Background: Both MUC1 and galectin-3 are overexpressed in various malignant tumors.
Results: Binding of galectin-3 to MUC1 enhances the phosphorylation of ERK1/2 and Akt, promoting tumor cell malignancy.
Conclusion: MUC1-mediated signal transduction occurs through direct binding of galectin-3 to MUC1.
Significance: Our findings will provide a new insight into the promotion of MUC1-mediated tumor cell malignancy.
Keywords: cancer; cell motility; cell proliferation; mucin 1, cell surface-associated (MUC1); signal transduction; galectin-3
Abstract
Both mucin 1 (MUC1) and galectin-3 are known to be overexpressed in various malignant tumors and associated with a poor prognosis. It has been extensively reported that MUC1 is involved in potentiation of growth factor-dependent signal transduction. Because some carbohydrate moieties carried on MUC1 change to preferable ones for binding of galectin-3 in cancer cells, we speculated that MUC1-mediated signaling may occur through direct binding of galectin-3. Immunochemical studies showed that the distribution of galectin-3 coincided with that of MUC1 in various human tumor tissues but not in human nonmalignant tissues, and the level of galectin-3 retained on the surface of various cancer cells paralleled that of MUC1. Treatment of MUC1-expressing cells with galectin-3 induced phosphorylation of ERK1/2 and Akt following enhanced phosphorylation of MUC1 C-terminal domain, consistently promoting tumor cell malignancy. It is also noted that this enhanced phosphorylation occurred independently of EGF receptor-mediated signaling in both EGF receptor- and MUC1-expressing cells, and multivalency of galectin-3 was important for initiation of MUC1-mediated signaling. Expectedly, both silencing of endogenous galectin-3 and treatment with galectin-3 antagonists down-regulated cell proliferation of MUC1-expressing cells. These results suggest that the binding of galectin-3 to MUC1 plays a key role in MUC1-mediated signaling. Thus, constitutive activation of MUC1-mediated signaling in an autocrine/paracrine manner caused by ligation of galectin-3 promotes uncontrolled tumor cell malignancy. This signaling may be another MUC1-mediated pathway and function in parallel with a growth factor-dependent MUC1-mediated signaling pathway.
Introduction
Mucin 1 (MUC1),2 one of the transmembrane mucins, is a heavily O-glycosylated protein that protrudes from the apical membrane of cells (1, 2). MUC1 is synthesized as a single polypeptide and autocleaved in the endoplasmic reticulum into two subunits (N- and C-terminal subunits, respectively) that form a stable heterodimeric complex held together through a noncovalent interaction on the cell surface (3, 4). The N-terminal domain (MUC1-ND), which is a major part of the MUC1 extracellular domain, contains variable numbers of 20 amino acid tandem repeats including five serine/threonine residues that are possible O-glycosylation sites (5, 6). The C-terminal domain (MUC1-CD) consists of a short extracellular domain, a transmembrane domain, and a cytoplasmic tail, and the MUC1-CD is involved in not only anchoring of the MUC1-ND to the membrane but also various signaling events. In malignant tumors, MUC1 is aberrantly overexpressed and distributed on the entire cell surface because of loss of polarity, and its expression level is correlated with a poor prognosis (1, 2). It has been reported that MUC1 is associated with several growth factor receptors and potentiates growth factor-dependent signaling through the MUC1-CD (2, 7); however, in those studies, MUC1-CD but not MUC1-ND has been focused on. The MUC1-ND has an extended structure because it carries a large number of sialylated O-glycans. In addition, O-glycans carried on the MUC1-ND in cancer cells are altered, so an increase of short oligosaccharides such as Thomsen-Friedenreich antigen (known as TF or T antigen; Galβ1–3GalNAcα-Ser/Thr) has been found (8, 9). These oligosaccharides carried on the MUC1-ND are expected to act as a scaffold for the binding of galectins.
Galectins, one of the animal lectin groups, are defined by their ability to bind to β-galactoside-containing oligosaccharides through an evolutionarily conserved carbohydrate recognition domain (10). In the galectin family, galectin-3 is a chimera type galectin and comprises one carbohydrate recognition domain and non-lectin domain, which consists of an N-terminal domain and a collagen-like linker region rich in glycine, tyrosine, and proline residues (11). Galectin-3 is mainly distributed in the cytoplasm, nuclei, and mitochondria (12, 13) and is involved in multiple cellular functions such as anti-apoptosis, cell proliferation, and pre-mRNA splicing (14–16). Despite lacking a signal peptide, galectin-3 is also secreted outside the cell via a nonclassical pathway and thus is found on the cell surface and/or in the extracellular space (17, 18). It is known that the expression level and distribution of galectin-3 are changed in various human tumors (10, 19). In addition, the level of galectin-3 is also correlated with tumor progression, and the concentration of circulating galectin-3 in the serum of cancer patients has been reported to be higher than that in healthy individuals (20, 21). The secreted extracellular galectin-3 binds to a wide array of glycoproteins and glycolipids on the cell surface and in the extracellular matrix, which play biological roles such as in angiogenesis and cell adhesion (10, 22, 23). Furthermore, because galectin-3 can act as a bivalent or multivalent ligand (24, 25), it can cross-link cell surface glycoconjugates, which, like many other receptor-ligand systems, can trigger a cascade of transmembrane signaling events (26). It was recently reported that exogenous galectin-3 activates a calcium-sensitive MAPK signaling pathway (27).
In regard to the relationship between MUC1 and galectins, it has been reported that MUC1 is a natural receptor for endogenous galectins such as galectin-3. The interaction of galectin-3 with MUC1 induces polarization of MUC1 on the cell surface and resultant exposure of cell adhesion molecules, leading to homotypic aggregation of cancer cells or adhesion of cancer cells to endothelial cells (28–30). However, these studies have provided no insights into the downstream signaling by MUC1 after the binding of galectin-3. Studies on signaling through the direct binding of endogenous ligands to MUC1 have been limited except in the case of some ligands, such as ICAM-1 (intercellular adhesion molecule 1) and Siglec-9 (sialic acid-binding Ig-like lectin 9) (31–33).
In this study, we demonstrated that galectin-3 was similarly distributed with MUC1 in various human tumor tissues and on the surface of cancer cells and that extracellular galectin-3 retained on the cell surface approximately paralleled the level of MUC1, suggesting that galectin-3 actually binds to MUC1 on the cell surface. The binding of galectin-3 to MUC1 activated the MAPK and PI3K/Akt signaling pathways, leading to enhancement of cell proliferation and motility. Consistently, proliferation and motility of galectin-3 shRNA-treated cells were down-modulated. This galectin-3-triggered signaling was markedly reduced by using monovalent galectin-3 produced by treatment with collagenase, suggesting that multivalency of galectin-3 is important for initiation of galectin-3-triggered MUC1-mediated signaling. In addition, an EGFR inhibitor had no effect on these signaling pathways. These results suggest that galectin-3 may play a significant role as a potential endogenous ligand for activation of MUC1-mediated signaling in an autocrine/paracrine manner and that this signaling may be another MUC1-mediated pathway and function in parallel with a growth factor-dependent MUC1-mediated signaling pathway.
Experimental Procedures
Antibodies and Reagents
Mouse anti-MUC1-ND, Armenian hamster anti-MUC1-CD, rabbit anti-galectin-1 (for Western blotting), and rabbit anti-galectin-1 (for immunocytochemistry) antibodies were purchased from BD Biosciences (San Jose, CA), Lab Vision (Fremont, CA), Abcam (Cambridge, UK), and GeneTex (Irvine, CA), respectively. Goat anti-galectin-3, rabbit anti-EGFR, and biotin-conjugated mouse anti-phosphotyrosine antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-phospho-ERK1/2, rabbit anti-ERK1/2, rabbit anti-phospho-Akt, and rabbit anti-Akt antibodies were from Cell Signaling Technology (Beverly, MA). Mouse anti-β-actin antibodies and collagenase type VII were from Sigma-Aldrich. Recombinant human galectin-3 and EGFR/ErbB-2/ErbB-4 inhibitor were from Pepro Tech (Rocky Hill, NJ) and Merck Millipore, respectively.
Cell Culture and Cell Transfection
HCT116 (a human colon cancer cell line), A549 (a human lung cancer cell line), and SKOV3 (a human ovary cancer cell line) cells were obtained from the American Type Culture Collection (Manassas, VA). Human MUC1 gene transfectants (HCT116/MUC1 and A549/MUC1) and the respective control cells (HCT116/Mock and A549/Mock) were prepared as described previously (34). Human MUC1 gene knockdown cells (SKOV3/Si-1 and -2) and MUC1-expressing cells (SKOV3/Scr) were generated as described previously (35). Human galectin-3 gene knockdown HCT116/MUC1 cells (HCT116/MUC1-Gal-3/Si) and control cells (HCT116/MUC1-Scr) were generated by introducing human galectin-3 shRNA and scrambled shRNA vectors (InvivoGen, San Diego, CA), respectively, into HCT116/MUC1 cells. HCT116/Mock, HCT116/MUC1, HCT116/MUC1-Scr, HCT116/MUC1-Gal-3/Si, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells were cultured in DMEM containing 10% heat-inactivated FBS (HI-FBS), 4 mm l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. A549/Mock and A549/MUC1 cells were maintained in F-12K medium (American Type Culture Collection) containing 10% HI-FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Preparation of Total RNA and DNA Microarray Analysis
Preparation of total RNA and DNA microarray analysis were performed as described previously (35).
Preparation of Cell Lysates
Cells were sonicated in cell lysis buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, and a protease inhibitor mixture; Nacalai Tesque, Kyoto, Japan) and then centrifuged. The supernatant was used as a whole cell lysate.
Cell Surface Biotinylation and Preparation of Cell Surface Proteins
Subconfluent cells were washed with PBS, and then the cell surface was labeled with biotin using EZ-Link Sulfo-NHS-Biotin (Thermo Scientific, Rockford, IL) at 4 °C according to the manufacturer's protocol. After quenching with 100 mm glycine in PBS, the cells were solubilized as described above. Biotin-labeled cell surface proteins were collected with streptavidin-SepharoseTM high performance (GE Healthcare).
Immunoprecipitation and Western Blotting
Lysates prepared from HCT116/MUC1 cells as described above were incubated with anti-galectin-3 antibodies or control IgG, and subsequently immune complexes were collected with PureProteomeTM protein G magnetic beads (Merck Millipore).
