Background: Cholesterol crystals are involved in various chronic inflammatory diseases; however, direct pattern recognition receptors (PRRs) recognizing cholesterol crystals have not been identified.
Results: We purified endogenous components interacting with human Mincle and identified cholesterol.
Conclusion: Direct interaction of human Mincle with cholesterol crystals activates innate immune responses.
Significance: Human Mincle could be a therapeutic target of chronic inflammation.
Keywords: cholesterol-binding protein, dendritic cell, inflammation, innate immunity, pattern recognition receptor (PRR), C-type lectin receptor, cholesterol crystals
Abstract
C-type lectin receptors (CLRs) are an emerging family of pattern recognition receptors that recognizes pathogens or damaged tissue to trigger innate immune responses. However, endogenous ligands for CLRs are not fully understood. In this study, we sought to identify an endogenous ligand(s) for human macrophage-inducible C-type lectin (hMincle). A particular fraction of lipid extracts from liver selectively activated reporter cells expressing hMincle. MS analysis determined the chemical structure of the active component as cholesterol. Purified cholesterol in plate-coated and crystalized forms activates reporter cells expressing hMincle but not murine Mincle (mMincle). Cholesterol crystals are known to activate immune cells and induce inflammatory responses through lysosomal damage. However, direct innate immune receptors for cholesterol crystals have not been identified. Murine macrophages transfected with hMincle responded to cholesterol crystals by producing pro-inflammatory cytokines. Human dendritic cells expressed a set of inflammatory genes in response to cholesterol crystals, and this was inhibited by anti-human Mincle. Importantly, other related CLRs did not bind cholesterol crystals, whereas other steroids were not recognized by hMincle. These results suggest that cholesterol crystals are an endogenous ligand for hMincle and that they activate innate immune responses.
Introduction
Pattern recognition receptors (PRRs)2 play vital roles in innate immune responses by recognizing pathogen-associated molecular patterns such as nucleotides, lipids, carbohydrates, proteins, and other components of pathogens as non-self ligands (1). PRRs consist of several groups of receptor subfamilies including Toll-like receptors (TLRs), retinoic acid-inducible gene-I-like receptors (RLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), and C-type lectin receptors (CLRs) (2, 3). In addition to pathogen-associated molecular patterns, PRRs sense damage-associated molecular patterns (DAMPs) exposed or released by necrotic cells (4). Among them, CLRs share at least one conserved carbohydrate recognition domain through which a variety of ligands are recognized (5). Most commonly, CLRs bind a carbohydrate moiety on various ligands. However, recent accumulating evidence suggests that CLRs recognize a wide variety of ligands including proteins, lipids, and crystals (5, 6).
Macrophage-inducible C-type lectin (Mincle, also called Clec4e or Clecsf9) is a CLR expressed on activated macrophages and dendritic cells (DCs) (7). We have previously demonstrated that Mincle associates with the FcRγ chain, an immunoreceptor tyrosine-based activation motif (ITAM)-containing adaptor protein, to transmit signals (8). We have also shown that Mincle recognizes glycolipid ligand, such as trehalose 6,6′-dimycolate (TDM), the most immunostimulatory component of mycobacteria, as well as glyceroglycolipids from various Malassezia species (9, 10). In addition to pathogens, Mincle recognizes dying cells (8); however, it remains unclear whether Mincle recognizes lipids derived from self as DAMPs.
Cholesterol is an essential membrane component for the maintenance of cell integrity and fluidity and for a wide variety of biological activities (11). However, it has long been recognized that excessive intake of cholesterol leads to hypercholesterolemia and results in the formation of cholesterol crystals (12, 13). Cholesterol crystals are present in atherosclerotic plaques and are released into blood upon plaque rupture. The most serious symptoms triggered by the crystals have long been considered to be the formation of embolus in small vessels, causing ischemia and inflammation (14). Although cholesterol crystal deposition was previously considered to be a feature of advanced atherosclerosis, a recent study shows that cholesterol crystals are present in early atherosclerotic lesions (15). In addition, they can directly trigger inflammatory responses by inducing IL-1β and IL-1α through the activation of NLRP3 inflammasomes (15–18). As such, cholesterol crystals have been recently defined to be one of DAMPs (14, 15, 19). However, the direct recognition of cholesterol crystals by PRRs is not fully understood, although several proteins are reported to interact with cholesterol (20–23). In this study, we identify cholesterol crystals as endogenous ligands for hMincle and find that this interaction activates myeloid cells to produce pro-inflammatory molecules.
Experimental Procedures
Reagents
Cholesterol (C75209), cortisone (C2755), progesterone (P8783), estradiol (E8875), Testosterone (T1500), aldosterone (A9477), ergosterol (E5610), TDM (T3034), and LPS (L4516) were purchased from Sigma-Aldrich. Total lipid extract from bovine liver (181104C), dehydroepiandrosterone (DHEA) (700087P), cholesteryl ester 14:0 (110859P), 16:0 (110862P), 18:0 (110866P), 20:0 (110870P), and 22:0 (110875P), sitosterol (700095P), desmosterol (700060P), and cholestanoic acid (700070P) were purchased from Avanti. The Screen-Well® Fatty Acid Library BML-2803 Version 1.0 was purchased from Enzo Life Sciences. 1-(Trimethylsilyl) imidazole was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Monosodium urate crystals were purchased from InvivoGen. Cholesterol crystals were prepared as described previously (16). Briefly, cholesterol dissolved in 95% ethanol was heated to 60 °C and then left at room temperature to form crystals. Cholesterol crystals were suspended in PBS.
Cells
2B4-NFAT-GFP reporter cells expressing various C-type lectin receptors were prepared as described previously (8). For FcRγ-coupled receptors, the reporter cells expressing FcRγ alone were used as control. RAW-Blue cells were purchased from InvivoGen and were further transfected with hMincle using retrovirus-mediated gene transfer (pMX-IRES-hCD8). For preparation of human monocyte-derived DCs, human peripheral blood mononuclear cells were isolated from peripheral blood of healthy donors by Lymphocyte Separation Solution (d = 1.077) (Nacalai Tesque) gradient centrifugation. Human CD14+ monocytes were purified from peripheral blood mononuclear cells using anti-human CD14 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Dendritic cells were obtained from CD14+ monocytes after culture in RPMI 1640 supplemented with 10% FBS, non-essential amino acid, 10 ng/ml human GM-CSF, and 10 ng/ml human IL-4 for 7 days. For cell stimulation, lipid ligands were solubilized in chloroform:methanol (C:M), diluted with 2-propanol, and used as plate-coated form.
Preparation of Lipophilic Fraction
Bovine liver was treated with C:M (2:1, v/v), and soluble extract was further fractionated by high performance thin-layer chromatography (HPTLC). Each fraction was collected from the TLC plate and dissolved in C:M, followed by dilution with 2-propanol for cell stimulation.
In Vitro Stimulation Assay
Each 2-propanol-diluted lipid was added at 20 μl to a well of a 96-well plate followed by evaporation of the solvent as described previously (9). For Ab blocking, cells were stimulated with cholesterol crystals or the plate-coated form of various lipids for 18–24 h in the presence or absence of anti-human Mincle mAb 13D10-H11 (rat IgG1, κ) (24). Isotype-matched control mAb (clone, R3-34) was purchased from BD Biosciences. Reporter activity was determined by flow cytometry (2B4-NFAT-GFP cells). Concentration of murine TNF and human IL-1β was measured by the ELISA kit (BD Pharmingen).
