Background: Mutations in nucleotide binding domain 1 of cystic fibrosis (CF) transmembrane conductance regulator cause severe CF.
Results: Correctors rescue trafficking of ΔF508 by altering interaction with Hsp27 and −40.
Conclusion: Rescue of trafficking mutants can be accomplished by combining correctors from different classes.
Significance: ΔF508 is the most common mutation. Information on the mechanism of corrector action is critical for treating the majority of patients.
Keywords: ABC transporter, chaperonin, electrophysiology, heat shock protein (HSP), lung, membrane, mutant, protein degradation, protein folding, protein processing
Abstract
Correcting the processing of ΔF508-CFTR, the most common mutation in cystic fibrosis, is the major goal in the development of new therapies for this disease. Here, we determined whether ΔF508 could be rescued by a combination of small-molecule correctors, and identified the mechanism by which correctors rescue the trafficking mutant of cystic fibrosis transmembrane conductance regulator (CFTR). We transfected COS-7 cells with ΔF508, created HEK-293 stably expressing ΔF508, and utilized CFBE41o− cell lines stably transduced with ΔF508. As shown previously, ΔF508 expressed less protein, was unstable at physiological temperature, and rapidly degraded. When the cells were treated with the combination C18 + C4 the mature C-band was expressed at the cell surface. After treatment with C18 + C4, we saw a lower rate of protein disappearance after translation was stopped with cycloheximide. To understand how this rescue occurs, we evaluated the change in the binding of proteins involved in endoplasmic reticulum-associated degradation, such as Hsp27 (HspB1) and Hsp40 (DnaJ). We saw a dramatic reduction in binding to heat shock proteins 27 and 40 following combined corrector therapy. siRNA experiments confirmed that a reduction in Hsp27 or Hsp40 rescued CFTR in the ΔF508 mutant, but the rescue was not additive or synergistic with C4 + 18 treatment, indicating these correctors shared a common pathway for rescue involving a network of endoplasmic reticulum-associated degradation proteins.
Introduction
Cystic fibrosis (CF)3 is a lethal autosomal recessive genetic illness caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which is translated into a defective protein, with loss of function (1). CFTR is a chloride ion channel located at the apical membrane of epithelial tissues (e.g. the sweat glands, pancreas, and respiratory, intestinal, and reproductive tracts) and is responsible for salt and fluid balance of mucosal surface fluids. A reduction in CFTR function leads to an increase in the concentration of chloride in sweat, a loss or reduction in exocrine pancreatic activity, and the accumulation of thick, viscous mucus in airways (2). CF patients present with recurrent pulmonary infections, together with lung inflammation and fibrosis, all of which lead to respiratory failure, as well as with pancreatic insufficiency, which may be associated with diabetes mellitus (3). Therefore, CF patients require many treatments to reduce their complications and overcome their debilitating symptoms. During the last few decades, the average rate of survival of CF patients has increased as a result of early diagnosis and the development of more efficient therapies (4). However, there is no intervention to restore the primary defect in the CFTR trafficking mutants, and improvements are still needed to reduce the burden of the required treatments and increase patients' life expectancy.
CFTR is a member of the ATP-binding cassette (ABC) family and is composed of two transmembrane domains, two nucleotide binding domains (NBDs), and a unique regulatory domain (5, 6). There are more than 1,900 mutations described in CFTR. The most common mutation is ΔF508, found in NBD1, which affects about 90% of the CF patients (1). ΔF508-CFTR is a partially glycosylated and misfolded protein that is retained in the endoplasmic reticulum (ER) and degraded by the proteasome, precluding the delivery of the CFTR molecule to the cell surface (8).
The impact of the missing phenylalanine at position 508 (ΔF508) on CFTR has been studied intensely (9), (10). Surprisingly, the ΔF508 mutation at first glance has very little effect on the overall structure of the domain. However, a deeper look reveals effects throughout much of the entire CFTR molecule, including a reduced thermal stability of NBD1, altered interactions with the intracellular loops, and an altered stability of NBD2 (11). The functional effects of this mutation are 2-fold: arrested processing in the ER and reduced channel activity, both of which must be rescued to produce a clinical benefit to patients.
Many attempts have been made to devise ways to rescue ΔF508-CFTR. These strategies have included transcomplementation (12–14), in which truncated versions of CFTR can act as molecular chaperones and rescue ΔF508-CFTR. Alternatively, chemical correctors have been identified that act on ΔF508-CFTR either directly or indirectly to attenuate the deleterious effects of the disease (15–17). Among these, VX-770, a potentiator, has been shown to activate CFTR current in mutations, such as G551D, in which the inactive protein is present at the plasma membrane (18). Furthermore, the corrector VX-809 has been able to rescue the trafficking in ΔF508-CFTR and produce a gain in channel activity (19), although its clinical benefit has been shown to be limited (20). Thus, there is still an unmet need for improved therapies and new correctors. In addition, the mechanism by which CFTR is rescued by small molecules is still unclear. The goal of the present work was to evaluate the effect of correctors combination on ΔF508 and to explore the mechanism of action of the best correctors in rescuing the delivery of CFTR to the cell surface.
Materials and Methods
Cell Culture
The African green monkey fibroblast-like cell line COS-7 (catalog CRL-1651, ATCC) was maintained in Dulbecco's modified Eagle's medium (DMEM, Life Technologies) high glucose (1× glucose), supplemented with 10% fetal bovine serum (FBS, Invitrogen), penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C in a humidified incubator in 5% CO2. To evaluate the temperature correction, cells were maintained at 27 °C for 24 h before being harvested. Flp-In human embryonic kidney (HEK)-293 cells (catalog CRL-1573, Life Technologies) cultured in DMEM containing 10% FBS with penicillin (100 units/ml), streptomycin (100 μg/ml), and Zeocin (100 μg/ml) at 37 °C were used to generate the stably transfected ΔF508 mutant cell lines. CFBE41o− cells stably transfected with ΔF508 (gift of Erik Sorscher) and WT CFTR (gift of Dieter Gruenert) were maintained in minimum essential Eagle's medium (Invitrogen) with 10% FBS, penicillin/streptomycin, l-glutamate (200 mm), and puromycin (5 μg/ml, Sigma).