The immunoprecipitates or cell lysates were subjected to SDS-PAGE, followed by Western blotting and incubation with primary antibodies (anti-MUC1-ND, anti-MUC1-CD, anti-galectin-1, anti-galectin-3, anti-β-actin, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-Akt, anti-Akt, anti-EGFR, or biotin-conjugated anti-phosphotyrosine antibodies). After incubation with HRP-conjugated secondary antibodies, the bands were visualized using chemiluminescence, and in some cases the intensity of bands was determined with ImageJ software (National Institutes of Health, Bethesda, MD). Biotin-conjugated anti-phosphotyrosine antibodies were detected by incubation of the membranes with HRP-conjugated streptavidin.
Immunochemical and Hematoxylin and Eosin Staining
Immunochemical and hematoxylin and eosin staining was performed as described previously (35). Briefly, sections of paraffin-embedded tumor and nonmalignant tissues were deparaffinized. After antigen retrieval and blocking, MUC1-ND and galectin-3 were detected by using anti-MUC1-ND and anti-galectin-3 antibodies and each fluorescence-conjugated secondary antibody. Specimens of tumor and adjacent nonmalignant tissues were obtained from cancer patients in accordance with a protocol approved by Osaka City University.
Immunocytochemistry
To determine the distributions of MUC1, galectin-3, and galectin-1 on the cell surface, after blocking with 1% BSA in PBS, cells were incubated with anti-MUC1-ND, anti-galectin-3, and anti-galectin-1 antibodies at 4 °C for 2 h. After washing with cold PBS, the cells were stained with fluorescence-conjugated secondary antibodies at 4 °C for 1 h. After washing with cold PBS, the cells were fixed with 4% paraformaldehyde in PBS and stained with DAPI. For intracellular immunostaining, cells were fixed with 4% paraformaldehyde in PBS at room temperature and then permeabilized with PBS containing 5% BSA and 0.1% Triton X-100 at room temperature for 30 min. Thereafter, the cells were incubated with primary antibodies and subsequently with fluorescence-conjugated secondary antibodies and DAPI at room temperature. Images were obtained by confocal fluorescence microscopy (Leica, Mannheim, Germany).
Preparation of Monovalent Galectin-3
Recombinant galectin-3 was digested with collagenase type VII in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 10 mm CaCl2 at 37 °C for 2 h basically according to Herrmann et al. (11). Thereafter, collagenase was removed from the reaction solution including the fragments of carbohydrate recognition domain and N-terminal domain using Amicon® ultra centrifugal filters with a molecular mass cutoff of 50 kDa (Merck Millipore). The solution that passed through the filters was concentrated and replaced with PBS using Amicon® ultra centrifugal filters with a molecular mass of 10 kDa (Merck Millipore) and then used as cleaved monovalent galectin-3.
Treatment of Cells with Galectin-3
Prior to experiments, the following procedures were performed to exclude galectin-3 on the cell surface. Cells were cultured in medium containing 10% HI-FBS and 30 mm lactose. After washing with serum-free medium containing 30 mm lactose, the cells were incubated in the same medium for 3 h. After washing with serum-free medium, the cells were harvested with cell dissociation buffer, enzyme-free, and Hanks' balanced salt solution (Invitrogen), and then suspended in serum-free medium. In some cases, an EGFR inhibitor or DMSO was added to the cell suspension, followed by incubation at 37 °C for 1 h.
Thereafter, the cell suspension was divided equally and subsequently incubated with recombinant galectin-3 or PBS at 37 °C for 10 min. The cells were dissolved in SDS-PAGE sample buffer and then subjected to SDS-PAGE, followed by Western blotting as described above.
Phosphorylation of MUC1-CD
After exclusion of galectin-3 from the cell surface as described above, cells were incubated with recombinant galectin-3 or PBS at 37 °C for 4 min. After centrifugation and discarding of the supernatants, the cell pellets were solubilized with cell lysis buffer containing a phosphatase inhibitor mixture (Nacalai Tesque). Thereafter, MUC1 was immunoprecipitated from the lysates with anti-MUC1-ND antibodies, and then the immunoprecipitates were subjected to SDS-PAGE, followed by Western blotting as described above.
MTT Assays
Cells were seeded into 96-well plates in triplicate and cultured until 72 h. The experiment was repeated using at least two independent cell preparations. After adding the MTT solution (Nacalai Tesque), the plates were incubated for 2 h. After removal of the medium, the resulting formazan crystals were dissolved in DMSO. The absorbance of each well was measured using a plate reader at 570 nm with a reference wavelength of 630 nm.
Anchorage-dependent Clonogenic Assays
These assays were performed basically according to Mahanta et al. (36). Cells were seeded into 60-mm dishes and cultured for 2 weeks. After washing with PBS, the cells were fixed with 4% paraformaldehyde in PBS at room temperature for 20 min. After washing with distilled water, the cells were stained with 0.5% crystal violet in 70% methanol at room temperature for 20 min, followed by washing with distilled water and drying. The dye in the cells was extracted with a 10% acetic acid solution for 20 min, and then the absorbance at 590 nm was determined.
Wound Healing Assays
Cells were seeded into a culture insert (Ibidi, Martinsried, Germany) and then incubated for 24 h. After removal of the culture insert, which created a cell-free gap between the cell patches, images were taken (0 h), and the cells were cultured in medium containing 10% HI-FBS and 10 mm hydroxyurea for 20 h (control and gene manipulated HCT116 cells) or 7 h (control and gene manipulated SKOV3 cells). In some experiments, recombinant galectin-3 was added to the medium described above, and then the cells were cultured. Images were taken at individual times (7 and 20 h), and then the level of cell motility was assessed by measuring the cell-free area with Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Synthesis of Glycopolymers
Artificial glycopolymers were synthesized as described previously (37).
Binding of Galectin-3 to Glycopolymers
Artificial glycopolymers were coated on Universal-BINDTM 96-well plates (Corning Inc., Corning, NY) in triplicate at room temperature for 1 h, followed by immobilization using UV illumination. After washing with PBS and subsequent blocking with 2.5% BSA in PBS at room temperature for 2 h, serially diluted recombinant galectin-3 (0–2.5 μg/ml) was added to each well, followed by incubation at room temperature for 1 h. After washing with 0.05% Tween 20 in PBS, anti-galectin-3 antibodies were added to the wells. After incubation at room temperature for 2 h, bound anti-galectin-3 antibodies were detected with HRP-conjugated secondary antibodies and a 1-StepTM Ultra TMB-ELISA Substrate (Thermo Scientific), and finally the absorbance at 450 nm was measured.
Effects of Glycopolymers on Cell Proliferation
Cells were seeded into 96-well plates and cultured in medium containing 0.5% HI-FBS and artificial glycopolymers. After incubation for 72 h, MTT assays were performed as described above.
Statistical Analysis
Unless otherwise stated, statistically analyzed experiments were performed using three independent cell preparations. Differences between two groups were analyzed statistically using the two-tailed Student's t test. Differences among three or more groups were analyzed by analysis of variance, followed by the Tukey-Kramer or Dunnett's test.
Results
MUC1 Is Similarly Distributed with Galectin-3 on the Surface of Cancer Cells and in Human Tumor Tissues
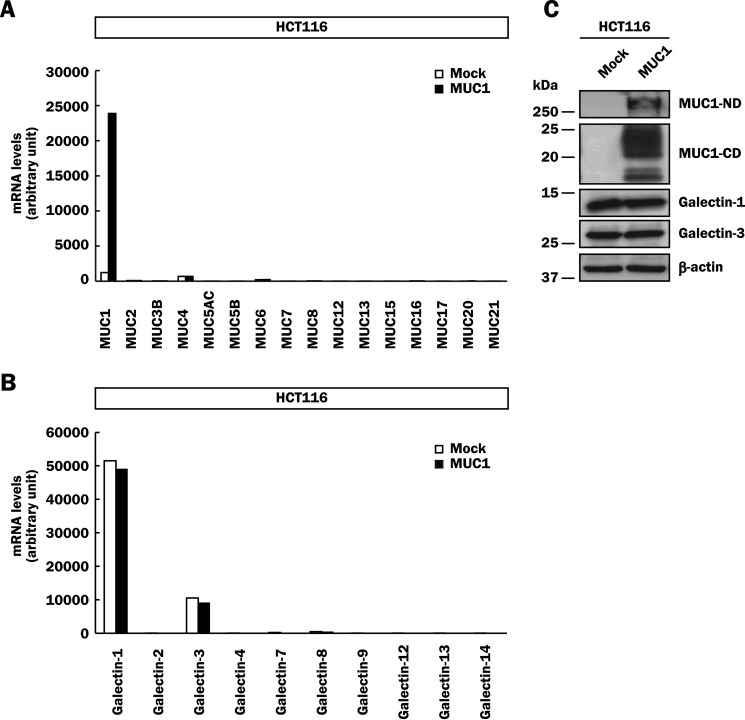
To date, many mucin and galectin family members have been identified in mammals (2, 10). Aberrant overexpression of MUC1 is observed in various malignant tumor cells, and MUC1 is potentially a natural receptor for endogenous galectin-3. To investigate the function of MUC1, we mainly used a human colon cancer cell line, HCT116 cells. Because DNA microarray analysis demonstrated that HCT116 cells expressed negligible levels of membrane-bound and secreted mucins including MUC1 (Fig. 1A), we generated MUC1-expressing cells by introducing human MUC1cDNA into HCT116 cells (HCT116/MUC1) and confirmed the expression of MUC1 by DNA microarray analysis. Of the galectin family, galectin-1 and -3 were mainly expressed in HCT116 cells, and the substantial levels of their mRNAs were not affected by MUC1cDNA introduction (Fig. 1B). To confirm the expression levels of these proteins, lysates of HCT116/MUC1 and its control cells (HCT116/Mock) were subjected to SDS-PAGE, followed by Western blotting. In agreement with the results of DNA microarray analysis, MUC1 was detected as bands of MUC1-ND and -CD with molecular masses of ∼250 and 15–25 kDa, respectively, in HCT116/MUC1 cells but not in HCT116/Mock cells (Fig. 1C). The multiple bands of MUC1-CD are considered to be due to different levels of N-glycosylation, as described previously (38). It has been reported that MUC1 suppresses the expression of microRNA against galectin-3 gene in some cancer cells, resulting in up-regulation of galectin-3 expression (38). However, there was no significant difference in the protein level of galectin-3 between HCT116/Mock and HCT116/MUC1 cells (Fig. 1C). Kharbanda et al. (39) reported similar results, i.e. that the expression of galectin-3 has no relationship with that of MUC1 in a human lung cancer cell line, H1975 cells.
FIGURE 1.
