Background: The role of calcium-regulated kinases in lineage specific cardiac progenitor cells (CPCs) is unknown.
Results: Nuclear calcium/calmodulin-dependent kinase II δB isoform (CaMKIIδB) is up-regulated during cardiac progenitor commitment, which facilitates survival and differentiation of CPCs.
Conclusion: Augmentation of CaMKIIδB in CPCs enhances differentiation into the cardiomyogenic lineages.
Significance: The ability of CaMKIIδB to enhance cardiomyogenesis maybe relevant for regenerative-based therapies.
Keywords: Ca2+/calmodulin-dependent protein kinase II (CaMKII), cell death, cell differentiation, histone deacetylase 4 (HDAC4), stem cells
Abstract
Ca2+/Calmodulin-dependent protein kinase II (CaMKII) signaling in the heart regulates cardiomyocyte contractility and growth in response to elevated intracellular Ca2+. The δB isoform of CaMKII is the predominant nuclear splice variant in the adult heart and regulates cardiomyocyte hypertrophic gene expression by signaling to the histone deacetylase HDAC4. However, the role of CaMKIIδ in cardiac progenitor cells (CPCs) has not been previously explored. During post-natal growth endogenous CPCs display primarily cytosolic CaMKIIδ, which localizes to the nuclear compartment of CPCs after myocardial infarction injury. CPCs undergoing early differentiation in vitro increase levels of CaMKIIδB in the nuclear compartment where the kinase may contribute to the regulation of CPC commitment. CPCs modified with lentiviral-based constructs to overexpress CaMKIIδB (CPCeδB) have reduced proliferative rate compared with CPCs expressing eGFP alone (CPCe). Additionally, stable expression of CaMKIIδB promotes distinct morphological changes such as increased cell surface area and length of cells compared with CPCe. CPCeδB are resistant to oxidative stress induced by hydrogen peroxide (H2O2) relative to CPCe, whereas knockdown of CaMKIIδB resulted in an up-regulation of cell death and cellular senescence markers compared with scrambled treated controls. Dexamethasone (Dex) treatment increased mRNA and protein expression of cardiomyogenic markers cardiac troponin T and α-smooth muscle actin in CPCeδB compared with CPCe, suggesting increased differentiation. Therefore, CaMKIIδB may serve as a novel modulatory protein to enhance CPC survival and commitment into the cardiac and smooth muscle lineages.
Introduction
Cardiac regeneration, homeostatic, or after acute myocardial damage, is in part supported by the migration of stem and progenitor cells from the bone marrow and endogenous cardiac niches (1, 2). Stem cells with cardiomyogenic potential were identified based on expression of the receptor tyrosine kinase c-kit and termed as cardiac stem cells (CSCs)2 present in early cardiac development and in the adult heart (3, 4). Importantly, c-kit+ cells are up-regulated temporally after myocardial damage by undergoing proliferation and commitment toward the cardiomyogenic lineage confirmed by genetic lineage tracing (5, 6). CSCs isolated and expanded ex vivo acquire cardiac specific transcription factors and are referred to as cardiac progenitor cells (CPCs) (7). CPCs exhibit properties of self-renewal and multipotency and can give rise to cardiomyocytes, endothelial, and smooth muscle lineages in vitro (8). The clinical relevancy of CPCs has been further validated by isolation of stem cells from human cardiac tissue used in the Stem Cell Infusion in Patients with Ischemic Cardiomyopathy (SCIPIO) Phase I clinical trial (9). However, the intrinsic mechanisms involved in the regulation of CPC survival, proliferation and direct cardiomyogenic commitment have not been elucidated.
Calcium (Ca2+) is an integral second messenger, regulating cellular processes such as cellular survival, proliferation, growth, and differentiation (10). Increases in intracellular Ca2+ bind to calmodulin, which then activates Ca2+/calmodulin-dependent serine/threonine kinase, a class of enzymes known as CaMKs (11). CaMKII is the predominant enzyme expressed in cardiac tissue and can be activated with oxidative stress following cardiac injury (12). Chronic up-regulation of the kinase results in cardiomyocyte hypertrophy leading to cardiac failure in mouse models (13, 14). CaMKIIδ, the main isoform expressed in the heart, is elevated in heart failure samples implicating CaMKII in the regulation of proper cardiomyocyte contractility (15, 16). However, the distinct role of CaMKII and the main cardiac δ isoforms in resident CPCs has not been previously addressed.
CaMKIIδB and CaMKIIδC are the predominant splice variants described in the adult myocardium. CaMKIIδB localization remains differentiated from CaMKIIδC because of a nuclear-localized sequence. Yet CaMKIIδB expression is not exclusive to the nucleus as the CaMKII holoenzyme is formed by a majority of δ subunits (17, 18). Nuclear CaMKIIδ (B isoform) regulates cellular growth through indirect de-repression of myocyte enhancer factor 2 (MEF2) after phosphorylation and inactivation of the histone deacetylase 4 (HDAC4) (18–20). Furthermore, CaMKIIδB has been shown to promote cellular protection by binding to the transcription factor GATA4 and indirectly inhibiting the expression of inflammatory genes (21–23).
CaMKIIδB regulates vascular smooth muscle cell migration, proliferation, and growth suggesting kinase activity is not limited to cardiomyocytes (24, 25). CaMKII is linked to the regulation of proliferation and differentiation of embryonic stem cells after inhibition of Class II HDACs (26). CaMKIIδB phosphorylation of HDAC4 induces translocation to the cytosol, thereby relieving its inhibitory action and allowing transcription of genes involved in cell cycle arrest and lineage specific differentiation in a variety of stem cells (18–20, 27–29). Currently the use of HDAC inhibitors such as Trichostatin A and 5-aza cytidine are used to increase the efficiency of reprogramming and differentiation of stem cells, supporting the role of HDACs in maintaining pluripotency and proliferation (27). Therefore, this study aims to characterize a CaMKIIδB-dependent mechanism of cardiac progenitor survival and cardiogenic commitment.
Experimental Procedures
Cardiac Progenitor Cell Isolation
Adult CPCs were isolated from 12-week-old FVB male mice as previously described (30).
Induction of Differentiation
CPCs were cultured in full medium as previously described (30) and used as a control. Differentiation was induced by culturing CPCs in α-minimal essential media (α-MEM) supplemented with 10% fetal bovine serum (FBS) with the addition of 10 nm dexamethasone (Dex) for 6 days. Treatment of CPCs with α-MEM/10% FBS without Dex was used as an additional control.
Lentiviral Constructs and Cell Transduction
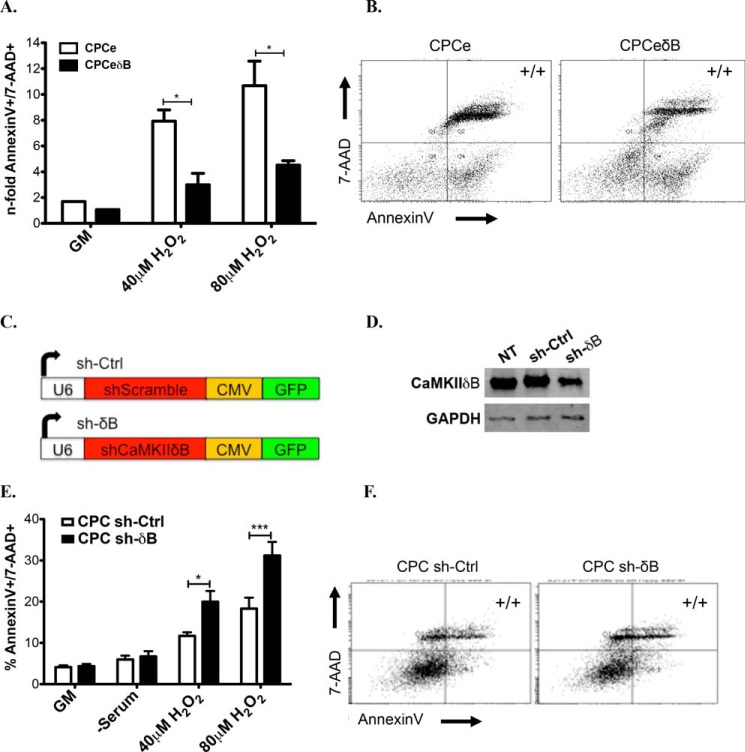
Bicistronic lentiviral constructs were created to overexpress a HA-tagged CAMKIIδ gene under control of a myeloproliferative sarcoma virus LTR-negative control region deleted promoter and enhanced green fluorescence protein (eGFP) driven off a vIRES. The control lentivirus drives expression of eGFP alone. Transduction of CPCs with bicistronic lentiviruses expressing eGFP or HA-CaMKIIδB-eGFP was performed with a multiplicity of infection of 10. Cells were allowed 48 h to express eGFP (CPCe) or HA-CaMKIIδB-eGFP (CPCeδB), then purified after fluorescence activated cell sorting (FACS) by placing one-cell per well of a 96-micro plate to allow for clonal expansion. Three CPCe and five CPCeδB clones were derived. Silencing lentiviral constructs utilized the U6 promoter to drive expression of small hairpin targeted RNA. Two shRNAs were created, a scrambled control and sh toward CaMKIIδB. Both constructs had an inserted cytomegalovirus (CMV) eGFP cassette to fluorescently label transduced cells. Transduction with sh- scrambled control (sh-Ctrl) or sh to CaMKIIδB (sh-δB) was performed with a MOI of 10 and allowed 48 h for knockdown before purifying eGFP-positive cells by FACS.
Evaluation of Cell Morphology
Images of cultured CPCs were obtained on a Leica DMIL microscope and outlines were traced using ImageJ software as previously described (31).
