Abstract
Three potassium channels have been associated with primary aldosteronism (PA) in rodents and humans: KCNK3 (TASK-1), KCNK9 (TASK-3), and KCNJ5 (Kir3.4). Mice with deficiency in Kcnk3 and Kcnk9 have elevated aldosterone production and blood pressure. In humans, adrenal tumors with somatic mutations in KCNJ5 cause PA. However, there are very few reports on the expression patterns of these genes in humans versus rodents. Herein, we compared human and rat mRNA expression (by quantitative real-time polymerase chain reaction (qPCR) and protein levels (by immunohistochemistry) across three tissues (adrenal, brain, heart) and two laser-captured adrenal zones (zona glomerulosa, zona fasciculata). Our findings show that expression patterns of KCNK3, KCNK9, and KCNJ5 are inconsistent between rats and humans across both tissues and adrenal zones. Thus, species variation in the expression of PA-related potassium channels indicates an evolutionary divergence in their role in regulating adrenal aldosterone production.
Keywords: aldosterone, primary aldosteronism, potassium (K+), zona glomerulosa, zona fasciculata
1. Introduction
1.1. Primary aldosteronism (PA)
Aldosterone controls sodium homeostasis, and its physiological production in the adrenal zona glomerulosa (ZG) is tightly controlled by the renin-angiotensin-aldosterone-system. However, in about 8% of hypertensive patients, aldosterone is autonomously produced due to aldosterone-producing adenoma (APA) or idiopathic hyperaldosteronism, both of which are major causes of primary aldosteronism (PA) (Chao et al., 2013; Young, 2007). It is important to detect and treat PA in hypertensive patients, since aldosterone excess in PA patients is associated with a higher likelihood of cardiovascular complications (Milliez et al., 2005).
1.2. KCNK3 and KCNK9 (TASK-1 and TASK-3)
Circulating potassium (K+) is well recognized as one of the major regulators of ZG aldosterone production. ZG cells respond to small changes in circulating K+ because they are depolarized by K+ and selectively permeable to K+, such that they effectively act as K+ electrodes (Guagliardo et al., 2012). Therefore, K+ channels significantly influence ZG cell membrane potential. Intriguingly, it has been suggested that each species may utilize a different set of K+ channels to maintain membrane potential in ZG cells (Guagliardo et al., 2012). KCNK3, or TWIK-related acid-sensitive K+ channel (TASK)-1, and KCNK9 (TASK-3) are both members of the KCNK family of two-pore-forming domain K+ channels, which are also known as K+-selective leak channels (Goldstein et al., 2001). Members of this channel family produce weak rectifying currents and are generally active at resting cell membrane potentials. These channels generate background (or “leak”) K+ conductances that play a key role in maintaining negative membrane potential (Bayliss et al., 2003). A recent study demonstrated that human KCNK5 (TASK-2) mRNA and protein have lower expression in APAs compared with the normal human adrenal cortex, and that H295R cells transfected with a KCNK5 dominant-negative mutants increased CYP11B2 expression and aldosterone production (Lenzini et al., 2014). Furthermore, other studies have shown that mice lacking Kcnk3 (Heitzmann et al., 2008), Kcnk9 (Guagliardo et al., 2012; Penton et al., 2012), or both Kcnk3 and Kcnk9 (Davies et al., 2008) in the adrenal develop PA. From these findings, it has been hypothesized that KCNK3 and KCNK9 may play an important role in PA in humans; however, expression patterns of these K+ channels have not previously been characterized in the human adrenal gland to this date.
1.3. KCNJ5 (Kir3.4)
Potassium inwardly-rectifying channel, subfamily J, member 5 (KCNJ5 or Kir3.4) is a G protein-activated inwardly-rectifying K+ (Kir) channel, forming tetrameric complexes of two membrane-spanning domains. Like other Kir channels, KCNJ5 regulates cell membrane potential (Hibino et al., 2010). Recently, KCNJ5 gene somatic mutations have been found in human APAs (Choi et al., 2011). KCNJ5 may have an important role in physiological and pathological aldosterone production because KCNJ5 protein is strongly expressed in the human ZG and in APA (Choi et al., 2011; Monticone et al., 2012). Furthermore, it was reported that normal (non-mutated) KCNJ5 participated in AngII-stimulated aldosterone production in the human adrenocortical cell line HAC15 (Oki et al., 2012). However, despite these studies in humans, detailed analyses of KCNJ5 expression in mouse and rat adrenal glands have not been described.
1.4. Summary of this study
In this study, K+ channel (KCNK3, KCNK9, KCNJ5) expression patterns were compared between two species: humans and rats. By quantitative real-time polymerase chain reaction (qPCR) and immunohistochemical analyses, we characterized mRNA and protein expression levels across three tissues (adrenal, brain, heart) and three adrenal zones [ZG, zona fasciculata (ZF), medulla]. Intriguingly, our results demonstrated a divergence between humans and rats in the expression of PA-related K+ channels.
2. Materials and Methods
2.1. Human tissue samples
For human tissue comparison, human tissue RNA samples (four adrenal, three brain, and three heart) were purchased from Clontech (Mountain View, CA), BioChain Institute (Newark, CA), System Biosciences (Mountain View, CA), and Becton, Dickinson and Company (Franklin Lakes, New Jersey). For human KCNK9 qPCR, two additional brain RNA samples were used. RNA was then reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA).
