortic dissection).
Patients with the following inclusion criteria were entered in the study: having CKD as defined by the K/DOQI guidelines [2], not yet on dialysis, having had stable renal function within the last 3 months (<5 ml/min/1.73 m2 change in GFR), and no change in medication in the preceding 3 months.
Exclusion criteria were diabetes mellitus, atrial fibrillation, known left ventricular dysfunction (ejection fraction <55%), signs and symptoms of congestive heart failure, pregnancy or lactation, significant valvular or coronary heart disease, cardiac arrhythmia or conduction defects, systemic diseases, proteinuria in the nephrotic range (<3.0 g/day), and use of sedative or hypnotic drugs or any other drugs potentially affecting blood pressure during ambulatory monitoring (e.g. corticosteroids).
Cause of Renal Impairment
The cause of CKD was assessed by reviewing the clinical history and investigations. Patients were classified as ‘hypertensive/glomerulosclerosis’ if they had no clear evidence of active renal disease but had a history of hypertensive diseases and positive urinary protein excretion without casts.
The underlying etiologies of CKD in our study patients included chronic glomerulonephritis (61 patients, 55%), hypertensive nephrosclerosis (26 patients, 24%), and other diseases (23 patients, 21%).
All patients were treated with antihypertensive drugs including renin-angiotensin inhibitors, and BP was well-controlled in all patients.
BP Measurements
BP was not measured in the clinic, and the patients were given instructions on how to measure and record their own BP at home. BP measurements were recorded at least twice a week at home in the sitting position – once in the morning before breakfast within 30 min of awakening, and once in the evening just before dinner [11]. Home BP measurements were made using the HEM 401C (Omron Life Science Co. Ltd., Tokyo, Japan), a semi-automatic device that operates on the cuff-oscillometric principle and generates a digital display of systolic and diastolic BP and pulse rate [12]. Usually, 20 recorded values of both systolic and diastolic BP were averaged as monthly BP; target home BP was 130/85 mm Hg or lower and home BP measurements were encouraged.
Measures of PWV
PWV was measured using an automatic waveform analyzer (form PWV/ABI; Omron Colin, Co. Ltd., Komaki, Japan). All individuals were examined after having rested in the supine position for at least 5 min. The methods for PWV measurements were described elsewhere [13,14].
Laboratory Measurements
Serum creatinine, 24-hour urinary excretion of creatinine and protein, and hematologic and serum tests, including urea, uric acid, blood urea nitrogen and electrolytes among others, were obtained at the beginning of the observation period, during every month of follow-up and at the end of the observation period.
Estimated GFR (eGFR) was calculated using a modified three-variable equation for eGFR in Japanese patients: eGFR = 194 × age-0.287 × sCr-1.094 (× 0.739, if female), where sCr = serum creatinine [15].
Total Care of CKD Patients
The selection of an antihypertensive agent depended on physician preference. All patients were treated with antihypertensive drugs including renin-angiotensin inhibitors, and BP was well-controlled in all patients [1,16].
Subjects were treated with recombinant human erythropoietin as necessary, and their hemoglobin levels were maintained between 11 and 12 g/dl [17]. They were given oral iron supplementation if diagnosed with iron deficiency.
Subjects with parathyroid hormone levels greater than 300 pg/ml were treated with 1,25(OH)2D3 and CaCO3 supplements, while patients with levels lower than 70 pg/ml were treated with CaCO3 to reduce the degree of hyperphosphatemia. Doses were adjusted based on serum levels of calcium and phosphate. Lipid-lowering drugs, primarily statin derivatives, were administered if serum cholesterol levels exceeded 240 mg/dl [18].
Statistical Analysis
Statistical analyses were performed using JMP software, version 9 (JMP, a business unit of SAS, Cary, N.C. USA). Correlations between various characteristics were determined using Pearson's correlation test. p < 0.05 was considered significant. Patient event-free curves were calculated by the Kaplan-Meier life table analysis method, and differences between the groups were evaluated by the log rank test.
