Abstract
Background
Cancer cells survival depends on glucose metabolism and ATP. Inhibiting glucose metabolism is a possible anticancer treatment. The phosphorylation of 2-deoxy-D-glucose (2-DG), which is a glycogen analogue, seriously affects the normal glycometabolism phosphorylation process, leading to ATP consumption. Studies showed that 2-DG could regulate RIP and c-FLIP. This paper aimed to investigate the effect of 2-DG on RIP and c-FLIP expression in HepG2 and Hep3B cells, further illustrating the effect and mechanism of 2-DG regulating RIP and c-FLIP expression on liver cancer cell apoptosis induced by TRAIL.
Material/Methods
RIP and c-FLIP gene silencing HepG2 and Hep3B cell models were established by siRNA and detected by Western blot. Cell viability was determined by MTT and apoptosis rate was measured by flow cytometry. JC-1 fluorescent probe was used to test mitochondrial membrane potential.
Results
2-DG or TRAIL alone significantly reduced HepG2 and Hep3B cell survival rate and promoted apoptosis. Compared with the single TRAIL treatment group, the combination of 2-DG and TRAIL could reduce cell survival rate, increase apoptosis rate, and decease mitochondrial membrane potential, which is dependent on Caspases. 2-DG can inhibit RIP and c-FLIP expression, leading to increased TRAIL-induced HepG2 and Hep3B cells apoptosis.
Conclusions
2-DG can down-regulate RIP and c-FLIP expression, and change Caspases activities to increase the liver cancer cell apoptosis induced by TRAIL.
MeSH Keywords: CASP8 and FADD-Like Apoptosis Regulating Protein; CCAAT-Binding Factor; Cellulose 1,4-beta-Cellobiosidase; Neuroectodermal Tumors, Primitive, Peripheral
Background
Liver cancer is one of the most common malignant tumors, with high morbidity and mortality [1,2]. It was confirmed that cancer cells survival depends on glucose metabolism, and inhibiting glycometabolism process might be an anti-cancer strategy [3,4]. 2-deoxy-D-glucose (2-DG) is a type of glycogen analogue phosphorylated by hexokinase. Its accumulation in the cells disrupts normal phosphorylation process of glucose metabolism. 2-DG can inhibit cell growth in a variety of cancers and increase chemotherapy drugs’ curative effect [5]. 2-DG can also restrain protein glycosylation, leading to increase endoplasmic reticulum tension and activate protein synthesis reaction [6]. In breast cancer, gastric cancer, and ovarian cancer, 2-DG is an important material to regulate receptor interacting protein (RIP) and cellular caspase 8 (FLICE)-like inhibitory protein (cFLIP) [7,8].
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor family. It became a new resource for anti-cancer drugs because of its function in selective inducing cancer cells apoptosis [9,10]. TRAIL is highly expressed in several organs, such as spleen, lymph nodes, small intestine, prostate, and placenta. It also can be found in immune cells, such as natural killer cells, monocytes, B cells, and dendritic cells [11–13]. TRAIL can selectively kill heterogeneous tumors without damaging normal tissues [14]. TRAIL induces apoptosis mainly through binding with TRAIL receptors -1 and -2. After activating Caspase-8, it could activate the Caspases system directly or indirectly through the mitochondrial apoptosis pathway [15,16]. It was reported that TRAIL receptor expression level and a variety of regulatory mechanism in cells are closely related to cellular sensitivity to apoptosis induced by TRAIL. Materials that can induce apoptosis mainly include c-FLIP and pro-apoptotic and anti-apoptotic Bcl-2 family members and IAP family members (including XIAP, cIAP-1, and cIAP-2) [17]. XIAP can bind with Caspase-3/9 to inhibit their activity, while cIAP-1 and cIAP-2 could protect cells induced by TRAIL through RIP ubiquitin. Death receptor complex recruited to Caspase-8 when cIAP-1 and cIAP-2 were inhibited by Smac/DIABLO, leading to Caspase-8 activation and apoptosis [18]. Therefore, RIP is not only the effector of tumor necrosis factor signaling pathway, but also an essential factor in TNF-induced NF-κB activation. RIP can inhibit apoptosis and promote cell proliferation through activating NF-κB, eventually promoting cancer formation [19, 20]. This study tried to investigate the effect of 2-DG on RIP and c-FLIP expression in liver cancer cells to illustrate the mechanism of 2-DG regulating RIP and c-FLIP effect on TRAIL-induced liver cancer cell apoptosis. The study can not only help further understanding the mechanism of reversing TRAIL resistance, but also provide a basis for future clinical application of TRAIL.