Proliferation Assay and Cell Doubling Time
Cell proliferation was determined using the CyQuant Direct Cell Proliferation Assay (Life Technologies) according to the manufacturer's instruction. CPCs were plated at a density of 500 cells in 100 μl of full growth medium per well of a 96-well flat bottom microplate. Assay was conducted on day of plating, day 2, day 4, and day 6 followed by the addition of 100 μl of CyQUANT direct green fluorescent nucleic acid stain in each well and incubated for 60 min. Green fluorescence intensity was measured at 495 nm using a plate reader (Tecan) and represented as fold change to the fluorescence intensity measured on the day of plating (day 0). Subsequent measurements were determined at the indicated days. Population doubling times were calculated using the readings from CyQuant Direct Proliferation Assay and use of a population doubling time online calculator.
Cell Cycle Analysis
Cells were fixed in 70% ethanol and stored overnight at −20 °C. Cell cycle distribution was analyzed by staining with propidium iodide (PI) plus RNase staining buffer (BD Biosciences) at 37 °C for 15 min. Analysis was performed using BD FACSCanto and data processed by FlowJo software to determine relative distributions of cells in G1 and G2/M phases of the cell cycle.
MTT Assay
CPCe and CPCeδB were subjected to a MTT colorimetric reducing assay, which analyzes (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), a yellow tetrazole being reduced to purple formazan in cells. This assay was used to determine metabolic activity between cell lines. In this assay 500 cells were plated per well in a 96-well flat bottom plate incubated with 10 μl of 5 mg/ml MTT reagent for 4 h. Reaction was stopped with 100 μl of diluted HCL for lysis overnight. The MTT assay was performed on the day of plating, 3 and 6 days after plating in CPC growth medium, α-MEM/10% FBS alone or α-MEM/10% FBS with Dex. Absorbance was read at 595 nm with a plate reader (Tecan).
Cell Death Assay
CPCs were plated in a 6-well dish (80,000 cells per well) and incubated in starvation medium (growth factor and FBS-depleted medium) for 18 h. The cells were then treated with either 40 or 80 μm hydrogen peroxide for 4 h. Analysis of cell death was initiated by collecting cells in suspension and adherent cells and pelleting at 1600 rpm for 5 min. The pellet was then resuspended in Annexin V binding buffer and incubated with Annexin V (1:40) from BD Biosciences combined with 7-amino-actinomycin D (7-AAD) staining to label apoptotic and necrotic cells, respectively. Data were acquired on a FACS Canto (BD Biosciences) and analyzed with FACS Diva software (BD Biosciences). Cell death was quantitated by co-labeling of Annexin V and 7-AAD and represented as a fold change or percentage relative to cells in starvation medium alone.
Immunoblot Analyses
Protein lysates from whole hearts and cultured cells were prepared in sample buffer as previously described (31). Protein lysates from homogenized tissue were incubated 1:1 with sample buffer. CPCs were either lysed directly with sample buffer or were fractionated into nuclear and cytosolic compartments according to manufacturer's protocol prior to incubating in sample buffer (PARIS Kit, Life Technologies). All protein samples for Western blot analysis were separated on a 4–12% Bis-Tris mini-gel (Life Technologies) and transferred to a polyvinylidene difluoride membrane. Membranes were blocked for 1 h with 10% milk in TBST (1% Tris-buffered saline/0.1% Tween) and then probed with primary antibody overnight in milk. Next day blots were washed with TBST buffer and incubated in secondary antibodies in milk for 1.5 h. Primary antibodies for Western blot are as follows: rabbit anti-CaMKIIδ (1:15000; UC Davis, Dr. Donald Bers), mouse anti-HDAC4 (1:1,000; Cell Signaling #5392)rabbit anti-phospho-HDAC4 (Ser-632)/HDAC5 (Ser-661)/HDAC7 (Ser-486) (1:1000; Cell Signaling #3424) goat anti-GFP (1:1000; Rockland 600-101-215), mouse anti-HA tag (1:200; Santa Cruz Biotechnology, Inc. sc-7392), mouse anti-p16 (1:200, Santa Cruz Biotechnology, Inc. F-9 and F-12 clones sc-55600, sc-1661), mouse anti-p53 (1:500, Abcam ab26), rabbit anti-α-sarcomeric actinin (1:500; Abcam ab72592), mouse anti-α-smooth muscle actin (1:1000; Sigma Aldrich a2547), rabbit anti-Tie2 (1:100; Santa Cruz Biotechnology Inc. sc-9026), mouse anti-mouse β-actin (1:500; Santa Cruz Biotechnology Inc sc-81178), rabbit anti-Mef2c (1:500; Cell Signaling #5030), rabbit anti-Cyclin B1 (1:500; Cell Signaling #12231), rabbit anti-Cyclin D1 (1:1000; Cell Signaling #2978), rabbit anti-phospho histone H3 (1:1000, Life Technologies 44–1190G), mouse anti-GAPDH (1:3000; Millipore AB2302), and rabbit anti-Lamin A (C-terminal) (1:1000; Sigma Aldrich L1293).
Immunocytochemistry
CPCs were plated at a density of 15,000 per well of a two-chamber permanox slide. Cells were then fixed in 4% paraformaldehyde for 60 min then washed in phosphate-buffered saline (PBS). Cells were permeabilized in 0.1% Triton X in PBS for 2 min and washed twice with PBS. Cells were then blocked in 10% horse serum in PBS for 1 h and then incubated with primary antibody overnight. Next day, cells were washed in PBS and incubated with secondary antibody for 1.5 h. Cells were imaged on a Leica TCS SP2 confocal microscope. Primary antibodies are as follows: rabbit anti-CaMKIIδ (1:1500; UC Davis, Dr. Donald Bers) and rat-anti tubulin (1:50, Harlan).
Immunohistochemistry
Heart sections were deparaffinized, and antigens were retrieved in 1 mmol/liter citrate (pH 6.0), followed by a 1-h block in 1% Tris/NaC1 plus blocking reagent (Perkin Elmer) or TNB. Primary antibodies were incubated overnight. Slides were washed in 1% Tris/NaC1 (TN) followed by secondary antibody incubation for 2 h. Cells were washed after secondary antibodies in TN followed by a final wash containing DAPI (1:10,000 in TN for 10 min to stain for nuclei). Primary antibodies are as follows: goat anti-CD117 (c-kit) (1:100, R&D Systems), mouse anti-cardiac troponin T (1:100, Abcam). CD117 required tyramide amplification (Perkin Elmer).
In Situ Detection of CaMKIIδ in CPCs
c-kit cells were identified in paraffin heart sections after immunohistochemical staining and use of a Leica TCS SP8 confocal microscope. In the Leica software, a measuring tool was used to create a region of interest around the border of a c-kit+ cell and the nucleus separately. The mean fluorescence value was measured and used to represent the relative expression levels of CaMKIIδ in the whole cell and the nuclear compartment of c-kit+ cells. Mean fluorescence was normalized to area of interest.
RNA Extraction and Quantitative Real Time PCR
Reverse transcriptase was performed using protocol described for iScript cDNA Synthesis Kit (BIORAD). cDNA was diluted 1:100 in nuclease-free molecular grade water before performing qRT-PCR. cDNA was incubated with primers at 100 nm concentration. Real time PCR was performed using iQ SYBR Green (Bio-Rad) following the manufacturer's protocol. The fold change in gene expression was determined using the ddCt method. CaMKIIδB and CaMKIIδC forward and reverse primers were used as previously described (22). Primer sequences are as follows: cardiac troponin t, forward 5′-ACCCTCAGGCTCAGGTTCA-3′ and reverse 5′-GTGTGCAGTCCCTGTTCAGA-3′; α-smooth muscle actin mouse forward 5′-GTTCAGTGGTGCCTCTGTCA-3′ and reverse 5′-ACTGGGACGACATGGAAAAG-3′; Mef2c, forward 5′-CGGTGTCGTCAGTTGTATGG-3′ and reverse 5′-TGCAGTAGATATGCGGCTTG-3′; Bcl-2, forward 5′-AGGAGCAGGTGCCTACAAGA-3′ and reverse 5′-GCATTTTCCCACCACTGTCT-3′; 18S forward 5′-CGAGCCGCCTGGATACC-3′ and reverse 5′-CATGGCCTCAGTTCCGAAAA-3′.
Experimental Animals
The review board of the Institutional Animal Care and Use Committee at San Diego State University approved all animal protocols and studies.
Statistical Analysis
All data are expressed as mean ± S.E. Statistical analyses were done using unpaired Student's t test, one way-ANOVA with Tukey post-tests or using two way ANOVA with Bonferroni post-tests using Graph Pad Prism v5.0. A value of p < 0.05 was considered statistically significant.