Identification of Cholesterol
Fraction #7-15 was subjected to normal-phase HPLC (column, Cosmosil 5SLII; mobile phase, n-hexane/2-propanol (99/1); detection, λ210), and the peak was identified to represent cholesterol based on the result of the subsequent mass analysis (micrOTOF II, Bruker). Cholesterol in Fraction #7-15: tR (min) = 8.95, m/z = 409.3318 [M+Na]+, calculated for C27H46NaO, Δ milli mass unit (mmu) = 12.3. Standard cholesterol: tR (min) = 8.92, m/z = 409.3394 [M+Na]+, Δmmu = 4.7. The GC-MS analysis of the trimethylsilyl (TMS) sterols was undertaken using a Shimadzu QP-2010 GC-MS system (Kyoto, Japan), operating in electron ionization mode with an ionization energy of 70 eV. The instrument was equipped with an INERTCAP-5MS/SIL capillary column (30 m, inner diameter 0.25 mm, GL Sciences, Tokyo, Japan) with helium as carrier gas at a 0.66 ml/min flow rate. Column temperature was initially kept for 2 min at 250 °C, gradually increased to 280 °C at a rate of 5.0 °C/min, and then increased to 280 °C and kept for 20 min. The injector and interface were set to 300 and 250 °C, respectively. The gas chromatograph operated in the split-less mode. The mass spectrum was monitored starting at m/z 40 and ending at m/z 600, with a scan interval of 1.0 s and a threshold of 1000, and the solvent cut was set to 2 min. The sterol sample was heated with 1-(trimethylsilyl) imidazole (Tokyo Chemical Industry Co., Ltd.)/pyridine (1/1, 0.1 ml) for 1 h at 60 °C to give the TMS ether of sterol. The injection solution (1 ml) was TMS sterols in n-hexane.
Ig Fusion Protein
The extracellular domain of human Mincle (amino acids 46–219) was fused to the hIgG1 Fc region and prepared as described previously (10).
In Vitro Mincle Binding Assay
2 μg/ml hIgG1-Fc (Ig) and hMincle-Ig in binding buffer (20 mm Tris-HCl, 150 mm NaCl, 1 mm CaCl2, 2 mm MgCl2, pH 7.0) was incubated with 0.1 nmol/0.32 cm2 of plate-coated cholesterol. Bound protein was detected with anti-hIgG-HRP followed by the addition of colorimetric substrate. Peroxidase activity was measured by spectrophotometer.
DNA Microarray
Total RNA was isolated by RNeasy (Qiagen). DNA microarray analysis was performed using the Human Gene 1.0 ST array (Affymetrix).
Quantitative PCR
Total RNA was isolated by Sepasol-RNA I Super G (Nacalai Tesque). 1 μg of RNA sample was used for reverse transcription and synthesis of cDNA with ReverTra Ace qPCR RT Master Mix (TOYOBO). Real-time PCR was performed by using THUNDERBIRD SYBR qPCR mix (TOYOBO) and ABI PRISM 7000 (Applied Biosystems). All primers for specific target genes are listed in Table 1.
TABLE 1.
Primers used for quantitative PCR in Fig. 6B
| Gene | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| INHBA | GGGTCCACATAATACCAACCTCA | CCTGGCCTTCAAGTACCAGC |
| LPL | TCAGCTGTGTCTTCAGGGG | CTCCAGAGTCTGACCGCCT |
| AREG | GTGGTGCTGTCGCTCTTGATA | CCCCAGAAAATGGTTCACGCT |
| CLEC5A | AGGTGGCGTTGGATCAACAA | TTAGGCCAATGGTCGCACAG |
| DCSTAMP | CGCTGCCTCCTGGATTATCAC | AAGCTCTTTGCCCTTAGGTTG |
| CSF1 | TGGCGAGCAGGAGTATCAC | AGGTCTCCATCTGACTGTCAAT |
| SLC28A3 | CAAAGCAGGGAGCACACAAAC | ACAGGCCGAAATCACCATAAC |
| CCL4 | GCTTGCTTCTTTTGGTTTGG | CTTTTCTTACACCGCGAGGA |
| TIMP3 | CATGTGCAGTACATCCATACGG | CATCATAGACGCGACCTGTCA |
| TM4SF19 | CAAGGCTCAGTCCCAGGATA | CCCATTCTGATTCTTGACACC |
Molecular Simulation
Docking studies of the cholesterol and hMincle were performed by the MOE (Molecular Operating Environment) software using hMincle co-crystallized with citrate (Protein Data Bank (PDB) ID: 3WH2) as a template.
Statistical Analysis
An unpaired two-tailed Student's t test was used for all the statistical analyses.
Results
Identification of Cholesterol as an Endogenous Ligand of Mincle
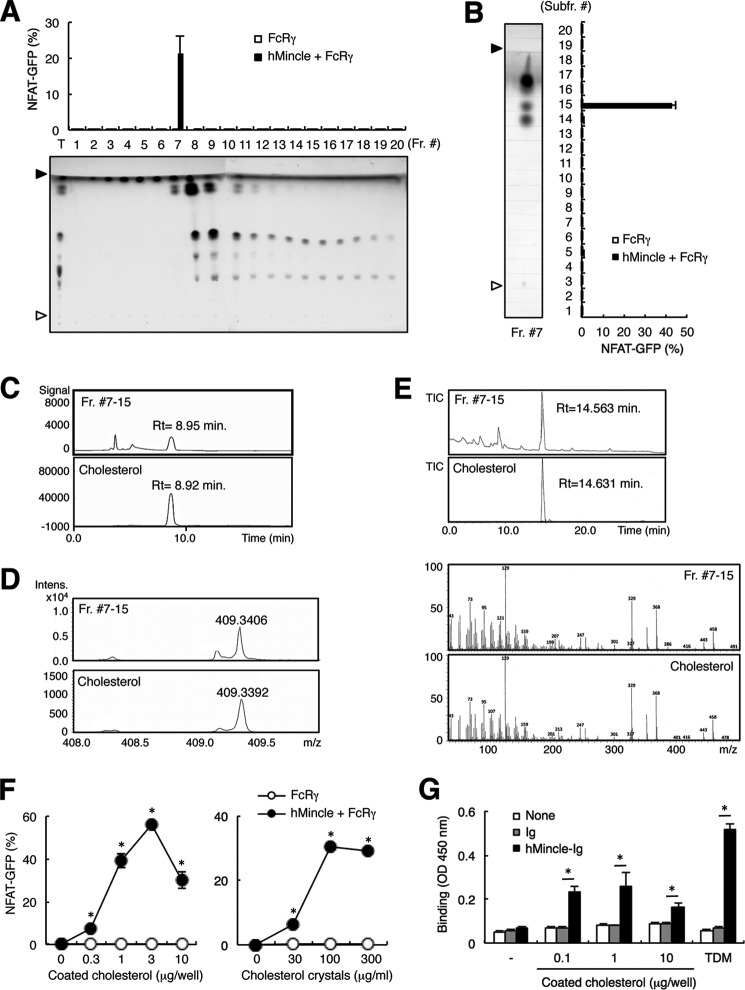
To investigate whether hMincle recognizes endogenous lipids, we first separated lipid extracts of liver into 20 fractions by silica gel column chromatography and evaluated each fraction using an NFAT-GFP reporter assay. Reporter cells transfected with FcRγ alone or together with hMincle were stimulated with each fraction in plate-coated form. Activity from these fractions showed a strong peak in Fraction #7 (Fig. 1A). We then analyzed Fraction #7 by means of HPTLC, separating it into 20 subfractions. Reporter cells expressing hMincle selectively responded to Fraction #7-15 with relatively high Rf values (Fig. 1B), suggesting that hMincle binds to low polarity lipid(s). Further analyses by HPLC and mass spectrometry revealed that the major component of Fraction #7-15 is cholesterol (Fig. 1, C and D). The cholesterol structure was also confirmed by GC-MS after esterification using TMS (Fig. 1E). Indeed, purified cholesterol in plate-coated and crystalized forms activated reporter cells expressing hMincle (Fig. 1F). We further confirmed that hMincle directly binds to coated cholesterol by using soluble hMincle-Ig fusion protein (Fig. 1G). Collectively, these results suggest that hMincle is capable of interacting with cholesterol crystals.