Material and Methods
Main reagents
HepG2 cell line was bought from Shanghai Chuanxiang Biological Technology Co., LTD. Hep3B cell line was from Shanghai Bioleaf Biological Co., LTD. The 2-DG was from Sigma. Soluble recombinant TRAIL was from PeporTehc. The PI apoptosis detection kit was from the Keygen. The Caspase-8 detection kit was from Wuhan Boster Company. Caspase-3, RIP, and c-FLIP antibodies were bought from Abcam. β-actin and Caspase-8 antibodies were from Santa Cruz.
Cell culture
HepG2 and Hep3B cells were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum, 100 kU/L penicillin, and 100 mg/L streptomycin in a humid atmosphere containing 5% CO2 at 37°C.
c-FLIP and RIP siRNA
siRNA sequence was designed based on c-FLIP and RIP genetic sequence in the GeneBank and synthesized by QIAGEN. The sequences used were as follows. c-FLIP siRNA: forward, 5′-UCGGGGACUUGGCUGAACUUU-3′; reverse, 5′-AGUU CAGCCAAGUCCCCGACA-3′. RIP siRNA: forward, 5′-CCACUAG UCUGACGGAUAAUU-3′; reverse, 5′-UUAUCCGUCAGACUA UGGUAA-3′. We constructed recombinant plasmid pRNAT-U6/Neo/siRNA-c-FLIP and pRNAT-U6/Neo/siRNA-RIP to get better transfection effect. 7×105 HepG2 or Hep3B cells were seeded in a 6-well plate. Empty vector, pRNAT-U6/Neo, pRNAT-U6/Neo/siRNA-c-FLIP, and pRNAT-U6/Neo/siRNA-RIP were transfected to the HepG2 and Hep3B cells through Lipofectamine 2000. After 24 h, Western blot was used to detect c-FLIP and RIP protein expression to determine the transfection efficacy.
MTT assay
2-DG and TRAIL cytotoxic effects on liver cancer cell line HepG2 and Hep3B were determined 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT). 1×104 HepG2 and Hep3B cells were seeded in 96-well plate for 24, 48, or 96 h. 20 μl 5 mg/mL MTT solution was added to each well and incubated for 4 h. We added 150 μl DMSO to each cell for 30 min at room temperature, and the plate was detected at 570 nm to get the absorbance value (optical density, OD).
Mitochondrial membrane potential detection
Mitochondrial membrane potential was detected by JC-1. 2×105 HepG2 and Hep3B cells were seeded in a 6-cell plate. After treated with 10 mM 2-DG or 200 ng/ml TRAIL for 24 h, the cells were added with 1 ml JC-1 dyeing liquid at 37°C for 20 min. Then the cells were washed twice by buffer and incubated in medium to be observed under a laser confocal microscope.
Cell apoptosis detection
Cell apoptosis was measured by propidium iodine (PI). 4×104 HepG2 and Hep3B cells were seeded in a 96-cell plate. After being treated with 10 mM 2-DG or 200 ng/ml TRAIL for 24 h, the cells were collected and added with 5 μl V-FITC and 5 μl PI for 15 min in the dark. Then the cells were detected by flow cytometry.
Western blot
After transfection, the cells were digested with lysis buffer. Total protein was separated by denaturing 10% SDS-polyacrylamide gel electrophoresis. After being incubated with c-FLIP, RIP, DR4, DR5, FADD, Caspase-3/8/9, ERK1/2, and DFF45 primary antibodies, the membrane was detected with chemiluminescence and calculated with Image J software. Protein levels were normalized to β-actin and changes were determined.