2.2. Human adrenal collection for laser capture microdissection (LCM) and immunohistochemistry (IHC)
Use of the human tissues for LCM and IHC was approved by the Institutional Review Board of Georgia Regents University. After obtaining informed consents from the families, normal human adrenal samples were collected from renal transplantation donors at Georgia Regents University. Extracted adrenals were immediately transferred into cold Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, 1:1; Gibco, NY). Then, the adrenal glands were cleaned of adherent tissues and one piece each of 3–4 mm-thick adrenal was frozen in embedding media (Tissue-Tek Optimum Cutting Temperature (O.C.T.) compound; Sakura Finetek U.S.A, Inc., Torrance, CA) for LCM or fixed in 10% formaldehyde, embedded in paraffin, and serially sectioned in 5 um sections onto glass microscope slides for IHC, as previously reported (Nishimoto et al., 2012). A total of nine human adrenals were used for LCM (six male, two female): Donor Adrenal Normal (DAN) 24 (male, 30 years old), #28 (female, 43 years old), #31 (male, 29 years old), #32(male, 17 years old), #45 (female, 37 years old), #47 (male, 46 years old), #48 (male, 39 years old), #50 (male, 43 years old), and #55 (male, 19 years old). Hypertension data, unfortunately, is not available for these deceased donors. Frozen adrenal glands in O.C.T. compound were cut in 7 μm serial sections onto Superfrost Plus Microscope Slides (Fisher Scientific, Loughborough, UK) or PEN-Membrane Slides (Leica, Germany). These sections were stained with cresyl violet (Sigma, Rochester, NY) by following the manufacturer’s ribonuclease-free protocol in the HistoGene LCM Frozen Section Staining Kit (Molecular Devices, Sunnyvale, CA); the xylene wash step was excluded when staining PEN-Membrane Slides. To collect enriched populations of aldosterone-producing ZG cells, the first of the five to seven serially sectioned slides was immunostained for CYP11B2 as a reference, and five to seven layers of CYP11B2 positive cells immediately beneath the capsule were carefully laser captured from the adjacent unstained sections. ZF cells were captured from lipid-rich cells in the middle layer. Adrenal medulla cells were also captured. RNAs from ZG, ZF, and medulla cells were isolated using the PicoPure RNA isolation kit (Molecular Devices) as previously described (Nishimoto et al., 2012). Total RNA (1–10ng) from ZG, ZF, and medulla samples was submitted to the University of Michigan DNA Sequencing Core (UMDSC) MicroArray Core Facility for amplified cDNA preparation. RNA amplification and reverse transcription were performed using the Ovation Pico WTA System V2 (NuGEN Technologies, Inc., San Carlos, CA). The cDNA was then purified using the QIAquick PCR Purification Kit (Qiagen, Germantown, Maryland) and used for qPCR analyses.
2.3. Rat tissue samples
All rat procedures carried out were reviewed and approved by the University of Michigan’s University Committee on the Use and Care of Animals or the Georgia Regents University Institutional Animal Care and Use Committee. For tissue comparison, 5 male and 4 female Sprague-Dawley (SD) rats were acquired from the Rodent Recycling Program at the University of Michigan Medical School. These male and female rats were sacrificed by rapid decapitation at an age of average ± S.E.M: 10.2 ± 1.1 and 10.9 ± 1.1 weeks, respectively. Whole adrenal tissue, segments of brain cortex, and segments of heart left ventricle were extracted, cleaned of surrounding connective tissues, and homogenized with 600uL of RLT Buffer Plus (QIAGEN, Germantown, Maryland) containing 1% beta-mercaptoethanol (SIGMA Life Science, St. Louis, MO) as samples of ‘adrenal’, ‘brain’, and ‘heart’, respectively. RNA from the homogenized tissue was isolated using the RNeasy Plus Mini Kit (QIAGEN) and reverse transcribed using the High Capacity cDNA Reverse Transcription Kit.
2.4. Rat adrenal collection for LCM and IHC
As previously described, five eleven-week-old male SD rats (Harlan) on a normal sodium (NS) diet were sacrificed, and their adrenal glands were extracted and frozen in O.C.T. compound for LCM or fixed in 4% paraformaldehyde and paraffin-embedded for IHC. Cells from rat ZG and ZF cells were laser captured and amplified cDNA was prepared (Nishimoto et al., 2012).
2.5. Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed using cDNA from human and rat tissues and amplified cDNA from laser captured ZG and ZF cells from human and rat adrenal gland (see 2.1. to 2.3.). For qPCR, either 5 ng (human/rat tissues) of cDNA or 1 ng (human/rat isolated ZG/ZF) of amplified cDNA was mixed with Fast Universal PCR Master Mix (Applied Biosystems) and primer/probe mix specific for each gene, except for human KCNK9. Human KCNK9 primers were mixed with cDNA as mentioned above and SYBR Green PCR master mix (Applied Biosystems). In instances where 1 ng of the isolated adrenal zones showed no detectable signal, we repeated the reactions using 5 ng of template. Except for human CYP11B2 and human KCNK9, all primer/probe mixes were purchased from Applied Biosystems: human KCNK3, Hs00605529_m1; human KCNJ5, Hs00168476_m1, rat Cyp11b2, Rn02396730_g1; rat Kcnk3, Rn04223042_m1; rat Kcnk9, Rn00755967_m1; and rat Kcnj5, Rn01789221_mH. For human CYP11B2 qPCR, primer/Taqman probe mix was prepared as previously reported (Ye et al., 2007). Human KCNK9 was amplified using specific primers designed in the 3′ UTR of human KCNK9 transcript variant 1 (NM_001282534); forward 5′-ACC TTT CCA GCC AGA CAG AG – 3′ and reverse 5′ – AAG GAG GAG ATC CAT GCT TC – 3′. Peptidylprolyl isomerase A (PPIA, cyclophilin A) transcript was used for normalization of sample loading (Hs99999904_m1 for human cDNA and Rn03302269_gH for rat cDNA).