FIGURE 1.
hMincle recognizes plate-coated cholesterol. A, the bovine liver lipid extracts were eluted with 2 ml of C:M by silica gel column chromatography and fractionated into 20 fractions, each containing 100 μl. Each fraction was coated onto a plate to stimulate NFAT-GFP reporter cells expressing FcRγ alone or together with hMincle. The NFAT-GFP induction was analyzed by flow cytometry (upper). Each fraction was analyzed by HPTLC developed with chloroform-methanol-water (65:25:4, v/v/v). The HPTLC plate was stained by copper acetate-phosphoric acid (lower). Open and closed arrowheads show the origin and solvent fronts, respectively. T, total lipid extracts. Fr. #, fraction number. B, Fraction #7 was analyzed by HPTLC (C:M = 8:2, v/v) followed by copper acetate-phosphoric acid stain (left) and further divided into 20 subfractions (Subfr.). Open and closed arrowheads show the origin and solvent fronts, respectively. Reporter cells expressing FcRγ alone or together with hMincle were stimulated with plate-coated form in each fraction for 18 h. The NFAT-GFP induction was analyzed by flow cytometry (right). C, HPLC chromatogram of Fraction #7-15 (Fr. #7-15) (upper) and cholesterol (lower). Rt, retention time. D, positive mode electrospray ionization-TOF-MS spectra of Fraction #7-15 (upper) and cholesterol (lower). Intens., intensity. E, GC-MS analysis of Fraction #7-15 and cholesterol after esterification using TMS. The samples were subjected to GC-MS. The results are as follows: standard cholesterol TMS ether, (min) = 14.6, m/z = 458 (M+), 443 (M-15), 368, 329, 129 (base peak); cholesterol TMS-ether derived from liver, (min) = 14.6, m/z = 458 (M+), 443 (M-15), 368, 329, 129 (base peak). Upper, total ion current (TIC) chromatograms; lower, electron ionization mass spectrometry spectra. F, reporter cells expressing hMincle and FcRγ were stimulated with the plate-bound form of cholesterol (left) or cholesterol crystals (right) for 18 h. G, hIgG1-Fc (Ig) or hMincle-Ig was incubated with plate-coated cholesterol. Bound protein was detected with anti-hIgG-HRP followed by the addition of colorimetric substrate. Peroxidase activity was measured by spectrophotometer. Data are representative of two (C–E and G) or three (A, B, and F) separate experiments. Data are presented as mean ± S.D. *, p < 0.05 (F and G).
Human Mincle Selectively Binds to Cholesterol
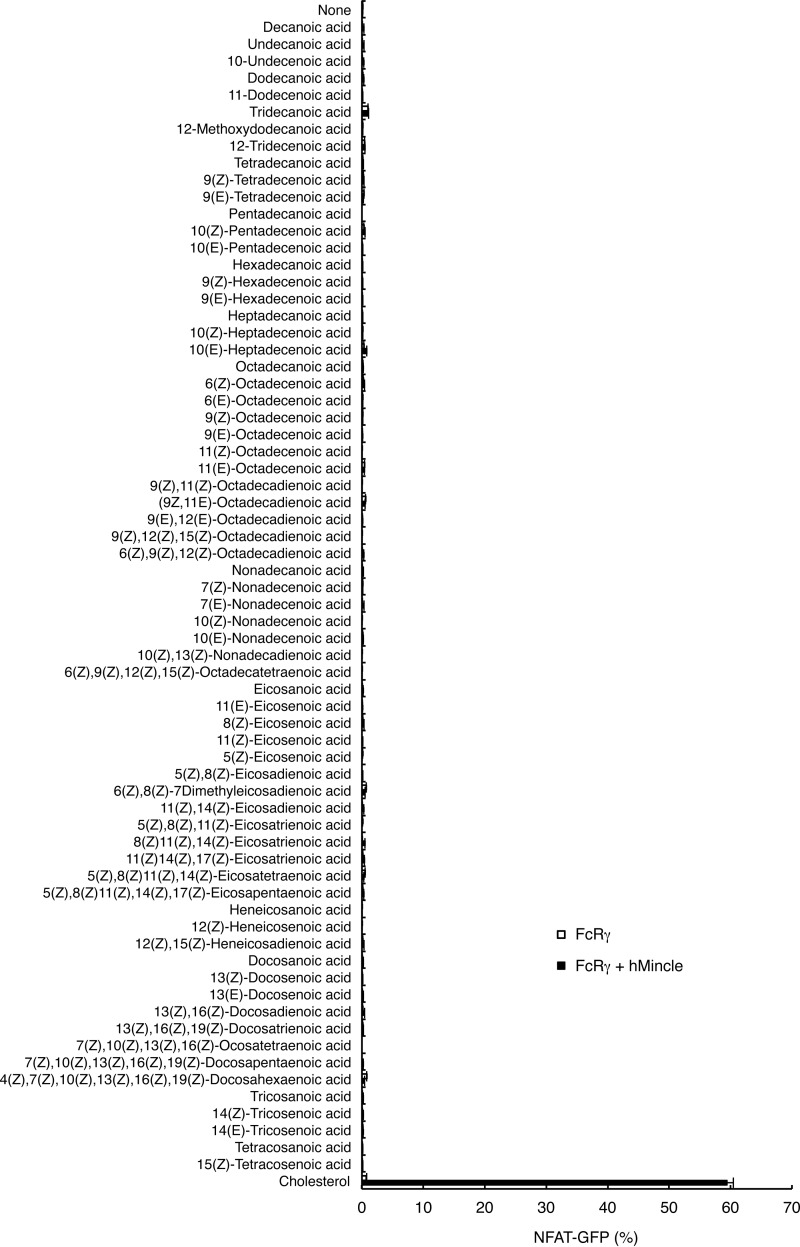
We next investigated whether hMincle recognizes other lipids. To compare the ligand activity of lipids having different properties under the same conditions, we stimulated reporter cells with plate-bound forms of various lipids. However, none of 68 fatty acids tested was able to induce NFAT activation (Fig. 2).
FIGURE 2.
hMincle did not react to fatty acids. Reporter cells expressing FcRγ alone or together with hMincle were stimulated with 10 nmol/well of fatty acids (Screen-Well® Fatty Acid Library BML-2803 Version 1.0) and plate-coated cholesterol (10 nmol/well) for 18 h. The NFAT-GFP induction was analyzed by flow cytometry. Data are representative of three independent experiments.
