Background: Bacteriophage-mediated seroconversion by glucosylation is currently unknown for O-antigens synthesized by ABC transporter-dependent pathways.
Results: Raoultella terrigena O-antigen is modified with a glucose side chain when expressed in E. coli K-12.
Conclusion: The ABC transporter-dependent pathway poses no intrinsic mechanistic barrier to phage-mediated glucosylation.
Significance: O-antigen glucosylation has implications for evolution of antigenic diversity and vaccine development.
Keywords: ABC transporter, bacteriophage, biosynthesis, glycosylation, lipopolysaccharide (LPS), nuclear magnetic resonance (NMR), outer membrane, polysaccharide, O-antigen
Abstract
Lysogenic bacteriophages may encode enzymes that modify the structures of lipopolysaccharide O-antigen glycans, altering the structure of the bacteriophage receptor and resulting in serotype conversion. This can enhance virulence and has implications for antigenic diversity and vaccine development. Side chain glucosylation is a common modification strategy found in a number of bacterial species. To date, glucosylation has only been observed in O-antigens synthesized by Wzy-dependent pathways, one of the two most prevalent O-antigen synthesis systems. Here we exploited a heterologous system to study the glucosylation potential of a model O-antigen produced in an ATP-binding cassette (ABC) transporter-dependent system. Although O-antigen production is cryptic in Escherichia coli K-12, because of a mutation in the synthesis genes, it possesses a prophage glucosylation cluster, which modifies the GlcNAc residue in an α-l-Rha-(1→3)-d-GlcNAc motif found in the original O16 antigen. Raoultella terrigena ATCC 33257 produces an O-antigen possessing the same disaccharide motif, but its assembly uses an ABC transporter-dependent system. E. coli harboring the R. terrigena O-antigen biosynthesis genes produced an O-antigen displaying reduced reactivity toward antisera raised against the native R. terrigena repeat structure, indicative of an altered chemical structure. Structural determination using NMR revealed the addition of glucose side chains to the repeat units. O-antigen modification was dependent on a functional ABC transporter, consistent with modification in the periplasm, and was eliminated by deletion of the glucosylation genes from the E. coli chromosome, restoring native level antisera sensitivity and structure. There are therefore no intrinsic mechanistic barriers for bacteriophage-mediated O-antigen glucosylation in ABC transporter-dependent pathways.
Introduction
Bacterial surfaces possess a range of different macromolecules that contain complex carbohydrates. These glycoconjugates mediate contact with the external environment andrepresent a remarkably diverse spectrum of carbohydrate structures. Significant progress has been made in establishing their precise structures and modes of assembly. As an example, there are more than 180 chemically and serotypically distinct glycans, giving rise to LPS O-antigens in Escherichia coli, and the structures, genes, and biosynthetic enzymes have been catalogued (1–4). Diversity in glycoconjugate structures results from recombination events within genetic loci responsible for the synthesis of polysaccharides to generate a new structure and by acquisition of unlinked genes whose products modify existing structures. One mechanism of diversification, illustrated by certain O-antigens in the genera Escherichia, Salmonella, and Shigella, involves the addition of a side branch glucose to the main chain of the glycan (see Fig. 1). The genes responsible for glucosylation are encoded by the genomes of lysogenic bacteriophages, so phage infection leads to serotype conversion. Glucosylation has a particularly profound effect on serotype in Shigella. Of 15 recognized serotypes, all but two contain the same glycan backbone (serotype Y). Diversity arises mainly from glucosylation and/or O-acetylation of this backbone (reviewed in Ref. 5), but other modifications are also possible (6, 7). Depending on the serotype, any of the sugars in the tetrasaccharide repeat unit may be modified. Salmonella isolates can contain up to four glucosylation systems, modifying different parts of the O-antigen structures (8). Clearly, these modification processes have a significant impact on diversity.
FIGURE 1.

Structures of the O-polysaccharide repeat units. The component sugars are glucose (Glc), 2-acetylamino-2-deoxyglucose (N-acetylglucosamine; GlcNAc), and 6-deoxymannose (rhamnose; Rha).
O-antigens currently known to be subject to glucosylation are united by a common assembly mechanism, known as the Wzy-dependent pathway (reviewed in Ref. 9). In this process, repeat units of the O-antigen are built at the cytoplasmic face of the cell membrane on an undecaprenol diphosphate (Und-PP)4 lipid carrier. A transporter (Wzx) then exports these lipid-linked intermediates to the external face of the membrane, where they provide substrates for the pathway-defining polymerase (Wzy). The polymerization reaction involves transfer of a growing glycan chain from its Und-PP carrier to the nonreducing end of the incoming Und-PP-linked repeat unit. After polymerization, the O-antigen is transferred to a lipid A core acceptor, and the completed molecule is translocated to the outer membrane (10). The glucosylation modification reaction was first reported in the seroconversion of Salmonella O serotypes. Although the activated initial donor of the glucose residue is UDP-glucose, the direct donor is undecaprenol phosphate-glucose (11–14). Early studies established an association between glucosylation modification and lysogenic bacteriophages (13, 15), and the same is true in Shigella (5). It is now known that the modification reaction requires acquisition of only three genes. The GtrB enzyme synthesizes undecaprenol phosphate-Glc, and GtrA is the putative exporter for the lipid-linked donor; the corresponding genes are conserved across glucosylation systems. A third gene (designated gtrC or gtr*) is variable and encodes a serotype-specific glucosyltransferase (16, 17).
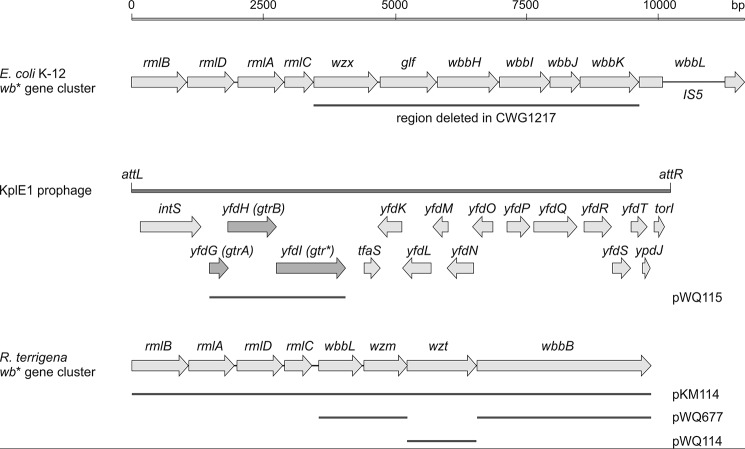
To date, glucosylation machinery of this type is only known to modify O-antigens assembled by the well distributed Wzy-dependent processes. However, many O-antigens follow a different synthetic pathway, defined by the involvement of an ATP-binding cassette (ABC) transporter, composed of dimers of both the nucleotide-binding domain (NBD) polypeptide, which is designated Wzt, and of the transmembrane domain (TMD), known as Wzm (18). In this pathway, the O-antigens are still assembled on an Und-PP acceptor, but polymerization is completed in the cytoplasm by the sequential action of glycosyltransferase enzymes. Once complete, the Und-PP-linked glycan is exported to the periplasm by the ABC transporter. The nascent O-antigen is then transferred to the lipid A core molecule and translocated to the outer membrane by processes that operate independently of the mode of O-antigen synthesis. The association of glucosylation modification with the Wzy-dependent pathway may reflect the limits to which systems have been characterized at a bioinformatics and biochemical level or may result from the glycosylation system being critically dependent on elements of the Wzy-dependent machinery. Here, we examine the possibility that the ABC transporter-dependent pathway provides an intrinsic mechanistic barrier for the glucosylation process by examining the potential for the native E. coli K-12 glucosylation machinery encoded by prophage KplE1 (19) to modify target residues supplied by a heterologous ABC transporter-dependent O-antigen from Raoultella terrigena ATCC 33257 (see Fig. 2). This O-antigen is built up of repeat units containing an α-l-Rha-(1→3)-d-GlcNAc disaccharide, and this represents a motif that is modifiable by glucosylation systems in both E. coli K-12 and Shigella flexneri (see Fig. 1).
