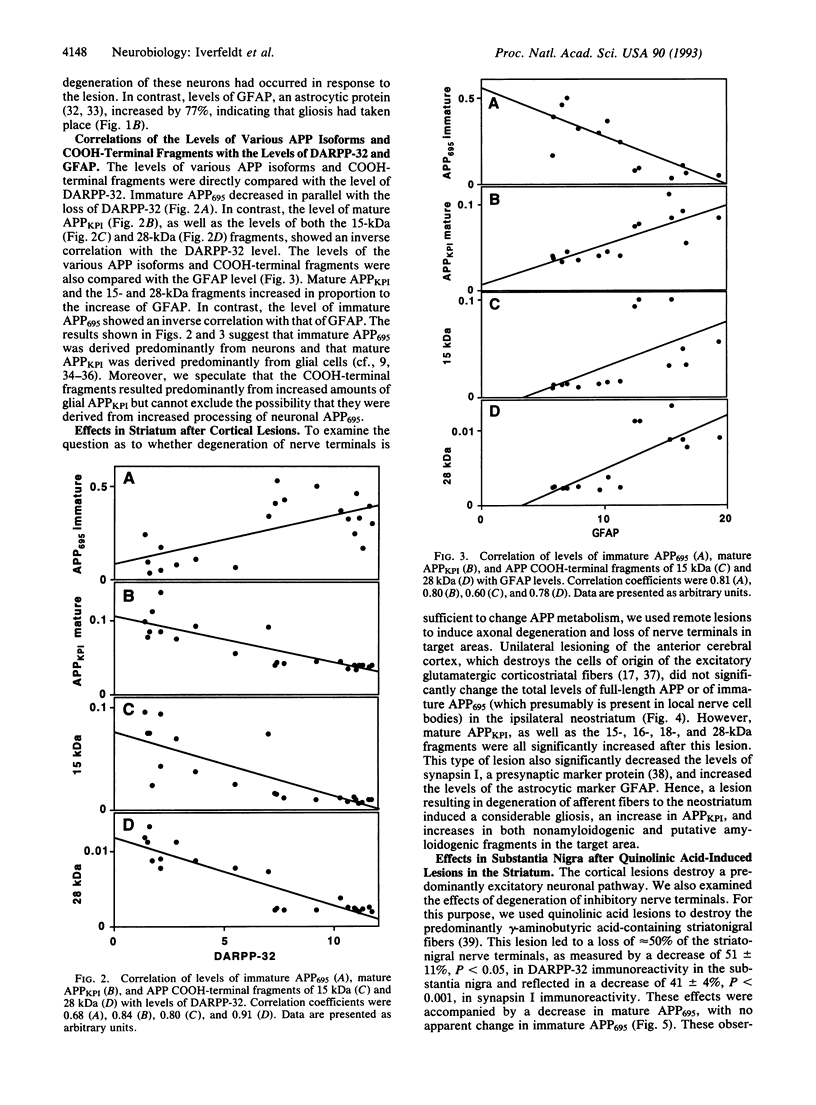
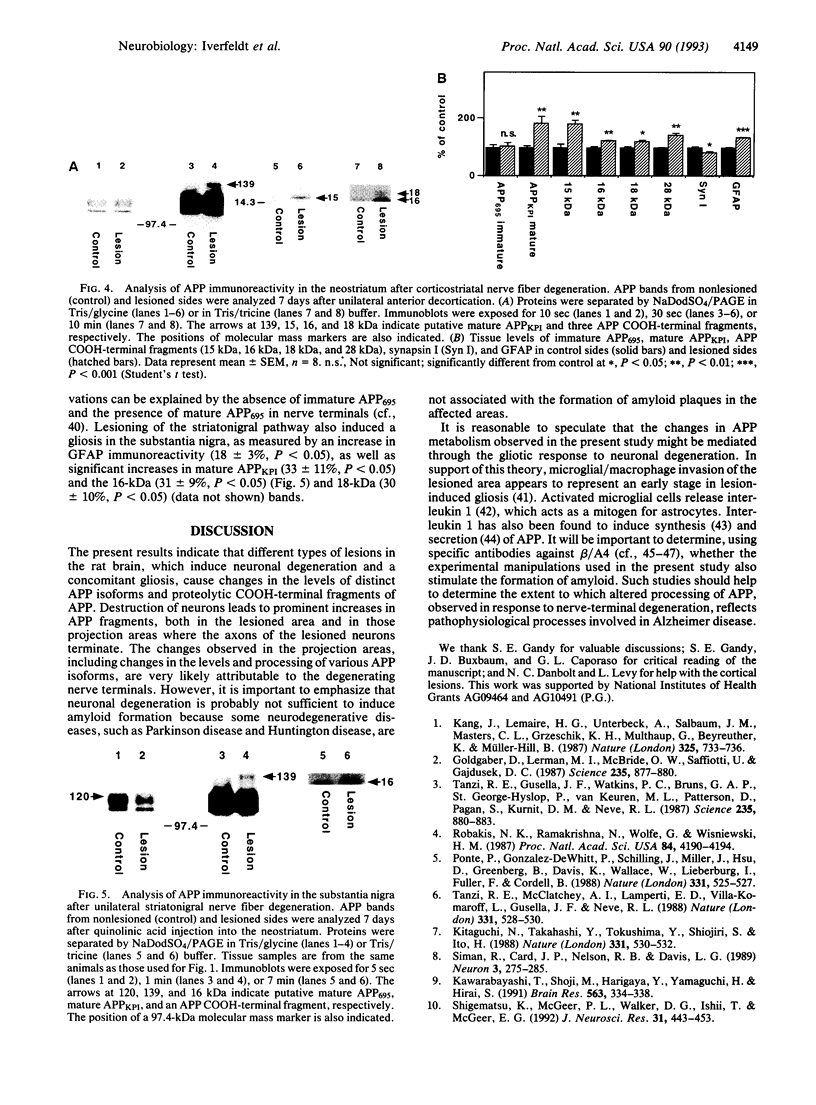
Abstract
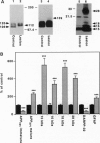
In the brains of individuals with Alzheimer disease, senile plaques containing aggregates of beta-amyloid peptide, derived from the beta-amyloid precursor protein (APP), are seen in association with degenerating nerve terminals. It is not known whether the degenerating nerve terminals cause the formation of these aggregates or whether beta-amyloid peptide in the aggregates causes nerve-terminal degeneration. In the present study of rat brain, degeneration either of local neurons or of nerve terminals caused decreased levels of a neuron-enriched isoform of APP, increased levels of a glia-enriched isoform of APP, and increased levels of potentially amyloidogenic, as well as nonamyloidogenic, COOH-terminal fragments of APP. Our results demonstrate that neuronal degeneration affects APP processing and suggest that it may contribute to amyloid formation in mammalian brain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso R. P., Ramabhadran T. V., Unterbeck A. J., Greengard P. Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Oishi M., Chen H. I., Pinkas-Kramarski R., Jaffe E. A., Gandy S. E., Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Ramabhadran T. V., Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Benfenati F., Valtorta F., Greengard P. The synapsins. Annu Rev Cell Biol. 1990;6:433–460. doi: 10.1146/annurev.cb.06.110190.002245. [DOI] [PubMed] [Google Scholar]
- Divac I., Fonnum F., Storm-Mathisen J. High affinity uptake of glutamate in terminals of corticostriatal axons. Nature. 1977 Mar 24;266(5600):377–378. doi: 10.1038/266377a0. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Gaul G., Dutly F., Frei K., Foguet M., Lübbert H., Paul G. APP RNA splicing is not affected by differentiation of neurons and glia in culture. FEBS Lett. 1992 Aug 3;307(3):329–332. doi: 10.1016/0014-5793(92)80706-m. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986 Aug;6(8):2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D., Harris H. W., Hla T., Maciag T., Donnelly R. J., Jacobsen J. S., Vitek M. P., Gajdusek D. C. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Selkoe D. J. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991 Dec;11(12):3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Isacson O., Fischer W., Wictorin K., Dawbarn D., Björklund A. Astroglial response in the excitotoxically lesioned neostriatum and its projection areas in the rat. Neuroscience. 1987 Mar;20(3):1043–1056. doi: 10.1016/0306-4522(87)90262-4. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T., Shoji M., Harigaya Y., Yamaguchi H., Hirai S. Expression of APP in the early stage of brain damage. Brain Res. 1991 Nov 1;563(1-2):334–338. doi: 10.1016/0006-8993(91)91558-i. [DOI] [PubMed] [Google Scholar]