Expression of the mucin and galectin family in HCT116 cells. A and B, mRNA levels of the mucin (A) and galectin (B) family in HCT116/Mock and HCT116/MUC1 cells were determined by DNA microarray analysis. C, lysates of HCT116/Mock and HCT116/MUC1 cells were subjected to SDS-PAGE, followed by Western blotting and detection with anti-MUC1-ND, anti-MUC1-CD, anti-galectin-1, anti-galectin-3, and anti-β-actin antibodies. β-Actin was used as a loading control.
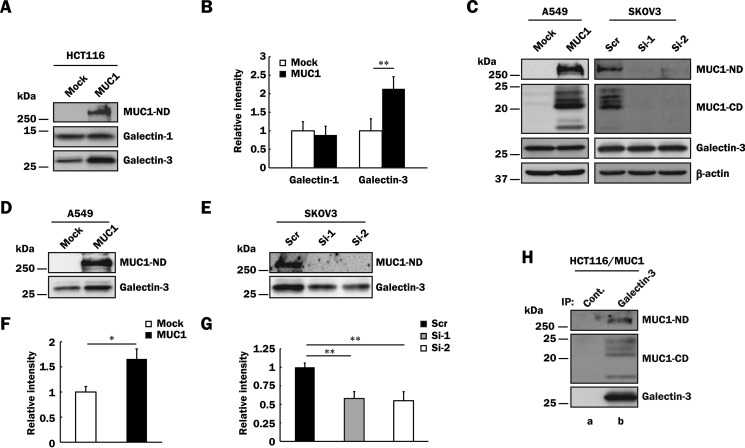
It is well known that galectins are present both intracellularly and extracellularly, and extracellular galectins bind to cell surface glycoconjugates bearing suitable β-galactoside-containing oligosaccharides (10). To determine the levels of MUC1 and galectins on the surface of HCT116/Mock and HCT116/MUC1 cells, the surface of both cell types was labeled with biotin, and then biotin-labeled cell surface proteins were precipitated from the lysates with streptavidin-Sepharose. The precipitates were subjected to SDS-PAGE, followed by Western blotting and detection with anti-MUC1-ND, anti-galectin-1, and anti-galectin-3 antibodies. As shown in Fig. 2 (A and B), a higher level of biotin-labeled galectin-3 was detected in HCT116/MUC1 cells as compared with in HCT116/Mock cells, whereas that of biotin-labeled galectin-1 on the surface of HCT116/MUC1 cells was similar to that of HCT116/Mock cells. These results suggest that galectin-3 but not galectin-1 is retained on the cell surface through its binding to MUC1. In this context, it has been reported that although the galectin family exhibits high affinity for β-galactoside-containing carbohydrate moieties such as TF antigen, its affinity for each β-galactoside-containing carbohydrate moiety differs among galectins (40, 41). In the case of TF antigen, galectin-3 exhibits a higher affinity than galectin-1 does (41, 42). Therefore, although both galectin-1 and -3 may be secreted extracellularly, as shown in Fig. 2 (A and B), galectin-3 is likely to preferentially bind to TF antigen carried on the MUC1 extracellular domain. To examine expression of TF antigen carried on MUC1, peanut agglutinin, a TF antigen-binding lectin-conjugated agarose, was added to the lysates of HCT116/MUC1 cells, and then the peanut agglutinin-binding glycoconjugates pulled down from the lysates were subjected to SDS-PAGE, followed by Western blotting. Expectedly, MUC1 was detected in the peanut agglutinin-binding glycoconjugates (data not shown), indicating that MUC1 core protein carries preferable carbohydrate moieties for the binding of galectin-3.
FIGURE 2.
Binding of galectin-3 to MUC1 in various MUC1-expressing cells. A, HCT116/Mock and HCT116/MUC1 cells were treated with biotin as described under “Experimental Procedures,” and the cell surface proteins were precipitated from the lysates with streptavidin-Sepharose. The precipitates were subjected to SDS-PAGE, followed by Western blotting and detection with anti-MUC1-ND, anti-galectin-1, and anti-galectin-3 antibodies. B, the levels of galectin-1 and -3 on the cell surface were determined by measuring the intensities of their bands in Fig. 2A with ImageJ. The respective intensities of galectin-1 and -3 in control cells were taken as 1 (means ± S.D., n = 3). **, p < 0.01. C, lysates of A549/Mock, A549/MUC1, SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells were subjected to SDS-PAGE, followed by Western blotting as described in Fig. 1C. β-Actin was used as a loading control. D and E, A549/Mock, A549/MUC1 (D), SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 (E) cells were labeled with biotin, and then MUC1-ND and galectin-3 on the cell surface were detected as described in Fig. 2A. F and G, the intensity of galectin-3 in Fig. 2 (D and E) was determined and normalized as described in Fig. 2B (means ± S.D., n = 3). *, p < 0.05; **, p < 0.01. H, galectin-3 was immunoprecipitated (IP) from lysates of HCT116/MUC1 cells with anti-galectin-3 antibodies (lane b) or control IgG (lane a) and then subjected to SDS-PAGE, followed by Western blotting and detection with anti-MUC1-ND, anti-MUC1-CD, and anti-galectin-3 antibodies.
Next, to examine whether or not the binding of galectin-3 to MUC1 on the cell surface is also observed in other human cancer cell lines, we detected biotin-labeled cell surface galectin-3 as described above using A549 and SKOV3 cells, which are derived from a human lung cancer and a human ovary cancer, respectively. Because A549 and SKOV3 cells express a low level and a considerable level of MUC1, respectively, MUC1-expressing cells (A549/MUC1 and SKOV3/Scr) and the respective control cells (A549/Mock, SKOV3/Si-1 and -2) were prepared as described under “Experimental Procedures.” Although there was no significant difference in the level of endogenous galectin-3 between MUC1-expressing cells and the respective control cells (Fig. 2C), as expected, a higher level of galectin-3 on the cell surface was detected in MUC1-expressing cells as compared with in the respective control cells (Fig. 2, D–G). To further confirm the binding of galectin-3 to MUC1, co-immunoprecipitation assays were performed using HCT116/MUC1 cells. Galectin-3 was immunoprecipitated from HCT116/MUC1 cell lysates and subjected to SDS-PAGE, followed by Western blotting. As shown in Fig. 2H, MUC1 was co-immunoprecipitated with galectin-3, and both MUC1-ND and -CD were detected, probably because of the tight association of both domains even if galectin-3 bound to either MUC1-ND or -CD.
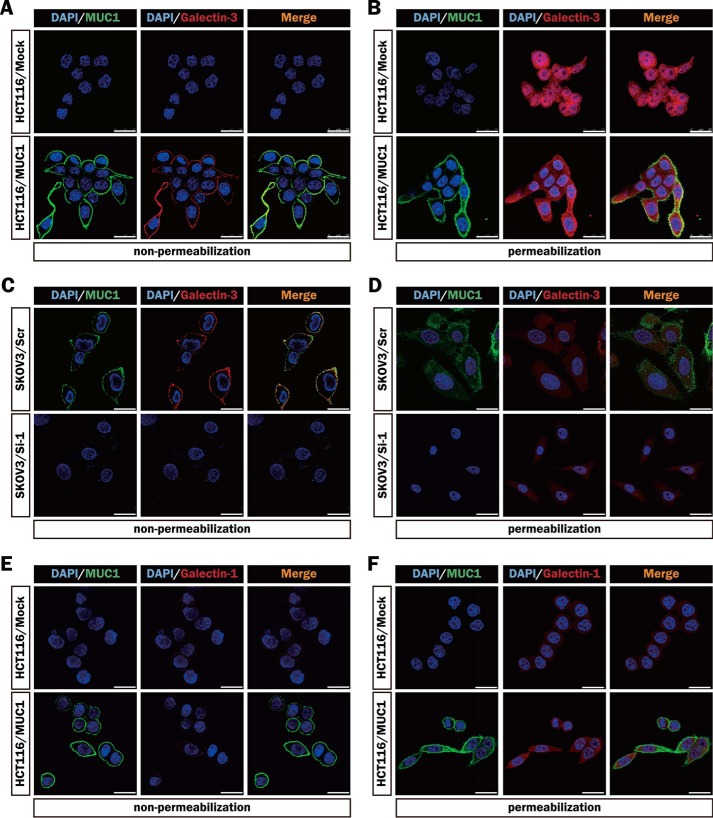
Furthermore, we stained MUC1-expressing cells (HCT116/MUC1 and SKOV3/Scr) and the respective control cells (HCT116/Mock and SKOV3/Si-1) immunocytochemically using anti-MUC1-ND and anti-galectin-3 antibodies to observe the distributions of MUC1 and galectin-3. Although MUC1 was detected on the entire surface of MUC1-expressing cells, its distribution was not completely uniform (Fig. 3, A and C). The uneven distribution of galectin-3 on the cell surface seems to parallel that of MUC1, suggesting the association of galectin-3 with MUC1 on the cell surface. On the other hand, a similar level of cytoplasmic galectin-3 was detected under permeabilized conditions in each cell type (Fig. 3, B and D). Immunocytochemical staining of HCT116/Mock and HCT116/MUC1 cells using anti-MUC1-ND and anti-galectin-1 antibodies demonstrated the same level and distribution of galectin-1 on the surface of both cell types as well as those in the cytoplasm regardless of the presence or absence of MUC1 (Fig. 3, E and F). These results are consistent with the biochemical data in Fig. 2.
FIGURE 3.
Distributions of MUC1, galectin-3, and galectin-1 in various MUC1-expressing cells. A, C, and E, MUC1 and galectin-3 on the surface of HCT116/Mock, HCT116/MUC1 (A), SKOV3/Scr, and SKOV3/Si-1 (C) cells, or MUC1 and galectin-1 on the surface of HCT116/Mock and HCT116/MUC1 (E) cells were detected immunocytochemically using the combinations of anti-MUC1-ND and Alexa Fluor 488-conjugated secondary antibodies (green), and anti-galectin-3 or anti-galectin-1 and Alexa Fluor 594-conjugated secondary antibodies (red), respectively. B, D, and F, after the cells had been permeabilized as described under “Experimental Procedures,” MUC1 and galectin-3 in HCT116/Mock, HCT116/MUC1 (B), SKOV3/Scr and SKOV3/Si-1 (D) cells, or MUC1 and galectin-1 in HCT116/Mock and HCT116/MUC1 (F) cells were detected as described above. The nuclei were stained with DAPI (blue). Image magnification is ×630. Scale bars, 25 μm.