FIGURE 2.
Organization of the O-antigen biosynthesis genes in E. coli. Shown are the wb* O-antigen biosynthesis gene clusters from E. coli K-12 (GenBankTM accession number NC_010473) and R. terrigena ATCC 33257 (GenBankTM accession number AY376146), as well as the KplE1 prophage (also named CPS-53) in the E. coli K-12 genome, encoding the three Gtr proteins required for glucosylation of O-antigens.
Experimental Procedures
Bacterial Strains and Growth Conditions
Bacterial cultures were grown with aeration at 37 or 30 °C in LB base (Invitrogen), supplemented with appropriate antibiotics (100 μg ml−1 ampicillin, 34 μg ml−1 chloramphenicol, 100 μg ml−1 kanamycin). R. terrigena ATCC 33257 was a gift from U. Mamat (Research Centre Borstel, Leibnitz Centre for Medicine and Biosciences, Borstel, Germany). The E. coli strains used in this study are derivatives of strain TOP10 (F−, mcrA, Δ(mrr-hsdRMS-mcrBC), φ80, lacZΔM15, ΔlacX74, deoR, nupG, recA1, araD139, Δ(ara-leu)7697, galU, galK, rpsL(Strr), endA1) from Invitrogen. Deletion mutagenesis was performed using the λ-red recombination system (20). To remove part of the K-12 wb* gene cluster in E. coli Top10 to create E. coli CWG1217 (Δwzx-wbbK), pKD4 (21) was used as a template to amplify the kanamycin resistance cassette using oligonucleotide primers containing 50 nucleotides of homology with wzx and wbbK genes (identified by lowercase); the primer sequences were 5′-acgaataaattatctttaagaagaaacgttatatatctggctgtcgttcaGTGTAGGCTGGAGCTGCTTC-3′ and 5′-atgttcttcagtaataaaattaactagttcatcaaacccaactaatacatCATATGAATATCCTCCTTAG-3′. Electrocompetent cells of E. coli Top10 containing pSIM6 (22) were transformed with the linear PCR product, and after recovery overnight with aeration at 30 °C, mutants were selected on LB agar containing 15 μg ml−1 kanamycin grown for 2 days at 30 °C. The correct deletion was confirmed by a series of diagnostic PCR amplification reactions using primers with sequences located outside and within the deleted regions. Sensitivity to ampicillin indicated loss of pSIM6.
To construct E. coli CWG1218 (Δwzx-wbbK ΔgtrA), the chloramphenicol acetyltransferase gene was amplified from pKD3 (21) using oligonucleotide primers OL1019 (5′-aagacttggatgatagacttcattcctttgattattagctgatagaagaaGTGTAGGCTGGAGCTGCTTC-3′) and OL1020 (5′-aattggtatcgcttcttcttcattgaagacaggaactacaagagatatctCATATGAATATCCTCCTTAG-3′), containing ∼50-nucleotide extensions identical to thesequences flanking the target open reading frame, which is designated yfdG in E. coli DH10b (GenBankTM accession number NC_010473.1). The process for mutagenesis, confirmation, and curing of pSIM6 was the same as that described for the parent, CWG1217, except mutants were selected on LB agar containing 10 μg ml−1 chloramphenicol at 30 °C. Potential downstream (polarity) effects of the cassettes replacing gtrA or wzx-wbbK had no influence on the experimental strategy, so they were left in place in strains used to isolate polysaccharides for NMR experiments. However, they were subsequently excised using the pCP20 helper plasmid (21) to construct E. coli CWG1219 (Δwzx-wbbK ΔgtrA), to avoid conflicts in selectable markers for the Wzt titration and gtr complementation experiments.
DNA Methods
Custom oligonucleotide primers were obtained from Sigma. PCR amplification was performed using Pwo DNA polymerase (Roche Applied Science). The PureLink PCR purification kit (Invitrogen) was used to clean the PCR product. Plasmid DNA was purified from overnight cultures using the PureLink quick plasmid miniprep kit (Invitrogen). Chromosomal DNA was obtained using the PureLink genomic DNA mini kit (Invitrogen). Restriction digest and ligation reactions were performed according to the manufacturer's instructions. DNA sequencing was performed in the Genomics Facility of the Advanced Analysis Center at the University of Guelph.
Plasmid Constructs
pKM114 is a pMBL19 derivative containing the 10.3-kb R. terrigena wb* gene cluster, encoding production of the O-antigen, and was a gift from U. Mamat (Research Centre Borstel, Leibnitz Centre for Medicine and Biosciences, Borstel, Germany).
The wzt gene was PCR-amplified from pKM114 using primers designed to include an N-terminal FLAG tag in the gene product. The primer sequences (forward primer: 5′-gatcgaattcATGgattacaaggatgacgacgataagTCCTCTAATGAAATTGCTATCCAGGTCAC-3′; reverse primer: 5′-gatcaagcttTTACTCTCCATTCGAAATAATTTTCAACGGAG-3′) incorporated EcoRI and HindIII restriction sites (underlined) for cloning (chromosomal sequences are indicated by uppercase letters, and FLAG tag sequences are italicized). The PCR product was digested with EcoRI and HindIII and ligated into pWQ573 to generate pWQ114 (23). The vector contains an l-arabinose-inducible pBAD promoter (24) and a chloramphenicol resistance cassette.
The pWQ811 vector is a pMBL19 (25) derivative containing a tetracycline-inducible promoter and an ampicillin resistance cassette. It was generated by cloning the tetracycline-inducible operator tetRA into pMBL19. The tetRA genes were PCR-amplified from E. coli BL21 (DE3) (forward primer, 5′-gtcgaagaattcatATGACTTTTCTCTATCACTGATAGGGAGTGG-3′; reverse primer, 5′-agaacagctagCATTTAGGTGGGTACGTTGGAGC-3′) and digested with EcoRI and NheI using the sites incorporated into the primers. The kanamycin resistance cassette from pBSL15 (26) was amplified (forward primer, 5′-aaaaaaattaataagcttgcatgctgcagtcgactagtCGTTGCTGTCGCTAGCTTCAC-3′; reverse primer, 5′-aaaaaagaattccatggtacccgggatcctctagactagtGTTGCTGTCGCGAACCCCAG-3′), digested with EcoRI and AseI (end cohesive with NdeI digest) and ligated into NdeI/NheI-digested pMBL19 concurrently with the tetRA digest. This construct was digested with SpeI and self-ligated to remove the kanamycin selection marker.
pWQ677 contains wbbL-wzm-rbs-wbbB cloned into pWQ811. The added rbs (ribosome binding site) is required for expression of WbbB in this construct. wbbL-wzm-rbs (forward primer, 5′-aaaaaagaattctATGACTTATGAAGCAATGAAGCC-3′; reverse primer, 5′-aaaaaaaaactagtggtaccaacctcctagaggaCATCAGCAAATCCC-3′; a ribosome binding site is indicated in italics) and wbbB (forward primer, 5′-aaaaaagctagcaggaggttggtaccATGCTGGCTGTATTTTTACCTCCC-3′; reverse primer, 5′-aaaaaaaagcttccatggaCTAGCGGTTGCGCTTAAACTCC-3′) were PCR-amplified from pKM114. The PCR fragments were ligated individually into pWQ811, using the EcoRI and KpnI sites for wbbL-wzm-rbs and the KpnI and HindIII sites for wbbB.