2.6. IHC
IHC was performed for human and rat serial tissue sections using pH 9 Target Retrieval Solution (Dako, Glostrup, Denmark), an EnVision horseradish peroxidase-labeled secondary antibody (Dako), and 3, 3′-diaminobenzidine (brown color) for visualization, as previously reported (Nishimoto et al., 2012). For positive control tissue, a rat heart 4% paraformaldehyde-fixed paraffin-embedded section (see 2.4) was used. For all tissues, negative controls were run omitting the primary antibody from the protocol. Primary antibodies, dilutions, and suppliers are listed in Table 1.
Table 1.
Primary antibodies used in IHC, human and rat
| Target | Reference number/Citation | Source | Dilution | |
|---|---|---|---|---|
| HUMAN | CYP11B2 | (Gomez-Sanchez et al., 2014) | Dr. Celso Gomez-Sanchez, M.D. (Univ. of Mississippi Medical Center) | 1:100 |
| KCNK3 (TASK-1) | NBP1-83070 | Novus Biologicals | 1:100 | |
| KCNK9 (TASK-3) | 37678 | Signalway Antibody | 1:1000 | |
| KCNJ5 (Kir3.4) | HPA017353 | SIGMA Life Science | 1:100 | |
| RAT | CYP11B2 | (MacKenzie et al., 2000) | Dr. Celso Gomez-Sanchez, M.D. (Univ. of Mississippi Medical Center) | 1:200 |
| KCNK3 (TASK-1) | NBP1-83070 | Novus Biologicals | 1:1000 | |
| KCNK9 (TASK-3) | LS-B3980 | LifeSpan Biosciences | 1:1000 | |
| KCNJ5 (Kir3.4) | HPA017353 | Sigma-Aldrich | 1:200 |
2.7. Statistics for qPCR
The 2−ΔΔCt method was used to calculate fold changes (Livak and Schmittgen, 2001). DNA levels that were not detectable by RT-PCR were assigned a Ct value of 40 for analysis, and the S.E.M. of fold change was calculated based on delta delta Ct values. Statistical differences were determined either by student’s t-test across adrenal zones or by one-way ANOVA across tissues using delta Ct values in SigmaPlot 12.5 software (Systat Software, Inc), as previously reported (Nishimoto et al., 2014). P values below 0.05 were considered statistically significant.
3. Results
3.1. Tissue and zone confirmation
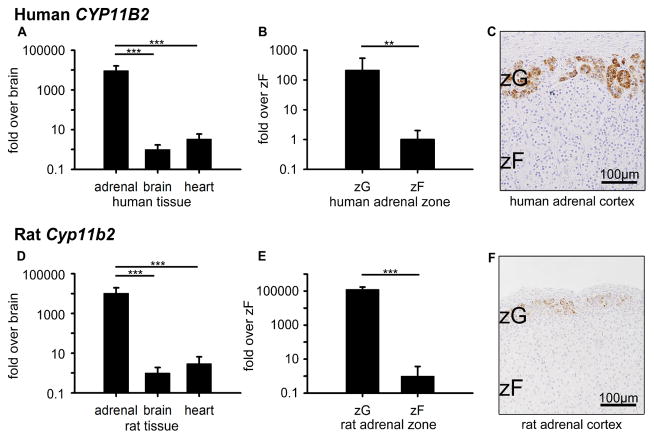
In order to confirm the aldosterone-producing ability in whole adrenal tissue and ZG, we first compared CYP11B2 expression levels in three tissues (adrenal, brain, and heart) and two zones (ZG and ZF). In tissue comparisons, CYP11B2 mRNA in human adrenal, expressed as [mean ± S.E.M.], was: 9414.5 ± 6944.6-fold and 2788.6 ± 2057.0-fold higher than that in brain (1.0 ± 0.7, p<0.001) and heart (1.0 ±0.8, p<0.001), respectively (Figure 1A). In human zone comparisons, CYP11B2 in ZG was 207.4 ± 330.2-fold higher than that in ZF (1.0 ± 1.0, p<0.01) (Figure 1B). Consistent with CYP11B2 mRNA expression, IHC showed distinct CYP11B2 protein expression in the ZG, as previously reported (Nishimoto et al., 2010) (Figure 1C). As expected, the rat tissue and adrenal zone expression was similar to that in humans. Cyp11b2 mRNA in rat adrenal was 10287.9 ± 9377.0-fold and 3466.7 ± 3159.8-fold higher than those in brain (1.0 ± 0.9, p<0.001) and heart (1.0 ± 1.2, p<0.001), respectively (Figure 1D). In rat zone comparisons, Cyp11b2 in ZG was 125914.3 ± 45735.1-fold higher than that in ZF (1.0 ± 2.7, p<0.001) (Figure 1E). CYP11B2 protein was clearly expressed in the ZG, as previously reported (Mitani et al., 1994) (Figure 1E). In summary, CYP11B2 expression in human and rat tissues and zones was confirmed, ensuring that tissue collection and laser capturing were accurate.