Gene Silencing
siRNA-mediated silencing was carried out against Hsp40 (10 nm, catalog sc-35599, Santa Cruz) and Hsp27 (10 nm, catalog 6526, Cell Signaling) in the HEK-293 or CFBE41o− cell lines for 72 h. Transfections were performed using INTERFERin (Polyplus Transfection) according to the manufacturer's instructions.
Generation of Stably Transfected HEK-293 Cell Lines
The ΔF508-CFTR mutant clone was subcloned into the pcDNA5-FRT expression vector to generate stably transfected cells. Flp-In HEK-293 cells were transfected with pcDNA5/FRT carrying CFTR ΔF508 according to the manufacturer's protocol (Flp-InTM System, Life Technologies). After transfection, the medium was changed to DMEM with 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and hygromycin (100 μg/ml). The hygromycin-resistant foci were isolated, expanded, and then analyzed for the expression of CFTR by immunoblotting.
Treatments
Small-molecule correctors were applied for 16 h to assess the effect of rescuing CFTR. C3, C4, and C18 were obtained from the CFFT panel library. The compounds were used alone or in combinations of two. The stability of the CFTR protein was analyzed via cycloheximide treatment (25 μg/ml, Sigma). Cells were harvested 1, 2, 4, 6, or 8 h after translation was stopped with cycloheximide.
Surface Protein Biotinylation of CFTR
The plasma membrane proteins of HEK-293 cells stably expressing ΔF508 was submitted to EZ-LinkTM sulfo-NHS-SS-biotin (Thermo Scientific) for 30 min at 4 °C. The cells were then washed gently three times with glycine quenching buffer (200 mm glycine and 25 mm Tris-HCl, pH 8.0, in Dulbecco's phosphate-buffered saline plus Ca2+ and Mg2+) and solubilized in lysis buffer (50 mm Tris-HCl, pH 7.4, with 150 mm NaCl and 1% Nonidet P-40 with protease inhibitors). The lysates were rotated for 30 min at 4 °C, then centrifuged at 14,000 × g for 20 min at 4 °C. The total amount of cellular protein was determined using the Protein Assay Dye Reagent (Bio-Rad). The cell-surface proteins were isolated from the total lysate (2,000 μg) by incubation with NeutrAvidin Plus UltraLink Resin (Thermo Scientific) for 45 min at 4 °C (25 μg of protein/1 μl of beads). After a brief centrifugation, the supernatant was discarded, and the beads were washed five times with lysis buffer. The bound proteins were eluted with 2× Laemmli sample buffer with 5% β-mercaptoethanol. The eluted proteins were subjected to SDS-PAGE and immunoblotting as described below.
Immunoblotting
The cells were harvested and solubilized in lysis buffer as described above, supplemented with a protease inhibitor mixture (catalog 78429, Thermo Scientific). The cell lysates were centrifuged at 14,000 × g for 20 min at 4 °C to pellet the insoluble material. The supernatants (30–50 μg of proteins) were subjected to 10% SDS-PAGE and immunoblotting, followed by enhanced chemiluminescence (SuperSignal West Dura Extended Duration Substrate, catalog number 34075, Thermo Scientific). The chemiluminescent signal on the PVDF (polyvinylidene difluoride) membrane (Bio-Rad) was directly captured by a FujiFilm LAS-4000 plus system with a cooled CCD camera. CFTR protein was detected with monoclonal anti-human CFTR (217; 1:1,000) antibody (provided by Dr. J. Riordan, Department of Biochemistry and Biophysics and Cystic Fibrosis Center of North Carolina). Ezrin, used as a loading control, was detected with monoclonal antibody (1:10,000; Santa Cruz Biotechnology). To detect the chaperones, the membranes were incubated with primary antibodies anti-Hsp40 (1:1,000) and anti-Hsp27 (1:500). Image Gauge version 3.2 software (Fuji Film) was used for quantification of the bands.
Immunopreciptation
The proteins were extracted, and protein concentration was measured as described above. The protein lysates (2,000 μg) were then rotated with 80 μl of protein A/G-agarose beads (Santa Cruz Biotechnology) and 5 μg of anti-CFTR antibody (M3A7, Millipore) for 4 h at 4 °C. A/G beads were washed four times with lysis buffer supplemented with protease inhibitor. Sample buffer (2×) with 5% β-mercaptoethanol was added 1:1 with the A/G beads. The protein samples were used for immunoblotting as described above.
Short-circuit Currents
The short-circuit currents (Isc) were measured in Ussing-type chambers (Physiological Instruments, San Diego, CA). Confluent cystic fibrosis bronchial epithelial cells (CFBE) stably expressing ΔF508-CFTR or WT-CFTR were seeded onto 12-mm diameter Costar® SnapwellTM cell culture inserts (Corning Costar, Acton, MA; 3801) and cultured for 7 days to establish polarized monolayers. Once monolayers reach a considered relevant resistance, inserts were mounted in an Ussing-type chamber and bathed in solutions (see below) maintained at 37 °C and stirred by bubbling with 95% O2, 5% CO2. Corrector treatments (C4, C18, or C4 + C18; 10 μm) were added to the CFBE cell culture medium 16 h before experiments, Hsp27 siRNA (10 nm) were added 72 h before. Short-circuit current (Isc) was measured by voltage clamping the transepithelial voltage across the monolayers to 0 mV with a multichannel voltage-current clamp amplifier (model VCC MC6, Physiologic Instruments). Transepithelial resistance was measured by periodically applying a 5-mV bipolar voltage pulse, recording the deflection response in short-circuit current (Isc), and applying Ohm's law. The apical bath solution contained (in mm): 120 sodium gluconate, 2 CaCl2, 5 KCl, 2 MgCl2, 10 Hepes, 10 d-glucose. The basolateral bath solution contained (in mm): 120 NaCl, 2 CaCl2, 5 KCl, 2 MgCl2, 10 Hepes, 10 d-glucose (adjusted to pH 7.3 with NaOH). The pharmacological agent amiloride (100 μm) was added to inhibit apical Na+ absorption through ENaC. Following an equilibration period, the baseline Isc was recorded. To activate CFTR, the adenylate cyclase activator forskolin (10 μm) and the tyrosine kinase inhibitor genistein (30 μm) were added sequentially to the apical and basolateral bath solutions. Thiazolidonone CFTR inhibitor CFTRinh172 (10 μm) was added to inhibit Isc, indicating that the measured current was CFTR-mediated chloride transport.