The gtr gene cluster from E. coli Δwzx-wbbK was PCR-amplified from genomic DNA (forward primer, 5′-taatggtaccACAGCAAGTATCGAT-3′; reverse primer, 5′-ttaggatccCGCAATTCTATCAGGAG-3′) following a gtr-cloning strategy described elsewhere (27)). PCR fragments were cleaved with KpnI and ligated into pWQ573 cleaved with KpnI and SmaI to produce pWQ115. This plasmid contains a fragment carrying the gtrA, gtrB and gtr* genes for complementation because the chromosomal deletion of gtrA also eliminated expression of the overlapping gtrB gene.
SDS-PAGE and Immunoblotting
To examine LPS in whole cell lysates, equivalent amounts of cells (determined by optical density at 600 nm), were solubilized in SDS-PAGE loading buffer and heated to 100 °C for 10 min. Samples were then treated with proteinase K prior to SDS-PAGE with 12% polyacrylamide resolving gels in Tris-glycine buffer (28, 29). LPS was visualized with silver staining (30). For O-antigen immunoblot analysis, LPS was transferred to nitrocellulose membranes (Protran; PerkinElmer Life Sciences) and probed with rabbit antiserum generated against Klebsiella pneumoniae O12 formalin-fixed whole cells. The K. pneumoniae O12 antigen is structurally and serologically identical to the O-antigen in R. terrigena ATCC 33257 (31). Goat anti-rabbit secondary antibody conjugated to alkaline phosphatase (Cedarlane Laboratories) was used, and the immunoblot was developed with 5-bromo-4-chloro-3-indoyl phosphate and nitroblue tetrazolium (Roche Applied Science). For Western blot analysis, samples were solubilized in SDS-PAGE loading buffer, heated to 100 °C for 10 min, and subjected to SDS-PAGE. Protein was transferred to nitrocellulose membrane and probed with mouse polyclonal anti-FLAG antibodies (Sigma-Aldrich). Goat anti-rabbit secondary antibody conjugated to alkaline phosphatase (Cedarlane Laboratories) was used as previously described.
Titration of Wzt
Glucose-supplemented (0.4%) overnight cultures of E. coli CWG1217 (Δwzx-wbbK) and CWG1219 (Δwzx-wbbK ΔgtrA) transformed with both pWQ114 and pWQ677 were diluted 1/100 and grown to an A600 of ∼0.8 following induction of wbbL-wzm-wbbB expression by adding 2.5 ng ml−1 anhydrotetrocycline. At the same time, expression of wzt from pWQ114 was varied by repression with 0.4% glucose or induction with a range of (0.002–0.2%) l-arabinose concentrations. LPS and Wzt levels were analyzed by SDS-PAGE, using a 12% resolving gel, and immunoblotting.
Complementation of the gtr Mutation
CWG1219 was transformed with pKM114 and pWQ115. The cultures were grown overnight, and LPS was examined by SDS-PAGE and immunoblotting.
Isolation of LPS and O-polysaccharide
Cultures of E. coli CWG1217 (Δwzx-wbbK) and CWG1218 (Δwzx-wbbK ΔgtrA) transformed with pKM114 were grown at 37 °C overnight. Cells were harvested by centrifugation, washed with distilled water, and lyophilized. LPS was isolated by hot phenol-water extraction (32), followed by dialysis of the extract without separation of the phenol and water phases, and freed from insoluble material by centrifugation. The resulting crude LPS solution was concentrated and purified by precipitation of proteins and nucleic acids with cold aqueous 50% CCl3CO2H (33). After centrifugation, the supernatant was dialyzed against distilled water and lyophilized. The O-polysaccharide was released by heating an LPS sample (150 mg) with 2% acetic acid at 100 °C for 2 h. The lipid precipitate was removed by centrifugation, and the carbohydrate-containing supernatant was fractionated on a Sephadex G-50 superfine column (2.5 cm × 75 cm) in 50 mm pyridinium acetate buffer (pH 4.5) at a flow rate of 0.6 ml min−1. Elution was monitored with a differential refractometer (Knauer).
Nuclear Magnetic Resonance Spectroscopy
NMR studies were performed in the Advanced Analysis Centre at the University of Guelph. Polysaccharide samples were deuterium-exchanged by lyophilizing twice from 99.9% D2O and then examined as solutions in 99.96% D2O. NMR spectra were recorded at 35 °C on a Bruker Avance II 600 MHz spectrometer equipped with a cryoprobe, using internal sodium 3-trimethylsilylpropanoate-2,2,3,3-d4 (δH 0, δC −1.6) as a reference. Two-dimensional experiments were performed using standard Bruker software, and the Bruker TopSpin 2.1 program was used to acquire and process the NMR data. Mixing times of 100 and 200 ms were used in total correlation spectroscopy and NOESY experiments, respectively. The heteronuclear multiple-bond correlation spectroscopy experiment was optimized for the JH,C coupling constant 8 Hz.
Results
Generation of Recombinant Strains Expressing the O-antigen from R. terrigena ATCC 33257
The O-antigen of R. terrigena ATCC 33257 was selected to probe the ability of a phage-mediated glucosylation system to interact with an ABC transporter-dependent O-antigen biosynthesis pathway. The repeating structure of this O-antigen possesses an α-l-Rha-(1→3)-d-GlcNAc disaccharide motif that is subject to glucosylation in E. coli O16 and S. flexneri 4a (Fig. 1). The R. terrigena O-antigen gene cluster cloned in pKM114 (31) contains eight genes involved in O-antigen formation (Fig. 2). The same O-antigen biosynthesis genes are present in K. pneumoniae O12 (34). The rmlBADC genes encode the enzymes for production of the dTDP-l-rhamnose precursor (35) and wbbL encodes a rhamnosyltransferase (34). Homologues of these genes are found in the O-antigen biosynthesis gene locus in E. coli K-12 (including Top10). Located downstream of wbbL are the wzm and wzt genes encoding the TMD and NBDs of the ABC transporter, respectively. The predicted wbbB gene product possesses domains with homology to glycosyltransferases sharing homology with GT1 and GT25 families, as well as a putative β-Kdo transferase resembling KpsS and KpsC from E. coli capsule systems (36). The R. terrigena O-antigen shares the same repeating unit (and serological cross-reactivity) with the K. pneumoniae O12 antigen, where the same repeat unit polysaccharide is terminated with a single β-Kdo residue at the nonreducing terminus (37). Signals consistent with this terminator were reported in the NMR spectrum of R. terrigena O-antigen (31). The sequence data are consistent with WbbB participating in both chain extension and chain termination, but this remains to be confirmed biochemically, and the precise activities giving rise to the O-antigen backbone are not central to the current study.
Although E. coli K-12 strains lack inherent O-antigen synthesis because of mutation(s) in the biosynthesis gene cluster (38), the ancestral O-antigen was serotype O16 (39) (Fig. 1). To exclude any interference between R. terrigena and E. coli glycosyltransferases and ensure that a homogeneous glycan backbone was synthesized, pKM114 was introduced into E. coli CWG1217 (Δwzx-wbbK), which lacks all of the glycosyltransferase activities, transport (Wzx) and polymerization machinery (Wzy) encoded by the chromosomal O-antigen biosynthesis locus.
Differential Reactivities of Native R. terrigena LPS and LPS from the Recombinant Strains with O-specific Antibodies
Whole cell lysate LPS samples were probed in immunoblots with antibodies raised against the K. pneumoniae O12 antigen, which is identical to the O-antigen from R. terrigena ATCC 33257. The immunoreactivity of LPS in CWG1217 (Δwzx-wbbK) containing pKM114 was dramatically reduced in comparison to R. terrigena ATCC 33257 whole cell lysate, despite the evident signal in the corresponding silver-stained LPS gel, suggesting an alteration in O-antigen structure (Fig. 3). This anomaly was noted previously in a different E. coli background, but the molecular basis was not examined (31).