Results
CaMKIIδ Is Expressed in CPCs during Post-natal Growth and Is Up-regulated after Pathological Stress
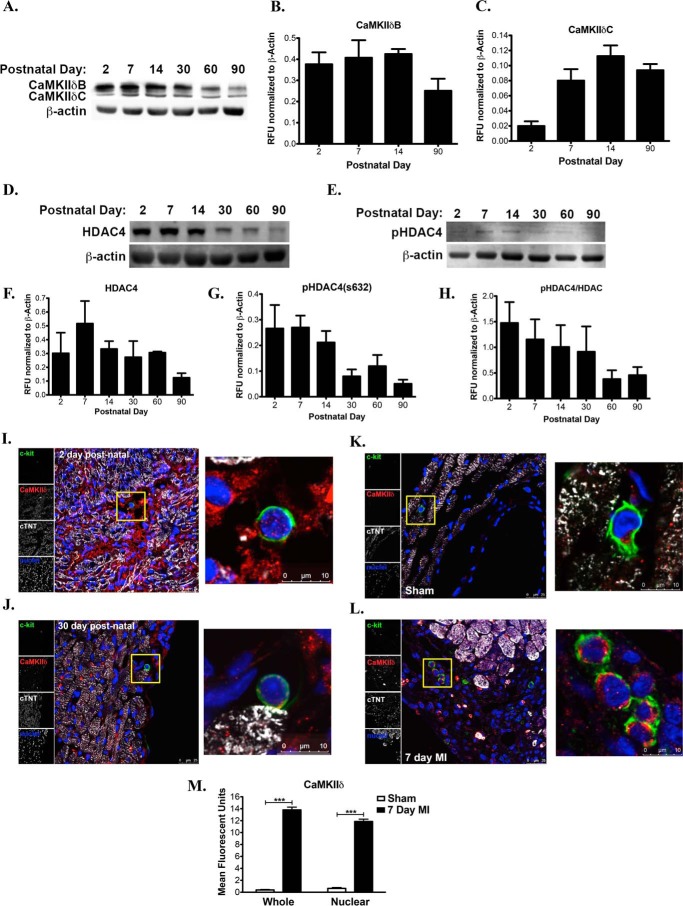
To validate levels of CaMKIIδ isoforms during different post-natal stages, immunoblot and immunohistochemical analysis was performed using whole heart lysates and heart tissue sections from mouse origin. CaMKIIδB protein expression is elevated in hearts from post-natal day 2 through 30 and decreases with progression into adulthood (Fig. 1, A and B). CaMKIIδC protein levels steadily increase with hypertrophic growth maintaining protein expression in the adult mouse heart (Fig. 1, A and C). Since both CaMKIIδB and CaMKIIδC are known to inhibit HDAC4 activity by phosphorylation on the serine 632 (20). We assessed total and phosphorylated HDAC4 in the developing heart. HDAC4 and phosphorylated HDAC4 on the serine 632 (pHDAC4) decrease with aging consistent with the role of HDAC4 to suppress growth in cardiomyocytes (Fig. 1, D–G). Normalized pHDAC4 to total HDAC4 is highest in the early post-natal heart (2-day-old heart) and decreases by 2-fold in the adult heart (Fig. 1H). These data show that increased phosphorylation of HDAC4 is critical during neonatal growth, which is followed by dephosphorylating in the post-mitotic heart (32).
FIGURE 1.
CaMKIIδ is expressed in CPCs during post-natal growth and is up-regulated after pathological stress. A, CaMKIIδB (top band) and CaMKIIδC (bottom band) protein expression in whole mouse heart lysates at increasing postnatal days. B, quantitation of CaMKIIδB and C, CaMKIIδC expression levels represented as relative fluorescent units (RFU) normalized to β-actin. D, total HDAC4 levels in the post-natal mouse heart. E, phosphorylated HDAC4 on the serine 632 in the post-natal and adult mouse heart. F, quantitation of HDAC4 expression normalized to β-actin represented as RFU. G, quantitation of pHDAC4 expression normalized to β-actin represented as RFU. H, phosphorylated HDAC4 over total HDAC4 in the post-natal and adult mouse heart. I, 2-day-old and J, 30-day-old hearts visualized for expression and localization of CaMKIIδ in myocytes and c-kit+ CPCs. K, CaMKIIδ labeling in CPCs in sham-operated mice or L, following MI surgery for 7 days. M, quantitation of CaMKIIδ expression and localization (whole or nuclear) in endogenous CPCs in sham and 7 day MI-treated mice. Mice are 3 months of age prior to MI surgery. Cardiac troponin T (cTNT) and TO-PRO-3 iodide were used to label myocardium and nuclei, respectively. ***, p < 0.0001 Sham versus 7 Day MI.
The increased presence of c-kit+ CPCs during post-natal growth coincides with a burst in cardiomyocyte proliferation and hypertrophic growth (33). To determine if endogenous c-kit+ CPCs express CaMKIIδ isoforms in vivo, CPCs were co-labeled with a pan CaMKIIδ antibody. CaMKIIδ protein is detected in cardiomyocytes during an early postnatal day heart (Fig. 1I) and is down regulated in a 30-day-old heart based on immunohistochemical analysis (Fig. 1J). At the same post-natal time points, CaMKIIδ protein was detected in c-kit labeled CPCs at low levels (Fig. 1, I and J). CPCs up regulate both cytoplasmic and nuclear CaMKIIδB 7 days after myocardial infarction (MI) relative to c-kit cells discovered in 3-month-old sham surgery controls (Fig. 1, K–M). Overall, differential localization and expression of CaMKIIδ in CPCs in vivo suggests relevance of this kinase in stem cells after pathological stress and possibly during early commitment.
CaMKIIδB Localizes to the Nucleus and Inhibits HDAC4 in CPCs Undergoing Cardiogenic Commitment
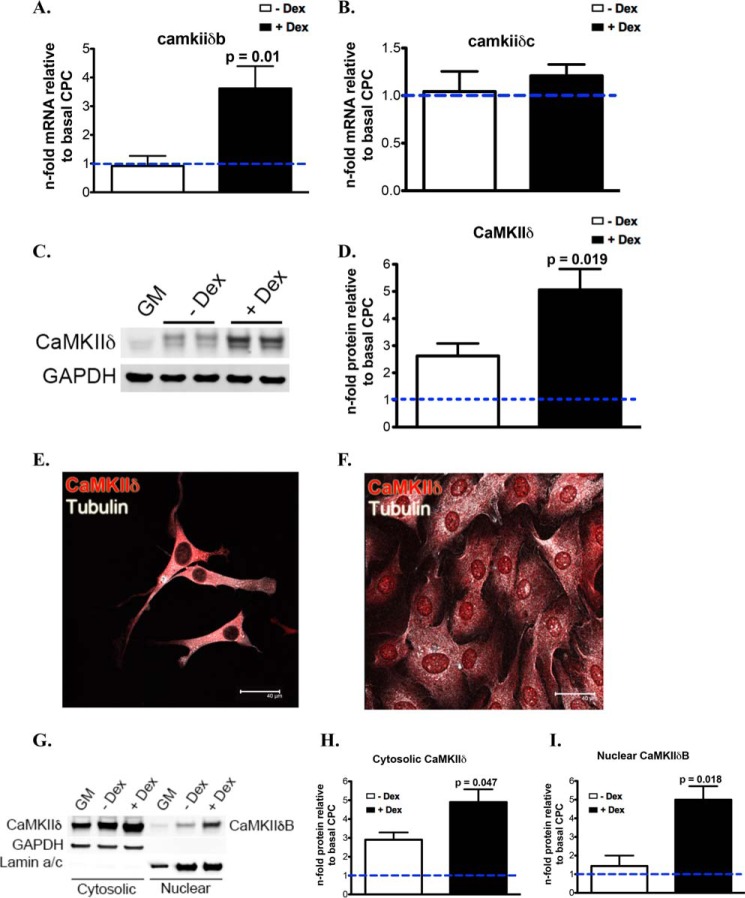
To investigate CaMKIIδ expression and localization in CPCs during basal and differentiation conditions, CPCs were isolated from the adult myocardium based on established protocols (33). CPC commitment is characterized by decreased proliferation and increased cellular size, concomitant with expression of proteins of the cardiomyogenic lineage following Dex treatment (34). Relative to basal levels, CaMKIIδB mRNA levels are up-regulated in CPCs treated with Dex (Fig. 2A), whereas CaMKIIδC mRNA levels remain unchanged when comparing cells treated without and with Dex (Fig. 2B). Following stimulation with Dex, CPCs have increases in both CaMKIIδ isoforms in whole cell lysates visualized after immunoblotting (Fig. 2, C and D). CaMKIIδ is primarily localized in the cytosol of CPCs during regular growth medium conditions (Fig. 2E), and is up-regulated after differentiation along with a marked presence of CaMKIIδB in the nucleus of CPCs confirmed by immunocytochemistry (Fig. 2C, 2D, and 2F). Furthermore, fractionated CPCs were analyzed for CaMKIIδ in the cytoplasmic and nuclear compartments of the cells, confirming that CaMKIIδB localizes in the nucleus during commitment of CPCs (Fig. 2, G–I).
FIGURE 2.
CaMKIIδB localization to the nucleus is increased in CPCs during commitment. A, CaMKIIδB and B, CaMKIIδC mRNA in CPCs with and without dexamethasone (Dex) stimulation for 7 days represented as a fold change relative to CPCs maintained in growth medium (GM). C, CaMKIIδ protein levels in whole cell lysates with and without Dex stimulation. D, quantitation of total CaMKIIδ levels relative to GM-treated CPCs. E, CaMKIIδ protein is primarily cytosolic in CPCs without differentiation stimulus. F, CaMKIIδ increases in expression and localizes to the nuclear compartment of CPCs after 6 days of Dex treatment. G, CaMKIIδ expression in the cytosolic and nuclear fractions of CPCs after induction with differentiation medium. H, quantitation of cytosolic CaMKIIδ and I, nuclear CaMKIIδB. Graphs are represented as a fold change relative to basal CPCs in cytosolic and nuclear fractions. GAPDH is probed as a loading control for immunoblotting. p values compare CPCs in 10% FBS α-MEM without Dex to CPCs treated with Dex.