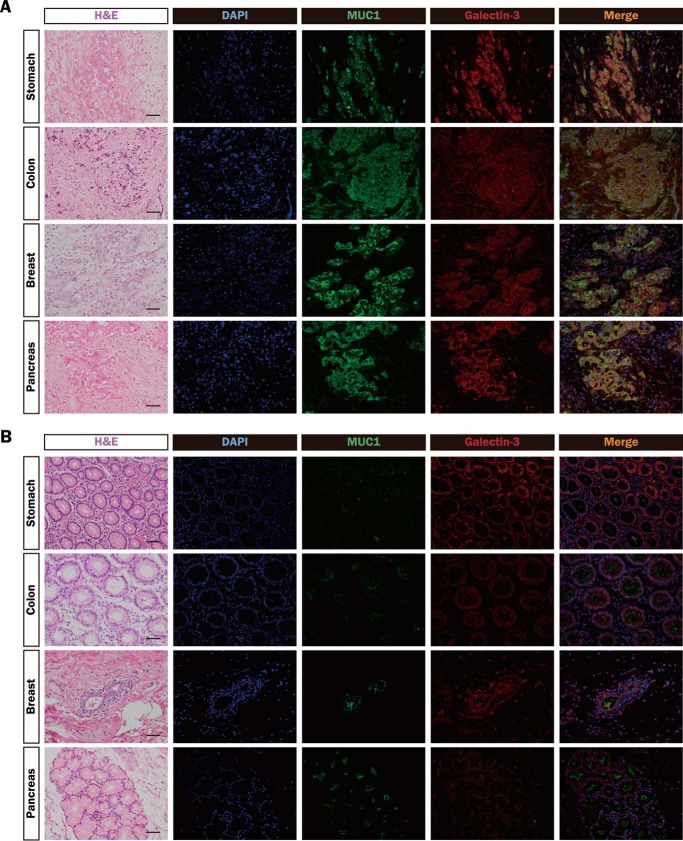
Next, to confirm that MUC1 and galectin-3 are actually distributed similarly in human tumor tissues, human tumor and nonmalignant tissues (stomach, colon, breast, and pancreas) were immunostained with anti-MUC1-ND and anti-galectin-3 antibodies. The distribution of galectin-3 coincided with that of MUC1 in tumor tissues but not in nonmalignant tissues (Fig. 4, A and B), suggesting that some endogenous galectin-3 is actually associated with MUC1 in tumor tissues.
FIGURE 4.
Distributions of MUC1 and galectin-3 in various tumor and nonmalignant tissues. A and B, sections of paraffin-embedded human tumor (A) and nonmalignant (B) tissues (stomach, colon, breast, and pancreas) were stained with hematoxylin and eosin (H&E), DAPI (blue), and the same combinations of antibodies as described in Fig. 3 (MUC1-ND, green; galectin-3, red). Image magnification is ×200. Scale bars, 100 μm.
Treatment of MUC1-expressing Cells with Galectin-3 Induces MUC1-mediated Signaling
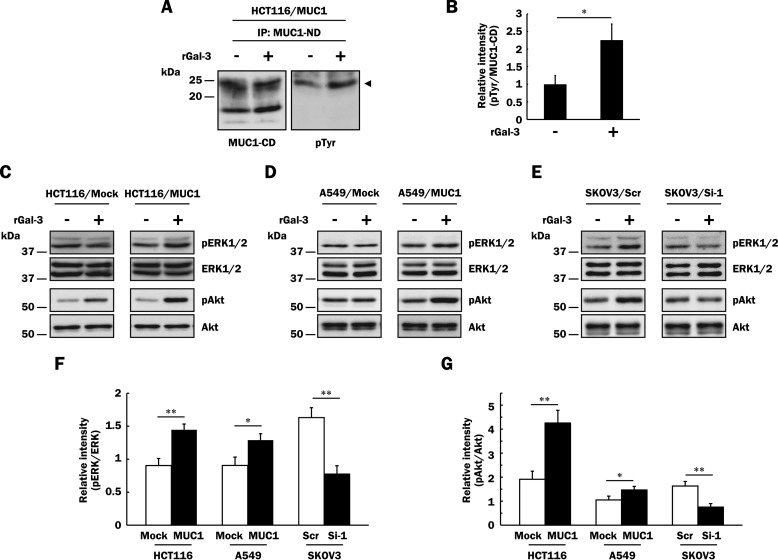
It has been extensively demonstrated that MUC1 is associated with growth factor receptors in some cancer cells and potentiates growth factor-dependent signal transduction. However, little is known about the signal transduction through direct ligation of MUC1 with endogenous ligands including galectin-3. Extracellular galectin-3 has been implicated in biological processes such as cell-cell adhesion. Because galectin-3 can cross-link and lattice MUC1, it is expected that galectin-3 can induce MUC1-mediated signaling. It has been reported that phosphorylation of tyrosine residues of MUC1-CD is involved in multiple signal transduction pathways (2). First, we investigated phosphorylation of MUC1-CD after treatment with galectin-3. To exclude the possible influence of endogenous galectin-3 and growth factors included in serum and/or produced by the cells, HCT116/MUC1 cells were washed with lactose-containing medium, and subsequently the cells were preincubated in the same medium. Thereafter, they were treated with or without galectin-3 as described under “Experimental Procedures,” and MUC1 immunoprecipitated from each cell lysate was subjected to SDS-PAGE, followed by Western blotting and detection with biotin-conjugated anti-phosphotyrosine and anti-MUC1-CD antibodies. Among several bands of MUC1-CD on SDS-PAGE, only a species with a molecular mass of ∼25 kDa was phosphorylated, and the level of phosphorylation increased ∼2.3-fold on treatment with galectin-3 (Fig. 5, A and B), indicating that the binding of galectin-3 to MUC1 triggers the phosphorylation of the tyrosine residues of MUC1-CD. Li et al. (34) also showed that only a species with a molecular mass of ∼25 kDa was phosphorylated during growth factor-initiated signaling. Regarding this experiment, surface proteins of HCT116/MUC1 cells were labeled with biotin as described above, and MUC1 was immunoprecipitated from the cell lysates. The immunoprecipitates were subjected to SDS-PAGE, followed by Western blotting and detection with HRP-conjugated streptavidin. It was MUC1-CD with a molecular mass of ∼25 kDa that was labeled with biotin (data not shown), indicating that a 25-kDa species is expressed on the cell surface and phosphorylated, others maybe being intermediate forms during intracellular transport. Next, to determine whether or not phosphorylation of MUC1-CD leads to activation of signaling cascades, HCT116/Mock and HCT116/MUC1 cells were treated with or without galectin-3 as described under “Experimental Procedures,” and the lysates were subjected to SDS-PAGE, followed by Western blotting and detection of phosphorylated ERK1/2 and Akt. Although phosphorylated ERK1 was under the detectable level, phosphorylation of ERK2 was elevated ∼1.5-fold on treatment with galectin-3 in HCT116/MUC1 cells but not in HCT116/Mock cells (Fig. 5, C and F). It is also noted that phosphorylation of Akt was enhanced more prominently on treatment with galectin-3 in HCT116/MUC1 cells (∼4-fold) than in HCT116/Mock cells (∼2-fold) (Fig. 5, C and G), suggesting that although galectin-3-initiated MUC1-mediated signaling mainly contributes to enhancement of Akt phosphorylation, other unknown membrane glycoproteins may also be involved in galectin-3-induced Akt phosphorylation in HCT116/Mock and HCT116/MUC1 cells. Similarly, phosphorylation of ERK1/2 and Akt was enhanced more prominently in other MUC1-expressing cells (A549/MUC1 and SKOV3/Scr) as compared with in the respective control cells (Fig. 5, D–G). These results suggest that the binding of galectin-3 to MUC1 initiates signaling cascades.
FIGURE 5.
Enhanced phosphorylation of ERK1/2 and Akt through the binding of galectin-3 to MUC1. A, after galectin-3 retained on the cell surface had been excluded as described under “Experimental Procedures,” HCT116/MUC1 cells were treated with recombinant galectin-3 (40 μg/ml, rGal-3 +) or PBS (vehicle, rGal-3 −) for 4 min, and then MUC1 was immunoprecipitated (IP) from lysates of HCT116/MUC1 cells with anti-MUC1-ND antibodies. The immunoprecipitates were subjected to SDS-PAGE, followed by Western blotting and detection with biotin-conjugated anti-phosphotyrosine (pTyr) and anti-MUC1-CD antibodies. The arrowhead in the right panel indicates tyrosine-phosphorylated MUC1-CD. B, the intensities of the bands in Fig. 5A were determined with ImageJ. The ratio of tyrosine-phosphorylated MUC1-CD (pTyr) to MUC1-CD in galectin-3-treated HCT116/MUC1 cells was determined when that in non-galectin-3-treated HCT116/MUC1 cells was taken as 1 (means ± S.D., n = 3). *, p < 0.05. C–E, after exclusion of galectin-3 from the cell surface as described above, HCT116/Mock, HCT116/MUC1 (C), A549/Mock, A549/MUC1 (D), SKOV3/Scr and SKOV3/Si-1 (E) cells were treated with recombinant galectin-3 (40 μg/ml, rGal-3 +) or PBS (vehicle, rGal-3 −) for 10 min, and then the lysates were subjected to SDS-PAGE, followed by Western blotting and detection with anti-phospho-ERK1/2 (pERK1/2), anti-ERK1/2, anti-phospho-Akt (pAkt), and anti-Akt antibodies. F and G, the intensities of the bands in Fig. 5 (C–E) were determined with ImageJ. Each histogram shows the ratios of pERK1/2 to ERK1/2 (F) and pAkt to Akt (G) in each galectin-3-treated cell type when the ratios obtained from each non-galectin-3-treated cell type were taken as 1 (means ± S.D., n = 3). *, p < 0.05; **, p < 0.01.