Data acquisition and analysis were done with the Acquire and Analyze Data Acquisition System (Physiologic Instruments, version 2.3). Data are expressed as the CFTRinh172 sensitive short-circuit current (ΔIsc) calculated by subtracting the Isc measurement after CFTRinh172 treatment from the values corresponding to the plateau phase reached after addition of genistein on forskolin-stimulated Isc.
Statistical Assays
Statistical comparisons were made by using one-way analysis of variance followed by Tukey's test or an unpaired Student's t test (Prism 5.0, GraphPad Software). All data are present as mean ± S.E. All measurements were done at least three times, and the values were considered significant at p < 0.05.
Results
Expression and Temperature Correction of ΔF508
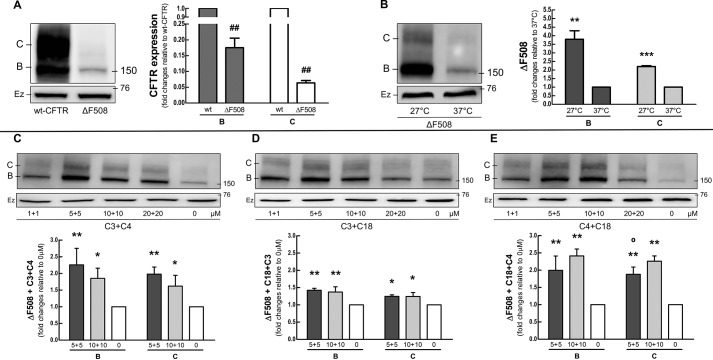
To confirm how ΔF508 affects CFTR, we transiently expressed it in COS-7 cells (Fig. 1A). As expected ΔF508 showed a lower total protein expression than wild-type CFTR (WT-CFTR). As is well known for ΔF508 (21), incomplete folding is responsible for the reduced protein levels resulting from ER-associated degradation (ERAD). To take this initial characterization one step forward, we incubated cells at low temperature (27 °C). Both the immature B and mature C bands of ΔF508 increased significantly when the cells were grown at the reduced temperature (Fig. 1B). This shows clearly that ΔF508 is more stable at reduced temperature than at human physiological temperature (37 °C).
FIGURE 1.
COS-7 cells were transfected with 4 μg of WT-CFTR or ΔF508. A, expression of ΔF508-CFTR. Immunoblots and graphs comparing the expression of bands B and C in the ΔF508 mutant and WT-CFTR. Versus WT: ##, p < 0.01. B, temperature correction of ΔF508. Immunoblots and graphs comparing the expression of CFTR in cells with ΔF508 incubated in 27 or 37 °C. Versus 37 °C: **, p < 0.01; ***, p < 0.001. C-E, correcting ΔF508-CFTR. Cells were treated 16 h with increasing doses of C3 + C4 (C), C18 + C3 (D), and C18 + C4 (E). Versus 0 μm: *, p < 0.05; **, p < 0.01. Versus 10 μm: ○, p < 0.05 (n = 3). Ezrin (Ez) was used as a loading control.
Correction of ΔF508-CFTR Mutations with Small Molecules
Correctors have been investigated as a possible therapeutic strategy to restore the trafficking and function of ΔF508-CFTR. We tested C3 and C18, discovered by Vertex Pharmaceuticals (22); and C4, discovered by Verkman and collaborators (15). C3 and C18, both class I correctors, are thought to stabilize NBD1-transmembrane domain 1/2 interfaces. C4 has been assigned to class II and is hypothesized to restore the stability of NBD2 or its interfaces with other domains of CFTR (23). The ΔF508 mutation (Fig. 1, C–E) responded to C3 + C4 or C18 + C4 with a ≈2–2.5-fold increase in both the immature B and mature C bands, depending on the dose and corrector. This response was greater than that observed for the combined C18 + C3.
The Combination of C18 and C4 Increases the Stability of ΔF508 and Allows It Be Delivered to the Cell Surface
Previous studies have shown that the mature C-band of WT-CFTR is a relatively stable protein with a long half-life (12, 24). To assess the rate of disappearance of ΔF508 as an indication of its rate of degradation, we treated cells containing ΔF508 with cycloheximide to block protein synthesis. With cycloheximide treatment, very little C band was expressed from the ΔF508 mutant, and the C band that was expressed disappeared rapidly following the exposure to the cycloheximide (Fig. 2A). We next tested the rate of disappearance following a combined treatment with C18 and C4 (Fig. 2B). As indicated above, the combined treatment resulted in a substantial increase in the amount of both bands B and C. For ΔF508, combined corrector therapy increased the amount of band C present at 8 h when compared with immature band B, indicating that the combined therapy was effective in stabilizing the mature band (Fig. 2C).
FIGURE 2.
A and B, the combination of C18 and C4 reduces the rate of disappearance of ΔF508. Immunoblotting of HEK-293 cells stably transfected with ΔF508, without or with C18 + C4 (10 μm) treatment for 16 h. Cells were treated with cycloheximide (CHX, 25 μg/ml) and harvested at the time points indicated (n = 3). C, quantification of data from Fig. 2A. B, versus 0 h no C18 + C4: *, p < 0.05; **, p < 0.01. Versus 0 h + C18 + C4: ##, p < 0.01. Bars represent the densitometry quantification of immature band B (white) and mature band C (dark bars). D and E, the combination of C18 and C4 rescues NBD1 mutants to the cell surface. Biotinylation of the surface proteins was performed in HEK-293 cells stably transfected with ΔF508, with or without C18 + C4 (10 μm) treatment for 16 h. F, the presence of band C on the surface expression was evaluated 2, 4, and 6 h after stopping the translation of ΔF508, with C18 + C4 treatment (n = 3). Ezrin (Ez), an intracellular protein, was used as a loading control. The absence of ezrin binding to biotin is evidence that the cellular membrane is intact and biotin did not leak into the cell. This is verification that our assay is measuring only proteins in the plasma membrane such as CFTR.