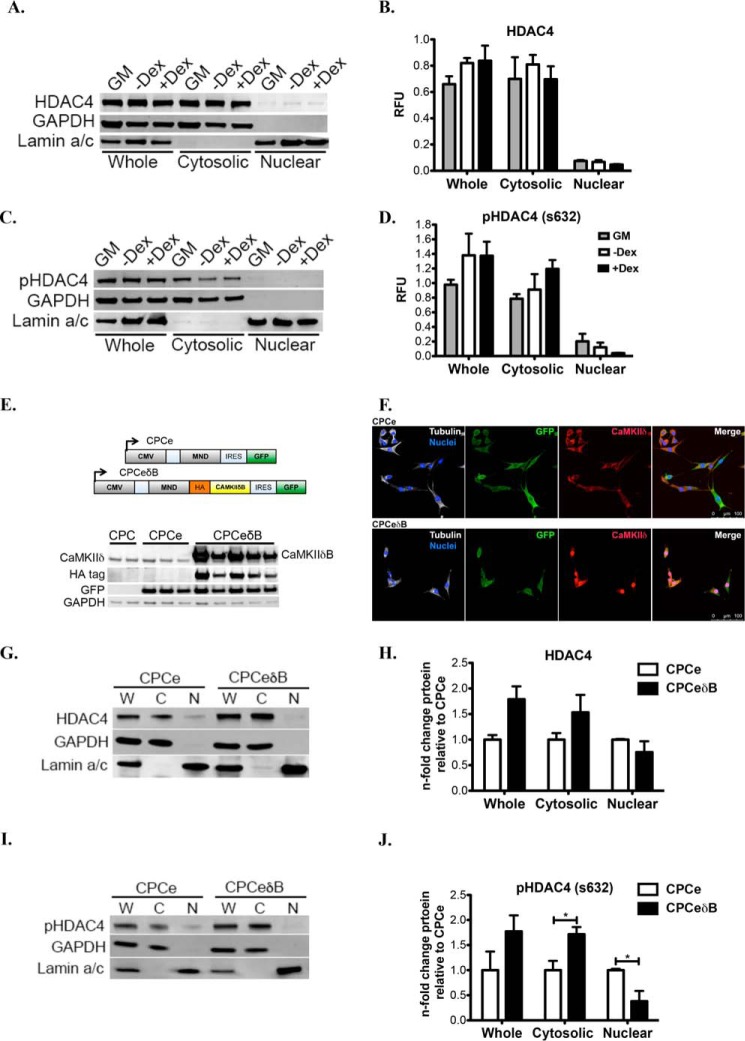
CaMKIIδB-dependent regulation of HDAC4 has been previously reported (19, 20); therefore, we attempted to investigate if this also occurs in our isolated CPCs. Inactivated HDAC4 (pHDAC4) protein trended toward an increase in the cytoplasmic fraction of CPCs treated with differentiation medium (Fig. 3, C and D), indicating a correlative role during differentiation to increase CaMKIIδB in the nucleus and de-repress genes necessary for growth and differentiation. However, total HDAC levels were not significantly changed in CPCs without and with Dex treatment (Fig. 3, A and B). To validate that HDAC4 is a target of CaMKIIδ in CPCs, we used a lentivirus to overexpress CaMKIIδB (CPCeδB) confirmed by protein analysis to detect CaMKIIδ, the HA tag and green fluorescent protein (GFP) (Fig. 3, E and F). CPCs expressing GFP alone was used as a control (CPCe) (Fig. 3, E and F). Although total HDAC4 levels were unchanged in CPCe relative to CPCeδB (Fig. 3, G and H), CPCeδB have increased cytosolic pHDAC4 relative to CPCe, correlating with a decreased level of pHDAC4 in the nuclear compartment (Fig. 3, I and J). These results suggest that CaMKIIδB overexpression inactivates HDAC4.
FIGURE 3.
CaMKIIδB localizes to the nucleus and inhibits HDAC4 in CPCs undergoing cardiogenic commitment. A, immunoblot of total HDAC4 and protein levels in whole, cytosolic and nuclear fractions of CPCs treated with GM, −Dex, or + Dex supplemented medium. B, quantitation of total HDAC4. C, immunoblot of pHDAC4 (s632) protein levels in whole, cytosolic, and nuclear fractions of CPCs treated with GM, −Dex, or + Dex-supplemented medium. D, quantitation of pHDAC4 (s632). Immunoblots are presented as a fold change relative to CPCs in GM after normalization to GAPDH or Lamin A (C terminus). E, lentiviral constructs to establish CPCe and CPCeδB lines (top). CPCeδB lines overexpress CaMKIIδB as well as the HA tag. CPCe overexpress eGFP. GAPDH was probed as a loading control (bottom). F, CPCe (top) and CPCeδB (bottom) fluorescent images. Cells were stained with antibodies toward GFP and CAMKIIδ. Cell morphology and nuclei were visualized by staining for tubulin and with DAPI, respectively. G, CPCe and CPCeδB lines probed for total HDAC4 levels by immunoblot and H, quantitation in whole cell lysates, cytosolic and nuclear fractions. I, phosphorylated HDAC4 on the serine 632 protein levels in CPCe or CPCeδB by immunoblot and J, quantitated from whole cell lysates,cytosolic, and nuclear fractions. Immunoblots are probed with GAPDH to normalize for protein loading of the whole and cytosolic lysates. Lamin A (C terminus) antibody was utilized to normalize for loading of the nuclear fraction. Total and phosphorylated HDAC4 are represented as a fold change relative to CPCe. *, p < 0.05 CPCe versus CPCeδB. W, whole cell; C, cytosolic fraction; and N, nuclear fraction.
Nuclear CaMKIIδ Overexpression in CPCs Promotes Increases in Cellular Size and Decreased Proliferation
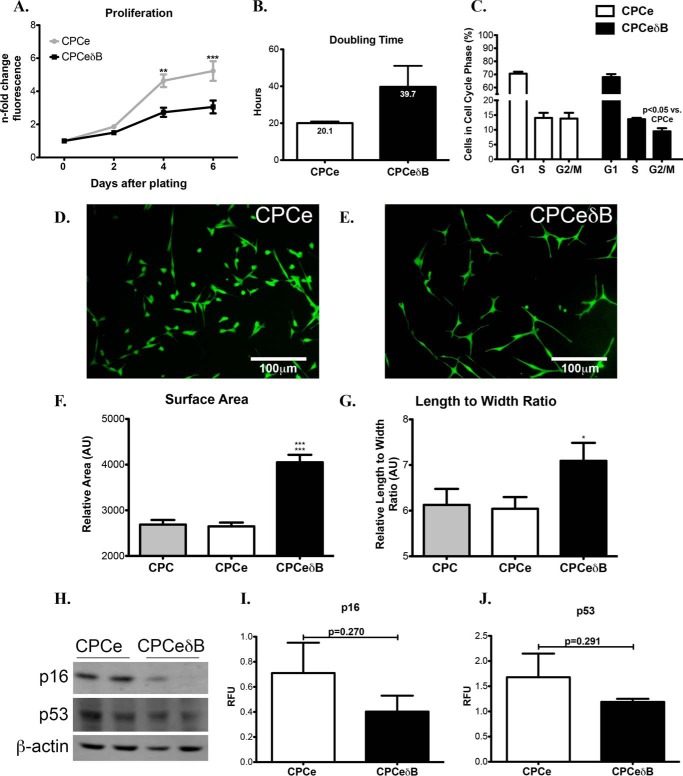
Overexpression of CaMKIIδB in CPCs presented phenotypic characteristics that are relevant to increased cardiomyogenic differentiation (35). In support of this, CPCeδB show decreased proliferative potential (Fig. 4A) and increased cell doubling time (Fig. 4B) compared with CPCe. CPCeδB are less present in the G2/M phase of the cell cycle compared with CPCe (Fig. 4C). Interestingly, CPCeδB have increased cellular size based on measurement of cell surface area (Fig. 4F) and cell length to width ratio (Fig. 4G) during basal growth conditions relative to CPCe (Fig. 4, D–G). Cellular senescence was not evident in CPCeδB based on p53 or p16 protein levels (Fig. 4, H–J). Therefore, the decreased proliferative rate and larger cell morphology exhibited by CPCeδB is not due to increased senescence or irreversible cell cycle arrest.
FIGURE 4.
Nuclear CaMKIIδ overexpression in CPCs promotes increases in cellular size and decreased proliferation. A, CPCeδB has decreased proliferative capacity relative to CPCe based on a fluorescent nucleic acid stain measured at 2, 4, and 6 days after seeding equal cell numbers. ** and *** p < 0.01 and p < 0.0001 CPCeδB relative to CPCe. B, CPCeδB populations shows an increased doubling time relative to CPCe. C, cell cycle analysis using flow cytometry in CPCe and CPCeδB. D, fluorescent images of CPCe and E, CPCeδB populations in growth media. F, CPCeδB shows an increase in relative cell surface area. ***, p < 0.0001 CPCeδB relative to CPC and CPCe. G, CPCeδB shows an increase in length to width ratio relative to non-modified CPCs and CPCe. *, p < 0.01 CPCeδB relative to CPCe. H, expression of senescence markers p16 and p53 by immunoblot. β-Actin was probed as a loading control. I, quantitation of p16 and J, p53 in CPCe and CPCeδB populations.