FIGURE 3.
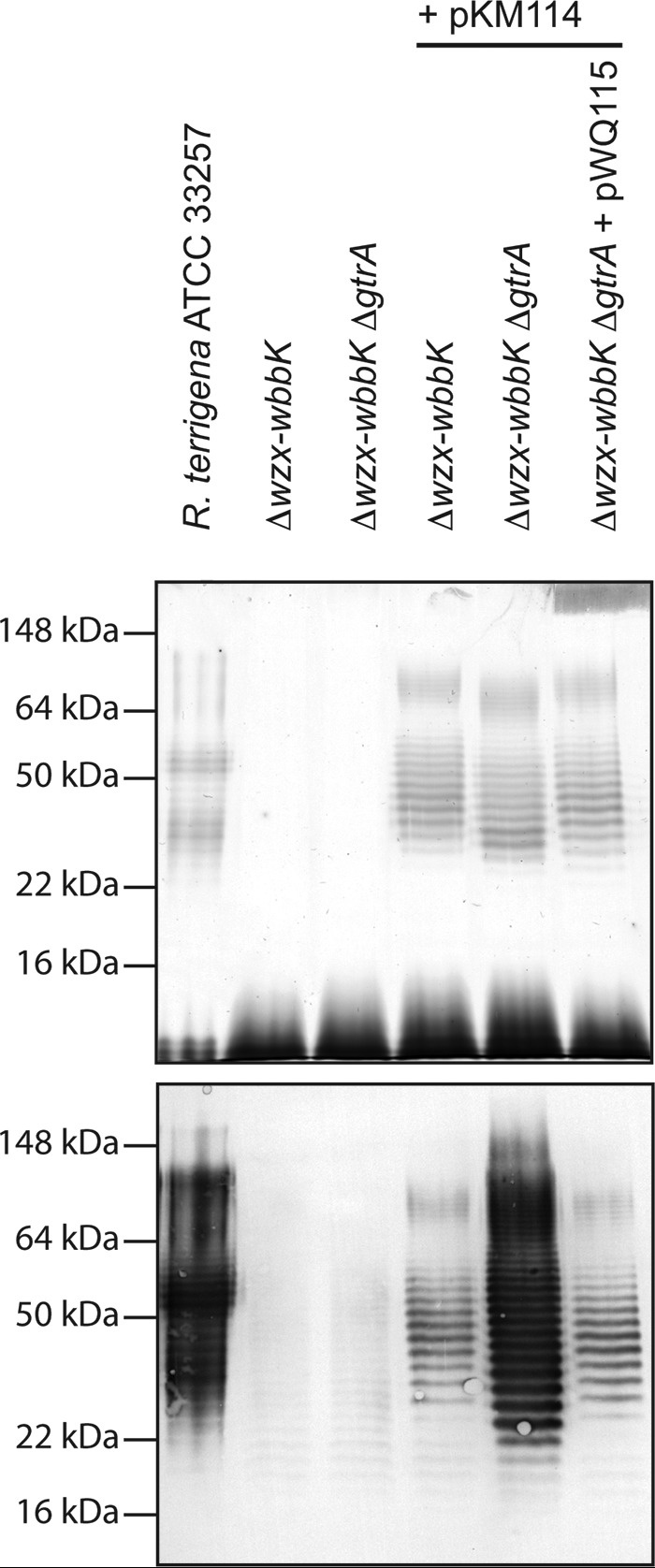
Reaction of LPS from R. terrigena ATCC 33257 and E. coli recombinant strains with antibodies raised against the K. pneumoniae O12 antigen. The figure shows the silver-stained SDS-PAGE LPS profile (upper panel) and the corresponding immunoblot (lower panel). The samples were proteinase K-treated whole cell lysates. The markers indicated on the left are protein standards to allow comparison of LPS migrations in different backgrounds.
Differential Antibody Reactivity Depends on Bacteriophage-derived Glucosylation Genes
Addition of the glucosyl side chains in the O16 antigen of E. coli K-12 has been attributed to prophage-mediated modification (5). Examination of the deposited genome of E. coli DH10b (the parent of Top10) (GenBankTM accession number NC_010473.1) revealed a three-ORF cluster with high sequence identity to S. flexneri bacteriophage (gtr) glucosylation clusters, belonging to the prophage KplE1 (19). The λ-red recombination system was used to remove the first gene in this cluster, a gtrA homologue annotated yfdG, which overlaps with the first four nucleotides of the gtrB homologue, yfdH. GtrA performs an essential step in glucosylation (17). Whole cell lysates from E. coli CWG1219 (Δwzx-wbbK ΔgtrA) and its gtrA+ parent, CWG1217, transformed with pKM114 showed the production of equivalent amounts of O-antigen-substituted LPS, as judged by silver stain. However, LPS from the CWG1219 (Δwzx-wbbK ΔgtrA) background reacted significantly more strongly with O12 antisera (Fig. 3). Reintroduction of the gtr cluster (on pWQ115) into CWG1219 resulted in decreased immunoreactivity, similar to the LPS from the gtrA+ CWG1217 background. Notably, the banding of O-antigen-substituted LPS molecules is out of register in gtrA+ and gtrA− backgrounds, as expected from the increased size of modified repeat units. We therefore concluded that the decreased immunoreactivity toward the heterologous O-antigen may be due to glucosylation of the polysaccharide chain by a lysogenic bacteriophage-derived gtr cluster within the E. coli K-12 chromosome.
Elucidation of the Structures of R. terrigena O-antigens Expressed in E. coli Mutants
LPS was isolated from E. coli CWG1217 (Δwzx-wbbK) and CWG1218 (Δwzx-wbbK ΔgtrA) transformants, both harboring pKM114. Purified LPS samples were degraded with 2% AcOH, and the released polysaccharides were isolated by gel chromatography on Sephadex G-50 for NMR spectroscopy.
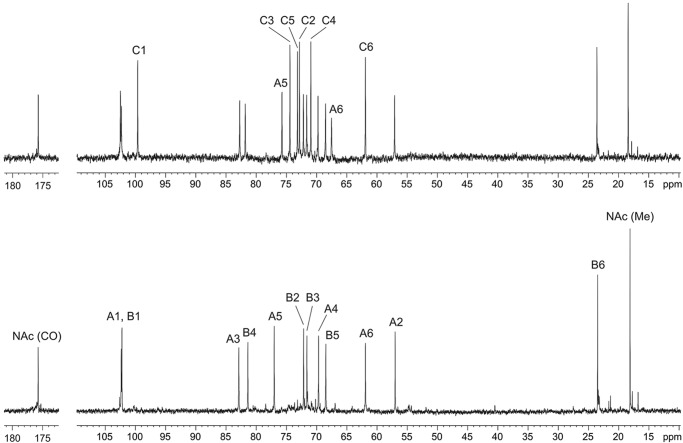
Analysis of 1H and 13C NMR spectra (Fig. 4, bottom panel, and Table 1), as well as the 1H,13C heteronuclear single-quantum coherence spectrum of the O-antigen expressed in CWG1218 (Δwzx-wbbK ΔgtrA) and comparison with data reported previously (31), confirmed that it possesses a disaccharide repeat unit structurally identical to that of the R. terrigena ATCC 33257 and K. pneumoniae O12 O-antigen (Fig. 1). This finding is in agreement with the observed wild-type level reactivity of the recombinant LPS with anti-K. pneumoniae O12 antibodies (Fig. 3).
FIGURE 4.
13C NMR spectra of the O-polysaccharides. The spectra represent samples from E. coli CWG1217 (Δwzx-wbbK) (top panel) and CWG1218 (Δwzx-wbbK ΔgtrA) (bottom panel) harboring pKM114. The numbers refer to carbons in sugar residues designated as shown in Table 1. The displacement of the A6 and A5 signals (C-6 and C-5 of GlcNAc) is due to positive α-effect on the linkage carbon and negative β-effect on the neighboring carbon and indicates the position of glucosylation.