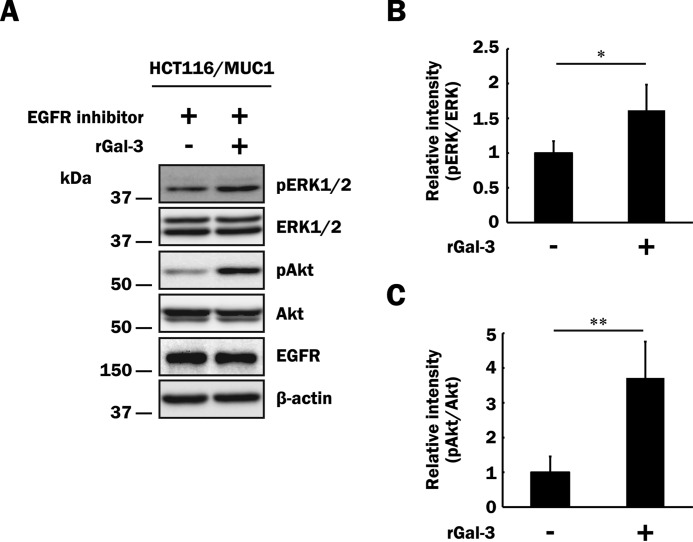
So far, many reports have demonstrated that MUC1-mediated signaling originates indirectly through ligation of growth factors such as EGF and FGF with receptors that are associated with MUC1 on the cell surface (2, 34, 38, 43, 44). Because HCT116/MUC1 cells express EGFR, as described previously (33), we next performed the same experiment as described above in the presence of an EGFR inhibitor. Prior to investigating the influence of the EGFR inhibitor on MUC1-mediated signaling through the binding of galectin-3 to MUC1, we confirmed the effect of the EGFR inhibitor using HCT116/MUC1 cells pretreated with or without the inhibitor. The cells were treated with EGF in the presence or absence of the EGFR inhibitor, and total (i.e. phosphorylated and unphosphorylated) EGFR was immunoprecipitated with anti-EGFR antibodies. The immunoprecipitates were subjected to SDS-PAGE, followed by Western blotting and detection with biotin-conjugated anti-phosphotyrosine and anti-EGFR antibodies. Phosphorylation of EGFR was completely inhibited by treatment with the EGFR inhibitor in HCT116/MUC1 cells (data not shown). In the presence of the EGFR inhibitor, HCT116/MUC1 cells were treated with or without galectin-3, and then phosphorylation of ERK1/2 and Akt was examined as described above. Phosphorylation of ERK1/2 and Akt was elevated on treatment with galectin-3 even in the presence of the EGFR inhibitor, as well as in its absence (Fig. 6), indicating that phosphorylation of ERK1/2 and Akt is enhanced through MUC1-mediated signaling pathways triggered by galectin-3 but not EGFR-related events.
FIGURE 6.
Influence of an EGFR inhibitor on phosphorylation of ERK1/2 and Akt through the binding of galectin-3 to MUC1. A, after exclusion of galectin-3 from the cell surface as described in Fig. 5A, HCT116/MUC1 cells were preincubated with an EGFR inhibitor (10 μm) for 1 h and subsequently treated with recombinant galectin-3 (40 μg/ml, rGal-3 +) or PBS (vehicle, rGal-3 −) for 10 min. Phosphorylated ERK1/2, total ERK1/2, phosphorylated Akt, and total Akt were determined as described in Fig. 5 (C–E). Concomitantly, EGFR and β-actin were also determined. B and C, the intensities of the bands in Fig. 6A were determined as described in Fig. 5 (F and G). The ratios obtained from non-galectin-3-treated HCT116/MUC1 cells were taken as 1 (means ± S.D., n = 4). *, p < 0.05; **, p < 0.01.
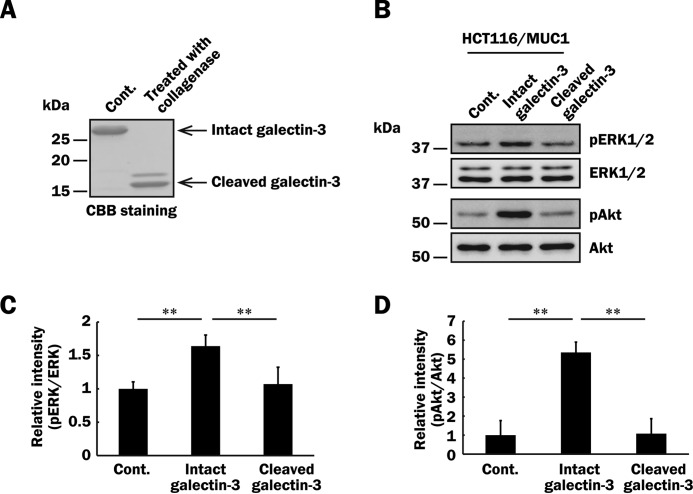
MUC1-mediated signaling seems to be initiated by cross-linking of MUC1 through the binding of galectin-3. Thus, multivalency of galectin-3 may be necessary to initiate MUC1-mediated signaling. Oligomerization of galectin-3 through association of its N-terminal domain can be abolished by its cleavage from the carbohydrate recognition domain by treatment with collagenase type VII (11, 45). To determine whether or not multivalency of galectin-3 is important for MUC1-mediated signaling, we prepared cleaved galectin-3 without the ability of oligomerization (Fig. 7A). As shown in Fig. 7 (B–D), phosphorylation of ERK1/2 and Akt induced by treatment with intact galectin-3 was markedly reduced by using cleaved galectin-3 instead of intact galectin-3. These results indicate that multivalency of galectin-3 is important for initiation of MUC1-mediated signaling.
FIGURE 7.
Effect of galectin-3 multivalency on phosphorylation of ERK1/2 and Akt through the binding of galectin-3 to MUC1. A, galectin-3 treated with collagenase type VII (cleaved) or PBS (intact) as described under “Experimental Procedures” was subjected to SDS-PAGE, followed by Coomassie Brilliant Blue staining. B, after exclusion of galectin-3 from the cell surface and subsequent preincubation with the EGFR inhibitor as described in Fig. 6A, HCT116/MUC1 cells were treated with intact galectin-3, cleaved galectin-3 (40 μg/ml, respectively), or PBS (vehicle) for 10 min, and then phosphorylated ERK1/2, total ERK1/2, phosphorylated Akt, and total Akt were determined as described in Fig. 5 (C–E). C and D, the intensities of the bands in Fig. 7B were determined as described in Fig. 5 (F and G). The ratios obtained from PBS-treated HCT116/MUC1 cells were taken as 1 (means ± S.D., n = 3). **, p < 0.01.
MUC1-mediated Signaling Initiated by Galectin-3 Enhances Cell Proliferation and Motility
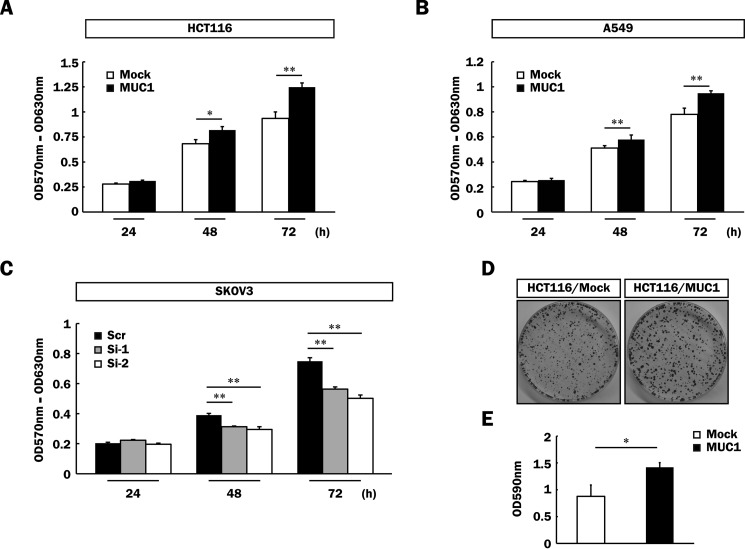
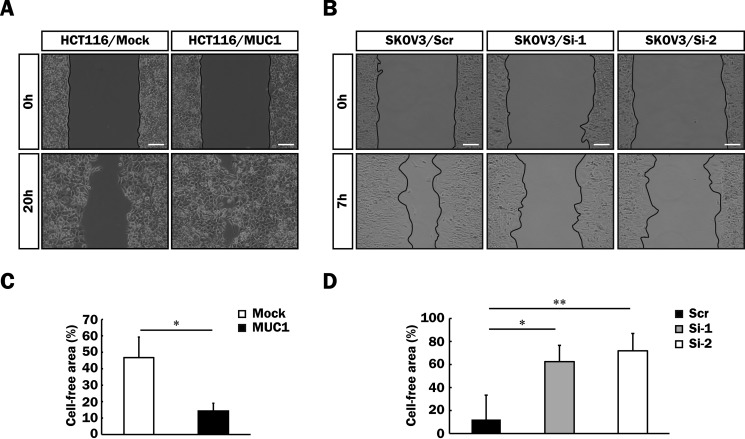
It is generally known that activation of the MAPK and PI3K/Akt signaling pathways induces various cellular functions such as cell proliferation and motility (46, 47). To determine whether or not MUC1-mediated signaling triggered by the binding of galectin-3 promotes malignancy, proliferation of MUC1-expressing cells (HCT116/MUC1, A549/MUC1, and SKOV3/Scr), and the respective control cells was examined by MTT assays. Proliferation of HCT116/MUC1 and A549/MUC1 cells was facilitated by forced expression of MUC1 as compared with that of the respective control cells (Fig. 8, A and B). In contrast, proliferation of SKOV3/Si-1 and -2 cells was down-regulated by silencing of MUC1 expression as compared with that of SKOV3/Scr cells (Fig. 8C). Anchorage-dependent clonogenic assays were also performed using HCT116/Mock and HCT116/MUC1 cells, and similar results were obtained (Fig. 8, D and E). Next, we further investigated cell motility by means of wound healing assays using the cells described above. To exclude the possibility that cell proliferation influences the assessment of cell motility, these cells were treated with hydroxyurea, which suppresses cell proliferation through inhibition of DNA synthesis. Prior to performing wound healing assays, the effective concentration of hydroxyurea that inhibits the proliferation of these cells was determined by BrdU assays (data not shown). Cell motility was also enhanced by forced expression of MUC1 in HCT116 cells (Fig. 9, A and C) and decreased by silencing of MUC1 in SKOV3 cells (Fig. 9, B and D), suggesting the correlation of cell motility with the expression level of MUC1.
FIGURE 8.
Enhancement of cell proliferation of various MUC1-expressing cells. A–C, HCT116/Mock and HCT116/MUC1 (5 × 103 cells) (A), A549/Mock and A549/MUC1 (2 × 103 cells) (B), and SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 cells (2 × 103 cells) (C) were plated and cultured for 24, 48, and 72 h. The level of cell proliferation was assessed by MTT assays (means ± S.D., n = 3). *, p < 0.05; **, p < 0.01. D, HCT116/Mock and HCT116/MUC1 cells (5 × 103 cells) were plated and cultured for 2 weeks. The cells thereafter were fixed and stained with crystal violet as described under “Experimental Procedures.” E, the dye was extracted from the cells as described under “Experimental Procedures,” and its level was determined by measuring the absorbance at 590 nm (means ± S.D., n = 3). *, p < 0.05.