RT-PCR
The cDNA was synthesized with 1 μg RNA from the samples by TRIzol. The primers were synthesized by Shanghai Boshang Biotechnology Co., LTD. XIAP forward primer: 5′-CGCCC TAGGCACCAGGGTGTG-3′, reverse primer: 5′-TCGGTGAGCAGCACA GGGTG-3′; c-IAP forward primer: 5′-GCCCAACCGCGCTGAGTACA -3′, reverse primer: 5′-TGCCTTCTATGGCCTCCACGA -3′; β-actin forward primer: 5′-GACATGCCGCCTGGAGAAAC-3′, reverse primer: 5′-AGCCCAGGATGCCCTTTAGT-3′. The cycling conditions consisted of an initial, single cycle of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 15 s at 60°C, and 30 s at 72°C.
Statistical analysis
All statistical analyses were performed using SPSS17.0 software (SPSS Inc., USA). Differences between multiple groups were analyzed by one-way ANOVA and Duncan’s multiple range test. Numerical data were presented as means and standard deviation (±SD). P<0.05 was considered as a significant difference.
Results
2-DG enhanced HepG2 and Hep3B cells apoptosis induced by TRAIL
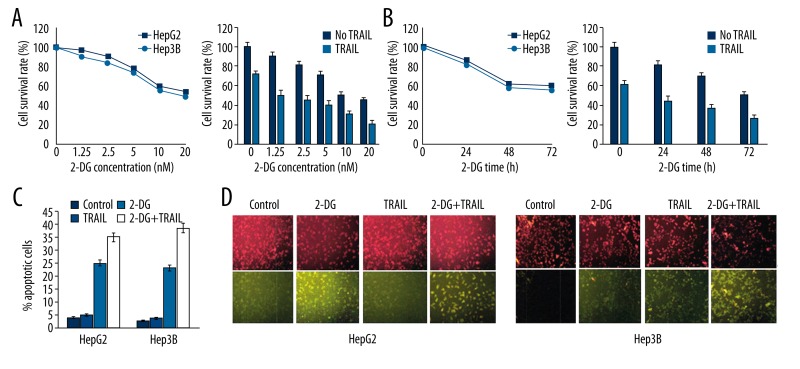
HepG2 and Hep3B cell apoptosis rates were tested after being treated with 2-DG or TRAIL. It was found that single 2-DG or TRAIL reduced cell survival rate, and 2-DG group exhibited dose- and time-dependent effects (Figure 1A). Compared with the single TRAIL treatment group, cell survival rate decreased after treatment by 2-DG and TRAIL together (Figure 1B). Flow cytometry results showed that single 2-DG or TRAIL obviously promoted apoptosis (Table 1). Compared with the single treatment group, two liver cancer cells presented higher apoptosis rate after treatment by 2-DG and TRAIL together (Figure 1C). In addition, we used fluorescent probe JC-1 to detect the change of mitochondrial membrane potential. JC-1 showed green when the mitochondrial membrane was damaged, while it showed red in normal cells. Results revealed that liver cancer cells had obvious combined mitochondrial membrane damage after treatment by 2-DG and TRAIL (Figure 1D).
Figure 1.
2-DG enhanced HepG2 and Hep3B cells apoptosis induced by TRAIL.
Table 1.
2-DG enhanced HepG2 and Hep3B cells apoptosis induced by TRAIL (%).
| Control | 2-DG | TRAIL | 2-DG+ TRAIL | |
|---|---|---|---|---|
| HepG2 | 4.75±0.29 | 5.16±0.22 | 24.57±2.26* | 36.51±6.83*,# |
| Hep3B | 3.83±0.51 | 4.81±0.36 | 23.48±3.17* | 38.24±5.92*,# |
P<0.05, compared with control;
P<0.05, compared with 2-DG or TRAIL group.
2-DG enhanced TRAIL induced HepG2 and Hep3B cell apoptosis dependent on Caspases
To investigate Caspase’s role in 2-DG enhanced cell apoptosis induced by TRAIL, we used Caspases inhibitor z-VAD-FMK. Table 2 shows that the HepG2 and Hep3B cell apoptosis rate decreased after treatment with z-VAD-FMK compared with 2-DG or TRAIL treatment, indicating that 2-DG enhanced TRAIL-induced HepG2 and Hep3B cell apoptosis dependent on Caspases.