The major goal in the development of new therapies for CF is correction of the processing defect that interferes with the delivery of CFTR to the plasma membrane (Fig. 2D). Toward this end, the combination of C18 + C4 was able to increase the surface expression of ΔF508 (Fig. 2E). Importantly, treatment of ΔF508 with C18 + C4 resulted in the surface expression of the mature C band remaining stable for 6 h after protein translation was stopped (Fig. 2F).
The Combination of Correctors Rescues ΔF508 Function
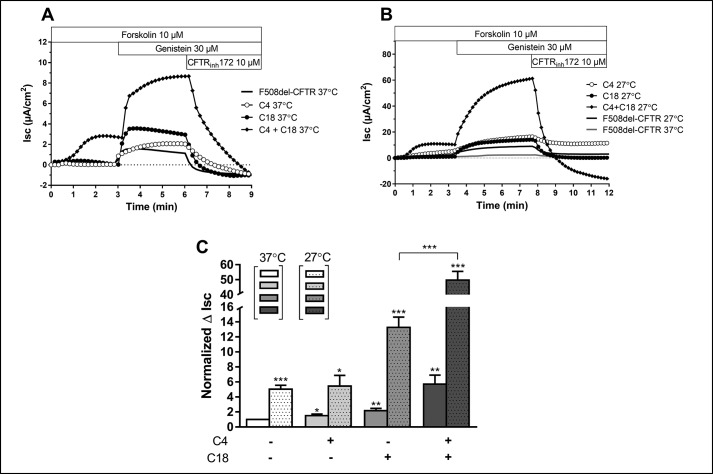
The ultimate answer to the question of how these correctors function resides within the answer of how well they restore CFTR chloride channel function, assayed here by measuring the short-circuit current in polarized monolayers of CFBE41o− cells stably expressing ΔF508-CFTR. Consistent with the increase in maturation to C band and increase in surface expression and stability induced the combination of correctors is restoration of function. Fig. 3 shows that when cells are grown at 37 °C, that C4 and C18 applied individually result in an approximate 2-fold increase in Isc. The combination of C4 + 18 results in ∼5-fold increase in Isc when compared with the basal current. Growing cells at reduced temperature has a large effect on the correction induced by C18, which increases to 3-fold when compared with the basal current. The most profound change at reduced temperature is noted when C4 + 18 are combined. The combination is highly synergistic when cells are grown at reduced temperature, increasing the Isc ∼10-fold when compared with the basal current.
FIGURE 3.
Effect of low temperature incubation on corrected CFTR-mediated Cl− secretion in CFBE F508del-CFTR cells after treatment with C4, C18, or the combination C4 + C18. Original short-circuit current recordings (A) in untreated cells or after incubation of C4, C18, and C4 + C18 (10 μm, 16 h) in cells kept at 37 °C, or at 27 °C (B) as indicated. C, corresponding ΔIsc response. Data are expressed as the CFTRinh172 sensitive short-circuit current (ΔIsc) calculated by subtracting the Isc after CFTRinh172 treatment from the peak forskolin/genistein-stimulated Isc. Dots are associated with a 27 °C incubation. Statistical significance is presented as follows: ns, no significant difference; *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 5–9 for each condition) compared with control condition (n = 8 for 37 °C and 5 for 27 °C). Amiloride (100 μm) was kept during the duration of the whole experiment to avoid interference of ENaC-mediated Na+ currents.
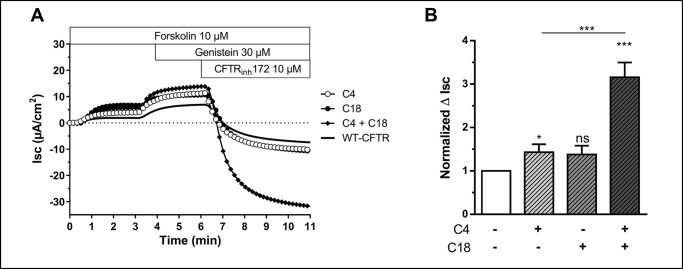
For comparison, Fig. 4 shows the Isc for CFBE41o− cells stably transfected with WT-CFTR. Note that the combination of correctors also rescue WT-CFTR. By comparing control currents generated from WT-CFTR to those of ΔF508 rescued by the combination C4 + 18 one can readily see that the combination of correctors can rescue ΔF508 to approximately WT function.
FIGURE 4.
Effect of small molecule corrector treatment C4, C18, and the combination C4 + C18 on CFTR-mediated Cl− secretion in CFBE cells stably expressing WT-CFTR. A, original short-circuit current recording in untreated cells or after incubation of C4, C18, C4 + C18 (10 μm, 16 h, 37 °C) as indicated. B, corresponding ΔIsc response. Data are expressed as the CFTRinh172 sensitive short-circuit current (ΔIsc) calculated by subtracting the Isc after CFTRinh172 treatment from the peak forskolin/genistein-stimulated Isc. Statistical significance is presented as follows: ns, no significant difference; *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 7 for each condition) compared with control condition (n = 7). Amiloride (100 μm) was kept during the duration of the whole experiment to avoid interference of ENaC-mediated Na+ currents. (WT cells are a gift from Dieter Gruenert (7).)