TABLE 1.
1H and 13C NMR chemical shifts (δ, ppm)
| Sugar residue | C-1, H-1 | C-2, H-2 | C-3, H-3 | C-4, H-4 | C-5, H-5 | C-6, H-6 (6a, 6b) | |
|---|---|---|---|---|---|---|---|
| R. terrigena O-polysaccharide produced in E. coli CWG1217 (Δwzx-wbbK) | |||||||
| →3)-β-d-GlcpNAc-(1→a | A | 102.5, 4.82 | 57.0, 3.80 | 82.7, 3.61 | 69.7, 3.60 | 75.7, 3.62 | 67.5, 3.78, 3.94 |
| →4)-α-l-Rhap-(1→ | B | 102.3, 4.85 | 72.2, 3.74 | 71.6, 3.81 | 81.8, 3.59 | 68.4, 3.99 | 18.4, 1.26 |
| α-d-Glcp-(1→ | C | 99.6, 4.96 | 72.8, 3.55 | 74.4, 3.74 | 70.9, 3.41 | 73.1, 3.69 | 61.8, 3.75; 3.84 |
| R. terrigena O-polysaccharide produced in E. coli CWG1218 (Δwzx-wbbK ΔgtrA) | |||||||
| →3)-β-d-GlcpNAc-(1→a | A | 102.2, 4.80 | 57.0, 3.78 | 82.9, 3.60 | 69.6, 3.50 | 77.0, 3.42 | 61.9, 3.75, 3.91 |
| →4)-α-l-Rhap-(1→ | B | 102.3, 4.85 | 72.1, 3.74 | 71.6, 3.81 | 81.4, 3.60 | 68.4, 3.99 | 18.1, 1.27 |
a Signals for NAc groups are at δH 2.05, δC 23.5 (CH3), and 175.7 (CO).
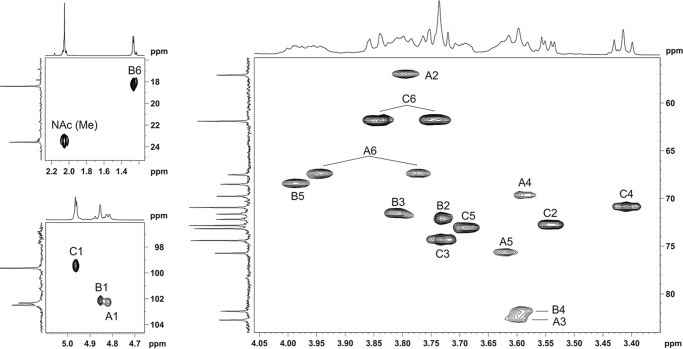
In contrast, the 13C NMR spectrum of the O-antigen expressed in E. coli CWG1217 (Δwzx-wbbK) demonstrated a regular structure with a trisaccharide repeat unit (Fig. 4, top panel). The spectrum contained signals for three sugar residues, including those for anomeric carbons at δ 99.6, 102.3, and 102.5; one nitrogen-bearing carbon at δ 57.0, one H3C-CH group at δ 18.4; two HOCH2-C groups at δ 61.8 and 67.5; and one N-acetyl group at δ 23.5 (CH3) and 175.7 (CO). The 1H NMR spectrum showed signals for three anomeric protons at δ 4.82, 4.85, and 4.96; one C-CH3 group at δ 1.26; one N-acetyl group at δ 2.05; and other protons in the region δ 3.41–3.94 (Table 1). All signals in the NMR spectra were assigned by using two-dimensional 1H,1H correlation spectroscopy, total correlation spectroscopy, NOESY (supplemental Figs. S1–S3), 1H,13C heteronuclear single-quantum coherence (Fig. 5), and heteronuclear multiple-bond correlation spectroscopy experiments (supplemental Fig. S4) (Table 1). Analysis of 1H and 13C NMR chemical shifts and comparison with data on free monosaccharides reported previously (40), intraresidue H,H and H,C correlations, and the coupling constants revealed spin systems for β-GlcN (residue A), α-Rha (residue B), and α-Glc (residue C), all in the pyranose form. The presence of a GlcN H-2/NAc CO correlation at δ 3.80/175.7 observed in the heteronuclear multiple-bond correlation spectroscopy experiment indicated that GlcN residues are N-acetylated (supplemental Fig. S4).
FIGURE 5.
Partial 1H,13C heteronuclear single-quantum coherence spectrum of the O-polysaccharide of E. coli CWG1217 Δwzx-wbbK harboring the R. terrigena O-antigen gene cluster. The corresponding parts of the 1H and 13C NMR spectra are shown along the axes. The numbers refer to H/C pairs in sugar residues, designated as shown in Table 1.
The glycosylation pattern in the repeat unit was inferred from significant downfield displacements of the signals for the linkage carbons, C-3 and C-6 of β-GlcNAc and C-4 of α-Rha (at δC 82.7, 67.5, and δC 81.8, respectively), compared with their positions at δC 74.8, 61.8, and 73.2, in the corresponding nonsubstituted monosaccharides (40). The chemical shifts for C-2 to C-6 of α-Glc revealed no significant differences, indicating that glucose occupies the terminal position in a side chain. Finally, the monosaccharide sequence in the repeat unit was determined by the following inter-residue cross-peaks between anomeric protons and linkage carbons in the heteronuclear multiple-bond correlation spectroscopy spectrum: GlcNAc H-1/Rha C-4, Rha H1/GlcNAc C-3, and Glc H-1/GlcNAc C-6 (supplemental Fig. S4).
Thus, the O-antigen expressed in E. coli CWG1217 Δwzx-wbbK has the structure shown in Fig. 1. It differs from R. terrigena O-antigen by the presence of α-Glc residue attached to C-6 of GlcNAc, which is the same position observed in the E. coli O16 antigen. No minor signals that would correspond to nonglucosylated polysaccharide were observed in the NMR spectra.
O-antigen Modification Is Dependent on a Functional ABC Transporter
A key feature of the phage-mediated glucosylation reaction is its periplasmic location. To confirm the same location in the recombinant bacteria, we varied the amount of expression of the wzt gene (encoding the NBD of the ABC transporter) to control the flow of completed O-antigen across the inner membrane to the expected site of glucosylation and examined the effect on immunoreactivity. In E. coli K-12 hosts, the transport of an O-antigen produced by this type of pathway is dependent on the cognate ABC transporter (18). E. coli CWG1217 (Δwzx-wbbK) and CWG1219 (Δwzx-wbbK ΔgtrA) were transformed with pWQ677 carrying wbbL-wzm-wbbB under the control of the Tet promoter and pWQ114 where expression of a N-terminally FLAG-tagged version of Wzt was regulated by the arabinose-inducible pBAD promoter. Expression of the biosynthesis genes was induced with a constant amount of anhydrotetracycline, whereas wzt was induced with varying l-arabinose. In the presence of 0.4% glucose, wzt (NBD) expression was repressed, and the silver-stained gel revealed no detectable high molecular weight laddering indicative of O-antigen-substituted lipid A core in either CWG1217 or CWG1219 (Fig. 6, top panel). In the absence of transport, cytosolic Und-PP-linked glycan is expected to accumulate, but these molecules are not seen in silver-stained profiles. The Und-PP-linked intermediates seen under these conditions show strong reactivity with the antibody in the immunoblot, consistent with the absence of glucosylation (Fig. 6, middle panel). Induction of wzt expression led to detectable amounts of FLAG-Wzt in the cell lysates (Fig. 6, bottom panel) and the formation of O-antigen-substituted lipid A core in both strain backgrounds. Comparison of the silver-stained profiles shows the individual bands of O-antigen-containing LPS molecules in CWG1217 and CWG1219 are not in register, as expected for molecules that differ in the presence or absence of glucose residues. Following activation of transport in E. coli CWG1217 (Δwzx-wbbK), the immunoreactive signal, consisting of both Und-PP and lipid A core-linked glycan, is reduced. This is the expected outcome if modification of reactive nonglucosylated intermediates occurs in the periplasm prior to their transition into the poorly reactive mature LPS containing glucosylated O-antigen. In contrast, the high level of immunoreactivity of the glycans in CWG1219 (Δwzx-wbbK ΔgtrA) remained unchanged when transport and completion of the LPS molecule were activated. These observations are therefore entirely consistent with the currently understood mechanism and location of gtr glucosylation.