Figure 1.
CYP11B2 expression in human (A–C) and rat (D–F). (A, D) Quantitative RT-PCR analysis for CYP11B2 mRNA across adrenal, brain, and heart tissues. (B, E) Quantitative RT-PCR analysis for CYP11B2 mRNA across ZG and ZF adrenal zones. Values represent mean ± S.E.M. *P<0.05, **P<0.01, ***P<0.001. (C, F) Immunohistochemical analyses of CYP11B2 protein localization in ZG and ZF adrenal zones, plus hematoxylin nuclear counterstain.
3.2. KCNK3 (TASK-1) expression in human and rat adrenal glands
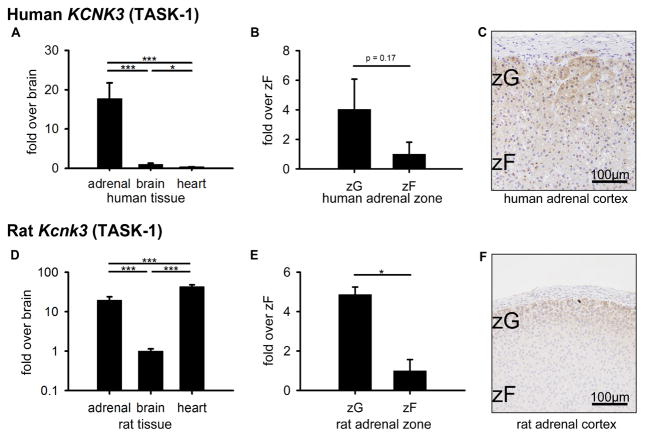
Kcnk3 expression levels have been partially described in mouse (Davies et al., 2008), but have not been characterized in the human or rat adrenal gland. KCNK3 mRNA in human adrenal was 17.7 ± 4.0-fold and 44.3 ± 10.0-fold higher than those in brain (1.0 ± 0.3, p<0.001) and heart (1.0 ± 0.1, p<0.001), respectively (Figure 2A). In human zone comparisons, KCNK3 in ZG was 4.0 ± 2.0-fold higher than in ZF (1.0 ± 0.8-fold, p=0.17) (Figure 2B). Consistent with KCNK3 mRNA expression, KCNK3 protein displayed slightly higher expression in ZG compared to ZF (Figure 2C). In rat tissue comparisons, Kcnk3 mRNA was also high in adrenal but higher in heart. Kcnk3 in adrenal was 19.6 ± 4.3-fold (p<0.001) and in heart was 43.0 ± 5.1-fold (p<0.001) higher than that in brain (1.0 ± 0.1) (Figure 2D). In rat zone comparisons, Kcnk3 in ZG was 4.9 ± 0.4-fold higher than that in ZF (1.0 ± 0.6, p<0.05) (Figure 2E), which differs from a mouse analysis in a previous report (Davies et al., 2008). Consistent with mRNA expression patterns, KCNK3 protein was expressed in the ZG (Figure 2F). Thus, the expression patterns of KCNK3 in human and rat were clearly determined. We found similar KCNK3 expression patterns between human and rat adrenals.
Figure 2.
KCNK3 (TASK-1) expression in human (A–C) and rat (D–F). (A, D) Quantitative RT-PCR analysis for KCNK3 mRNA across adrenal, brain, and heart tissues. (B, E) Quantitative RT-PCR analysis for KCNK3 mRNA across ZG and ZF adrenal zones. Values represent mean ± S.E.M. *P<0.05, **P<0.01, ***P<0.001. (C, F) Immunohistochemical analyses of KCNK3 protein localization in ZG and ZF adrenal zones, plus hematoxylin nuclear counterstain.