Univariate analysis for decline in renal function as evaluated by eGFR and for increase in PWV was used to assess the relationship between the difference in eGFR or the increase in PWV and selected variables. Values are given as means ± SD. The significance of the differences in the data of laboratory and tonometry variables between the baseline values and those after 10 years was assessed by paired t tests. p values <0.05 were regarded as statistically significant.
Results
One hundred and ten patients with CKD stages 3-5 were enrolled in this study. Their baseline characteristics are summarized in table 1.
Table 1.
Baseline characteristics of the patients with CKD stages 3-5 (n = 110)
| Age, years | 57.8 ± 10.6 |
| Gender (male/female) | 72/38 |
| eGFR, ml/min/1.73 m2 | 25.5 ± 17.5 |
| Systolic BP, mm Hg | 139.6 ± 15.6 |
| Diastolic BP, mm Hg | 82.6 ± 7.1 |
| Heart rate, beats/min | 72.3 ± 8.6 |
| cfPWV, cm/s | 518.9 ± 153.2 |
| baPWV, cm/s | 1,672.2 ± 209.8 |
| Serum albumin, g/dl | 4.0 ± 0.4 |
| Hemoglobin, g/dl | 11.2 ± 0.9 |
| Total cholesterol, mg/dl | 201.2 ± 36.3 |
| Phosphate, mg/dl | 3.9 ± 1.9 |
| Calcium, mg/dl | 9.0 ± 1.0 |
| Urinary protein excretion, g/gCr | 1.23 ± 0.92 |
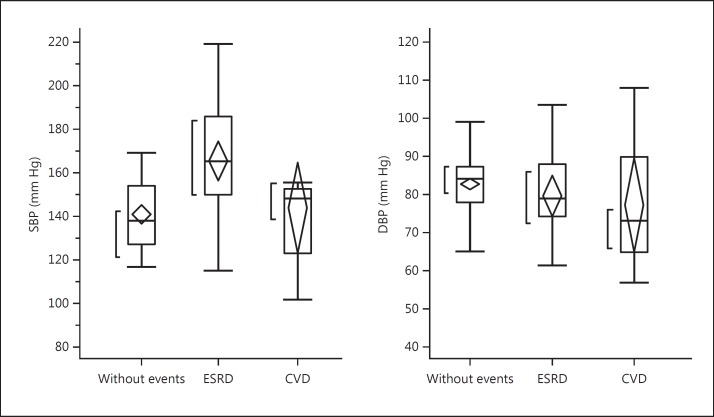
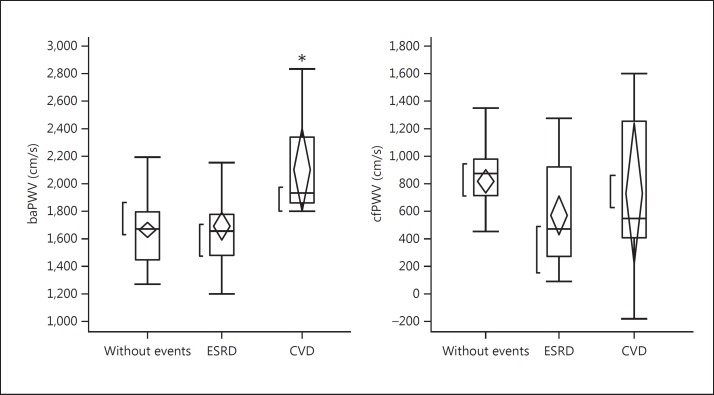
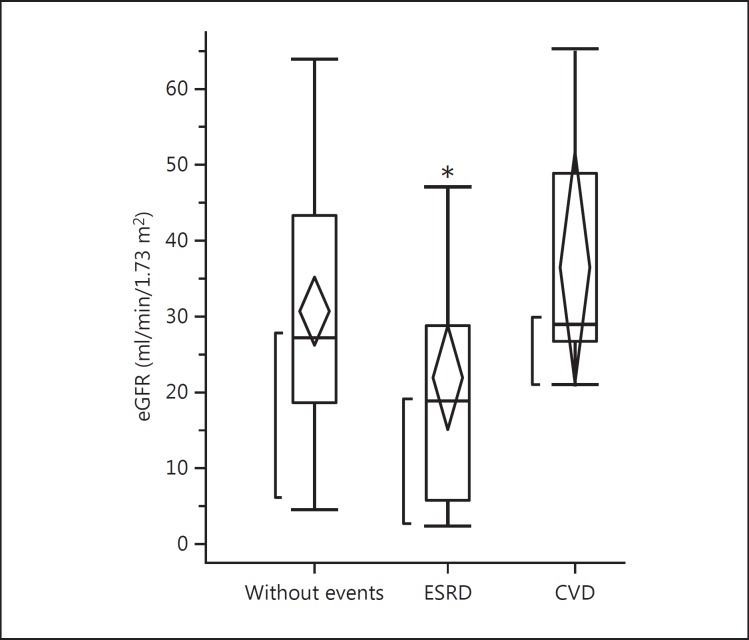
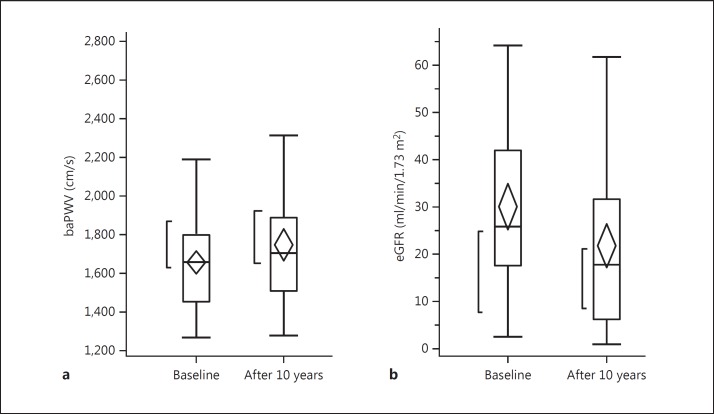
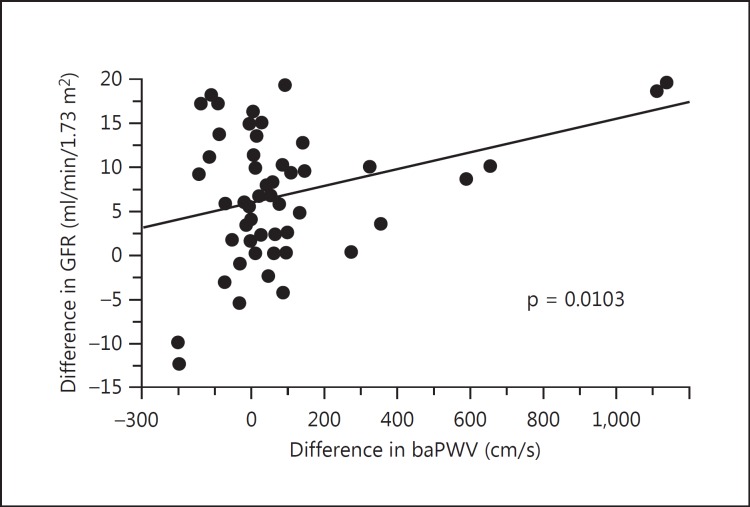
Twenty-nine patients (26%) received renal replacement therapy, 12 patients (11%) developed cardiovascular diseases (CVDs), 5 patients were found to have neoplasm, and 9 patients dropped out of the study during the 10-year observation period. The remaining 55 patients had no events and were observed over the 10-year period. The comparison of laboratory findings at baseline among 3 groups is shown in table 2. There were no significant differences in laboratory findings among these 3 groups. In figures 1, 2, 3, baseline hemodynamic and eGFR data of the 3 groups are compared. The baseline eGFR in the patients who developed end-stage renal disease (ESRD) was significantly lower, and the baseline baPWV in the patients who developed CVD was significantly higher. In tables 3 and 4, the hemodynamic and laboratory findings in the 55 patients who were followed for 10 years are shown. During the 10-year period, the hemodynamic data except baPWV and the laboratory findings except eGFR did not show any significant changes. However, the baPWV was significantly higher and the eGFR was significantly lower at the end of the 10-year observation period compared with the respective baseline values (p < 0.01 and p < 0.05, respectively; fig. 4a, b). There was a significant positive correlation between the difference in eGFR and the difference in baPWV over the 10-year observation period (p = 0.01; fig. 5).