FIGURE 6.
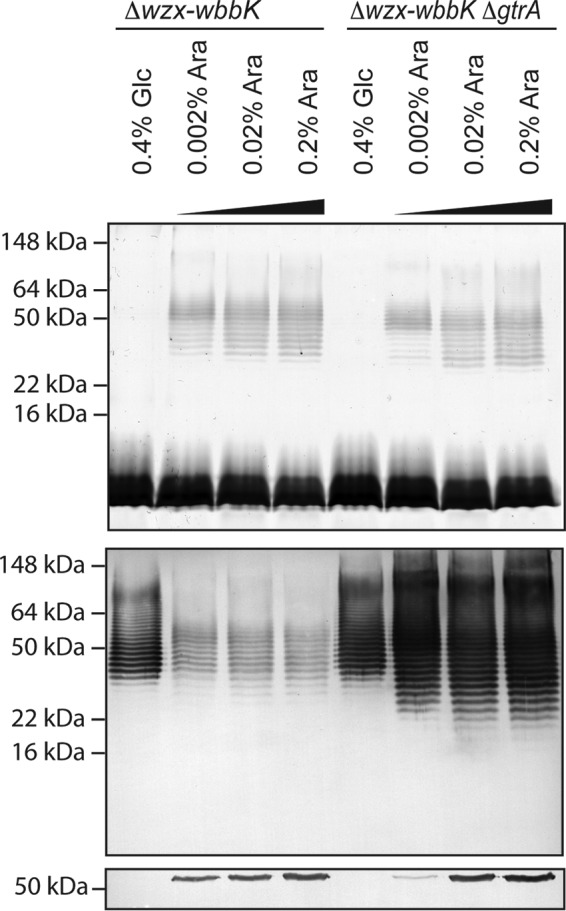
O-antigen modification is dependent on a functional ABC transporter. The samples were proteinase K-treated whole cell lysates of E. coli CWG1217 (Δwzx-wbbK) and CWG1219 (Δwzx-wbbK ΔgtrA) transformed with pWQ677 (carrying wbbL-wzm-wbbB) and pWQ114 (encoding FLAG-Wzt). Gene expression was induced with 2.5 ng ml−1 anhydrotetracycline to give a constant level of the biosynthesis enzymes. The amount of FLAG-Wzt (NBD) was varied by repression with glucose or induction with different concentrations of l-arabinose. The presence of the O12 antigen in LPS and Und-PP-linked glycans was assessed using silver stain (top panel) and immunoblotting (middle panel) of proteinase K-treated whole cell lysates. The silver stain profile detects only lipid-A linked O-antigen, whereas the immunoblot detects O-antigen linked to both lipid-A and UndPP. The Western immunoblot of FLAG-tagged Wzt confirmed the presence of the NBD (bottom panel). The markers indicated on the left are protein standards to allow comparison of LPS migrations in different backgrounds.
Discussion
Bacteriophage-encoded activities play an important role in serological diversity in Gram-negative bacteria. However, attributing O-antigen glucosylation to lysogenic bacteriophages requires both a solved glycan structure and identification of the context and content of the O-antigen biosynthesis loci. Although these types of comprehensive data are available for some species (e.g. E. coli, Salmonella, Shigella), this is not the case for many other bacteria. In all of the known examples, the target O-antigen for glucosylation is synthesized in a Wzy-dependent pathway. This study exploited a heterologous system, to provide the proof of principle that an ABC transporter-dependent mechanism poses no intrinsic mechanistic barrier to phage-mediated glucosylation and could potentially occur in natural isolates or in vaccine strains producing recombinant O-antigens. Glucosylation was dependent on genes encoded by a prophage in E. coli K-12, and the O-antigen was found to be α-glucosylated at the position C-6 of the GlcNAc residues in the O-antigen repeat unit, the same site seen in the E. coli O16 antigen. That is the same position modified in the O-antigen of S. flexneri isolates infected with lysogenic phage SfIV (5) (Fig. 1).
Given its effect on serotype, glucosylation is an important consideration in vaccine strategies (41, 42). Molecular modeling studies have established that glucosylation alters the conformation of Shigella O-antigens. In general, it affects the conformation of the nearest backbone linkage, but the influence varies, depending on the precise site modified (41). In some serotypes, glucosylation can lead to a substantial (up to 50%) physical shortening of the O-antigen chain (43). In many Gram-negative bacteria, the O-antigen is a critical determinant in resistance to serum killing (44). Although glucosylation does not appear to change this role in Salmonella and Shigella (43, 45, 46), other cellular properties are certainly impacted in a species-specific manner. In Shigella, glucosylation leads to greater invasion, which is correlated to enhanced function and exposure of the type 3 secretion system (43), and elevated acid tolerance (47). In Salmonella, glucosylation is associated with virulent isolates (48) but is not a stable property leading to antigenic (form) variation in the O serotype (45, 46, 49). Glucosylation is induced by exposure to macrophages, and although not required for invasion and systemic spread, it appears to enhance long term colonization (46).
The results presented here raise interesting questions about the glucosylation process itself. Biosynthetic experiments suggested that the acceptor for the modification reaction is the Und-PP-linked O-antigen polymerization product rather than the individual Und-PP-linked repeat units (12). Consistent with this proposal, the LPS products of wzy mutants, which contain a single O-repeat unit, are not modified in Salmonella (50, 51). Likewise, the first repeat unit of S. flexneri O-antigen is not subject to glucosylation (7, 53, 54). However, in bacteria producing full-length LPS forms, both glucosylated and nonglycosylated glycans can be produced by the same culture, and when they were separated, the modification was confined to LPS molecules with O-antigens exceeding ∼6 repeat units in length (55). In Wzy-dependent systems, growth of the glycan chain occurs one repeat unit at a time at the reducing terminus, building on new Und-PP-linked intermediates delivered by Wzx. The distribution of glucosyl modifications has been interpreted as reflecting a process where the catalytic site of the GtrC/Gtr* enzyme (mediating the final transfer) has no access to shorter polymerization products or lacks sufficient affinity for those products to allow modification. Only when the glycan chain reaches a particular size would it become a substrate for modification. The structure of the recombinant glucosylated O-antigen described here is not consistent with a large region of unmodified material, but NMR analysis lacks the sensitivity to reveal one or two unmodified repeat units at the reducing terminus of long chains. Each O-antigen chain retains part of the core oligosaccharide at its reducing terminus, which is released from lipid A with acetic acid. Signals for the core region are not evident because the NMR spectra are dominated by signals from the repeat units of long chains, and the same would apply to a core oligosaccharide linked to one or two unmodified core-proximal repeat units. In Salmonella, the extent of the unmodified region was determined by comparing mobilities of LPS molecules bearing very short O-antigen chains by SDS-PAGE in the presence or absence of glucosylation. Unfortunately, the R. terrigena assembly pathway precludes a similar approach because it involves a chain termination mechanism designed to generate only longer glycan chains, where the limits of SDS-PAGE resolution preclude such comparisons (Fig. 6). The underlying strategy for this type of chain length regulation has recently been resolved for E. coli O9a antigen, a prototype for the ABC transporter-dependent pathway (56, 57). What is clear from NMR spectra is that the recombinant system in E. coli lacks the phase variation that results in a combination of modified and unmodified chains in some Salmonella isolates (8, 55).