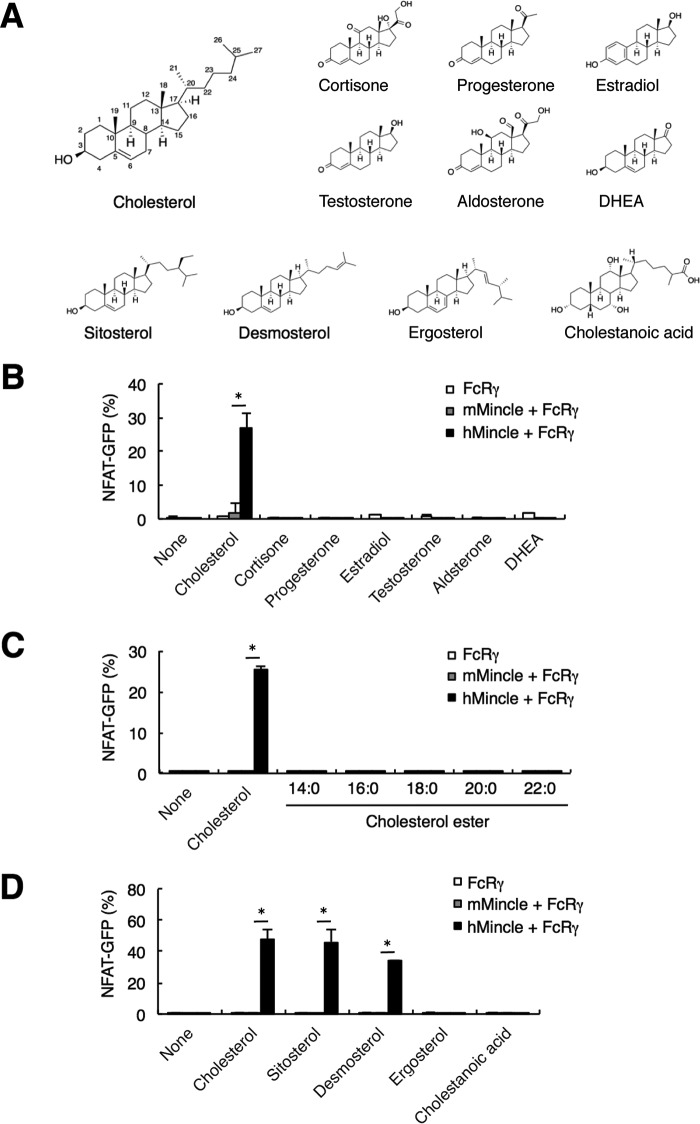
Natural steroid hormones are synthesized from cholesterol, and their core structures are similar to that of cholesterol (Fig. 3A). We therefore asked whether hMincle and mMincle recognize plate-coated steroid hormones. Of note, only cholesterol, but not cortisone, progesterone, estradiol, testosterone, aldosterone, and DHEA, showed ligand activity of hMincle but not mMincle (Fig. 3B).
FIGURE 3.
hMincle selectively responds to cholesterol. A, chemical structures of cholesterol, steroid hormones, and sterols. B, reactivity of hMincle to cortisone, progesterone, estradiol, testosterone, aldosterone, and DHEA. Each steroid hormone was coated onto a plate (1 μg/well) to stimulate reporter cells expressing FcRγ alone or together with mMincle or hMincle for 18 h. The NFAT-GFP induction was analyzed by flow cytometry. C, responsiveness of hMincle to cholesteryl esters. Plate-coated cholesteryl esters (5 μg/well) were co-cultured with reporter cells expressing FcRγ alone or together with mMincle or hMincle for 18 h. Each ester differs in the length of fatty acid (14:0,16:0, 18:0, 20:0, and 22:0). D, reactivity of hMincle to sterols. Reporter cells expressing FcRγ alone or together with mMincle or hMincle were stimulated with the indicated sterols in a plate-coated form for 18 h. Data are representative of three independent experiments (B–D). Data are presented as mean ± S.D. *, p < 0.05 (B–D).
Most ingested cholesterol is present in the esterified form; however, cholesterol esters with various lengths of saturated fatty acids had no activity in this assay (Fig. 3C). The fungal sterol, ergosterol, also had no activity when tested, whereas sitosterol, a plant sterol, and desmosterol, an intermediate of cholesterol synthesis, showed substantial activity with hMincle (Fig. 3D). Thus, among various steroids, hMincle selectively binds to cholesterol and closely related sterols. These results also suggest that a hydroxyl residue at C3 and an alkyl chain at C17 appear to be required for recognition by hMincle (Fig. 3A).
Receptor Specificity for Cholesterol Crystals among C-type Lectin Receptors
Mincle and MCL (also called Clec4d or Clecsf8), but not other CLRs, have a patch of hydrophobic amino acids that may be responsible for binding the lipid moiety of TDM (24–26). We therefore assessed whether MCL interacts with cholesterol crystals using MCL-expressing reporter cells and found that it did not (Fig. 4A). In addition, none of the other CLRs closely related to Mincle, such as Dectin-1 and Dectin-2, recognized cholesterol crystals (Fig. 4B).
FIGURE 4.
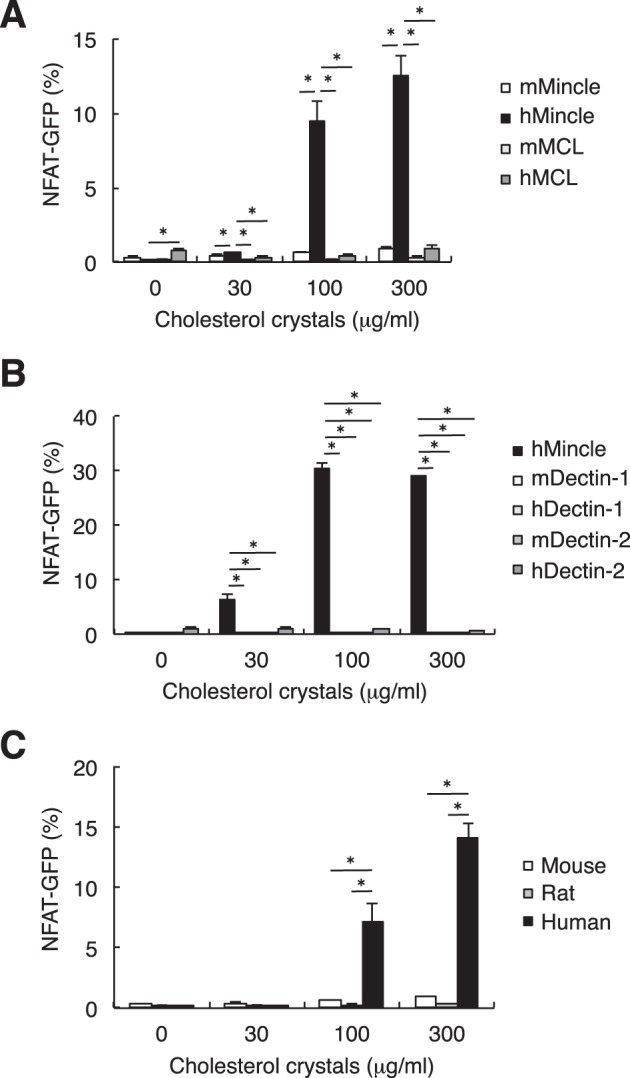
Selective binding of hMincle to cholesterol crystals. A, reporter cells expressing mMincle, hMincle, mouse MCL (mMCL), or human MCL (hMCL) were stimulated, and the NFAT-GFP induction was analyzed by flow cytometry. B, reporter cells expressing the indicated CLRs were co-cultured with cholesterol crystals for 18 h. C, reporter cells expressing FcRγ together with human, mouse, or rat Mincle were stimulated with cholesterol crystals for 18 h. The NFAT-GFP induction was analyzed by flow cytometry. All data are representative of three separate experiments and presented as mean ± S.D. *, p < 0.05.