CaMKIIδB Overexpression in CPCs Enhances Expression of Pro-survival and Cardiac Lineage Commitment Markers and Down-regulates Proteins Involved in Mitotic Progression
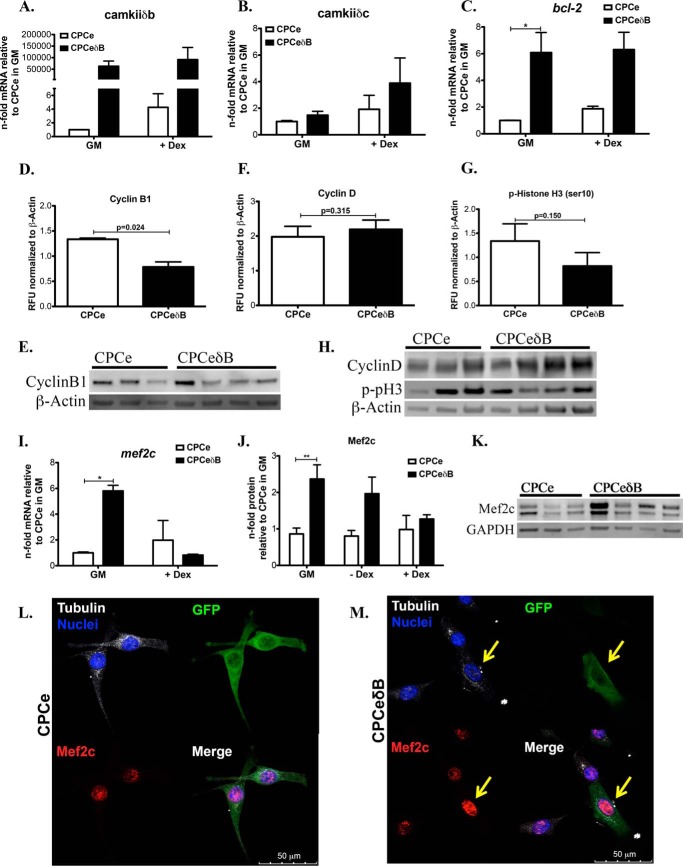
Overexpression of CaMKIIδB in CPCs was additionally confirmed by qRT-PCR, whereas the levels of CaMKIIδB are highly expressed during growth conditions and remained stable after the addition of Dex (Fig. 5A). CaMKIIδB overexpression did not induce CaMKIIδC during growth conditions, but was marginally increased with addition of Dex (Fig. 5B). CPCeδB express higher levels of Bcl-2 mRNA before and after the addition of a differentiation stimulus (Fig. 5C), supporting the role of CaMKIIδB in pro-survival signaling in CPCs. Next, we wanted to associate downstream molecules related to decreased proliferation in CPCeδB. Cell cycle regulator cyclin B1, a protein involved in mitotic progression, was significantly down regulated in CPCeδB (Fig. 5, D and E). Cyclin D and phosphohistone H3, a marker of the G1 phase of the cell cycle and mitosis, respectively, were unchanged in CPCeδB compared with CPCe (Fig. 5, F–H). Mef2c cardiac transcription factor expression in CPCs supports progression of myocyte progenitor precursor cells in to cells of the cardiomyogenic lineage (36). Additionally, CaMKIIδB overexpression is known to enhance the up-regulation of Mef2c to promote growth and maturation of cardiomyocytes (18). As expected, Mef2c gene expression in CPCeδB was increased and is consistent with elevated protein expression during basal cell culture conditions compared with CPCe (Fig. 5, I–M). Mechanistically, CaMKIIδB expression promotes cell survival and cardiac lineage commitment through the up-regulation of Bcl-2 and Mef2c. Furthermore, CaMKIIδB down-regulates cyclins that are associated with mitotic division leading to a slower growth rate.
FIGURE 5.
CaMKIIδB overexpression in CPCs enhances expression of pro-survival and cardiac lineage commitment markers and down-regulates proteins involved in mitotic progression. A, CaMKIIδB; B, CaMKIIδC; C, Bcl-2 gene expression in CPCe and CPCeδB during growth conditions or with the addition of dex. Values are represented as n-fold mRNA relative to CPCe in GM and after normalization to ribosomal 18S. D, cyclin B1 protein quantitation. E, cyclin B1 protein immunoblot representation. F, cyclin D and G, p- histone H3 (Ser-10) quantitation. H, cyclin D and p-Histone H3 immunoblot representation. Values are represented as RFU after normalizing to loading control β-Actin. I. Mef2c gene expression in CPCe and CPCeδB during growth conditions or with the addition of dex. Values are represented as n-fold mRNA relative to CPCe in GM and after normalization to ribosomal 18S. *, p < 0.05. J, Mef2c protein expression in CPCs cultured in GM, medium without the addition of dex or with the addition of dex. Values are represented as n-fold mRNA relative to CPCe in GM and after normalization to loading control GAPDH. **, p < 0.01. K, immunoblot representation of Mef2c in CPCe and CPCeδB maintained in GM conditions alone, quantitation in J. n = 3 CPCe and n = 4 C CPCeδB were analyzed for immunoblotting. L and M, Mef2c protein intensity within nuclei of CPCe and CPCeδB. M, CPCeδB identified by GFP staining express higher Mef2c in the nucleus. Cell morphology and nuclei were visualized by staining for tubulin and with DAPI, respectively.
CaMKIIδB Overexpression in CPCs Increases Cell Commitment Toward the Cardiomyogenic Lineage
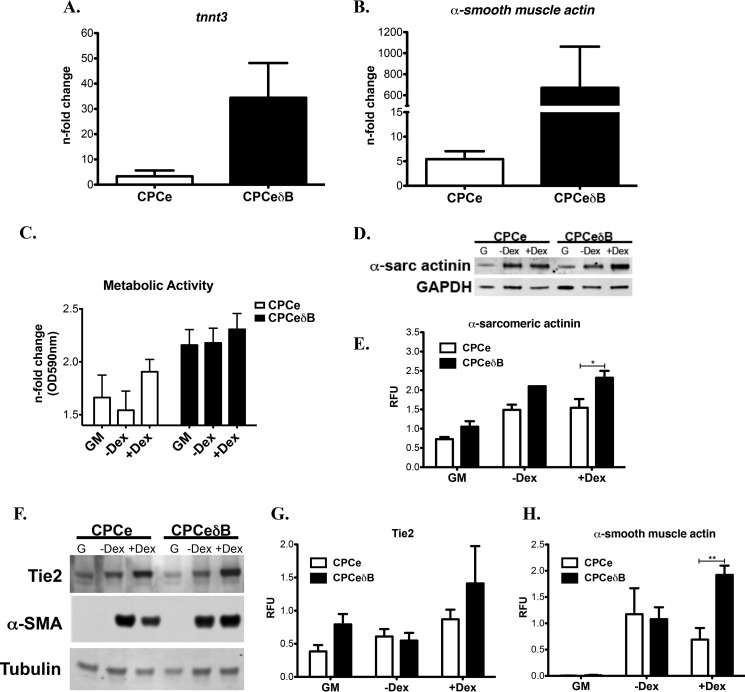
CPCeδB treated with Dex for 6 days shows a strong up-regulation of cardiac and smooth muscle markers such as cardiac troponin T (tnnt3, cTNT) and α-smooth muscle actin (α-SMA) based on mRNA levels (Fig. 6, A and B). Consistent with increased differentiation phenotype, CPCeδB have elevated metabolic activity during growth conditions and during differentiation, where CPCe has increased metabolic activity only with the addition of Dex (Fig. 6C). Furthermore, cardiac protein marker α-sarcomeric actinin was up-regulated in Dex treated CPCeδB (Fig. 6, D and E). CPCeδB commitment to the vascular endothelial and smooth muscle differentiation is increased relative to CPCe based on immunoblot analysis of endothelial marker Tie2 (Fig. 6, F and G) and smooth muscle marker α-SMA (Fig. 6, F and H). These data indicate that CaMKIIδB can drive the differentiation of CPCs and enhance the transition to mature cells of the cardiac, endothelial, and smooth muscle lineages.
FIGURE 6.
CaMKIIδB overexpression in CPCs increases cell commitment toward the cardiomyogenic lineage. A, CPCeδB stimulated with Dex for 6 days show increased expression of cardiogenic marker cardiac troponin T (tnnt3) and B. α-smooth muscle actin based on mRNA expression. C, cardiac marker α-sarcomeric expression and D, quantitation in CPCe and CPCeδB in normal growth conditions (G), −Dex and +Dex. E, metabolic activity under GM, −Dex, or +Dex growth conditions. F, endothelial (Tie2) and smooth muscle (α-SMA) were probed in CPCe and CPCeδB grown in normal growth conditions (G), −Dex or +Dex. G, quantitation of Tie2 and H. α-SMA, which is up-regulated in Dex-treated CPCeδB. Values are represented as relative fluorescent units (RFU) normalized to GAPDH or tubulin. *, p < 0.05 and **, p < 0.01 CPCe versus CPCeδB.
CaMKIIδB Expression in CPCs Antagonizes Apoptotic Cell Death after Oxidative Stress Stimuli
Expression of CaMKIIδB prevents apoptosis in cardiomyocytes following doxorubicin or hydrogen peroxide (H2O2) stimulation (21, 22). Overexpression of CaMKIIδB in CPCs similarly improves survival after 40 μm and 80 μm H2O2 induced death compared with CPCe (Fig. 7, A–C). To establish a pro-survival role of CaMKIIδB in CPCs, a small hairpin RNA to CaMKIIδB (sh-δB) was used to decrease the expression of the kinase before stimulation with oxidative stress (Fig. 7D). CPCs with reduced levels of CaMKIIδB had increased susceptibility to cell death at increasing concentrations of H2O2 (Fig. 7, E–F). Collectively, CaMKIIδB plays an integral role in early commitment of CPCs while enhancing cell survival during oxidative stress.
FIGURE 7.
CaMKIIδB expression in CPCs antagonizes apoptotic cell death after oxidative stress stimuli. A, quantitation of Annexin V and 7-AAD double positive cells (to label for cells in late apoptosis) in B. CPCe and C, CPCeδB after incubation in growth medium (GM), 40 or 80 μm H2O2. Values are represented as a fold change relative to GM-treated cells. D, schematic of constructs (top) and confirmation of knockdown of CaMKIIδB in CPCs relative to controls (NT: non-treated and sh-Ctrl). E, quantitation of late apoptosis in CPCs transfected with F, small hairpin (sh) control and G, Sh to knockdown CaMKIIδB and subjected to GM, 40, or 80 μm H2O2. Values are represented as a percentage based on flow cytometric analysis.