Table 2.
2-DG enhanced TRAIL induced HepG2 and Hep3B cell apoptosis dependent on Caspases (%).
| 2-DG | 2-DG+z-VAD | TRAIL | TRAIL+z-VAD | 2-DG+TRAIL | 2-DG+TRAIL+ z-VAD | |
|---|---|---|---|---|---|---|
| HepG2 | 5.16±0.22 | 3.11±0.17 | 24.57±2.26 | 16.13±3.19* | 36.51±6.83* | 22.11±5.62# |
| Hep3B | 4.81±0.36 | 3.65±0.21 | 23.48±3.17 | 15.78±2.68* | 38.24±5.9* | 23.43±4.76# |
P<0.05, compared with 2-DG or TRAIL group;
P<0.05, compared with 2-DG+TRAIL group.
2-DG impact on RIP and c-FLIP expression in HepG2 and Hep3B cells
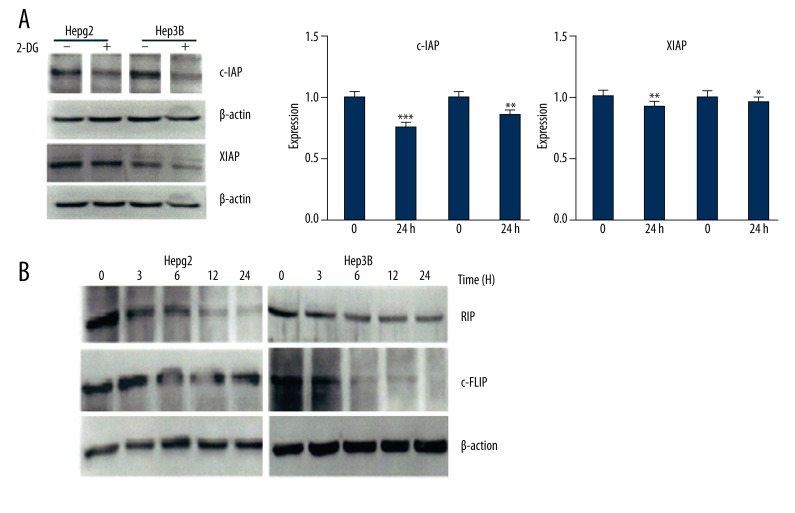
RT-PCR and Western blot showed that XIAP and c-IAP mRNA and protein expression in HepG2 and Hep3B cells significantly decreased after treatment with 2-DG for 24 h (Figure 2A), and RIP and c-FLIP protein also declined (Figure 2B), suggesting that 2-DG inhibits XIAP, c-IAP, RIP, and c-FLIP expression in HepG2 and Hep3B cells.
Figure 2.
2-DG impact on RIP and c-FLIP expression in HepG2 and Hep3B cells.
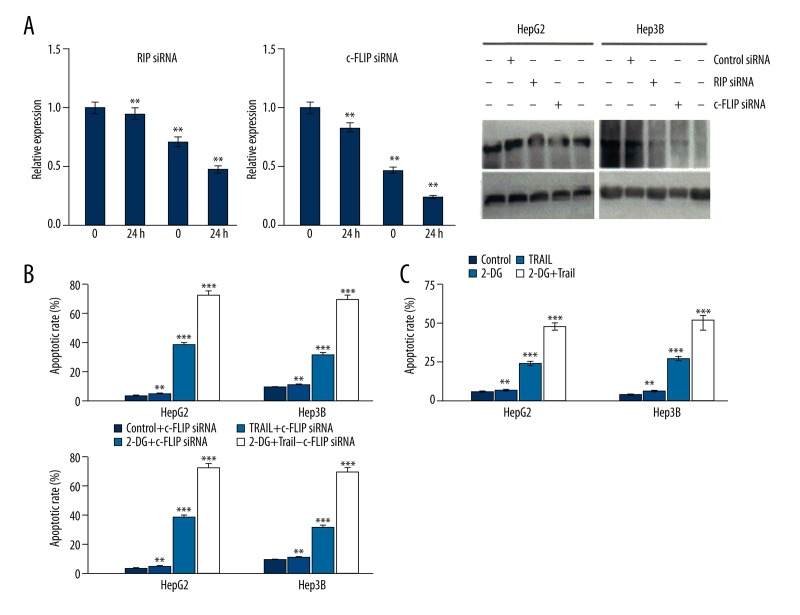
Down-regulating RIP and c-FLIP can enhance HepG2 and Hep3B cell apoptosis induced by TRAIL
We further investigate the role of RIP and c-FLIP in cell apoptosis induced by TRAIL. As shown in Figure 3A, down-regulating RIP and c-FLIP expression significantly enhanced HepG2 and Hep3B cell apoptosis induced by TRAIL or 2-DG, while combined treatment showed higher apoptosis rate than in the TRAIL treatment group (Figure 3B). The TRAIL-induced HepG2 and Hep3B cells apoptosis rate increased obviously after 2-DG reduced RIP and c-FLIP expression (Figure 3C).