We also examined the capability of Mincle from different species to bind cholesterol crystals. Human Mincle responded to cholesterol crystals, whereas mouse and rat Mincle did not (Fig. 4C). Taken together, among ITAM-coupled CLRs expressed in mammals, human Mincle can uniquely interact with cholesterol crystals.
hMincle Confers Murine Macrophages with Sensitivity to Cholesterol Crystals
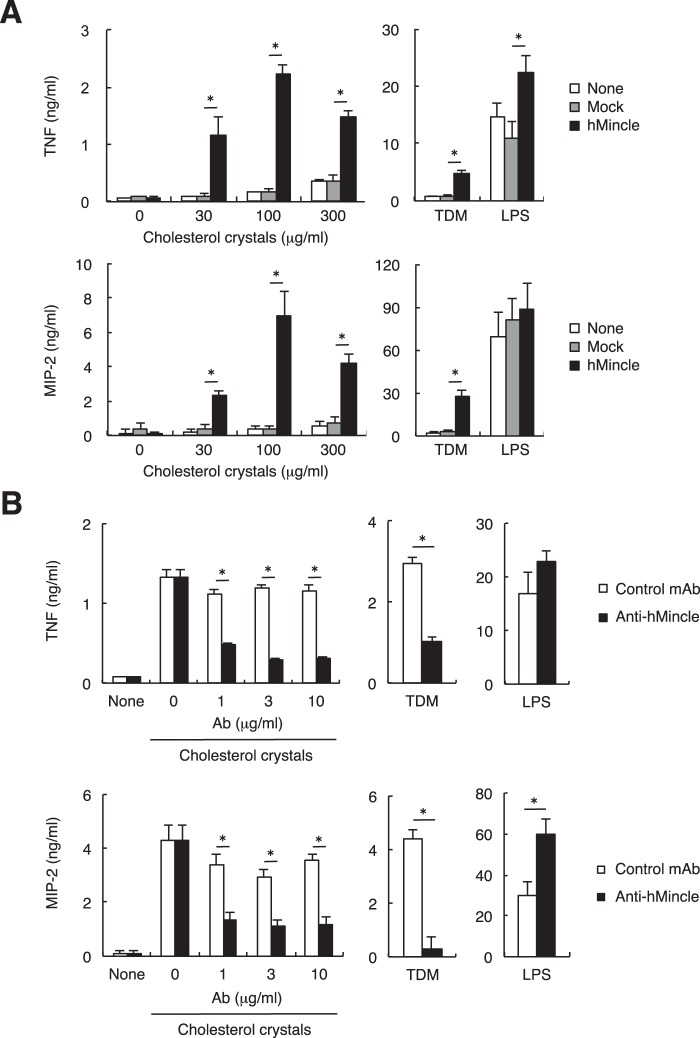
To identify cellular responses triggered by the hMincle-cholesterol interaction in myeloid cells, we examined production of proinflammatory cytokines, as these outcomes are independent of inflammasome activation (27). Consistent with the fact that mMincle did not recognize cholesterol, murine macrophage Raw cells, which express mMincle and functional FcRγ signaling pathway, were not activated by cholesterol crystals as assessed by secretion of pro-inflammatory cytokines. However, upon transfection with hMincle, Raw cells responded to cholesterol crystals by producing TNF and MIP-2 (Fig. 5A). The secretion of these cytokines was impaired in the presence of anti-hMincle blocking mAb (Fig. 5B). These results suggest that expression of hMincle in murine myeloid cells confers sensitivity to cholesterol crystals, resulting in pro-inflammatory responses.
FIGURE 5.
Interaction of hMincle with cholesterol crystals induces the production of pro-inflammatory cytokines. A, RAW-Blue cells expressing hMincle or parental cell lines were stimulated with cholesterol crystals, LPS (10 ng/ml), or TDM (100 ng/well). Supernatants were collected after 24 h of culture. Concentrations of TNF and MIP-2 were determined by ELISA. B, RAW-Blue cells expressing hMincle were stimulated with cholesterol crystals (100 μg/ml), LPS (10 ng/ml), or TDM (100 ng/ml) for 24 h in the presence or absence of the indicated amount of anti-hMincle antibody (anti-hMincle) or isotype control antibody (R3-34) (control mAb). Concentrations of TNF and MIP-2 were determined by ELISA. All data are representative of two independent experiments and presented as mean ± S.D. *, p < 0.05.
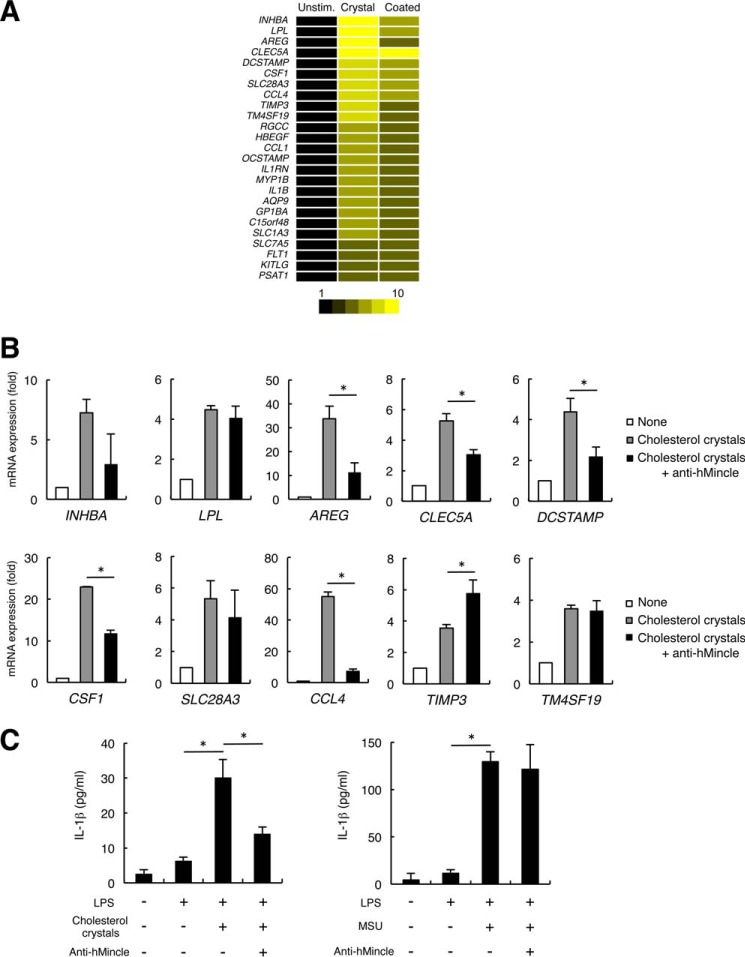
Cholesterol Crystals Induce Transcription of Inflammatory Genes through hMincle
We then examined gene expression in human DCs in response to cholesterol. Human monocyte-derived DCs (hMo-DCs), which express endogenous hMincle, were stimulated with plate-coated cholesterol or cholesterol crystals. Microarray analyses revealed that similar sets of pro-inflammatory genes including CLRs, chemokines, and cytokines were markedly up-regulated upon stimulation with the coated and crystalized forms of cholesterol (Fig. 6A). Although cholesterol crystals are known to activate inflammasomes through organelle damages (15, 16), inflammasome activation does not entirely account for the up-regulation in expression of these genes (28). Indeed, the induction of such genes, CLEC5A, CCL4, CSF1, AREG, and DCSTAMP, was impaired in the presence of anti-hMincle mAb (Fig. 6B). These results suggest that cholesterol crystals activate human DCs to induce pro-inflammatory molecules through Mincle. Cholesterol crystals were reported to induce mature IL-1β production through inflammasome activation (15–18). We then examined the role of hMincle in this process. hMo-DCs produced IL-1β by the stimulation with cholesterol crystals in the presence of LPS; however, this was substantially inhibited by anti-hMincle mAb (Fig. 6C, left). In contrast, anti-hMincle mAb did not affect IL-1β secretion induced by another inflammasome activator, monosodium urate crystals (Fig. 6C, right), which did not bind hMincle (data not shown). These results suggest that the interaction of hMincle with cholesterol crystals contributes to the secretion of mature IL-1β.