The Combination of C18 and C4 Alters the Binding of CFTR to Proteins Involved in Quality Control
The protein translation, folding, and trafficking of CFTR is complex, involving several chaperones (25). It is well known that when mutant CFTR fails to fold properly, chaperones assist the cell in targeting the mutant molecules for degradation (25). Less known is how the correctors alter the interaction of the mutant CFTR with proteins involved in its quality control. To address this open question, we conducted co-immunoprecipitation studies with two chaperones: Hsp27 and -40. Hsp27 (HspB1), a member of the small heat shock protein family, is thought to guard against protein aggregation during stress (26). Hsp27 is well known to be involved in the degradation of ΔF508-CFTR (27) by ligating it with the small ubiquitin-like modifier SUMO-2 (28). Hsp40 (DnaJ) sequesters misfolded CFTR for ERAD (29) and it also acts as a co-chaperone for Hsp70.
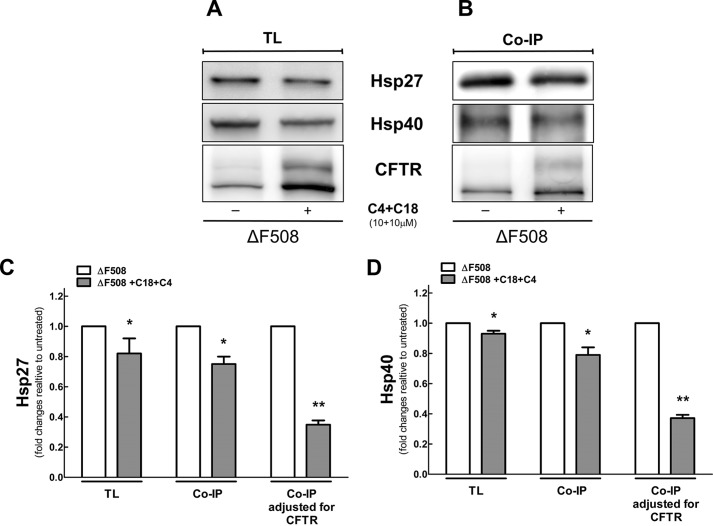
Fig. 5 shows that combined corrector treatment with C4 + C18 reduced the steady state level of Hsp27 and had a dramatic effect to reduce its binding to ΔF508. Combined therapy had a smaller effect on the steady state levels of Hsp40 but also reduced its binding to CFTR.
FIGURE 5.
A, total lysate (TL) level of ERAD proteins and CFTR in the ΔF508. HEK-293 cell lines stably expressing ΔF508 were treated with C18 and C4 for 16 h. Protein samples were used for immunoblotting and incubated with different primary antibodies as presented in B. The combination of C18 and C4 affects the binding of Hsp27 and -40 to ΔF508. HEK-293 cells were stably transfected with ΔF508 and treated with C18 + C4 (10 μm) for 16 h. CFTR was immunoprecipitated (IP) with M3A7 (anti-CFTR antibody) and incubated with A/G beads for 4 h at 4 °C. Immunoblotting was performed, and samples were incubated with different primary antibodies as shown in C and D. Quantification of data for Hsp27 and -40, respectively. Versus untreated: *, p < 0.05; **, p < 0.01 (n = 4). Please note that there is an ∼20% reduction on the pull-down of both chaperones after treatment. However, when the increase in CFTR is taken into account there is a large reduction in the binding of both chaperones to CFTR.
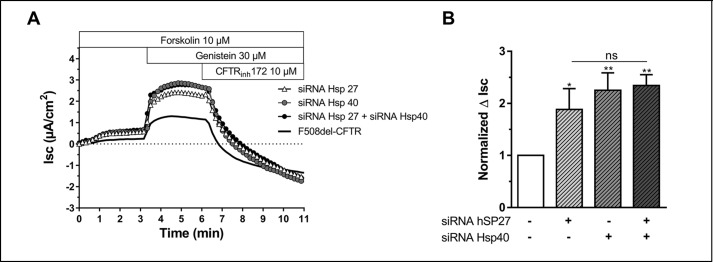
Because the largest effects were noted with Hsp27 and -40, we took these experiments one step further by using siRNA to reduce the expression of Hsp27 and -40 either individually or in combination. We found that reducing the amount of either one present rescued the C band of the ΔF508, but the effect was no greater when both chaperones were knocked down together (Fig. 6). Short-circuit current experiments corroborated these results (Fig. 7). Silencing either chaperone increased the short-circuit current by ∼2-fold but again no greater effect was noted when they were knocked down together. These results indicate that both of these chaperones are important for the rescue of the ΔF508 mutant. The lack of additivity or synergy may indicate that they target the same pathway to rescue the mutants.
FIGURE 6.
A, effect of Hsp40 and Hsp27 knockdown on the ΔF508-CFTR mutant. Hsp40 siRNA (10 nm) and Hsp27 siRNA (10 nm) were transfected into cell lines stably expressing ΔF508-CFTR for 72 h. B, data summarizing the densitometry measurements of immunoblots from total protein lysate samples. Versus untreated: **, p < 0.01 (n = 4). Ezrin (Ez) was used as a loading control.
FIGURE 7.
Effect of double knockdown Hsp27 and Hsp40 on CFTR-mediated Cl− secretion in CFBE F508del-CFTR cells. A, original short-circuit current recording in untreated cells or after incubation with siRNA Hsp27, siRNA Hsp40, or the combination siRNA Hsp27 + siRNA Hsp 40 (72 h, 37 °C). B, corresponding ΔIsc response. Data were expressed as the CFTRinh172 sensitive short-circuit current (ΔIsc) calculated by subtracting the Isc after CFTRinh172 treatment from the peak forskolin/genistein-stimulated Isc. Statistical significance was presented as follows: ns, no significant difference; *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 4–8 for each condition) compared with control condition (n = 4). Amiloride (100 μm) was kept during the duration of the whole experiment to avoid interference of ENaC-mediated Na+ currents.