Figure 3.
Down-regulating RIP and c-FLIP can enhance HepG2 and Hep3B cell apoptosis induced by TRAIL.
Discussion
Tumor cell survival depends on glycolysis metabolism. They need to absorb glucose and glycolysis at high speed to survive. Thus, glycolysis inhibitors become a potential anti-cancer drug [2]. 2-DG is a synthesized glucose analogue, which is not only an endoplasmic reticulum stress inhibitor, but also is a hexokinase inhibitor. It can suppress cancer cell uptake glycogen to inhibit cancer cell growth and metabolism and has been considered as a potential anticancer agent [3]. Studies have confirmed that there is a certain correlation between 2-DG and TRAIL. 2-DG can up-regulate GRP78 and TRAIL-R2 expression in melanoma cells. It can promote cell apoptosis induced by TRAIL, but its mechanism is still unclear [4]. We used liver cancer cells to investigate the effect of 2-DG on RIP and c-FLIP expression, and its role in cell apoptosis induced by TRAIL.
Apoptosis is also called programmed cell death, and it can maintain normal cell development and homeostasis. It can eliminate the cells unable to repair, mutation, aging, or infected by pathogens. Cytokines, virus, and antitumor drugs can induce cell apoptosis [21,22]. TRAIL is an important ligand of the tumor necrosis factor (TNF) family that can induce cancer cells apoptosis with no effect on normal cells [23,24]. TRAIL inducing cell apoptosis through the death receptor pathway is an important antitumor mechanism. After binding with TRAIL receptor, TRAIL may cause FADD domain combined with pro-Caspase-8 to form DISC [25]. Pro-Caspase-8 became the activated form in DISC that can activate Caspase-9. It further activated Caspase-3 and DNA fragment factor 45 (DFF45) to promote apoptosis occurrence. In this process, some molecules, such as c-FLIP, RIP, and TRADD, were recruited to DISC to participate in apoptosis regulation [26,27]. This study confirmed that 2-DG and TRAIL reduced cell mitochondrial membrane potential and induced cell apoptosis, and that 2-DG depended on Caspases to enhance cell apoptosis induced by TRAIL.
c-FLIP is a kind of apoptosis-inhibiting protein similar to Caspase-8 [28]. Research confirmed that c-FLIP can bind with FADD to suppress Caspase-8 activation and restrain cell apoptosis [29,30]. Therefore, overexpressed c-FLIP can inhibit tumor cell apoptosis. Stanger et al. found that overexpressed RIP can activate NF-κB signaling pathway by yeast two-hybrid analysis [31]. Recent studies suggested that cancer cell c-FLIP and RIP expression levels are closely related to Caspase-8 activation, and overexpressed c-FLIP or RIP may suppress Caspase-8 activation [32]. Several studies have confirmed that RIP and c-FLIP activity were closely related to caspase-8 [33]. ER stress can regulate multiple caspases, including caspase-8 [34]. As an inhibitor of ER stress, 2-DG may affect RIP and c-FLIP expression through caspase-8. Further research is needed on the impact of 2-DG on caspase-8 activity and to explore its effect on RIP and c-FLIP. Our results showed that the c-FLIP and RIP are up-regulated in liver cancer cell HepG2 and Hep3B, and that 2-DG treatment can significantly decrease c-FLIP and RIP protein expression. In the HepG2 and Hep3B cells with RIP, c-FLIP gene silencing or down-regulation by 2-DG, the TRAIL-induced cell apoptosis rate increased significantly.