FIGURE 6.
Human DCs express inflammatory genes through hMincle in response to cholesterol crystals. A, hMo-DCs were stimulated with a plate-coated form of cholesterol or cholesterol crystals for 8 h. Gene expression was analyzed by DNA microarray. Unstim., unstimulated. B, hMo-DCs were stimulated with cholesterol crystals for 8 h in the presence or absence of anti-hMincle antibody. Gene expression was analyzed by quantitative PCR. C, hMo-DCs were stimulated with LPS (100 ng/ml) alone or in combination with cholesterol crystals (300 μg/ml) (left) or monosodium urate (MSU, 250 μg/ml) (right) in the presence or absence of anti-hMincle mAb for 2 days. The concentration of IL-1β was determined by ELISA. Data are representative of two independent experiments (A and C) or one experiment (B). Data are presented as mean ± S.D. *, p < 0.05 (B and C).
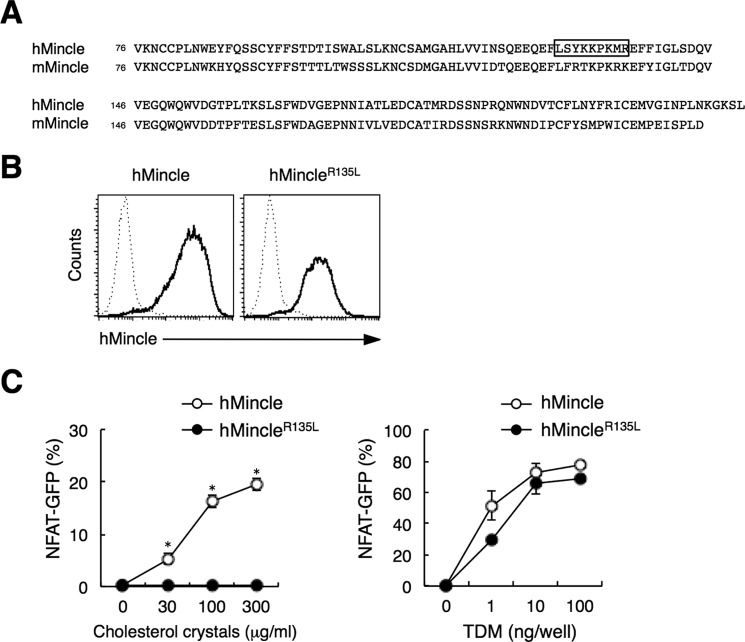
hMincle Binds to Cholesterol Crystals through Its Cholesterol-interacting Motif
It has been reported that several proteins interact with cholesterol through a cholesterol recognition/interaction amino acid consensus (CRAC) motif ((L/V)X1–5YX1–5(R/K)) (29–31). The particular amino acid sequence matching this motif is found in hMincle (LXY129XXXXXR) but not in mMincle (Fig. 7A). Replacement of Arg in this motif with Leu is known to abolish cholesterol binding (29). We therefore established reporter cell lines expressing hMincle mutants into which we introduced mutations in the CRAC-like sequence, and then assessed the effect of the mutations on cholesterol recognition ability. Although the mutant in which Arg135 was replaced with Leu (hMincleR135L) expressed normally on the cell surface (Fig. 7B), the hMincleR135L mutant failed to recognize cholesterol crystals (Fig. 7C, left). Importantly, this mutant retained the ability to recognize TDM at a level comparable with wild-type hMincle (Fig. 7C, right). These results suggest that the CRAC-like motif within hMincle determines the ability to interact with cholesterol.
FIGURE 7.
hMincle interacts with cholesterol crystals through its CRAC-like sequence. A, sequence alignment of the carbohydrate recognition domain of hMincle and mMincle. The CRAC-like sequence is boxed. B, cell surface expressions of hMincle or hMincleR135L. Reporter cells expressing hMincle or hMincleR135L mutant were left unstained (dotted line) or stained with anti-hMincle mAb (solid line), and then analyzed by flow cytometer. C, reporter cells expressing hMincle or mutant were stimulated with the indicated amount of cholesterol crystals (left) or TDM (right) for 18 h. The NFAT-GFP induction was analyzed by flow cytometry. Data are presented as mean ± S.D. *, p < 0.05. Data are representative of three separate experiments (B and C).
Discussion
In this study, we demonstrate that hMincle directly binds to cholesterol crystals and activates innate immune responses. Cholesterol crystals are metabolic danger signals that trigger NLRP3 inflammasome activation and subsequent IL-1β secretion through multiple pathways (15–18, 32). hMincle is likely to be involved in IL-1β secretion mainly by inducing pro-IL-1β (Fig. 6A) via NF-κB activation. Given that ITAM-mediated signals activate NLRP3 in a Syk-dependent manner (33, 34), Mincle may also promote cleavage of pro-IL-1β through NLRP3 activation as well as the induction of pro-IL-1β, as suggested previously (35–37). Cholesterol crystals can therefore activate two different families of innate sensors, NLRs and CLRs, which may crosstalk with each other. Indeed, the blockade of hMincle impaired IL-1β secretion even in the presence of LPS (Fig. 6C). Similarly, another inflammatory crystal, monosodium urate, is recognized by both NLRP3 and a CLR, Clec12a (6). Thus, CLRs may have a tendency to bind to the “crystal form” of endogenous components in addition to recognizing well established carbohydrate-containing compounds. The structural basis and specificity of the recognition of “non-sugar” crystals by CLRs are also important issues to be clarified in the future.
In addition to pro-inflammatory genes, we found that genes related to lipid metabolism were also up-regulated by plate-coated and crystal forms of cholesterol (Fig. 6A). However, the induction of one such gene, lipoprotein lipase (LPL), was not affected by the presence of anti-hMincle. This may suggest the involvement of other cholesterol sensor(s) in DCs that act independently of hMincle.