3.3. KCNK9 (TASK-3) expression in human and rat adrenal glands
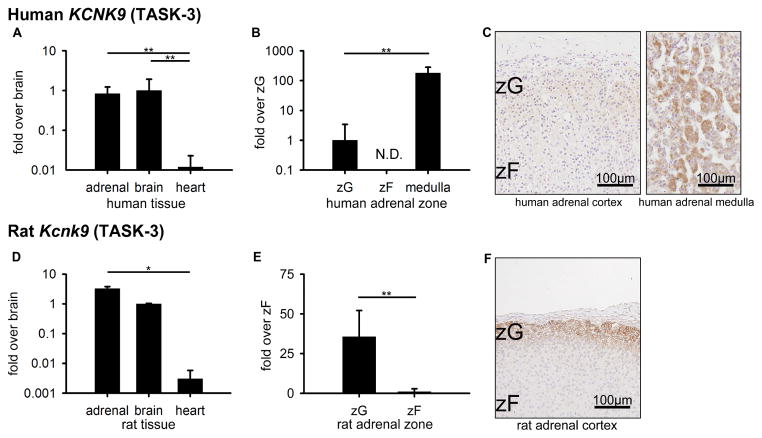
Similar to Kcnk3, Kcnk9 expression levels have been partially described in mouse (Davies et al., 2008) but have not been previously characterized in rat or human adrenal. In human tissue comparisons, KCNK9 was highest in brain at 83.7 ± 78.0-fold and 1.2 ± 1.1-fold higher than heart (1.0 ± 0.9, p<0.01) and adrenal (1.0 ± 0.3, p=0.826), respectively, but not significantly different from the adrenal (Figure 3A). In human zone comparisons, KCNK9 was highest in the medulla, at 177.5 ± 92.5-fold higher than ZG (1.0 ± 1.4, p<0.01), and not detectable in ZF (Figure 3B). Consistent with the mRNA expression patterns, KCNK9 protein was detected in human adrenal medulla (Figure 3C). Of note, the IHC images of the adrenal cortex and adrenal medulla in Figure 3C were taken from the same adrenal section. Thus, these tissue regions were processed by IHC under the same conditions. In contrast, for rat tissue, although the expression level was not different between adrenal and brain, Kcnk9 mRNA in adrenal (1073.9 ± 190.5-fold) was higher than that in heart (1.0 ± 0.9, p<0.05) (Figure 3D). Furthermore, rat zone comparisons showed that Kcnk9 levels in ZG were 35.6 ± 6.9-fold above ZF (1.0 ± 0.5, p<0.01) (Figure 3E). Consistently, IHC displayed high KCNK9 protein expression in rat ZG (Figure 3F). In summary, KCNK9 expression patterns differed between human and rat adrenal.
Figure 3.
KCNK9 (TASK-3) expression in human (A–C) and rat (D–F). (A, D) Quantitative RT-PCR analysis for KCNK9 mRNA across adrenal, brain, and heart tissues. (B, E) Quantitative RT-PCR analysis for KCNK9 mRNA across ZG, ZF, and adrenal medulla. Values represent mean ± S.E.M. *P<0.05, **P<0.01, ***P<0.001. (N.D.) Not detectable by quantitative RT-PCR. (C) Immunohistochemical analyses of KCNK9 protein localization in human ZG and ZF adrenal zones and human adrenal medulla (positive control), plus hematoxylin nuclear counterstain. Note: the immunohistochemistry was performed in human ZG, ZF, and medulla simultaneously under the same conditions. (F) Immunohistochemical analysis of KCNK9 protein localization in rat ZG and ZF adrenal zones, plus hematoxylin nuclear counterstain.
3.4. KCNJ5 (Kir3.4) expression in human and rat adrenal glands
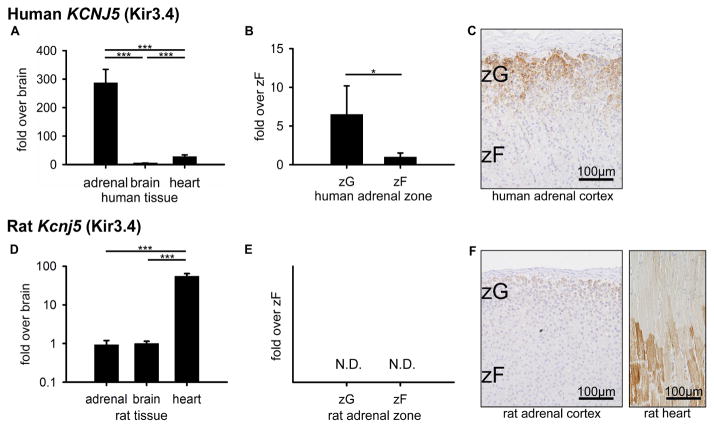
After the important discovery of KCNJ5 mutations in human aldosterone-producing adenomas (Choi et al., 2011), a number of human KCNJ5 studies have been performed, including reports on KCNJ5 localization by IHC (Monticone et al., 2012). However, KCNJ5 expression in the mouse and rat adrenal gland has not previously been characterized. In human tissue, KCNJ5 mRNA was highest in adrenal at 287.3-fold and 10.2 ± 1.7-fold higher than brain (1.0 ± 0.7, p<0.001) and heart (1.0 ± 0.2, p<0.001), respectively (Figure 4A). Human zone comparisons showed 6.5 ± 3.7-fold higher KCNJ5 levels in ZG above ZF (1.0 ± 0.5, p<0.05) (Figure 4B). Consistent with mRNA expression patterns and previous reports, KCNJ5 protein was expressed in human ZG (Figure 4C). On the other hand, rat tissue comparisons showed Kcnj5 mRNA highest in heart at 59.7 ± 9.9-fold and 55.2 ± 9.1-fold higher than adrenal (1.0 ± 0.3, p<0.001) and brain (1.0 ± 0.1, p<0.001), respectively (Figure 4D). Furthermore, in rat zonal comparisons, Kcnj5 mRNA was not detectable in either ZG or ZF (Figure 4E). IHC was consistent, showing distinct KCNJ5 expression in heart tissue and minimal signal in the ZG and ZF. Faint signal is considered to be non-specific background staining (Figure 4F). Of note, the IHC in the Figure 4F was simultaneously performed in rat adrenal and heart under the same conditions. In general, KCNJ5 expression patterns differed between human and rat across both tissues and adrenal zones.