Conclusions
2-DG can down-regulate RIP and c-FLIP expression, thus increasing the liver cancer cell apoptosis induced by TRAIL. Therefore, 2-DG might be an important molecular target for inducing liver cancer cell apoptosis and liver cancer gene therapy.
Footnotes
Source of support: Departmental sources
References
- 1.Iwazawa J, Ohue S, Hashimoto N, Mitani T. Local tumor progression following lipiodol-based targeted chemoembolization of hepatocellular carcinoma: a retrospective comparison of miriplatin and epirubicin. Cancer Manag Res. 2012;4:113–19. doi: 10.2147/CMAR.S30431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee IJ, Seong J. The optimal selection of radiotherapy treatment for hepatocellular carcinoma. Gut Liver. 2012;6:139–48. doi: 10.5009/gnl.2012.6.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 4.Porporato PE, Dhup S, Dadhich RK, et al. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaushik N, Lee SJ, Choi TG, et al. Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells. Sci Rep. 2015;5:8726. doi: 10.1038/srep08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Li J, Wang F, et al. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355:176–83. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Xi H, Kurtoglu M, Lampidis TJ. The wonders of 2-deoxy-D-glucose. IUBMB Life. 2014;66:110–21. doi: 10.1002/iub.1251. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Jiang CC, Lavis CJ, et al. 2-Deoxy-D-glucose enhances TRAIL-induced apoptosis in human melanoma cells through XBP-1-mediated up-regulation of TRAIL-R2. Mol Cancer. 2009;8:122. doi: 10.1186/1476-4598-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braun FK, Mathur R, Sehgal L, et al. Inhibition of methyltransferases accelerates degradation of cFLIP and sensitizes B-cell lymphoma cells to TRAIL-induced apoptosis. PLoS One. 2015;10:e0117994. doi: 10.1371/journal.pone.0117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu D, Xia SL, Shao XX, et al. Association of ulcerative colitis with TNF-related apoptosis inducing ligand (TRAIL) gene polymorphisms and plasma soluble TRAIL levels in Chinese Han population. Eur Rev Med Pharmacol Sci. 2015;19:467–76. [PubMed] [Google Scholar]
- 11.Park MR, Kim SG, Cho IA, et al. Licochalcone-A induces intrinsic and extrinsic apoptosis via ERK1/2 and p38 phosphorylation-mediated TRAIL expression in head and neck squamous carcinoma FaDu cells. Food Chem Toxicol. 2015;77:34–43. doi: 10.1016/j.fct.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Damasio DC, Nolte S, Polak LP, et al. The lectin BJcuL induces apoptosis through TRAIL expression, caspase cascade activation and mitochondrial membrane permeability in a human colon adenocarcinoma cell line. Toxicon. 2014;90:299–307. doi: 10.1016/j.toxicon.2014.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Balzarolo M, Watzl C, Medema JP, Wolkers MC. NAB2 and EGR-1 exert opposite roles in regulating TRAIL expression in human Natural Killer cells. Immunol Lett. 2013;151:61–67. doi: 10.1016/j.imlet.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Raulf N, El-Attar R, Kulms D, et al. Differential response of head and neck cancer cell lines to TRAIL or Smac mimetics is associated with the cellular levels and activity of caspase-8 and caspase-10. Br J Cancer. 2014;111:1955–64. doi: 10.1038/bjc.2014.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laussmann MA, Passante E, Hellwig CT, et al. Proteasome inhibition can impair caspase-8 activation upon submaximal stimulation of apoptotic tumor necrosis factor-related apoptosis inducing ligand (TRAIL) signaling. J Biol Chem. 2012;287:14402–11. doi: 10.1074/jbc.M111.304378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Hu X, Qu X, et al. Cetuximab enhances TRAIL-induced gastric cancer cell apoptosis by promoting DISC formation in lipid rafts. Biochem Biophys Res Commun. 2013;439:285–90. doi: 10.1016/j.bbrc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Gordy C, Liang J, Pua H, He YW. c-FLIP protects eosinophils from TNF-alpha-mediated cell death in vivo. PLoS One. 2014;9:e107724. doi: 10.1371/journal.pone.0107724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang F, Wei X, Leng X, et al. C-FLIP(L) contributes to TRAIL resistance in HER2-positive breast cancer. Biochem Biophys Res Commun. 2014;450:267–73. doi: 10.1016/j.bbrc.2014.05.106. [DOI] [PubMed] [Google Scholar]