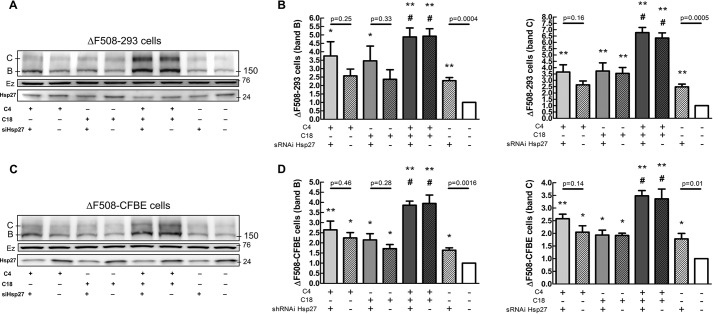
To take this investigation even further, we examined the interaction between correction via Hsp27 knockdown and the effects of correctors C4 and C18 in CFBE cells stably expressing CFTR on both ΔF508 protein (Fig. 8) and currents (Fig. 9). Both show that combined C4 + C18 corrector treatment for ΔF508 was much more effective than treatment with either of these correctors alone. Importantly, knocking down Hsp27 did not enhance the effect of the combined C4 + C18 corrector treatment. Thus, the corrector combination therapy and Hsp27 knockdown was neither additive nor synergistic. Taken together, our findings support the idea that combined treatment with C18 and C4 stabilizes the ΔF508 CFTR protein, reducing its binding to ERAD proteins and allowing CFTR to be addressed to the plasma membrane.
FIGURE 8.
A, effect of Hsp27 knockdown and correctors on the ΔF508-CFTR mutant. HEK-293 cells stably expressing ΔF508 were transfected with siRNA Hsp27 (10 nm) for 72 h. Subsequently, cells were treated with C4 (10 μm) and C18 (10 μm) for 16 h. B, data summarizing the densitometry measurements of immunoblots. Data are normalized to untreated (white bars). Versus untreated: *, p < 0.05, **, p < 0.01. Versus siRNA Hsp27: #, p < 0.05 (n = 4). C, CFBE cells stably expressing ΔF508 were transfected with siRNA Hsp27 (10 nm) for 72 h. Subsequently, cells were treated with C4 (10 μm) and C18 (10 μm) for 16 h. D, data summarizing the densitometry measurements of immunoblots. Data are normalized to untreated (white bars). Versus untreated: *, p < 0.05; **, p < 0.01. Versus siRNA Hsp27: #, p < 0.05 (n = 4). Ezrin (Ez) was used as a loading control.
FIGURE 9.
Summary of Ussing chamber experiments performed to evaluate rescue of CFTR-mediated Cl− secretion across the apical plasma membrane in CFBE F508del-CFTR cells after small molecule corrector treatments or after Hsp27 knockdown. A, original short-circuit current recording in untreated cells or after incubation with siRNA Hsp 27 (72 h) in untreated cells or after treatment with C4, C18, and C4 + C18 (10 μm, 16 h). All cells were kept at 37 °C. B, corresponding ΔIsc response. Data are expressed as the CFTRinh172 sensitive short-circuit current (ΔIsc) calculated by subtracting the Isc after CFTRinh172 treatment from the peak forskolin/genistein-stimulated Isc. Statistical significance was presented as follows: ns, no significant difference; *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 8–13 for each condition) compared with control condition (n = 12). Amiloride (100 μm) was kept during the duration of the whole experiment to avoid interference of ENaC-mediated Na+ currents.
Discussion
Many advances have occurred to improve the quality of life of CF patients and minimize their symptoms (4); however, these measures are still unable to restore normal function. The goal for CF researchers is to identify ways to correct the folding of mutated CFTR and deliver the functional protein to the cell surface, thereby further improving the lives of CF patients. To date, several pharmacological compounds have been investigated by high-throughput screening that may have the capacity to rectify the trafficking of misfolded proteins (16, 18, 19, 23). VX-809, which elicits a striking restoration of CFTR expression and function in vitro (19), is being evaluated for efficacy and safety in clinical trials (20). However, despite this in vitro progress, only limited clinical benefit was noted when VX-809 was used by itself in a Phase IIa clinical study in ΔF508-homozygous patients (20). Thus, a combination of correctors could be the key to achieving therapeutic levels of CFTR expression.
To address this problem, we focused on combining correctors as a strategy for rescue of ΔF508 (5). As shown previously (30), ΔF508 expressed less protein than did WT-CFTR. Our data show clearly that when correctors from different classes (I and II, i.e. C4 and C18) were applied together, we saw that both protein trafficking and function of ΔF508 were rescued. Given that these two classes of compounds have different ways of rescuing mutant CFTR, it is clear from our data that to obtain a large enough rescue of the mutant protein, either a new corrector or a combination of correctors may have to be applied to correct the NBD1-transmembrane domain interactions and the stability of the NBD1 domain for the therapy to reach a therapeutic level. The data are very promising in that a combination of correctors has the potential of increasing ΔF508 function to that generated by WT-CFTR. If the correct therapeutic combination is found the data suggests that patients bearing the ΔF508 mutation have the chance to be restored to a healthy life.
Because the combined treatment with C18 and C4 was able to rescue the ΔF508 mutation in CFTR, we asked whether this combination of correctors would restore trafficking to the plasma membrane. We indeed demonstrate that the combined therapy not only rescued protein to the plasma membrane but also increased the residence time there. It is well known that growing cells at a reduced temperature rescues protein at the cell surface. However, the temperature-rescued ΔF508-CFTR at the cell surface is not stable, and it is rapidly removed from the plasma membrane. The observation that we were able to rescue both the trafficking and residence time in the plasma membrane with the combined therapy indicates that this therapy stabilizes CFTR.
Previous studies as well as the experiments reported here indicate that ΔF508 creates an unstable protein. When a mutant protein fails to acquire its native conformation, the ER arrests its biosynthesis and sends it for degradation via the proteasome. Severely misfolded proteins that cannot be degraded by the proteasome are sent instead to the aggresome for degradation by autophagy (8, 12–14, 31). It is well known that several proteins, the ensemble referred to as a proteostatic network, play a role in ERAD (32). According to Roth and Balch (33), this network includes chaperones/co-chaperones, the GroEL/TCP1-ring complex/chaperonin family of folding machines, tetratricopeptide repeat domain containing proteins, proteins that modulate oxidative folding, and degradation components comprising both the cytosolic ubiquitin-proteasome and membrane-linked autophagy-lysosome systems. In our work, we probed a subset of this network by assessing the binding of the chaperones Hsp27 and -40. We asked how combined corrector therapy would affect the binding of these proteins to mutant CFTR. Our results showed that combined treatment with C4 + C18 resulted in a profound reduction in the binding of these chaperones to ΔF508.