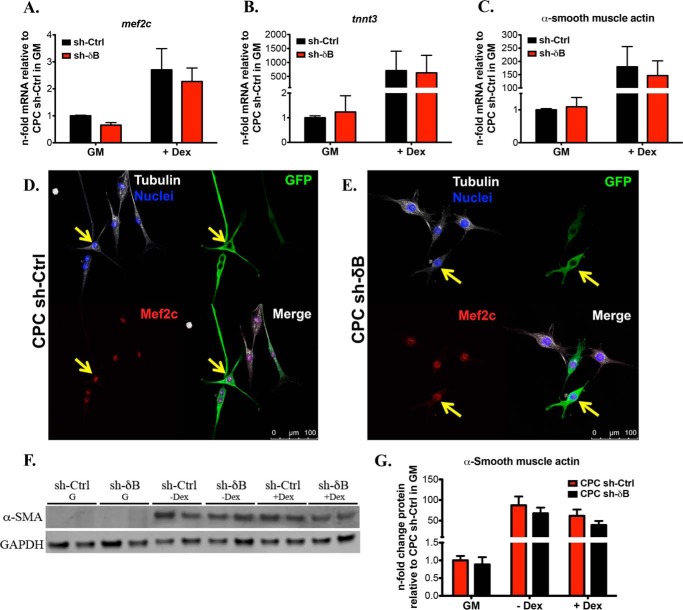
Knockdown of CaMKIIδB Does Not Impair Commitment of CPCs Toward the Cardiac or Smooth Muscle Phenotype
Next we determined if silencing of CaMKIIδB impacts CPC ability to undergo cardiac lineage commitment. Overall, CaMKIIδB knockdown did not result in any significant differences in Mef2c, cTNT or α-SMA gene expression patterns compared with CPC sh-Ctrl (Fig. 8, A–C). Furthermore, Mef2c and α-SMA protein levels were unaltered in CPC sh-δB based on fluorescent microscopy and immunoblotting (Fig. 8, D-G). These data suggest that CaMKIIδB is not required for cardiac-specific lineage commitment of CPCs.
FIGURE 8.
Knockdown of CaMKIIδB does not impair commitment of CPCs toward the cardiac or smooth muscle phenotype. A, cardiac markers Mef2c; B, cardiac troponin T (tnnt3); and C, smooth muscle marker α-smooth muscle actin gene expression in CPC sh-Ctrl and CPC sh-δB during growth conditions and after the addition of dex. Values are represented as fold change mRNA relative to CPC sh-Ctrl in GM after normalizing to ribosomal 18. D, CPC sh-Ctrl and E, CPC sh-δB fluorescent images after staining for Mef2c protein. Cells were maintained in GM. Cells were stained with antibodies toward GFP to identify cells that were successfully transduced with the sh-based lentivirus. Cell morphology and nuclei were visualized by staining for tubulin and with DAPI, respectively. F, immunoblotting for α-smooth muscle actin in CPC sh-Ctrl and CPC sh-δB in GM, medium without dex, or medium with dex. G, quantitation of α-smooth muscle actin represented as a fold change relative to CPC sh-Ctrl in GM and after normalizing to GAPDH.
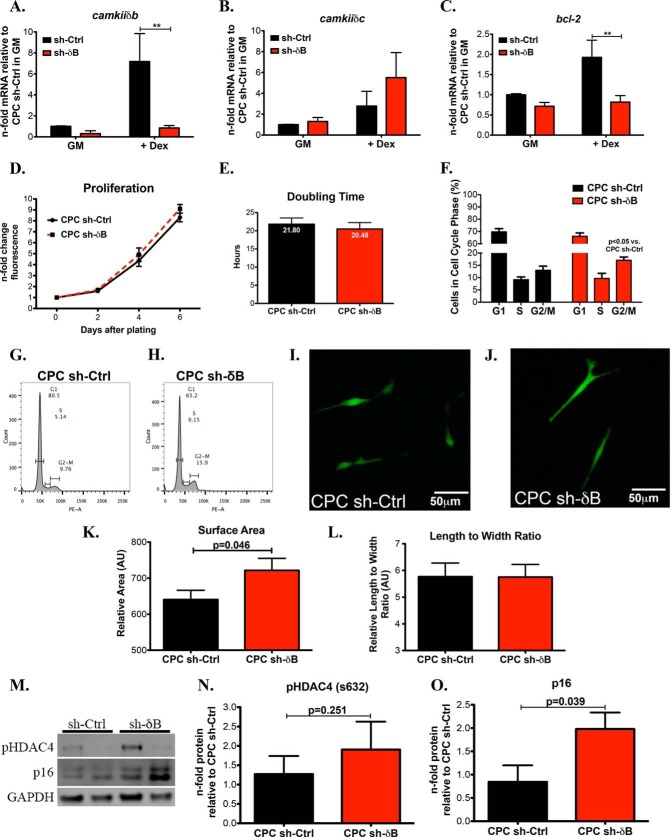
Knockdown of CaMKIIδB in CPCs Prevents the Up-regulation of Pro-apoptotic Molecules and Promotes Properties of Cellular Senescence
Lentiviral-mediated knockdown of CaMKIIδB was further confirmed by qRT-PCR in CPCs subjected to growth conditions (Fig. 9A). Furthermore, Dex induced up-regulation of CaMKIIδB was blunted in CPC sh-δB confirming the effectiveness of our knockdown strategy (Fig. 9A). As expected, CPC sh-Ctrl increased CaMKIIδB after Dex treatment (Fig. 9A). CaMKIIδC was not significantly increased after knockdown of CaMKIIδB, however there was an observed increased trend of CaMKIIδC in CPCs treated with sh-δB compared with controls (Fig. 9B). Bcl-2 gene expression was induced after Dex treatment in CPC sh-Ctrl but this effect was abrogated after silencing of CaMKIIδB (Fig. 9C). Proliferation was not significantly different between CPC sh-δB and CPC sh-Ctrl (Fig. 9, D and E). In contrast to the overexpression of CaMKIIδB, sh-δB-treated cells were increasingly in the G2/M phase of the cell cycle (Fig. 9, F–H). Length to width ratio (spindle shaped morphology) was similarly observed between the two groups (9I, 9J, and 9L). However, CPC sh-δB displayed an increased cellular size as evidenced by fluorescence microscopy and cell surface area quantitation (Fig. 9, I, J, and K). Knockdown of CaMKIIδB did not appear to significantly affect the phosphorylated status of HDAC4 (Fig. 9, M and N). Since cell proliferation was unchanged yet the percentage of G2/M cells was increased in CPC sh-δB, we assessed for markers of cellular senescence after silencing of CaMKIIδB. Indeed, p16 was up-regulated in CPCs sh-δB (Fig. 9, M and O) suggesting that the increased presence of CPC sh-δB in the mitotic phase of the cell cycle maybe due to increased cell cycle arrest.
FIGURE 9.
Knockdown of CaMKIIδB in CPCs prevents the up-regulation of pro-apoptotic molecules and promotes properties of cellular senescence. A, CaMKIIδB; B, CaMKIIδC; and C, Bcl-2 gene expression in CPC sh-Ctrl and CPC sh-δB during growth conditions or with the addition of dex. Values are represented as n-fold mRNA relative to CPC sh-Ctrl in GM and after normalization to ribosomal 18S. **, p < 0.01. D, proliferation measured using a direct nucleic acid fluorescent dye. Fluorescent values are represented as a fold change relative to day of plating (Day 0). E, cell doubling time in hours. F, cell cycle analysis using propidium iodide to determine percentage of cells in the different stages of the cell cycle analyzed by flow cytometry. G, cell cycle images of CPC sh-Ctrl and H. CPC sh-δB. I, fluorescent images of CPC sh-Ctrl; and J, CPC sh-δB. K, CPC sh-Ctrl and CPC sh-δB cell surface area and L, length to width ratio represented as arbitrary units. M, immunoblot representation of pHDAC4 on the serine 632 and p16. N, quantitation of pHDAC4 and O. Senescence marker p16. Values are represented as a fold change relative to CPC sh-Ctrl and after normalizing to GAPDH.
Discussion
There is debate as to the stem cell type that is needed to treat myocardial damage. CPCs, although limited, have been validated as a cell type to treat heart disease due to their cardiomyogenic potential (6, 9). In this study, we present a canonical calcium-signaling cascade implicated in growth and survival of CPCs by CaMKII. CaMKIIδ isoforms are studied in the context of maladaptive hypertrophy in transgenic mice (13). However, recent studies have implicated CaMKIIδB to reduce expression of inflammatory factors in a global CaMKIIδ knock-out model, indicating a protective role of the δB kinase in the cardiac context after pathological damage (23). Until now, it remained unknown whether CaMKIIδ isoforms are present in resident CPCs although expression is reported in smooth muscle cells in the heart (24, 25). Our study is the first to identify nuclear translocation of the CaMKIIδB isoform during lineage commitment of CPCs (Fig. 1 and 2). Furthermore, overexpression of CaMKIIδ supports distinct morphological changes and increases differentiation accompanied by decreased cell cycle progression (Figs. 3 and 4). Consistent with cardiomyocyte data, CaMKIIδB confers survival in CPCs, which is abrogated when δB expression is reduced and subjected to oxidative stress stimuli (Fig. 6). Collectively, this data supports the ability of CPCs to acquire growth and differentiation phenotypes regulated by CaMKIIδ signaling to the nucleus.
Currently, there is interest in identifying proteins and signaling cascades in CPCs related to mature cardiomyocytes. During basal conditions, CPCs express the β2-adrenergic receptor (β2-AR) regulating stem cell proliferation but acquire the mature β1-AR after co-culture with cardiomyocytes (36). Ca2+ in fetal CSCs supports cellular growth, proliferation and commitment (37). Inositol-1,4,5-triphosphate (IP3) receptors on the sarcoplasmic reticulum induce spontaneous Ca2+ oscillations in mouse and human CPCs (37, 38). CPCs are validated to not only express IP3 receptors, but also purinergic G protein-coupled receptors (P2Y) and SERCA2, which are functionally stimulated and activated after introduction of Ca2+ and/or ATP (38, 39). Furthermore, IP3 receptors promote an influx of Ca2+ in the nucleus activating CaMKII/MEF2 and cellular growth (40). In Fig. 1, expression levels of CaMKIIδ are increased and localized to the nucleus during acute stress, indicating that a short-term stimulus is sufficient to prime cardiac commitment of stem cells. This was further validated in isolated CPCs, as our differentiation protocol induced nuclear accumulation of CaMKIIδB (Fig. 2), correlating with inactivated p-HDAC4 in the cytosolic fraction (Fig. 3). These studies suggest that the transitory fate of CPCs during cell division and differentiation can be defined by a complex interplay of calcium-regulated molecules prior to acquiring cardiomyogenic fate.