Table 2.
Baseline laboratory findings
| Variables | Progression of renal dysfunction (n = 55) | ESRD (n = 29) | CVD (n = 17) |
|---|---|---|---|
| Serum albumin, g/dl | 4.1 ± 0.4 | 3.9 ± 0.8 | 4.1 ± 0.4 |
| Hemoglobin, g/dl | 11.4 ± 1.0 | 10.6 ± 1.5 | 11.4 ± 1.4 |
| Total cholesterol, mg/dl | 196.3 ± 35.5 | 187.2 ± 43.3 | 219.2 ± 42.7 |
| Phosphate, mg/dl | 3.9 ± 1.9 | 4.1 ± 1.8 | 3.8 ± 1.3 |
| Calcium, mg/dl | 9.0 ± 1.3 | 9.6 ± 1.2 | 9.2 ± 1.0 |
| Urinary protein excretion, g/gCr | 1.56 ± 0.82 | 1.78 ± 1.79 | 0.96 ± 0.66 |
Fig. 1.
Comparison of baseline values of systolic and diastolic BP among patients who had no events (n = 55), patients who developed ESRD (n = 29), and patients who had events (cardiovascular events or neoplasm) (n = 17)during the 10-year period. SBP = Systolic BP; DBP = diastolic BP. Abbreviations are the same in the following figures.
Fig. 2.
Comparison of baseline values of baPWV and cfPWV among patients who had no events (n = 55), patients who developed ESRD (n = 29), and patients who had events (cardiovascular events or neoplasm) (n = 17) during the 10-year period. baPWV was significantly lower in patients who had cardiovascular events than in the other two groups. There was no difference in cfPWV among the 3 groups. * p < 0.05.
Fig. 3.
Comparison of baseline values of eGFR among patients who had no events (n = 55), patients who developed ESRD (n = 29), and patients who had events (cardiovascular events or neoplasm) (n = 17) during the 10-year period. * p < 0.05.
Table 3.
Comparison of hemodynamic factors at the start and at the end of the 10-year observation period
| At the start (n = 55) | After 10 years (n = 55) | |
|---|---|---|
| Systolic BP | 136.1 ± 15.6 | 137.5 ± 14.9 |
| Diastolic BP | 77.0 ± 7.1 | 77.6 ± 11.2 |
| PP | 59.0 ± 12.8 | 59.4 ± 13.7 |
| Heart rate | 74.1 ± 10.9 | 75.6 ± 9.6 |
| cfPWV | 918.9 ± 153.2 | 939.4 ± 133.2 |
Data on the 55 patients who were observed over the entire 10-year study period are shown.
Table 4.
Comparison of laboratory findings at the start and at the end of the 10-year observation period
| At the start (n = 55) | After 10 years (n = 55) | |
|---|---|---|
| Serum albumin, g/dl | 4.1 ± 0.4 | 4.0 ± 0.5 |
| Hemoglobin, g/dl | 11.4 ± 1.0 | 11.2 ± 0.9 |
| Total cholesterol, mg/dl | 201.2 ± 36.3 | 180.4 ± 29.6 |
| Phosphate, mg/dl | 3.9 ± 1.9 | 3.6 ± 1.5 |
| Calcium, mg/dl | 9.0 ± 1.0 | 9.2 ± 0.7 |
| Urinary protein excretion, g/gCr | 1.56 ± 0.82 | 1.66 ± 0.97 |
Data on the 55 patients who were observed over the entire 10-year study period are shown.
Fig. 4.