The WaaL enzyme that ligates nascent O-antigens to lipid A is specific for Und-PP-linked glycan donors, and its mechanism and architecture are conserved across species (58). This enzyme is unaffected by the pathway of O-antigen biosynthesis. For example, in addition to native Wzy-dependent O-antigens, E. coli K-12 hosts effectively ligate the products of ABC transporter pathways. This property is shown here with the R. terrigena glycan and in previous reports with different O-antigens of Klebsiella (34, 52). Although the preceding steps may vary, immediately prior to ligation the Und-PP-linked glycans from either of the assembly strategies are similarly confined to the interface of the cytoplasmic membrane and the periplasm. This presumably offers comparable access of the glucosylation machinery to its glycan substrate but rules out any essential targeted interaction of Gtr proteins with specific components of an O-antigen assembly pathway.
In conclusion, the ABC transporter-dependent pathway provides no intrinsic mechanistic barrier for bacteriophage-derived modification systems. This possibility must now be considered in studies correlating O-antigen structures with genetic determinants and in the use of recombinant strains in vaccine strategies.
Author Contributions
E. M., O. G. O., J. D. K., and C. W. conceived the study. E. M., O. G. O., and J. D. K. performed the experiments. E. M., O. G. O., J. D. K., and C. W. analyzed and interpreted the data. E. M., O. G. O., and C. W. wrote the manuscript, and J. D. K. provided input for the final draft.
Supplementary Material
Acknowledgment
We thank U. Mamat (Research Centre Borstel, Leibnitz Centre for Medicine and Biosciences, Borstel, Germany) for generously providing R. terrigena ATCC 33257 and pKM114.
This work was supported by funding (to C. W.) from the Natural Sciences and Engineering Research Council of Canada. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1–S4.
- Und-PP
- undecaprenol diphosphate
- ABC
- ATP-binding cassette
- NBD
- nucleotide-binding domain.
References
- 1. Stenutz R., Weintraub A., Widmalm G. (2006) The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30, 382–403 [DOI] [PubMed] [Google Scholar]
- 2. Rojas-Macias M. A., Ståhle J., Lütteke T., Widmalm G. (2015) Development of the ECODAB into a relational database for Escherichia coli O-antigens and other bacterial polysaccharides. Glycobiology 25, 341–347 [DOI] [PubMed] [Google Scholar]
- 3. Iguchi A., Iyoda S., Kikuchi T., Ogura Y., Katsura K., Ohnishi M., Hayashi T., Thomson N. R. (2015) A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Res. 22, 101–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lundborg M., Modhukur V., Widmalm G. (2010) Glycosyltransferase functions of E. coli O-antigens. Glycobiology. 20, 366–368 [DOI] [PubMed] [Google Scholar]
- 5. Allison G. E., Verma N. K. (2000) Serotype-converting bacteriophages and O-antigen modification in Shigella flexneri. Trends Microbiol. 8, 17–23 [DOI] [PubMed] [Google Scholar]
- 6. Sun Q., Knirel Y. A., Lan R., Wang J., Senchenkova S. N., Shashkov A. S., Wang Y., Wang Y., Luo X., Xu J. (2014) Dissemination and serotype modification potential of pSFxv_2, an O-antigen PEtN modification plasmid in Shigella flexneri. Glycobiology. 24, 305–313 [DOI] [PubMed] [Google Scholar]
- 7. Knirel Y. A., Sun Q., Senchenkova S. N., Perepelov A. V., Shashkov A. S., Xu J. (2015) O-Antigen modifications providing antigenic diversity of Shigella flexneri and underlying genetic mechanisms. Biochemistry (Moscow) 80, 901–914 [DOI] [PubMed] [Google Scholar]
- 8. Davies M. R., Broadbent S. E., Harris S. R., Thomson N. R., van der Woude M. W. (2013) Horizontally acquired glycosyltransferase operons drive Salmonellae lipopolysaccharide diversity. PLoS Genet. 9, e1003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raetz C. R., Whitfield C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitfield C., Trent M. S. (2014) Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 83, 99–128 [DOI] [PubMed] [Google Scholar]
- 11. Nikaido H., Nikaido K., Nakae T., Mäkelä P. H. (1971) Glucosylation of lipopolysaccharide in Salmonella: biosynthesis of O antigen factor 122: I. over-all reaction. J. Biol. Chem. 246, 3902–3911 [PubMed] [Google Scholar]
- 12. Takeshita M., Mäkelä P. H. (1971) Glucosylation of lipopolysaccharide in Salmonella: biosynthesis of O antigen factor 122. III. The presence of 122 determinants in haptenic polysaccharides. J. Biol. Chem. 246, 3920–3927 [PubMed] [Google Scholar]
- 13. Wright A. (1971) Mechanism of conversion of the Salmonella O antigen by bacteriophage ϵ34. J. Bacteriol. 105, 927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sasaki T., Uchida T., Kurahashi K. (1974) Glucosylation of O-antigen in Salmonella carrying ϵ15 and ϵ34 phages. J. Biol. Chem. 249, 761–772 [PubMed] [Google Scholar]
- 15. Stocker B. A. D., Staub A. M., Tinelli R., Kopacka B. (1960) Immunochemical studies on Salmonella: IV. study of antigen 1 present in two Salmonella belonging to the B and E4 groups. Ann. Inst. Pasteur (Paris) 98, 505–523 [PubMed] [Google Scholar]
- 16. Mavris M., Manning P. A., Morona R. (1997) Mechanism of bacteriophage SfII-mediated serotype conversion in Shigella flexneri. Mol. Microbiol. 26, 939–950 [DOI] [PubMed] [Google Scholar]
- 17. Guan S., Bastin D. A., Verma N. K. (1999) Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology 145, 1263–1273 [DOI] [PubMed] [Google Scholar]
- 18. Cuthbertson L., Kos V., Whitfield C. (2010) ABC transporters involved in export of cell surface glycoconjugates. Microbiol. Mol. Biol. Rev. 74, 341–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panis G., Méjean V., Ansaldi M. (2007) Control and regulation of KplE1 prophage site-specific recombination: a new recombination module analyzed. J. Biol. Chem. 282, 21798–21809 [DOI] [PubMed] [Google Scholar]
- 20. Sawitzke J. A., Thomason L. C., Costantino N., Bubunenko M., Datta S., Court D. L. (2007) Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 421, 171–199 [DOI] [PubMed] [Google Scholar]
- 21. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Datta S., Costantino N., Court D. L. (2006) A set of recombineering plasmids for Gram-negative bacteria. Gene. 379, 109–115 [DOI] [PubMed] [Google Scholar]
- 23. Greenfield L. K., Richards M. R., Vinogradov E., Wakarchuk W. W., Lowary T. L., Whitfield C. (2012) Domain organization of the polymerizing mannosyltransferases involved in synthesis of the Escherichia coli O8 and O9a lipopolysaccharide O-antigens. J. Biol. Chem. 287, 38135–38149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakano Y., Yoshida Y., Yamashita Y., Koga T. (1995) Construction of a series of pACYC-derived plasmid vectors. Gene. 162, 157–158 [DOI] [PubMed] [Google Scholar]
- 26. Alexeyev M. F. (1995) Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques 18, 52–56 [PubMed] [Google Scholar]
- 27. Adams M. M., Allison G. E., Verma N. K. (2001) Type IV O antigen modification genes in the genome of Shigella flexneri NCTC 8296. Microbiology 147, 851–860 [DOI] [PubMed] [Google Scholar]