CPCs under genetic modification with mature cardiomyocyte genes increase our knowledge as to the potential of stem cells to promote cardiac repair after adoptive transfer in the heart. CPCs express Ca2+ receptors and pumps, however, expression of the Ryanodine receptors and β1-AR are not present in CPCs indicating that these cells hold a primitive molecular cardiac signature (39). Related to our study, CPCs transfected with a siRNA targeting HDAC4, and therefore inhibiting the repression of growth genes, increased differentiation of CPCs in vivo supporting myocardial regeneration (29). In cardiomyocytes, HDAC4 and HDAC5 form a complex to inhibit MEF2 and serum response factor elements (18, 19). Inactivation of Class IIa HDACs by CaMKII and protein kinase D promotes shuttling of HDAC4 from the nuclear compartment by the chaperone 14-3-3 (28, 42). Our data show a decrease of nuclear p-HDAC4 in our non-modified CPCs (Fig. 3). Conversely, p-HDAC was increased in the cytosol of Dex treated CPCs or CPCeδB (Fig. 3). However, the results were modest indicating that additional factors affect the kinase activity of CaMKII and/or inhibition of Class IIa HDACs. In contrast, HDAC inhibition, including inhibition of Class I HDACs, has been shown to decrease the proliferative and differentiation potential of mesenchymal stem cells indicating that HDACs are essential for maintenance of proper stem cell function (43).
We evaluated whether overexpression of CaMKIIδB can enhance the therapeutic potential of CPCs. Here a lentiviral protocol was employed and characteristics such as increased cellular size and decreased proliferation were immediately apparent after overexpression of CaMKIIδB (Fig. 4). To delineate a CaMKIIδB-dependent role to enhance stem cell survival relative to the CaMKIIδC isoform, we subjected CPCs to oxidative stress and observed a decrease in late apoptotic cells (6-fold reduction) relative to control CPCs (Fig. 7). Anti-apoptotic molecule Bcl-2 is highly up-regulated in CPCeδB (6-fold) (Fig. 5), which has been shown to facilitate survival of cardiomyocytes as CaMKIIδB facilitates GATA4 binding to the Bcl-2 promoter (22). Silencing of CaMKIIδB increases CPC susceptibility to apoptosis (Fig. 7), although Bcl-2 levels were similar to controls (Fig. 9). Interestingly, CaMKIIδC mRNA was fairly increased in CPC sh-δB a factor that may promote cell death during oxidative stress (Fig. 9). The nuclear localization sequence in CaMKIIδB allows for unique influence on nuclear signaling such as proliferation, survival, and growth compared with the δC isoform (44). Cardiomyocyte apoptosis and sensitivity to stress stimuli is increased in cardiomyocytes with elevated cytosolic CaMKIIδ due to the deregulation of calcium signaling (45). Our data, and in accordance with the literature, confirms that CaMKIIδB is essential in CPC survival possibly through increased Bcl-2 (22).
Differentiation of CPCs is profiled by a series of morphological and molecular features including increases in cellular size, slowed proliferation and up-regulation of markers consistent with mature cell types (34). CaMKIIδB expression in CPCs resulted in decreased cyclin B1, a mitotic regulator that is down regulated in differentiated cells (Fig. 5) (46). These data are consistent with decreased proliferation and reduced presence of cells in the G2/M phase of the cell cycle in CPCeδB (Fig. 4). In contrast to our overexpression strategy, transduction with sh-δB did not change the proliferative rate of CPCs relative to controls. Rather, silencing promoted the accumulation of cells in the G2/M phase of the cell cycle (Fig. 9). In addition to the unique cell cycle status of CPC sh-δB, cells exhibited increased cell size (Fig. 9). In fact the knockdown of CaMKIIδB significantly increased p16 expression indicative of mitotic arrest induced senescence. Additionally, G2/M arrest is associated with increased cell death consistent with the observed phenotype of CPCs after silencing of CaMKIIδB (47).
Mef2c, is a calcium-dependent transcription factor and regulator of cardiac muscle differentiation and development (48, 49). Additionally, transfection of non-myocytes with Mef2c and GATA4 enhances expression of mature cardiac genes (41). We observed a marked increase in nuclear Mef2c in CPCeδB, which supports the up-regulation of mature cardiac and smooth muscle markers α-sarcomeric actinin, cTNT and α-SMA observed in Fig. 6. Differentiation was not significantly altered relative to control cells after transfection of CPCs with shRNA to CaMKIIδB (Fig. 8). This indicates that CaMKIIδB is not required for growth and commitment of CPCs, and it is possible that there are alternative compensatory mechanisms that sustain CPC self-renewal capabilities in the absence of CaMKIIδB. In particular the knockdown studies brings interests to the differential impact of silencing and/or overexpressing specific δ isoforms as they each play diverse roles in the entire cell. Currently, identification of calcium-associated proteins brings validation to CPC origin and potency status and supports the potential of CPCs as a cardiac regenerative population.
Author Contributions
P. Q. designed and conducted a majority of the experiments outlined in the paper, analyzed the results and wrote the paper. N. H. performed Western blots and analysis concerning total and phosphorylated HDAC, identifying markers of senescence and proteins associated with differentiation, and assisted in editing of the paper. J. D. C. and K. M. B. performed Western blots, RNA isolations, and qRT-PCR analysis. J. M. E. conducted Western blots. B. J. W. performed qRT-PCRs. L. O. created the lentiviruses used in this study. D. M. B. supplied the CAMKIIδ antibody and provided critical feedback and editing of the manuscript. M. A. S. provided critical feedback on experimental design and editing of the manuscript. C. P. conceived the idea, designed experiments, and wrote/edited the paper with P. Q.
Acknowledgments
We thank all the members of the Sussman laboratory for critical feedback on the data and the writing of the manuscript. We acknowledge the SDSU Flow Cytometry Core facility, the director Dr. Roland Wolkowicz, and core manager Cameron Smurthwaite for assistance. We also acknowledge and thank Dr. Donald Bers from the University of California, Davis for sending us the CaMKIIδ antibody.
This work was supported by National Institutes of Health Grant F31HL117623 (to P. Q.), Rees Stealy Research Foundation, and SDSU Heart Institute Fellowship and Achievement Rewards for College Scientists Scholarship. This work was also supported by American Heart Association Grant 15BGIA23010047 (to N. H.), by National Institutes of Health Grants R01HL067245, R37HL091102, R01HL105759, R01HL113647, R01HL117163, P01HL085577, and R01HL122525 as well as an award from the Fondation Leducq Transatlantic Network (to M. A. S.), and by King Abdulaziz City for Science and Technology (Grant No. KACST 10-BIO 1350-20 and 13-MED456-20, to C. P.). Mark A. Sussman is a founder and co-owner of CardioCreate Inc. All other authors declare that they have no conflicts of interest with the contents of this article.
- CSC
- cardiac stem cell
- α-SMA
- α-smooth muscle actin
- CaMKII
- calcium/calmodulin kinase II
- CPC
- cardiac progenitor cell
- CPCe
- cardiac progenitor cells overexpressing eGFP
- CPCeδB
- cardiac progenitor cells overexpressing CaMKIIδB
- cTNT
- cardiac troponin T
- Dex
- dexamethasone
- GATA4
- GATA-binding protein 4
- HDAC4
- histone deacetylase 4
- pHDAC4
- phosphorylated histone deacetylase 4 on serine 632
- MEF2
- myocyte-specific enhancer factor 2
- sh-Ctrl
- small hairpin control lentivirus
- sh-δB
- small hairpin targeting CaMKIIδB lentivirus.