FIGURE 9.
Enhancement of cell motility of various MUC1-expressing cells. A and B, a cell-free area as to HCT116/Mock, HCT116/MUC1 (A), SKOV3/Scr, SKOV3/Si-1, and SKOV3/Si-2 (B) cells was created with culture inserts as described under “Experimental Procedures,” and then the cells were cultured for 20 h (A) or 7 h (B) in the presence of hydroxyurea (10 mm). Images were taken immediately after removal of the culture inserts (0 h) and after the incubation times described above (20 or 7 h). Image magnification is ×100. Scale bars, 100 μm. C and D, the percentage of the cell-free area was expressed as the percentage of the cell-free area at 20 (C) or 7 h (D) when the cell-free area at 0 h was taken as 100% (means ± S.D., n = 3). *, p < 0.05; **, p < 0.01.
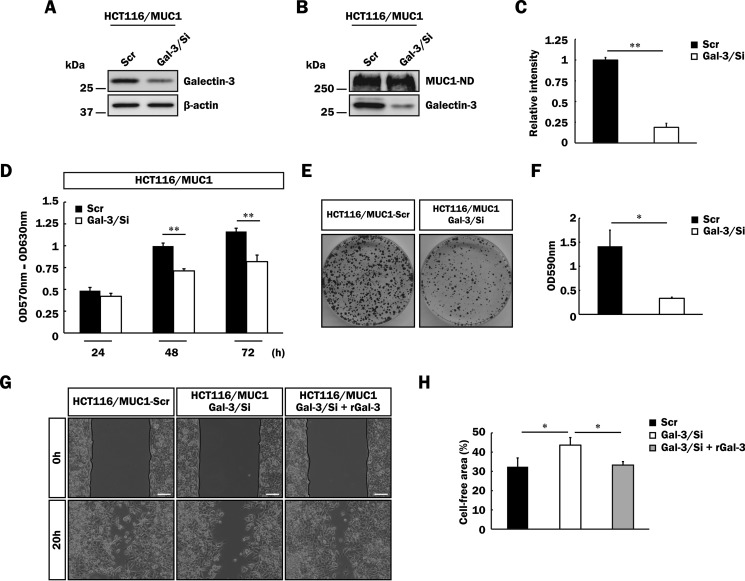
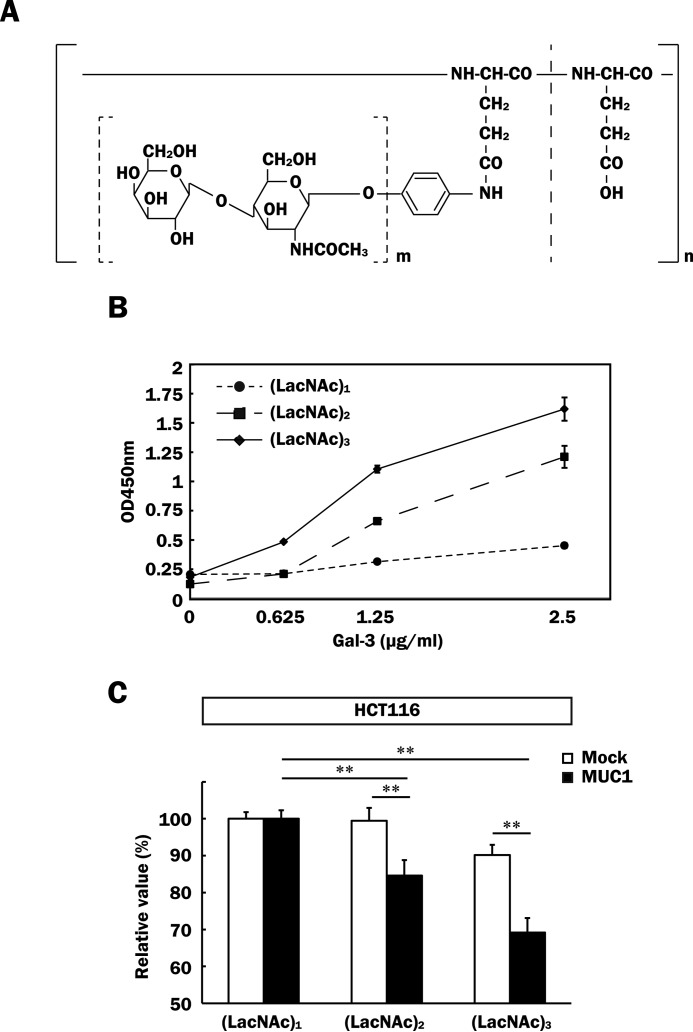
To further determine whether or not endogenous galectin-3 is related to promotion of malignancy in MUC1-expressing cells, we generated HCT116/MUC1 cells expressing a lower level of galectin-3 by treatment of MCT116/MUC1 cells with galectin-3 shRNA (HCT116/MUC1-Gal-3/Si). Reduction of the galectin-3 expression in HCT116/MUC1-Gal-3/Si cells as compared with in control cells (HCT116/MUC1-Scr) was confirmed by SDS-PAGE and Western blotting (Fig. 10A). Next, both cell types were labeled with biotin to estimate the level of galectin-3 retained on the cell surface as described in Fig. 2. As shown in Fig. 10 (B and C), galectin-3 retained on the cell surface was markedly decreased in HCT116/MUC1-Gal-3/Si cells. Furthermore, expectedly, galectin-3-silent cells proliferated more slowly than control cells did (Fig. 10, D–F), and cell motility was also down-modulated by silencing of galectin-3 (Fig. 10, G and H). It is also noted that down-modulation of cell motility of HCT116/MUC1-Gal-3/Si cells recovered to the level of control cells on treatment with exogenous galectin-3 (Fig. 10, G and H). Taking these results together, we speculated that inhibition of the binding of galectin-3 to MUC1 by antagonists may suppress the promotion of tumor cell malignancy. To examine this possibility, we used galectin-3 antagonists, i.e. glycopolymers carrying several repeats of N-acetyllactosamine (LacNAc), which exhibit high affinity for galectin-3. Because it has been reported that the binding affinity of LacNAc for galectin-3 is dependent on the number of repetitive LacNAcs (40), we prepared three kinds of artificial glycopolymers carrying single, tandem, and triplet LacNAc repeats (Fig. 11A), and then the binding affinity of galectin-3 for these glycopolymers was measured by using plates coated with each glycopolymer. In agreement with the previous report, the binding of galectin-3 to glycopolymers increased according to the number of repetitive LacNAcs (Fig. 11B). Next, we examined the effects of these glycopolymers on cell proliferation. As shown in Fig. 11C, cell proliferation was inhibited depending on the number of repetitive LacNAcs, and proliferation of HCT116/MUC1 cells was more markedly inhibited than that of HCT116/Mock cells. These findings suggest that galectin-3 bound to MUC1 is excluded by glycopolymers, and thereby cell proliferation is inhibited. Partial exclusion of galectin-3 from the cell surface by treatment with the glycopolymer carrying a triplet LacNAc repeat was confirmed by flow cytometry (data not shown). With respect to the inhibitory effect, the glycopolymer carrying a triplet LacNAc repeat had a low inhibitory effect even on the proliferation of HCT116/Mock cells, maybe because of suppression of galectin-3-induced Akt phosphorylation through the binding of galectin-3 to unknown glycoprotein receptors related to cell growth, as shown in Fig. 5 (C and G).
FIGURE 10.
Effect of galectin-3 silencing on malignancy of MUC1-expressing cells. A, lysates of HCT116/MUC1-Scr and HCT116/MUC1-Gal-3/Si cells were subjected to SDS-PAGE, followed by Western blotting as described in Fig. 1C. β-Actin was used as a loading control. B, biotin-labeled MUC1-ND and galectin-3 on the surface of HCT116/MUC1-Scr and HCT116/MUC1-Gal-3/Si cells were detected as described in Fig. 2A. C, the level of galectin-3 on the cell surface was determined by measuring the intensities of the bands in Fig. 10B as described in Fig. 2B (means ± S.D., n = 3). **, p < 0.01. D, HCT116/MUC1-Scr and HCT116/MUC1-Gal-3/Si cells (1 × 104 cells) were plated, and the proliferation level was determined as described in Fig. 8 (A–C) (means ± S.D., n = 3). **, p < 0.01. E and F, HCT116/MUC1-Scr and HCT116/MUC1-Gal-3/Si cells (5 × 103 cells) were plated, and then anchorage-dependent cell proliferation was evaluated by measuring the absorbance as described in Fig. 8 (D and E) (means ± S.D., n = 3). *, p < 0.05. G and H, HCT116/MUC1-Scr and HCT116/MUC1-Gal-3/Si cells were precultured using culture inserts. After creating a gap, the cells were further incubated for 20 h under the same conditions as described in Fig. 9 (A and B). HCT116/MUC1-Gal-3/Si cells were also cultured in the presence of recombinant galectin-3 (rGal-3) (40 μg/ml), and their cell motilities were assessed as described above. The percentage of the cell-free area was calculated as described in Fig. 9 (C and D) (means ± S.D., n = 3). *, p < 0.05. Image magnification is ×100. Scale bars, 100 μm.
FIGURE 11.
Inhibitory effects of galectin-3 antagonists on cell proliferation. A, schematic structures of artificial glycopolymers. m indicates the number of repetitive LacNAcs (m = 1–3), and n indicates the degree of polymerization of glutamic acid residues (n = 467). B, each glycopolymer (0.5 μm) was immobilized on a plate as described under “Experimental Procedures.” The binding affinity of galectin-3 for each glycopolymer was measured by plate assays (means ± S.D., n = 3). C, HCT116/Mock and HCT116/MUC1 cells (1 × 104 cells) were cultured in medium containing 0.5% HI-FBS and each glycopolymer (0.1 μm) for 72 h. The effects of the glycopolymers on cell proliferation were determined by MTT assays. The absorbance observed for each (LacNAc)1-treated cell was taken as 100% (means ± S.D., n = 4). **, p < 0.01. (LacNAc)1, glycopolymer carrying a single LacNAc repeat; (LacNAc)2, glycopolymer carrying a tandem LacNAc repeat; (LacNAc)3, glycopolymer carrying a triplet LacNAc repeat.
Discussion
It has been reported that elevated levels of MUC1 and galectin-3 are associated with a poor prognosis in a variety of malignant tumors. However, little is known about the cooperative functions of these molecules except for the effect on cell adhesion through the binding of galectin-3 to MUC1.