Figure 4.
KCNJ5 (Kir3.4) expression in human and rat. (A, D) Quantitative RT-PCR analysis for KCNJ5 mRNA across adrenal, brain, and heart tissues. (B, E) Quantitative RT-PCR analysis for KCNJ5 mRNA across ZG and ZF adrenal zones. Values represent mean ± S.E.M. *P<0.05, **P<0.01, ***P<0.001. (N.D.) Not detectable by quantitative RT-PCR. (C) Immunohistochemical analyses of KCNJ5 protein localization in human ZG and ZF adrenal zones, plus hematoxylin nuclear counterstain. (F) Immunohistochemical analysis of KCNJ5 protein localization in rat ZG and ZF adrenal zones and rat heart tissue (positive control), plus hematoxylin nuclear counterstain. Note: the immunohistochemistry was performed in rat adrenal and rat heart simultaneously under the same conditions.
4. Discussion
4.1. Summary and impact
Adrenal aldosterone production is tightly regulated by angiotensin II and circulating K+. Both agonists act through intracellular calcium signaling pathways to stimulate adrenal aldosterone biosynthesis and release. Recent studies have demonstrated that gene mutations in several K+ channels alter intracellular calcium signaling, leading to excess adrenal aldosterone production. These include mouse genetic deletion of Kcnk3 and Kcnk9 and human germline or somatic mutations in the ion selectivity filter of KCNJ5. Because of the importance of these channels in rodents and humans, we undertook the current study in mRNA and protein expression patterns of KCNK3, KCNK9, and KCNJ5 in rat and human adrenal. Our findings suggest that species differences should be considered when studying the role of these channels in adrenal aldosterone production.
4.2. KCNK3 and KCNK9 (TASK-1 and TASK-3)
4.2.1. Rodent
KCNK3 and KCNK9 have recently been identified as important regulators of K+ conductance related to the maintenance of ZG cell membrane potential. Genetic deletion of Kcnk3 and/or Kcnk9 in mice removes an important background K+ current that leads to elevated adrenal aldosterone production and a model of PA. Furthermore, Kcnk3 and Kcnk9 knockout mice did not exhibit suppression of aldosterone production with dietary sodium loading. In normal mice, Kcnk3 mRNA was localized to the ZG and ZF, while Kcnk9 mRNA was expressed primarily in the ZG and medulla (Davies et al., 2008). In our immunohistochemical analyses of rats, KCNK3 protein was localized primarily in the ZG and outer ZF, and KCNK9 protein was localized in the ZG. We also found that Kcnk3 mRNA was expressed at significantly higher levels in the ZG compared with the ZF, suggesting a role in aldosterone production in rats, as has previously been demonstrated in mice. Furthermore, we saw no sex difference in rat adrenal gland Kcnk3 or Kcnk9 expression (Supplemental Figure 1). In addition, we determined that Kcnk9 mRNA is expressed in the ZG at significantly higher levels than those of the ZF, consistent with previous demonstrations of the key regulatory role of KCNK9 protein in K+ conductance in ZG cells. In summary, our zonation studies demonstrate that increased KCNK3 and KCNK9 expression in the ZG is consistent between mice and rats.
4.2.2. Human
The role of KCNK3 in human adrenal aldosterone production is not clear. Little is known regarding KCNK3 expression in the human adrenal other than in comparison to KCNK family members. These studies suggest that KCNK3 is more highly expressed compared with other KCNK members. Microarray analysis has shown that this pattern holds true in not only normal adrenals, but also in APA and H295R adrenal cells. Genetic knockdown of KCNK3 in adrenal H295R cells resulted in upregulation of steroidogenic acute regulatory (StAR) protein and aldosterone synthase. These cells exhibited increased intracellular calcium, resulting in CaMK activation and increased CYP11B2 expression (Nogueira et al., 2010). As for other KCNK family members, lower levels of KCNK5 have been associated with APA and increased aldosterone synthesis (Lenzini et al., 2014), suggesting that KCNK5 plays an important role in adrenal aldosterone production. Our expression studies of KCNK3 in the adrenal cortex suggest a trend towards higher expression in ZG versus ZF in human, based on higher (but not statistically significant) mRNA fold expression and apparent stronger immunohistochemical staining in ZG versus ZF. On the other hand, KCNK9 was not detected in human ZF, while positive and significant expression was observed in the medulla and in brain tissue. However, the relatively low mRNA expression of KCNK9 transcripts in the ZG, especially compared with the medulla, suggests a possibly lesser role in adrenal aldosterone production.
4.2.3. Species Comparison
KCNK3 expression patterns appear similar between human and rat adrenals, while KCNK9 expression patterns are inconsistent between rodents and humans. In rats, Kcnk3 mRNA expression levels in ZG cells are higher than those in ZF, and similarly in humans, there is a possible trend towards higher KCNK3 mRNA expression levels in the ZG compared to the ZF. Our studies localized rat KCNK3 protein mainly to ZG and outer ZF cells, while previous studies showed mouse Kcnk3 mRNA throughout the adrenal cortex. Based on our findings, the tissue distributions for KCNK3 and KCNK9 among adrenal, brain, and heart differ somewhat between rats and humans. Furthermore, absolute levels of KCNK9 transcripts appear to be higher in the rat adrenal than in the human adrenal (Supplemental Figure 2). Within the adrenal, Kcnk9 in both rats and mice is primarily expressed in ZG, with little expression in ZF. Humans, on the other hand, have undetectable levels of KCNK9 in ZF and relatively low levels in ZG, suggesting an important aldosterone-regulating role of KCNK9 in rodents but possibly a lesser role in humans.