References
- 1. Orlic D., Kajstura J., Chimenti S., Limana F., Jakoniuk I., Quaini F., Nadal-Ginard B., Bodine D. M., Leri A., Anversa P. (2001) Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. U.S.A. 98, 10344–10349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rota M., Padin-Iruegas M. E., Misao Y., De Angelis A., Maestroni S., Ferreira-Martins J., Fiumana E., Rastaldo R., Arcarese M. L., Mitchell T. S., Boni A., Bolli R., Urbanek K., Hosoda T., Anversa P., Leri A., Kajstura J. (2008) Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ. Res. 103, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tallini Y. N., Greene K. S., Craven M., Spealman A., Breitbach M., Smith J., Fisher P. J., Steffey M., Hesse M., Doran R. M., Woods A., Singh B., Yen A., Fleischmann B. K., Kotlikoff M. I. (2009) c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc. Natl. Acad. Sci. U.S.A. 106, 1808–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaruba M. M., Soonpaa M., Reuter S., Field L. J. (2010) Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation 121, 1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ellison G. M., Vicinanza C., Smith A. J., Aquila I., Leone A., Waring C. D., Henning B. J., Stirparo G. G., Papait R., Scarfò M., Agosti V., Viglietto G., Condorelli G., Indolfi C., Ottolenghi S., Torella D., Nadal-Ginard B. (2013) Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 [DOI] [PubMed] [Google Scholar]
- 6. van Berlo J. H., Kanisicak O., Maillet M., Vagnozzi R. J., Karch J., Lin S. C., Middleton R. C., Marbán E., Molkentin J. D. (2014) c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leri A., Kajstura J., Anversa P. (2011) Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ. Res. 109, 941–961 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Fischer K. M., Cottage C. T., Wu W., Din S., Gude N. A., Avitabile D., Quijada P., Collins B. L., Fransioli J., Sussman M. A. (2009) Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 120, 2077–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolli R., Chugh A. R., D'Amario D., Loughran J. H., Stoddard M. F., Ikram S., Beache G. M., Wagner S. G., Leri A., Hosoda T., Sanada F., Elmore J. B., Goichberg P., Cappetta D., Solankhi N. K., Fahsah I., Rokosh D. G., Slaughter M. S., Kajstura J., Anversa P. (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Volkers M., Rohde D., Goodman C., Most P. (2010) S100A1: a regulator of striated muscle sarcoplasmic reticulum Ca2+ handling, sarcomeric, and mitochondrial function. J. Biomed. Biotechnol. 10.1155/2010/178614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hook S. S., Means A. R. (2001) Ca(2+)/CaM-dependent kinases: from activation to function. Annu. Rev. Pharmacol. Toxicol. 41, 471–505 [DOI] [PubMed] [Google Scholar]
- 12. Luczak E. D., Anderson M. E. (2014) CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 73, 112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mishra S., Ling H., Grimm M., Zhang T., Bers D. M., Brown J. H. (2010) Cardiac hypertrophy and heart failure development through Gq and CaM kinase II signaling. J. Cardiovasc. Pharmacol. 56, 598–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sossalla S., Fluschnik N., Schotola H., Ort K. R., Neef S., Schulte T., Wittköpper K., Renner A., Schmitto J. D., Gummert J., El-Armouche A., Hasenfuss G., Maier L. S. (2010) Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ. Res. 107, 1150–1161 [DOI] [PubMed] [Google Scholar]
- 15. Fischer T. H., Eiringhaus J., Dybkova N., Förster A., Herting J., Kleinwächter A., Ljubojevic S., Schmitto J. D., Streckfuss-Bömeke K., Renner A., Gummert J., Hasenfuss G., Maier L. S., Sossalla S. (2014) Ca(2+) /calmodulin-dependent protein kinase II equally induces sarcoplasmic reticulum Ca(2+) leak in human ischaemic and dilated cardiomyopathy. Eur. J. Heart Failure 16, 1292–1300 [DOI] [PubMed] [Google Scholar]
- 16. Awad S., Al-Haffar K. M., Marashly Q., Quijada P., Kunhi M., Al-Yacoub N., Wade F. S., Mohammed S. F., Al-Dayel F., Sutherland G., Assiri A., Sussman M., Bers D., Al-Habeeb W., Poizat C. (2014) Control of Histone H3 Phosphorylation by CaMKII in Response to Hemodynamic Cardiac Stress. J. Pathol. 235, 606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srinivasan M., Edman C. F., Schulman H. (1994) Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J. Cell Biol. 126, 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang T., Kohlhaas M., Backs J., Mishra S., Phillips W., Dybkova N., Chang S., Ling H., Bers D. M., Maier L. S., Olson E. N., Brown J. H. (2007) CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J. Biol. Chem. 282, 35078–35087 [DOI] [PubMed] [Google Scholar]
- 19. Little G. H., Bai Y., Williams T., Poizat C. (2007) Nuclear calcium/calmodulin-dependent protein kinase IIδ preferentially transmits signals to histone deacetylase 4 in cardiac cells. J. Biol. Chem. 282, 7219–7231 [DOI] [PubMed] [Google Scholar]
- 20. Backs J., Song K., Bezprozvannaya S., Chang S., Olson E. N. (2006) CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J. Clin. Investig. 116, 1853–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peng W., Zhang Y., Zheng M., Cheng H., Zhu W., Cao C. M., Xiao R. P. (2010) Cardioprotection by CaMKII-δB is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ. Res. 106, 102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little G. H., Saw A., Bai Y., Dow J., Marjoram P., Simkhovich B., Leeka J., Kedes L., Kloner R. A., Poizat C. (2009) Critical role of nuclear calcium/calmodulin-dependent protein kinase IIδB in cardiomyocyte survival in cardiomyopathy. J. Biol. Chem. 284, 24857–24868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ling H., Gray C. B., Zambon A. C., Grimm M., Gu Y., Dalton N., Purcell N. H., Peterson K., Brown J. H. (2013) Ca2+/Calmodulin-dependent protein kinase IIδ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ. Res. 112, 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li H., Li W., Gupta A. K., Mohler P. J., Anderson M. E., Grumbach I. M. (2010) Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 298, H688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott J. A., Xie L., Li H., Li W., He J. B., Sanders P. N., Carter A. B., Backs J., Anderson M. E., Grumbach I. M. (2012) The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am. J. Physiol. Heart Circ. Physiol. 302, H1953–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoch B., Wobus A. M., Krause E. G., Karczewski P. (2000) Δ-Ca(2+)/calmodulin-dependent protein kinase II expression pattern in adult mouse heart and cardiogenic differentiation of embryonic stem cells. J. Cell. Biochem. 79, 293–300 [PubMed] [Google Scholar]
- 27. Sun G., Fu C., Shen C., Shi Y. (2011) Histone deacetylases in neural stem cells and induced pluripotent stem cells. J. Biomed. Biotechnol. 10.1155/2011/835968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clocchiatti A., Florean C., Brancolini C. (2011) Class IIa HDACs: from important roles in differentiation to possible implications in tumourigenesis. J. Cell Mol. Med. 15, 1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang L. X., DeNicola M., Qin X., Du J., Ma J., Tina Zhao Y., Zhuang S., Liu P. Y., Wei L., Qin G., Tang Y., Zhao T. C. (2014) Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. Am. J. Physiol. Cell Physiol. 307, C358–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Konstandin M. H., Toko H., Gastelum G. M., Quijada P., De La Torre A., Quintana M., Collins B., Din S., Avitabile D., Völkers M., Gude N., Fässler R., Sussman M. A. (2013) Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ. Res. 113, 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hariharan N., Quijada P., Mohsin S., Joyo A., Samse K., Monsanto M., De La Torre A., Avitabile D., Ormachea L., McGregor M. J., Tsai E. J., Sussman M. A. (2015) Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. J. Am. College Cardiol. 65, 133–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Porrello E. R., Olson E. N. (2014) A neonatal blueprint for cardiac regeneration. Stem Cell Res. 13, 556–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fransioli J., Bailey B., Gude N. A., Cottage C. T., Muraski J. A., Emmanuel G., Wu W., Alvarez R., Rubio M., Ottolenghi S., Schaefer E., Sussman M. A. (2008) Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells 26, 1315–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bailey B., Izarra A., Alvarez R., Fischer K. M., Cottage C. T., Quijada P., Díez-Juan A., Sussman M. A. (2009) Cardiac stem cell genetic engineering using the αMHC promoter. Regenerative Med. 4, 823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohsin S., Khan M., Nguyen J., Alkatib M., Siddiqi S., Hariharan N., Wallach K., Monsanto M., Gude N., Dembitsky W., Sussman M. A. (2013) Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ. Res. 113, 1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan M., Mohsin S., Avitabile D., Siddiqi S., Nguyen J., Wallach K., Quijada P., McGregor M., Gude N., Alvarez R., Tilley D. G., Koch W. J., Sussman M. A. (2013) β-Adrenergic regulation of cardiac progenitor cell death versus survival and proliferation. Circ. Res. 112, 476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferreira-Martins J., Ogórek B., Cappetta D., Matsuda A., Signore S., D'Amario D., Kostyla J., Steadman E., Ide-Iwata N., Sanada F., Iaffaldano G., Ottolenghi S., Hosoda T., Leri A., Kajstura J., Anversa P., Rota M. (2012) Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ. Res. 110, 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Ferreira-Martins J., Rondon-Clavo C., Tugal D., Korn J. A., Rizzi R., Padin-Iruegas M. E., Ottolenghi S., De Angelis A., Urbanek K., Ide-Iwata N., D'Amario D., Hosoda T., Leri A., Kajstura J., Anversa P., Rota M. (2009) Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ. Res. 105, 764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ellison G. M., Torella D., Karakikes I., Purushothaman S., Curcio A., Gasparri C., Indolfi C., Cable N. T., Goldspink D. F., Nadal-Ginard B. (2007) Acute β-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J. Biol. Chem. 282, 11397–11409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gómez A. M., Ruiz-Hurtado G., Benitah J. P., Domínguez-Rodríguez A. (2013) Ca(2+) fluxes involvement in gene expression during cardiac hypertrophy. Curr. Vasc. Pharmacol. 11, 497–506 [DOI] [PubMed] [Google Scholar]
- 41. Inagawa K., Miyamoto K., Yamakawa H., Muraoka N., Sadahiro T., Umei T., Wada R., Katsumata Y., Kaneda R., Nakade K., Kurihara C., Obata Y., Miyake K., Fukuda K., Ieda M. (2012) Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ. Res. 111, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 42. Harrison B. C., Huynh K., Lundgaard G. L., Helmke S. M., Perryman M. B., McKinsey T. A. (2010) Protein kinase C-related kinase targets nuclear localization signals in a subset of class IIa histone deacetylases. FEBS Lett. 584, 1103–1110 [DOI] [PubMed] [Google Scholar]
- 43. Lee S., Park J. R., Seo M. S., Roh K. H., Park S. B., Hwang J. W., Sun B., Seo K., Lee Y. S., Kang S. K., Jung J. W., Kang K. S. (2009) Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 42, 711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edman C. F., Schulman H. (1994) Identification and characterization of ΔB-CaM kinase and ΔC-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim. Biophys. Acta 1221, 89–101 [DOI] [PubMed] [Google Scholar]
- 45. Zhu W., Woo A. Y., Yang D., Cheng H., Crow M. T., Xiao R. P. (2007) Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J. Biol. Chem. 282, 10833–10839 [DOI] [PubMed] [Google Scholar]
- 46. Savatier P., Lapillonne H., van Grunsven L. A., Rudkin B. B., Samarut J. (1996) Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene 12, 309–322 [PubMed] [Google Scholar]
- 47. Galvin K. E., Ye H., Erstad D. J., Feddersen R., Wetmore C. (2008) Gli1 induces G2/M arrest and apoptosis in hippocampal but not tumor-derived neural stem cells. Stem Cells 26, 1027–1036 [DOI] [PubMed] [Google Scholar]
- 48. McKinsey T. A., Zhang C. L., Olson E. N. (2002) MEF2: a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 27, 40–47 [DOI] [PubMed] [Google Scholar]
- 49. Lin Q., Schwarz J., Bucana C., Olson E. N. (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276, 1404–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]