By means of DNA microarray analysis, it was demonstrated that HCT116 cells expressed negligible levels of mucins including MUC1 (Fig. 1A). Therefore, we mainly used HCT116/Mock and HCT116/MUC1 cells throughout all experiments because the biological functions of MUC1 through the binding of galectins may be attenuated by other mucins, which have a potential for binding to galectins, as well as MUC1. Expression of MUC1 was confirmed, and the same levels of galectin-1 and -3 were expressed in HCT116/Mock and HCT116/MUC1 cells (Fig. 1C), but interestingly, it was galectin-3 but not galectin-1 that bound more prominently to the surface of HCT116/MUC1 cells than that of HCT116/Mock cells (Fig. 2, A and B). Furthermore, the distribution of galectin-3 almost completely coincided with that of MUC1 on the surface of cancer cells and in tumor tissues (Figs. 3 and 4A). In nonmalignant tissues, as shown in Fig. 4B, MUC1 was detected slightly, and its distribution seemed to be different from that of galectin-3, probably because of limited distribution of MUC1 on the apical surface and/or nonexpression of TF antigen carried on MUC1 in nonmalignant cells.
In related studies, it has been reported that the binding of galectin-3 to MUC1 leads to enhancement of metastasis and loss of anoikis (28–30). These phenomena are brought about by the polarization of MUC1 on the cell surface and eventual exposure of adhesion molecules, but not by activation of signal transduction. However, as shown in Fig. 5, treatment of HCT116/MUC1 cells with galectin-3 induced phosphorylation of MUC1-CD and subsequent phosphorylation of ERK1/2 and Akt. Galectin-3-induced phosphorylation of ERK1/2 and Akt increased in a dose-dependent manner (data not shown), and microgram levels of galectin-3 were necessary to enhance the phosphorylation of ERK1/2 and Akt in HCT116/MUC1 cells. Zhao et al. (29) reported that the concentration of circulating galectin-3 in the serum of patients with colorectal cancer increased up to 5 μg/ml. In tumor microenvironments, galectin-3 is produced not only by the cancer cells but also by the peritumoral inflammatory and stromal cells and is concentrated on the cell surface and/or local matrix, raising the local concentration of galectin-3 to above that detected in the serum of cancer patients. Thus, we speculate that the level of exogenously added galectin-3 in this study may be similar to that of endogenous galectin-3 in tumor microenvironments. In relation to these findings, Ramasamy et al. (38) demonstrated that galectin-3 enhances the interaction between MUC1 and EGFR, resulting in enhancement of the EGF-dependent MUC1-mediated signaling pathway. In contrast, Merlin et al. (48) reported that silencing of galectin-3 facilitates the interaction between MUC1 and EGFR, leading to promotion of EGF-dependent MUC1-mediated signaling. This discrepancy has not been clarified yet; however, in any case, growth factors such as EGF are essential for initiation of MUC1-mediated signaling. To determine whether or not galectin-3-induced phosphorylation occurs despite the absence of EGF and/or EGFR, we examined the effect of galectin-3 on phosphorylation of ERK1/2 and Akt using cells cultured in serum-free medium and in the presence of an EGFR inhibitor, which inhibits its autophosphorylation. Phosphorylation of ERK1/2 and Akt was elevated on treatment with galectin-3 independently of both EGF stimulation and EGFR tyrosine phosphorylation (Fig. 6). In addition, we previously reported that EGFR was not co-immunoprecipitated with MUC1 in HCT116/MUC1 cells (33). Thus, these findings suggest that phosphorylation of MUC1-CD by galectin-3 and subsequent phosphorylation of ERK1/2 and Akt occur independently of EGFR. In addition, because the EGFR inhibitor used in this study suppresses not only EGFR but also ErbB2 (HER2) and ErbB4 (HER4) activation, at least EGFR, ErbB2, and ErbB4 are not directly involved in the phosphorylation of MUC1. However, there remains a possibility that galectin-3 acts as a mediator of interactions between other receptor tyrosine kinases and MUC1, followed by activation of the MAPK and PI3K/Akt signaling pathways.
The MUC1-CD cytoplasmic tail, which comprises 72 amino acids, contains seven conserved tyrosine residues, each one being phosphorylated by different (receptor) tyrosine kinases (2). Phosphorylation of the YTNP and YHPM motifs containing Tyr-60 and Tyr-20, respectively, leads to the MAPK and PI3K/Akt signaling pathways through the binding of the Src homology 2 domain of an adaptor protein, Grb2 (growth factor receptor-bound protein 2), and that of the PI3K p85 subunit to the corresponding motifs, respectively (49–52). Although the phosphorylated sites of MUC1-CD on treatment with galectin-3 have not been elucidated yet, the same tyrosine residues of MUC1-CD may be phosphorylated, and common downstream events between the EGF- and galectin-3-initiated pathways may occur to enhance the MAPK and PI3K/Akt signaling pathways, although MUC1-CD is phosphorylated by different mechanisms in each pathway. In addition, Tyr-46 contained in the YEKV motif is phosphorylated by several tyrosine kinases including EGFR, leading to recruitment of β-catenin to MUC1-CD (34). In this context, we previously demonstrated that recruitment of β-catenin to MUC1-CD was enhanced on treatment with Siglec-9 in HCT116/MUC1 cells and with galectin-3 in 3T3/MUC1 cells (33, 53). With respect to the recruitment of β-catenin, it is also speculated that galectin-3-triggered signaling leads to phosphorylation of the same tyrosine residues of MUC1-CD as EGF-triggered signaling does, and after recruitment of β-catenin, the same pathway may occur in both signaling events. These results are consistent with the fact that similar downstream events after phosphorylation of MUC1-CD on treatment with galectin-3 occurred even in the presence of the EGFR inhibitor.
When galectin-3 is present freely or binds to a monovalent binding site, the major form of galectin-3 is monomeric (54). On the other hand, when galectin-3 binds to multivalent binding sites, galectin-3 behaves as a multivalent lectin by forming oligomers through association of the galectin-3 N-terminal domain (24, 25). MUC1 seems to be one of the most preferable glycoproteins for oligomerizing galectin-3 because O-glycans, which are the binding sites of galectin-3, may be expressed repeatedly on the tandem repeats of the MUC1 core protein. Furthermore, it is an extremely high molecular glycoprotein and easily accessible to galectin-3 because of its rod-like structure, which is longer (200–500 nm) than typical cell surface adhesion molecules (∼30 nm) (55, 56), and the binding affinity of galectin-3 for oligosaccharides carried on proteins including MUC1 increases dramatically by clustering of its proteins (57). The significant role of the MUC1 extracellular domain has also been demonstrated by the report that MUC1-mediated signaling is dependent on the number of tandem repeats (58). From another point of view, because homooligomerization of MUC1-CD through interaction of a juxtamembrane region, the CQC motif, in MUC1-CD plays a crucial role in MUC1-mediated signal transduction (2, 39, 52, 59), clustering of MUC1 through the binding of galectin-3 to MUC1 may assist oligomerization of MUC1-CD.
Tumor malignant phenotypes such as cell growth and motility changed with the levels of MUC1 and galectin-3 expression, indicating that MUC1 and galectin-3 play a significant cooperative role in promoting tumor cell malignancy. Being consistent with these results, galectin-3 antagonists, synthetic glycopolymers containing LacNAc, were effective in down-regulating proliferation of MUC1-expressing cells. Interestingly, several reports have demonstrated that other galectin-3 antagonists, i.e. GCS-100 and lactulose-l-leucine, which are oligosaccharides modified from citrus pectin and synthetic glycoamine, respectively, inhibit several cellular functions in multiple myeloma and prostate cancer cells (60–62).
Thus, constitutive activation of MUC1-mediated signaling in an autocrine/paracrine manner caused by ligation of galectin-3 promotes uncontrolled tumor cell malignancy. This may be an alternative MUC1-mediated pathway that promotes tumor cell malignancy even though growth factors such as EGF and FGF and/or their receptors are absent and/or nonfunctional.
Author Contributions
Y. M., K. A., and H. N. designed the study. Y. M. and H. N. wrote the paper. Y. M. and K. A. carried out the experiments. M. Y., T. S., and K. H. prepared the specimens of tumor and adjacent nonmalignant tissues and analyzed them pathologically. T. M. designed and synthesized the artificial glycopolymers.
This work was supported by a grants-in-aid of the Fugaku Trust for Medical Research and the Private University Strategic Research Foundation Support Program. The authors declare that they have no conflicts of interest with the contents of this article.
- MUC1
- mucin 1
- MUC1-ND
- MUC1 N-terminal domain
- MUC1-CD
- MUC1 C-terminal domain
- EGFR
- EGF receptor
- TF
- Thomsen-Friedenreich
- Gal
- galactose
- GalNAc
- N-acetylgalactosamine
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- LacNAc
- N-acetyllactosamine
- HI-FBS
- heat-inactivated FBS.