Cholesterol crystal deposition has been thought to arise at a later phase of atherosclerosis (38). In contrast, it was recently reported that small cholesterol crystals can be the primary inflammatory stimuli in early atherosclerotic lesions (15). It therefore remains to be determined whether hMincle initiates inflammation or aggravates already ongoing inflammation in response to cholesterol crystals under pathological conditions.
Cholesterol crystals, but not steroid hormones or cholesteryl esters, are recognized by hMincle. Although they have a similar core structure, only cholesterol has a hydrophobic iso-octyl side chain at C-17. We showed that hMincle does not respond to DHEA, which lacks a hydrophobic side chain at C-17 (Fig. 3B), suggesting that this side chain might be important for the interaction of cholesterol with hMincle. Despite the existence of the same hydrophobic iso-octyl side chain at C-17, cholesteryl esters did not activate reporter cells. In cholesteryl esters, the 3β-hydroxyl group at the C-3 position of cholesterol is esterified to a fatty acid. Thus, the 3β-hydroxyl group may also determine the binding of Mincle to cholesterol. The results from sitosterol, ergosterol, and desmosterol suggest that a single bond between C-7 and C-8 in ring B is required for the activity. Taken together, a steroid backbone with two characteristics, alkyl chain at C-17 and hydroxyl residue at C-3, may be a minimal common feature for binding to hMincle. Alternatively, but not mutually exclusively, as a relatively high concentration of cholesterol crystals is required for the activation of reporter cells, currently we cannot exclude the possibility that a small amount of “modified” cholesterol derivatives or any particular forms of cholesterol crystals may be acting as an active ligand.
To obtain a sufficient amount of candidate lipids, we first used lipid extracts concentrated from bovine liver in this study. We also detected cholesterol as an active ligand from murine liver extract (data not shown). Therefore, a series of procedures of extraction, purification, and identification established in this study may be a useful method to discover novel endogenous ligands (DAMPs) that are released or modified upon damage from various tissues. Besides, this method would be also applicable for identifying de novo-synthesized DAMPs during cellular damages or diseases from damaged tissues or patient samples.
The clear species differences between rodents and human imply that Mincle might have acquired reactivity to cholesterol during evolution (Fig. 4C). In agreement with this assumption, we found that Mincle from various primates and guinea pig has a CRAC consensus sequence at a position corresponding to that of hMincle, whereas neither mouse nor rat Mincle has such sequences (data not shown).
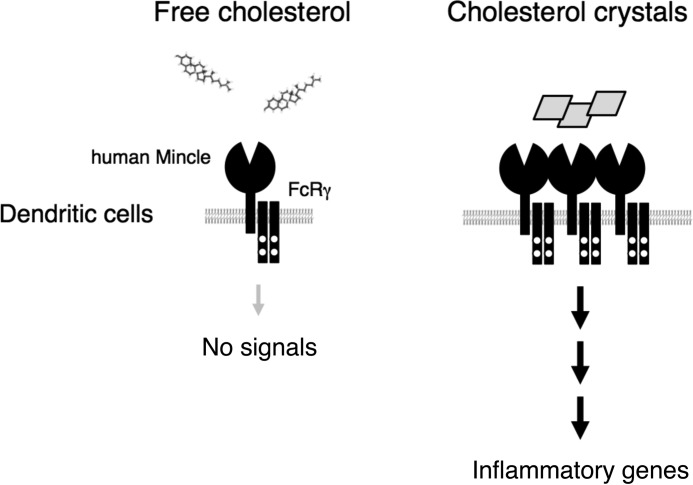
It is still unknown whether ligand recognition through the CRAC consensus sequence results in efficient receptor engagement. According to the simulation based on the crystal structure of hMincle, the CRAC-like sequence appears to form a pocket that is suitable to interact with cholesterol at a different site from that for TDM (data not shown) (24, 26). These findings suggest that the multivalent form of cholesterol, such as cholesterol crystals or coated cholesterol, could induce multimerization of hMincle in a mechanism similar to multivalent TDM in bacterial cell wall or the plate-coated form (9) (Fig. 8).
FIGURE 8.
Schematic representation of the stimulatory effect of cholesterol crystals through hMincle. Left, free or membrane-buried forms of cholesterol are not recognized by Mincle. Right, only multimerized forms of cholesterol, such as coated or crystalized forms, can activate myeloid cells through hMincle.
As cholesterol is a highly hydrophobic compound, one might argue that the interaction with hMincle is mediated by aberrant hydrophobic interactions between cholesterol and the hydrophobic portion of the protein. However, we show that other related CLRs did not bind to cholesterol, and hMincle did not interact with other hydrophobic lipid derivatives. Consistent with the fact that cholesterol is normally buried within the physiological membrane, reporter cells expressing hMincle were not activated by interacting with adjacent cells unless crystalized forms were added. Furthermore, the addition of liposomes containing cholesterol or LDLs did not activate reporter cells (data not shown). The specific recognition of cholesterol crystal by hMincle implies that hMincle is involved in a wide variety of physiological and pathological responses induced by cholesterol crystals. As murine Mincle did not interact with cholesterol, more detailed studies are needed to address this issue by establishing humanized animal models, such as hMincle knock-in mice.
To the best of our knowledge, human Mincle is the first surface PRR to be demonstrated to recognize cholesterol crystals and activate innate immunity. As excessive cholesterol and its crystals are closely related to various metabolic disorders and chronic inflammation, this finding may indicate the blockade of human Mincle as a possible therapeutic option in such diseases.
Author Contributions
R. K., E. I., and T. M. performed and analyzed the experiments and wrote the paper. M. O. wrote the paper. T. I. provided the materials. S. Y. designed the experiments and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank I. Matsunaga, M. Sugita, K. Yamasaki, S. Torigoe, and K. Toyonaga for discussions and the Laboratory for Technical Support at the Medical Institute of Bioregulation for DNA sequence and microarray analyses.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (40312946) and Ono Medical Research Foundation (to S. Y.). The authors declare that they have no conflicts of interest with the contents of this article.
- PRR
- pattern recognition receptor
- NLR
- nucleotide-binding oligomerization domain-like receptor
- CLR
- C-type lectin receptor
- DAMP
- damage-associated molecular pattern
- hMincle
- human Mincle
- murine Mincle
- mMincle
- MCL
- macrophage C-type lectin
- DC
- dendritic cell
- hMo-DC
- human monocyte-derived DC
- ITAM
- immunoreceptor tyrosine-based activation motif
- TDM
- trehalose 6,6′-dimycolate
- FcR
- Fc receptor
- TMS
- trimethylsilyl
- NFAT
- nuclear factor of activated T-cells
- C:M
- chloroform:methanol
- HPTLC
- high performance thin-layer chromatography
- CRAC
- cholesterol recognition/interaction amino acid consensus
- Ig
- hIgG1-Fc.