- 28. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 29. Hitchcock P. J., Brown T. M. (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsai C. M., Frasch C. E. (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119, 115–119 [DOI] [PubMed] [Google Scholar]
- 31. Mertens K., Müller-Loennies S., Stengel P., Podschun R., Hansen D. S., Mamat U. (2010) Antiserum against Raoultella terrigena ATCC 33257 identifies a large number of Raoultella and Klebsiella clinical isolates as serotype O12. Innate Immun. 16, 366–380 [DOI] [PubMed] [Google Scholar]
- 32. Westphal O. (1965) Bacterial lipopolysaccharide-extraction with phenol water and further application of procedure. Methods Carbohydr. Chem. 1, 83–91 [Google Scholar]
- 33. Kul'shin V. A., Iakovlev A. P., Avaeva S. N., Dmitriev B. A. (1987) Improved method of lipopolysaccharide isolation from Gram-negative bacteria. Mol. Gen. Mikrobiol. Virusol. 5, 44–46 [PubMed] [Google Scholar]
- 34. Izquierdo L., Merino S., Regué M., Rodriguez F., Tomás J. M. (2003) Synthesis of a Klebsiella pneumoniae O-antigen heteropolysaccharide (O12) requires an ABC 2 transporter. J. Bacteriol. 185, 1634–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Macpherson D. F., Manning P. A., Morona R. (1994) Characterization of the dTDP-rhamnose biosynthetic genes encoded in the rfb locus of Shigella flexneri. Mol. Microbiol. 11, 281–292 [DOI] [PubMed] [Google Scholar]
- 36. Willis L. M., Whitfield C. (2013) KpsC and KpsS are retaining 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc. Natl. Acad. Sci. U.S.A. 110, 20753–20758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinogradov E., Frirdich E., MacLean L. L., Perry M. B., Petersen B. O., Duus J. Ø., Whitfield C. (2002) Structures of lipopolysaccharides from Klebsiella pneumoniae. Eluicidation of the structure of the linkage region between core and polysaccharide O chain and identification of the residues at the non-reducing termini of the O chains. J. Biol. Chem. 277, 25070–25081 [DOI] [PubMed] [Google Scholar]
- 38. Liu D., Reeves P. R. (1994) Escherichia coli K12 regains its O antigen. Microbiology 140, 49–57 [DOI] [PubMed] [Google Scholar]
- 39. Stevenson G., Neal B., Liu D., Hobbs M., Packer N. H., Batley M., Redmond J. W., Lindquist L., Reeves P. (1994) Structure of the O-antigen of Escherichia coli K-12 and the sequence of Its rfb gene cluster. J. Bacteriol. 176, 4144–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jansson P. E., Kenne L., Widmalm G. (1991) CASPER: a computer program used for structural analysis of carbohydrates. J. Chem. Inf. Comput. Sci. 31, 508–516 [DOI] [PubMed] [Google Scholar]
- 41. Theillet F.-X., Simenel C., Guerreiro C., Phalipon A., Mulard L. A., Delepierre M. (2011) Effects of backbone substitutions on the conformational behavior of Shigella flexneri O-antigens: implications for vaccine strategy. Glycobiology. 21, 109–121 [DOI] [PubMed] [Google Scholar]
- 42. Rondini S., Micoli F., Lanzilao L., Gavini M., Alfini R., Brandt C., Clare S., Mastroeni P., Saul A., MacLennan C. A. (2015) Design of glycoconjugate vaccines against invasive African Salmonella enterica serovar Typhimurium. Infect. Immun. 83, 996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. West N. P., Sansonetti P., Mounier J., Exley R. M., Parsot C., Guadagnini S., Prévost M.-C., Prochnicka-Chalufour A., Delepierre M., Tanguy M., Tang C. M. (2005) Optimization of virulence functions through glucosylation of Shigella LPS. Science 307, 1313–1317 [DOI] [PubMed] [Google Scholar]
- 44. Lerouge I., Vanderleyden J. (2002) O-antigen structural variation: mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol. Rev. 26, 17–47 [DOI] [PubMed] [Google Scholar]
- 45. Mäkelä P. H., Hovi M., Saxén H., Valtonen M., Valtonen V. (1988) Salmonella, complement and mouse macrophages. Immunol. Lett. 19, 217–222 [DOI] [PubMed] [Google Scholar]
- 46. Bogomolnaya L. M., Santiviago C. A., Yang H.-J., Baumler A. J., Andrews-Polymenis H. L. (2008) “Form variation” of the O12 antigen is critical for persistence of Salmonella typhimurium in the murine intestine. Mol. Microbiol. 70, 1105–1119 [DOI] [PubMed] [Google Scholar]
- 47. Martinić M., Hoare A., Contreras I., Alvarez S. A. (2011) Contribution of the lipopolysaccharide to resistance of Shigella flexneri 2a to extreme acidity. PLoS One 6, e25557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rahman M. M., Guard-Petter J., Carlson R. W. (1997) A virulent isolate of Salmonella enteritidis produces a Salmonella typhi-like lipopolysaccharide. J. Bacteriol. 179, 2126–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mäkelä P. H. (1966) Genetic determination of the O antigens of Salmonella groups B (4,5,12) and C1 (6,7). J. Bacteriol. 91, 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hämmerling G., Lüderitz O., Westphal O. (1970) Structural investigations on the core polysaccharide of Salmonella typhimurium and the mode of attachment of the O-specific chains. Eur. J. Biochem. 15, 48–56 [DOI] [PubMed] [Google Scholar]
- 51. Rudén U., Mäkelä P. H. (1974) O-acetylation and glucosylation of lipopolysaccharide in hybrids between Salmonella groups B and C2. Eur. J. Biochem. 48, 11–20 [DOI] [PubMed] [Google Scholar]
- 52. Kos V., Cuthbertson L., Whitfield C. (2009) The Klebsiella pneumoniae O2a antigen defines a second mechanism for O antigen ATP-binding cassette transporters. J. Biol. Chem. 284, 2947–2956 [DOI] [PubMed] [Google Scholar]
- 53. Kondakova A. N., Vinogradov E. V., Shekht M. E., Markina A. A., Lindner B., L'vov V. L., Aparin P. G., Knirel Y. A. (2010) Structure of the oligosaccharide region (core) of the lipopolysaccharides of Shigella flexneri types 2a and 5b. Bioorg. Khim. 36, 429–432 [DOI] [PubMed] [Google Scholar]
- 54. Kubler-Kielb J., Vinogradov E., Mocca C., Pozsgay V., Coxon B., Robbins J. B., Schneerson R. (2010) Immunochemical studies of Shigella flexneri 2a and 6, and Shigella dysenteriae type 1 O-specific polysaccharide-core fragments and their protein conjugates as vaccine candidates. Carbohydr. Res. 345, 1600–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Helander I. M., Moran A. P., Mäkelä P. H. (1992) Separation of two lipopolysaccharide populations with different contents of O-antigen factor 122 in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 6, 2857–2862 [DOI] [PubMed] [Google Scholar]
- 56. King J. D., Berry S., Clarke B. R., Morris R. J., Whitfield C. (2014) Lipopolysaccharide O antigen size distribution is determined by a chain extension complex of variable stoichiometry in Escherichia coli O9a. Proc. Natl. Acad. Sci. U.S.A. 111, 6407–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hagelueken G., Clarke B. R., Huang H., Tuukkanen A., Danciu I., Svergun D. I., Hussain R., Liu H., Whitfield C., Naismith J. H. (2015) A coiled-coil domain acts as a molecular ruler to regulate O-antigen chain length in lipopolysaccharide. Nat. Struct. Mol. Biol. 22, 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruan X., Loyola D. E., Marolda C. L., Perez-Donoso J. M., Valvano M. A. (2012) The WaaL O-antigen ligase has features in common with metal ion-dependent inverting glycosyltransferases. Glycobiology 22, 288–299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.