Changes in baPWV (a) and eGFR (b). There was a significant change in eGFR and baPWV between the start and the end of the 10-year observation period (n = 55).
Fig. 5.
Correlation between the change in eGFR and the change in baPWV at the start and at the end of the 10-year observation.
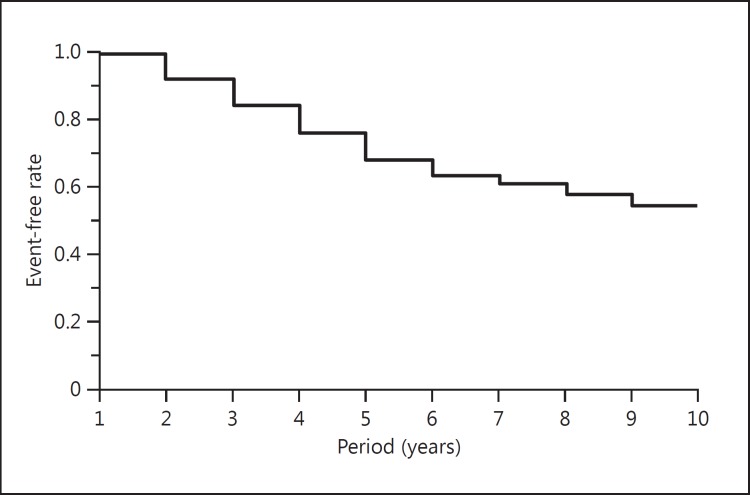
The Kaplan-Meier curve of the patients is shown in figure 6. The incidence-free rates at 5 and 10 years were 68 and 54%, respectively.
Fig. 6.
Kaplan-Meier event-free curve. The cumulative event-free rates at 5 years and 10 years were 68 and 54%, respectively.
Discussion
The present study provides the first direct evidence in CKD patients that measures of aortic stiffness are predictors of fatal and nonfatal CVD events, extending the available evidence from the dialysis population to CKD patients.
In this study, 25% of the participating patients developed ESRD during the 10-year observation period. At the start of the observation period, the baseline value of eGFR was significantly lower in the ESRD patients than in the other patients, indicating that the lower the eGFR, the more rapidly the patients developed ESRD. This is generally accepted by nephrologists. Decline in renal function has been evaluated by the surrogate endpoint of doubling of the serum creatinine concentration from the baseline level, or the ‘hard’ endpoint of death or the development of ESRD. The latter hard endpoint is usually determined by the starting time of renal replacement therapy. In the present study, the average baseline value of eGFR was 25.5 ml/min/1.73 m2. At this level of renal dysfunction, more than two thirds of patients are assumed to develop ESRD during the subsequent 5 years [19]. Compared with the generally accepted ratio for the development of ESRD, treatment at our clinic slowed the decline in renal function.
It has been controversial what factors determine or are associated with decline in renal function. Previously, Chue et al. [20] reported that serum phosphate, but not PWV, independently predicted decline in renal function in patients with early CKD. In contrast, Kim et al. [21] reported that pulse pressure (PP) was an independent risk factor for rapid decline in kidney function in populations with relatively preserved kidney function. In our present findings, the baseline level of eGFR was the sole determinant factor. Other variables related with hemodynamics such as systolic BP, PP, and PWV were not found to be determinant factors for decline in renal function. Ng et al. [22] have recently demonstrated that in CKD patients most of the variability in central PP could be explained by peripheral PP. Compared with the previous reports, our observation period was relatively longer. In the short term, several hemodynamic factors such as systolic BP, PP and PWV may play some roles; however, in the long term, since these factors are modulated by increases in doses and/or in the number of antihypertensive drugs, the effects of such hemodynamic factors would be cancelled.