4.3. KCNJ5
4.3.1. Rodent
KCNJ5, particularly in humans, has been recently pinpointed as a key regulator of ZG cell membrane potential, and several mutations in the channel have been linked to adrenal disorders. However, its role in rodent adrenal aldosterone production has not been determined. The role of KCNJ5 in mice has been previously studied in the heart (Liang et al., 2014; Mesirca et al., 2013) and in a mouse cardiac muscle cell line (Nobles et al., 2010). In rats, KCNJ5 has also been studied in cardiac tissue (Atkinson et al., 2013; Bingen et al., 2013), and in kidney tissue of obese rats (Kang et al., 2013). However, to date, no studies have investigated the role of KCNJ5 in the adrenal cortex or in aldosterone regulation in rodents. Our study suggests that, at least in rats, Kcnj5 mRNA and KCNJ5 protein are not expressed in the adrenal cortex. This suggests that KCNJ5 does not play a role in adrenal steroid production in rats.
4.3.2. Human
KCNJ5 and other K+ channels maintain the negative membrane potential of human ZG cells under basal conditions. Mutations in and near the selectivity filter of KCNJ5 result in loss of selectivity, leading to sodium entry, membrane depolarization, calcium mobilization, and aldosterone synthesis. 30–60% of APAs have mutations in the selectivity filter of KCNJ5 (Gomez-Sanchez and Oki, 2014). KCNJ5 overexpression in the HAC15 adrenocortical cell line downregulated membrane voltage, intracellular calcium, and CYP11B2 mRNA. Furthermore, activation of KCNJ5 blocked angiotensin II-stimulated membrane voltage and suppressed aldosterone production (Oki et al., 2012). In agreement with earlier data, KCNJ5 protein was expressed in both ZG and the outer ZF, with higher KCNJ5 mRNA expression in ZG. These findings are consistent with the previously established role of KCNJ5 in the regulation of aldosterone production, including effects of mutations.
4.3.3. Species Comparison
While Kcnj5 mRNA is expressed at undetectable levels in rat adrenal ZG and ZF, KCNJ5 in humans is more strongly expressed in ZG compared with ZF, and its key role in normal and pathological aldosterone production has been described in many reports.
4.4. Overall Summary
The current study supports the finding that while several KCNK family members (especially KCNK9) appear to dominate regulation of ZG K+ currents in mice and rats, KCNJ5 appears to play this important role in humans. Our data reveal distinctly different expression patterns of these K+ channels between humans and rats. Species variation in the expression patterns of these K+ channels highlights a possible evolutionary divergence in their regulation of aldosterone production. Furthermore, this inconsistency across species suggests that rodents may not be ideal models for some aspects of human adrenal disease, including the role of KCNJ5 in normal or pathologic production of aldosterone production.
Supplementary Material
Quantitative RT-PCR analysis of Cyp11b2, Kcnk3, Kcnk9, and Kcnj5 mRNA expression in rat adrenal, brain, and heart by sex. No differences were observed between male and female mRNA levels. Fold changes are compared to the average mRNA levels of male and female rats combined. Values represent mean ± S.E.M.
Relative mRNA expression patterns of KCNK9 in human and rat determined by semi-quantitative RT-PCR. (A) KCNK9 mRNA expression patterns among rat brain, rat adrenal, human brain, and human adrenal. (B) Comparison of KCNK9 mRNA expression between rat and human adrenals (n=4 for each).
Highlights.
We examine three potassium channels related to primary aldosteronism in humans and rats.
Expression patterns of primary aldosteronism-related potassium channels differ between these species.
Species variation should be considered when studying the role of these channels.