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., Ito H. Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature. 1988 Feb 11;331(6156):530–532. doi: 10.1038/331530a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Sisodia S. S., Archer D. R., Martin L. J., Weidemann A., Beyreuther K., Fischer P., Masters C. L., Price D. L. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König G., Mönning U., Czech C., Prior R., Banati R., Schreiter-Gasser U., Bauer J., Masters C. L., Beyreuther K. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J Biol Chem. 1992 May 25;267(15):10804–10809. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Löffler J., Huber G. Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. J Neurochem. 1992 Oct;59(4):1316–1324. doi: 10.1111/j.1471-4159.1992.tb08443.x. [DOI] [PubMed] [Google Scholar]
- Marty S., Dusart I., Peschanski M. Glial changes following an excitotoxic lesion in the CNS--I. Microglia/macrophages. Neuroscience. 1991;45(3):529–539. doi: 10.1016/0306-4522(91)90268-s. [DOI] [PubMed] [Google Scholar]
- Mönning U., König G., Banati R. B., Mechler H., Czech C., Gehrmann J., Schreiter-Gasser U., Masters C. L., Beyreuther K. Alzheimer beta A4-amyloid protein precursor in immunocompetent cells. J Biol Chem. 1992 Nov 25;267(33):23950–23956. [PubMed] [Google Scholar]
- Nordstedt C., Gandy S. E., Alafuzoff I., Caporaso G. L., Iverfeldt K., Grebb J. A., Winblad B., Greengard P. Alzheimer beta/A4 amyloid precursor protein in human brain: aging-associated increases in holoprotein and in a proteolytic fragment. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8910–8914. doi: 10.1073/pnas.88.20.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet C. C., Miller P. E., Hemmings H. C., Jr, Walaas S. I., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984 Jan;4(1):111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gonzalez-DeWhitt P., Schilling J., Miller J., Hsu D., Greenberg B., Davis K., Wallace W., Lieberburg I., Fuller F. A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature. 1988 Feb 11;331(6156):525–527. doi: 10.1038/331525a0. [DOI] [PubMed] [Google Scholar]