It is well known that the morbidity and mortality of CVD in patients with CKD are high and the presence of CKD worsens the outcomes of CVD [23]; however, it still remains uncertain what factors predict the occurrence of CVD. CKD-associated risks for CVD such as dyslipidemia, systemic inflammation and vascular calcification have been proposed [3,24]. Among these factors, arterial stiffness is one of the strongest predictors of CVD in CKD patients [25,26,27]. In the present study, CKD patients who suffered from CVD during the 10-year observation period had a higher baseline PWV, indicating that PWV possibly predicts CVD event. Arterial stiffness increases progressively with the decline in renal function [6]. Based on the results of the present study, baPWV but not cfPWV might be an important predictor of CVD. Since it is generally acknowledged that cfPWV represents a measure of aortic stiffness and baPWV represents a measure of both central and peripheral arterial stiffness, central as well as peripheral arterial stiffness is associated with increased risk of CVD. This has a two-fold meaning: under well-controlled BP, PWV changes gradually, and total arterial stiffness might be a more important predictor of CVD than central aortic stiffness in CKD patients.
There are potential limitations in our study. Although prospective, it is observational and cross-sectional in design and therefore subject to potential residual confounding. Secondly, our study is limited by the small number of patients. Thirdly, this study was performed at a single renal center; thus, the generalizability of the findings to other populations, including other sites, is unknown. This might cancel the variable factors from other studies conducted at multiple centers.
Overall, during the 10-year observation period, 25% of the participating patients developed ESRD. This figure is not high compared with that observed in general clinical practice in Japan [28]. Also, 12 patients suffered from CVD during the 10-year period, indicating that BP control is most important for CKD patients [29]. Moreover, even under well-controlled BP, PWV should be monitored carefully.
Fourthly, we illustrated the Kaplan-Meier curve including all events: renal replacement therapy, CVDs and neoplasm. In Japan, the causes of death due to CVD are less compared with those of neoplasm. There is only 15% of death due to CVD. Besides, this holds true in CKD patients.
In the present study, we did not calculate the death rate, because no sudden death was noted and some patients died during hospitalization, while others died after the introduction of renal replacement therapy. In such situations, it was very difficult to determine when exactly the patients had died.
In conclusion, it is suggested that with moderate progression of renal dysfunction and under well-controlled BP, peripheral but not central arterial stiffness is possibly one of the strongest predictors of CVD in patients with CKD stages 3-5.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgement
Mrs Sachiko Nakazato, a secretary, calculated the data and typed the manuscript.
References
- 1.Suzuki H, Saruta T. An overview of blood pressure regulation associated with the kidney. Contrib Nephrol. 2004;143:1–15. doi: 10.1159/000078708. [DOI] [PubMed] [Google Scholar]
- 2.K/DOQI clinical practice guidelines for chronic kidney disease evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Sigrist MK, Taal MW, Bungay P, McIntyre CW. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 5.Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P. Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967–974. doi: 10.1681/ASN.2010080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D'Agostino RB, Levy D, Vasan RS. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111:1370–1376. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 7.Townsend RR, Wimmer NJ, Chirinos JA, Parsa A, Weir M, Perumal K, Lash JP, Chen J, Steigerwalt SP, Flack J, Go AS, Rafey M, Rahman M, Sheridan A, Gadegbeku CA, Robinson NA, Joffe M. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–289. doi: 10.1038/ajh.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–1457. doi: 10.1161/HYPERTENSIONAHA.112.197210. [DOI] [PubMed] [Google Scholar]