These results are consistent with the theory that a proteostatic network directs the processing of CFTR and that a corrector therapy must affect this network. The additional question raised is whether the combined therapy with C4 + 18 achieved the processing of mutant CFTR to the cell surface as noted here. There are two possibilities: one is that the combined therapy stabilized the mutant CFTR directly, and this stabilization affected the network. Alternately, the combined therapy affected the network, thereby releasing the NBD1 mutants from biosynthetic arrest in the ER. To test these two possibilities, we knocked down two of the heat shock proteins, Hsp27 and -40, either individually or in combination; these were the chaperones whose binding was most affected by the C4 + C18 treatment. We found that knocking down Hsp27 or -40 individually or in combination rescued the NBD1 mutant, but the effect of combined knockdown was not additive or synergistic. Knocking down Hsp27 or -40 individually or in combination was as effective in rescuing ΔF508-CFTR as was the combination of the corrector compounds C4 + 18. Importantly, the knockdown was neither additive nor synergistic with C4 + C18. Although our data do not establish this point conclusively, they suggest that combined corrector therapy operates by acting on the central instability of the NBD1 mutants, by reduced binding of chaperones/co-chaperones, such as Hsp27 and -40. What is clear is that the proteostatic network is affected dramatically by the combined therapy, which stabilizes the NBD1 mutant, ΔF508, and allows it to be processed to the cell surface and restores its function. Given the emerging data that a combined treatment will be necessary for therapeutic rescue of ΔF508-CFTR, and given the intense effort in this arena, in the future many new compounds that rescue ΔF508-CFTR are expected to be developed. In light of our results it is clear that a combination of correctors similar to C4 + 18 would be particularly effective.
In conclusion, the findings of this study show that combination therapy can rescue the ΔF508-CFTR mutant to a greater extent than single correctors. In addition, the combination of correctors (C4 + C18) decreased the binding to two proteins involved in ERAD (Hsp27 and -40) and allowed the stabilized CFTR to reach the plasma membrane.
Author Contributions
M. L. P., C. B., I.S., and L. C. were involved in conducting the experiments; L. C. made substantial contributions to conception and design, data analysis, and interpretation of data; W. B. G. made substantial contributions, data analysis, and interpretation of data; M. M. was involved in review of the data. M. L. P., W. B. G., and L. C. wrote the manuscript. All authors approved the manuscript.
Acknowledgment
We acknowledge Debbie McClellan for editing the manuscript.
This work was supported by the United States CF Foundation and the National Council for Scientific and Technological Development (CNPq/Program Science without Borders), Brazil (to M. L. -P.). The authors declare that they have no conflicts of interest with the contents of this article.
- CF
- cystic fibrosis
- CFTR
- CF transmembrane conductance regulator
- NBD
- nucleotide binding domain
- ER
- endoplasmic reticulum
- CFBE
- cystic fibrosis bronchial epithelial
- ENaC
- epithelial Na+ channel
- ERAD
- ER-associated degradation.
References
- 1. Riordan J. R., Rommens J. M., Kerem B. S., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L., Drumm M. L., Iannuzzi M. C., Collins F. S., Tsui L. C. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073; Correction (1989) Science245, 1347 [DOI] [PubMed] [Google Scholar]
- 2. Quinton P. M. (1990) Cystic fibrosis: a disease in electrolyte transport. FASEB J. 4, 2709–2717 [DOI] [PubMed] [Google Scholar]
- 3. Mickle J. E., Cutting G. R. (1998) Clinical implications of cystic fibrosis transmembrane conductance regulator mutations. Clin. Chest Med. 19, 443–458 [DOI] [PubMed] [Google Scholar]
- 4. Cohen-Cymberknoh M., Shoseyov D., Kerem E. (2011) Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am. J. Respir. Crit Care Med. 183, 1463–1471 [DOI] [PubMed] [Google Scholar]
- 5. Lewis H. A., Buchanan S. G., Burley S. K., Conners K., Dickey M., Dorwart M., Fowler R., Gao X., Guggino W. B., Hendrickson W. A., Hunt J. F., Kearins M. C., Lorimer D., Maloney P. C., Post K. W., Rajashankar K. R., Rutter M. E., Sauder J. M., Shriver S., Thibodeau P. H., Thomas P. J., Zhang M., Zhao X., Emtage S. (2004) Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 23, 282–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riordan J. R. (2008) CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 [DOI] [PubMed] [Google Scholar]
- 7. Illek B., Lei D., Fischer H., Gruenert D. C. (2010) Sensitivity of chloride efflux vs. transepithelial measurements in mixed CF and normal airway epithelial cell populations. Cell Physiol. Biochem. 26, 983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen T. J., Loo M. A., Pind S., Williams D. B., Goldberg A. L., Riordan J. R. (1995) Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83, 129–135 [DOI] [PubMed] [Google Scholar]
- 9. Lewis H. A., Wang C., Zhao X., Hamuro Y., Conners K., Kearins M. C., Lu F., Sauder J. M., Molnar K. S., Coales S. J., Maloney P. C., Guggino W. B., Wetmore D. R., Weber P. C., Hunt J. F. (2010) Structure and dynamics of NBD1 from CFTR characterized using crystallography and hydrogen/deuterium exchange mass spectrometry. J. Mol. Biol. 396, 406–430 [DOI] [PubMed] [Google Scholar]
- 10. Lewis H. A., Zhao X., Wang C., Sauder J. M., Rooney I., Noland B. W., Lorimer D., Kearins M. C., Conners K., Condon B., Maloney P. C., Guggino W. B., Hunt J. F., Emtage S. (2005) Impact of the ΔF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J. Biol. Chem. 280, 1346–1353 [DOI] [PubMed] [Google Scholar]