References
- 1. Geijtenbeek T. B., Gringhuis S. I. (2009) Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson M. J., Sancho D., Slack E. C., LeibundGut-Landmann S., Reis e Sousa C. (2006) Myeloid C-type lectins in innate immunity. Nat. Immunol. 7, 1258–1265 [DOI] [PubMed] [Google Scholar]
- 3. Takeuchi O., Akira S. (2010) Pattern recognition receptors and inflammation. Cell 140, 805–820 [DOI] [PubMed] [Google Scholar]
- 4. Sancho D., Reis e Sousa C. (2013) Sensing of cell death by myeloid C-type lectin receptors. Curr. Opin. Immunol. 25, 46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Osorio F., Reis e Sousa C. (2011) Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 34, 651–664 [DOI] [PubMed] [Google Scholar]
- 6. Neumann K., Castiñeiras-Vilariño M., Höckendorf U., Hannesschläger N., Lemeer S., Kupka D., Meyermann S., Lech M., Anders H. J., Kuster B., Busch D. H., Gewies A., Naumann R., Groß O., Ruland J. (2014) Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity 40, 389–399 [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto M., Tanaka T., Kaisho T., Sanjo H., Copeland N. G., Gilbert D. J., Jenkins N. A., Akira S. (1999) A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 163, 5039–5048 [PubMed] [Google Scholar]
- 8. Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. (2008) Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 9. Ishikawa E., Ishikawa T., Morita Y. S., Toyonaga K., Yamada H., Takeuchi O., Kinoshita T., Akira S., Yoshikai Y., Yamasaki S. (2009) Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206, 2879–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., Tateno H., Uno J., Hirabayashi J., Mikami Y., Takeda K., Akira S., Saito T. (2009) C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. U.S.A. 106, 1897–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabas I. (2002) Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J. Clin. Invest. 110, 905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ory D. S. (2004) Nuclear receptor signaling in the control of cholesterol homeostasis: have the orphans found a home? Circ. Res. 95, 660–670 [DOI] [PubMed] [Google Scholar]
- 13. Saric M., Kronzon I. (2011) Cholesterol embolization syndrome. Curr. Opin. Cardiol. 26, 472–479 [DOI] [PubMed] [Google Scholar]
- 14. Lusis A. J. (2000) Atherosclerosis. Nature 407, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., Wright S. D., Hornung V., Latz E. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajamäki K., Lappalainen J., Oörni K., Välimäki E., Matikainen S., Kovanen P. T., Eklund K. K. (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5, e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freigang S., Ampenberger F., Spohn G., Heer S., Shamshiev A. T., Kisielow J., Hersberger M., Yamamoto M., Bachmann M. F., Kopf M. (2011) Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur. J. Immunol. 41, 2040–2051 [DOI] [PubMed] [Google Scholar]
- 18. Freigang S., Ampenberger F., Weiss A., Kanneganti T. D., Iwakura Y., Hersberger M., Kopf M. (2013) Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat. Immunol. 14, 1045–1053 [DOI] [PubMed] [Google Scholar]
- 19. Lim R. S., Suhalim J. L., Miyazaki-Anzai S., Miyazaki M., Levi M., Potma E. O., Tromberg B. J. (2011) Identification of cholesterol crystals in plaques of atherosclerotic mice using hyperspectral CARS imaging. J. Lipid Res. 52, 2177–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stewart C. R., Stuart L. M., Wilkinson K., van Gils J. M., Deng J., Halle A., Rayner K. J., Boyer L., Zhong R., Frazier W. A., Lacy-Hulbert A., El Khoury J., Golenbock D. T., Moore K. J. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheedy F. J., Grebe A., Rayner K. J., Kalantari P., Ramkhelawon B., Carpenter S. B., Becker C. E., Ediriweera H. N., Mullick A. E., Golenbock D. T., Stuart L. M., Latz E., Fitzgerald K. A., Moore K. J. (2013) CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bae Y. S., Lee J. H., Choi S. H., Kim S., Almazan F., Witztum J. L., Miller Y. I. (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 104, 210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore K. J., Sheedy F. J., Fisher E. A. (2013) Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furukawa A., Kamishikiryo J., Mori D., Toyonaga K., Okabe Y., Toji A., Kanda R., Miyake Y., Ose T., Yamasaki S., Maenaka K. (2013) Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc. Natl. Acad. Sci. U.S.A. 110, 17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feinberg H., Jégouzo S. A., Rowntree T. J., Guan Y., Brash M. A., Taylor M. E., Weis W. I., Drickamer K. (2013) Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 288, 28457–28465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jégouzo S. A., Harding E. C., Acton O., Rex M. J., Fadden A. J., Taylor M. E., Drickamer K. (2014) Defining the conformation of human mincle that interacts with mycobacterial trehalose dimycolate. Glycobiology 24, 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanneganti T. D., Ozören N., Body-Malapel M., Amer A., Park J. H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E. P., Akira S., Núñez G. (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440, 233–236 [DOI] [PubMed] [Google Scholar]
- 28. Hasegawa M., Imamura R., Motani K., Nishiuchi T., Matsumoto N., Kinoshita T., Suda T. (2009) Mechanism and repertoire of ASC-mediated gene expression. J. Immunol. 182, 7655–7662 [DOI] [PubMed] [Google Scholar]
- 29. Li H., Papadopoulos V. (1998) Peripheral-type benzodiazepine receptor function in cholesterol transport: identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 139, 4991–4997 [DOI] [PubMed] [Google Scholar]
- 30. Epand R. M. (2006) Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 45, 279–294 [DOI] [PubMed] [Google Scholar]
- 31. Gimpl G. (2010) Cholesterol-protein interaction: methods and cholesterol reporter molecules. Subcell. Biochem. 51, 1–45 [DOI] [PubMed] [Google Scholar]
- 32. Tall A. R., Yvan-Charvet L. (2015) Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gross O., Poeck H., Bscheider M., Dostert C., Hannesschläger N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., Mocsai A., Tschopp J., Ruland J. (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436 [DOI] [PubMed] [Google Scholar]
- 34. Yasukawa S., Miyazaki Y., Yoshii C., Nakaya M., Ozaki N., Toda S., Kuroda E., Ishibashi K., Yasuda T., Natsuaki Y., Mi-ichi F., Iizasa E., Nakahara T., Yamazaki M., Kabashima K., Iwakura Y., Takai T., Saito T., Kurosaki T., Malissen B., Ohno N., Furue M., Yoshida H., Hara H. (2014) An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat. Commun. 5, 3755. [DOI] [PubMed] [Google Scholar]
- 35. Desel C., Werninghaus K., Ritter M., Jozefowski K., Wenzel J., Russkamp N., Schleicher U., Christensen D., Wirtz S., Kirschning C., Agger E. M., Prazeres da Costa C., Lang R. (2013) The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One 8, e53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schweneker K., Gorka O., Schweneker M., Poeck H., Tschopp J., Peschel C., Ruland J., Gross O. (2013) The mycobacterial cord factor adjuvant analogue trehalose-6,6′-dibehenate (TDB) activates the Nlrp3 inflammasome. Immunobiology 218, 664–673 [DOI] [PubMed] [Google Scholar]
- 37. Shenderov K., Barber D. L., Mayer-Barber K. D., Gurcha S. S., Jankovic D., Feng C. G., Oland S., Hieny S., Caspar P., Yamasaki S., Lin X., Ting J. P., Trinchieri G., Besra G. S., Cerundolo V., Sher A. (2013) Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through Mincle/CARD9 signaling and the inflammasome. J. Immunol. 190, 5722–5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stary H. C., Chandler A. B., Dinsmore R. E., Fuster V., Glagov S., Insull W. Jr., Rosenfeld M. E., Schwartz C. J., Wagner W. D., Wissler R. W. (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92, 1355–1374 [DOI] [PubMed] [Google Scholar]