- 19.Tomasella A, Blangy A, Brancolini C. A receptor-interacting protein 1 (RIP1)-independent necrotic death under the control of protein phosphatase PP2A that involves the reorganization of actin cytoskeleton and the action of cofilin-1. J Biol Chem. 2014;289:25699–710. doi: 10.1074/jbc.M114.575134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YF, He W, Zhang C, et al. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 2014;225:445–53. doi: 10.1016/j.toxlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Wu ZZ, Li M, Sheng W. [Study on the molecular mechanism of lingual epithelial cell apotosis and its related genes in different tongue furs]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:986–88. [in Chinese] [PubMed] [Google Scholar]
- 22.Cheung TH, Chung TK, Lo KW, et al. Apotosis-related proteins in cervical intraepithelial neoplasia and squamous cell carcinoma of the cervix. Gynecol Oncol. 2002;86:14–18. doi: 10.1006/gyno.2002.6655. [DOI] [PubMed] [Google Scholar]
- 23.Kelly MM, Hoel BD, Voelkel-Johnson C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biol Ther. 2002;1:520–27. doi: 10.4161/cbt.1.5.169. [DOI] [PubMed] [Google Scholar]
- 24.Guiho R, Biteau K, Heymann D, Redini F. TRAIL-based therapy in pediatric bone tumors: how to overcome resistance. Future Oncol. 2015;11:535–42. doi: 10.2217/fon.14.293. [DOI] [PubMed] [Google Scholar]
- 25.Loreto C, Almeida LE, Migliore MR, et al. TRAIL, DR5 and caspase 3-dependent apoptosis in vessels of diseased human temporomandibular joint disc. An immunohistochemical study. Eur J Histochem. 2010;54:e40. doi: 10.4081/ejh.2010.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonardi R, Almeida LE, Trevilatto PC, Loreto C. Occurrence and regional distribution of TRAIL and DR5 on temporomandibular joint discs: comparison of disc derangement with and without reduction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:244–51. doi: 10.1016/j.tripleo.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Jayarama S, Ganesh L, et al. Akt-phosphorylated mitogen-activated kinase-activating death domain protein (MADD) inhibits TRAIL-induced apoptosis by blocking Fas-associated death domain (FADD) association with death receptor 4. J Biol Chem. 2010;285:22713–22. doi: 10.1074/jbc.M110.105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Sun SY. c-FLIP links mTORC2 to apoptosis. Oncoscience. 2014;1:306–7. doi: 10.18632/oncoscience.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang EY, Jeong MS, Park SY, Jang SB. Evidence of complex formation between FADD and c-FLIP death effector domains for the death inducing signaling complex. BMB Rep. 2014;47:488–93. doi: 10.5483/BMBRep.2014.47.9.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safa AR. Roles of c-FLIP in apoptosis, necroptosis, and autophagy. J Carcinog Mutagen. 2013;(Suppl 6) doi: 10.4172/2157-2518.S6-003. pi: 003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanger BZ, Leder P, Lee TH, et al. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–23. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 32.Yin X, Krikorian P, Logan T, Csizmadia V. Induction of RIP-2 kinase by proinflammatory cytokines is mediated via NF-kappaB signaling pathways and involves a novel feed-forward regulatory mechanism. Mol Cell Biochem. 2010;333:251–59. doi: 10.1007/s11010-009-0226-y. [DOI] [PubMed] [Google Scholar]
- 33.Weinlich R, Oberst A, Dillon CP, et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 2013;5:340–48. doi: 10.1016/j.celrep.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimbo A, Fujita E, Kouroku Y, et al. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp Cell Res. 2003;283:156–66. doi: 10.1016/s0014-4827(02)00033-2. [DOI] [PubMed] [Google Scholar]