- 11. Mendoza J. L., Schmidt A., Li Q., Nuvaga E., Barrett T., Bridges R. J., Feranchak A. P., Brautigam C. A., Thomas P. J. (2012) Requirements for efficient correction of ΔF508 CFTR revealed by analyses of evolved sequences. Cell 148, 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cebotaru L., Vij N., Ciobanu I., Wright J., Flotte T., Guggino W. B. (2008) Cystic fibrosis transmembrane regulator missing the first four transmembrane segments increases wild type and ΔF508 processing. J. Biol. Chem. 283, 21926–21933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cebotaru L., Woodward O., Cebotaru V., Guggino W. B. (2013) Transcomplementation by a truncation mutant of CFTR enhances ΔF508 processing through a biomolecualr interaction. J. Biol. Chem. 288, 10505–10512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cebotaru L., Rapino D., Cebotaru V., Guggino W. B. (2014) Correcting the cystic fibrosis disease mutant, A455E CFTR. PLoS One 9, e85183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedemonte N., Lukacs G. L., Du K., Caci E., Zegarra-Moran O., Galietta L. J., Verkman A. S. (2005) Small-molecule correctors of defective ΔF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Invest. 115, 2564–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Accurso F. J., Rowe S. M., Clancy J. P., Boyle M. P., Dunitz J. M., Durie P. R., Sagel S. D., Hornick D. B., Konstan M. W., Donaldson S. H., Moss R. B., Pilewski J. M., Rubenstein R. C., Uluer A. Z., Aitken M. L., Freedman S. D., Rose L. M., Mayer-Hamblett N., Dong Q., Zha J., Stone A. J., Olson E. R., Ordoñez C. L., Campbell P. W., Ashlock M. A., Ramsey B. W. (2010) Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N. Engl. J. Med. 363, 1991–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amaral M. D., Farinha C. M. (2013) Rescuing mutant CFTR: a multi-task approach to a better outcome in treating cystic fibrosis. Curr. Pharm. Des 19, 3497–3508 [DOI] [PubMed] [Google Scholar]
- 18. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Cao D., Neuberger T., Turnbull A., Singh A., Joubran J., Hazlewood A., Zhou J., McCartney J., Arumugam V., Decker C., Yang J., Young C., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. (2009) Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. U.S.A. 106, 18825–18830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Goor F., Hadida S., Grootenhuis P. D., Burton B., Stack J. H., Straley K. S., Decker C. J., Miller M., McCartney J., Olson E. R., Wine J. J., Frizzell R. A., Ashlock M., Negulescu P. A. (2011) Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. U.S.A. 108, 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clancy J. P., Rowe S. M., Accurso F. J., Aitken M. L., Amin R. S., Ashlock M. A., Ballmann M., Boyle M. P., Bronsveld I., Campbell P. W., De Boeck K., Donaldson S. H., Dorkin H. L., Dunitz J. M., Durie P. R., Jain M., Leonard A., McCoy K. S., Moss R. B., Pilewski J. M., Rosenbluth D. B., Rubenstein R. C., Schechter M. S., Botfield M., Ordoñez C. L., Spencer-Green G. T., Vernillet L., Wisseh S., Yen K., Konstan M. W. (2012) Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67, 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoelen H., Kleizen B., Schmidt A., Richardson J., Charitou P., Thomas P. J., Braakman I. (2010) The primary folding defect and rescue of ΔF508 CFTR emerge during translation of the mutant domain. PLoS One 5, e15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Goor F., Straley K. S., Cao D., González J., Hadida S., Hazlewood A., Joubran J., Knapp T., Makings L. R., Miller M., Neuberger T., Olson E., Panchenko V., Rader J., Singh A., Stack J. H., Tung R., Grootenhuis P. D., Negulescu P. (2006) Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L1117-L1130 [DOI] [PubMed] [Google Scholar]
- 23. Okiyoneda T., Veit G., Dekkers J. F., Bagdany M., Soya N., Xu H., Roldan A., Verkman A. S., Kurth M., Simon A., Hegedus T., Beekman J. M., Lukacs G. L. (2013) Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat. Chem. Biol. 9, 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lukacs G. L., Chang X. B., Bear C., Kartner N., Mohamed A., Riordan J. R., Grinstein S. (1993) The ΔF508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane: determination of functional half-lives on transfected cells. J. Biol. Chem. 268, 21592–21598 [PubMed] [Google Scholar]
- 25. Amaral M. D. (2004) CFTR and chaperones: processing and degradation. J. Mol. Neurosci. 23, 41–48 [DOI] [PubMed] [Google Scholar]
- 26. Kim Y. E., Hipp M. S., Bracher A., Hayer-Hartl M., Hartl F. U. (2013) Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355 [DOI] [PubMed] [Google Scholar]
- 27. Ahner A., Nakatsukasa K., Zhang H., Frizzell R. A., Brodsky J. L. (2007) Small heat-shock proteins select ΔF508-CFTR for endoplasmic reticulum-associated degradation. Mol. Biol. Cell 18, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahner A., Gong X., Schmidt B. Z., Peters K. W., Rabeh W. M., Thibodeau P. H., Lukacs G. L., Frizzell R. A. (2013) Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol. Biol. Cell 24, 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farinha C. M., Nogueira P., Mendes F., Penque D., Amaral M. D. (2002) The human DnaJ homologue (Hdj)-1/heat-shock protein (Hsp) 40 co-chaperone is required for the in vivo stabilization of the cystic fibrosis transmembrane conductance regulator by Hsp70. Biochem. J. 366, 797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rich D. P., Gregory R. J., Cheng S. H., Smith A. E., Welsh M. J. (1993) Effect of deletion mutations on the function of CFTR chloride channels. Receptors Channels 1, 221–232 [PubMed] [Google Scholar]
- 31. Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most of cystic fibrosis. Cell 63, 827–834 [DOI] [PubMed] [Google Scholar]
- 32. Balch W. E., Roth D. M., Hutt D. M. (2011) Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harbor Perspect. Biol. 3, pii:1004499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roth D. M., Balch W. E. (2011) Modeling general proteostasis: proteome balance in health and disease. Curr. Opin. Cell Biol. 23, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]