- Potempska A., Styles J., Mehta P., Kim K. S., Miller D. L. Purification and tissue level of the beta-amyloid peptide precursor of rat brain. J Biol Chem. 1991 May 5;266(13):8464–8469. [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Podlisny M. B., Joachim C. L., Vickers E. A., Lee G., Fritz L. C., Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shigematsu K., McGeer P. L., Walker D. G., Ishii T., McGeer E. G. Reactive microglia/macrophages phagocytose amyloid precursor protein produced by neurons following neural damage. J Neurosci Res. 1992 Mar;31(3):443–453. doi: 10.1002/jnr.490310306. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Siman R., Card J. P., Nelson R. B., Davis L. G. Expression of beta-amyloid precursor protein in reactive astrocytes following neuronal damage. Neuron. 1989 Sep;3(3):275–285. doi: 10.1016/0896-6273(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Czernik A. J., Kao H. T., Takei K., Johnston P. A., Horiuchi A., Kanazir S. D., Wagner M. A., Perin M. S., De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989 Sep 29;245(4925):1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., McClatchey A. I., Lamperti E. D., Villa-Komaroff L., Gusella J. F., Neve R. L. Protease inhibitor domain encoded by an amyloid protein precursor mRNA associated with Alzheimer's disease. Nature. 1988 Feb 11;331(6156):528–530. doi: 10.1038/331528a0. [DOI] [PubMed] [Google Scholar]
- Walaas I. Biochemical evidence for overlapping neocortical and allocortical glutamate projections to the nucleus accumbens and rostral caudatoputamen in the rat brain. Neuroscience. 1981;6(3):399–405. doi: 10.1016/0306-4522(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Girault J. A., Greengard P. Localization of cyclic GMP-dependent protein kinase in rat basal ganglia neurons. J Mol Neurosci. 1989;1(4):243–250. [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in the rat brain. J Neurosci. 1984 Jan;4(1):84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. C., Bragin V., Robakis N. K., Sambamurti K., VanderPutten D., Merril C. R., Davis K. L., Santucci A. C., Haroutunian V. Increased biosynthesis of Alzheimer amyloid precursor protein in the cerebral cortex of rats with lesions of the nucleus basalis of Meynert. Brain Res Mol Brain Res. 1991 May;10(2):173–178. doi: 10.1016/0169-328x(91)90108-a. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wunderlich D., Lee A., Fracasso R. P., Mierz D. V., Bayney R. M., Ramabhadran T. V. Use of recombinant fusion proteins for generation and rapid characterization of monoclonal antibodies. Application to the Kunitz domain of human beta amyloid precursor protein. J Immunol Methods. 1992 Feb 14;147(1):1–11. doi: 10.1016/s0022-1759(12)80022-6. [DOI] [PubMed] [Google Scholar]