- 9.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 10.Wang MC, Tsai WC, Chen JY, Cheng MF, Huang JJ. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton) 2007;12:591–597. doi: 10.1111/j.1440-1797.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 11.Imai Y, Otsuka K, Kawano Y, Shimada K, Hayashi H, Tochikubo O, Miyakawa M, Fukiyama K. Japanese society of hypertension (JSH) guidelines for self-monitoring of blood pressure at home. Hypertens Res. 2003;26:771–782. doi: 10.1291/hypres.26.771. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Moriwaki K, Nakamoto H, Sugahara S, Kanno Y, Okada H. Blood pressure reduction in the morning yields beneficial effects on progression of chronic renal insufficiency with regression of left ventricular hypertrophy. Clin Exp Hypertens. 2002;24:51–63. doi: 10.1081/ceh-100108715. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Nakamoto H, Okada H, Sugahara S, Kanno Y. A selective angiotensin receptor antagonist, Valsartan, produced regression of left ventricular hypertrophy associated with a reduction of arterial stiffness. Adv Perit Dial. 2003;19:59–66. [PubMed] [Google Scholar]
- 14.Suzuki H, Dogi M, Takenaka T. Various approaches for vascular health in elderly women. Clin Exp Hypertens. 2013;35:295–299. doi: 10.3109/10641963.2013.780068. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H. Angiotensin type 1 receptor blockers in chronic kidney disease. Contrib Nephrol. 2004;143:159–166. doi: 10.1159/000078719. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda A, Kitaoka T, Suzuki H. Effect of hemoglobin target on progression of chronic kidney disease. J Nephrol Ther. 2014;S1:006. [Google Scholar]
- 18.Suzuki H, Watanabe Y, Kumagai H, Shuto H. Comparative efficacy and adverse effects of the addition of ezetimibe to statin versus statin titration in chronic kidney disease patients. Ther Adv Cardiovasc Dis. 2013;7:306–315. doi: 10.1177/1753944713513222. [DOI] [PubMed] [Google Scholar]
- 19.Rosansky SJ, Glassock RJ. Is a decline in estimated GFR an appropriate surrogate end point for renoprotection drug trials? Kidney Int. 2014;85:723–727. doi: 10.1038/ki.2013.506. [DOI] [PubMed] [Google Scholar]
- 20.Chue CD, Edwards NC, Davis LJ, Steeds RP, Townend JN, Ferro CJ. Serum phosphate but not pulse wave velocity predicts decline in renal function in patients with early chronic kidney disease. Nephrol Dial Transplant. 2011;26:2576–2582. doi: 10.1093/ndt/gfq787. [DOI] [PubMed] [Google Scholar]
- 21.Kim CS, Kim HY, Kang YU, Choi JS, Bae EH, Ma SK, Kim SW. Association of pulse wave velocity and pulse pressure with decline in kidney function. J Clin Hypertens (Greenwich) 2014;16:372–377. doi: 10.1111/jch.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng KP, Moody WE, Chue CD, Edwards NC, Savage T, Tomson CR, Steeds RP, Townend JN, Ferro CJ. Central pulse pressure in patients with chronic kidney disease and in renal transplant recipients. J Hum Hypertens. 2014;28:180–185. doi: 10.1038/jhh.2013.71. [DOI] [PubMed] [Google Scholar]
- 23.Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 24.Gusbeth-Tatomir P, Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. Kidney Blood Press Res. 2007;30:97–107. doi: 10.1159/000100905. [DOI] [PubMed] [Google Scholar]
- 25.Georgianos PI, Sarafidis PA, Malindretos P, Nikolaidis P, Lasaridis AN. Hemodialysis reduces augmentation index but not aortic or brachial pulse wave velocity in dialysis-requiring patients. Am J Nephrol. 2011;34:407–414. doi: 10.1159/000331700. [DOI] [PubMed] [Google Scholar]
- 26.Ohishi M, Tatara Y, Ito N, Takeya Y, Onishi M, Maekawa Y, Kato N, Kamide K, Rakugi H. The combination of chronic kidney disease and increased arterial stiffness is a predictor for stroke and cardiovascular disease in hypertensive patients. Hypertens Res. 2011;34:1209–1215. doi: 10.1038/hr.2011.117. [DOI] [PubMed] [Google Scholar]
- 27.Taal MW. Arterial stiffness in chronic kidney disease: an update. Curr Opin Nephrol Hypertens. 2014;23:169–173. doi: 10.1097/01.mnh.0000441153.40072.e0. [DOI] [PubMed] [Google Scholar]
- 28.Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A. Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol. 2010;14:558–570. doi: 10.1007/s10157-010-0328-6. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R. Antihypertensive agents and arterial stiffness: relevance to reducing cardiovascular risk in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2007;16:409–415. doi: 10.1097/MNH.0b013e3282063b86. [DOI] [PubMed] [Google Scholar]