Acknowledgments
Dr. Celso Gomez-Sanchez for providing human and rat CYP11B2 antibodies. Financial support from the National Institute of Diabetes and Digestive and Kidney Diseases DK043140 (to W.E.R), and a fellowships to K. Nishimoto. from the Federation of National Public Service Personnel Mutual Aid Associations and the Tachikawa Hospital, Japan. K. Nanba is supported by American Heart Association (14POST20020003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson AJ, Logantha SJ, Hao G, Yanni J, Fedorenko O, Sinha A, Gilbert SH, Benson AP, Buckley DL, Anderson RH, Boyett MR, Dobrzynski H. Functional, anatomical, and molecular investigation of the cardiac conduction system and arrhythmogenic atrioventricular ring tissue in the rat heart. J Am Heart Assoc. 2013;2:e000246. doi: 10.1161/JAHA.113.000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–19. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Bingen BO, Neshati Z, Askar SF, Kazbanov IV, Ypey DL, Panfilov AV, Schalij MJ, de Vries AA, Pijnappels DA. Atrium-specific Kir3.x determines inducibility, dynamics, and termination of fibrillation by regulating restitution-driven alternans. Circulation. 2013;128:2732–44. doi: 10.1161/CIRCULATIONAHA.113.005019. [DOI] [PubMed] [Google Scholar]
- Chao CT, Wu VC, Kuo CC, Lin YH, Chang CC, Chueh SJ, Wu KD, Pimenta E, Stowasser M. Diagnosis and management of primary aldosteronism: an updated review. Ann Med. 2013;45:375–83. doi: 10.3109/07853890.2013.785234. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–72. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A. 2008;105:2203–8. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–84. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Oki K. Minireview: potassium channels and aldosterone dysregulation: is primary aldosteronism a potassium channelopathy? Endocrinology. 2014;155:47–55. doi: 10.1210/en.2013-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–7. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardo NA, Yao J, Hu C, Barrett PQ. Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology. 2012;153:3579–86. doi: 10.1210/en.2012-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagliardo NA, Yao J, Hu C, Schertz EM, Tyson DA, Carey RM, Bayliss DA, Barrett PQ. TASK-3 channel deletion in mice recapitulates low-renin essential hypertension. Hypertension. 2012;59:999–1005. doi: 10.1161/HYPERTENSIONAHA.111.189662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. The EMBO journal. 2008;27:179–87. doi: 10.1038/sj.emboj.7601934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Kang YA, Hu YR, Gao L, Yang H, Li NF. Expression of GIRK4 gene in kidney tissues of obese rat. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2013;35:36–9. doi: 10.3881/j.issn.1000-503X.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Lenzini L, Caroccia B, Campos AG, Fassina A, Belloni AS, Seccia TM, Kuppusamy M, Ferraro S, Skander G, Bader M, Rainey WE, Rossi GP. Lower expression of the TWIK-related acid-sensitive K+ channel 2 (TASK-2) gene is a hallmark of aldosterone-producing adenoma causing human primary aldosteronism. J Clin Endocrinol Metab. 2014;99:E674–82. doi: 10.1210/jc.2013-2900. [DOI] [PubMed] [Google Scholar]
- Liang B, Nissen JD, Laursen M, Wang X, Skibsbye L, Hearing MC, Andersen MN, Rasmussen HB, Wickman K, Grunnet M, Olesen SP, Jespersen T. G-protein-coupled inward rectifier potassium current contributes to ventricular repolarization. Cardiovasc Res. 2014;101:175–84. doi: 10.1093/cvr/cvt240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods (San Diego, Calif) 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E. Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol. 2000;24:321–8. doi: 10.1677/jme.0.0240321. [DOI] [PubMed] [Google Scholar]
- Mesirca P, Marger L, Toyoda F, Rizzetto R, Audoubert M, Dubel S, Torrente AG, Difrancesco ML, Muller JC, Leoni AL, Couette B, Nargeot J, Clapham DE, Wickman K, Mangoni ME. The G-protein-gated K+ channel, IKACh, is required for regulation of pacemaker activity and recovery of resting heart rate after sympathetic stimulation. J Gen Physiol. 2013;142:113–26. doi: 10.1085/jgp.201310996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–8. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 1994;135:431–8. doi: 10.1210/endo.135.1.8013381. [DOI] [PubMed] [Google Scholar]
- Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE. Effect of KCNJ5 Mutations on Gene Expression in Aldosterone-Producing Adenomas and Adrenocortical Cells. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2011-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Harris RB, Rainey WE, Seki T. Sodium Deficiency Regulates Rat Adrenal Zona Glomerulosa Gene Expression. Endocrinology. 2014:en20131999. doi: 10.1210/en.2013-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Rigsby CS, Wang T, Mukai K, Gomez-Sanchez CE, Rainey WE, Seki T. Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology. 2012;153:1755–63. doi: 10.1210/en.2011-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles M, Sebastian S, Tinker A. HL-1 cells express an inwardly rectifying K+ current activated via muscarinic receptors comparable to that in mouse atrial myocytes. Pflugers Arch. 2010;460:99–108. doi: 10.1007/s00424-010-0799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira EF, Gerry D, Mantero F, Mariniello B, Rainey WE. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin Endocrinol (Oxf) 2010;73:22–9. doi: 10.1111/j.1365-2265.2009.03738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE. The potassium channel, Kir3.4 participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology. 2012;153:4328–35. doi: 10.1210/en.2012-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton D, Bandulik S, Schweda F, Haubs S, Tauber P, Reichold M, Cong LD, El Wakil A, Budde T, Lesage F, Lalli E, Zennaro MC, Warth R, Barhanin J. Task3 potassium channel gene invalidation causes low renin and salt-sensitive arterial hypertension. Endocrinology. 2012;153:4740–8. doi: 10.1210/en.2012-1527. [DOI] [PubMed] [Google Scholar]
- Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195:39–48. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) 2007;66:607–18. doi: 10.1111/j.1365-2265.2007.02775.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitative RT-PCR analysis of Cyp11b2, Kcnk3, Kcnk9, and Kcnj5 mRNA expression in rat adrenal, brain, and heart by sex. No differences were observed between male and female mRNA levels. Fold changes are compared to the average mRNA levels of male and female rats combined. Values represent mean ± S.E.M.
Relative mRNA expression patterns of KCNK9 in human and rat determined by semi-quantitative RT-PCR. (A) KCNK9 mRNA expression patterns among rat brain, rat adrenal, human brain, and human adrenal. (B) Comparison of KCNK9 mRNA expression between rat and human adrenals (n=4 for each).