References
- 1.Kufe D., Inghirami G., Abe M., Hayes D., Justi-Wheeler H., and Schlom J. (1984) Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma 3, 223–232 [DOI] [PubMed] [Google Scholar]
- 2.Kufe D. (2009) Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer 9, 874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligtenberg M. J., Kruijshaar L., Buijs F., van Meijer M., Litvinov S. V., and Hilkens J. (1992) Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 267, 6171–6177 [PubMed] [Google Scholar]
- 4.Parry S., Silverman H. S., McDermott K., Willis A., Hollingsworth M. A., and Harris A. (2001) Identification of MUC1 proteolytic cleavage sites in vivo. Biochem. Biophys. Res. Commun. 283, 715–720 [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui J., Abe M., Hayes D., Shani E., Yunis E., and Kufe D. (1988) Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc. Natl. Acad. Sci. U.S.A. 85, 2320–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gendler S., Taylor-Papadimitriou J., Duhig T., Rothbard J., and Burchell J. (1988) A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J. Biol. Chem. 263, 12820–12823 [PubMed] [Google Scholar]
- 7.Senapati S., Das S., and Batra S. K. (2010) Mucin-interacting proteins: from function to therapeutics. Trends Biochem. Sci. 35, 236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd K. O., Burchell J., Kudryashov V., Yin B. W., and Taylor-Papadimitriou J. (1996) Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J. Biol. Chem. 271, 33325–33334 [DOI] [PubMed] [Google Scholar]
- 9.Brockhausen I. (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1473, 67–95 [DOI] [PubMed] [Google Scholar]
- 10.Liu F. T., and Rabinovich G. A. (2005) Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J., Turck C. W., Atchison R. E., Huflejt M. E., Poulter L., Gitt M. A., Burlingame A. L., Barondes S. H., and Leffler H. (1993) Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-, tyrosine-rich sequence with bacterial and tissue collagenase. J. Biol. Chem. 268, 26704–26711 [PubMed] [Google Scholar]
- 12.Davidson P. J., Davis M. J., Patterson R. J., Ripoche M. A., Poirier F., and Wang J. L. (2002) Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology 12, 329–337 [DOI] [PubMed] [Google Scholar]
- 13.Yu F., Finley R. L. Jr., Raz A., and Kim H. R. (2002) Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J. Biol. Chem. 277, 15819–15827 [DOI] [PubMed] [Google Scholar]
- 14.Yang R. Y., Hsu D. K., and Liu F. T. (1996) Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 93, 6737–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honjo Y., Nangia-Makker P., Inohara H., and Raz A. (2001) Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin. Cancer Res. 7, 661–668 [PubMed] [Google Scholar]
- 16.Dagher S. F., Wang J. L., and Patterson R. J. (1995) Identification of galectin-3 as a factor in pre-mRNA splicing. Proc. Natl. Acad. Sci. U.S.A. 92, 1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato S., and Hughes R. C. (1994) Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 269, 4424–4430 [PubMed] [Google Scholar]
- 18.Hughes R. C. (1999) Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta 1473, 172–185 [DOI] [PubMed] [Google Scholar]
- 19.van den Brûle F., Califice S., and Castronovo V. (2004) Expression of galectins in cancer: a critical review. Glycoconj. J. 19, 537–542 [DOI] [PubMed] [Google Scholar]
- 20.Iurisci I., Tinari N., Natoli C., Angelucci D., Cianchetti E., and Iacobelli S. (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 6, 1389–1393 [PubMed] [Google Scholar]
- 21.Saussez S., Lorfevre F., Lequeux T., Laurent G., Chantrain G., Vertongen F., Toubeau G., Decaestecker C., and Kiss R. (2008) The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 44, 86–93 [DOI] [PubMed] [Google Scholar]
- 22.Nangia-Makker P., Honjo Y., Sarvis R., Akahani S., Hogan V., Pienta K. J., and Raz A. (2000) Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 156, 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochieng J., Leite-Browning M. L., and Warfield P. (1998) Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 246, 788–791 [DOI] [PubMed] [Google Scholar]
- 24.Yang R. Y., Hill P. N., Hsu D. K., and Liu F. T. (1998) Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry 37, 4086–4092 [DOI] [PubMed] [Google Scholar]
- 25.Ahmad N., Gabius H. J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., and Brewer C. F. (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 [DOI] [PubMed] [Google Scholar]
- 26.Nieminen J., Kuno A., Hirabayashi J., and Sato S. (2007) Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J. Biol. Chem. 282, 1374–1383 [DOI] [PubMed] [Google Scholar]
- 27.Gao X., Balan V., Tai G., and Raz A. (2014) Galectin-3 induces cell migration via a calcium-sensitive MAPK/ERK1/2 pathway. Oncotarget 5, 2077–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L. G., Andrews N., Zhao Q., McKean D., Williams J. F., Connor L. J., Gerasimenko O. V., Hilkens J., Hirabayashi J., Kasai K., and Rhodes J. M. (2007) Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 282, 773–781 [DOI] [PubMed] [Google Scholar]
- 29.Zhao Q., Guo X., Nash G. B., Stone P. C., Hilkens J., Rhodes J. M., and Yu L. G. (2009) Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 69, 6799–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q., Barclay M., Hilkens J., Guo X., Barrow H., Rhodes J. M., and Yu L. G. (2010) Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 9, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahn J. J., Shen Q., Mah B. K., and Hugh J. C. (2004) MUC1 initiates a calcium signal after ligation by intercellular adhesion molecule-1. J. Biol. Chem. 279, 29386–29390 [DOI] [PubMed] [Google Scholar]
- 32.Shen Q., Rahn J. J., Zhang J., Gunasekera N., Sun X., Shaw A. R., Hendzel M. J., Hoffman P., Bernier A., and Hugh J. C. (2008) MUC1 initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal protrusive motility after ligating intercellular adhesion molecule-1. Mol. Cancer Res. 6, 555–567 [DOI] [PubMed] [Google Scholar]
- 33.Tanida S., Akita K., Ishida A., Mori Y., Toda M., Inoue M., Ohta M., Yashiro M., Sawada T., Hirakawa K., and Nakada H. (2013) Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J. Biol. Chem. 288, 31842–31852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Ren J., Yu W., Li Q., Kuwahara H., Yin L., Carraway K. L. 3rd, and Kufe D. (2001) The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and β-catenin. J. Biol. Chem. 276, 35239–35242 [DOI] [PubMed] [Google Scholar]
- 35.Mori Y., Akita K., Tanida S., Ishida A., Toda M., Inoue M., Yashiro M., Sawada T., Hirakawa K., and Nakada H. (2014) MUC1 protein induces urokinase-type plasminogen activator (uPA) by forming a complex with NF-κB p65 transcription factor and binding to the uPA promoter, leading to enhanced invasiveness of cancer cells. J. Biol. Chem. 289, 35193–35204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahanta S., Fessler S. P., Park J., and Bamdad C. (2008) A minimal fragment of MUC1 mediates growth of cancer cells. PLoS One 3, e2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidari K. I., Murata T., Yoshida K., Takahashi Y., Minamijima Y. H., Miwa Y., Adachi S., Ogata M., Usui T., Suzuki Y., and Suzuki T. (2008) Chemoenzymatic synthesis, characterization, and application of glycopolymers carrying lactosamine repeats as entry inhibitors against influenza virus infection. Glycobiology 18, 779–788 [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy S., Duraisamy S., Barbashov S., Kawano T., Kharbanda S., and Kufe D. (2007) The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol. Cell 27, 992–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharbanda A., Rajabi H., Jin C., Tchaicha J., Kikuchi E., Wong K. K., and Kufe D. (2014) Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clin. Cancer Res. 20, 5423–5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W. E., Yagi F., and Kasai K. (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 [DOI] [PubMed] [Google Scholar]
- 41.Sparrow C. P., Leffler H., and Barondes S. H. (1987) Multiple soluble β-galactoside-binding lectins from human lung. J. Biol. Chem. 262, 7383–7390 [PubMed] [Google Scholar]
- 42.Bian C. F., Zhang Y., Sun H., Li D. F., and Wang D. C. (2011) Structural basis for distinct binding properties of the human galectins to Thomsen-Friedenreich antigen. PLoS One 6, e25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder J. A., Thompson M. C., Gardner M. M., and Gendler S. J. (2001) Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 276, 13057–13064 [DOI] [PubMed] [Google Scholar]
- 44.Ren J., Raina D., Chen W., Li G., Huang L., and Kufe D. (2006) MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol. Cancer Res. 4, 873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John C. M., Leffler H., Kahl-Knutsson B., Svensson I., and Jarvis G. A. (2003) Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin. Cancer Res. 9, 2374–2383 [PubMed] [Google Scholar]
- 46.Dhillon A. S., Hagan S., Rath O., and Kolch W. (2007) MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290 [DOI] [PubMed] [Google Scholar]
- 47.Engelman J. A. (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9, 550–562 [DOI] [PubMed] [Google Scholar]
- 48.Merlin J., Stechly L., de Beaucé S., Monté D., Leteurtre E., van Seuningen I., Huet G., and Pigny P. (2011) Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene 30, 2514–2525 [DOI] [PubMed] [Google Scholar]
- 49.Pandey P., Kharbanda S., and Kufe D. (1995) Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res. 55, 4000–4003 [PubMed] [Google Scholar]
- 50.Kinlough C. L., Poland P. A., Bruns J. B., Harkleroad K. L., and Hughey R. P. (2004) MUC1 membrane trafficking is modulated by multiple interactions. J. Biol. Chem. 279, 53071–53077 [DOI] [PubMed] [Google Scholar]
- 51.Kato K., Lu W., Kai H., Kim K. C. (2007) Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L686–L692 [DOI] [PubMed] [Google Scholar]
- 52.Raina D., Kosugi M., Ahmad R., Panchamoorthy G., Rajabi H., Alam M., Shimamura T., Shapiro G. I., Supko J., Kharbanda S., and Kufe D. (2011) Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol. Cancer Ther. 10, 806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanida S., Mori Y., Ishida A., Akita K., and Nakada H. (2014) Galectin-3 binds to MUC1-N-terminal domain and triggers recruitment of β-catenin in MUC1-expressing mouse 3T3 cells. Biochim. Biophys. Acta 1840, 1790–1797 [DOI] [PubMed] [Google Scholar]
- 54.Morris S., Ahmad N., André S., Kaltner H., Gabius H. J., Brenowitz M., and Brewer F. (2004) Quaternary solution structures of galectins-1, -3, and -7. Glycobiology 14, 293–300 [DOI] [PubMed] [Google Scholar]
- 55.Bramwell M. E., Wiseman G., and Shotton D. M. (1986) Electron-microscopic studies of the CA antigen, epitectin. J. Cell Sci. 86, 249–261 [DOI] [PubMed] [Google Scholar]
- 56.Becker J. W., Erickson H. P., Hoffman S., Cunningham B. A., and Edelman G. M. (1989) Topology of cell adhesion molecules. Proc. Natl. Acad. Sci. U.S.A. 86, 1088–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dam T. K., Gabius H. J., André S., Kaltner H., Lensch M., and Brewer C. F. (2005) Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 44, 12564–12571 [DOI] [PubMed] [Google Scholar]
- 58.Cascio S., Zhang L., and Finn O. J. (2011) MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-κB p65 and binding to cytokine promoters: importance of extracellular domain. J. Biol. Chem. 286, 42248–42256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raina D., Ahmad R., Rajabi H., Panchamoorthy G., Kharbanda S., and Kufe D. (2012) Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. Int. J. Oncol. 40, 1643–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chauhan D., Li G., Podar K., Hideshima T., Neri P., He D., Mitsiades N., Richardson P., Chang Y., Schindler J., Carver B., and Anderson K. C. (2005) A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 65, 8350–8358 [DOI] [PubMed] [Google Scholar]
- 61.Streetly M. J., Maharaj L., Joel S., Schey S. A., Gribben J. G., and Cotter F. E. (2010) GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood 115, 3939–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glinskii O. V., Sud S., Mossine V. V., Mawhinney T. P., Anthony D. C., Glinsky G. V., Pienta K. J., and Glinsky V. V. (2012) Inhibition of prostate cancer bone metastasis by synthetic TF antigen mimic/galectin-3 inhibitor lactulose-l-leucine. Neoplasia 14, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]